Abstract
Blockade of osteoclast (OC) activity efficiently decreases tumor burden and associated bone erosion in immune-compromised animals bearing human osteolytic cancers. Here we show that modulation of anti-tumor T cell responses alters tumor growth in bone regardless of OC status, using genetic and pharmacologic models. PLCγ2−/− mice, with dysfunctional OCs and impaired dendritic cell (DC)-mediated T cell activation, had increased bone tumor burden despite protection from bone loss. In contrast, Lyn−/− mice, with more numerous OCs and a hyperactive myeloid population leading to increased T cell responses, had reduced tumor growth in bone despite enhanced osteolysis. The unexpected tumor/bone phenotype observed in PLCγ2−/− and Lyn−/− mice was transplantable, suggesting the involvement of an immune component. Consistent with this hypothesis, T cell activation diminished skeletal metastasis while T cell depletion enhanced it, even in the presence of Zoledronic acid, a potent anti-resorptive agent. Importantly, injection of antigen-specific wild-type cytotoxic CD8+ T cells in PLCγ2−/− mice or CD8+ T cell depletion in Lyn−/− mice normalized tumor growth in bone.
Our findings demonstrate the important contribution of CD8+ T cells in the regulation of bone metastases regardless of OC status, thus including T cells as critical regulators of tumor growth in bone.
Introduction
Bone metastases represent a serious complication of many cancers, including breast, prostate, and lung tumors, as well as multiple myeloma. In the bone marrow, there is a mutual positive interaction between osteoclasts (OCs) and cancer cells known as the vicious cycle (1). Growth factors secreted by the tumor cells, such as Receptor Activator of NF-κB-Ligand (RANKL), stimulate the bone resorptive activity of OCs. In turn, bone stored factors including Transforming Growth Factor (TGFβ) are released and enhance tumor growth (1). Experimental evidence suggests that OCs play a central role in modulating the tumor/bone vicious cycle. Antagonizing RANKL, using recombinant osteoprotegerin (Fc-OPG) or soluble anti-RANKL antibody (Ab), reduces OC number and significantly decreases tumor burden in murine models of breast cancer bone metastasis and multiple myeloma (2). Similarly, treatment with Zoledronic Acid (ZOL), a N-bisphosphonate compound highly effective in inducing OC apoptosis and suppressing bone resorption, protects mice injected with human breast cancer cells from tumor growth in bone (3). Based on these findings, the OC is now the principal therapeutic target for bone metastases (4). However, not all patients with bone metastases respond well to anti-resorptive therapy, and one third develops further skeletal-related events within 2 years of initiating these therapies (5). Thus, the results from clinical studies suggest that other bone marrow residing cells, in addition to OCs, could be regulating tumor growth in bone.
The bone microenvironment is a reservoir of several immune cell types. Memory T cells have been found in the bone marrow of patients with breast cancer, implicating them in cancer immune surveillance (6). Interestingly, some of the therapies aimed at disrupting the mutual interaction between cancer cells and OCs also have immunomodulatory effects. For example, TGFβ, which is released into the bone marrow microenvironment by the resorptive OC, inhibits T cell proliferation, natural killer (NK) cell function and antigen presentation (7). Thus, blockade of TGFβ at sites of metastases may locally activate T cell function, initiating an anti-tumor immune response. ZOL, in addition to its anti-resorptive effect, can activate cytotoxic gamma/delta T cells (8) and inhibit certain populations of myeloid derived cells with T cell suppressor abilities. Unfortunately, to date, the contribution of T cells in modulating the tumor/bone vicious cycle has not been evaluated since most models of bone metastases use human breast cancer cells injected into immune compromised mice.
The aim of the present study is to examine the relative contribution of immune cells and OCs in the tumor/bone vicious cycle using a syngenic mouse model. We turned to the B16 melanoma model of bone metastases because these cells 1) grow in C57BL/6 immune competent mice, and 2) metastasize to bone following intra-cardiac injection. B16 is a relatively poorly immunogenic cell line, although it can induce a modest but specific T cell response (9). Thus, by using B16 cells we can take advantage of genetically manipulated mice with specific immune phenotypes. Phospholipase C gamma (PLCγ) 2 is an enzyme converting PIP2 into DAG and IP3 leading to activation of PKC and calcium pathways. PLCγ2−/− mice have broadly compromised immune responses due to impaired B cell development, NK cell cytotoxic activity and DC-mediated antigen presentation leading to defective T cell activation (10–13). Furthermore, PLCγ2−/− mice are osteopetrotic due to reduced OC number and functionality (14) (15). Lyn is a Src family member mainly involved in down modulation of several intracellular pathways, including PLCγ2 activation (16). Lyn−/− mice have increased B cell-mediated immune responses, expanded macrophages, mast cells and DCs (17) (18). Due to a hyperactive myeloid population, T cell responses are also enhanced, and Lyn−/− mice develop autoimmunity with age (19). Furthermore, Lyn−/− mice have decreased bone mass and more numerous OCs due to enhanced RANKL signaling and PLCγ phosphorylation (16, 20).
Because PLCγ2 deficiency impacts OC formation and function, we expected that PLCγ2−/− mice would have decreased bone tumor burden following B16 tumor inoculation. Furthermore, we would have anticipated increased tumor growth in bone in Lyn−/− mice based on their hyperactive OC phenotype. In contrast, we found that PLCγ2−/− mice are more susceptible to tumor growth in bone, despite their OC defect, while Lyn−/− mice display significant inhibition of tumor growth in bone, in the face of more numerous OCs. Despite the lack of PLCγ2 and Lyn expression in T cells, aberrant myeloid-mediated T cell activation is responsible for these unexpected findings. Our data demonstrate that CD8+ T cells modulate tumor growth in bone regardless of genetic or pharmacological OC inhibition, thus expanding the current tumor/bone vicious cycle model to include an immune component in addition to the OC.
Material and Methods
Tumor Cells and animal models of bone metastases
B16 mouse melanoma cells were obtained from Dr. David Fisher (Harvard Medical School). These cells have been characterized by expression arrays and western blotting for melanocyte markers like melanin, tyrosinase, c-kit and microphthlamia transcription factor. Pigmented tumor formation in mice further confirmed that B16 are melanoma cells. This assay is routinely used in the lab for their characterization and it was performed within the last few weeks.
Firefly-conjugated B16 cells (B16-FL) were injected intratibially (IT), intracardiac (LV) or subcutaneously (sc) in 6 week old, female mice ((21) and supportive material and methods). For CD4+ or CD8+ T cell depletion we injected 100µg/100µl YTS191.1.2 or YTS169.4.2.1 mAb, respectively, starting 1 day prior to tumor inoculation and continuing every other day for the duration of the experiment. ZOL (0.75µg/mouse) was administered s.c. 10 and 4 days prior to tumor inoculation and mice were sacrificed 2 weeks later. This dosing of ZOL was designed to produce drug levels similar to those achieved with the clinical dosing regimen of 4 mg Zometa® for the treatment of bone metastases (22).
Bone histomorphometry
Mouse tibias were decalcified and processed for bone histomorphometry as described (21). Since PLCγ2−/− and Lyn−/− mice have different basal bone mass compared to WT, we calculated bone loss as percentage of BV/TV relative to their no-tumor controls (arbitrarily set at 100%) using Bioquant Osteo (Nashville TN) .
Bone marrow transplantation
5 week old, female C57BL/6 mice were lethally irradiated using a 137Cs source with 900 rads to generate recipient mice. Bone marrow was harvested from 6-week-old, female PLCγ2−/− and Lyn−/− mice or WT littermates, suspended in PBS and 200µl containing 106 cells were injected into the lateral tail vein of recipient mice to generate PLCγ2−/− and Lyn−/− radiation chimeras and WT controls.
DC generation
DCs were generated from bone marrow progenitor cells from 5–8 week old C57BL/6 mice, as previously described ((23) and supportive material and methods). DCs were matured with 1µg/ml LPS overnight and upregulation of mature DC markers confirmed by FACS.
T cell adoptive transfer
CD8+ T cell were magnetically isolated from WT mice vaccinated with GP10025–33 (10µg/ml) (Biosythesis, TX) pulsed DCs using CD8+ T cell isolation kit (Myltenyi Biotec). Reactivity of GP100 specific CD8+ T cells was confirmed in vitro by IFNγ release following peptide stimulation. 1×106 CD8+ T cells plus 200µl of IL-2 (2000U/ml) (Chemicon, CA), were adoptively transferred into tumor-bearing (IT injected) PLCγ2−/− mice via tail vein. Subsequently, GP10025–33 peptide pulsed (4hrs), LPS-matured DCs were given subcutaneously on the day of the adoptive transfer to further activate GP100 specific CD8+ T cells. Tumor-bearing WT and PLCγ2−/− mice receiving IL-2 alone were used as controls. Tumor growth was monitored on days 8, 10 and 12 by BLI.
Detection of GP-10025–33 specific CD8+ T cell
Spleens were isolated from B16-FL s.c. injected mice. Splenocytes were cultured with 10µg/ml GP-10025–33 peptide in the presence of Golgistop (BD), to inhibit cytokine secretion. After 8 hours, samples were stained with α-CD8-FITC Ab (BD), fixed, permeabilized using Cytofix/Cytoperm kit (BD), co-stained with PE-conjugated α-IFNγ Ab and analyzed by FACS.
Cytometric Bead array
5×104 splenocytes from B16-FL s.c. injected mice were plated in a 96 well/plate with 200 µl of RPMI-1640 and 10% mouse serum. Cells were restimulated with irradiated B16-FL (2500 Rad) for 3 days. Supernatants were harvested and IFNγ production was assessed by FACS analysis using the TH1/TH2 assay kit (BD) according to manufacture procedures.
Statistical Analysis
For each in vivo experiment 4–6 mice per group were used. Experiments were done in triplicate and analyzed using Student's t-test. In calculating two-tailed significance levels for equality of means, equal variances were assumed for the two populations. Results were considered significant at p<0.05 and are indicated with an asterisk (*).
Results
PLCγ2−/− mice have increased bone tumor burden despite defective OC function
Deletion of PLCγ2 in mice results in an osteopetrotic phenotype due to OC defects (14) (15). Based on the tumor/bone vicious cycle model in which OC activity is required to sustain tumor growth in bone, we predicted that PLCγ2−/− mice would be protected from bone metastases. To test this hypothesis, we injected luciferase labeled B16 cells (B16-FL) intratibially (IT), to test tumor growth in bone, or into the left cardiac ventricle (LV), to test tumor tropism to bone, of 6 week old PLCγ2−/− and wild-type (WT) littermates. Presence of bone tumors was measured by bioluminescence imaging (BLI) 8 and 10 days after tumor inoculation, and by histological examination on day 12. Unexpectedly, despite the OC defect, PLCγ2−/− mice showed significantly increased tumor burden in bone (Fig.1A). Histological analysis confirmed the increase in tumor area in PLCγ2−/− bones (Fig.1B–C). These results were also replicated using non-labeled parental B16 cells (not shown), indicating that presence of the luciferase was not responsible for the altered tumor growth.
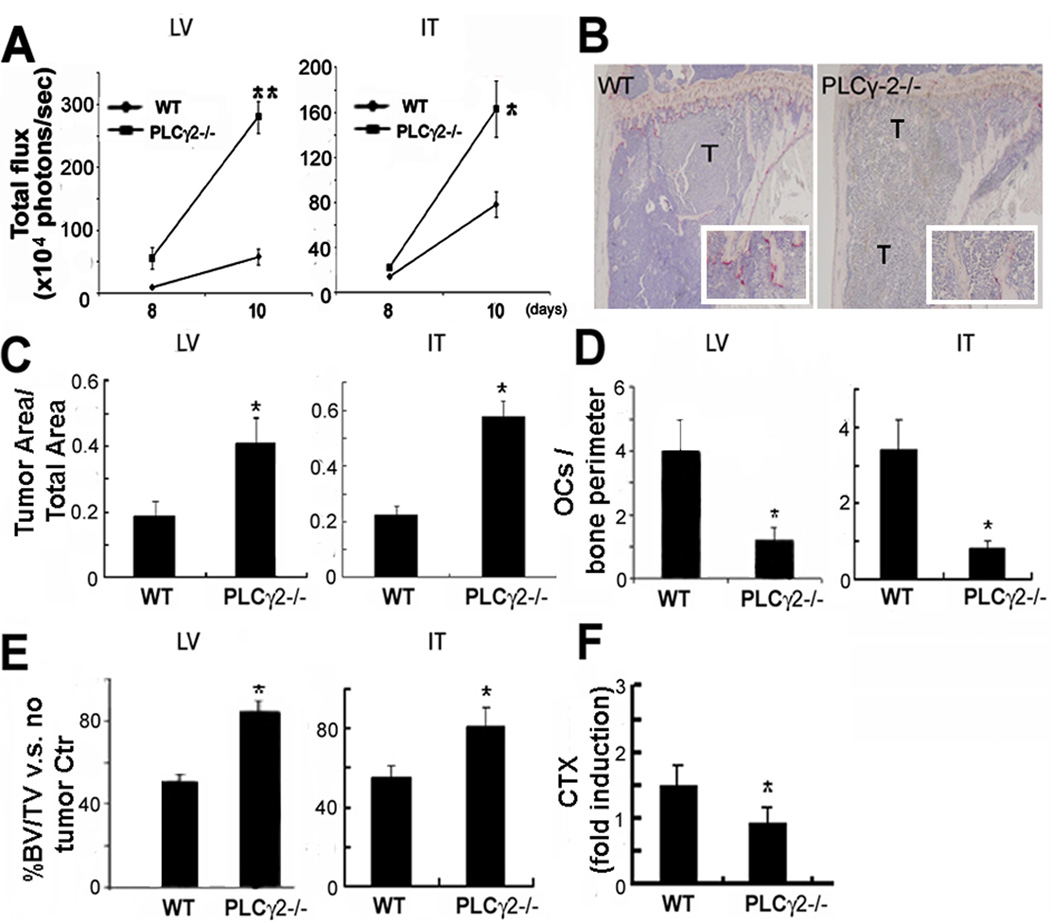
Fig. 1.
PLCγ2−/− mice display increased tumor burden in bone, but not tumor-associated bone loss. B16-FL cells were injected LV or IT into 6 week old WT and PLCγ2−/− littermates. (A) BLI at day 8 and 10 showed higher tumor burden in PLCγ2−/− mice (LV:**, p<0.001 vs WT mice, IT:*, p<0.05 vs WT mice, N=4). (B) TRAP stained sections from WT and PLCγ2−/− tibias showed OCs (red cells in 2.5× magnification insert) and tumor cells (T). (C) Histological analysis of tumor area/total bone area showed a significant increase in tumor growth in PLCγ2−/− mice (*, p<0.05 vs WT) despite decreased OC number/bone perimeter (D) (*, p<0.05 vs WT). (E) Bone loss was calculated as percentage of BV/TV relative to no-tumor controls. While WT mice underwent 50% bone loss after tumor injection, only less than 20% bone loss occurred in PLCγ2−/− mice (*, p<0.05 vs WT). (F) Increased bone loss in WT mice was further confirmed by higher CTX levels. Data are expressed as fold induction from baseline, arbitrarily set as 1, and significant lower CTX levels in PLCγ2−/− mice are indicated (*, p < 0.05 vs WT, N=5 for WT and N=4 for PLCγ2−/−).
Interestingly, consistent with impaired basal osteoclastogenesis, tumor-bearing PLCγ2−/− animals continued to display a 3 fold decrease in OC number compared to equally treated WT littermates (Fig.1D). Despite the increase in tumor burden, PLCγ2−/− mice were still protected from tumor-associated bone destruction as determined by over 80% remaining trabecular bone volume/total bone volume (BV/TV) relative to their no-tumor controls. In contrast, tumor-bearing WT mice displayed about 50% reduction in BV/TV (Fig.1E). Decreased tumor associated bone erosion in PLCγ2−/− mice was further confirmed by low serum levels of Collagen type I fragment (CTX), a marker of in vivo OC activity (Fig.1F). Thus, our data demonstrate that OC deficiency is not sufficient to prevent tumor growth in bone of PLCγ2−/− mice.
Increased bone tumor burden in PLCγ2−/− mice is transplantable
Since PLCγ2−/− mice have broadly compromised myeloid and NK cell functions (10–13), we explored whether the increased bone tumor burden in PLCγ2−/− mice was mediated by cells of the hematopoietic compartment. We transplanted bone marrow cells from WT or PLCγ2−/− donors into lethally irradiated 5 week-old C57BL/6 WT mice. Three weeks post-transplantation, B16-FL cells were inoculated LV. Similar to PLCγ2−/− mice, tumor growth in bone was increased in WT mice carrying PLCγ2−/− marrow cells (PLCγ2−/−>WT) as determined by BLI (Fig.2A) and histological analysis (Fig.2B–C). PLCγ2−/−>WT transplanted mice were protected from tumor-induced bone loss and the number of OCs per bone perimeter was about 4 fold less compared to WT>WT transplants (Fig.2D–E). Based on the dysfunctional PLCγ2−/− OC phenotype, these findings indicate that cells of hematopoietic origin, other than OCs, contributed to enhance tumor growth in bone of PLCγ2−/− mice.
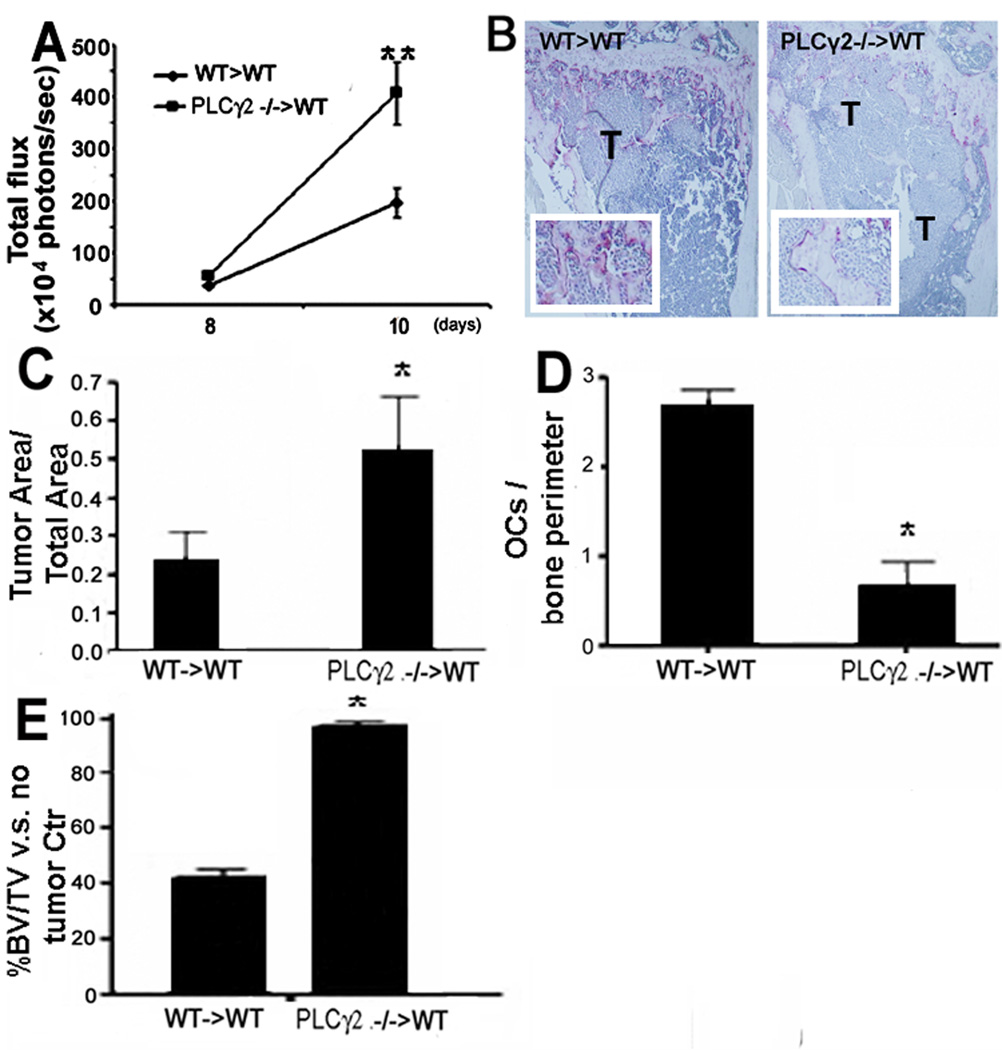
Fig. 2.
Increased bone tumor burden in PLCγ2−/− mice is transplantable. Three weeks post-transplantation, B16-FL were injected LV into WT mice transplanted with WT (WT>WT) or with PLCγ2−/− (PLCγ2−/−>WT) bone marrow cells. (A–B) BLI and TRAP stained long bone sections showed increased tumor growth in bone of PLCγ2−/−>WT (**, p<0.01 vs WT>WT mice, N=6; OCs are visualized in insert 2.5× magnification). Histomorphometry in PLCγ2−/−>WT transplanted animals revealed (C) increased tumor area/total bone area (*, p<0.05 vs WT>WT mice), (D) reduced OC number (*, p<0.05 vs WT>WT mice) and (E) reduced tumor-induced bone loss (*, p<0.05 vs WT>WT mice).
Lyn−/− mice are protected from bone tumor growth despite increased OC responsiveness
To determine if an aberrant immune condition might contribute to regulation of tumor growth in bone regardless of the OC status, we turned to Lyn−/− mice, characterized by enhanced myeloid cell and OC functionality (17) (18) (19). In contrast to PLCγ2−/− animals, Lyn−/− mice displayed significant inhibition of tumor growth in bone following either intratibial or intracardiac tumor inoculation (Fig.3A–B). Analysis of tumor area/total bone area confirmed a 4 fold decrease in tumor burden in Lyn−/− mice (Fig.3C). However, despite the significantly reduced bone tumor burden, Lyn−/− mice had increased OC numbers and showed as much tumor-associated bone loss as WT mice (Fig.3D–E). Consistent with this finding, Lyn−/− mice had elevated serum CTX levels (Fig.3F). These data indicated that increased OC responsiveness is not sufficient to enhance tumor growth in bone of Lyn−/− mice.
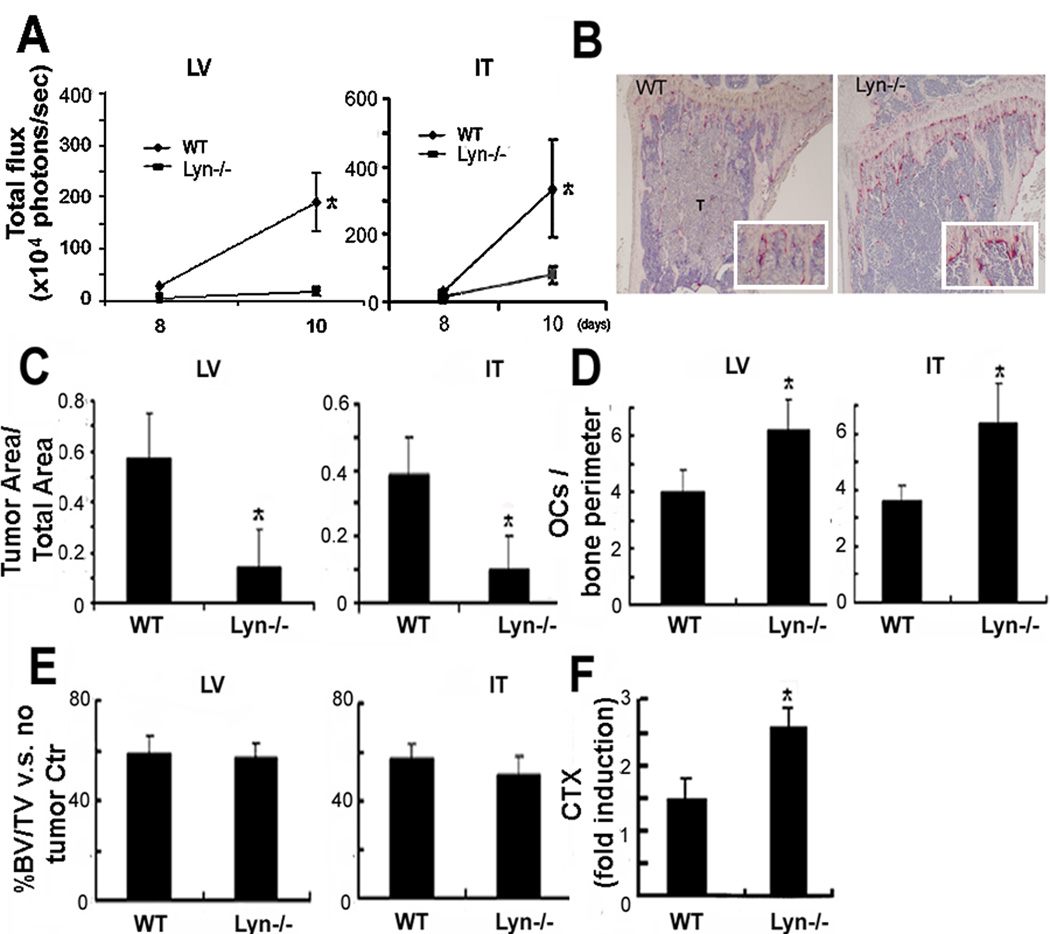
Fig. 3.
Lyn−/− animals are protected from tumor burden in bone, but not from bone loss. (A–B) BLI and TRAP stained long bones showed reduced tumor growth (T) in bones of Lyn−/− mice inoculated LV or IT with B16-FL cells (*, p<0.05 vs WT mice, N=6). (C) Histomorphometry showed decreased tumor area/total bone area in Lyn−/− mice (*, p<0.05 vs WT mice, N=6), despite (D) increased OC numbers (*, p<0.05 vs WT mice, N=6; insert 2.5× magnification) and (E) 50% bone loss calculated as percentage BV/TV relative to no-tumor controls. (F) CTX serum levels showed higher OC activity in Lyn−/− mice compared to WT (*, p<0.05 vs WT mice, N=5 for WT and N=4 for Lyn−/−).
Reduction in bone tumor burden in Lyn−/− is transplantable
To determine if cells of the hematopoietic compartment were responsible for reduced bone tumor burden in Lyn−/− mice, we performed bone marrow transplants, in which WT recipient mice carried WT (WT>WT) or Lyn−/− (Lyn−/−>WT) marrow cells. We found that Lyn−/−>WT transplanted animals were protected from B16-FL tumor growth in bone as determined by BLI (Fig.4A) and histological analysis (Fig.4B). Similarly to Lyn−/− mice, Lyn−/−>WT transplants displayed disproportionate bone loss for their tumor burden, showing similar OC number and percentage of BV/TV as WT>WT transplants despite the 4 fold decrease in bone tumor burden (Fig.4C–E). Due to the hyperactive Lyn−/− OC phenotype, these data indicate that cells of hematopoietic origin, other than OCs, contribute to reduce tumor growth in bone of Lyn−/− mice.
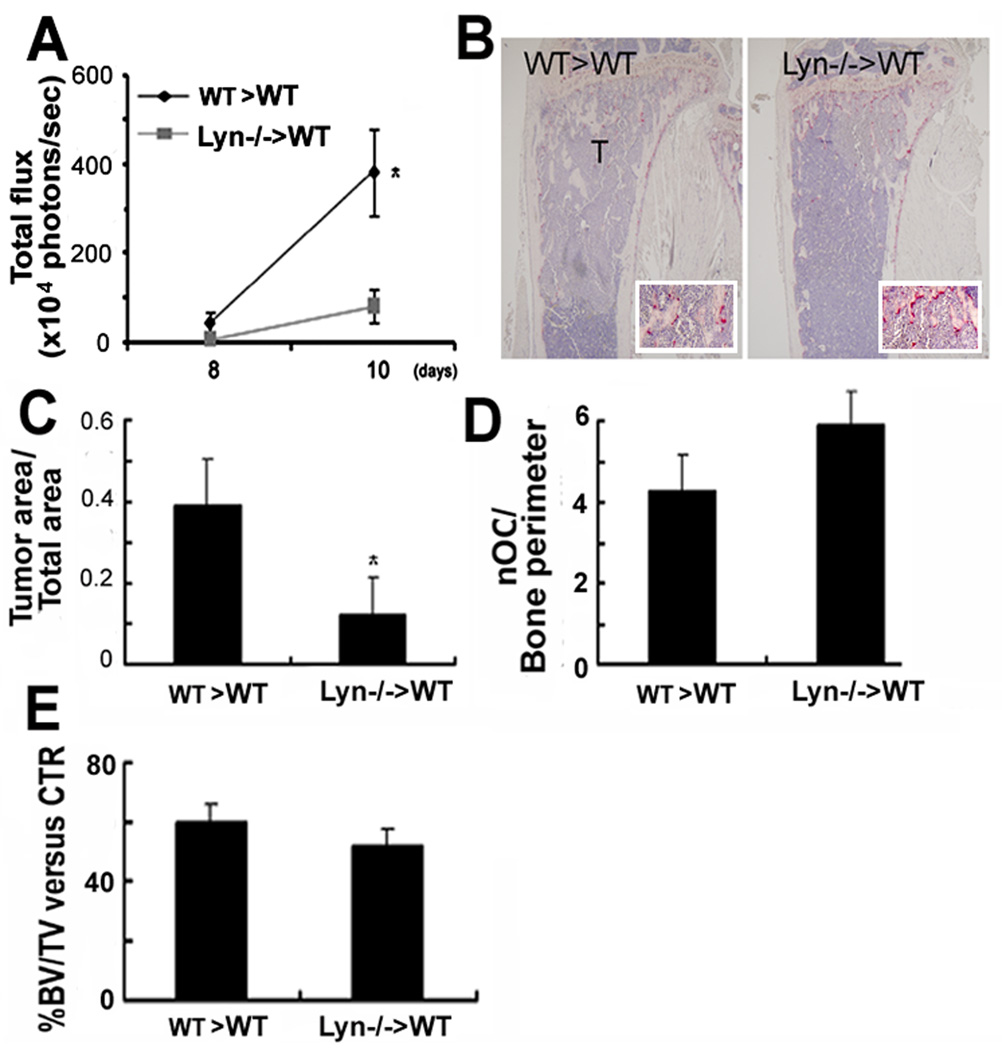
Fig. 4.
Reduction in bone tumor burden in Lyn−/− is transplantable. Three weeks post-transplantation, B16-FL were injected LV into WT mice transplanted with WT (WT>WT) or Lyn−/− (Lyn−/−>WT) bone marrow cells. (A) BLI and (B) histology showed reduced tumor cells (T) in Lyn−/−>WT mice (*, p<0.05 vs WT>WT mice, N=4; insert 2.5× magnification). (C) Histomorphometry showed reduction in tumor area/total bone area in Lyn−/−>WT mice (*, p<0.05 vs WT> WT mice, N=4), but (D–E) WT>WT and Lyn−/−>WT mice showed similar differences in OC number and in the percentage BV/TV relative to no-tumor-bearing mice.
Modulation of T cell activation alters tumor burden in bone independent of OC activity
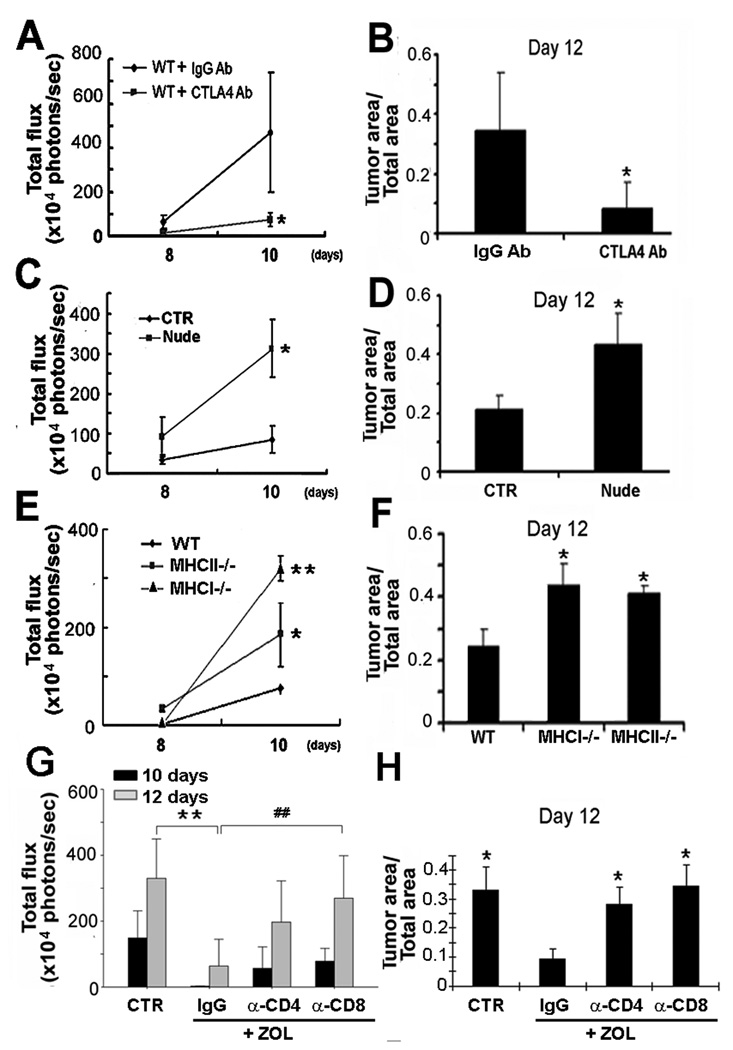
T cells are known modulators of anti-tumor immune responses (24). To determine if T cells might be regulating tumor growth in bone, we injected WT mice with the α-CTLA4 Ab, which enhances T cell proliferation and/or function (25), following intracardiac inoculation of B16-FL. α-CTLA4 Ab significantly suppressed tumor growth in bone by BLI and histomorphometric analysis (Fig.5A–B). Conversely, Nude mice, which lack all T cell subsets, had enhanced tumor growth in bone compared to control animals (Fig.5C). Histological analysis confirmed an abundance of tumor cells in the bones of Nude mice compared to their controls (Fig.5D). To determine which T cell subset might be regulating tumor growth in bone, we turned to MHCI−/− mice, which do not have CD8+ T cells, and MHCII−/− mice, which lack CD4+ T cells. Absence of either CD4+ or CD8+ T cells significantly increased B16-FL tumor growth in bone following intracardiac inoculation. MHCI−/− mice had > 4 fold and MHCII−/− mice had >2.5 fold increase in bone tumor burden by day 10 compared to WT mice (Fig.5E–F).
Fig. 5.
T cells modulate tumor growth in bone. (A–B) BLI and histology of tumor area/total bone area showed reduced tumor growth in WT mice with α-CTLA4 Ab (T cell activating Ab) compared to isotype IgG (*, p<0.05 vs WT mice, N=4). (C–D) BLI and histology of Nude mice injected LV with B16-FL showed increased tumor growth in bone compared to controls (CTR) (*, p<0.05 vs CTR mice, N=4). (E–F) BLI and histology in WT, MHCI−/− and MHCII−/− mice injected LV with B16-FL showed enhanced tumor growth in bone in the absence of specific T cell subsets (**, p<0.01 vs WT mice:*, p<0.05 vs WT mice, N=4). (G–H) BLI and tumor area/total area showed that α-CD4+ and α-CD8+ Abs reduced the anti-tumor effect of ZOL compared to WT treated with ZOL (**p<0.02 IgG vs CTR and ##p<0.02 IgG vs α-CD8).
Since the tumor/bone vicious cycle model considers OC activity to be central for tumor proliferation in bone, we sought to determine the relative contribution of OCs and T cells in this process. We tested the efficacy of ZOL anti-resorptive treatment in protecting from tumor growth in bone in mice depleted of CD4+ or CD8+ T cells. WT mice were treated with ZOL 10 and 4 days prior to B16-FL intratibial inoculation and α-CD4+ or α-CD8+ T cell depleting Abs were administered one day prior and every two days after injection of cancer cells. Efficiency of T cell depletion was confirmed at the end of the experiment by FACS analysis. Despite blockade of tumor-associated bone loss (supportive information 1), we found that ZOL anti-tumor effect was significantly reduced in CD8+, and to a lesser extent in CD4+, T cell depleted mice compared to WT mice receiving the anti-resorptive treatment (Fig.5G–H). Thus, T cells modulate tumor growth in bone regardless of the OC status.
T cell abnormalities are responsible for tumor growth in bone of PLCγ2−/− and Lyn−/− mice
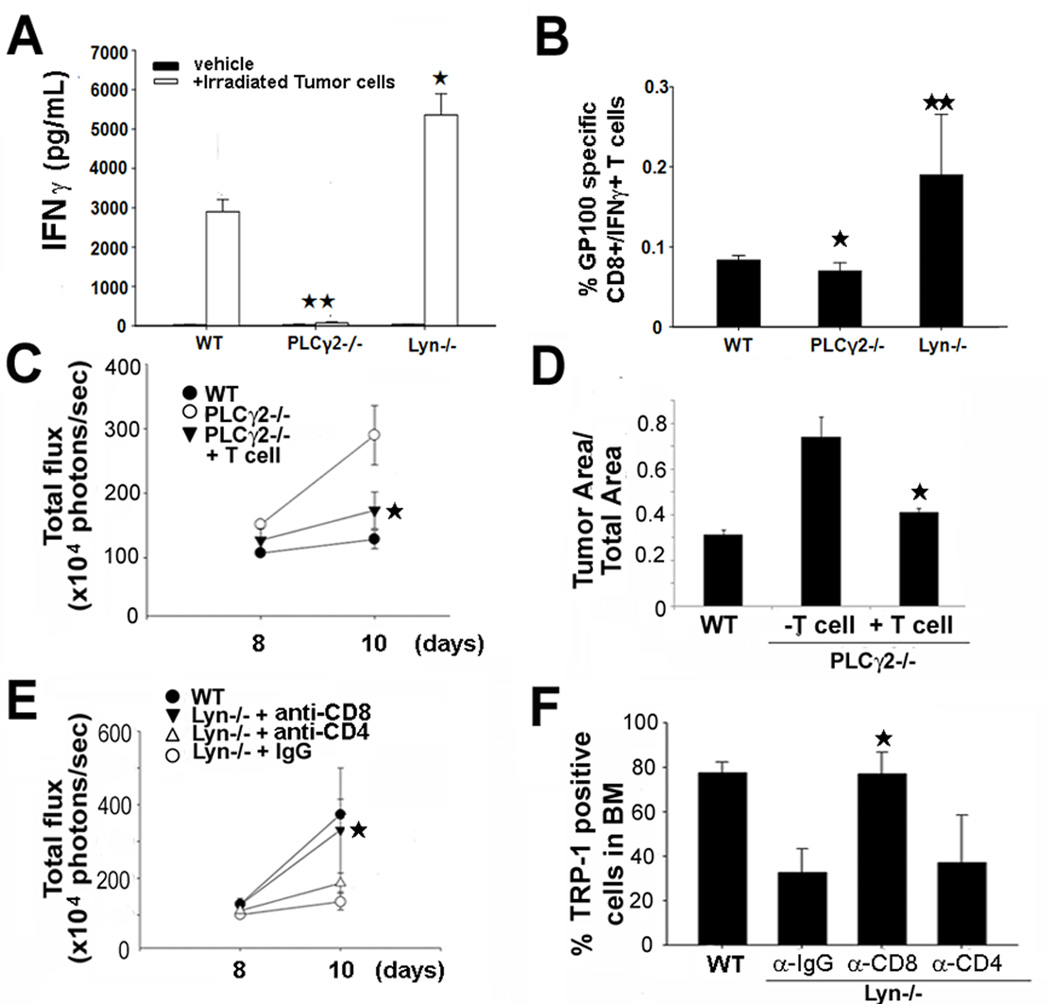
Despite a lack of PLCγ2 and Lyn expression in T cells, myeloid cell abnormalities (impaired DC function in PLCγ2−/− (23), myeloid hyperactivity in Lyn−/− mice (19)) could induce aberrant T cell-mediated anti-tumor responses. To determine presence of activated T cells in tumor-bearing mice, we isolated spleens from PLCγ2−/− and Lyn−/− mice two weeks after B16-FL subcutaneous (s.c) inoculation. We examined IFNγ release by activated T cells, restimulated in vitro for 3 days with irradiated B16-FL cells. This assay showed impaired IFNγ release in PLCγ2−/− and hyper production in Lyn−/− cultures compared to WT (Fig.6A). IFNγ release was also observed in response to parental B16 cells (not shown). To further determine whether the anti-tumor T cell response was specific to the B16 cell line, splenocytes isolated from tumor-bearing mice were stimulated for 8 hours with tumor antigen GP100, expressed by B16 cells. FACS analysis showed that the percentage of CD8+/IFNγ producing PLCγ2−/− T cells responding to GP100 was significant less than WT, while in Lyn−/− mice it was two-fold more than WT (Fig.6B), supporting the conclusion that PLCγ2−/− and Lyn−/− mice have altered T cell responses to tumor.
Fig. 6.
T cell transfer protects while T cell depletion enhances tumor growth in bone of PLCγ2−/− and Lyn−/− mice, respectively. (A) IFNγ production was measured in splenocytes from tumor-bearing WT, PLCγ2−/− and Lyn−/− mice restimulated with irradiated B16-FL cells in vitro. IFNγ levels were reduced in PLCγ2−/− mice and increased in Lyn−/− mice (*p<0.05 Lyn−/− vs WT; **p<0.01 PLCγ2−/− vs WT, N=3). (B) Percentage of GP100 specific CD8+/IFNγ producing T cells was determined by FACS in spleen of WT, PLCγ2−/− and Lyn−/− tumor-bearing mice. T cell activation was reduced in PLCγ2−/− mice, while increased in Lyn−/− animals (*, p<0.05 PLCγ2−/− vs WT;**, p<0.01 Lyn−/− vs WT N=3). (C–D) BLI and histology showed decreased bone tumor burden in PLCγ2−/− mice adoptively transferred with WT CD8+ T cells versus PLCγ2−/− controls (*, p<0.05 vs PLCγ2−/− mice, N=5). (E–F) BLI and percentage of B16-FL cells in bone, determined by FACS using α-TRP-1 Ab to detect B16 cells, was performed in Lyn−/− mice injected with IgG control (CTR), α-CD8 or α-CD4 Abs. Data showed that CD8+ T cell depletion enhanced tumor growth in bone of Lyn−/− mice (*, p<0.05 α-CD8 vs α-IgG Ab, N=5).
To further determine whether T cell abnormalities were responsible for increased bone tumor burden in PLCγ2−/− mice, we adoptively transferred tumor-specific cytotoxic CD8+ T cells into PLCγ2−/− mice. Since B16 melanoma cells express the antigen GP100, we generated GP100-reactive CD8+ T cells by injecting twice GP10025–33 peptide-pulsed mature DCs into WT mice. Two weeks later, CD8+ T cells were isolated from spleens and their functionality was confirmed in vitro in response to the antigen (supportive information 2). GP100-reactive CD8+ T cells were then adoptively transferred into PLCγ2−/− mice intratibially injected with B16-FL cells, along with GP100 pulsed WT DCs and IL-2. Mice injected with IL-2 alone were used as controls. IL-2 itself did not affect tumor growth in this model (supportive information 3). Adoptive transfer of GP100-reactive CD8+ T cells reduced tumor burden in PLCγ2−/− mice compared to null mice receiving IL-2 alone (Fig.6C–D). Conversely, Ab-mediated CD8+ T cell depletion in tumor-bearing Lyn−/− mice enhanced tumor burden in bone by BLI, although CD4+ depletion did not (Fig 6E). To simultaneously measure tumor burden and confirm CD4/CD8 depletion, we performed FACS analysis on tumor-bearing bones and found that CD8+ depletion led to more tumor growth in bone marrow, as determined by TRP-1+ staining, a surface marker of B16 cells (Fig.6F). Thus, CD8+ T cells contributed to the regulation of tumor growth in bone in PLCγ2−/− and Lyn−/− mice regardless of OC functionality.
Discussion
Animal studies using human breast cancer cells injected in immune compromised mice strongly support a tumor/bone vicious cycle model in which blockade of OC function can reduce tumor growth in bone and prevent associated bone loss (26) (27). ZOL, a potent inhibitor of OC activity, is widely used in the clinic to treat patients with bone metastases. ZOL protects form tumor-induced bone loss and reduces skeletal complications such as bone pain, pathological fractures, bone surgery, and hypercalcemia. Unfortunately, one-third of patients with bone metastases who respond to ZOL treatment develop further skeletal-related events within 2 years of initiating the anti-resorptive therapy (5). Therefore, the tumor/bone vicious cycle model must be revisited to include other bone marrow derived cells, in addition to the OCs that can participate in restraining or encouraging the expansion of tumor in the bone microenvironment, thus altering the efficacy of anti-resorptive therapies. By using immune competent mice we now demonstrate that alterations in T cell functions can bypass the requirement for OCs in tumor growth within bone and reduce the efficiency of ZOL treatment.
PLCγ2 plays a critical role in bone homeostasis and immune function(12, 13, 15, 23, 28–30). Previous studies using a tumor rejection assay, in which MHC-I deficient susceptible target cells were injected intraperitoneally in WT or PLCγ2−/− mice, suggested compromised tumor elimination in PLCγ2−/− mice (31). Consistent with this finding, we observed increased s.c tumor growth and elevated metastatic dissemination following intra-venous injection of B16-FL cells, demonstrating broadly impaired anti-tumor immune responses (supportive information 4). However, in bone, the OC resorptive activity is thought to be central in regulating metastatic dissemination and tumor burden (1). By measuring direct tumor growth in bone or tropism of tumor cells to bone, we demonstrate that PLCγ2−/− OC defect is not sufficient to prevent bone metastases, thus challenging the current tumor/bone vicious cycle model.
Mirroring the PLCγ2 phenotype, deletion of Lyn, a negative regulator of PLCγ activity, leads to increased immune activation (18, 19, 32, 33). In agreement with a hyperactive immune condition, Lyn−/− mice are protected form s.c. tumor growth and metastatic dissemination. Lyn−/− mice are also more prone to bone loss due to increased OC number (20). While more numerous OCs are expected to create a highly favorable environment for tumor growth in bone, our data demonstrate that increased immune activation is sufficient to counteract the OC-mediated pro-tumor effect, thus protecting Lyn−/− mice from bone metastases.
Immune cells have emerged as significant regulators of primary cancer development as well as metastasis into ectopic tissues. Tumor antigens are presented to naïve T cells, leading to activation of CD4+ and CD8+ T cells. Subsequently, tumor-specific T cells home to tumor sites, where they kill antigen-positive tumor cells (34). However, tumor cells can also escape the immune system, developing the ability to grow in an immunologically intact host. Several factors released by tumor cells can directly or indirectly induce T cell immune suppression. TGFβ, released by OCs (35), has positive proliferative effects on tumor cells but also very potent anti-inflammatory activity (7). Thus, the anti-tumor effects of OC blockade and/or inhibition of TGFβ pathway could, at least in part, depend on interfering with T cell-mediated anti-tumor responses. We now demonstrate that global T cell deficiency increases, while T cell activation protects from growth of B16-FL cells in bone. CD8+, and to a lesser extent CD4+ T cells, both have anti-tumor capacities. However, T cell manipulations do not seem to affect the capacity of the cancer cells to reach bone, presumably because T cell activation occurs after the cells have arrived in the bone microenvironment.
Importantly, we found that tumor growth in bone in CD8+ T cell depleted mice occurs independently of ZOL-mediated OC blockade. This finding is in apparent contradiction with previous reports showing that ZOL reduces bone tumor burden in immune compromised animals. However, a direct comparison between the anti-tumor effect of ZOL in immune competent and immune deficient mice has never been reported. ZOL is effective in decreasing tumor burden in T cell deficient mice compared to untreated immune deficient controls. However, the anti tumor effect of ZOL in CD8+ T cell depleted mice is significantly reduced compared to immune competent WT animals treated with the anti-resorptive agent. This observation has important clinical relevance since ZOL is the current treatment of choice for patients with bone metastases. Although ZOL has been shown to successfully reduce incidence of bone metastases, not all patients respond well (5). Considering that cancer patients often have immune imbalances, our data suggest that reduced T cell anti-tumor immune responses could be the responsible for tumor growth in bone despite ZOL-mediated OC blockade.
Altered T cell activation is also central to the tumor/bone phenotype observed in PLCγ2−/− and Lyn−/− mice. Although B16 is a poorly immunogenic cell line, it has been reported that it can induce a modest anti-tumor T cell response (9). We further confirmed this observation by identifying CD8+ T cells that can respond to the endogenous B16 antigen, GP100. Specifically, we observed enhanced GP100-specific CD8+ T cells in tumor-bearing Lyn−/− mice but a blunted response in PLCγ2−/− animals. Release of IFNγ by these tumor specific CD8+ T cells was also enhanced in Lyn−/− mice, while barely detectable in PLCγ2−/− animals. Importantly, depletion of CD8+ T cells restores tumor growth in bone in Lyn−/− mice, while adoptive transfer of tumor-specific WT CD8+ T cells into PLCγ2−/− mice reduces bone tumor burden.
Despite not being expressed in T cells, both PLCγ2 and Lyn indirectly govern T cell activation by modulating myeloid cell functions. Defective DC-mediated antigen presentation (13, 23) likely underlies the reduced number of tumor specific CD8+ T cell in PLCγ2−/− mice. Because of impaired DC functions, PLCγ2−/− mice are protected from antigen-induced arthritis, an inflammatory condition strongly dependent on T cell activation (23). It is very likely that DC defects impair T cell activation in PLCγ2−/− tumor-bearing mice, thus enhancing bone tumor burden even in the absence of functional OCs. An additional explanation for the tumor phenotype in PLCγ2−/− mice could be defective NK cell cytotoxicity (12). It is unlikely that the NK cell abnormalities are the primarily responsible for increased tumor growth in these mice, since NK cell depletion in WT mice can only slightly increase bone tumor burden without reaching the levels observed in PLCγ2−/− animals (supportive information 5). Conversely, Lyn−/− mice have expanded macrophage and DC populations (18, 36), and hyperactive myeloid cells which augmented T cell responses and IFNγ production (19). Indeed, we found that tumor-bearing Lyn−/− mice display increased tumor antigen-specific CD8+ T cells in spleen compared to WT. Therefore, similar to PLCγ2 deficiency, absence of Lyn in the myeloid population is likely to be responsible for enhanced T cell anti-tumor responses.
This is the first report analyzing the relative contribution of bone and immune cells in development of bone metastases. We now provide compelling evidence that a condition of immune deficiency can interfere with the anti-tumor effects of OC blockade. We used both genetic models of immune and OC modulation (Lyn and PLCγ2), as well as pharmacological inhibition of OCs and T cells. These findings suggest that the immune status can affect the efficacy of anti-resorptive treatments in reducing bone metastases, and emphasize the need to further explore the beneficial effects of combined T cell stimulation and anti-resorptive therapies in patients with bone metastases.
Supplementary Material
Acknowledgements
We gratefully thank Tonia Thompson for research administration and Karon Hertlein for secretarial support (Department of Orthopaedics, Washington University). This work was supported by NIH to RF (RO1 AR53628) and ARRA to RF (63181), NIH to DVN (AR052705 and EB007568), AH (T32HL007088), and KW (R01 52152) and the Barnes-Jewish Foundation to DVN.
Abbreviation and symbols
- PLCγ2
Phospholipase C gamma 2
- OC
osteoclast
- DC
dendritic cell
- NK
natural killer
- LV
left cardiac ventricle
- IT
intratibial
- s.c.
subcutaneous
- BLI
bioluminescence imaging
- BV/TV
trabecular bone volume/total bone volume
- ZOL
Zoledronic acid
- TRAP
tartrate acid phosphatase
- Ab
antibody
- CTX
collagen type I fragments
- IFNγ
interferon gamma
References
- 1.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 2.Vanderkerken K, De Leenheer E, Shipman C, et al. Recombinant Osteoprotegerin Decreases Tumor Burden and Increases Survival in a Murine Model of Multiple Myeloma. Cancer Research. 2003;63:287–289. [PubMed] [Google Scholar]
- 3.Hirbe AC, Roelofs AJ, Floyd DH, et al. The bisphosphonate zoledronic acid decreases tumor growth in bone in mice with defective osteoclasts. Bone. 2009;44:908–916. doi: 10.1016/j.bone.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roodman GD. Mechanisms of Bone Metastasis. New England Journal of Medicine. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 5.Pavlakis NSR, Stockler M. Bisphosphonates for breast cancer. Cochrane Database of Systematic Revie. 2005 doi: 10.1002/14651858.CD003474.pub2. ws. [DOI] [PubMed] [Google Scholar]
- 6.Feuerer M, Rocha M, Bai L, et al. Enrichment of memory T cells and other profound immunological changes in the bone marrow from untreated breast cancer patients. Int J Cancer. 2001;92:96–105. [PubMed] [Google Scholar]
- 7.Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res. 2007;13:5262–5270. doi: 10.1158/1078-0432.CCR-07-1157. [DOI] [PubMed] [Google Scholar]
- 8.Schilbach K, Geiselhart A, Handgretinger R. Induction of proliferation and augmented cytotoxicity of gammadelta T lymphocytes by bisphosphonate clodronate. Blood. 2001;97:2917–2918. doi: 10.1182/blood.v97.9.2917. [DOI] [PubMed] [Google Scholar]
- 9.Iwai Y, Terawaki S, Honjo T. PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int Immunol. 2005;17:133–144. doi: 10.1093/intimm/dxh194. [DOI] [PubMed] [Google Scholar]
- 10.Wang D, Feng J, Wen R, et al. Phospholipase Cgamma2 is essential in the functions of B cell and several Fc receptors. Immunity. 2000;13:25–35. doi: 10.1016/s1074-7613(00)00005-4. [DOI] [PubMed] [Google Scholar]
- 11.Aki D, Minoda Y, Yoshida H, et al. Peptidoglycan and lipopolysaccharide activate PLCgamma2, leading to enhanced cytokine production in macrophages and dendritic cells. Genes Cells. 2008;13:199–208. doi: 10.1111/j.1365-2443.2007.01159.x. [DOI] [PubMed] [Google Scholar]
- 12.Tassi I, Presti R, Kim S, Yokoyama WM, Gilfillan S, Colonna M. Phospholipase C-gamma 2 is a critical signaling mediator for murine NK cell activating receptors. J Immunol. 2005;175:749–754. doi: 10.4049/jimmunol.175.2.749. [DOI] [PubMed] [Google Scholar]
- 13.Tassi I, Cella M, Castro I, Gilfillan S, Khan WN, Colonna M. Requirement of phospholipase C-gamma2 (PLCgamma2) for Dectin-1-induced antigen presentation and induction of TH1/TH17 polarization. Eur J Immunol. 2009;39:1369–1378. doi: 10.1002/eji.200839313. [DOI] [PubMed] [Google Scholar]
- 14.Mao D, Epple H, Uthgenannt B, Novack DV, Faccio R. PLC{gamma}2 regulates osteoclastogenesis via its interaction with ITAM proteins and GAB2. J Clin Invest. 2006;116:2869–2879. doi: 10.1172/JCI28775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epple H, Cremasco V, Zhang K, Mao D, Longmore GD, Faccio R. PLCgamma2 modulates integrin signaling in the osteoclast by affecting the localization and activation of Src kinase. Mol Cell Biol. 2008 doi: 10.1128/MCB.00259-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoon SH, Lee Y, Kim HJ, et al. Lyn inhibits osteoclast differentiation by interfering with PLCgamma1-mediated Ca2+ signaling. FEBS Lett. 2009;583:1164–1170. doi: 10.1016/j.febslet.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Nishizumi H, Taniuchi I, Yamanashi Y, et al. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity. 1995;3:549–560. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 18.Chu C-L, Lowell CA. The Lyn Tyrosine Kinase Differentially Regulates Dendritic Cell Generation and Maturation. Journal of Immunology. 2005;175:2880–2889. doi: 10.4049/jimmunol.175.5.2880. [DOI] [PubMed] [Google Scholar]
- 19.Scapini P, Hu Y, Chu C-L, et al. Myeloid cells, BAFF, and IFN-Î3 establish an inflammatory loop that exacerbates autoimmunity in Lyn-deficient mice. The Journal of Experimental Medicine. 2010;207:1757–1773. doi: 10.1084/jem.20100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HJ, Zhang K, Zhang L, Ross FP, Teitelbaum SL, Faccio R. The Src family kinase, Lyn, suppresses osteoclastogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0806963106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirbe AC, Uluckan O, Morgan EA, et al. Granulocyte colony-stimulating factor enhances bone tumor growth in mice in an osteoclast-dependent manner. Blood. 2007;109:3424–3431. doi: 10.1182/blood-2006-09-048686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rauch DA, Hurchla MA, Harding JC, et al. The ARF Tumor Suppressor Regulates Bone Remodeling and Osteosarcoma Development in Mice. PLoS ONE. 5:e15755. doi: 10.1371/journal.pone.0015755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cremasco V, Benasciutti E, Cella M, Kisseleva M, Croke M, Faccio R. Phospholipase C Gamma 2 Is Critical for Development of a Murine Model of Inflammatory Arthritis by Affecting Actin Dynamics in Dendritic Cells. PLoS ONE. 2009;5:e8909. doi: 10.1371/journal.pone.0008909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leen AM, Rooney CM, Foster AE. Improving T cell therapy for cancer. Annu Rev Immunol. 2007;25:243–265. doi: 10.1146/annurev.immunol.25.022106.141527. [DOI] [PubMed] [Google Scholar]
- 25.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat Immunol. 2002;3:611–618. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki A, Boyce BF, Story B, et al. Bisphosphonate risedronate reduces metastatic human breast cancer burden in bone in nude mice. Cancer Research. 1995;55:3551–3557. [PubMed] [Google Scholar]
- 27.Peyruchaud O, Serre CM, NicAmhlaoibh R, Fournier P, Clezardin P. Angiostatin inhibits bone metastasis formation in nude mice through a direct anti-osteoclastic activity. J Biol Chem. 2003;278:45826–45832. doi: 10.1074/jbc.M309024200. [DOI] [PubMed] [Google Scholar]
- 28.Cremasco V, Graham DB, Novack DV, Swat W, Faccio R. Vav/Phospholipase Cgamma2-mediated control of a neutrophil-dependent murine model of rheumatoid arthritis. Arthritis Rheum. 2008;58:2712–2722. doi: 10.1002/art.23757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham DB, Robertson CM, Bautista J, et al. Neutrophil-mediated oxidative burst and host defense are controlled by a Vav-PLCgamma2 signaling axis in mice. J Clin Invest. 2007;117:3445–3452. doi: 10.1172/JCI32729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hashimoto A, Takeda K, Inaba M, et al. Cutting edge: essential role of phospholipase C-gamma 2 in B cell development and function. J Immunol. 2000;165:1738–1742. doi: 10.4049/jimmunol.165.4.1738. [DOI] [PubMed] [Google Scholar]
- 31.Caraux A, Kim N, Bell SE, et al. Phospholipase C-gamma2 is essential for NK cell cytotoxicity and innate immunity to malignant and virally infected cells. Blood. 2006;107:994–1002. doi: 10.1182/blood-2005-06-2428. [DOI] [PubMed] [Google Scholar]
- 32.Smith KG, Tarlinton DM, Doody GM, Hibbs ML, Fearon DT. Inhibition of the B cell by CD22: a requirement for Lyn. J Exp Med. 1998;187:807–811. doi: 10.1084/jem.187.5.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishizumi H, Horikawa K, Mlinaric-Rascan I, Yamamoto T. A double-edged kinase Lyn: a positive and negative regulator for antigen receptor-mediated signals. J Exp Med. 1998;187:1343–1348. doi: 10.1084/jem.187.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 35.Guise TA. Molecular mechanisms of osteolytic bone metastases. Cancer. 2000;88:2892–2898. doi: 10.1002/1097-0142(20000615)88:12+<2892::aid-cncr2>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 36.Harder KW, Quilici C, Naik E, et al. Perturbed myelo/erythropoiesis in Lyn-deficient mice is similar to that in mice lacking the inhibitory phosphatases SHP-1 and SHIP-1. Blood. 2004;104:3901–3910. doi: 10.1182/blood-2003-12-4396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.