Abstract
The nervous system must dynamically represent sensory information in order for animals to perceive and operate within a complex, changing environment. Receptive field plasticity in the auditory cortex allows cortical networks to organize around salient features of the sensory environment during postnatal development, and then subsequently refine these representations depending on behavioral context later in life. Here we review the major features of auditory cortical receptive field plasticity in young and adult animals, focusing on modifications to frequency tuning of synaptic inputs. Alteration in the patterns of acoustic input, including sensory deprivation and tonal exposure, leads to rapid adjustments of excitatory and inhibitory strengths that collectively determine the suprathreshold tuning curves of cortical neurons. Long-term cortical plasticity also requires co-activation of subcortical neuromodulatory control nuclei such as the cholinergic nucleus basalis, particularly in adults. Regardless of developmental stage, regulation of inhibition seems to be a general mechanism by which changes in sensory experience and neuromodulatory state can remodel cortical receptive fields. We discuss recent findings suggesting that the microdynamics of synaptic receptive field plasticity unfold as a multi-phase set of distinct phenomena, initiated by disrupting the balance between excitation and inhibition, and eventually leading to wide-scale changes to many synapses throughout the cortex. These changes are coordinated to enhance the representations of newly-significant stimuli, possibly for improved signal processing and language learning in humans.
Keywords: auditory cortex, development, excitatory-inhibitory balance, hearing loss, neuromodulation, receptive field, synaptic plasticity
1. Introduction
In the auditory system, neurons are tuned to various acoustic properties and parameters such as sound frequency, intensity, or repetition rate. The receptive fields and tuning preferences of auditory cells to these variables are not necessarily fixed, but can be changed depending on the forms of sensory experience, during neonatal development, throughout adulthood, and after hearing loss or other forms of auditory system pathology. As the entire receptive field of a particular neuron can be high dimensional and difficult or impossible to completely characterize in high detail, here we use shifts in excitatory and inhibitory frequency tuning in the rodent primary auditory cortex (AI) as a model for investigating the general phenomenology, mechanisms, and functional consequences of synaptic receptive field plasticity (i.e., modification of the tuning properties of synaptic inputs onto a sensory neuron).
Most previous studies of cortical receptive field organization and plasticity have relied on extracellular recordings of spike output or local field potentials. However, recent advances in understanding the organization and dynamics of cortical circuits have been obtained using intracellular techniques such as in vivo whole-cell voltage-clamp recording. With this method, it is possible to measure the excitatory and inhibitory currents activated by sensory stimulation, and use these data to compute tuning curves for tone-evoked synaptic conductances. While changes in tuning curves can be registered in terms of suprathreshold spiking activity, much of this plasticity seems to be due to adjustment of the often-subthreshold synaptic inputs that lead to spike generation; thus, examination of synaptic modifications is a natural level for investigation of cortical receptive field plasticity. Additionally, it is becoming increasingly apparent that inhibitory circuits are themselves plastic and strongly govern the modification of excitatory synapses. In many of these cases, inhibition is affected independently of excitation in a complex, dynamic manner. Therefore, in order to build predictive models and improve therapeutic treatments for auditory pathologies such as tinnitus or deafness, intracellular electrophysiological recordings are required to characterize the changes to cortical networks after episodes of learning or injury, as this technique is currently the only available method for directly measuring inhibitory synaptic transmission.
In this review, we first describe the relation between synaptic inputs, spiking output, and the tonotopic organization of AI as a whole. We then discuss studies of changes to AI synapses that occur in response to peripheral damage both in vitro and in vivo. While hearing loss or manipulations of the sensory environment can also affect subcortical processing by upstream stations (Sanes and Constantine-Paton, 1983; Willott, 2005), and thus indirectly change the synaptic drive onto cortical neurons, a growing body of evidence indicates that modifications to cortical circuits are initiated earlier and endure for longer than changes elsewhere within the auditory pathway (Ma and Suga, 2005; Froemke et al., 2007). In the remainder of this review, we focus on developmental and adult synaptic receptive field modifications induced by patterned stimulation and sensory exposure. We hypothesize that, although there are important differences between critical period plasticity and adult plasticity, there may also be a conserved set of basic mechanisms for long-term reorganization of excitatory and inhibitory cortical inputs in the intact brain.
2. Synaptic and spiking AI frequency tuning curves
Much has been learned about the organization and plasticity of cortical networks from extracellular recording of neuronal action potentials. These spiking receptive fields are a complex function of synaptic inputs, intrinsic ion channel activity (especially the activation threshold for Na+ channels), and dendritic processing (Hirsch, 2003; Huberman et al., 2008; Nowak et al., 2010). Each of these components can be regulated and modified in ways that are still being experimentally determined (Losonczy et al., 2008; Feldman, 2009; Dorrn et al., 2010), making it challenging to predict how perturbations in the patterns of sensory experience lead to changes in neural circuitry. However, to a first approximation, the organization of suprathreshold spiking tuning curves is governed by the strengths and kinetics of excitatory and inhibitory synapses, at least in young adult and adult animals (Monier et al., 2003; Wehr and Zador, 2003; Zhang et al., 2003; Dorrn et al., 2010). For this reason, we focus here on the synaptic basis of receptive field plasticity in terms of input strength, although undoubtedly other factors that influence postsynaptic integration- directly or indirectly- also play important roles in shaping the tuning properties of cortical neurons (Häusser and Mel, 2003).
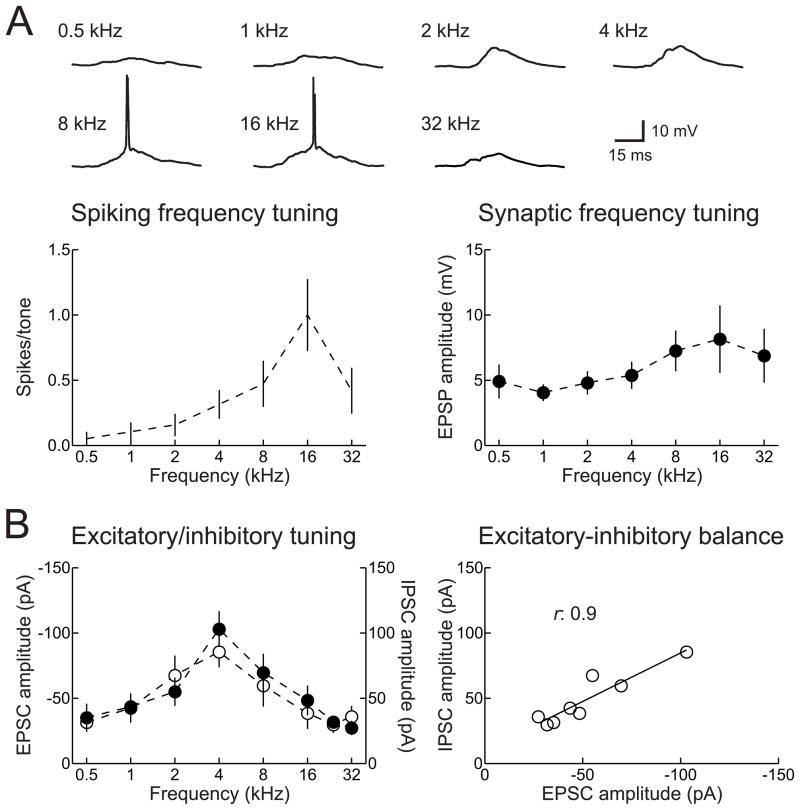
Similar to other stations along the mammalian auditory pathway, AI neurons are generally tuned to sound frequency (Fig. 1A). While many neurons have a clear preference for pure tones of a specific frequency (the ‘best frequency’), the tuning widths, best frequencies, and overall response rates depend strongly on sound level. Neurons in adult rat AI, for example, can exhibit broad sub- and suprathreshold tuning at moderate to high intensities, potentially spanning much of the total cochlear frequency range (Sally and Kelly, 1988; Zhang et al., 2003; Metherate et al., 2005). Regardless of bandwidth, the spiking tuning curve of a neuron (Fig. 1A, left) is necessarily a subset of synaptic tuning (Fig. 1A, right).
Figure 1.
Spiking and synaptic frequency tuning curves of adult rat AI. A, Example current-clamp recording of spikes and excitatory postsynaptic potentials (EPSPs) from an AI neuron. Top, representative tone-evoked responses. Bottom left, spiking tuning curve of this neuron. Bottom right, excitatory synaptic tuning curve of this cell. B, Example voltage-clamp recording of excitatory and inhibitory postsynaptic currents (EPSCs and IPSCs) from a different adult rat AI neuron. Left, synaptic frequency tuning. Right, correlation between peak excitatory and inhibitory responses across tone frequencies.
A major feature of synaptic receptive fields in adult cat, rat, and mouse, is that the relative strengths of excitatory and inhibitory responses to brief pure tones are proportional across tone frequency (Fig. 1B). Importantly, inhibition lags excitation by several milliseconds, allowing excitatory events to evoke one or more spikes with high temporal fidelity before being terminated by a corresponding degree of co-tuned inhibition (Volkov and Galazjuk, 1991; Wehr and Zador, 2003; Tan and Wehr, 2009). This phase delay for inhibition is likely due to the architecture of rodent thalamocortical circuity, in that there are few if any direct inhibitory projections to AI from the medial geniculate body (MGB), the lemniscal auditory thalamus (Winer, 1992). It remains to be determined whether this relationship holds for integration of synaptic inputs during ongoing sounds, especially with relatively shallow modulation rates, although recent compelling data from Zador and colleagues indicate that excitatory and inhibitory conductances seem to be co-regulated over time periods of milliseconds to seconds (Wehr and Zador, 2005; Asari and Zador, 2009).
It is also unclear what specific inputs and cell types contribute to these tone-evoked responses. There is a wide diversity of cortical GABAergic interneurons, with various firing properties, dendritic projection patterns, and dynamics of short-term plasticity (Petilla Interneuron Nomenclature Group, 2008). This suggests that different inhibitory cell types might be recruited by distinct patterns of sensory stimulation, contributing differentially to inhibitory responses as measured locally in the dendrites or globally at the soma. Likewise, it is also unknown to what degree thalamic or intracortical excitatory inputs contribute to net excitation evoked by tones or other stimuli. Cortical injections of muscimol, a GABAA receptor agonist, effectively eliminate intracortical contributions to synaptic receptive fields, sparing thalamocortical input. This method was found to reduce the bandwidth of frequency-intensity receptive fields, but spared characteristic frequency responses (Kaur et al., 2004). Thus thalamic inputs may be relatively sharply tuned, while intracortical excitation broadens tuning curves and contributes preferentially to responses away from best frequency. In contrast, Liu et al. (2007) attempted to isolate thalamic inputs using muscimol in combination with a GABAB receptor antagonist, to prevent reduction of presynaptic transmitter release at thalamocortical afferents (e.g., by activation of GABAB receptors on thalamic presynaptic terminals) while simultaneously reducing intracortical excitation. They found that tuning curve bandwidth was left intact, suggesting that thalamic input largely determines the overall extent of subthreshold frequency tuning curves (although this then leaves the question of the functional contributions of the extensive set of intracortical connections). Regardless of the anatomical basis of synaptic receptive fields, the relative connection strengths of thalamic and intracortical inputs can be changed by various forms of experience, with intracortical synapses seemingly expressing a higher degree of plasticity than thalamic inputs. After deafferentation of the peripheral sensory apparatus, either through whisker trimming (Diamond et al., 1994) or monocular deprivation (Trachtenberg et al., 2000), changes to cortical responses and receptive fields occur first in layers 2/3 and 5 before being detected in thalamorecipient layer 4. Moreover, long-term modifications to tone-evoked synaptic responses in adult rat AI can be induced by pairing sensory stimulation with neuromodulatory release; this procedure affects intracortical but not thalamcortical inputs onto cortical neurons (Froemke et al., 2007).
3. Sensory deprivation modifies AI synapses
Experience-dependent changes to AI synaptic circuitry have principally been studied in two main ways: after sensory deprivation and in response to patterned stimulation. In each case, cortical synapses are most susceptible to manipulations or loss of sensory input during developmental critical periods (Katz and Shatz, 1996; Buonomano and Merzenich, 1998; Hensch 2005). Auditory cortical critical periods usually last for a few days or weeks, beginning with hearing onset, and can occur at distinct ages for different receptive field properties or across cortical sectors (Chang et al., 2005; de Villers-Sidani et al., 2007; Razak and Fuzessery, 2007; de Villers-Sidani et al., 2008; Insanally et al., 2009; Sanes and Bao, 2009; Popescu and Polley, 2010). In humans, deafness during this time, even if ameliorated with devices such as hearing aids or cochlear implants later in life, can lead to profound impairments in speech and language comprehension (Eisenberg, 2007; Zeitler et al., 2008).
Developmental hearing loss, either sensorineural or conductive, has a number of effects on synaptic transmission throughout the auditory system (Takesian et al., 2009). Studies in brain slices have revealed that auditory neurons become more excitable after bilateral hearing loss (Fig. 2A). For example, when the cochlea is surgically ablated during early postnatal development, the mean strengths of excitatory synapses in the gerbil auditory midbrain and cortex are increased (Vale and Sanes, 2002; Kotak et al., 2005). Conversely, the amplitudes of inhibitory events are decreased in both cortex and inferior colliculus after deafening (Takesian et al., 2009), possibly due to a loss of presynaptic GABAergic terminals and a reduction in the number of postsynaptic GABA receptors (Sarro et al., 2008). These synergistic changes in excitation and inhibition after loss of afferent input presumably increase the overall excitability along the central auditory pathway, in a manner reminiscent of the synaptic adjustments described in the visual cortex after neonatal monocular deprivation (Maffei et al., 2004). Such plasticity might also account for the lower activation and perceptual thresholds for cochlear implant use in deafened animals (Snyder et al., 1990; Raggio and Schreiner, 1999), and could be related to the etiology of tinnitus after noise exposure (Eggermont et al., 2004).
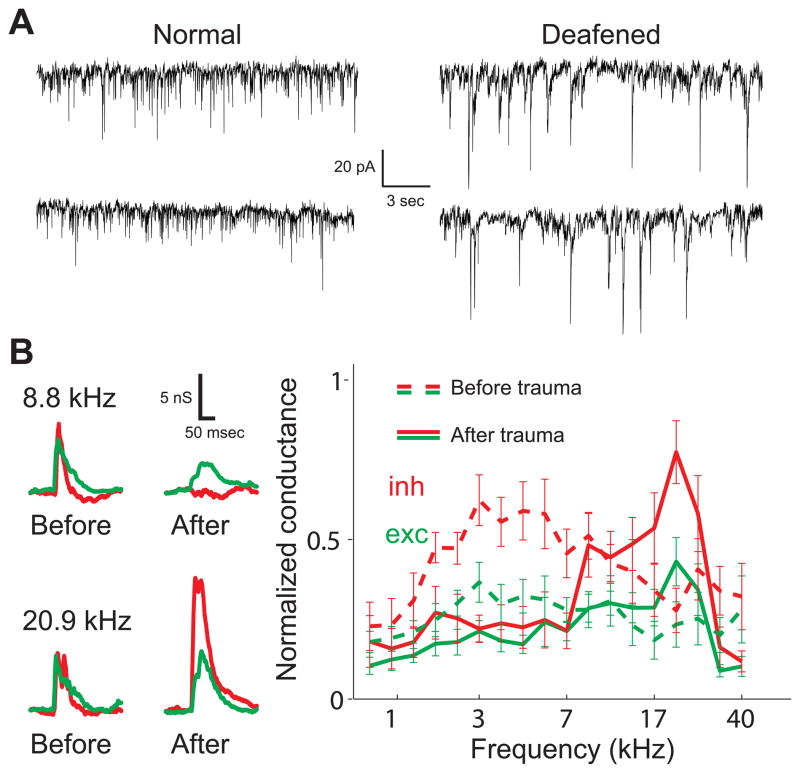
Figure 2.
Peripheral injury modifies AI synaptic strength. A, Spontaneous excitatory currents recorded from neurons in gerbil AI in vitro. Left, spontaneous activity in a normal-hearing animal. Right, spontaneous activity from an animal suffering from sensorineural hearing loss, induced by bilateral cochlear ablation at P10. Cortical excitatory events are larger on average. Adapted from Kotak et al. (2005). B, Brief acoustic trauma with 10 minutes of high-intensity tonal exposure induces synaptic modifications across frequency tuning curves in vivo. Left, Tone-evoked synaptic conductances recorded in young adult rat AI before and after acoustic trauma with 20 kHz tonal presentation. Excitatory (green) and inhibitory (red) responses at 8.8 kHz are reduced after trauma, while inhibitory responses evoked by 20.9 kHz tones are enhanced. Right, average frequency tuning before (dashed lines) and after (solid lines) acoustic trauma for 12 neurons. Adapted from Scholl and Wehr (2008).
The effects of hearing loss have also been studied on shorter time scales. A technically-impressive recent study characterized the changes to excitatory and inhibitory inputs in young adult rat AI in vivo immediately after a brief episode of high intensity noise exposure (Scholl and Wehr, 2008). Ten minutes of acoustic trauma with continuous tonal exposure at 110–120 dB sound pressure level (SPL) led to an increase in thresholds of auditory brainstem responses by almost 50 dB, indicating that significant hearing loss had been induced by this procedure. This induced a set of long-lasting synaptic modifications distributed across frequency tuning curves, as measured with whole-cell voltage-clamp recordings from AI neurons in anesthetized animals. Changes to tone-evoked excitatory and inhibitory synaptic strengths were rapidly expressed (within a few minutes) and endured for the duration of the recordings. These synaptic adjustments led to shifts in the best frequencies of excitation and inhibition, a disruption of excitatory-inhibitory balance (Fig. 2B), and prolonged the time course of membrane potential responses. As a consequence, synaptic frequency tuning curves became broader and the temporal precision of AI responses was degraded.
Although these changes were complex, Scholl and Wehr (2008) were able to identify several consistent features of synaptic receptive field modification induced by acoustic trauma. First, relative to the frequency of the traumatic tone, inhibitory responses evoked by lower frequency tones (2–3 octaves away) were reduced. However, within an octave or so of the traumatic tone frequency, inhibitory responses were greatly enhanced both for higher and lower frequency stimuli. Second, excitatory responses were only modestly reduced, or sometimes slightly enhanced in peri-traumatic regions where inhibition was increased. Finally, although spontaneous firing rates were unaffected, previous subthreshold inputs could become suprathreshold, leading to a small but significant shift in best frequency by ~1/3 octave and an overall broader spiking receptive field.
These changes in AI synaptic and spiking receptive fields are similar to the effects of deafferentation in the visual (Gilbert and Wiesel, 1992; Eysel et al., 1999) and somatosensory systems (Calford and Tweedale, 1988; He et al., 2004), in that peripheral damage leads almost immediately to network-wide reorganization of cortical receptive fields synaptic circuitry driven largely by disinhibition. It remains to be determined to what degree these changes are pathological or compensatory. Furthermore, it is unclear whether trauma- and deprivation-induced changes in cortical responses reflect modification of intracortical synapses themselves or are predominantly inherited from alterations of subcortical stations. However, the uncoupling between excitatory and inhibitory synaptic strength in vivo and in vitro indicates that, at least in part, some of these changes occur directly within the cortex.
4. Developmental plasticity of AI maps and receptive fields
In rodent AI, adult representations of sound frequency and intensity profoundly depend on the properties of the acoustic environment in early postnatal life. The critical period for AI frequency tuning begins immediately at hearing onset, but the duration and offset seem to be controlled by the patterns of sensory experience. Days of repetitive stimulation with pure tones of a given frequency or within a frequency range leads to an enlarged representation of those presented frequencies within characteristic frequency maps of young rats, but only when exposure occurs during the second postnatal week (de Villers-Sidani et al., 2007; Insanally et al., 2009). Conversely, unmodulated stimuli such as continual white noise (Chang and Merzenich 2003) or continual pure tones (Zhou et al., 2008) prevent tonotopic organization from emerging in AI, apparently keeping the cortex in an unrefined yet still plastic state.
Developmental changes of cortical frequency tuning at the spiking level are paralleled by modifications of the underlying excitatory and inhibitory synaptic receptive fields. In rodent AI, synaptic maturation occurs between postnatal day (P) 12 to P21 (Oswald and Reyes, 2008; Dorrn et al., 2010). Excitatory inputs are tuned for sound frequency by approximately P14 (Fig. 3A), likely as a consequence of activity-dependent but experience-independent pre-patterning that occurs before the rodent auditory system becomes functional (Froemke and Jones, 2010). Inhibitory inputs are initially present but untuned at hearing onset, gradually becoming tuned and proportional in strength to excitation across the frequency range of rodent hearing (Dorrn et al., 2010). In this way, developmental sensory experience throughout the same period (P12–P21) leads to calibration of synaptic circuitry and formation of excitatory-inhibitory balance (Fig. 3B,C). As a consequence, the spiking output of individual AI cells is initially unreliable, imprecise, and at longer latency than in adults (Dorrn et al., 2010; Yuan et al., 2010), leading to irregular or disorganized AI spiking receptive fields and characteristic frequency maps in neonatal rats (de Villers-Sidani et al., 2007). As cortical inhibitory circuits become shaped by activity and experience, spike timing precision and receptive field structure substantially increase. It is important to note that the overall amplitude of tone-evoked inhibitory responses in rat AI has been found to be approximately the same in young and older animals (Dorrn et al., 2010; Sun et al., 2010), in contrast to the gradual strengthening of inhibition that seems to occur in the rodent visual cortex (Hensch, 2005).
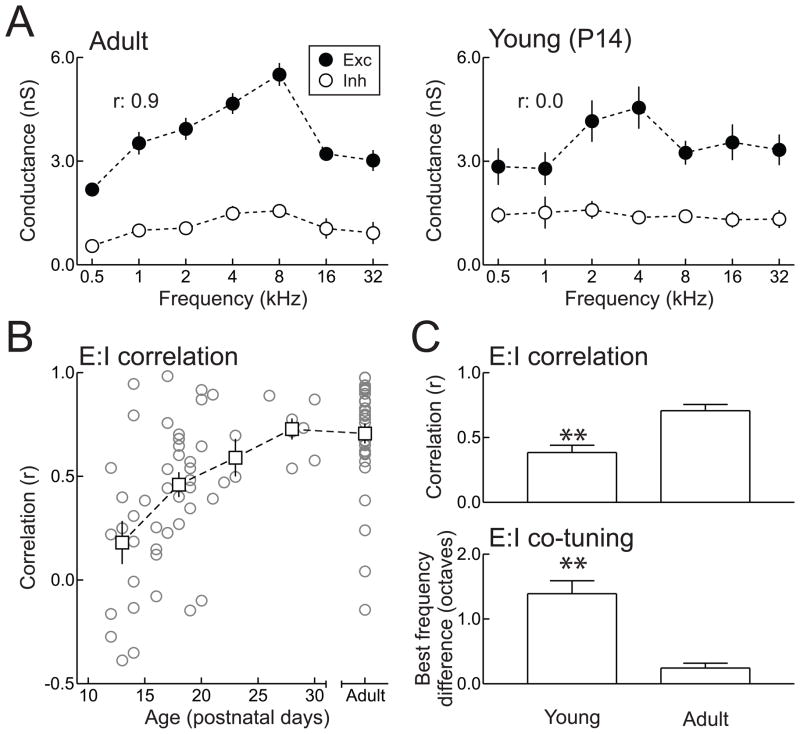
Figure 3.
Synaptic frequency tuning in adult and developing rat AI. A, Example in vivo whole-cell recordings from neurons in adult (left) and P14 (right) animals. Tone-evoked excitation and inhibition are balanced in adult AI (r: 0.9; left) but imbalanced in neonatal in AI due to untuned inhibition (r: 0.0; right). B, Increase of excitatory-inhibitory balance during the AI critical period. At the end of the second postnatal week, excitation and inhibition were uncorrelated. By the end of the third week, the correlation improved, and by the end of the first month, the correlation was similar to that measured in adult animals. C, Summary of changes to excitatory-inhibitory balance during development. Top, mean correlation between excitation and inhibition in young (P12–21) and adult animals. Bottom, mean difference in excitatory and inhibitory best frequencies in young and adult animals. Adapted from Dorrn et al. (2010).
A fraction of neurons in young AI appear to have highly tuned and balanced excitatory and inhibitory synaptic receptive fields, equivalent to that observed in adult AI (Dorrn et al., 2010; Sun et al., 2010). It is currently unknown why some cells in young rat AI display unusually high correlation and co-tuning between excitatory and inhibitory frequency tuning. However, existing data suggest a few possibilities. For one, Sun et al. (2010) show that at threshold, excitatory and inhibitory tuning are mismatched by approximately one octave. However, while thresholds of AI neurons are higher during development than in adults, these thresholds are still considerably low (~30–45 dB SPL) throughout P12–P14, and at adult levels of ~ 20 dB SPL thereafter (de Villers-Sidani et al., 2007); these thresholds are far from the sound intensities of 70 dB SPL used in Dorrn et al. (2010) to assess excitatory-inhibitory balance in young AI.
A more likely explanation is that there are particular spatial regions of AI in which excitation and inhibition become precociously co-tuned. These cells may be localized specifically within cortical layer 4, which is specifically where Sun et al. (2010) concentrated their recordings, suggesting that development of cortical synaptic receptive fields might be heterochronic. In such a model, thalamorecipient neurons and cell assemblies would mature first, before inhibitory inputs of downstream cells and networks successively become co-tuned to match the statistics of excitatory inputs. Alternatively, or in addition, it is possible that specific subregions of AI are pre-balanced by hearing onset, and that over the auditory cortical critical period, surrounding sectors are progressively integrated into AI. This may be analogous to (or even account for) the experimental reports that the electrophysiologically-defined AI tonotopic map begins to form around a central mid-to-high-frequency sector in P11 rats, and expands in size to include outlying regions during the second and third weeks of postnatal development (Zhang et al., 2001; de Villers-Sidani et al., 2007), despite the observation that thalamic innervation of overall AI is likely complete before hearing onset (Lund and Mustari, 1977). It is plausible that in these earliest well-tuned regions of the AI map, average excitatory-inhibitory balance is at mature levels, while in surrounding, poorly-tuned regions, excitatory-inhibitory balance is much lower.
Regardless of the specific receptive field properties of individual cells in developing AI, over a substantial population of recordings, excitatory and inhibitory frequency tuning is uncorrelated on average between P12–P16. Excitation and inhibition progressively become balanced until reaching mature levels around P25–P30 (Fig. 3B). However, even in adult AI, excitatory-inhibitory balance is a statistical property of the cortex, with some cells having uncorrelated or anti-correlated synaptic frequency tuning (Fig. 3B). This is similar to results from the visual cortex, where in vivo intracellular recording studies have revealed untuned or cross-tuned inhibitory inputs (Ferster, 1986; Douglas et al., 1991; Pei et al., 1991; Schummers et al., 2002; Monier et al., 2003; Sohya et al, 2007). Finally, we predict that in older animals, excitatory-inhibitory balance breaks down again, decreasing the temporal precision and spectral selectivity of AI neurons in the aged brain (Turner et al., 2005). However, rather than resulting from strong but untuned inhibitory circuitry (as in developing AI), this may instead be due to weakening of cortical inhibition and loss of GABAergic cells, including parvalbumin-positive interneurons (Caspary et al., 2008; de Villers-Sidani et al., 2010).
The maturation of inhibitory frequency tuning and excitatory-inhibitory balance in AI can be accelerated by certain types of developmental auditory experience (Dorrn et al., 2010). A few minutes of patterned stimulation with repetitive, pulsed pure tones of a given frequency modifies excitatory and inhibitory synapses, such that synaptic tuning curves rapidly shift towards the presented tone frequency (Fig. 4A). This form of receptive field plasticity is spectrally and temporally complex, seemingly involving coordinated changes orchestrated across multiple inputs over minutes to hours. First, excitatory and inhibitory responses evoked by tones of the presented frequency are potentiated (Fig. 4B, top). Second, enhancement at these inputs seems to spread to spectrally-proximal inputs within one octave of the presented frequency (Fig. 4B, top). Finally, responses to the original best frequencies of excitation and inhibition are suppressed (Fig. 4B, bottom). Collectively, these long-term synaptic modifications substantially increase the correlation between excitatory and inhibitory frequency tuning curves. Although induced by a few minutes of patterned tonal stimulation, synaptic modifications and enhanced excitatory-inhibitory balance are maintained for over an hour (Fig. 4C). Moreover, these changes can persist for much longer if additional patterned stimulation is provided, and prevent future episodes of patterned stimulation from inducing other changes to synaptic receptive fields (i.e., effectively bring the critical period for AI frequency tuning to a close). Importantly, the changes to inputs other than that presented during patterned stimulation were predominantly responsible for the increase to excitatory-inhibitory balance (Dorrn et al., 2010).
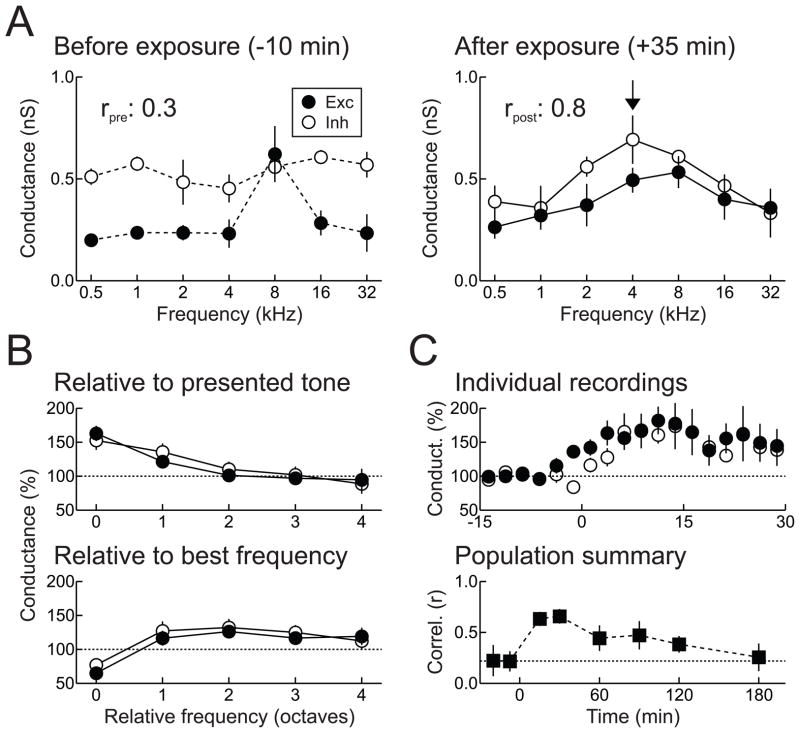
Figure 4.
Developmental sensory experience increases excitatory-inhibitory balance. A, In vivo whole-cell voltage-clamp recording from a young (P18) AI neuron. Left, before patterned stimulation, excitatory-inhibitory correlation is low (rpre: 0.27). Right, synaptic frequency tuning in the same cell ~35 minutes after patterned stimulation with 4 kHz tones (arrow). Excitation and inhibition are both potentiated at 4 kHz and the original best frequencies are depressed, increasing excitatory-inhibitory correlation (rpost: 0.82). B, Patterned stimulation induces long-term synaptic modifications across multiple inputs. Top, relative to the presented tone, significant enhancements of excitation and inhibition are also induced one octave away. Bottom, patterned stimulation also specifically depresses responses at the original best frequencies. C, Time course of synaptic receptive field modifications induced by patterned tonal stimulation. Top, changes in excitation and inhibition in 12 individual whole-cell recordings. Bottom, change in correlation coefficients measured over multiple cells (56 time points from 26 neurons in 15 animals) after a single episode of patterned stimulation. Adapted from Dorrn et al., 2010.
These synaptic changes induced by patterned stimulation seem somewhat similar to the set of modifications described by Scholl and Wehr (2008) after acoustic trauma, although the effects on spike generation are qualitatively different. Acoustic trauma was found to delay membrane potential responses, while patterned stimulation accelerates responses and improves spike timing precision at the presented frequency (Dorrn et al., 2010). Other types of disruptive or noxious stimuli also impair the development of cortical excitatory-inhibitory balance, including white noise stimulation (Chang et al., 2005; Dorrn et al., 2010) and perinatal exposure to environmental toxins (Kenet et al., 2007). In contrast to patterned tonal stimulation, continual or pulsed white noise stimulation for an equivalent exposure period did not improve the correlation between tone-evoked excitation and inhibition (Dorrn et al., 2010). Other forms of neonatal sensory exposure with different statistics have not yet been investigated at the synaptic level.
It is unclear what mechanisms contribute to the formation of cortical synaptic receptive fields and excitatory-inhibitory balance. In the young rat visual cortex, pairing visual stimulation with postsynaptic spiking strengthened sensory-evoked excitatory responses, in a manner that depended both on the pairing interval and postsynaptic Ca2+ influx (Meliza and Dan, 2006). This suggests that spike-timing-dependent synaptic modifications, similar to those characterized in vitro throughout the cortex and elsewhere in the central nervous system (Markram et al., 1997; Froemke and Dan, 2002; Tzounopoulos et al., 2004; Feldman, 2009), are induced by repetitive patterned stimulation in developing AI. However, given that spike timing is imprecise in young AI, synaptic plasticity induced by patterned stimulation may instead depend on local ‘hotspots’ of excitability where the excitation-inhibition ratio is particularly high. In each case, though, we predict that whenever sensory stimuli are paired with strong postsynaptic depolarization, NMDA receptors are activated, leading to increases in intracellular Ca2+ and subsequent long-term changes in synaptic strength.
Thus early in life, environmental factors including the patterns of acoustic experience control the strengths of excitatory and inhibitory synapses, which in turn govern the organization of receptive fields, the output of cortical circuitry, and the perception of auditory stimuli. The perceptual consequences of changes to sensory representations are not always obvious, and requires more investigation by studies combining auditory exposure, behavioral training, and in vivo electrophysiology. In an important study, it was shown that young rats exposed to several days of patterned stimulation had enlarged cortical representations around the presented frequency, but these animals were impaired in perceptual discrimination of this over-represented frequency. By contrast, discrimination of nearby under-represented frequencies was substantially improved (Han et al, 2007). It remains to be determined how other forms of developmental experience affect sensory perception, and how the shapes and relative structures of tuning profiles are related to detection and discriminative abilities.
5. Neuromodulation and synaptic receptive field plasticity in adult AI
After the end of the critical period, passive sensory stimulation is generally insufficient for long-term synaptic modifications and persistent changes in the organization of AI receptive fields. Instead, adult cortical plasticity seems to depend more strongly on stimulus history and internal state variables such as arousal level and motivation. This behavioral context is often conveyed by activation of subcortical modulatory systems that directly project to AI, e.g., the cholinergic nucleus basalis (Rasmusson, 2000; Weinberger, 2007) or the noradrenergic locus coeruleus (Edeline et al., 2010). Whereas neuromodulation can influence developmental plasticity as well as adult plasticity (Bear and Singer, 1986; Gu, 2002; Giocomo and Hasselmo, 2007; Seol et al., 2007), the modes of modulatory release may be fundamentally different in neonatal and mature brains. Specifically, the cortical modulatory milieu may be permissive for plasticity in the young cortex (perhaps due to tonic activation of modulatory centers), but in the adult brain, modulation occurs more phasically, in a manner that is tightly linked to changes in behavioral context or internal drives. This hypothesis is supported by the observations that passive exposure to patterned stimulation requires co-activation of neuromodulatory centers, like the cholinergic basal forebrain, to induce receptive field modifications in adult cortex (Bakin and Weinberger, 1996; Kilgard and Merzenich, 1998; Froemke et al., 2007), but patterned stimulation alone is sufficient for cortical receptive field plasticity in young animals (de Villers-Sidani et al., 2007; Insanally et al., 2009; Dorrn et al., 2010). Regardless, antagonists of neuromodulator receptors or lesions of modulatory nuclei have long been known to block developmental forms of cortical plasticity such as ocular dominance plasticity in the cat visual cortex (Bear and Singer, 1986), and neuromodulator antagonists also prevent induction of some forms of long-term synaptic plasticity in cortical slices (Choi et al., 2005).
Acetylcholine plays a central role in arousal, selective attention, and modulation of cortical responses (Mesulam, 1998; Weinberger, 1998; Yan and Zhang, 2005; Disney et al., 2007; Froemke et al., 2007; Parikh et al., 2007; Herrero et al., 2008; Silver et al., 2008; Goard and Dan 2009). Cholinergic modulation has a wide range of effects on cortical neurons, but a consistent observation is increased excitability (Woody and Gruen, 1987) and suppression of intracortical synaptic transmission (Xiang et al., 1998; Metherate et al., 2005; Sarter and Parikh, 2005), including release of GABA from cortical interneurons (Kruglikov and Rudy, 2008). Extracellular recording studies in vivo have shown that pairing pure tones of a specific frequency with electrical stimulation of nucleus basalis induces large, long-lasting enhancements of spontaneous and tone-evoked spiking (Bakin and Weinberger, 1996; Rasmusson and Dykes, 1988; Kilgard and Merzenich, 1998). Although electrical stimulation of nucleus basalis should activate a heterogeneous population of projection neurons, including those that release acetylcholine, glutamate, GABA, and various peptides (Henny and Jones, 2008; Lin and Nicolelis, 2008), pharmacological evidence indicates that cortical muscarinic acetylcholine receptors are specifically required for the long-term effects on AI receptive fields of this pairing procedure (Bakin and Weinberger, 1996; Froemke et al., 2007).
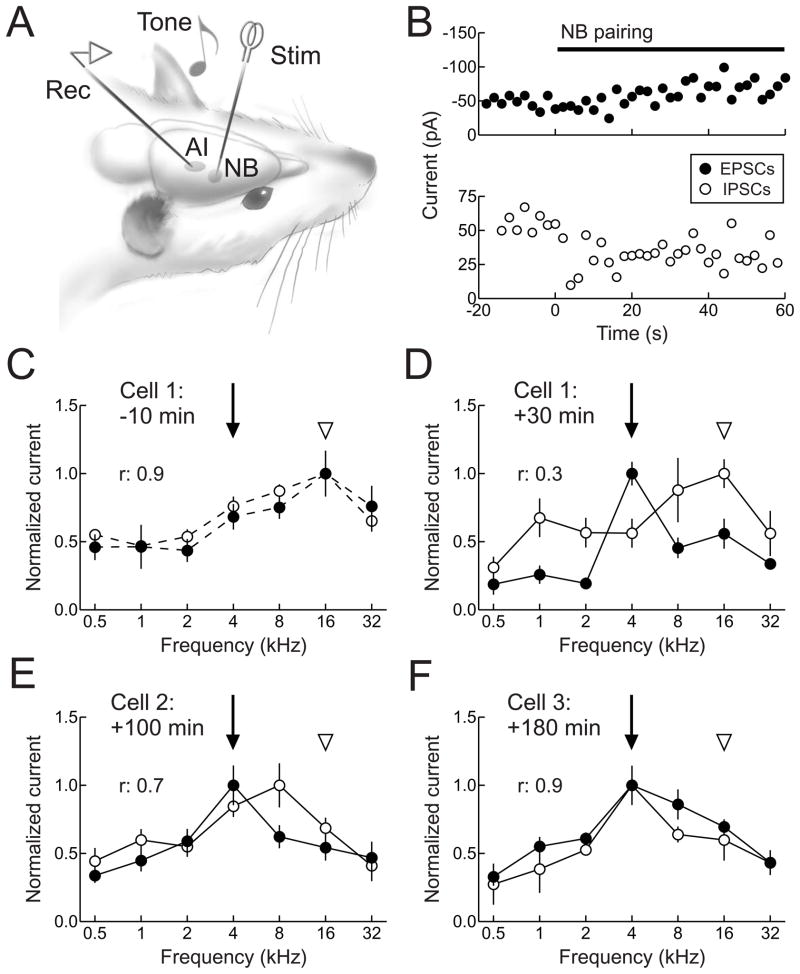
Intracellular recordings in vivo have revealed the mechanisms by which stimulation of the nucleus basalis neuromodulatory system activates cortical networks (Metherate et al., 1992; Metherate and Ashe, 1993) and enables receptive field plasticity (Froemke et al., 2007). In these latter experiments, in vivo whole-cell voltage-clamp recordings were obtained from neurons in anesthetized adult rat AI (Fig. 5A), and excitatory and inhibitory synaptic frequency tuning profiles were initially measured (Fig. 5B,C). Afterwards, tones of a specific non-preferred frequency were paired with electrical stimulation of nucleus basalis. Several seconds after the start of pairing, there was a large suppression of inhibitory events evoked by the paired tone, followed by a more gradual enhancement of tone-evoked excitation (Fig. 5B,D). These changes were long-lasting, persisting at least 20 minutes or more after the end of the pairing procedure (Fig. 5D–F). While nucleus basalis stimulation has immediate effects on both thalamocortical and intracortical transmission, longer-term synaptic modifications appear to be specific to intracortical connections and not to the primary thalamic input to AI (Metherate and Ashe, 1993; Froemke et al., 2007).
Figure 5.
Synaptic receptive field plasticity in adult rat AI. A, Experimental setup. A stimulation electrode was acutely implanted in nucleus basalis and whole-cell recordings were obtained from AI neurons. Synaptic responses to pure tones were recorded in voltage-clamp. B, Rapid changes to tone-evoked inhibition (open circles) and excitation (filled circles) during nucleus basalis stimulation. Top, Example of suppression of inhibition during pairing. Solid line, duration of nucleus basalis stimulation. Bottom, Example of enhancement of excitation during pairing. C, Synaptic frequency tuning of excitation and inhibition for the first cell 10 minutes prior to pairing 4 kHz tones (arrow) with nucleus basalis stimulation. Note the initial balance (r: 0.9) and co-tuning of excitation and inhibition (original best frequencies of both are 16 kHz, arrowhead). D, Frequency tuning of the same cell in B, recorded 30 minutes after NB pairing. Excitability at 4 kHz was increased due to the enhancement of excitation and suppression of inhibition after pairing, while overall excitatory-inhibitory balance was reduced (r: 0.3). E, A second cell from the same region of AI, recorded 100 minutes after pairing. 4 kHz remained the best frequency for excitation, while 8 kHz became the best frequency of inhibition, leading to an improvement in excitatory-inhibitory balance (r: 0.7). F, A third cell from same region of AI, recorded 180 minutes after pairing. The paired frequency was the best frequency for both excitation and inhibition, and the excitatory-inhibitory balance was restored (r: 0.9). Adapted from Froemke et al. (2007).
Due to the cooperative effects of suppression of inhibition and enhancement of excitation, nucleus basalis pairing disrupted excitatory-inhibitory balance in adult AI (Fig. 5B–E). Over a longer time period of several hours, however, synaptic modifications continually evolved, with inhibition progressively increasing to a higher level than before, eventually re-balancing the persistent increase of excitation at the paired frequency (Fig. 5F). These results indicate that the dynamics of inhibitory transmission could serve as a cortical memory trace of the relatively brief pairing episode (Froemke et al., 2007). The duration of input-selective disinhibition may permit self-reorganization of AI receptive fields, emphasizing the new preference for paired stimuli in a manner independent of further evoked neuromodulator release. Under natural conditions, this memory trace could represent sensory objects or events that have acquired new behavioral meaning, or might be similar to the sorts of cortical changes that occur during perceptual learning, especially for those tasks requiring focal attention and sensory discrimination. In this way, neuromodulatory systems allow cortical networks to selectively respond to important or novel stimuli, and appropriately update internal models of the external world.
Transient, focal suppression of inhibition may be a general mechanism for induction of receptive field modification in the adult cortex. During developmental critical periods, the high level of plasticity may be due to a less-refined inhibitory tone or imbalance between excitation and inhibition (Hensch 2004; Chang et al., 2005; Dorrn et al., 2010), permissive for alterations of cortical networks by passive stimuli. In adult cortex, however, receptive field plasticity also requires activation of neuromodulator systems, reflecting the importance of behavioral context in associative learning and memory provided by subcortical systems (Weinberger, 2007). This is further demonstrated by a series of studies from Fritz and colleagues (Fritz et al., 2003; Fritz et al., 2005), using single-unit recordings in AI of head-restrained behaving ferrets. Receptive fields of AI neurons were powerfully modified after behavioral conditioning. Excitatory and suppressive subregions of spectrotemporal receptive fields evoked by specific stimuli were altered when those stimuli were followed by tail-shock. The predominant changes to spectrotemporal receptive fields were increases of excitatory regions and reductions of suppressive regions around the conditioned tone (Fritz et al., 2003), strikingly similar to the synaptic effects of nucleus basalis pairing in anesthetized adult rats (Froemke et al., 2007). Future work will be required to determine if developmental forms of synaptic receptive field plasticity also depend in some way on neuromodulatory systems.
6. A mechanistic model for long-term cortical synaptic receptive field plasticity
These recent data from in vivo intracellular recordings help connect previous extracellular experiments of receptive field plasticity to a large in vitro literature on the mechanisms of long-term synaptic plasticity. Taken together, these studies suggest a general model of plasticity by which changes to sensory experience affect and remodel cortical circuits. In particular, we hypothesize that there are three core phases to the microdynamics of cortical synaptic receptive field plasticity: an initial disinhibition and stimulus-selective enhancement of excitation, followed by network-wide reorganization of excitatory inputs, concluded by a protracted period of inhibitory plasticity in order to balance (or rebalance) excitation and inhibition (Fig. 6). We find evidence of this progression from recordings made in rat AI following noise-induced hearing loss (Scholl and Wehr, 2008), after pairing a tone with electrical stimulation of the cholinergic basal forebrain (Froemke et al., 2007), and in response to passive patterned stimulation early in life (Dorrn et al., 2010). We emphasize that many of the details of this model remain to be determined, but it may prove useful as a framework or working hypothesis for the design of future experiments.
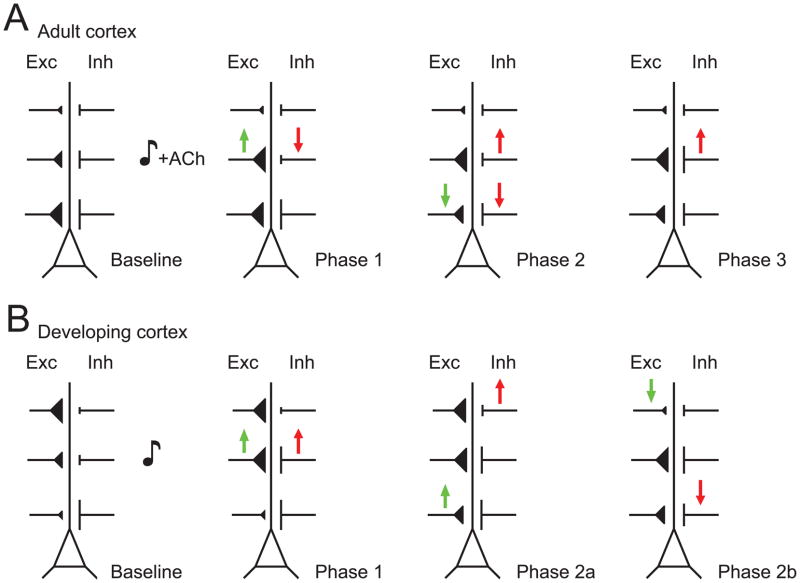
Figure 6.
Spatiotemporal dynamics of synaptic receptive field plasticity in rat AI neurons. A, Progression of synaptic modifications in adult rat AI after pairing electrical basal forebrain stimulation with tonal presentation. Initially, the strength of excitatory (‘Exc’) and inhibitory (‘Inh’) events are correlated across frequencies (‘Baseline’). During pairing, inhibition at the paired frequency is reduced in a manner that depends on cortical acetylcholine receptors, while NMDA receptor-dependent LTP is induced at excitatory inputs evoked by the paired frequency (‘Phase 1’). Green arrows represent changes in excitatory strength, while red arrows represent changes in inhibitory strength; size and dendritic locations of synaptic inputs are simply illustrative. Approximately thirty minutes after pairing, excitation and inhibition at the original best frequency are depressed, while inhibition at the paired input begins to recover (‘Phase 2’). Finally, inhibition at the paired frequency increases to a new, higher level to re-balance enhanced excitation (‘Phase 3’). B, Synaptic modifications in P12–P21 rats after patterned tonal stimulation. Before patterned stimulation, excitation and inhibition are imbalanced (‘Baseline’). At the presented input, both excitation and inhibition are enhanced for minutes to hours (‘Phase 1’). Potentiation seems to spread to inputs activated within one octave of the presented tone frequency (‘Phase 2a’), while the strengths of excitation and inhibition at their respective best frequencies is reduced (‘Phase 2b’).
In this scheme, the initial factor that controls the induction of cortical plasticity is the mechanism of disinhibition. Inhibition has long been known to limit the induction of excitatory synaptic plasticity by preventing activation of NMDA receptors, subsequent postsynaptic Ca2+ influx, and the biochemical signal transduction pathways that lead to expression and consolidation of long-term synaptic modifications (Feldman, 2009). Artola et al. (1990) used different concentrations of the GABAA receptor antagonist bicuculline to demonstrated that, in slices of rat visual cortex, 50 Hz tetanic stimulation did not induce long-term changes in synaptic strength when inhibitory circuitry was intact, but induced long-term depression (LTD) for a low concentration of bicuculline and long-term potentiation (LTP) for a higher concentration. Thus the relative level of inhibitory control in cortical circuits can control the sign, magnitude, and presence or absence of synaptic modifications. However, synaptic receptive field plasticity is a complex process with several aspects beyond LTP of various excitatory synapses. What phenomena have disinhibitory effects in local cortical circuits to initiate synaptic modifications, and what other changes occur within cortical networks as a consequence?
In the adult auditory cortex, delayed and balanced inhibition allows tone-evoked excitation, dominated by AMPA receptor activation, to produce a transient spiking response (Wehr and Zador, 2003), but brief enough to prevent effective depolarization of NMDA receptors. This organization- perhaps the normative processing mode of adult cortex- allows sensory information to be rapidly computed without necessarily modifying the existing circuitry. Nevertheless, there may be many ways to effectively disinhibit adult cortical networks and/or activate NMDA receptors to induce LTP. Several different neuromodulators, including acetylcholine, seem to reduce GABAergic transmission in cortex (Froemke et al., 2007; Kruglikov and Rudy, 2008). We postulate that this neuromodulator-based disinhibition allows for remodeling of synaptic receptive fields, when paired with specific sensory stimuli (Fig. 6A, ‘Phase 1’). In some cases though, cholinergic activity or neuromodulation in general may not be required for adult cortical receptive field plasticity (Ramanathan et al., 2009). For example, repetitive pairing of a non-preferred stimulus with a preferred stimulus effectively shifts AI frequency tuning curves in anesthetized and awake ferrets, in a manner that depends on the precise timing between the two tones (Dahmen et al., 2008), analogously to spike-timing-dependent plasticity (Froemke et al., 2006; Meliza and Dan, 2006; Feldman 2009). Here, the second of the two stimuli during the pairing procedure might overcome an inhibitory threshold to provide sufficient depolarization for NMDA receptor activation. Alternatively, certain forms of repetitive stimulation might selectively reduce inhibitory transmission relative to excitation if the dynamics of short-term excitatory and inhibitory plasticity are distinct.
During development, however, the average total strengths of excitation and inhibition are approximately balanced overall (Sun et al., 2010), but are locally imbalanced. This suggests that there may be particular ‘hotspots’ in neonatal AI naturally sensitive to repetitive, patterned stimulation with pure tones (Dorrn et al., 2010), and these locations within the cortical network will be the first sites of modification during patterned tonal stimulation (Fig. 6B, ‘Phase 1’). In particular, if inhibition is globally balanced with excitation, but locally imbalanced, this would lead to particular spatial locations within AI in which the excitatory-inhibitory ratio is unusually high. A slight change in the amount of excitatory input, as during patterned stimulation, might then be sufficient to strongly depolarize these cells, gate NMDA receptors, and set in motion the mechanisms of long-term synaptic modification. Neuromodulation may be important for developmental plasticity, as lesions of the cholinergic and noradrenergic system together prevent ocular dominance shifts after monocular deprivation (Bear and Singer, 1986), and neuromodulator agonists can gate spike-timing-dependent plasticity in slices of rodent visual cortex (Seol et al., 2007). Changes to the inputs or firing patterns of modulatory centers might be an important step in closing developmental critical periods, along with expression of molecules such as the cholinergic prototoxin Lynx1 that act as regulatory elements over adult cortical plasticity (Morishita et al., 2010).
During both adult and developmental forms of synaptic receptive field plasticity in rat AI, excitatory synapses tuned to the repetitively-presented stimulus are enhanced within approximately one minute (Froemke et al., 2007; Dorrn et al., 2010). This form of LTP enabled by cholinergic modulation is highly stimulus-specific in adult AI, but is less selective in young AI; nearby stimuli within one octave are also enhanced after patterned stimulation (Dorrn et al., 2010). There is precedent for spreading of LTP to neighboring synapses within tens of microns (Fig. 6B, ‘Phase 2a’), possibly by release of extracellular or intracellular messengers (Engert and Bonhoeffer, 1997; Harvey and Svoboda, 2007). Another possibility is that in the developing nervous system, there is intrinsically less stimulus specificity in the set of afferents activated by patterned stimulation, such that inputs within an octave of the presented stimulus are also reliably engaged by repetitive tonal exposure.
Changes to the presented input alone may not be sufficient to allow cortical networks to differentially respond to new or updated sensory information. Therefore, modifications to other inputs may also be important to take advantage of changes to cortical representations. One consistent observation in studies of receptive field plasticity is a reduction in the responses to the original best stimuli (Bakin and Weinberger, 1996; Froemke et al., 2007; Dahmen et al., 2008; Scholl and Wehr, 2008; Dorrn et al., 2010). We have found that, regardless of position within the tuning curve, these initially-preferred inputs are selectively depressed over tens of minutes, both in adult (Fig. 6A, ‘Phase 2’) and developing rat AI (Fig. 6B, ‘Phase 2b’), and this process is considerably slower than the expression of LTP at the paired or repetitively presented stimulus (Froemke et al., 2007; Dorrn et al., 2010). As a number of theoretical studies have emphasized the importance of tuning curve shape for information processing (Pouget et al., 1999; Zhang and Sejnowski, 1999), these coordinated positive and negative changes in excitatory inputs might not only help conserve net excitation, but also preserve the general structure of synaptic receptive fields, shifting the peak rather than distorting or flattening cortical tuning curves.
While this LTD of the original best stimulus could be related to the types of homeostatic synaptic scaling documented in the visual cortex (Desai et al., 2002), we think that instead, given the high degree of stimulus specificity and relatively fast dynamics, original best stimulus depression may be a form of heterosynaptic LTD (Scanziani et al., 1996; Royer and Paré, 2003). The mechanisms of best stimulus depression remain to be determined, especially the mechanisms by which AI neurons and cell assemblies are able to identify and selectively downregulate their particular local maxima. We note that, although the relative excitatory-inhibitory ratio may play a predominant role in AI plasticity (Froemke et al., 2007) and ocular dominance plasticity in developing visual cortex (Hensch, 2005; Southwell et al., 2010), these canonical examples of cortical reorganization are fundamentally different. In particular, shifts of AI frequency tuning begin with enhancement of a weaker input followed by a delayed reduction of the original best input; conversely, ocular dominance shifts are initiated by suppression of the originally preferred, deprived input followed by strengthening of the weaker, spared input (Frenkel and Bear, 2004). Rather than reflecting a basic difference between the visual and auditory systems, these opposite synaptic dynamics are probably related to the precise manipulation of sensory input in each case: monocular deprivation leads to a shift away from the deprived input, while repetitive tonal exposure leads to a shift towards the over-represented stimulus.
Finally, after excitatory tuning curves have shifted to prefer the paired or repetitively presented stimuli, inhibitory inputs are adjusted in proportion to excitation (Fig. 6A, ‘Phase 3’). This balancing of synaptic circuitry seems to unfold over minutes to hours, and in adult AI, does not occur in absence of sensory experience (Froemke et al., 2007), indicating that specific patterns of activity are required to guide reorganization of cortical microcircuitry. After nucleus basalis pairing, the direct cholinergic suppression of tone-evoked inhibition at the paired input is converted into an intermediate-term depression that lasts roughly 10–30 minutes, before progressively increasing to match the rapid increase of excitation evoked by the same stimulus. Similar orchestration of excitatory LTP and inhibitory LTD has been previously described in vitro (Lu et al., 2000; Ivenshitz and Segal, 2006), but little is known about this process in vivo, or how this inhibitory depression is then transformed into an enduring potentiation. Upregulation of BDNF release (Huang et al., 1999) or activity-dependent transcription factors such as Npas4 (Lin et al., 2008), which increases GABA receptor expression after postsynaptic increases in intracellular Ca2+ concentration, are two likely candidates important for balancing excitatory and inhibitory inputs. An important avenue for future research will be to determine the set points for excitatory-inhibitory balance, and how inhibitory circuitry is locally calibrated with high precision across subregions of synaptic receptive fields.
7. Conclusion
Intracellular recordings in vivo have been essential for describing the dynamics of modifications to cortical microcircuitry at the synaptic level. During development, perturbations in the sensory environment drive changes in synaptic strength, organizing cortical receptive fields around the statistics of sensory inputs. In the adult brain, receptive field plasticity is controlled by behavioral context and motivational state, acting through neuromodulators such as acetylcholine and noradrenaline to gate long-term changes in excitatory and inhibitory synaptic receptive fields, perhaps through a common disinhibitory and/or NMDA receptor signaling pathway. In each case, an extensive set of positive and negative adjustments are coordinated across multiple synaptic inputs, to update cortical representations of the external world and ensure that excitatory inputs are balanced by a proportional amount of inhibition.
It remains unclear how distinct elements of cortical networks and subcortical neuromodulatory systems are recruited by various forms of sensation, experience, and internal drive for the control of synaptic modifications, circuit dynamics, perception, and cognition. It will be important to determine the contributions to AI synaptic receptive fields not only from local intracortical connections and the MGB, but also from higher cortical areas including prefrontal cortex. In addition, recordings from subcortical nuclei in awake animals will be necessary to understand which feedback and modulatory inputs are activated under different behavioral contexts. A number of different neuromodulators have disinhibitory effects (Kruglikov and Rudy, 2008), but others- particularly noradrenalin (Edeline et al., 2010) and dopamine (Bao et al., 2001)- may act in other ways or on other elements of cortical networks, including glial cells and vascular processes. Modifications to non-neuronal aspects of the nervous system might be crucial for consolidating changes to cortical circuitry or ensuring that such changes are organized over considerable distances.
Careful analysis of the dynamics of synaptic receptive field modifications will be critical for understanding the key mechanisms and putative behavioral consequences of cortical plasticity. Chronic disruption of excitatory-inhibitory balance is also postulated to play a role in neuropathological conditions such as epilepsy and autism spectrum disorders, as well as hearing loss, tinnitus, and language impairments (Rubenstein and Merzenich, 2003). The ability to selectively increase or decrease specific inputs, through some combination of behavioral training, pharmacological approaches, and more invasive techniques (e.g., electrical stimulation or cell transplantation), provides a powerful means to potentially remediate a large number of nervous system disorders and improve cognitive functions, given an appreciation of the diverse mechanisms engaged during cortical remodeling.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Artola A, Bröcher C, Singer W. Different voltage-dependent thresholds for inducing long-term depression and long-term potentiation in slices of rat visual cortex. Nature. 1990;347:69–72. doi: 10.1038/347069a0. [DOI] [PubMed] [Google Scholar]
- Asari H, Zador AM. Long-lasting context dependence constrains neural encoding models in rodent auditory cortex. Journal of Neurophysiology. 2009;102:2638–2656. doi: 10.1152/jn.00577.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atencio CA, Schreiner CE. Spectrotemporal processing differences between auditory cortical fast-spiking and regular-spiking neurons. Journal of Neuroscience. 2008;28:3897–3910. doi: 10.1523/JNEUROSCI.5366-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chan VT, Merzenich MM. Cortical remodeling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- Bear MF, Singer W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature. 1986;320:172–176. doi: 10.1038/320172a0. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annual Reviews in Neuroscience. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Calford MB, Tweedale R. Immediate and chronic changes in responses of somatosensory cortex in adult flying-fox after digit amputation. Nature. 1988;332:446–448. doi: 10.1038/332446a0. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. Journal of Experimental Biology. 2008;211:1781–1791. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EF, Merzenich MM. Environmental noise retards auditory cortical development. Science. 2003;300:498–502. doi: 10.1126/science.1082163. [DOI] [PubMed] [Google Scholar]
- Chang EF, Bao S, Imaizumi K, Schreiner CE, Merzenich MM. Development of spectral and temporal response selectivity in the auditory cortex. Procedures of the National Academy of Science U S A. 2005;102:16460–16465. doi: 10.1073/pnas.0508239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SY, et al. Multiple receptors coupled to phospholipase C gate long-term depression in visual cortex. Journal of Neuroscience. 2005;25:11433–11443. doi: 10.1523/JNEUROSCI.4084-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmen JC, Hartley DE, King AJ. Stimulus-timing-dependent plasticity of cortical frequency representation. Journal of Neuroscience. 2008;28:13629–13639. doi: 10.1523/JNEUROSCI.4429-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Rocha J, Marchetti C, Schiff M, Reyes AD. Linking the response properties of cells in auditory cortex with network architecture: cotuning versus lateral inhibition. Journal of Neuroscience. 2008;28:9151–9163. doi: 10.1523/JNEUROSCI.1789-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villers-Sidani E, Chang EF, Bao S, Merzenich MM. Critical period window for spectral tuning defined in the primary auditory cortex (A1) in the rat. Journal of Neuroscience. 2007;27:180–189. doi: 10.1523/JNEUROSCI.3227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villers-Sidani E, Alzghoul L, Zhou X, Simpson KL, Lin RC, Merzenich MM. Recovery of functional and structural age-related changes in the rat primary auditory cortex with operant training. Procedures of the National Academy of Science U S A. 2010;107:13900–13905. doi: 10.1073/pnas.1007885107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nature Neuroscience. 2002;5:783–789. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- Diamond ME, Huang W, Ebner FF. Laminar comparison of somatosensory cortical plasticity. Science. 1994;265:1885–1888. doi: 10.1126/science.8091215. [DOI] [PubMed] [Google Scholar]
- Disney AA, Aoki C, Hawken MJ. Gain modulation by nicotine in macaque v1. Neuron. 2007;56:701–713. doi: 10.1016/j.neuron.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrn AL, Yuan K, Barker AJ, Schreiner CE, Froemke RC. Developmental sensory experience balances cortical excitation and inhibition. Nature. 2010;465:932–936. doi: 10.1038/nature09119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RJ, Martin KA. A functional microcircuit in cat visual cortex. Journal of Physiology. 1991;440:659–696. doi: 10.1113/jphysiol.1991.sp018733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeline JM, Manunta Y, Hennevin E. Induction of selective plasticity in the frequency tuning of auditory cortex and auditory thalamus neurons by locus coeruleus stimulation. Hearing Research. 2010 doi: 10.1016/j.heares.2010.08.005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends in Neuroscience. 2004;27:676–682. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Eisenberg LS. Current state of knowledge: speech recognition and production in children with hearing impairment. Ear and Hearing. 2007;28:766–772. doi: 10.1097/AUD.0b013e318157f01f. [DOI] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T. Synapse specificity of long-term potentiation breaks down at short distances. Nature. 1997;388:279–284. doi: 10.1038/40870. [DOI] [PubMed] [Google Scholar]
- Eysel UT, Schweigart G, Mittmann T, Eyding D, Qu Y, Vandesande F, Orban G, Arckens L. Reorganization in the visual cortex after retinal and cortical damage. Restorative Neurology and Neuroscience. 1999;15:153–164. [PubMed] [Google Scholar]
- Feldman DE. Synaptic mechanisms for plasticity in neocortex. Annual Review in Neuroscience. 2009;32:33–55. doi: 10.1146/annurev.neuro.051508.135516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D. Orientation selectivity of synaptic potentials in neurons of cat primary visual cortex. Journal of Neuroscience. 1986;6:1284–1301. doi: 10.1523/JNEUROSCI.06-05-01284.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel MY, Bear MF. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron. 2004;44:917–923. doi: 10.1016/j.neuron.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Fritz J, Shamma S, Elhilali M, Klein D. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nature Neuroscience. 2003;6:1216–1223. doi: 10.1038/nn1141. [DOI] [PubMed] [Google Scholar]
- Fritz J, Elhilali M, Shamma S. Differential dynamic plasticity of A1 receptive fields during multiple spectral tasks. Journal of Neuroscience. 2005;25:7623–7635. doi: 10.1523/JNEUROSCI.1318-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Dan Y. Spike-timing-dependent synaptic modification induced by natural spike trains. Nature. 2002;416:433–438. doi: 10.1038/416433a. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Tsay IA, Raad M, Long JD, Dan Y. Contribution of individual spikes in burst-induced long-term synaptic modification. Journal of Neurophysiology. 2006;95:1620–1629. doi: 10.1152/jn.00910.2005. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field plasticity. Nature. 2007;450:425–429. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Jones BJ. Development of auditory cortical synaptic receptive fields. Neuroscience and Biobehavioral Reviews. 2010 doi: 10.1016/j.neubiorev.2011.02.006. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Schreiner CE. Synaptic plasticity as a cortical coding scheme. Developmental Neuropsychology. 2010 doi: 10.1016/j.conb.2015.10.003. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. Receptive field dynamics in adult primary visual cortex. Nature. 1992;356:150–152. doi: 10.1038/356150a0. [DOI] [PubMed] [Google Scholar]
- Giocomo LM, Hasselmo ME. Neuromodulation by glutamate and acetylcholine can change circuit dynamics by regulating the relative influence of afferent input and excitatory feedback. Molecular Neurobiology. 2007;36:184–200. doi: 10.1007/s12035-007-0032-z. [DOI] [PubMed] [Google Scholar]
- Goard M, Dan Y. Basal forebrain activation enhances cortical coding of natural scenes. Nature Neuroscience. 2009;12:1444–1449. doi: 10.1038/nn.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience. 2002;111:815–835. doi: 10.1016/s0306-4522(02)00026-x. [DOI] [PubMed] [Google Scholar]
- Han Y, Kover H, Insanally M, Semerdjian JH, Bao S. Early experience impairs perceptual discrimination. Nature Neuroscience. 2007;10:1191–1197. doi: 10.1038/nn1941. [DOI] [PubMed] [Google Scholar]
- Harvey CD, Svoboda K. Locally dynamic synaptic learning rules in pyramidal neuron dendrites. Nature. 2007;450:1195–1200. doi: 10.1038/nature06416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häusser M, Mel B. Dendrites: bug or feature? Current Opinion in Neurobiology. 2003;3:372–383. doi: 10.1016/s0959-4388(03)00075-8. [DOI] [PubMed] [Google Scholar]
- He HY, Rasmusson DD, Quinlan EM. Progressive elevations in AMPA and GABAA receptor levels in deafferented somatosensory cortex. Journal of Neurochemistry. 2004;90:1186–1193. doi: 10.1111/j.1471-4159.2004.02590.x. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annual Reviews in Neuroscience. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nature Reviews in Neuroscience. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Henny P, Jones BE. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. European Journal of Neuroscience. 2008;27:654–670. doi: 10.1111/j.1460-9568.2008.06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero JL, Roberts MJ, Delicato LS, Gieselmann MA, Dayan P, Thiele A. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature. 2008;454:1110–1114. doi: 10.1038/nature07141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highley MJ, Contreras D. Balanced excitation and inhibition determine spike timing during frequency adaptation. Journal of Neuroscience. 2006;26:448–457. doi: 10.1523/JNEUROSCI.3506-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JA. Synaptic physiology and receptive field structure in the early visual pathway of the cat. Cerebral Cortex. 2003;13:63–69. doi: 10.1093/cercor/13.1.63. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. Journal of Physiology. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annual Reviews in Neuroscience. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insanally MN, Köver H, Kim H, Bao S. Feature-dependent sensitive periods in the development of complex sound representation. Journal of Neuroscience. 2009;29:5456–5462. doi: 10.1523/JNEUROSCI.5311-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivenshitz M, Segal M. Simultaneous NMDA-dependent long-term potentiation of EPSCs and long-term depression of IPSCs in cultured rat hippocampal neurons. Journal of Neuroscience. 2006;26:1199–1210. doi: 10.1523/JNEUROSCI.2964-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kaur S, Lazar R, Metherate R. Intracortical pathways determine breadth of subthreshold frequency receptive fields in primary auditory cortex. Journal of Neurophysiology. 2004;91:2551–2567. doi: 10.1152/jn.01121.2003. [DOI] [PubMed] [Google Scholar]
- Kenet T, Froemke RC, Schreiner CE, Pessah IN, Merzenich MM. Perinatal exposure to a noncoplanar polychlorinated biphenyl alters tonotopy, receptive fields, and plasticity in rat primary auditory cortex. Procedures of the National Academy of Science USA. 2007;104:7646–7651. doi: 10.1073/pnas.0701944104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kotak VC, Fujisawa S, Lee FA, Karthikeyan O, Aoki C, Sanes DH. Hearing loss raises excitability in the auditory cortex. Journal of Neuroscience. 2005;25:3908–3918. doi: 10.1523/JNEUROSCI.5169-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Takesian AE, Sanes DH. Hearing loss prevents the maturation of GABAergic transmission in the auditory cortex. Cerebral Cortex. 2008;18:2098–2108. doi: 10.1093/cercor/bhm233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruglikov I, Rudy B. Perisomatic GABA release and thalamocortical integration onto neocortical excitatory cells are regulated by neuromodulators. Neuron. 2008;58:911–924. doi: 10.1016/j.neuron.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BH, Wu GK, Arbuckle R, Tao HW, Zhang LI. Defining cortical frequency tuning with recurrent excitatory circuitry. Nature Neuroscience. 2007;10:1594–1600. doi: 10.1038/nn2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC, Nicolelis MA. Neuronal ensemble bursting in the basal forebrain encodes salience irrespective of valence. Neuron. 2008;59:138–149. doi: 10.1016/j.neuron.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, Hu LS, Malik AN, Greenberg ME. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008;455:1198–1204. doi: 10.1038/nature07319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonczy A, Makara JK, Magee JC. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature. 2008;452:436–441. doi: 10.1038/nature06725. [DOI] [PubMed] [Google Scholar]
- Lu YM, Mansuy IM, Kandel ER, Roder J. Calcineurin-mediated LTD of GABAergic inhibition underlies the increased excitability of CA1 neurons associated with LTP. Neuron. 2000;26:197–205. doi: 10.1016/s0896-6273(00)81150-2. [DOI] [PubMed] [Google Scholar]
- Lund RD, Mustari MJ. Development of the geniculocortical pathway in rats. Journal of Comparative Neurology. 1977;173:289–306. doi: 10.1002/cne.901730206. [DOI] [PubMed] [Google Scholar]
- Ma X, Suga N. Long-term cortical plasticity evoked by electrical stimulation and acetylcholine applied to the auditory cortex. Proceedings of the National Academy of Sciences, USA. 2005;102:9335–9340. doi: 10.1073/pnas.0503851102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nature Neuroscience. 2004;7:1353–1359. doi: 10.1038/nn1351. [DOI] [PubMed] [Google Scholar]
- Markram H, Lübke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- Meliza CD, Dan Y. Receptive-field modification in rat visual cortex induced by paired visual stimulation and single-cell spiking. Neuron. 2006;49:183–189. doi: 10.1016/j.neuron.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Some cholinergic themes related to Alzheimer’s disease: synaptology of the nucleus basalis, location of m2 receptors, interactions with amyloid metabolism, and perturbations of cortical plasticity. Journal of Physiology, Paris. 1998;92:293–298. doi: 10.1016/s0928-4257(98)80036-3. [DOI] [PubMed] [Google Scholar]
- Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. Journal of Neuroscience. 1992;12:4701–4711. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Nucleus basalis stimulation facilitates thalamocortical synaptic transmission in the rat auditory cortex. Synapse. 1993;14:132–143. doi: 10.1002/syn.890140206. [DOI] [PubMed] [Google Scholar]
- Metherate R, Kaur S, Kawai H, Lazar R, Liang K, Rose HJ. Spectral integration in auditory cortex: mechanisms and modulation. Hearing Research. 2005;206:146–158. doi: 10.1016/j.heares.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Monier C, Chavane F, Baudot P, Graham LJ, Frégnac Y. Orientation and direction selectivity of synaptic inputs in visual cortical neurons: a diversity of combinations produces spike tuning. Neuron. 2003;37:663–680. doi: 10.1016/s0896-6273(03)00064-3. [DOI] [PubMed] [Google Scholar]
- Morishita H, Miwa JM, Heintz N, Hensch TK. Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science. 2010;330:1238–1240. doi: 10.1126/science.1195320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle VB. Modality and topographic properties of single neurons of cat’s somatic sensory cortex. Journal of Neurophisiology. 1957;20:408–434. doi: 10.1152/jn.1957.20.4.408. [DOI] [PubMed] [Google Scholar]
- Nowak LG, Sanchez-Vives MV, McCormick DA. Spatial and temporal features of synaptic to discharge receptive field transformation in cat area 17. Journal of Neurophysiology. 2010;103:677–697. doi: 10.1152/jn.90946.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald AMM, Reyes AD. Maturation of intrinsic and synaptic properties of layer 2/3 pyramidal neurons in mouse auditory cortex. Journal of Neurophysiology. 2008;99:2998–3008. doi: 10.1152/jn.01160.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei X, Volgushev M, Vidyasagar TR, Creutzfeldt OD. Whole cell recording and conductance measurements in cat visual cortex in-vivo. Neuroreport. 1991;2:485–488. doi: 10.1097/00001756-199108000-00019. [DOI] [PubMed] [Google Scholar]
- Petilla Interneuron Nomenclature Group. Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsáki G, Cauli B, Defelipe J, Fairén A, Feldmeyer D, Fishell G, Fregnac Y, Freund TF, Gardner D, Gardner EP, Goldberg JH, Helmstaedter M, Hestrin S, Karube F, Kisvárday ZF, Lambolez B, Lewis DA, Marin O, Markram H, Muñoz A, Packer A, Petersen CC, Rockland KS, Rossier J, Rudy B, Somogyi P, Staiger JF, Tamas G, Thomson AM, Toledo-Rodriguez M, Wang Y, West DC, Yuste R. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nature Reviews in Neuroscience. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu MV, Polley DB. Monaural deprivation disrupts development of binaural selectivity in auditory midbrain and cortex. javascript:AL_get(this, ‘jour’, ‘Neuron.’); Neuron. 2010;65:718–731. doi: 10.1016/j.neuron.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouget A, Denève S, Ducom JC, Latham PE. Narrow versus wide tuning curves: What’s best for a population code? Neural Computation. 1999;11:85–90. doi: 10.1162/089976699300016818. [DOI] [PubMed] [Google Scholar]
- Raggio MW, Schreiner CE. Neuronal responses in cat primary auditory cortex to electrical cochlear stimulation. III. Activation patterns in short- and long-term deafness. Journal of Neurophysiology. 1999;82:3506–3526. doi: 10.1152/jn.1999.82.6.3506. [DOI] [PubMed] [Google Scholar]
- Ramanathan D, Tuszynski MH, Conner JM. The basal forebrain cholinergic system is required specifically for behaviorally mediated cortical map plasticity. Journal of Neuroscience. 2009;29:5992–6000. doi: 10.1523/JNEUROSCI.0230-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson DD, Dykes RW. Long-term enhancement of evoked potentials in cat somatosensory cortex produced by co-activation of the basal forebrain and cutaneous receptors. Experimental Brain Research. 1988;70:276–286. doi: 10.1007/BF00248353. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD. The role of acetylcholine in cortical synaptic plasticity. Behavioral Brain Research. 2000;115:205–218. doi: 10.1016/s0166-4328(00)00259-x. [DOI] [PubMed] [Google Scholar]
- Razak KA, Fuzessery ZM. Development of inhibitory mechanisms underlying selectivity for the rate and direction of frequency-modulated sweeps in the auditory cortex. Journal of Neuroscience. 2007;14:1769–1781. doi: 10.1523/JNEUROSCI.3851-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Paré D. Conservation of total synaptic weight through balanced synaptic depression and potentiation. Nature. 2003;422:518–522. doi: 10.1038/nature01530. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes, Brain and Behavior. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sally SL, Kelly JB. Organization of auditory cortex in the albino rat: sound frequency. Journal of Neurophysiology. 1988;59:1627–1638. doi: 10.1152/jn.1988.59.5.1627. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Constantine-Paton M. Altered activity patterns during development reduce neural tuning. Science. 1983;221:1183–1185. doi: 10.1126/science.6612332. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Bao S. Tuning up the developing auditory CNS. Current Opinion in Neurobiology. 2009;19:188–199. doi: 10.1016/j.conb.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarro EC, Kotak VC, Sanes DH, Aoki C. Hearing loss alters the subcellular distribution of presynaptic GAD and postsynaptic GABAA receptors in the auditory cortex. Cerebral Cortex. 2008;18:2855–2867. doi: 10.1093/cercor/bhn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Parikh V. Choline transporters, cholinergic transmission and cognition. Nature Reviews Neuroscience. 2005;6:48–56. doi: 10.1038/nrn1588. [DOI] [PubMed] [Google Scholar]
- Scanziani M, Malenka RC, Nicoll RA. Role of intercellular interactions in heterosynaptic long-term depression. Nature. 1996;380:446–450. doi: 10.1038/380446a0. [DOI] [PubMed] [Google Scholar]
- Scholl B, Wehr M. Disruption of balanced cortical excitation and inhibition by acoustic trauma. Journal of Neurophysiology. 2008;100:646–656. doi: 10.1152/jn.90406.2008. [DOI] [PubMed] [Google Scholar]
- Schummers J, Mariño J, Sur M. Synaptic integration by V1 neurons depends on location within the orientation map. Neuron. 2002;36:969–978. doi: 10.1016/s0896-6273(02)01012-7. [DOI] [PubMed] [Google Scholar]
- Seol GH, Ziburkus J, Huang S, Song L, Kim IT, Takamiya K, Huganir RL, Lee HK, Kirkwood A. Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity. Neuron. 2007;55:919–929. doi: 10.1016/j.neuron.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver MA, Shenhav A, D’Esposito M. Cholinergic enhancement reduces spatial spread of visual responses in human early visual cortex. Neuron. 2008;60:904–914. doi: 10.1016/j.neuron.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder RL, Rebscher SJ, Cao KL, Leake PA, Kelly K. Chronic intracochlear electrical stimulation in the neonatally deafened cat. I: Expansion of central representation. Hearing Research. 1990;50:7–33. doi: 10.1016/0378-5955(90)90030-s. [DOI] [PubMed] [Google Scholar]
- Sohya K, Kameyama K, Yanagawa Y, Obata K, Tsumoto T. GABAergic neurons are less selective to stimulus orientation than excitatory neurons in layer II/III of visual cortex, as revealed by in vivo functional Ca2+ imaging in transgenic mice. Journal of Neuroscience. 2007;27:2145–2149. doi: 10.1523/JNEUROSCI.4641-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwell DG, Froemke RC, Alvarez-Buylla A, Stryker MP, Gandhi SP. Cortical plasticity induced by inhibitory neuron transplantation. Science. 2010;327:1145–1148. doi: 10.1126/science.1183962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YJ, Wu GK, Liu BH, Li P, Zhou M, Xiao Z, Tao HW, Zhang LI. Fine-tuning of pre-balanced excitation and inhibition during auditory cortical development. Nature. 2010;465:927–931. doi: 10.1038/nature09079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AYY, Atencio CA, Polley DB, Merzenich MM, Schreiner CE. Unbalanced synaptic inhibition can create intensity-tuned auditory cortex neurons. Neuroscience. 2007;146:449–462. doi: 10.1016/j.neuroscience.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Tan AYY, Wehr M. Balanced tone-evoked synaptic excitation and inhibition in mouse auditory cortex. Neuroscience. 2009;163:1302–1315. doi: 10.1016/j.neuroscience.2009.07.032. [DOI] [PubMed] [Google Scholar]
- Takesian AE, Kotak VC, Sanes DH. Developmental hearing loss disrupts synaptic inhibition: implications for auditory processing. Future Neurology. 2009;4:331–349. doi: 10.2217/FNL.09.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachtenberg JT, Trepel C, Stryker MP. Rapid extragranular plasticity in the absence of thalamocortical plasticity in the developing primary visual cortex. Science. 2000;287:2029–2032. doi: 10.1126/science.287.5460.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzounopoulos T, Kim Y, Oertel D, Trussell LO. Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nature Neuroscience. 2004;7:719–725. doi: 10.1038/nn1272. [DOI] [PubMed] [Google Scholar]
- Turner JG, Hughes LF, Caspary DM. Affects of aging on receptive fields in rat primary auditory cortex layer V neurons. Journal of Neurophysiology. 2005;94:2738–2747. doi: 10.1152/jn.00362.2005. [DOI] [PubMed] [Google Scholar]
- Shen JX. Evidence for columnar organization in the auditory cortex of the mouse. Hearing Research. 1999;137:174–177. doi: 10.1016/s0378-5955(99)00149-5. [DOI] [PubMed] [Google Scholar]
- Vale C, Sanes DH. The effect of bilateral deafness on excitatory and inhibitory synaptic strength in the inferior colliculus. European Journal of Neuroscience. 2002;16:2394–2404. doi: 10.1046/j.1460-9568.2002.02302.x. [DOI] [PubMed] [Google Scholar]
- Volkov IO, Galazjuk AV. Formation of spike responses to sound tones in cat auditory cortex neurons: interaction of excitatory and inhibitory effects. Neuroscience. 1991;43:307–321. doi: 10.1016/0306-4522(91)90295-y. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–446. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Synaptic mechanisms of forward suppression in rat auditory cortex. Neuron. 2005;47:437–445. doi: 10.1016/j.neuron.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Physiological memory in primary auditory cortex: characteristics and mechanisms. Neurobiology of Learning and Memory. 1998;70:226–251. doi: 10.1006/nlme.1998.3850. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Auditory associative memory and representational plasticity in the primary auditory cortex. Hearing Research. 2007;229:54–68. doi: 10.1016/j.heares.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott JF. Hearing loss and the inferior colliculus. In: Winer JA, Schreiner CE, editors. The Inferior Colliculus. Springer-Verlag; New York: 2005. pp. 585–602. [Google Scholar]
- Winer JA. The functional architecture of the medial geniculate body and the primary auditory cortex. In: Webster DB, Popper AN, Fay RR, editors. Springer Handbook of Auditory Research, volume 1, The Mammalian Auditory Pathway: Neuroanatomy. Springer-Verlag; New York: 1992. pp. 222–409. [Google Scholar]
- Woody CD, Gruen E. Acetylcholine reduces net outward currents measured in vivo with single electrode voltage clamp techniques in neurons of the motor cortex of cats. Brain Research. 1987;424:193–198. doi: 10.1016/0006-8993(87)91210-8. [DOI] [PubMed] [Google Scholar]
- Wu GK, Li P, Tao HW, Zhang LI. Nonmonotonic synaptic excitation and imbalanced inhibition underlying cortical intensity tuning. Neuron. 2006;52:705–715. doi: 10.1016/j.neuron.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA. Cholinergic switching within neocortical inhibitory networks. Science. 1998;281:985–988. doi: 10.1126/science.281.5379.985. [DOI] [PubMed] [Google Scholar]
- Yan J, Zhang Y. Sound-guided shaping of the receptive field in the mouse auditory cortex by basal forebrain activation. European Journal of Neuroscience. 2005;21:563–576. doi: 10.1111/j.1460-9568.2005.03878.x. [DOI] [PubMed] [Google Scholar]
- Ye CQ, Poo MM, Dan Y, Zhang XH. Synaptic mechanisms of direction selectivity in primary auditory cortex. Journal of Neuroscience. 2010;30:1861–1868. doi: 10.1523/JNEUROSCI.3088-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K, Dorrn AL, Barker AJ, Schreiner CE, Froemke RC. Developmental sensory experience balances cortical excitation and inhibition. Society for Neuroscience Abstracts. 2010:482.13. doi: 10.1038/nature09119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitler DM, Kessler MA, Terushkin V, Roland TJ, Jr, Svirsky MA, Lalwani AK, Waltzman SB. Speech perception benefits of sequential bilateral cochlear implantation in children and adults: a retrospective analysis. Otology and Neurotology. 2008;29:314–25. doi: 10.1097/mao.0b013e3181662cb5. [DOI] [PubMed] [Google Scholar]
- Zhang K, Sejnowski TJ. Neuronal tuning: To sharpen or broaden? Neural Computation. 1999;11:75–84. doi: 10.1162/089976699300016809. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Bao S, Merzenich MM. Persistent and specific influences of early acoustic environments on primary auditory cortex. Nature Neuroscience. 2001;4:1123–30. doi: 10.1038/nn745. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Tan AY, Schreiner CE, Merzenich MM. Topography and synaptic shaping of direction selectivity in primary auditory cortex. Nature. 2003;424:201–205. doi: 10.1038/nature01796. [DOI] [PubMed] [Google Scholar]
- Zhou X, Nagarajan N, Mossop BJ, Merzenich MM. Influences of un-modulated acoustic inputs on functional maturation and critical-period plasticity of the primary auditory cortex. Neuroscience. 2008;154:390–396. doi: 10.1016/j.neuroscience.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]