Abstract
A fully automated chiral capillary electrophoresis - tandem mass spectrometric method (CE-MS/MS) was developed for enantiomeric quantification of DOPA and its precursors, phenylalanine (Phe) and tyrosine (Tyr). To avoid MS source contamination, a negatively charged chiral selector, sulfated β-cyclodextrin (sulfated β-CD) that migrated away from the detector was used in combination with the partial filling technique. The six stereoisomers were simultaneously quantified in less than 12 min. Detection limits were 0.48 and 0.51 μM for L- and D-DOPA enantiomers, respectively. Assay reproducibility (RSD, n=6) were 4.43%, 3.15%, 4.91%, 5.16%, 3.96%, and 3.25% for L-/D-DOPA, L-/D-Tyr, and L-/D-Phe at 10.0 μM, respectively. Thanks to the high enantioseparation efficiency, detection of trace D-DOPA in L-/D-DOPA mixtures could be achieved. The assay was employed to study the metabolism of DOPA, a well known therapeutic drug for treating Parkinson’s disease. It was found that L-DOPA was metabolized effectively in PC-12 cells. About 88% of L-DOPA disappeared after incubation at a cell density of 2 × 106 cells/mL for 3 hrs. However, D-DOPA coexisting with L-DOPA in the incubation solution remained intact. The enantiospecific metabolism of DOPA in this neuronal model was demonstrated.
Keywords: Capillary electrophoresis, Mass spectrometry, Chiral separation, Analysis of enantiomeric purity, Enantioselective metabolism, DOPA
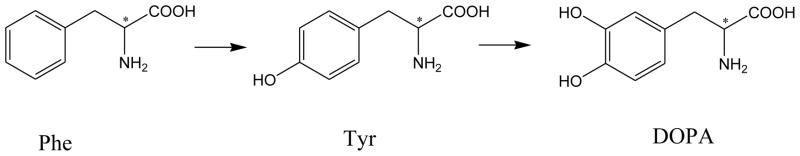
3,4-Dihydroxyphenylalanine (DOPA) is formed by hydroxylation of phenylalanine or tyrosine as shown in Figure 1. L-DOPA (levodopa) is the precursor to neurotransmitters, dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline) collectively known as catecholamines. In addition to its natural biological roles, L-DOPA is the most effective drug to treat Parkinson’s disease (PD) [1–2]. However, its antipode, D-DOPA, is inactive [3–4]. The biomedical significance has promoted the development of analytical methods for simultaneously quantifying stereoisomers of DOPA and its precursors. Many methods have been reported. These included high performance liquid chromatography (HPLC) [5–8], capillary electrophoresis (CE) [9–12], capillary electrokinetic chromatography (EKC) [13–14], and microchip electrophoresis (MCE) [15–16]. Most of these analytical methods deployed UV-detection which lacked sensitivity and the capability of peak identification.
Fig. 1.
Biosynthesis of DOPA.
Chiral CE coupled with mass spectrometry (CE-MS) is a powerful tool for analysis of enantiomers. On one hand, changing the chiral selector for separating a particular pair of enantiomers is easy to do in chiral CE. On the other hand, MS detection is a versatile detection technique allowing not only sensitive detection but also providing structural information for analytes. This information is very useful for the unambiguous identification and confirmation of components especially in real matrices [17–18]. However, only few chiral CE-MS methods have been developed for analysis of amino acids [19–21], and to the best of our knowledge there have been no reports on simultaneous quantification of DOPA, Phe, and Tyr enantiomers by using a CE-MS method.
In this study, we aimed to develop a CE-MS method for enantiomeric determination of DOPA and its precursors with high separation efficiency, peak identification capability, and assay sensitivity. Sulfated β-cyclodextrin (β-CD) was selected as the chiral selector since it was negatively charged and thus migrated away from the MS detector in a CE-MS separation. To avoid any potential contamination from the non-volatile chiral selector, the partial filling technique was deployed. The proposed method was preliminarily evaluated for the detection of trace D-DOPA present in bulk amounts of pharmaceutically active L-DOPA. In addition, the method was used to study DOPA metabolism in nerve cells.
Materials and methods
Chemicals and solutions
Sulfated β-CD, D-/L- DOPA, D-/L-Phe, and D-/L-Tyr were purchased from Sigma–Aldrich Chemical (St. Louis, MO, USA). All other reagents and chemicals used were of analytical reagent grade. Stock solutions (5.00 mM) of the enantiomeric chemicals were prepared in 5 mM hydrochloric acid and diluted appropriately with water on the day of use. All solutions were filtered through a 0.22 μm membrane filter prior to use.
CE-MS System
The system consisted of a CE instrument (7100, Agilent Technologies) and an ion trap mass spectrometer (LCQ Deca, ThermoFinnigan). A CE-MS adapter kit from Agilent Technologies was used for the coupling. All CE operations including capillary flush, chiral selector loading, sample injection, and separation were automated. The mass spectrometer was equipped with an ESI source and a syringe pump. It was operated in a positive ion mode. Multiple stage mass spectrometry (MS/MS) experiments were performed to isolate and fragment the targeted ions. The operating conditions of the MS detector were optimized with a solution of DOPA (5.0 μM) infused into the ESI-MS system with a syringe pump at a flow rate of 2μL/mL. Parameters were optimized using the autotune program. Data were collected and analyzed by using Xcalibur.
Chiral CE-MS Assay
Capillary was flushed with the CE running buffer for 3 min, and then the chiral selector solution was introduced into the capillary by pressure injection at 100 mbar for 50 s. A sample solution was injected at 50 mbar for 12 s. The capillary inlet end was placed in the CE running buffer vial and separation was started by applying a positive voltage. At the same time MS detection began (sheath liquid was automatically turned on by the mass spectrometer).
CE conditions: column, 75μm ID/190μm OD × 80 cm long fused-silica capillary; CE running buffer, 200 mM formic acid; chiral selector solution, 5.0 mM sulfated β-CD in CE running buffer; CE voltage, positive 30.0 kV; column temperature, 20 °C.
MS conditions: sheath liquid, 50% methanol in water containing 0.1% formic acid at 3μL/min; spray voltage, 4 kV; capillary temperature, 220°C; sheath gas, 20 arbitrary units (au); auxiliary gas, 0 au. For SRM experiments, normalized collision energy was set at 30 with an isolation width of 2.0 u, and the activation time was set at 30 ms.
Metabolic study of DOPA in PC-12 cells
PC-12 cells (obtained from Sigma-Aldrich) were cultured in complete RPMI medium supplemented with 10% heat inactivated horse serum and 5% FBS. Cells were routinely sub-cultured every 4–5 days. DOPA (either racemic or enantiomeric form, at a final concentration of 500 μM) was incubated with 5 mL PC-12 cell suspension (2 × 106 cells/mL) for 2 hours at 37 °C. After incubation, cells were removed by centrifugation. One mL of the medium was deproteinized by adding 300 μL of 30% trichloroacetic acid followed by centrifugation at 10000 g for 10 min. The supernatant was filtered through a 0.22 μm membrane filter and diluted 10 times with water prior to CE-MS analysis.
Results and discussion
Chiral CE-MS/MS separation of DOPA, Phe, and Tyr
A major challenge in the development of a chiral CE-MS method is the potential contamination of MS ionization source from the non-volatile chiral selector and other additives in CE running buffer. Several chiral selectors have been proven to be effective for resolving DOPA enantiomers in CE [9–16]. When used in CE-MS assays, a significant advantage of sulfated β-CD is that it migrates away from the MS detector since it’s negatively charged. To further ensure that the chiral selector not entering the MS detector, the partial filling technique [22–23] was deployed. That is, the chiral selector, sulfated β-CD, was introduced into the separation capillary by pressure injection for a certain time to partially fill the capillary prior to a sample injection. It was found that sulfated β-CD was effective not only for resolving DOPA, but also for resolving its precursors, Phe and Tyr. Concentrations of sulfated β-CD in the range of 1.0 to 25.0 mM were tested. Optimal separations in terms of enantiomeric resolution and migration time were obtained by using 5.0 mM sulfated β-CD. Formic acid was used as the electrolyte in the running buffer. It was preferable for CE-MS analysis of amino compounds because of its high volatility and strong acidity. Effects of formic acid concentration ranging from 0.01 to 1.0 M on the separation were investigated. Base-line resolution of DOPA, Tyr, and Phe was achieved when formic acid concentration was greater than 0.1 M. The electrophoretic current was found too high with a 1.0 M formic acid running buffer. Therefore, a running buffer containing 0.2 M formic acid was selected for further studies. The chiral selector solution consisted of 5.0 mM sulfated β-CD and 0.2 M formic acid. After studying the other CE and MS parameters, the selected conditions for the CE-MS assay were as follows: hydrodynamic introducing of the chiral selector solution for 50 s at 100 mbar; hydrodynamic injection of the sample for 12 s at 50 mbar; applying separation voltage of +30 kV; starting a sheath liquid flow rate at 3 μL/min and MS acquisition. All these operations were programmed and performed automatically. Figure 2 shows an electropherogram from separating a mixture of L-/D-DOPA, L-/D-Phe, and L-/D-Tyr (50.0 μM for each enantiomer). As can be seen, although the CE peaks of the six enantiomers overlapped to a certain degree, they were base-line separated in the extracted mass electropherograms (shown in Fig. 2B–D), demonstrating a great advantage of the CE-MS combination. MS2 spectra for DOPA, Phe, and Tyr were as follows: m/z 198 → 182, 152; m/z 182 → 165, 136; and m/z 166 → 149, 120, respectively (as shown in the insets). It was found that all the tested L-enantiomers eluted prior to the corresponding D-enantiomers, which suggested that the interaction between sulfated β-CD and D-enantiomers was stronger than that with L-enantiomers.
Fig. 2.
Electropherograms obtained from the separation of a mixture of L-/D-DOPA, L-/D-Phe, and L-/D-Tyr (50.0 μM for each enantiomer) by the proposed chiral CE-MS/MS method: (A) TIC of m/z 198, 182, and 166; (B) extracted mass electropherogram of m/z 198 → 181 for DOPA from (A); (C) extracted mass electropherogram of m/z 182 → 165 for Tyr from (A); (D) extracted mass electropherogram of m/z 166 → 120 for Phe from (A). Insets are MS2 spectra of DOPA, Tyr, and Phe, respectively. Chiral CE conditions: capillary, 80 cm × 75 μm i.d.; hydrodynamic introducing of chiral selector solution at 100 mbar for 50 s; chiral selector solution, 5 mM sulfated β-CD in 0.2 M formic acid; hydrodynamic injection of sample at 50 mbar for 12 s; CE running buffer, 0.2 M formic acid solution; separation voltage, +30 kV. MS detection conditions: sheath liquid, 50% methanol in water containing 0.1% formic acid at 3μL/min; ESI spray voltage, +4 kV; capillary temperature, 220°C; sheath gas, 20 arbitrary units (au); auxiliary gas, 0 au.
Analytical figures of merit
Under the optimized conditions, analytical figures of merit were studied for the proposed chiral CE-MS/MS method. Standard curves were prepared by analyzing a series of standard mixtures of DOPA, Phe, and Tyr at various concentrations. The calibration curves based on peak area versus analyte concentration showed a good linearity with correlation coefficient > 0.993 for all the enantiomers. The linear range was 2.5 – 200 μM. Detection limits (S/N =3) were estimated to be 0.48 and 0.51 μM for L-DOPA and D-DOPA, respectively. Assay reproducibility was determined by repeatedly analyzing a mixture of L-/D-DOPA, L-/D-Tyr, and L-/D-Phe (10.0 μM each enantiomer) for six times. Relative standard deviations (RSD) were 4.43%, 3.15%, 4.91%, 5.16%, 3.96%, and 3.25% for L-DOPA, D-DOPA, L-Tyr, D-Tyr, L-Phe, and D-Phe, respectively. Reproducibility of the migration times (RSD, n=6) were 1.40%, 1.57%, 1.50%, 1.70%, 1.66%, and 1.64% for L-DOPA, D-DOPA, L-Tyr, D-Tyr, L-Phe, and D-Phe, respectively. As far as we know, there have been no reports on simultaneous quantification of DOPA, Phe, and Tyr enantiomers by using a CE-MS method. The majority of chiral CE-based methods previously reported for DOPA enantiomeric quantification deployed UV detection. These methods had detection limits at the level of 0.5 μg/mL (or 2.5 μM) [9–12, 14, 24]. The present CE-MS method is not only more sensitive, but also offers the advantage of peak identification capability which is highly desired in analysis of complex biological samples.
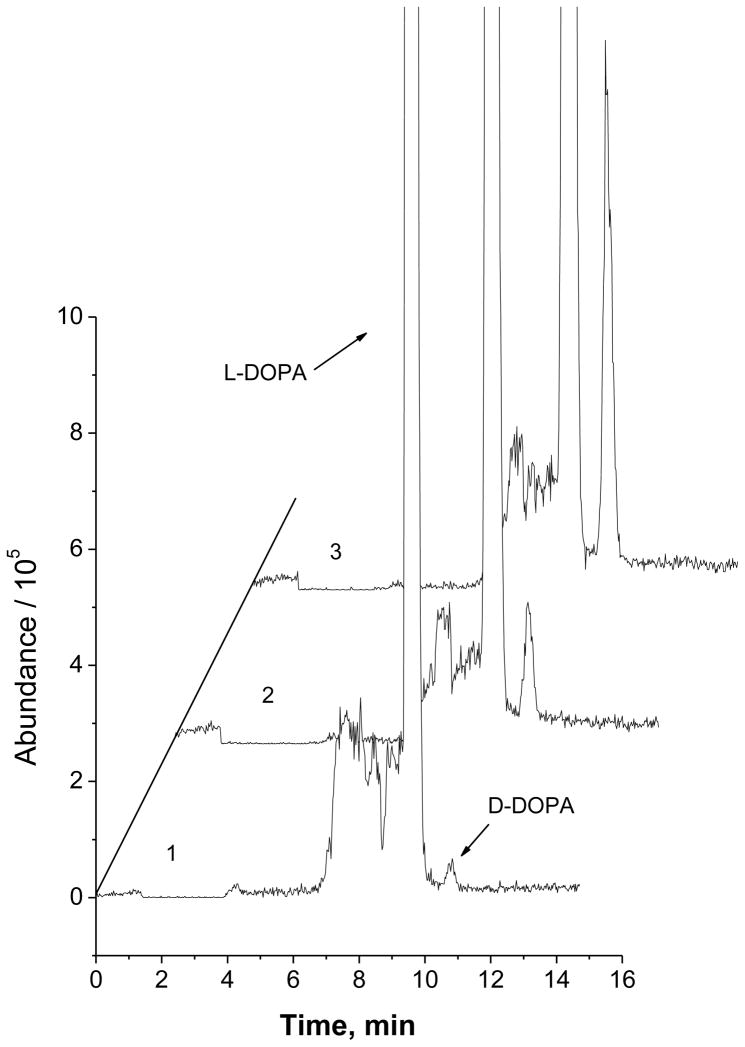
Determination of the enantiomeric purity of levodopa
Levodopa (i.e. L-DOPA) is used in the treatment of Parkinson’s disease and dopamine-responsive dystonia. L-DOPA is converted to dopamine in the brain by aromatic L-amino acid decarboxylase, also known as DOPA decarboxylase (DDC). Dextrodopa (i.e. D-DOPA) can not be converted to dopamine, and its existence may also cause side effects [9, 25]. However, there is a possibility that the therapeutic drug (levodopa) contains unwanted dextrodopa formed during the process of synthesis, formulation, or storage. A strict control of dextrodopa level in a levodopa formulation is significant and also an issue related to safe therapy. For this purpose, chiral CE-based methods [9, 26] were developed to assess the stereochemical purity of pharmaceutical preparations. These methods had a relative detection limit (i.e. the lowest mass percent of D-DOPA in a D-/L-DOPA mixture could be detected) of ~0.1%. The present chiral CE-MS method was also applied to detect trace amount of D-DOPA present in large quantity of L-DOPA. Figure 3 shows the electropherograms obtained from detection of 2.5 μM D-DOPA (curve 1), 10 μM D-DOPA (curve 2), and 40 μM D-DOPA (curve 3) co-existing with 500 μM L-DOPA. As can be seen, the peak of D-DOPA was well identified, and D-DOPA content could be unequivocally determined in each case. Analysis of a pharmaceutical preparation (Carbidopa-Levodopa 25–100 mg tablets, generic Sinemet) was carried out to determine D-/L-DOPA. Good agreements between results obtained and the nominal values labeled were observed for L-DOPA content. No D-DOPA (<0.1% D-DOPA/(D-DOPA + L-DOPA) at [L-DOPA] = 500.0 μM) was detected in this sample. The recovery of D-DOPA from this sample matrix were 103.4 ± 3.61 % and 99.1 ± 2.78 % (mean ± SD) at 0.5% and 5.0% (D-DOPA/(D-DOPA + L-DOPA)), respectively. These results indicated that the present method was useful for assessing the enantiomeric purity of levodopa in pharmaceutical formulations.
Fig. 3.
Electropherograms from enantiomeric purity analysis of levodopa: (1) 0.5 %, (2) 2.0%, and (3) 7.4% D-DOPA (calculated as D-DOPA/(D-DOPA + L-DOPA)). [L-DOPA] = 500.0 μM. Experimental conditions were as in Figure 2.
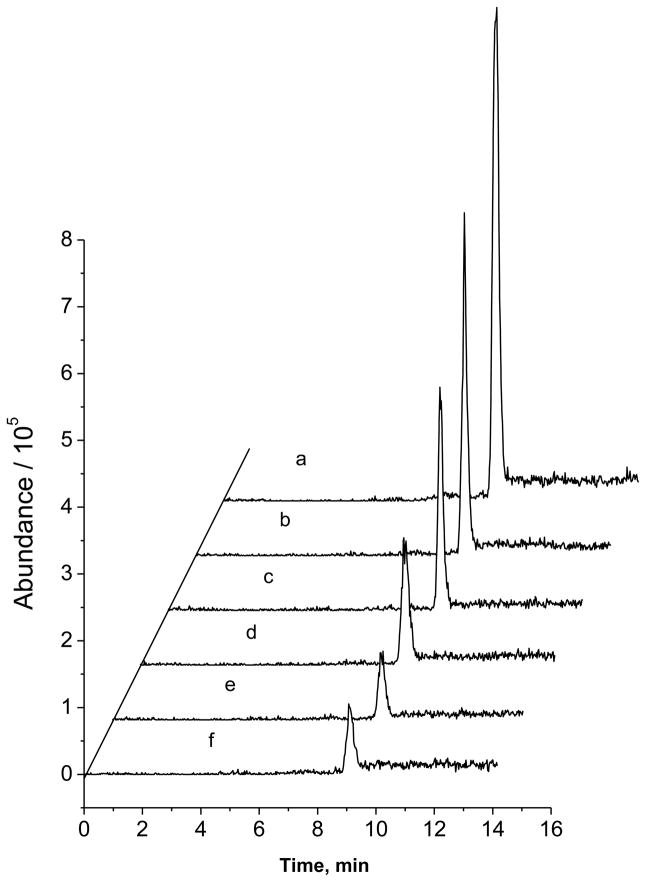
Enantioselective metabolism of DOPA in PC-12 cells
The proposed CE-MS/MS method was also applied in studies of DOPA metabolism in PC-12 cells, a widely used in vitro neuronal model. Assay accuracy and precision were assessed with this sample matrix. D-/L-DOPA were added to portions of a PC-12 cell homogenate at varying concentrations. These samples were then analyzed multiple times either within one day or in one week. The validation results (shown in Table 1) indicated that the method was accurate and reproducible. Figure 4a shows a typical electropherogram from separating racemic DOPA standard solution (50 μM each enantiomer). L-DOPA and D-DOPA were eluted at 9.0 and 10.1 min, respectively. From Fig. 4b&c, the peak area of L-DOPA from incubation with PC-12 cells decreased significantly compared with that from the incubation with the culture medium, suggesting that L-DOPA was metabolized effectively. Interestingly, it was safe to say that D-DOPA was not metabolized in PC-12 cells based on the almost identical peak areas of D-DOPA from the two incubation solutions (with or without PC-12 cells). It should be pointed out that all the peaks showed the typical MS spectrum of DOPA, that is, m/z 198 →181 and 152. Moreover, the peak identification was also verified by spiking the sample with authentic L-DOPA. To further investigate the metabolism of L-DOPA in this neuronal model, samples of the incubation solution were taken at different incubation times and analyzed. The electropherograms are shown in Fig. 5. As can be seen, L-DOPA concentration in the incubation solution decreased with the increase of incubation time. It was found that 88% of L-DOPA disappeared after incubation with PC-12 cells at a density of 2 × 106 cells/mL for 3 hrs. From a literature survey, little study has been done on DOPA metabolism in PC-12 cells, although L-DOPA toxicity to this cell line has been extensively studied [27–29]. The stereochemical preference in DOPA metabolism in PC-12 cells was demonstrated for the first time in this work.
Table 1.
Method Precision and Accuracy: Determination of D-/L-DOPA in PC-12 cell homogenate
| Intraday (n = 5)
| |||||||
|---|---|---|---|---|---|---|---|
| DOPA added (μM) | measured (μM) | accuracy (%) | precision (%RSD) | ||||
| D- | L- | D- | L- | D- | L- | D- | L- |
| 0.0 | 0.0 | ND | ND | ||||
| 10.0 | 100.0 | 9.6 | 102.1 | 96.0 | 102.1 | 3.9 | 1.7 |
| 50.0 | 50.0 | 50.2 | 50.7 | 100.4 | 101.4 | 1.3 | 2.1 |
| 100.0 | 10.0 | 98.5 | 10.3 | 98.5 | 103.0 | 1.1 | 2.8 |
| Interday (n = 5 in 7 days)
| |||||||
| DOPA added (μM) | measured (μM) | accuracy (%) | precision (%RSD) | ||||
| D- | L- | D- | L- | D- | L- | D- | L- |
| 0.0 | 0.0 | ND | ND | ||||
| 10.0 | 100.0 | 10.3 | 103.1 | 103.0 | 103.1 | 5.7 | 2.4 |
| 50.0 | 50.0 | 48.5 | 52.0 | 97.0 | 104.0 | 3.3 | 5.1 |
| 100.0 | 10.0 | 104.2 | 10.1 | 104.2 | 101.0 | 4.9 | 2.6 |
ND: not detected.
Fig. 4.

Electropherograms obtained from studying DOPA metabolism: (A) racemic DOPA standard solution (50.0 μM each enantiomer), (B) 500 μM racemic DOPA incubated with the culture medium for 2 hrs, and (C) 500 μM racemic DOPA incubated with PC-12 cells (2 × 106 cells/mL) for 2 hrs. Chiral CE-MS/MS conditions were as in Figure 2.
Fig. 5.
Typical electropherograms from analyzing an incubation solution of L-DOPA (at 500 μM) with PC-12 cells for different time: (a), 15 min; (b) 30 min: (c), 60 min; (d) 90 min; (e) 120 min; (e) 180 min. Chiral CE-MS/MS conditions were as in Figure 2.
Conclusion
A chiral CE-MS/MS method was developed for simultaneous enantiomeric quantification of DOPA and its precursors, Tyr and Phe. Use of a negatively charged chiral selector, i.e. sulfated β-CD, in combination with deploying the partially filling technique avoided potential problems associated with contamination of the MS detector. The proposed method was shown useful in assessing enantiomeric purity of levodopa, a therapeutic drug for treating Parkinson’s disease, for drug quality control purpose. In studying the metabolism of DOPA in PC-12 cells, the chiral CE-MS/MS analysis revealed that L-DOPA was effectively metabolized by PC-12 cells, but D-DOPA was not. To the best of our knowledge, this is the first demonstration of enantioselective metabolism of DOPA in the in vitro neuronal model.
Acknowledgments
Financial support from NIH grants (SC1 GM089557 and partially 2G12RR013459 RCMI at JSU) is gratefully acknowledged.
Abbreviations used
- CE
capillary electrophoresis
- MS
mass spectrometry
- ESI
electrospray ionization
- CD
cyclodextrin
- DOPA
3,4-Dihydroxyphenylalanine
- Tyr
tyrosine
- Phe
phenylalanine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Poewe W, Antonini A, Zijlmans JC, Burkhard PR, Vingerhoets F. Levodopa in the treatment of Parkinson’s disease: an old drug still going strong. Clin Interv Aging. 2010;5:229–238. doi: 10.2147/cia.s6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pezzoli G, Zini M. Levodopa in Parkinson’s disease: from the past to the future. Expert Opin Pharmacother. 2010;11:627–635. doi: 10.1517/14656561003598919. [DOI] [PubMed] [Google Scholar]
- 3.Alexander T, Sortwell CE, Sladek CD, Roth RH, Steece-Collier K. Comparison of neurotoxicity following repeated administration of l-dopa, d-dopa and dopamine to embryonic mesencephalic dopamine neurons in cultures derived from Fisher 344 and Sprague-Dawley donors. Cell Transplant. 1997;6:309–315. doi: 10.1177/096368979700600313. [DOI] [PubMed] [Google Scholar]
- 4.Spiers AS. Ineffectiveness of dextrodopa in chronic granulocytic leukaemia. Aust N Z J Med. 1974;4:475–478. doi: 10.1111/j.1445-5994.1974.tb03220.x. [DOI] [PubMed] [Google Scholar]
- 5.Török R, Berkecz R, Péter A. Enantioseparation of phenylalanine analogs on a quinine-based anion-exchanger chiral stationary phase: structure and temperature effects. J Sep Sci. 2006;29:2523. doi: 10.1002/jssc.200600100. [DOI] [PubMed] [Google Scholar]
- 6.McMurtrey K, Strawbridge C, McCoy J. HPLC resolution of the enantiomers of dihydroxyphenylalanine and selected salsolinol derivatives using sulfated beta-cyclodextrin. Enantiomer. 2000;5:377–383. [PubMed] [Google Scholar]
- 7.Wang J, Fang Y. Determination, purity assessment and chiral separation of levodopa methyl ester in bulk and formulation pharmaceuticals. Biomed Chromatogr. 2006;20:904–910. doi: 10.1002/bmc.617. [DOI] [PubMed] [Google Scholar]
- 8.Ghassempour A, Alizadeh R, Najafi NM, Karami A, Römpp A, Spengler B, Aboul-Enein HY. Crystalline degradation products of vancomycin as chiral stationary phase in microcolumn liquid chromatography. J Sep Sci. 2008;31:2339–2345. doi: 10.1002/jssc.200800185. [DOI] [PubMed] [Google Scholar]
- 9.Blanco M, Valverde I. Chiral and non chiral determination of Dopa by capillary electrophoresis. J Pharm Biomed Anal. 2003;31:431. doi: 10.1016/s0731-7085(02)00722-7. [DOI] [PubMed] [Google Scholar]
- 10.Dolezalová M, Fanali S. Enantiomeric separation of dihydroxyphenylalanine (DOPA), methyldihydroxyphenylalanine (MDOPA) and hydrazinomethyldihydroxyphenylalanine (CDOPA) by using capillary electrophoresis with sulfobutyl ether-beta-cyclodextrin as a chiral selector. Electrophoresis. 2000;21:3264. doi: 10.1002/1522-2683(20000901)21:15<3264::AID-ELPS3264>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 11.La S, Ahn S, Kim JH, Goto J, Choi OK, Kim KR. Enantioseparation of chiral aromatic amino acids by capillary electrophoresis in neutral and charged cyclodextrin selector modes. Electrophoresis. 2002;23:4123. doi: 10.1002/elps.200290030. [DOI] [PubMed] [Google Scholar]
- 12.Wongwan S, Hammitzsch-Wiedemann M, Scriba GKE. Determination of related substances of levodopa including the R-enantiomer by CE. Electrophoresis. 2009;30:3891–3897. doi: 10.1002/elps.200900060. [DOI] [PubMed] [Google Scholar]
- 13.Ream PJ, Suljak SW, Ewing AG, Han KA. Micellar electrokinetic capillary chromatography-electrochemical detection for analysis of biogenic amines in Drosophila melanogaster. Anal Chem. 2003;75:3972–3978. doi: 10.1021/ac034219i. [DOI] [PubMed] [Google Scholar]
- 14.Borst C, Holzgrabe U. Enantioseparation of dopa and related compounds by cyclodextrin-modified microemulsion electrokinetic chromatography. J Chromatogr A. 2008;1204:191–196. doi: 10.1016/j.chroma.2008.05.052. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz MA, Hauser PC. Chiral on-chip separations of neurotransmitters. Anal Chem. 2003;75:4691–4695. doi: 10.1021/ac030148b. [DOI] [PubMed] [Google Scholar]
- 16.Johirul M, Shiddiky A, Kim RE, Shim YB. Microchip capillary electrophoresis with a cellulose-DNA-modified screen-printed electrode for the analysis of neurotransmitters. Electrophoresis. 2005;26:3043–3052. doi: 10.1002/elps.200410438. [DOI] [PubMed] [Google Scholar]
- 17.Visky D, Jimidar I, Van Ael W, Vennekens T, Redlich D, De Smet M. Capillary electrophoresis-mass spectrometry in impurity profiling of pharmaceutical products. Electrophoresis. 2005;26:1541–1549. doi: 10.1002/elps.200410225. [DOI] [PubMed] [Google Scholar]
- 18.Simó C, García-Cañas V, Cifuentes A. Chiral CE-MS. Electrophoresis. 2010;31:1442–1456. doi: 10.1002/elps.200900673. [DOI] [PubMed] [Google Scholar]
- 19.Simó C, Rizzi A, Barbas C, Cifuentes A. Chiral capillary electrophoresis-mass spectrometry of amino acids in foods. Electrophoresis. 2005;26:1432–1441. doi: 10.1002/elps.200406199. [DOI] [PubMed] [Google Scholar]
- 20.Desiderio C, Iavarone F, Rossetti DV, Messana I, Castagnola M. Capillary electrophoresis-mass spectrometry for the analysis of amino acids. J Sep Sci. 2010;33:2385–2393. doi: 10.1002/jssc.201000171. [DOI] [PubMed] [Google Scholar]
- 21.Wakayama M, Aoki N, Sasaki H, Ohsugi R. Simultaneous analysis of amino acids and carboxylic acids by capillary electrophoresis-mass spectrometry using an acidic electrolyte and uncoated fused-silica capillary. Anal Chem. 2010;82:9967–76. doi: 10.1021/ac1019039. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka Y, Otsuka K, Terabe S. Separation of enantiomers by capillary electrophoresis-mass spectrometry employing a partial filling technique with a chiral crown ether. J Chromatogr A. 2000;875:323–330. doi: 10.1016/s0021-9673(99)01334-5. [DOI] [PubMed] [Google Scholar]
- 23.Rudaz S, Cherkaoui S, Gauvrit JY, Lantéri P, Veuthey JL. Experimental designs to investigate capillary electrophoresis-electrospray ionization-mass spectrometry enantioseparation with the partial-filling technique. Electrophoresis. 2001;22:3316–3326. doi: 10.1002/1522-2683(200109)22:15<3316::AID-ELPS3316>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 24.Ha PT, Van Schepdael A, Hauta-Aho T, Roets E, Hoogmartens J. Simultaneous determination of dopa and carbidopa enantiomers by capillary zone electrophoresis. Electrophoresis. 2002;23:3404–3409. doi: 10.1002/1522-2683(200210)23:19<3404::AID-ELPS3404>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Fang Y. Determination, purity assessment and chiral separation of levodopa methyl ester in bulk and formulation pharmaceuticals. Biomed Chromatogr. 2006;20:904–910. doi: 10.1002/bmc.617. [DOI] [PubMed] [Google Scholar]
- 26.Martínez-Gómez MA, Sagrado S, Villanueva-Camañas RM, Medina-Hernández MJ. Enantioseparation of phenotiazines by affinity electrokinetic chromatography using human serum albumin as chiral selector: application to enantiomeric quality control in pharmaceutical formulations. Anal Chim Acta. 2007;582:223–228. doi: 10.1016/j.aca.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 27.Vatassery GT, Smith WE, Quach HT. Effect of oxidative stress induced by L-dopa on endogenous antioxidants in PC-12 cells. Ann N Y Acad Sci. 2006;1074:330–336. doi: 10.1196/annals.1369.030. [DOI] [PubMed] [Google Scholar]
- 28.Lee JJ, Kim YM, Park SK, Lee MK. Effects of tributyltin chloride on L-DOPA-induced cytotoxicity in PC12 cells. Arch Pharm Res. 2006;29:645–650. doi: 10.1007/BF02968248. [DOI] [PubMed] [Google Scholar]
- 29.Oli RG, Fazeli G, Kuhn W, Walitza S, Gerlach M, Stopper H. No increased chromosomal damage in L-DOPA-treated patients with Parkinson’s disease: a pilot study. J Neural Transm. 2010;117:737–746. doi: 10.1007/s00702-010-0401-z. [DOI] [PubMed] [Google Scholar]