Abstract
The bacterial enzyme chondroitinase ABC (ChABC), which cleaves chondroitin sulfate glycosaminoglycan chains, can degrade inhibitory scar tissue formed following spinal cord injury, thereby promoting axonal growth and regeneration. However, delivering the active enzyme for prolonged periods presents practical limitations. To overcome these problems, we prepared a lentiviral vector (LV) encoding chondroitinase AC (Chase) together with the green fluorescent protein (GFP) reporter (Chase/LV) and demonstrated its expression and enzymatic activity in vitro and in vivo. Neural precursor cells infected with Chase/LV expressed the GFP reporter at levels that increased dramatically with time in culture. Enzymatic activity from the supernatant of the infected cells was demonstrated by dot blot assay using an antibody that recognizes the digested form of CSPG and was compared with the bacterial ChABC enzyme. Chick DRG cultures plated adjacent to the CSPG border and incubated with supernatant from Chase/LV-infected cells showed neurites growing into the CSPG area, a response similar to that after treatment with ChABC. In contrast, in control cultures, the neurites turned to avoid the inhibitory CSPG interface. Degradation of CSPG in these cultures was confirmed by specific CSPG antibodies. A single injection of Chase/LV into the spinal cord resulted in sustained secretion of the enzyme, whose activity was detected for 8 weeks by expression of GFP and evidence of the digested form of CSPG. This study demonstrates the efficacy of the Chase/LV vector and its potential as a therapeutic tool to reduce scar inhibition and promote axonal growth and repair following central nervous system injury.
Keywords: Chondroitin sulfate proteoglycan, chondroitinase, axonal growth, spinal cord injury, gene therapy
1. Introduction
Promoting axonal regeneration following injury to the adult mammalian central nervous system (CNS) remains a therapeutic challenge (Franz et al., 2011). A major obstacle to regeneration is the inhibitory environment that develops around the lesion area, which includes myelin proteins and the astroglial scar (Properzi et al., 2003; Sandvig et al., 2004; Silver and Miller, 2004). The scar formed after CNS injury by various neural and immune cells is a dense tissue that presents physical and chemical barriers for axonal growth and regeneration. Chondroitin sulfate proteoglycans (CSPGs), which are major inhibitory components of the scar, are extracellular matrix molecules with a central core protein attached to chondroitin sulfate (CS) and glycosaminoglycans (GAGs) (Bandtlow and Zimmermann, 2000), which are upregulated around the injury site in both brain and spinal cord (Asher et al., 2000; Jones et al., 2003a). The inhibitory effect of CSPGs on axonal regeneration is mediated mainly by the GAGs. The bacterial enzyme chondroitinase ABC (ChABC) can degrade the CSPGs by cleaving the GAG chains without altering the core protein structure. Treatment with ChABC promotes neurite outgrowth and axonal regeneration both in vitro and in vivo (Houle et al., 2006; McKeon et al., 1995; Moon et al., 2001; Yick et al., 2003). However, a single injection of ChABC may not be enough because of the rapid, significant decline of enzymatic activity at body temperature (Tester et al., 2007). In addition, delivery of ChABC by intrathecal injection may not reach the deeper regions in the CNS and may cause side effects (Jones and Tuszynski, 2001).
Gene therapy has often been used to facilitate the sustained delivery of therapeutic molecules by in vivo or ex vivo strategies (Hendriks et al., 2004). For example, viral vectors encoding neurotrophic factors have been used effectively to genetically modify various cells and graft them to promote axonal growth in models of spinal cord injury (Blesch and Tuszynski, 2007; Hendriks et al., 2004). Lentiviral vectors (LVs) offer a number of advantages for gene therapy because of their efficiency of infection and the low level of induced immune response in the host (Hendriks et al., 2007).
We prepared a LV encoding the secreted form of chondroitinase AC (Chase/LV) based on the mammalian construct of this enzyme (Curinga et al., 2007). The vector includes the green fluorescent protein (GFP) gene downstream from an internal ribosomal entry site sequence to better identify infected cells both in vitro and in vivo. We tested the enzymatic activity of the secreted Chase/LV in vitro and in vivo. Immunological and functional assays showed that Chase/LV released by infected neural precursor cells (NPCs) degraded CSPGs and promoted dorsal root ganglia (DRG) axonal growth into the CSPG interface. Injection of Chase/LV into the spinal cord showed that infected cells can be identified by GFP expression and correlated with the enzymatic activity, which is evident from 1 to 8 weeks after injection of the vector. We concluded that the Chase/LV vector can be a useful tool for promoting axonal regeneration, plasticity, and functional recovery in different models of CNS injuries.
2. Materials and methods
2.1. Construction of Chase lentivirus
The ChaseAC cDNA (Acorda Therapeutics, Hawthorne, NY, USA) was codon enhanced for mammalian gene expression and modified to contain an IgK leader for secretion and a flag epitope-tagged C-terminus as described previously (Curinga et al., 2007). The ChaseAC was cloned into a LV backbone to obtain the pLv.Chase plasmid. This vector was then modified to include the enhanced GFP reporter downstream of an internal ribosomal entry site derived from the pIRES2-eGFP (Clonetech Laboratories, Mountain View, CA, USA) to obtain the pLv.Chase. IRES.eGFP plasmid (Fig. 1). Virus was prepared by transfection of 293FT cells with the lentiviral plasmid using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) together with third generation lentiviral packaging plasmids, reaching 3.3 to 7 × 105 TU/μl. High-titer virus stocks were prepared at the Protein Expression Core of the Wistar Institute (Philadelphia, PA, USA).
Fig. 1.
Map of the Chase/LV. The CAG promoter drives the expression of Chase enzymatic activity followed by the downstream expression of eGFP using the internal ribosomal entry site (IRES). CAG promoter, CMV enhancer/chicken β-actin promoter; eGFP, enhanced green fluorescent protein; LTR, long terminal repeats; RSV, Rous sarcoma virus enhancer/promoter; WPRE, woodchuck posttranscriptional regulatory element.
2.2. Transduction of neural precursor cells
Neural precursor cells were isolated from embryonic day 13.5 transgenic Fischer 344 rats that express the human alkaline phosphatase reporter as described previously (Han et al., 2002, 2004; Lepore et al., 2004). Embryos were isolated in DMEM/F-12 (Invitrogen). Cords were dissociated using a 0.05% trypsin/EDTA (Invitrogen) solution for 20 min at 37°C. Cells were then plated in complete medium [DMEM/F-12, BSA (1 mg/ml; Sigma, St. Louis, MO), B27 (Invitrogen), basic fibroblast growth factor (bFGF) (20 ng/ml; Peprotech, Rocky Hill, NJ), penicillin–streptomycin (100 IU/ml; Invitrogen), N2 (10 μl/ml; Invitrogen); bFGF (10 ng/ml) and neurotrophin-3 (NT-3) (10 ng/ml; Peprotech)] on poly-L-lysine-coated (13.3 μg/ml; Sigma) and laminin-coated (20 μg/ml; Invitrogen) dishes. Cells were cultured for 2 to 3 days until they reached 80% confluence. Cells were then incubated with a mixture of Chase/LV (5 μl at 2.9 × 105 TU/μl), 5 ml of complete medium, and 5 μl of polybrene (8 mg/ml, Sigma) overnight. Medium was changed to complete medium the next day and every other day thereafter. All supernatants were collected and kept at −20°C for further use. Infected NPCs were examined daily until GFP expression was evident and then every other day for image analysis.
2.3. In vitro activity assay
Enzyme activity (dot blot assay, n=1): Nitrocellulose membranes were cut at 1 cm square and adsorbed with CSPG (cc117, 2.5 μg/2.5 μl; Millipore Billerica, MA) in a 24-well plate. Membranes were incubated in blocking buffer (5% milk phosphate-buffered saline) overnight at 4°C for 30 min to dry. Next day, membranes were washed with 0.05% Tween Tris-buffered saline twice, then incubated with the following solutions for 2 h at 37°C: 500 μl of supernatant from Chase/LV-infected NPCs, 500 μl of supernatant from normal NPCs, 500 μl of a mixture of commercially available bacterial ChABC (Sigma, 0.5 U in 500 μl of reaction buffer), and 500 μl of control chondroitinase reaction buffer (50 mM Tris, pH 8.0, 60 mM sodium acetate, and 0.02% bovine serum album). After they were washed 3 times with Tween Tris-buffered saline, membranes were incubated with the 3B3 antibody (1:1000; Seikagaku Biobusiness Corporation, Japan), followed by alkaline phosphatase-conjugated goat anti-mouse IgM (1:5000; Jackson ImmunoResearch, West Grove, PA, USA) for 1 h each. Membranes were developed for alkaline phosphatase reaction products with 100 mM Tris, 100 mM NaCl, and 50 mM MgCl2, pH 9.5, with 1.0 mg/ml nitroblue tetrazolium chloride, 0.1 mg/ml 5-bromo-4-chloro-indolyl-phosphate, and 5mM levamisole (Sigma). The reaction was stopped by washing membranes with dH2O.
2.4. Enzyme functional assay
Embryonic day 7 chicken DRG were cultured as explants to examine neurite outgrowth when exposed to a CSPG border. Coverslips were first precoated with 100 μg/ml poly-D-lysine (Sigma). CSPG borders were generated with 1-mm strips of filter paper (Fisherbrand, P5; Fisher Scientific, Pittsburgh, PA) saturated with 3 μl of 100-μg CSPG (CC117; Millipore). Following application of CSPG, the coverslips were coated with 25 μg/ml laminin (Invitrogen) for 1 h at 37°C. Following the preparation of substrata, coverslips were pretreated for 24 h with one of the following solutions (500 μl each): (1) supernatant from NPCs (n = 8); (2) supernatant from NPC/Chase/LV (n = 9); (3) ChABC enzyme (0.5 U) (n = 8). The explants were placed approximately 150 to 250 μm from the border of the CSPG with defined medium (F-12H with additives; Invitrogen) supplemented with 20 ng/ml nerve growth factor and mixed 1:1 with experimental solution as described above. Cultures were fixed for 24 h after DRG plating and double stained with anti-tubulin (Tuj1) to identify neurons (green; Covance, Princeton, NJ, USA); with CS-56 (red; Sigma) to identify CSPG; or with 3B3 (red; Seikagaku) to identify CSPG digestion.
2.5. In vivo expression
Adult female Sprague Dawley rats (225-250 g) were deeply anesthetized with a mixture of ketamine (25 mg/ml), xylazine (1.3 g/ml), and acepromazine (0.25 mg/ml). Laminectomy was performed at the cervical C3-4 level. Chase/LV (2 μl at 2.9 × 105 TU/μl) or GFP/LV (2 μl at 2 × 105 TU/μl) was injected into the spinal cord 1.5 mm lateral to the midline and 1 mm deep from the dura using a nanoliter injector (World Precision Instruments, Sarasota, FL, USA). Rats were killed 1 week after injection with GFP/LV (n = 1) and 1, 2, 4, and 8 weeks after injection with Chase/LV (n = 2 for 1, 8 weeks; n = 1 for 2, 4 weeks). Spinal cords were dissected and sectioned horizontally. Sections were stained with GFP (1:1500, Invitrogen) and 2B6 (1:500, Seikagaku) antibodies overnight at room temperature after treatment with 5% normal goat serum. Sections were incubated with goat anti-rabbit FITC and goat anti-mouse rhodamine (1:400, Jackson ImmunoResearch) for 2 h at room temperature. Sections were coverslipped with Vectashield (Vector Laboratories, Burlingame, CA, USA).
3. Results
NPCs were infected with Chase/LV overnight followed by a medium change next day and then every other day. GFP expression was first visible 3 days after infection, with few cells expressing GFP (Fig. 2A). The number of cells expressing GFP increased over time, with many cells showing GFP after 6 days (Fig. 2B) and more after 8 days, when the intensity of GFP expression had become high (Fig. 2C). The dot blot assay with the 3B3 antibody, which recognizes sulfated GAG stubs and therefore serves as a marker for successful digestion of CSPG, verified that CSPG was digested with the supernatant derived from NPCs infected with Chase/LVand cultured for 8 days (Fig. 2D, membrane 1) and the bacterial ChABC enzyme (Fig. 2D, membrane 2). In contrast, supernatant from uninfected NPC cultures (Fig. 2D, membrane 3) and the control ChABC reaction buffer (Fig. 2D, membrane 4) did not stain with the 3B3 antibody. These results indicate that neural stem cells can be genetically modified by the Chase/LV vector and secrete an active chondroitinase.
Fig. 2.
Immunological assay of Chase/LV properties. NPCs infected with Chase/LV showed few cells expressing GFP after 3 days (A). The number of cells expressing GFP increased at 6 days (B) and 8 days (C) in culture. A dot blot assay showed CSPG digestion in membranes treated with supernatant from NPCs expressing Chase/LV, where CSPG digestion was identified by the 3B3 antibody (D, blot 1). A similar pattern of digestion was obtained with the bacterial chondroitinase enzyme ChABC (D, blot3), whereas neither uninfected NPC culture medium nor control reaction buffer was positive for 3B3 staining (D, blot 2 and blot 4, respectively).
We next tested the Chase/LV-induced enzymatic activity in a functional assay of neurite outgrowth in vitro (Fig. 3A-F). Chick DRGs were dissected from day-7 embryos and cultured adjacent to an inhibitory CSPG border pretreated with various experimental media. The DRG were cultured for 24 h and then stained with Tuj and CS-56 or 3B3 antibodies to determine axonal growth from the DRG and the levels of and digestion of CSPG, respectively. Treatment with uninfected NPC culture medium as control allowed neurites from the DRG explant to grow in all directions except in the CSPG-rich region, where only a few short fibers were present, most of which turned away. Among the eight DRG cultures, all neurites from DRG explants turned at the border of the CSPG. The region of the CSPG was stained heavily by the CS-56 antibody (Fig. 3A, red), confirming the presence of CSPG, but was negative for 3B3 staining, indicating that no CSPG digestion had occurred (Fig. 3D). In contrast, CSPG treated with supernatant from NPCs infected with Chase/LV was partially digested, showing reduced CS-56 staining (Fig. 3B, red) and positive staining by 3B3 (Fig. 3E, red). In this case, neurites from the DRG explant grew in all directions, including the region that had the CSPG border. Analysis of nine DRG cultures showed that neurites from seven DRG explants consistently crossed the border of the CSPG and extended into the CSPG region. Neurites from two DRG explants showed 50% of the neurites across the border of the CSPG. As expected, the bacterial enzyme ChABC completely digested the CSPG; the area was therefore negative for CS-56 staining (Fig. 3C) and strongly positive for 3B3 staining (Fig. 3F, red). Neurites from the DRG explant grew in all directions, which was comparable to the growth observed with the enzyme produced by the Chase/LV vector. Analysis of eight DRG cultures showed that all the neurites crossed the border of the CSPG. These results demonstrate a functional outcome for the secreted chondroitinase produced by the Chase/LV vector with respect to reversing the inhibition of axonal growth by the CSPG environment.
Fig. 3.
Functional assay of Chase/LV properties. Coverslips precoated with poly-D-lysine were coating with CSPGs using a 1-mm thick strip of filter paper saturated with CSPG and then coated with laminin. Coverslips were pretreated for 24 h with one of following supernatants: (1) NPC culture medium; (2) NPC/Chase/LV; (3) ChABC enzyme. DRG from day 7 embryonic chicks were cultured at the border of the CSPG with defined medium, supplemented with 20 ng/ml nerve growth factor and mixed 1:1 with experimental supernatants. Cultures were fixed for 24 h after DRG plating and double stained with anti-tubulin (Tuj1) to identify neurons (green) together with CS-56 to identify CSPG or 3B3 to identify CSPG digestion. Chick DRG grew extensive neurites in all directions except in the CSPG-rich region (A, red area), which remained undigested (D, negative for 3B3 staining) when treated with culture medium. Although few fibers grew into the CSPG area, most of them turned to avoid the CSPG. However, when treated with medium obtained from NPCs infected with Chase/LV, CSPGs were partially digested (B, CS-56 staining, red, and E, 3B3 staining, red), thereby allowing DRG axons to grow into the digested CSPG region. Treatment with ChABC completely digested the CSPG (C, with CS-56 negative staining and strong 3B3 positive staining) and allowed DRG to grow in all directions.
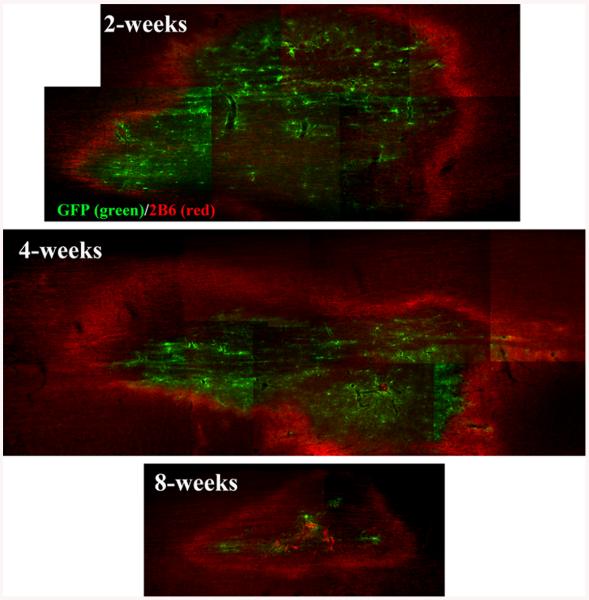
Finally, we examined whether direct injection in vivo of the Chase/LV vector will result in CSPG digestion by the enzyme secreted from infected host cells. Chase/LV or control GFP/LV vectors were injected into the intact cervical spinal cord, and the animals were analyzed with respect to GFP expression and digestion of CSPG. One week after injection of the vector, GFP expression was detected at the injection area in both Chase and GFP animals. Double staining with GFP and 2B6 was performed to localize the region of viral infection and to assess the enzymatic activity of Chase using the 2B6 antibody, which recognizes degradation products of CSPG. We found strong staining with 2B6 around the region of GFP expression in animals infected with Chase/LV (Fig. 4, right panels) but no 2B6 staining in animals infected with GFP/LV (Fig. 4, left panels). These results suggest that host cells infected with the Chase/LV vector secreted an active chondroitinase enzyme. Similar patterns of GFP/2B6 staining were found 2, 4, and 8 weeks after Chase/LV injection (Fig. 5), indicating that the Chase transgene enzyme was still expressed and remained active for up to 8 weeks in vivo. However, the expression levels were much lower at 8 weeks as indicated by reduced GFP and 2B6 staining.
Fig. 4.
Chase/LV expression in vivo. Chase/LV and GFP/LV were injected into the spinal cord and analyzed after1 week. Double staining with GFP (green) and 2B6 (red) showed that GFP was expressed in the spinal cords infected with GFP/LV or Chase/LV, but intense 2B6 staining around the GFP–positive area was present only in Chase/LV animals (right middle), not in GFP/LV animals (left middle). These data suggest that host cells infected with Chase/LV secrete an active enzyme that degrades CSPG.
Fig. 5.
Time course of Chase expression in vivo. At 2, 4, and 8 weeks after injection of Chase/LV into spinal cords, horizontal sections were double stained with 2B6 (red) and GFP (green). The analysis showed sustained CSPG digestion by Chase around GFP expressing cells from 2 weeks to 8 weeks after injection, suggesting that Chase/LV remained active in vivo for up to 8 weeks.
4. Discussion
CSPG is a major component of the extracellular matrix, serving to maintain the structural integrity of tissue. However, the upregulation of CSPGs around the lesion area following central and peripheral nervous system injuries (Jones et al., 2003b; Pindzola et al., 1993) results in the formation of a dense scar, which together with other inhibitory molecules, impedes axonal growth and regeneration and may also diminish conduction of intact axons (Hunanyan et al., 2010). Treatment with the bacterial enzyme ChABC after spinal cord injury degrades CSPG, reduces the scar, and promotes plasticity (Bradbury et al., 2002; Moon et al., 2001), thereby providing a promising therapeutic opportunity (Bradbury and Carter, 2010). However, the delivery of an active enzyme for prolonged periods presents practical limitations for effective clinical applications. For example, the use of ChABC by direct injection into the lesion area often requires repeated injections to effectively digest CSPG because ChABC is thermally sensitive, and the degrading activity of ChABC is significantly decreased at body temperature over time (Tester et al., 2007). Similarly, the prolonged delivery of ChABC using intrathecal catheters results in limited diffusion superficially and may be accompanied by side effects (Jones and Tuszynski, 2001).
Some of these limitations can be addressed by preparing a stable form of ChABC (Lee et al., 2010) or by using scaffolds for controlled delivery of the enzyme (Hyatt et al., 2010). Using gene therapy methods to deliver a secreted form of the enzyme by viral vectors is highly advantageous because it allows sustained, controlled delivery by direct injection to transduce host cells or by transplants of genetically modified cells that combine the benefits of the graft and the enzyme. In a previous study, an adenoviral vector encoding the Chase, was characterized with respect to its enzymatic activity and ability to reduce axonal inhibition in vitro (Curinga et al., 2007). The study showed that mammalian cells (human astrocytoma U373) secreted an enzymatically active enzyme but that cell supernatants had to be concentrated (29 X) to demonstrate the enzymatic activity. To facilitate the potential application of Chase by an effective gene therapy approach in vivo, the modified Chase with codon enhanced for mammalian gene expression was prepared in a lentiviral construct, which also contained a GFP reporter (Chase/LV); this process improved both the efficacy and the detection of the expression of Chase. Lentiviral vectors stably and efficiently transduce a wide range of dividing and nondividing cells, resulting in long-term expression of the transgene (Hendriks et al., 2004) with no immune response or detectable pathological effects (Baekelandt et al., 2003).
We demonstrated that NPCs and host cells in the spinal cord can be transduced by Chase/LV with GFP serving as a marker for cells expressing Chase. The genetic modification of NPC to express Chase is particularly important because NPC transplants have been used as a promising therapeutic approach in spinal cord injury; they resulted in a significant improvement of bladder and motor function in rats with a contusion injury (Mitsui et al., 2005) and show potential for neuronal cell replacement and connectivity (Bonner et al., 2011). Obtaining NPCs that secrete Chase will allow the use of these and other cells in patients with chronic injuries. The secreted form of Chase was present in the supernatant at relatively high concentrations and could be used without concentration to show CSPG digestion by immunological detection of sulfated GAG stubs and by a functional assay demonstrating DRG neurites growing into the CSPG interface. Both the immunological and the functional assays yielded comparable results with the bacterial enzyme ChABC. The convenience and efficacy of the vector were evident when a single injection of Chase/LV into the spinal cord transduced many host cells to express GFP and secrete Chase for up to 8 weeks. However, although the levels of GFP expression and Chase enzymatic activity remained high for at least 4 weeks, they were reduced at 8 weeks, showing the staining only in the epicenter of the injection area. Importantly, the in vivo data suggest that the secreted enzymatic activity of Chase, in contrast to the native bacterial ChABC, remained active at body temperature (Tester et al., 2007). These results are consistent with those from a previous study (Curinga et al., 2007) showing that the enzymatic activity of Chase secreted by mammalian cells was reduced at lower temperatures but was maintained at increased temperatures up to 39°C.
Our in vivo data show that host CNS cells can be effectively transduced to secrete an active form of the chondroitinase capable of digesting CSPG for prolonged periods using a single injection of the Chase/LV. Following CNS injuries, most of the upregulated CSPGs is produced by reactive astrocytes in the glial scar. Delivering Chase into these cells using the Chase/LV vector at and around the lesion will allow the sustained secretion of the enzyme without repeated injections or the use of infusion by an intrathecal catheter. This strategy has the potential to become a useful therapeutic tool for reducing scar inhibition and promoting axonal growth and repair following CNS injury.
Acknowledgements
A preliminary form of this work was presented at the Meeting of the Society for Neuroscience 2008 as an abstract “Engineering chondroitinase to enhance axon regeneration,” by GM Curinga, Y Jin, C Zhang, C Mashburn, DM Snow and GM Smith. The present study was funded by NIH (SP01NS055976) and the CHN Foundation #160746.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asher RA, Morgenstern DA, Fidler PS, Adcock KH, Oohira A, Braistead JE, et al. Neurocan is upregulated in injured brain and in cytokine-treated astrocytes. J Neurosci. 2000;20:2427–38. doi: 10.1523/JNEUROSCI.20-07-02427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekelandt V, Eggermont K, Michiels M, Nuttin B, Debyser Z. Optimized lentiviral vector production and purification procedure prevents immune response after transduction of mouse brain. Gene Ther. 2003;10:1933–40. doi: 10.1038/sj.gt.3302094. [DOI] [PubMed] [Google Scholar]
- Bandtlow CE, Zimmermann DR. Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol Rev. 2000;80:1267–90. doi: 10.1152/physrev.2000.80.4.1267. [DOI] [PubMed] [Google Scholar]
- Blesch A, Tuszynski MH. Transient growth factor delivery sustains regenerated axons after spinal cord injury. J Neurosci. 2007;27:10535–45. doi: 10.1523/JNEUROSCI.1903-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JF, Connors TM, Silverman WF, Kowalski DP, Lemay MA, Fischer I. Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. J Neurosci. 2011;31:4675–86. doi: 10.1523/JNEUROSCI.4130-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, Carter LM. Manipulating the glial scar: Chondroitinase ABC as a therapy for spinal cord injury. Brain Res Bull. 2011;84:306–16. doi: 10.1016/j.brainresbull.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–40. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Curinga GM, Snow DM, Mashburn C, Kohler K, Thobaben R, Caggiano AO, et al. Mammalian-produced chondroitinase AC mitigates axon inhibition by chondroitin sulfate proteoglycans. J Neurochem. 2007;102:275–88. doi: 10.1111/j.1471-4159.2007.04530.x. [DOI] [PubMed] [Google Scholar]
- Franz S, Weidner N, Blesch A. Gene therapy approaches to enhancing plasticity and regeneration after spinal cord injury. Exp Neurol. 2011 Jan 31; doi: 10.1016/j.expneurol.2011.01.015. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SS, Kang DY, Mujtaba T, Rao MS, Fischer I. Grafted lineage-restricted precursors differentiate exclusively into neurons in the adult spinal cord. Exp Neurol. 2002;177:360–75. doi: 10.1006/exnr.2002.7995. [DOI] [PubMed] [Google Scholar]
- Han SS, Liu Y, Tyler-Polsz C, Rao MS, Fischer I. Transplantation of glial-restricted precursor cells into the adult spinal cord: survival, glial-specific differentiation, and preferential migration in white matter. Glia. 2004;45:1–16. doi: 10.1002/glia.10282. [DOI] [PubMed] [Google Scholar]
- Hendriks WT, Eggers R, Carlstedt TP, Zaldumbide A, Tannemaat MR, Fallaux FJ, et al. Lentiviral vector-mediated reporter gene expression in avulsed spinal ventral root is short-term, but is prolonged using an immune “stealth” transgene. Restor Neurol Neurosci. 2007;25:585–99. [PubMed] [Google Scholar]
- Hendriks WT, Ruitenberg MJ, Blits B, Boer GJ, Verhaagen J. Viral vector-mediated gene transfer of neurotrophins to promote regeneration of the injured spinal cord. Prog Brain Res. 2004;146:451–76. doi: 10.1016/S0079-6123(03)46029-9. [DOI] [PubMed] [Google Scholar]
- Houle JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J. Combining an autologous peripheral nervous system “bridge” and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci. 2006;26:7405–15. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunanyan AS, Garcia-Alias G, Alessi V, Levine JM, Fawcett JW, Mendell LM, et al. Role of chondroitin sulfate proteoglycans in axonal conduction in mammalian spinal cord. J Neurosci. 2010;30:7761–9. doi: 10.1523/JNEUROSCI.4659-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt AJ, Wang D, Kwok JC, Fawcett JW, Martin KR. Controlled release of chondroitinase ABC from fibrin gel reduces the level of inhibitory glycosaminoglycan chains in lesioned spinal cord. J Control Release. 2010;147:24–9. doi: 10.1016/j.jconrel.2010.06.026. [DOI] [PubMed] [Google Scholar]
- Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol. 2003a;182:399–411. doi: 10.1016/s0014-4886(03)00087-6. [DOI] [PubMed] [Google Scholar]
- Jones LL, Sajed D, Tuszynski MH. Axonal regeneration through regions of chondroitin sulfate proteoglycan deposition after spinal cord injury: a balance of permissiveness and inhibition. J Neurosci. 2003b;23:9276–8. doi: 10.1523/JNEUROSCI.23-28-09276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LL, Tuszynski MH. Chronic intrathecal infusions after spinal cord injury cause scarring and compression. Microsc Res Tech. 2001;54:317–24. doi: 10.1002/jemt.1144. [DOI] [PubMed] [Google Scholar]
- Lee H, McKeon RJ, Bellamkonda RV. Sustained delivery of thermostabilized ChABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2010;107:3340–5. doi: 10.1073/pnas.0905437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore AC, Han SS, Tyler-Polsz CJ, Cai J, Rao MS, Fischer I. Differential fate of multipotent and lineage-restricted neural precursors following transplantation into the adult CNS. Neuron Glia Biol. 2004;1:113–26. doi: 10.1017/s1740925x04000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon RJ, Hoke A, Silver J. Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp Neurol. 1995;136:32–43. doi: 10.1006/exnr.1995.1081. [DOI] [PubMed] [Google Scholar]
- Mitsui T, Shumsky JS, Lepore AC, Murray M, Fischer I. Transplantation of neuronal and glial restricted precursors into contused spinal cord improves bladder and motor functions, decreases thermal hypersensitivity, and modifies intraspinal circuitry. J Neurosci. 2005;25:9624–36. doi: 10.1523/JNEUROSCI.2175-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon LD, Asher RA, Rhodes KE, Fawcett JW. Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nat Neurosci. 2001;4:465–66. doi: 10.1038/87415. [DOI] [PubMed] [Google Scholar]
- Pindzola RR, Doller C, Silver J. Putative inhibitory extracellular matrix molecules at the dorsal root entry zone of the spinal cord during development and after root and sciatic nerve lesions. Dev Biol. 1993;156:34–48. doi: 10.1006/dbio.1993.1057. [DOI] [PubMed] [Google Scholar]
- Properzi F, Asher RA, Fawcett JW. Chondroitin sulphate proteoglycans in the central nervous system: changes and synthesis after injury. Biochem Soc Trans. 2003;31:335–6. doi: 10.1042/bst0310335. [DOI] [PubMed] [Google Scholar]
- Sandvig A, Berry M, Barrett LB, Butt A, Logan A. Myelin-, reactive glia-, and scar-derived CNS axon growth inhibitors: expression, receptor signaling, and correlation with axon regeneration. Glia. 2004;46:225–51. doi: 10.1002/glia.10315. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–56. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Tester NJ, Plaas AH, Howland DR. Effect of body temperature on chondroitinase ABC’s ability to cleave chondroitin sulfate glycosaminoglycans. J Neurosci Res. 2007;85:1110–8. doi: 10.1002/jnr.21199. [DOI] [PubMed] [Google Scholar]
- Yick LW, Cheung PT, So KF, Wu W. Axonal regeneration of Clarke’s neurons beyond the spinal cord injury scar after treatment with chondroitinase ABC. Exp Neurol. 2003;182:160–8. doi: 10.1016/s0014-4886(02)00052-3. [DOI] [PubMed] [Google Scholar]