Abstract
Astrocytes are known to express the gap junction forming proteins connexin30 (Cx30) and connexin43 (Cx43), but it has remained controversial whether these cells also express connexin26 (Cx26). To further investigate this issue, we examined immunofluorescence labelling of glial connexins in wild-type vs transgenic mice with targeted deletion of Cx26 in neuronal and glial cells (Cx26fl/fl:Nestin-Cre mice). The Cx26 antibodies utilized specifically recognized Cx26 and lacked cross reaction with highly homologous Cx30, as demonstrated by immunoblotting and immunofluorescence in Cx26-transfected and Cx30-transfected C6 glioma cells. Punctate immunolabelling of Cx26 with these antibodies was observed in leptomeninges and subcortical brain regions. This labelling was absent in subcortical areas of Cx26fl/fl:Nestin-Cre mice, but persisted in leptomeningeal tissues of these mice, thereby distinguishing localization of Cx26 between parenchymal vs non-parenchymal tissue. In subcortical brain parenchyma, Cx26-positive puncta were often co-localized with astrocytic Cx43, and some were localized along astrocyte cell bodies and processes immunolabelled for glial fibrillary acidic protein (GFAP). Cx26-positive puncta were also co-localized with punctate labelling of Cx47 around oligodendrocyte somata. Comparisons of Cx26 labelling in rodent species revealed a lower density of Cx26-positive puncta and a more restricted distribution in subcortical regions of mouse compared with rat brain, perhaps partly explaining reported difficulties in detection of Cx26 in mouse brain parenchyma using antibodies or Cx26 gene reporters. These results support our earlier observations of Cx26 expression in astrocytes and its ultrastructural localization in individual gap junction plaques formed between astrocytes as well as in heterotypic gap junctions between astrocytes and oligodendrocytes.
Keywords: gap junctions, glial cells, connexin26
Introduction
Macroglial cells in the central nervous system (CNS) are highly interconnected by gap junctions, creating what has been described as a functional panglial network (Giaume & Theis, 2010; Rash, 2010; Maglione et al., 2010). Multiple gap junction-forming connexin proteins participate in the establishment of these networks, such that Cx30 and Cx43 expressed in astrocytes contribute to the formation of homotypic astrocyte-astrocyte (A/A) gap junctions as well as to heterotypic astrocyte-oligodendrocyte (A/O) gap junctions with Cx32 and Cx47 expressed in oligodendrocytes (Scherer et al., 1995; Li et al., 1997, 2008; Nagy & Rash, 2000; Altevogt et al., 2002; Altevogt & Paul, 2004; Nagy et al., 2004; Kleopa et al., 2004). Cx29 is also expressed in oligodendrocytes, but appears to be incapable of forming functional gap junctions (Altevogt et al., 2002; Ahn et al., 2008). The complex subcellular compartments as which macroglial cells form gap junctions and targeting of connexins to these compartments has been extensively investigated (Mugnaini, 1986; Yamamoto et al., 1990; Wolburg & Rohlmann, 1995; Rash et al., 1997, 2001a; Kamasawa et al., 2005; reviewed in Theis et al., 2005; Orthmann-Murphy et al., 2008; Rash, 2010). This body of knowledge has provided the basis for understanding numerous anatomical and physiological impairments resulting from glial connexin gene deletion in mice (Sutor et al., 2000; Menichella et al., 2003, 2006; Dere et al., 2003; Frisch et al., 2003; Theis et al., 2003; Odermatt et al., 2003; Wallraff et al., 2006, Rouach et al., 2008) and the basis for CNS diseases caused by mutations in glial connexin genes in humans (Bahr et al., 1999; Paulson et al., 2002; Uhlenberg et al., 2004; Orthmann-Murphy et al., 2007, 2008; Paznekas et al., 2003, 2009).
Identification of each of the connexins expressed by glial cells is particularly important given the possibility that loss or mutation of one connexin may be compensated to some extent by the presence or altered expression pattern of another at particular subcellular locations within the glial syncytium. In this regard, controversies surrounding the expression of Cx26 in astrocytes of rodent brain over the past decade have prevented focus of attention on possible contributions of this connexin to normal CNS function or to pathophysiology in CNS disease conditions. Thus, although well characterized in leptomeningeal cells (Spray et al., 1991; Rash et al., 2001b), data on expression, distribution and cellular localization of Cx26 in brain parenchyma has been surrounded with uncertainties; earlier disparate reports on this topic have been previously reviewed (Nagy et al., 2004). Our own comprehensive analyses of Cx26 in CNS parenchyma indicated lack of its expression in cerebral cortex, but showed moderate expression in subcortical brain regions, where it was found to be localized in A/A and O/A gap junctions (Nagy et al., 2001). Others have also reported the presence of Cx26 in brain parenchyma (Mercier & Hatton, 2001) and its localization at astrocyte gap junctions (Altevogt & Paul, 2004). However, Fillipov et al. (2003) have reported absence of β-galactosidase detection in astrocytes of transgenic mice that had one allele of the Cx26 coding sequence replaced with the LacZ reporter DNA.
Here, we again address the question of Cx26 expression in brain and clarify issues centered on specificity of the Cx26 antibodies that have been used to explore Cx26 localization in the CNS. Specifically, we conducted immunohistochemical studies with transgenic mice in which Cx26 was deleted in CNS cells using Cx26fl/fl:Nestin-Cre mice.
Materials and methods
Antibodies and animals
The connexin antibodies used in this study were obtained from Invitrogen/Zymed Laboratories (Carlsbad, CA, USA), including those against Cx26 (Cat No. 51-2800 and 33-5800) the specificities of which have been reported (Nagy et al., 2001). We have previously shown that two additionally available Cx26 antibodies cross react with Cx30 (Nagy et al., 2001), and were therefore not used in the present study. Polyclonal anti-glial fibrillary acidic protein (GFAP) was obtained from Invitrogen (Camarillo, CA, USA). Cx26fl/fl female mice (Cohen-Salmon et al., 2002) were mated to male mice expressing the Cre recombinase under control of the rat Nestin promoter and enhancer (Tronche et al., 1999). Female Cx26+/fl mice were bred to Cx26+/fl:Nestin-Cre male mice to obtain Cx26fl/fl:Nestin-Cre mice and control littermates. A total of eight wild-type adult mice, five Cx26fl/fl:Nestin-Cre adult mice (Cx26(−/−)), and one Cx26(+/−) heterozygous adult C57BL/6 mouse were used in this study. Animals were utilized according to approved protocols by the Central Animal Care Committee of University of Manitoba, with minimization of the numbers animals used.
Light microscopic immunofluorescence
Immunohistochemistry was performed according to standard protocols as we have previously described (Penes et al., 2005; Li et al., 2004). For studies involving Cx26fl/fl:Nestin-Cre mice and their wild-type littermates, animals were deeply anesthetized by peritoneal injection of a 0.9% NaCl, 10% Ketavet, 2% xylazine hydrochloride solution. The mice were subsequently transcardially perfused with 3 ml PBS and the brains were removed and placed for 20 min into fixative containing 0.16 M sodium phosphate buffer, pH 7.6, 2% freshly depolymerized paraformaldehye, and 0.2% picric acid. Brains were then transferred to cryoprotectant consisting of 10% sucrose in 25 mM sodium phosphate buffer, pH 7.4, and stored for a minimum of 24–72 h in this solution.
Tissue sections were cut on a cryostat at 10 µm thickness and then washed for 20 min in 50 mM Tris-HCl, pH 7.4, containing 1.5% sodium chloride (TBS) and 0.3% Triton X-100 (TBSTr). For immunolabelling of Cx26, sections were incubated in TBSTr for 24 h at 4°C with monoclonal or polyclonal anti-Cx26 at a concentration of 2–3 µg/ml. For double immunofluorescence labelling, sections were incubated simultaneously with two primary antibodies (a mouse monoclonal and a rabbit polyclonal: Cx26 + GFAP; Cx26 + Cx43; Cx26 + Cx30; Cx26 + Cx47) for 24 h at 4°C, then washed for 1 h in TBSTr, and incubated for 1.5 h at room temperature simultaneously with appropriate combinations of secondary antibodies, which included: FITC-conjugated horse anti-mouse IgG diluted 1:100 (Vector Laboratories, Burlingame, CA, USA), Cy3-conjugated goat anti-mouse IgG diluted 1:200 (Jackson ImmunoResearch Laboratories, West Grove, PA, USA), Alexa Flour 488-conjugated goat anti-rabbit IgG diluted 1:1000 (Molecular Probes, Eugene, Oregon) and Cy3-conjugated donkey anti-rabbit IgG diluted 1:200 (Jackson ImmunoResearch Laboratories). All antibodies were diluted in TBSTr containing 5% normal goat or normal donkey serum. After secondary antibody incubations, sections were washed in TBSTr for 20 min, then in 50 mM Tris-HCl buffer, pH 7.4 for 30 min, and covered with antifade medium and coverslipped. Control procedures included omission of one of the primary antibodies with inclusion of each of the secondary antibodies to establish absence of inappropriate cross-reactions between primary and secondary antibodies or between different combinations of secondary antibodies. Conventional and confocal immunofluorescence images were acquired on a Zeiss Axioskop2 fluorescence microscope using Axiovision 3.0 software (Carl Zeiss Canada, Toronto, Ontario, Canada) and on an Olympus Fluoview IX70 confocal microscope using Olympus Fluoview software (Olympus Canada, Inc., Markham, ON, Canada), and assembled using Adobe Photoshop CS (Adobe Systems, San Jose, CA, USA), CorelDraw Graphics Suite 12 (Corel Corporation, Ottawa, ON, Canada), and Northern Eclipse software (Empix Imaging, Mississauga, ON, Canada).
Cell culture and transfection
C6 rat glioma cells or C6 cells stably expressing Cx26 (C6-Cx26; gift from D.J. Belliveau, University of Western Ontario, London, Ontario, Canada) were grown in Dulbecco’s Modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and maintained in an incubator at 37°C with 5% CO2. C6 cells were transiently transfected with a mouse Cx30 expression plasmid (C6-Cx30; gift from R. Nickel, Centre for Auditory Research, The Ear Institute, University College London, London UK) or plasmid vector (C6-vector) using Lipofectamine 2000 reagent (Gibco BRL Life Technologies) and taken for analysis of protein expression 24 h post-transfection. For fixation of cultures, cells grown on glass coverslips were washed in sodium phosphate buffer containing 0.9% NaCl, pH 7.4 (PBS), fixed for 5 min in ice-cold 1% formaldehyde in PBS, rinsed in PBS and stored at 4°C. Light microscopic immunofluorescence of cultured cells was conducted as described above for tissue sections.
Western blotting
Animals were deeply anesthetized with an intraperitoneal injection of equithesin, decapitated, and thalamus and liver dissected at 4°C and flash frozen for storage at −80°C. Tissue was homogenized in 14 volumes of IP buffer (20 mM Tris-HCl, pH 8.0, 140 mM NaCl, 1% Triton X-100, 10% glycerol, 1 mM EGTA, 1.5 mM MgCl2) supplemented with 5 µl/ml protease inhibitor cocktail (Roche Diagnostics, Laval Canada) in a glass Teflon homogenizer and then briefly sonicated. For preparation of cell lysates, cells grown on 100 mm culture dishes were rinsed with PBS and then lysed in ice cold IP buffer and briefly sonicated. Protein concentrations for all samples were determined using a kit (BioRad Laboratories, Hercules CA). For immunoblotting, 30 µg of liver or thalamus per lane, or 15 µg per lane of C6-Cx26, C6-Cx30 or C6-vector cell lysate were diluted in loading buffer with 10% 2-mercaptoethanol and separated on 10% SDS-PAGE gels. Proteins were transferred to nitrocellulose membranes (0.2 µm) for 45 min at 100 volts in standard transfer buffer with 0.05% SDS. Following blocking for 60 min in TBS-Tw (20 mM Tris pH 8.0, 150 mM NaCl, and 0.2% Tween-20) containing 5% skim milk powder, immunoblots were incubated overnight at 4°C with 2 µg/ml anti-Cx26 Ab51-2800 or 2 µg/ml anti-Cx30 Ab71-2200 diluted in TBS-Tw containing 1% skim milk. After washing in TBS-Tw, incubation for 1 h at room temperature with horseradish peroxidase-conjugated goat anti-rabbit IgG diluted 1:5000 (Sigma) and final washes in TBS-Tw, immunoreactive bands were revealed by chemiluminescence (Amersham Biosciences Ltd., Baie d’Urfe Canada).
Results
Characterization of anti-Cx26 and anti-Cx30 antibodies
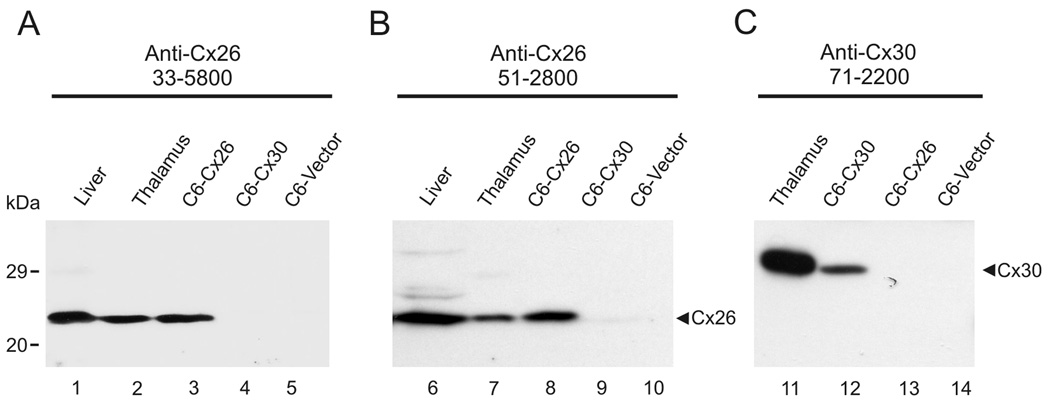
Due to the high amino acid sequence homology between Cx26 and Cx30, specific recognition of Cx26 using polyclonal and monoclonal anti-Cx26 Ab51-2800 and Ab33-5800 and recognition of Cx30 using polyclonal anti-Cx30 Ab71-2200 was confirmed by immunoblotting of tissues in which expression of these connexins was previously documented (Nagy et al., 1999, 2001) and in lysates from C6 glioma cells expressing these connexins. Both monoclonal and polyclonal anti-Cx26 antibodies detected a specific band migrating at ~26 kDa in total protein from liver (Fig. 1A,B, lanes 1,6), thalamus (Fig. 1A,B, lanes 2,7) and C6 cells stably expressing Cx26 (Fig. 1A,B, lanes 3,8), but not in lysates from C6 cells transiently transfected with Cx30 (Fig. 1A,B, lanes 4,9) or control vector-transfected C6 cells (Fig. 1A,B, lanes 5,10). The specificity of polyclonal anti-Cx30 was indicated in blots of total protein isolated from thalamus (Fig. 1C, lane 11) and from C6 cells transiently transfected with a mouse Cx30 expression plasmid (Fig. 1C, lane 12), where a band migrating at 30 kDa was detected, but which was absent in lysates from C6 cells stably expressing Cx26 (Fig. 1C, lane 13) and in control vector-transfected C6 cells (Fig. 1C, lane 14). The lack of anti-Cx26 reaction with Cx30 in the thalamus and in Cx30-transfected C6 cells, as shown by the absence of a band in protein isolates from these sources (Fig. 1A,B, lanes 2,7 and 3,8) that co-migrates with Cx30 (Fig. 1C, lanes 11 and 12), indicated that the Cx26 Ab33-5800 and Ab51-2800 antibodies did not cross-react with Cx30 in these preparations.
FIG. 1.
Immunoblot detection of Cx26 and Cx30 in homogenates of the liver and thalamus, and in lysates of C6 glioma cells transiently transfected with Cx30 (C6-Cx30) or stably expressing Cx26 (C6-Cx26). Lanes were loaded with 30 µg of protein from liver or thalamus, and with 15 µg of protein from C6 cells. (A,B) Anti-Cx26 antibodies Ab33-5800 (A) and Ab51-2800 (B) detect Cx26 migrating at 26 kDa in liver (lanes 1, 6), thalamus (lanes 2, 7) and C6-Cx26 cells (lanes 3, 8), which was absent in C6-Cx30 (lanes 4, 9) and in control vector-transfected C6 cells (lanes 5, 10). (C) Anti-Cx30 Ab71-2200 detected Cx30 migrating at 30 kDa in the thalamus (lane 11) and in C6-Cx30 cells (lane 12), but not in C6-Cx26 cells (lane 13) or in control vector-transfected C6 cells (C6-vector, lane 14).
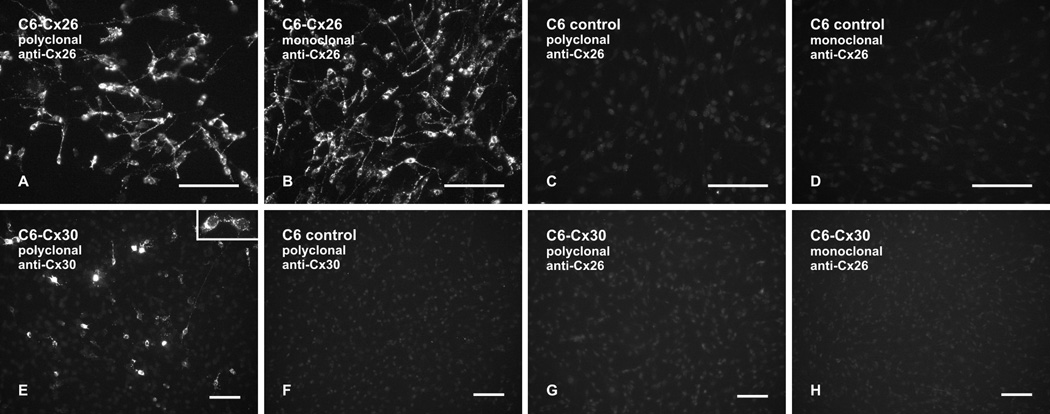
Immunofluorescence labelling specificity of anti-Cx26 and anti-Cx30 was tested using C6 glioma cells. In C6 cells stably expressing Cx26, both polyclonal and monoclonal anti-Cx26 (Ab51-2800 and Ab33-5800) produced robust immunolabelling of Cx26 that was localized intracellularly, along cell processes and at cell-cell contacts (Fig. 2A and B). Control C6 cells transfected with empty vector were devoid of labelling with these antibodies (Fig. 2C and D). In C6 cells transiently transfected with Cx30 and grown to near confluence, polyclonal anti-Cx30 produced moderate to intense immunolabelling of Cx30 that was localized intracellularly and punctate labelling that was localized at cell-cell contacts in cases where pairs of adjacent cells each displayed Cx30 expression (Fig. 2E). Similar results were obtained with monoclonal anti-Cx30 Ab71-2200 (not shown). Control vector-transfected C6 cells were devoid of immunolabelling with polyclonal anti-Cx30 (Fig. 2F) and with monoclonal anti-Cx30 (not shown). In Cx30-transfected sister cultures corresponding to that shown in Figure 2E, there was a total lack of Cx30 detection with either polyclonal anti-Cx26 (Fig. 2G) or monoclonal anti-Cx26 (Fig. 2H), indicating that these antibodies did not cross react with Cx30.
FIG. 2.
Immunofluorescence labelling of Cx26 and Cx30 in C6 glioma cell cultures. (A–D) C6 glioma cells stably expressing Cx26 show immunolabelling with polyclonal anti-Cx26 Ab51-2800 (A) and with monoclonal anti-Cx26 Ab33-5800 (B), and control non-transfected C6 cells show absence of labelling with these antibodies (C,D). (E,F) C6 glioma cells transiently transfected with Cx30 show intracellular labelling (E) and punctate labelling at cell-cell appositions (E, inset) with polyclonal anti-Cx30 Ab71-2200. Control non-transfected C6 cells show an absence of labelling with polyclonal anti-Cx30 Ab71-2200 (F). (G,H) C6 glioma cells transiently transfected with Cx30 show an absence of Cx30 detection with either polyclonal anti-Cx26 Ab51-2800 (G) or monoclonal anti-Cx26 Ab33-5800 (H). Scale bars: 100 µm.
Immunolabelling of Cx26 in wild-type and Cx26fl/fl:Nestin-Cre mice
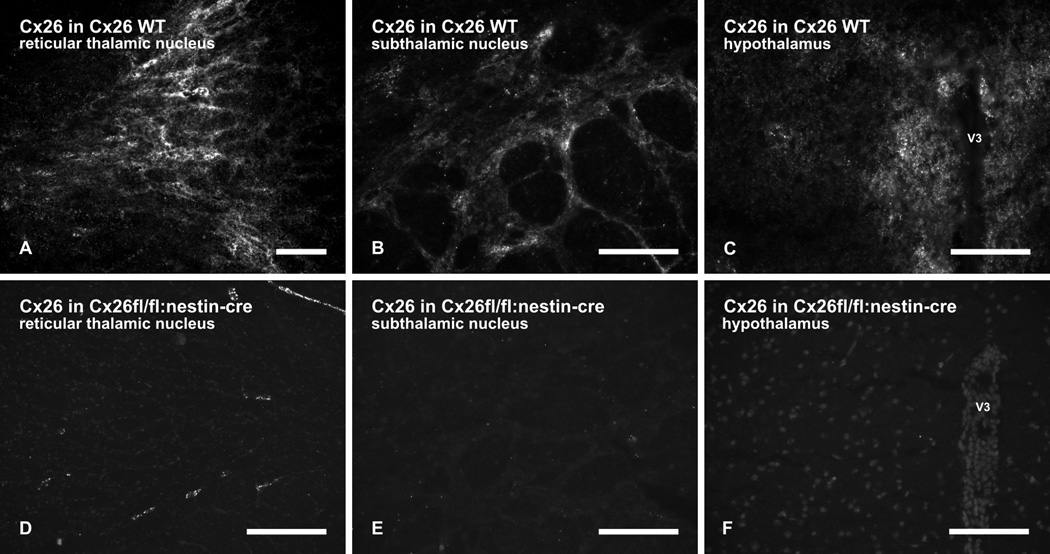
In addition to expression of Cx30 and Cx43 in astrocytes, we previously reported that Cx26 is also expressed in astrocytes located in subcortical regions of rat CNS, but not in those located in cerebral cortex (Nagy et al., 2001). Consistent with these earlier observations, polyclonal and monoclonal anti-Cx26 (Ab51-2800 and Ab33-5800) produced punctate immunofluorescence labelling in various subcortical regions of C57BL/6 mice, as shown with monoclonal anti-Cx26 Ab33-5800 in the reticular thalamic nucleus (Fig. 3A), subthalamic nucleus (Fig. 3B) and the hypothalamus (Fig. 3C). Similar labelling was also observed in other thalamic nuclei as well as midbrain and brainstem regions (not shown). Parenchyma of cerebral cortex was devoid of labelling for Cx26 (not shown), consistent with our previous observations in rat brain (Nagy et al., 2001), and Cx26 was also undetectable in mouse hippocampus (not shown). In Cx26fl/fl:Nestin-Cre mice, immunolabelling with these antibodies was totally absent in brain parenchyma, as shown with monoclonal anti-Cx26 Ab33-5800 in the reticular thalamic nucleus (Fig. 3D), the subthalamic nucleus (Fig. 3E) and the hypothalamus (3F). Patterns of immunolabelling for other glial connexins, including Cx30, Cx32, Cx43 and Cx47, were unchanged in the Cx26fl/fl:Nestin-Cre mice (not shown). Thus, the above anti-Cx26 antibodies specifically detect Cx26 in brain and lack cross reaction with other glial connexins. Although not quantified, it should be noted that the density of punctate labelling for Cx26 in subcortical regions of mice was lower than observed in subcortical areas of rat brain (Nagy et al., 2001). Although the Cx26fl/fl:Nestin-Cre mice appear not to exhibit any phenotypic abnormalities, they have not as yet been studied in the context of functional deficits. Any potential deficits would presumably be restricted to systems and cell types in which Nestin promoter-driven Cre recombinase would cause deletion of the Cx26 coding DNA.
FIG. 3.
Immunofluorescence labelling of Cx26 in brain regions of wild-type (WT) and Cx26fl/fl:Nestin-Cre adult mice. (A–C) Patches of moderate to dense punctate immunolabelling of Cx26 in wild-type mice is shown in the reticular thalamic nucleus (A), in the subthalamic nucleus (B) and the hypothalamus (C) in the vicinity of the third ventricle (V3). (D–F) Absence of immunolabelling in brain regions of Cx26fl/fl:Nestin-Cre mice is shown in the reticular thalamic nucleus (D), subthalamic nucleus (E) and hypothalamus (F). Some Cx26-puncta in D are associated with leptomeninges (see Fig. 5). Scale bars: A,C,F, 100 µm; B,D, 50 µm; E, 200 µm.
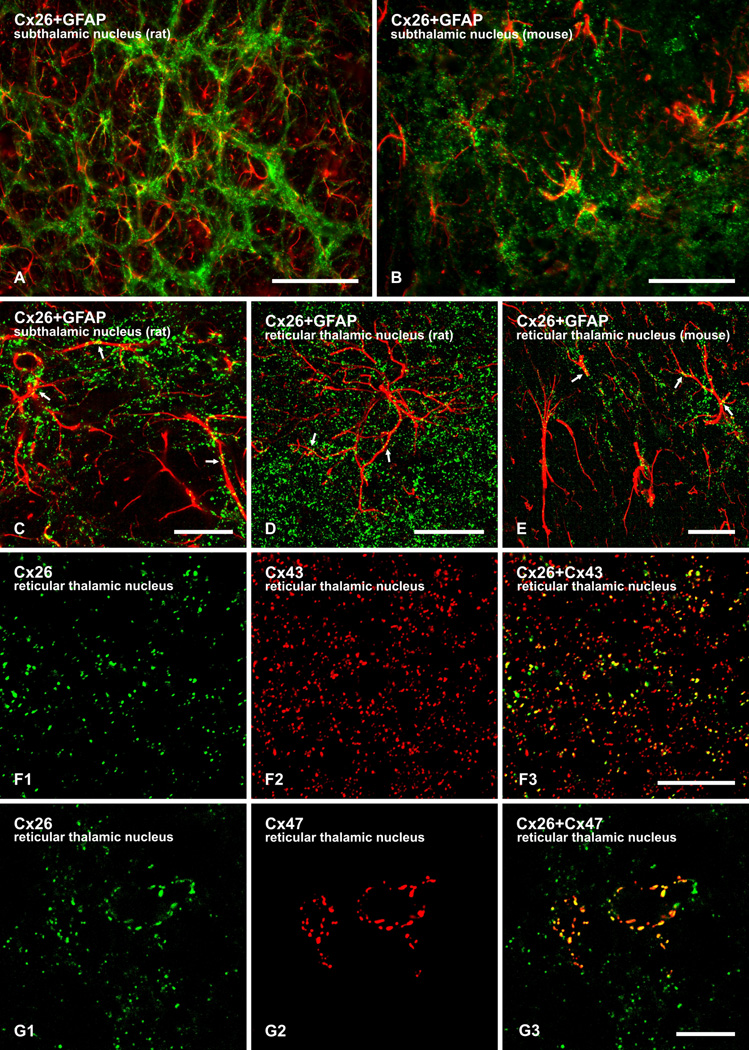
We and others have shown that a proportion of Cx26-positive puncta in rat brain is co-localized with Cx30 and Cx43 at A/A gap junctions (Nagy et al., 2001; Mercier & Hatton, 2001; Altevogt & Paul, 2004) as well as with Cx47 at A/O gap junctions (Nagy et al., 2001). To examine further the cellular localization of Cx26 detected in brain parenchyma, immunofluorescence labelling for Cx26 was conducted in combination with the astrocyte marker GFAP, notwithstanding that labelling for GFAP typically does not delineate the full expanse of astrocyte processes and that not all astrocytes, in particular, in the thalamus do express GFAP (Bushong et al., 2002; Barres 2008). In various subcortical brain regions, some immunolabelling for Cx26 was co-distributed with GFAP-positive astrocytes in both rat (Fig. 4A) and mouse (Fig. 4B) brain. Laser scanning confocal analyses revealed Cx26-positive puncta localized along GFAP-positive astrocyte cell bodies and processes, as shown in the subthalamic nucleus (Fig. 4C) and reticular thalamic nucleus (Fig. 4D) of rat brain and the reticular thalamic nucleus of mouse brain (Fig. 4E).
FIG. 4.
Immunofluorescence localization of labelling for Cx26 with GFAP, Cx43 and Cx47 in brain. (A,B) Low magnification overlays showing co-distribution of Cx26 (green) with GFAP-positive astrocytes (red) in rat (A) and mouse (B) brain. (C–E) Higher magnification confocal overlay images showing co-localization of punctate labelling for Cx26 (green) along GFAP-positive astrocyte processes (red) in the subthalamic nucleus (C) and reticular thalamic nucleus (D) of rat, and the reticular thalamic nucleus of mouse (E), with red/green overlap seen as yellow (arrows). (F) Laser scanning confocal double immunofluorescence labelling for Cx26 with Cx43 in mouse reticular thalamic nucleus, showing Cx26-positive puncta (F1) co-localized with Cx43-positive puncta (F2), as seen by yellow in overlays (F3). Laser scanning confocal double immunofluorescence labelling for Cx26 with Cx47 in mouse reticular thalamic nucleus, showing Cx26-positive puncta (G1) co-localized with Cx47-positive puncta (G2), as seen by yellow in overlays (G3). Scale bars: A, 100 µm; B, 50 µm; C–E, F,G, 10 µm.
As previously observed in rat (Nagy et al., 2001), double labelling for Cx26 in combination with Cx43 revealed substantial co-localization of the two connexins in subcortical brain regions, as shown in the reticular thalamic nucleus (Fig. 4F), providing support for the localization of Cx26 at glial gap junctions. Equally revealing was double immunofluorescence labelling for Cx26 in combination with Cx47. Expression of Cx47 is restricted exclusively to oligodendrocytes in rodent brain (Kleopa et al., 2004; Menichella et al., 2003; Odermatt et al., 2003), and we have previously shown that Cx47-positive puncta are heavily concentrated on the somata and initial processes of oligodendrocytes, allowing clear delineation and visualization of these cells even in the absence of oligodendrocyte cell body markers (Kamasawa et al., 2005; Li et al., 2008). In subcortical brain regions, punctate immunolabelling for Cx47 associated with oligodendrocytes and their initial processes was often co-localized with Cx26, as shown in the reticular thalamic nucleus (Fig. 4G).
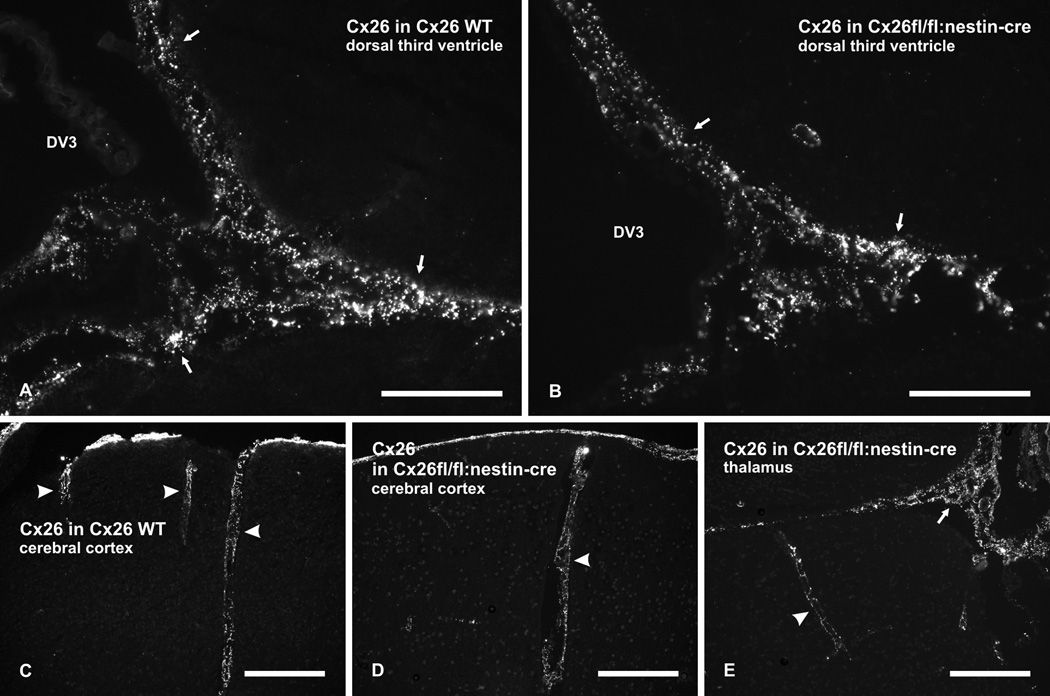
In wild-type mice, dense immunolabelling for Cx26 was associated with leptomeninges along brain ventricular surfaces, such as those at the dorsal aspect of the third ventricle (Fig. 5A), and along blood vessels penetrating into the brain, as shown in the cerebral cortex (Fig. 5C). This non-parenchymal expression of Cx26 was preserved in Cx26fl/fl:Nestin-Cre mice (Fig. 5B and 5D,E). These results indicate that Cx26-positive leptomeningeal cells had not expressed the Nestin driven Cre recombinase, although a 99% recombination efficacy was described for Nestin-Cre activity in mice and Nestin was first identified in neuroepithelial precursor cells of the rat (Dubois et al., 2006; Lendahl et al., 1990) Therefore Cx26 continues to be expressed in leptomeningeal cells, presumably at gap junctions between these cells. This fortuitous outcome allowed us to distinguish between parenchmal and non-parenchymal Cx26 expression, and revealed the more limited extent to which Cx26 associated with leptomeningeal tissues contributes to detection of Cx26 near brain surfaces.
FIG. 5.
Immunofluorescence labelling of Cx26 in leptomeninges of wild-type (WT) and Cx26fl/fl:Nestin-Cre adult mice. (A,B) Dense punctate immunolabelling for Cx26 is seen associated with leptomeninges covering the surface of the dorsal third ventricle (DV3) in wild-type mice (A, arrows), and is seen to be preserved in a similar region of Cx26fl/fl:Nestin-Cre mice (B, arrows). (C–E) Images showing immunolabelling for Cx26 associated with leptomeninges along blood vessels penetrating into the cerebral cortex of wild-type mice (C, arrowheads), and persistence of leptomeninges-associated labelling for Cx26 along blood vessels penetrating into the cerebral cortex (D, arrowheads) and the thalamus (E, arrowheads) in a region adjacent to the DV3 (E, arrows) of Cx26fl/fl:Nestin-Cre adult mice. Scale bars: A,B, 100 µm; C,D,E, 200 µm.
Discussion
Using ablation of antibody target in transgenic mice, which represents the “gold standard” for demonstrations of antibody specificity (Lorincz & Nusser, 2008), the present results validate our earlier observations on parenchmal Cx26 expression in adult rodent brain (Nagy et al., 2001). Further support for this parenchymal glial expression is provided here by localization of Cx26-puncta on GFAP-positive astrocytes, and co-localization of Cx26-puncta with two parenchymal connexins, astrocytic Cx43 and in particular oligodendrocytic Cx47, which is not present in leptomeninges. More direct evidence for cellular localization of gap junctions containing Cx26 in brain parenchyma has been previously provided by ultrastructural data on Cx26 using freeze-fracture replica immunogold labelling (FRIL) in rodent brain, where labelling for Cx26 in parenchymal CNS regions was found exclusively at A/A gap junctions and on the astrocyte side of A/O gap junctions (Nagy et al., 2001, 2004; Rash et al., 2001b, 2007). It should be noted, however, that in view of Nestin expression in neuroepithelial precursor cells (Dubois et al., 2006; Lendahl et al., 1990), it remains unclear whether these cells, or indeed any other type of cells, express Cx26 during early brain development.
Six factors have contributed to uncertainty and confusion regarding Cx26 expression in rodent brain. First, antibodies developed against Cx26 prior to the discovery of Cx30 were later found to cross react with Cx30 (Nagy et al., 1997, 1999, 2001). The high amino acid sequence similarity between Cx26 and Cx30 (Dahl et al., 1996) raised concerns that immunohistochemical results on Cx26 in brain simply reflect cross reaction of anti-Cx26 antibodies with Cx30. This problem was circumvented by development of antibodies that were demonstrated to be specific for Cx26; these antibodies were shown to recognize a Cx26 kDa protein in subcortical brain parenchyma, to label A/A and A/O gap junctions in brain, and to lack recognition of Cx30 in brain as shown by western blotting (Nagy et al., 2001). Further, the cerebral cortex showed moderate immunolabelling for Cx30, but anti-Cx26 Ab51-2800 and Ab33-5800 both fail to yield any detectable immunolabelling in cerebral cortical parenchyma (Nagy et al., 2001; and present results), indicating lack of cross reaction of these antibodies with Cx30. The specificity of these antibodies was confirmed here using cells transfected with Cx26. The sequence of thirteen amino acid residues against which anti-Cx26 Ab51-2800 and Ab33-5800 were generated shares only four residues in common with Cx30. In our experience in developing antibodies against individual connexins, such a low homology has never resulted in cross reactions where these antibodies detect an inappropriate connexin.
Second, development of antibodies specific for Cx26 vs Cx30 left no other choice than an epitope peptide target sequence in Cx26 containing adjacent lysines (KK), which are prone to cross linking upon fixation, potentially rendering the epitope undetectable with strong or prolonged fixation methods. Thus, we have emphasized the need for weak tissue fixation conditions for adequate detection of Cx26 in brain parenchyma (Nagy et al., 2004). Use of a stronger formaldehyde fixation protocol or longer duration of fixation in conjunction with these antibodies may still allow detection of, for example, the typically large Cx26-containing gap junctions associated with liver hepatocytes or the dense signals associated with leptomeninges, but could eliminate detection of the lower levels of Cx26 at the typically smaller gap junctions between astrocytes.
Third, the density of punctate immunofluorescence labelling for Cx26 that we previously reported in subcortical regions of rat brain was greater than that observed here in the corresponding regions of mouse brain. Whether this reflects differences in relative levels of Cx26 in these brain regions of the two species or differences in immunohistochemical detectability requires further analyses. In any case, controversies could arise when comparing results on Cx26 in mouse brain to those we previously reported in rat brain (Nagy et al., 2001) without regard to species differences in either connexin expression levels and/or technical limitations in connexin detection. The molecular basis for differences in Cx26 expression patterns in mouse and rat, and the impact of these differences on physiological functions of the glial syncytial network remain to be determined.
Fourth, leptomeningeal tissue covers not only brain surfaces but also extend from those surfaces along blood vessels penetrating into brain (Mercier & Hatton, 2001). It may therefore be considered that all Cx26 in brain is associated with leptomeninges, and any apparent parenchymal Cx26 has simply been misattributed as to cellular localization. However, immunofluorescence labelling for leptomeningeal Cx26 in both mouse and rat brain characteristically consists of large intensely labelled puncta, arrays of which along blood vessels in subcortical areas can often be distinguished from finer, more dense and randomly distributed Cx26-puncta that are not associated with blood vessels and that are presumably of parenchymal localization. The present result showing the persistence of Cx26 in leptomeninges of Cx26fl/fl:Nestin-Cre mice serves to resolve the extent to which detection of this connexin originates from leptomeningeal tissue, and provides a clear distinction between leptomeningeal Cx26 and the parenchymal Cx26 that undergoes gene deletion in these mice. The loss of Cx26 in astrocytes vs. the unaltered expression of Cx26 in leptomeninges of Cx26fl/fl:Nestin-Cre mice indicates lack of Nestin expression in Cx26-positive leptomeningeal cells and precursors. These mice thus provide a convenient means of distinguishing Cx26 localization to parenchymal astrocytes from its localization to meningeal projections into the brain, including sheaths of blood vessels and stroma of the choroid plexus, in which regard our results are consistent with observations by Mercier & Hatton (2001).
Fifth, perhaps the greatest doubts on reports of Cx26 expression in brain parenchyma were cast by the failure to detect β-galactosidase in astrocytes of transgenic mice that had one allele of the Cx26 coding sequence replaced by the LacZ reporter DNA (Filippov et al. (2003). The relatively weak expression of Cx26 by astrocytes in contrast to the strong signals found in leptomeninges may be an explanation for this effect. Fixation methods may result in partial loss of β-galactosidase function and weak expression of the reporter gene could be missed as a consequence. Alternatively, the Cx26lacZ allele may be an unreliable reporter for Cx26 expression particularly in astrocytes, in a manner analogous to the poor detection of β-galacosidase when using the Cx30lacZ allele as a reporter for Cx30 expression, at least in hippocampal astrocytes (Gosejacob et al., 2010).
And sixth, gap junctional coupling between hippocampal astrocytes is substantially reduced (50%) in mice with astrocyte directed deletion of Cx43, with the remaining coupling presumably mediated by gap junctions consisting of Cx30 in these cells (Theis et al., 2003). Deletion of both Cx43 and Cx30 in astrocytes completely eliminated tracer-coupling between these cells in hippocampus (Wallraff et al., 2006; (Gosejacob et al., 2010). Although this may be taken as evidence against the expression of a third connexin in astrocytes (i.e., Cx26), the lack of coupling between hippocampal astrocytes after Cx43 and Cx30 ablation is consistent with our observations of immunolabelling for Cx26 in subcortical parenchyma and absence of labelling for Cx26 in mouse hippocampus.
Connexin coupling partners at O/A gap junctions
Due to earlier uncertainties surrounding Cx26 expression in astrocytes, potential glial connexin coupling partners of Cx26 were not assessed (Orthmann-Murphy et al., 2008), but the present results now warrant consideration of this issue. It is known that Cx26 can form functional communicating channels with Cx30 (Yum et al., 2007) but not with Cx43 (White & Bruzzone, 1996; Gemel et al., 2004), suggesting that in addition to Cx30/Cx30 and Cx43/Cx43 channels at A/A gap junctions, astrocytes may also form junctions containing Cx26/Cx30 and Cx26/Cx26 in gray matter tissue. Regarding A/O gap junctions, it has recently been reported (Magnotti et al., 2011) that Cx47 can form functional communicating channels with Cx43 and Cx30 (see however Orthmann-Murphy et al., 2008) but not with Cx26. Thus, Cx26 at these junctions may form functional communicating channels with Cx32, as it does in liver and in transfected cells in vitro (Elfgang et al., 1995; Nelles et al., 1996). This possibility is supported by observations (Nagy et al., 2003b) that immunolabelling for Cx26 at A/O gap junctions was moderately reduced in the absence of its potential Cx32 coupling partner in Cx32 KO mice.
Acknowledgements
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) to J.I.N., by NIH grants NS31027, NS44010 and NS44395 to J.E.R., and by grants Wi 270/32-1 and SFB 645, B2 from the German Research Foundation to K.W. We thank B. McLean for excellent technical assistance.
Abbreviations
- aa
amino acids
- A/A
astrocyte-to-astrocyte
- Ab
antibody
- O/A
oligodendrocyte-to-astrocyte
- CNS
central nervous system
- Cx
connexin
- FITC
fluorescein isothiocyanate
- GFAP
glial fibrillary acidic protein
- IP buffer
immunoprecipitation buffer
- KO
knockout PB, phosphate buffer
- PBS
phosphate buffered saline
- SDS-PAGE
sodium dodecylsulphate polyacrylamide gel electrophoresis
- TBS
50 mM Tris-HCl, pH 7.4 with 1.5% sodium chloride
- TBSTr
50 mM Tris-HCl, pH 7.4, 1.5% NaCl, 0.3% Triton X-100
- TBSTw
20 mM Tris-HCl, pH 7.4, 150 mM NaCl with 0.2% Tween-20
- WT
wild-type
References
- Ahn M, Lee J, Gustafsson A, Enriquez A, Lancaster E, Sul J, Haydon PG, Paul DL, Huang Y, Abrams CK, Scherer SS. Cx29 and Cx32, two connexins expressed by myelinating glia, do not interact and are functionally distinct. J. Neurosci. Res. 2008;86:992–1006. doi: 10.1002/jnr.21561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altevogt BM, Kleopa KA, Postma FR, Scherer SS, Paul DL. Connexin29 is uniquely distributed within myelinating glial cells of the central and peripheral nervous system. J. Neurosci. 2002;22:6458–6470. doi: 10.1523/JNEUROSCI.22-15-06458.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altevogt BM, Paul DL. Four classes of intercellular channels between glial cells in the CNS. J. Neurosci. 2004;24:4313–4323. doi: 10.1523/JNEUROSCI.3303-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr M, Andres F, Timmerman V, Nelis ME, Van Broeckhoven C, Dichgans J. Central visual, acoustic and motor pathway involvement in Charcot-Marie-Tooth family with an asn205ser mutation in the connexin32 gene. J. Neurol. Neurosurg. Psych. 1999;66:202–206. doi: 10.1136/jnnp.66.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J. Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Salmon M, Ott T, Michel V, Hardelin JP, Perfettini I, Eybalin M, Wu T, Marcus DC, Wangemann P, Willecke K, Petit C. Targeted ablation of connexin26 in the inner ear epithelial gap junction network causes hearing impairment and cell death. Curr. Biol. 2002;12:1106–1111. doi: 10.1016/s0960-9822(02)00904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl E, Manthey D, Chen Y, Schwarz H-J, Chang S, Lalley PA, Nicholson BJ, Willecke K. Molecular cloning and functional expression of mouse connexin30, a gap junction gene highly expressed in adult brain and skin. J. Biol. Chem. 1996;271:17903–17910. doi: 10.1074/jbc.271.30.17903. [DOI] [PubMed] [Google Scholar]
- Dere E, De Souza-Silva MA, Frisch C, Teubner B, Söhl G, Willecke K, Huston JB. Connexin30-deficient mice show increased emotionality and decreased rearing activity in the open-field along with neurochemical changes. Eur. J. Neurosci. 2003;18:629–638. doi: 10.1046/j.1460-9568.2003.02784.x. [DOI] [PubMed] [Google Scholar]
- Dubois NC, Hofmann D, Kaloulis K, Bishop JM, Trumpp A. Nestin-Cre transgenic mouse line Nes-Cre1 mediates highly efficient Cre/loxP mediated recombination in the nervous system, kidney, and somite-derived tissues. Genesis. 2006;44:355–360. doi: 10.1002/dvg.20226. [DOI] [PubMed] [Google Scholar]
- Elfgang C, Eckert R, Lichtenberg-Fraté H, Butterweck A, Traub O, Klein RA, Hülser DF, Willecke K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J. Cell Biol. 1995;129:805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov MA, Hormuzdi SG, Fuchs EC, Monyer H. A reporter allele for investigating connexin26 gene expression in the mouse brain. Eur. J. Neurosci. 2003;18:3183–3192. doi: 10.1111/j.1460-9568.2003.03042.x. [DOI] [PubMed] [Google Scholar]
- Frisch C, Theis M, De Souza Silva MA, Dere E, Sohl G, Teubner B, Namestkova K, Willecke K, Huston JP. Mice with astrocyte-directed inactivation of connexin43 exhibit increased exploratory behaviour, impaired motor capacities, and changes in brain acetylcholine levels. Eur. J. Neurosci. 2003;18:2313–2318. doi: 10.1046/j.1460-9568.2003.02971.x. [DOI] [PubMed] [Google Scholar]
- Gemel J, Valiunas V, Brink PR, Beyer EC. Connexin43 and connexin26 form gap junctions, but not heteromeric channels in co-expressing cells. J. Cell Sci. 2004;117:2469–2480. doi: 10.1242/jcs.01084. [DOI] [PubMed] [Google Scholar]
- Giaume C, Theis M. Pharmacological and genetic approaches to study connexin-mediated channels in glial cells of the central nervous system. Brain Res. Rev. 2010;63:160–176. doi: 10.1016/j.brainresrev.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Gosejacob D, Dublin P, Bedner P, Huttmann K, Zhang J, Tress O, Willecke K, Pfrieger F, Steinhauser C, Theis M. Role of astroglial connexin30 in hippocampal gap junction coupling. Glia. 2010 doi: 10.1002/glia.21120. (In advance of print) [DOI] [PubMed] [Google Scholar]
- Kamasawa N, Sik A, Morita M, Yasumura T, Davidson KGV, Nagy JI, Rash JE. Connexin47 and connexin32 in gap junctions on oligodendrocyte somata, myelin sheaths, paranodal loops and Schmidt-Lanterman incisures: implications for ionic homeostasis and potassium siphoning. Neuroscience. 2005;136:65–86. doi: 10.1016/j.neuroscience.2005.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleopa AK, Orthmann JL, Enriquez A, Paul DL, Scherer SS. Unique distribution of the gap junction proteins connexin29, connexin32, and connexin47 in oligodendrocytes. Glia. 2004;47:346–357. doi: 10.1002/glia.20043. [DOI] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Li J, Hertzberg EL, Nagy JI. Connexin32 in oligodendrocytes and association with myelinated fibers in mouse and rat brain. J. Comp. Neurol. 1997;379:571–591. doi: 10.1002/(sici)1096-9861(19970324)379:4<571::aid-cne8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Li X, Ionescu AV, Lynn BD, Lu S, Kamasawa N, Morita M, Davidson KG, Yasumura T, Rash JE, Nagy JI. Connexin47, connexin29 and connexin32 co-expression in oligodendrocytes and Cx47 association with zonula occludens-1 (ZO-1) in mouse brain. Neuroscience. 2004;126:611–630. doi: 10.1016/j.neuroscience.2004.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Penes M, Odermatt B, Willecke K, Nagy JI. Ablation of Cx47 in transgenic mice leads to the loss of MUPP1, ZONAB and multiple connexins at oligodendrocyte-astrocyte gap junctions. Eur. J. Neurosci. 2008;28:1503–1517. doi: 10.1111/j.1460-9568.2008.06431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz A, Nusser Z. Specificity of immunoreactions: The importance of testing specificity in each method. J. Neurosci. 2008;28:9083–9086. doi: 10.1523/JNEUROSCI.2494-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglione M, Tress O, Haas B, Karram K, Trotter J, Willecke K, Kettenmann H. Oligodendrocytes in mouse corpus callosum are coupled via gap junction channels formed by connexin47 and connexin32. Glia. 2010;58:1104–1117. doi: 10.1002/glia.20991. [DOI] [PubMed] [Google Scholar]
- Magnotti L, Goodenough DA, Paul DL. Functional heterotypic interactions between astrocyte and oligodendrocyte connexins. Glia. 2011;59:26–34. doi: 10.1002/glia.21073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menichella DM, Goodenough DA, Sirkowski E, Scherer SS, Paul DL. Connexins are critical for normal myelination in the CNS. J. Neurosci. 2003;23:5963–5973. doi: 10.1523/JNEUROSCI.23-13-05963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menichella DM, Majdan M, Awatramani R, Goodenough DA, Sirkowski E, Scherer SS, Paul DL. Genetic and physiological evidence that oligodendrocyte gap junctions contribute to spatial buffering of potassium released during neuronal activity. J. Neurosci. 2006;26:10984–10991. doi: 10.1523/JNEUROSCI.0304-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F, Hatton GI. Connexin 26 and basic fibroblast growth factor are expressed primarily in the subpial and subependymal layers in adult brain parenchyma: Roles in stem cell proliferation and morphological plasticity. J. Comp. Neurol. 2001;431:88–104. doi: 10.1002/1096-9861(20010226)431:1<88::aid-cne1057>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Mugnaini E. Cell junctions of astrocytes, ependyma, and related cells in the mammalian central nervous system, with emphasis on the hypothesis of a generalized functional syncytium of supporting cells. In: Fedoroff S, Vernadakis A, editors. Astrocytes, Vol. I. New York: Academic Press; 1986. pp. 329–371. [Google Scholar]
- Nagy JI, Ochalski PAY, Li J, Hertzberg EL. Evidence for the co-localization of another connexin with connexin43 at astrocytic gap junctions in rat brain. Neuroscience. 1997;78:533–548. doi: 10.1016/s0306-4522(96)00584-2. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Patel D, Ochalski PAY, Stelmack GL. Connexin30 in rodent, cat and human brain: selective expression in gray matter astrocytes, co-localization with connexin43 at gap junctions and late developmental appearance. Neuroscience. 1999;88:447–468. doi: 10.1016/s0306-4522(98)00191-2. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Rash JE. Connexins and gap junctions of astrocytes and oligodendrocytes in the CNS. Brain Res. Rev. 2000;32:29–44. doi: 10.1016/s0165-0173(99)00066-1. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Li X, Rempel J, Stelmack G, Patel D, Staines WA, Yasumura T, Rash JE. Connexin26 in adult rodent CNS: demonstration at astrocytic gap junctions and co-localization with connexin30 and connexin43. J. Comp. Neurol. 2001;441:302–323. doi: 10.1002/cne.1414. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Ionescu AV, Lynn BD, Rash JE. Coupling of astrocyte connexins Cx26, Cx30, Cx43 to oligodendrocyte Cx29, Cx32, Cx47: Implications from normal and connexin32 knockout mice. Glia. 2003;44:205–218. doi: 10.1002/glia.10278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy JI, Dudek FE, Rash JE. Update on connexins and gap junctions in neurons and glia in the mammalian nervous system. Brain Res. Rev. 2004;47:191–215. doi: 10.1016/j.brainresrev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Nelles E, Bützler C, Jung D, Temme A, Gabriel H-D, Dahl U, Traub O, Stümpel F, Jungermann KJ, Zielasek, Toyka KV, Dermietzel R, Willecke K. Defective propagation of signals generated by sympathetic nerve stimulation in the liver of connexin32-deficient mice. Proc. Natl. Acad. Sci. USA. 1996;93:9565–9570. doi: 10.1073/pnas.93.18.9565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt B, Wellershaus K, Wallraff A, Seifert G, Degen J, Euwens C, Fuss B, Bussow H, Schilling K, Steinhauser C, Willecke K. Connexin 47 (Cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS. J. Neurosci. 2003;23:4549–4559. doi: 10.1523/JNEUROSCI.23-11-04549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orthmann-Murphy JL, Freidin M, Fischer E, Scherer SS, Abrams CK. Two distinct heterotypic channels mediate gap junction coupling between astrocyte and oligodendrocyte connexins. J. Neurosci. 2007;27:13949–13957. doi: 10.1523/JNEUROSCI.3395-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orthmann-Murphy JL, Abrams CK, Scherer SS. Gap junctions couple astrocytes and oligodendrocytes. J. Mol. Neurosci. 2008;35:101–116. doi: 10.1007/s12031-007-9027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paznekas WA, Boyadjiev SA, Shapiro RE, Daniels O, Wollnik B, Keegan CE, Innis JW, Dinulos B, Christian C, Hannibal MC, Jabs EW. Connexin43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am. J. Hum. Genet. 2003;72:408–418. doi: 10.1086/346090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paznekas WA, Karczeski B, Vermeer S, Lowry RB, Delatychi M, Laurence F, Koivisto PA, Maldergem LV, Boyadjiev SA, Bodurtha JN, Jabs EW. GJA1 mutations, variants, and connexin43 dysfunction as it relates to the oculodentodigital dysplasia phenotype. Hum. Mut. 2009;30:724–733. doi: 10.1002/humu.20958. [DOI] [PubMed] [Google Scholar]
- Paulson HL, Garbern JY, Hoban TF, Krajewski KM, Lewis RA, Fischbeck KH, Grossman RI, Lenkinski R, Kamholz JA, Shy ME. Transient central nervous system white matter abnormality in X-linked Charcot-Marie-Tooth Disease. Ann. Neurol. 2002;52:429–434. doi: 10.1002/ana.10305. [DOI] [PubMed] [Google Scholar]
- Penes M, Li X, Nagy JI. Expression of zonula occludens-1 (ZO-1) and the transcription factor ZO-1-associated nucleic acid-binding protein (ZONAB/MsY3) in glial cells and co-localization at oligodendrocyte and astrocyte gap junctions in mouse brain. Eur. J. Neurosci. 2005;22:404–418. doi: 10.1111/j.1460-9568.2005.04225.x. [DOI] [PubMed] [Google Scholar]
- Rash JE, Duffy HS, Dudek FE, Bilhartz BL, Whalen LR, Yasumura T. Grid-mapped freeze-fracture analysis of gap junctions in gray and white matter of adult rat central nervous system, with evidence for a 'pan-glial' syncytium that is not coupled to neurons. J. Comp. Neurol. 1997;388:265–292. doi: 10.1002/(sici)1096-9861(19971117)388:2<265::aid-cne6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Dudek FE, Nagy JI. Cell-specific expression of connexins and evidence of restricted gap junctional coupling between glial cells and between neurons. J. Neurosci. 2001a;15:1983–2000. doi: 10.1523/JNEUROSCI.21-06-01983.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Davidson K, Furman CS, Dudek FE, Nagy JI. Identification of cells expressing Cx43, Cx30, Cx26, Cx32 and Cx36 in gap junctions of rat brain and spinal cord. Cell Commun. Adhes. 2001b;8:315–320. doi: 10.3109/15419060109080745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Olson CO, Davidson KGV, Yasumura T, Kamasawa N, Nagy JI. Identification of connexins in gap junctions between neurons in rodent locus coeruleus. Neuroscience. 2007;147:938–956. doi: 10.1016/j.neuroscience.2007.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE. Molecular disruptions of the panglial syncytium block potassium siphoning and axonal saltatory conduction: pertinence to neuromyelitis optica and other demyelinating diseases of the central nervous system. Neuroscience. 2010;168:982–1008. doi: 10.1016/j.neuroscience.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- Scherer SS, Deschenes SM, Xu Y-T, Grinspan JB, Fischbeck KH, Paul DL. Connexin32 is a myelin-related protein in the PNS and CNS. J. Neurosci. 1995;15:8281–8294. doi: 10.1523/JNEUROSCI.15-12-08281.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray DC, Moreno AP, Kessler JA, Dermietzel R. Characterization of gap junctions between cultured leptomeningeal cells. Brain Res. 1991;568:1–14. doi: 10.1016/0006-8993(91)91373-9. [DOI] [PubMed] [Google Scholar]
- Sutor B, Schmolke C, Teubner B, Schirmer C, Willecke K. Myelination defects and neuronal hyperexcitability in the neocortex of connexin32-deficient mice. Cerebral Cortex. 2000;10:684–697. doi: 10.1093/cercor/10.7.684. [DOI] [PubMed] [Google Scholar]
- Theis M, Jauch R, Zhuo L, Speidel D, Wallraff A, Doring B, Frisch C, Sohl G, Teubner B, Euwens C, Huston J, Steinhauser C, Messing A, Heinemann U, Willecke K. Accelerated hippocampal spreading depression and enhanced locomotory activity in mice with astrocyte-directed inactivation of connexin43. J. Neurosci. 2003;23:766–776. doi: 10.1523/JNEUROSCI.23-03-00766.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis M, Söhl G, Eiberger J, Willecke K. Emerging complexities in identity and function of glial connexins. Trends Neurosci. 2005;28:188–195. doi: 10.1016/j.tins.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schütz G. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- Uhlenberg B, Schuelke M, Ruschendorf F, Ruf N, Kaindl AM, Henneke M, Thiele H, Stoltenburg-Didinger G, Aksu F, Topaloglu H, Nurnberg P, Hubner C, Weschke B, Gartner J. Mutations in the gene encoding gap junction protein α12 (connexin46.6) cause Pelizaeus-Merzbacher-Like disease. Am. J. Hum. Genet. 2004;75:251–260. doi: 10.1086/422763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallraff A, Köhling R, Heinemann U, Theis M, Willecke K, Steinhäuser C. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J. Neurosci. 2006;17:5438–5447. doi: 10.1523/JNEUROSCI.0037-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TW, Bruzzone R. Multiple connexin proteins in single intercellular channels: connexin compatibility and functional consequences. J. Bioenerg. Biomem. 1996;28:339–349. doi: 10.1007/BF02110110. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Rohlmann A. Structure-function relationships in gap junctions. Int. Rev. Cytol. 1995;157:315–373. doi: 10.1016/s0074-7696(08)62161-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Ochalski PAY, Hertzberg EL, Nagy JI. On the organization of astrocytic gap junctions in the brain as suggested by LM and EM immunocytochemistry of connexin43 expression. J. Comp. Neurol. 1990;302:853–883. doi: 10.1002/cne.903020414. [DOI] [PubMed] [Google Scholar]
- Yum SW, Zhang J, Valiunas V, Kanaporis G, Brink PR, White TW, Sherer SS. Human connexin26 and connexin30 form functional heteromeric and heterotypic channels. Am. J. Cell Physiol. 2007;293:C1032–C1048. doi: 10.1152/ajpcell.00011.2007. [DOI] [PubMed] [Google Scholar]