Abstract
Serotonin-6 (5-HT6) receptors are densely expressed in the dorsolateral striatum (DLS), a brain region linked to habits. Medications acting on the serotonergic system, including 5-HT6 receptors, can diminish habitual and repetitive behaviors associated with clinical syndromes such as Obsessive-Compulsive Disorder and may have implications for addiction as well. To examine the role of 5-HT6 receptors in the acquisition and persistence of habitual behavior, we manipulated 5-HT6 receptor expression in the DLS with herpes simplex virus vectors in combination with different behavioral procedures; control rats received a vector expressing enhanced green fluorescent protein. In one set of experiments, rats were tested under conditions that favor the acquisition of either discrete action-outcome responding or repetitive responding; increased 5-HT6 receptor expression in DLS did not alter learning in either paradigm. In the next experiment, rats were over-trained on fixed- then variable-interval schedules resulting in an escalation of lever pressing over sessions far in excess of that necessary to receive sucrose pellets. After training, rats received viral vector infusion into the DLS. Subsequently, half of each group underwent an omission contingency training session in which they received reinforcement for refraining from pressing the lever while the other half served as yoked controls. A probe session under extinction conditions was performed the following day. Only rats that received both the 5-HT6 vector and omission contingency training showed reduced lever pressing during the probe session. These results suggest that increasing 5-HT6 receptor signaling in the DLS facilitates behavioral flexibility in the face of changing contingencies.
Keywords: habitual responding, viral-mediated gene transfer, learning acquisition, serotonin
INTRODUCTION
Rodent dorsostriatum (primate caudate-putamen) contributes to reinforcement-learning and compulsive behavior. In particular, dorsolateral striatum (DLS) is involved in forming fixed patterns of repetitive responding (Packard & Knowlton, 2002). With training, goal-directed actions become reflexive habitual responses that allow quick and accurate expression of learned behaviors in response to environmental cues, replacing the need for more reflective time-consuming cognitive processes (Daw et al., 2005). However, some habits progress into pathologically compulsive stimulus-response (SR) behaviors insensitive to environmental change (Graybiel, 2008). Persistent compulsive SR behaviors are partly mediated by abnormal caudate-putamen activity in patients with Obsessive-Compulsive Disorder (OCD), substance abuse, and other behavioral control disorders (Vanderschuren & Everitt, 2005; van den Heuvel et al., 2010); rodent models of these disorders likewise demonstrate dorsostriatum involvement (Yin & Knowlton, 2006). Thus, behaviors can progress from goal-directed to habitual to compulsive, likely reflecting a shift from ventromedial to dorsolateral striatum control on behavior (Everitt & Robbins, 2005).
Rats over-trained on variable-interval reinforcement schedules establish SR behaviors inflexible to environmental change (Dickinson et al., 1983). These habitual behaviors lack the flexibility to update despite changes to the contingency required to earn rewards. An established model to assess habitual responding is the omission contingency procedure: rats over-trained on variable-intervals are rewarded for omitting behavioral responses previously earning reward (Davis & Bitterman, 1971), and are subsequently assessed during a probe session under extinction conditions. Rats unable to cease habitually responding display persistent behavioral responses (e.g. lever pressing) despite omission contingency training; an effect dependent on DLS activity during the omission contingency training session (Yin et al., 2006).
Alterations of serotonergic neurotransmission are associated with a variety of neuropsychiatric disorders demonstrating impulsivity and compulsivity (Fineberg et al., 2010), related behavioral features reliant on frontostriatal mechanisms (Chudasama & Robbins, 2006). In humans, serotonin enhancing drugs are used to treat OCD (Goddard et al., 2008; Soomro et al., 2008), whereas decreased serotonin levels increase impulsivity (Schweighofer et al., 2008). Similarly in rats, SSRIs decrease compulsive responding (Joel et al., 2004) whereas global serotonin depletion increases impulsivity on operant tasks (Winstanley et al., 2004). The dorsostriatum receives rich serotonergic afferents and numerous serotonin receptor subtypes are found on post-synaptic targets (Di Matteo et al., 2008); particularly abundant are 5-HT6 receptors (Roberts et al., 2002) in humans and rats more so than mice (Hirst et al., 2003). Several atypical antipsychotics with high affinity for 5-HT6 receptors (Roth et al., 1994) are frequently used as adjunctive agents to treat refractory OCD (Denys, 2006).
Given their CNS-exclusive expression and striatal enrichment, 5-HT6 receptors are a potentially therapeutic target for several psychiatric disorders (Woolley et al., 2004; Mitchell & Neumaier, 2005). 5-HT6 receptor antagonists improve performance on various learning and memory tasks in rats (Foley et al., 2004; Lieben et al., 2005; Mitchell et al., 2006; Marcos et al., 2008; Mitchell & Neumaier, 2008). Additionally, we have found that increased expression of 5-HT6 receptors using viral-mediated-gene-transfer interferes with reward-oriented and operant behaviors normally mediated by specific striatal subregions (Mitchell et al., 2007; Ferguson et al., 2008; Eskenazi & Neumaier, 2010). This strategy modulates the response to endogenously released serotonin without altering the spatial or temporal pattern of transmitter release. Since serotonin may oppose the effects of dopamine (Daw et al., 2002) which, in the DLS, facilitates habitual responding (Wickens et al., 2007), we hypothesized that increased DLS 5-HT6 receptor activity might aid in re-establishing behavioral flexibility after habitual behaviors are acquired. Therefore, we probed DLS activity with established models of both learning acquisition and habitual responding to assess the role of DLS 5-HT6 receptors. Here we provide the first evidence that increased DLS 5-HT6 receptor expression coupled with omission training decreases habitual responding, potentially revealing a new target for treating disorders wherein maladaptive habits become compulsive.
METHODS AND MATERIALS
Animal Use
Experimental procedures were approved by the University of Washington Institutional Animal Care and Use Committee and were conducted in accordance with National Institutes of Health guidelines. All efforts were made to minimize the number of animals used and to prevent their suffering. Male Long-Evans rats (Charles River, 250–275g at arrival; 90 total rats were used for this study; each rat was used in only one experiment, either Experiment 1a, 1b or 2) were habituated to the colony room (12 hour light/dark cycle, lights on at 6:00 AM, temperature set to 21°C, ad libitum rat chow except as indicated) and double-housed for five days before being handled. After this period, rats were single-housed and were handled daily for five days before being food restricted. Food restriction consisted of once daily feeding with 18 g of rat chow per rat at 5:30 PM at least a half hour after behavioral training/testing, in the colony room. Rats began food restriction five days before any behavioral procedure and continued on food restriction throughout training and testing unless otherwise specified (e.g. 24 hours before and after surgery). This mildly restricted diet produced ~5–10% reduction in the rate of weight gain compared to ad libitum fed rats.
Viral Vector
We used herpes simplex virus (HSV) viral vectors to manipulate 5-HT6 receptor expression, as previously described by our lab (Mitchell et al., 2007; Ferguson et al., 2008; Eskenazi & Neumaier, 2010). This HSV viral vector system infects neurons, not glia, and expresses transgene for about seven days (Barot et al., 2007; Ferguson et al., 2008). The experimental viral vector contains two cassettes, one that expresses the hemagglutinin (HA) epitope tagged 5-HT6 receptor driven by the HSV IE2/3 promoter and another that expresses enhanced green fluorescent protein (eGFP) driven by the cytomegalovirus promoter which serves as a way to locate virally infected neurons (Mitchell et al., 2007). Thus, all neurons that express eGFP using this vector also express the HA-tagged 5-HT6 receptor. A time-course showing increased expression of 5-HT6 receptors after intracerebral infusion of this viral vector in rats demonstrated that peak expression of transgenic 5-HT6 receptors occurs at day four post-infusion (Ferguson et al., 2008). The control viral vector expresses eGFP but not 5-HT6 receptors. These vectors will be referred to as 5-HT6/eGFP and eGFP-only respectively. Significant non-specific effects of transgene expression were not found in previous studies since eGFP-only controls learned tasks at the same rate as sham-operated controls, and learning effects of this 5-HT6 receptor-expressing vector were reversed by a selective 5-HT6 receptor antagonist (Mitchell et al., 2007; Ferguson et al., 2008).
Surgical procedures
Rats were removed from food restriction the night before surgery and were returned to food restriction 24 hours after surgery. Rats were anesthetized with isoflurane gas (1–3%) throughout the stereotaxic surgery and given buprenorphine analgesia (0.1 mg/kg, s.c.) pre-operatively. Bilateral bore holes were drilled above the coordinates: from bregma: A/P +0.7 mm, M/L ± 3.6 mm, D/V −4.0 mm. Using a 27 gauge long dental needle, two microliters of viral particles (~108 infective units/ml) per side were infused over 10 minutes at a rate of 200 nl/min, and the needle was left in place for an additional 5 minutes to minimize backflow before being slowly removed. This amount of viral vector was chosen based on previous studies to produce discrete infection at the target region (Neumaier et al., 2002; Mitchell et al., 2007).
Transcardial perfusions, tissue preparation and exclusion criteria
Rats were euthanized with an intraperitoneal injection (0.15 ml) of Beuthanasia-D (Schering-Plough), diluted 1:3 in sterile injectable saline (equivalent to pentobarbital 19 mg and phenytoin 2.5 mg). The procedure proceeded once the rat was unresponsive to paw pinch and upon absence of corneal reflex. Perfusions were performed with 100 mL phosphate buffered solution (PBS) followed by 200 mL of 4% paraformaldehyde, both 7.4 pH and kept on ice. Brains were removed and post-fixed in 4% paraformaldehyde for four hours then placed into PBS. Tissue sections were made on a Leica VT1000S vibrating blade microtome and mounted on slides and cover-slipped with Vectashield mounting medium (Vectorlabs, Burlingame, CA). Slides were visualized with a Nikon Eclipse E600 microscope with a HyQ FITC epi-fluorescence filter. Photomicrographs were taken with a Spot Insight Model #3.1.0 camera (Diagnostic Instruments Inc., USA) using Spot software version 4.5.9.5.
Injection accuracy was determined for each rat in a blinded fashion. Rats were excluded if eGFP-positive neurons were present in cortex or corpus callosum, ventral to −5.00 mm from brain surface, or medial to 3.00 mm from midline on either hemisphere. Of 180 injections (2 per rat for 90 rats), only 16 met exclusion criteria; thus, out of 90 rats, 16 were excluded from the study.
Detailed Behavioral Procedures
Equipment
Behavioral testing was conducted in standard rat modular test chambers (Med Associates, Georgia, VT, USA) equipped with two levers and two lights on either side of a pellet receptacle on one wall and a house-light on the opposite wall. All chambers were kept in sound-attenuating boxes (Med Associates) equipped with fans providing temperature regulation and white noise.
Experiment 1. Learning Acquisition
These behavioral paradigms are based on previous work from our lab (Eskenazi & Neumaier, 2010). Briefly, for these experiments, rats were handled for ten days and food restricted for five days before receiving intracerebral infusions of viral vector. Rats had their pre-test three days later and their test session on post-operative day four. Individual rats were trained on one of two behavioral tasks. Both tasks consisted of 100 trials on a fixed-interval of 20 seconds with the house-light on throughout the session. Animals were placed in the operant box for five minutes before the start of the session.
Experiment 1: Pretest
Three days post-operatively and one day prior to testing, rats were habituated to the test chamber for 30 minutes during which they were given thirty non-contingent pellets (45 mg sucrose pellets, Bioserv, Frenchtown, NJ, USA) on a variable-interval of 60 seconds, in the pellet receptacle inside the chamber with the house-light on. Any pellets remaining were placed into the rat’s home cage at the end of the session. Rats were then randomly assigned to Experiment 1a or 1b.
Experiment 1a: Inserting/Retracting (IR) lever pressing task
Each trial consisted of the presentation of a single lever (always on the same side), which inserted into the chamber on a fixed-interval of 20 seconds after which it remained extended for 10 seconds before retracting (i.e. a 20 second time-out period or intertrial interval). A successful lever press resulted in delivery of one pellet and the retraction of the lever for 20 seconds thereby preventing repetitive lever pressing. The session ended once 100 trials occurred. House-light and light above lever were continuously on throughout the session.
Experiment 1b: Continuously Extended (CE) lever pressing task
The house-light turned on and the two levers were extended and remained so during the entire session allowing for repetitive lever pressing. Presses on one lever were reinforced with sucrose pellets; however, after a pellet was delivered there was a 20 second unsignaled timeout during which lever presses upon the reinforced lever did not result in additional pellet delivery. Presses upon the other lever were never reinforced. A light above the reinforced lever remained on continuously, though there was no cue indicating the state of the lever (whether on timeout or not). The session ended once 100 pellets were obtained or 90 minutes elapsed, whichever occurred first.
Experiment 2: Habitual Responding
The protocol described below uses fixed- and variable-interval schedules of reinforcement that induce habitual lever pressing that is inflexible to changes in contingency, yet sensitive to pre-training lesions of the DLS (Yin et al., 2004) or muscimol-induced inactivation of the DLS during omission contingency training (Yin et al., 2006). The procedure produces a habitual compulsive-like pattern of responding, such that control animals (e.g. eGFP-only rats in our study) are resistant to the changed contingency during a single omission contingency training session, as indicated by performance on a subsequent probe session compared to yoked controls. Thus, the null hypothesis is that rats receiving the 5-HT6 viral vector will continue to lever press at high rates whether receiving omission contingency training or serving as yoked controls.
Over-training
After five days of handling and five days of food restriction, rats were acclimated to the operant chamber during a 30 minute session in which pellets were dispensed non-contingently on a variable-interval 60 second (VI60) schedule. Over the following four days rats were trained to lever press an active lever that was continuously extended throughout the entire session on a fixed-interval 20 second (FI20) schedule. That is, a lever press on the correct lever resulted in pellet delivery and created a 20 second time-out period. Sessions ended after 90 minutes elapsed or 100 pellets were earned whichever occurred first. There was an inactive lever present throughout the session; responses on this lever were recorded but were never reinforced. Rats then trained under the same conditions on a variable-interval 30 second schedule (VI30) for one day then a variable-interval 60 second schedule (VI60) for three days.
Surgery
After this over-training, rats were removed from food restriction and surgery was performed (as described above) two days later with viral vector assigned to groups balanced based on active lever press rates during the final pre-surgery VI60 session. 24 hours after surgery rats were returned to food restriction.
After surgery, rats were trained or tested sequentially in three ways as explained in further detail below: 1) re-trained on the pre-operative schedule of VI60 seconds, 2) trained during the omission contingency training session (or served as yoked control), and 3) tested on the final probe session.
Post-operative re-training
Two days after surgery rats underwent two more days of VI60 training. These re-training sessions were performed in order to test whether the 5-HT6/eGFP vector affected expression of the previously learned behavior (i.e. to test whether or not the vector would make the rats lose or forget the previously acquired behavior or alter their motor ability to perform the task).
Omission contingency training session
The omission contingency training session was performed in order to train the rats on either the omission or yoked contingency (described below) to allow for testing on the subsequent probe session. Based on their active lever press rate during the final post-surgery VI60 session, rats were assigned to either the omission contingency group or the yoked control group in a balanced fashion (i.e. so that average active lever press rate per group was even). The omission contingency training session was a 30 minute session in which failure to lever press (lever press omission) was reinforced; i.e., every lever press by a rat assigned to the omission group reset a count-down timer controlling sucrose pellet delivery to 20 seconds; thus, for every 20 seconds that passed without a lever press by the rat in the omission contingency group one pellet each was delivered to both the rat in the omission contingency group and to its yoked control rat. Lever presses performed by yoked controls did not affect pellet delivery; rather, yoked controls received pellets at the same time as did the rat in the omission contingency to which they were yoked.
The study was designed so that the omission contingency training session was conducted on post-operative day four because peak viral expression occurs at that time (Ferguson et al., 2008) and because it has been shown that manipulations of the dorsolateral striatum during the omission contingency training session are sufficient to disrupt habitual responding (Yin et al., 2006). The rats’ performance during the omission contingency training session is insufficient to analyze the impact of omission contingency versus yoked training because behavior is confounded by pellet delivery during the session. Therefore, a final probe session under extinction conditions was performed the next day, as described below.
Probe session
In order to reveal sensitivity to the omission contingency training session from the previous day’s training session, rats underwent a 10 minute session in which the houselight was on and both levers were extended in the operant chamber, but no pellets were delivered under any circumstance (i.e. extinction conditions); lever presses were recorded. The probe session is a reliable measure of rats’ drive to lever press per se since there are no pellets delivered during the session.
Data Analysis
Data from the behavioral sessions were collected using Med PC IV software. The number of sucrose pellets consumed during the pre-test, the number of sucrose pellets earned during the IR lever pressing task (Experiment 1a) or the CE lever pressing task (Experiment 1b) and the viral vector effect on established responding lever press rates (Experiment 2) were analyzed using two-tailed student's t-test. Within session time-binned data [i.e. number of non-reinforced active lever presses over the course of the CE lever pressing task session (Experiment 1b) and total number of active lever presses during the course of the omission contingency training session (Experiment 2)] were analyzed with Generalized Estimating Equation analysis with 5 minute and 3 minute bins respectively. Between-session data, (i.e. lever press rates between sessions during over-training for Experiment 2) were analyzed using 2-way repeated measures ANOVA with lever contingency (active/reinforced lever or inactive/never reinforced lever), and session as factors. Omission contingency training session and probe session lever presses were analyzed using 2-way ANOVA with viral vector (eGFP-only or 5-HT6/eGFP) and omission contingency training session behavioral contingency (omission or yoked) as factors. Both the omission contingency training session and the probe session data were normalized to the last day of training for each rat individually. Student's t-test and two-way ANOVA were performed using GraphPad Prism version 5.00 for Windows, GraphPad Software, San Diego, California, USA. Generalized Estimating Equation analysis was performed using SPSS version 17, Chicago, USA.
RESULTS
Stereotaxic injection accuracy
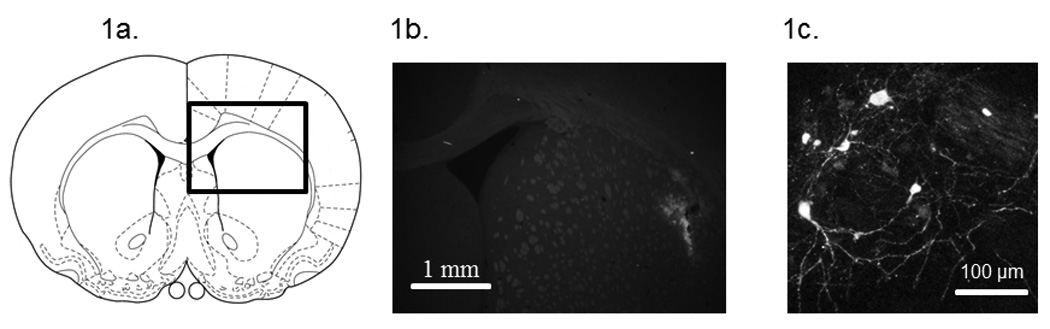
An illustration of the targeted region for viral vector infusion is shown in Figure 1a, and representative micrographs of eGFP positive cells in the area of infusion are shown in Figures 1b and 1c.
Figure 1.
Experiments 1a and 1b: Sucrose consumption during pre-test
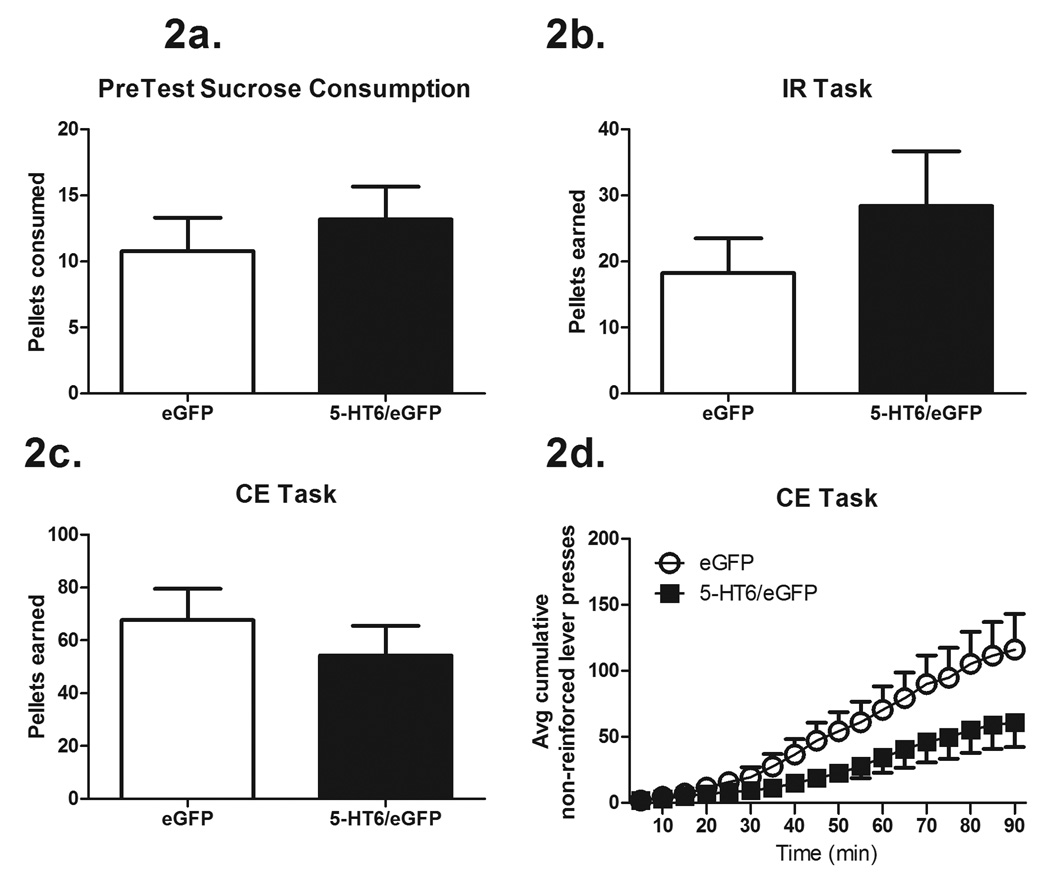
The number of pellets consumed during the pre-test was not affected by increased expression of 5-HT6 receptors in the DLS compared to eGFP-only controls (t34 = 0.67, P = 0.50, Figure 2a).
Figure 2.
Experiments 1a and 1b: Learning acquisition
Increased expression of 5-HT6 receptors in the DLS did not affect the number of sucrose pellets earned on the IR lever pressing task as compared to eGFP-only controls (t14 = 1.02, P = 0.32) indicating that performance on a discrete action-outcome task is not affected by increased 5-HT6 receptor signaling in the DLS (Figure 2b). In the CE lever pressing task, in which rats were able to lever press in a repetitive fashion, increased expression of 5-HT6 receptors in the DLS did not affect sucrose pellet acquisition (t18 = 0.81, P = 0.42, Figure 2c). However, there was a trend for rats with increased expression of 5-HT6 receptors in the DLS to make fewer non-reinforced lever presses on the active lever during the CE lever pressing task (Wald Chi-Square = 3.13, P = 0.07) (Figure 2d). There was not a similar trend through time on performance (pellet acquisition) between groups (eGFP-only vs 5-HT6/eGFP) during the IR lever pressing task (data not shown).
Experiment 2:
Over-training
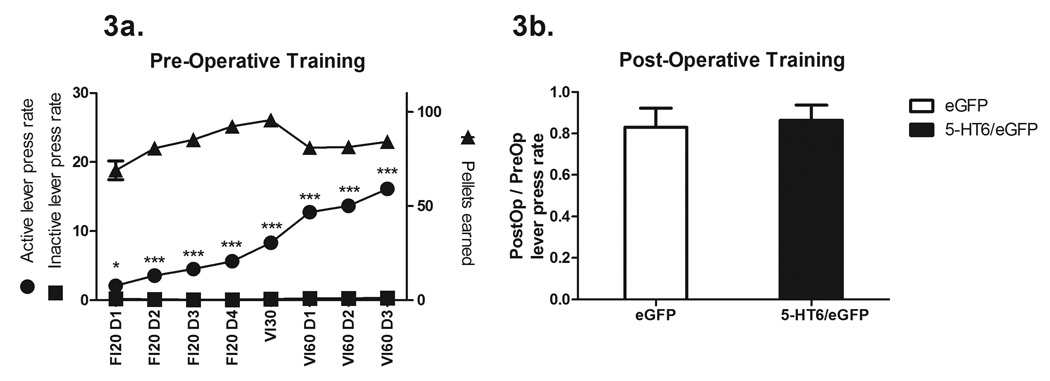
Performance of rats during training is shown in Figure 3a. There was a significant preference for lever presses on the lever associated with pellets (active lever) over lever presses on the lever that never earned a pellet (inactive lever) that significantly increased over time, (lever contingency F1,592 = 1409.83, P < 0.0001; training session F7,592 = 75.00, P < 0.001; interaction F7,592 = 70.87, P < 0.0001; post-hoc tests indicated that lever preference during Session 1 was significant (P < 0.05), as it was during all other sessions (P < 0.001)). It is important to note that even over the last three days of training on the same VI60 schedule of reinforcement, there was a significant escalation of lever presses upon the active lever even though the number of pellets earned remained stable across sessions.
Figure 3.
Post-operative re-training sessions
To determine whether there was an effect of viral vector treatment on established lever pressing under post-operative conditions prior to the omission contingency training session, we compared lever press rates from the last post-operative VI60 training session to the last pre-operative VI60 session. This allowed us to determine whether increased expression of 5-HT6 receptors affects either the retention of the acquired over-trained behavior or the motor ability to press the lever. There was no significant difference in the ratio of pre-operative over post-operative lever pressing in rats that received the eGFP-only viral vector versus rats that received the 5-HT6/eGFP viral vector, (t36 = 0.42, P = 0.67, Figure 3b), indicating that increased 5-HT6 receptor expression itself did not alter responding of the previously acquired task.
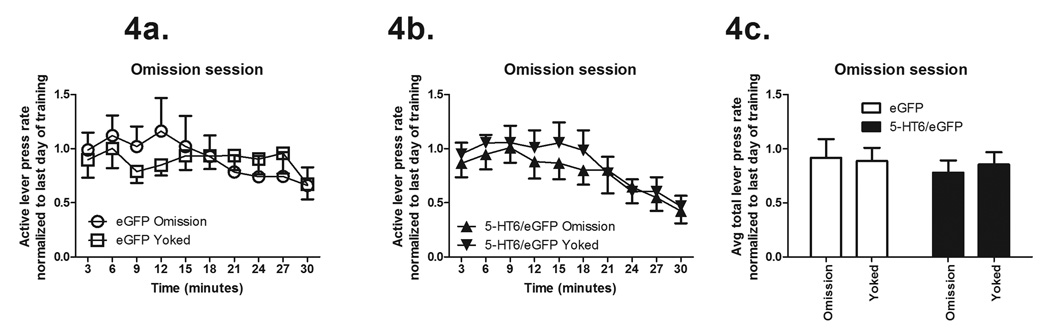
Omission contingency training session
Over the course of the 30 minute omission contingency training session, rats assigned to the omission contingency group received a pellet for each 20 seconds that passed in which they did not lever press; other rats served as yoked controls and received reinforcement at the same temporal pattern as rats in the omission contingency group, but their lever presses did not affect outcome. Lever press rates were compared to the last VI60 training session's lever press rate for that same rat. There was no effect of viral vector over the course of the session on active lever presses, (Figure 4a for eGFP-only rats and Figure 4b for 5-HT6/eGFP rats, comparing all groups: Wald Chi-Square 0.44, P = 0.50), nor of behavioral contingency over the course of the session on active lever presses (Wald Chi-Square = 0.04, P = 0.83). Total lever press rates during the omission contingency training session were normalized to the last day of training as shown in Figure 4c: there were also no differences between groups comparing total active lever presses, (viral vector F1,34 = 0.41, P = 0.52, behavioral contingency F1.34 = 0.03, P = 0.85, and interaction F1,34 = 0.16, P = 0.69).
Figure 4.
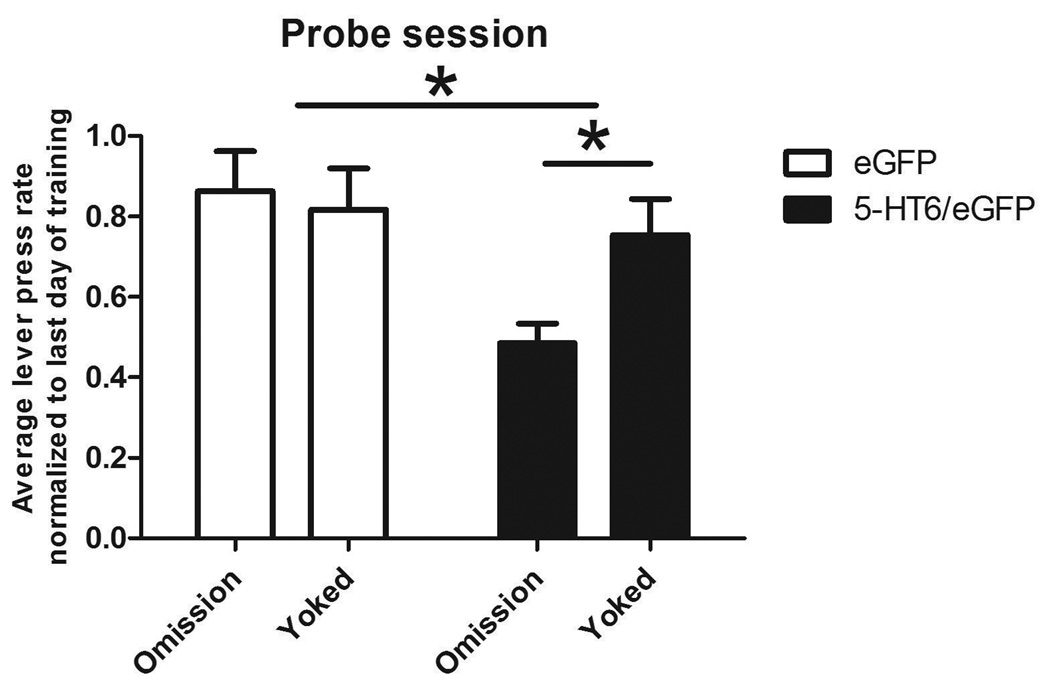
Probe session
In order to reveal whether the change in contingency during the omission contingency training session impacted subsequent habitual lever pressing, a probe session was performed the following day. Lever presses upon the lever that was previously reinforced were recorded (Figure 5). A two-way ANOVA revealed that there was an effect of viral vector treatment on lever presses during the probe session (F1,34 = 6.49, P = 0.016); post-hoc analysis showed that this was accounted for by rats undergoing the omission contingency (Bonferroni post-test, t =3.09, P < 0.01) but not the yoked contingency (Bonferroni post-test, t = 0.517, P > 0.05). This effect was specific to the lever previously reinforced, not to the other, inactive lever (data not shown).
Figure 5.
DISCUSSION
In this study we found that increased expression of 5-HT6 receptors in the dorsolateral striatum (DLS) facilitated behavioral flexibility by increasing the effectiveness of omission contingency training in reducing habitual lever pressing in over-trained rats on a subsequent probe test but had neither an effect on the initial acquisition of two variations of a simple operant behavior nor on sucrose pellet consumption in otherwise naïve rats. These findings are consistent with previous studies showing that enhanced serotonin signaling can decrease ‘compulsive’ lever pressing in rats (Joel et al., 2004) and extends those findings by validating 5-HT6 receptor signaling as one potential consequence of those effects and, additionally, demonstrates one region for these effects: the DLS. A recent study demonstrated a similar role for 5-HT6 receptors by showing that administration of the 5-HT6 receptor agonist WAY181187 facilitated extra-dimensional shifting on an attentional set-shifting paradigm, showing that 5-HT6 signaling enhanced behavioral flexibility on a well-trained task (Burnham et al., 2010). Our finding also agrees with the WAY-181187 dose-dependent decrease in ‘compulsive’ responding in a rodent model of Obsessive-Compulsive Disorder, schedule-induced polydipsia (Platt et al., 2008; Schechter et al., 2008). The present results suggest that 5-HT6 receptors specifically in the DLS are especially important for the effects of these receptors on habitual responding and potentially compulsive-like behaviors.
During the probe session, the decrease in habitual lever pressing in rats receiving increased DLS expression of 5-HT6 receptors was dependent upon omission contingency training from the previous day, as rats in the yoked group did not decrease lever press rates. Additionally, habitual responding was not altered by increased 5-HT6 receptor expression before the omission contingency training session (post-operative re-training sessions), indicating that increased 5-HT6 receptor expression alone did not abolish habitual responding but had to be paired with omission contingency training. Taken together, these findings indicate that the decrease in habitual responding is attributable to DLS 5-HT6 receptors facilitating adaptive behavioral flexibility in response to the change in contingency during the omission contingency training session.
Unlike the final probe test, behavior during the omission contingency training session is difficult to interpret because lever pressing has the potential to be modified by pellet delivery which confounds comparison across different omission/yoked pairs. For this reason, the final probe test, performed under extinction conditions, serves as a more reliable measure of rats’ drive to lever press. The fact that rats in the eGFP-only group continued to press at high rates during the final probe test whether assigned to the omission contingency or yoked control group the previous day demonstrates the inflexible habitual nature of lever pressing induced by the over-training sessions using variable-interval schedules of reinforcement, thus validating the behavioral model in accordance with previous work (Yin et al., 2006).
Increased 5-HT6 receptor expression in DLS had no effect on goal-directed learning acquisition during the IR and CE lever pressing tasks in naïve rats. This may reflect the limited role of DLS in the acquisition of these behaviors, since a number of previous studies have suggested the importance of the dorsomedial striatum in such learning paradigms (Yin et al., 2005a; Yin et al., 2005b). The null effect in this study of DLS 5-HT6 receptors is in contrast to the impairment in learning acquisition of simple operant tasks shown with increased expression of 5-HT6 receptors in other brain regions, namely the posterior dorsomedial striatum (Mitchell et al., 2007; Eskenazi & Neumaier, 2010). Our findings that increased expression of 5-HT6 receptors in the DLS affected neither learning acquisition of a goal-directed task nor consumption of a natural reward are also congruent with other research of the rodent striatum ascribing those roles to dorsomedial and ventral striatum respectively (Yin & Knowlton, 2006). Increased 5-HT6 expression in DLS did not alter the ability to learn simple operant tasks, as rats were still able to acquire pellets whether treated with the 5-HT6 receptor viral vector or the eGFP-only viral vector. Nonetheless, it is interesting to note the trend towards a less repetitive manner of lever pressing as indicated by comparing non-reinforced active lever presses throughout the session during the one session CE lever pressing task between eGFP-only and 5-HT6/eGFP rats since it suggests that 5-HT6 receptors in DLS may interfere with the early acquisition of excessive lever pressing even during a single session of training.
The current finding provides additional evidence that striatal 5-HT6 receptors act to modulate learning of and expression of reward and reinforcement behaviors in brain region specific manners, in line with previous work from our laboratory and others. Specifically, 5-HT6 receptor signaling interferes with the associative conditioning effects of cocaine as demonstrated by rats receiving 5-HT6 receptor vector infusion into nucleus accumbens failing to form conditioned place preference to cocaine (Ferguson et al., 2008). In the dorsomedial striatum, especially more posterior regions, infusion of 5-HT6 receptor expressing vectors interfere with the ability to acquire simple operant behaviors (Mitchell et al., 2007; Eskenazi & Neumaier, 2010). The present findings extend this view by showing a decrease in habitual responding when increased expression of 5-HT6 receptors in the DLS was paired with omission contingency training.
It is important to emphasize that DLS 5-HT6 receptor viral-mediated-gene transfer by itself did not decrease habitual responding acquired through over-training. This is demonstrated by both the fact that the 5-HT6/eGFP vector did not alter expression of the behavior on the post-operative re-training sessions, and also that habitual responding on the final probe test decreased only in the 5-HT6 omission rat group but not the 5-HT6 yoked rat group. This is relevant because it is important to differentiate between normal Stimulus Response (SR) behaviors that facilitate quick responses to stimuli and habitual responding that may progress to compulsions, persisting at a detriment to the animal. If an animal were to lose all SR behaviors then this too would be ultimately disadvantageous. To a certain degree our model addresses this point because habitual lever pressing behavior only decreases as a response to the 5-HT6/eGFP vector in DLS plus omission contingency training. This would be akin to combining a biological intervention with cognitive behavioral therapy. Therefore, our findings suggest that it is possible that manipulating 5-HT6 receptor function could be therapeutically relevant to treatment of compulsivity when used in conjunction with behavioral treatments. Thus, it is not a decrease in the expression of SR behaviors per se, but the increased cognitive flexibility from increased DLS 5-HT6 receptors that results in the subsequent decrease in habitual responding after omission contingency training that makes this finding compelling. As habitual responding and compulsivity are on a continuum, this may also have relevance specifically for disorders involving compulsive behaviors. For example, 5-HT6 receptors have recently been implicated in rat models of drug addiction (van Gaalen et al., 2010). Similarly, a recent report suggests that 5-HT6 receptors may be involved in enhancing flexibility involving extradimensional set-shifting, a task relevant for cognitive processes relevant to several neuropsychiatric illnesses (Burnham et al., 2010). The present report is the first to identify the dorsolateral striatum as a specific anatomical substrate for 5-HT6 receptor activity enhancing behavioral flexibility.
An alternative interpretation of our data is that the eGFP vector causes rats to fail to be sensitive to omission contingency training and that the 5-HT6/eGFP vector normalizes function. However, this seems unlikely for two reasons. First, similarly over-trained rats in the control groups of studies from other laboratories (over-trained on VI schedules) also fail to show sensitivity to omission contingency training (Yin et al., 2006). Thus, our eGFP control group’s behavior after omission contingency training is the same as that seen in the control groups of other studies without gene transfer. Second, in other studies published from our laboratory, rats treated with our eGFP vector behave identically to sham-operated and even un-operated control rats (Neumaier et al., 2002; Mitchell et al., 2007). Further, striatal neurons infected with this same eGFP vector had identical electrophysiological properties to non-infected neurons (Ferguson et al., 2010). Since these previous studies indicate that eGFP expression does not affect a range of behaviors related to (but not identical to) those used in this study, it seems unlikely that eGFP expression can explain our results.
In rats and humans, 5-HT6 receptors are expressed most abundantly in the striatum and at moderate levels in the cortex, cerebellum, hippocampus, and olfactory tubercle (Roberts et al., 2002). In the striatum, they are found on medium spiny neurons of both the direct and indirect pathway (striatonigral and striatopallidal pathways, respectively) (Ward & Dorsa, 1996) as well as certain striatal interneurons (Bonsi et al., 2007). Since 5-HT6 receptors have nanomolar affinity for serotonin (Monsma et al., 1993; Boess et al., 1997), they are likely to be activated during both low tonic and higher phasic conditions of serotonin release (Hirst et al., 2003), suggesting that 5-HT6 receptor activation is tuned to differences in tonic versus phasic release which may encode a dynamic range of serotonergic activity (Daw et al., 2002). Additionally, it has been proposed that serotonergic effects on the strengths of the direct and indirect pathways may be involved in balancing the time-scales of reinforcement learning (Doya, 2002); in fact, these same authors have recently discovered that central serotonergic neurons are activated while waiting for delayed rewards (Miyazaki et al., 2011a; Miyazaki et al., 2011b). This may be relevant to the present study in that rats undergoing omission contingency training must wait, rather than respond, i.e. lever press, in order to receive sucrose pellets.
Using computational modeling, it is predicted that serotonergic neuron firing rate, and, by extension, the pattern of serotonin release onto post-synaptic targets, plays an opponent role to dopamine in modulating operant behaviors by encoding an ongoing error prediction for punishment including negative punishment, e.g. absence of reward delivery (Daw et al., 2002). This view has recently been updated to allow that serotonin may interact with (rather than simply oppose) dopamine to help select appropriate behaviors to meet environmental demands (Cools et al., 2011) perhaps specifically by promoting the withholding of responses or promoting disengagement (presumably by preventing model-free reinforcement learning) (Boureau & Dayan, 2011). The present findings agree with these models in that enhanced serotonergic signaling facilitated learning to omit (withhold) a response of a behavior that had become habitual (as shown by the eGFP-only control animals).
The neuroanatomical substrates that these models are based on maintain that dopamine in the DLS facilitates habitual responding (Wickens et al., 2007) and that compulsive drug taking reflects a transition from ventral to dorsal striatal control over behavior (Everitt & Robbins, 2005). Since 5-HT6 receptors are coupled to Gαs and activate adenylate cyclase (Monsma et al., 1993) in both direct and indirect pathway medium spiny neurons which comprise 95% of the neurons in the DLS (Ward & Dorsa, 1996), we hypothesize that vigorous 5-HT6 receptor activation in DLS reduces the differential activation and inhibition of direct and indirect pathways by dopamine via D1 and D2 receptors, which are segregated in these pathways, respectively (Gerfen et al., 1990). Development of neuronal subtype specific viral vectors to drive expression in these specific subpopulations of neurons will help to investigate this issue further.
In conclusion, our work demonstrates a role for 5-HT6 receptor signaling in the dorsolateral striatum in facilitating behavioral flexibility of habitual responding in the face of changing contingencies in the environment. As habitual responding may be on a continuum in between goal-directed actions and compulsive behaviors, modulating 5-HT6 receptors may be a useful strategy for treating a variety of disorders that manifest with compulsive or habitually repetitive behaviors, such as Obsessive-Compulsive Disorder, Tourette syndrome, and addiction.
ACKNOWLEDGEMENTS
We would like to acknowledge the following sources of support: NIH Medical Scientist Training Program Grant 5 T32 GM07266 (DE), NIH Institutional Grant for Neurobiology 5 T32 GM07108 (DE), Achievement Rewards for College Scientists (DE) and NIH NIDA DA021273 (JFN). Thank you to Evan Carlos, Aaron Cohn and Aaron Mesnik-Greene for technical support.
ABBREVIATIONS
- 5-HT6
Serotonin 6 receptor
- CE
Continuously Extended lever pressing task
- DLS
Dorsolateral striatum
- eGFP
Enhanced green fluorescent protein
- FI
Fixed-interval reinforcement schedule
- HSV
Herpes-simplex virus
- IR
Inserting/Retracting lever pressing task
- MSN
Medium spiny neuron
- SR
Stimulus Response behaviors
- SSRI
Selective serotonin reuptake inhibitor
- VI
Variable-interval reinforcement schedule
REFERENCES
- Barot SK, Ferguson SM, Neumaier JF. 5-HT(1B) receptors in nucleus accumbens efferents enhance both rewarding and aversive effects of cocaine. Eur J Neurosci. 2007;25:3125–3131. doi: 10.1111/j.1460-9568.2007.05568.x. [DOI] [PubMed] [Google Scholar]
- Boess FG, Monsma FJ, Jr, Carolo C, Meyer V, Rudler A, Zwingelstein C, Sleight AJ. Functional and radioligand binding characterization of rat 5-HT6 receptors stably expressed in HEK293 cells. Neuropharmacology. 1997;36:713–720. doi: 10.1016/s0028-3908(97)00019-1. [DOI] [PubMed] [Google Scholar]
- Bonsi P, Cuomo D, Ding J, Sciamanna G, Ulrich S, Tscherter A, Bernardi G, Surmeier DJ, Pisani A. Endogenous serotonin excites striatal cholinergic interneurons via the activation of 5-HT 2C, 5-HT6, and 5-HT7 serotonin receptors: implications for extrapyramidal side effects of serotonin reuptake inhibitors. Neuropsychopharmacology. 2007;32:1840–1854. doi: 10.1038/sj.npp.1301294. [DOI] [PubMed] [Google Scholar]
- Boureau YL, Dayan P. Opponency revisited: competition and cooperation between dopamine and serotonin. Neuropsychopharmacology. 2011;36:74–97. doi: 10.1038/npp.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham KE, Baxter MG, Bainton JR, Southam E, Dawson LA, Bannerman DM, Sharp T. Activation of 5-HT(6) receptors facilitates attentional set shifting. Psychopharmacology (Berl) 2010;208:13–21. doi: 10.1007/s00213-009-1701-6. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Cools R, Nakamura K, Daw ND. Serotonin and dopamine: unifying affective, activational, and decision functions. Neuropsychopharmacology. 2011;36:98–113. doi: 10.1038/npp.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J, Bitterman ME. Differential reinforcement of other behavior (DRO): a yoked-control comparison. J Exp Anal Behav. 1971;15:237–241. doi: 10.1901/jeab.1971.15-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, Kakade S, Dayan P. Opponent interactions between serotonin and dopamine. Neural Netw. 2002;15:603–616. doi: 10.1016/s0893-6080(02)00052-7. [DOI] [PubMed] [Google Scholar]
- Daw ND, Niv Y, Dayan P. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nat Neurosci. 2005;8:1704–1711. doi: 10.1038/nn1560. [DOI] [PubMed] [Google Scholar]
- Denys D. Pharmacotherapy of obsessive-compulsive disorder and obsessive-compulsive spectrum disorders. Psychiatr Clin North Am. 2006;29:553–584. doi: 10.1016/j.psc.2006.02.013. xi. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Pierucci M, Esposito E, Crescimanno G, Benigno A, Di Giovanni G. Serotonin modulation of the basal ganglia circuitry: therapeutic implication for Parkinson's disease and other motor disorders. Prog Brain Res. 2008;172:423–463. doi: 10.1016/S0079-6123(08)00921-7. [DOI] [PubMed] [Google Scholar]
- Dickinson A, Nicholas DJ, Adams CD. The Effect of the Instrumental Training Contingency on Susceptibility to Reinforcer Devaluation. Q J Exp Psychol-B. 1983;35:35–51. [Google Scholar]
- Doya K. Metalearning and neuromodulation. Neural Netw. 2002;15:495–506. doi: 10.1016/s0893-6080(02)00044-8. [DOI] [PubMed] [Google Scholar]
- Eskenazi D, Neumaier JF. Increased expression of the 5-HT6 receptor by viral mediated gene transfer into posterior but not anterior dorsomedial striatum interferes with acquisition of a discrete action-outcome task. J Psychopharmacol. 2010 doi: 10.1177/0269881110388330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, Roth BL, Neumaier JF. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2010 doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Mitchell ES, Neumaier JF. Increased expression of 5-HT6 receptors in the nucleus accumbens blocks the rewarding but not psychomotor activating properties of cocaine. Biol Psychiatry. 2008;63:207–213. doi: 10.1016/j.biopsych.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, Sahakian BJ, Robbins TW, Bullmore ET, Hollander E. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology. 2010;35:591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley AG, Murphy KJ, Hirst WD, Gallagher HC, Hagan JJ, Upton N, Walsh FS, Regan CM. The 5-HT(6) receptor antagonist SB-271046 reverses scopolamine-disrupted consolidation of a passive avoidance task and ameliorates spatial task deficits in aged rats. Neuropsychopharmacology. 2004;29:93–100. doi: 10.1038/sj.npp.1300332. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Goddard AW, Shekhar A, Whiteman AF, McDougle CJ. Serotoninergic mechanisms in the treatment of obsessive-compulsive disorder. Drug Discov Today. 2008;13:325–332. doi: 10.1016/j.drudis.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Hirst WD, Abrahamsen B, Blaney FE, Calver AR, Aloj L, Price GW, Medhurst AD. Differences in the central nervous system distribution and pharmacology of the mouse 5-hydroxytryptamine-6 receptor compared with rat and human receptors investigated by radioligand binding, site-directed mutagenesis, and molecular modeling. Mol Pharmacol. 2003;64:1295–1308. doi: 10.1124/mol.64.6.1295. [DOI] [PubMed] [Google Scholar]
- Joel D, Ben-Amir E, Doljansky J, Flaisher S. 'Compulsive' lever-pressing in rats is attenuated by the serotonin re-uptake inhibitors paroxetine and fluvoxamine but not by the tricyclic antidepressant desipramine or the anxiolytic diazepam. Behav Pharmacol. 2004;15:241–252. [PubMed] [Google Scholar]
- Lieben CK, Blokland A, Sik A, Sung E, van Nieuwenhuizen P, Schreiber R. The selective 5-HT6 receptor antagonist Ro4368554 restores memory performance in cholinergic and serotonergic models of memory deficiency in the rat. Neuropsychopharmacology. 2005;30:2169–2179. doi: 10.1038/sj.npp.1300777. [DOI] [PubMed] [Google Scholar]
- Marcos B, Chuang TT, Gil-Bea FJ, Ramirez MJ. Effects of 5-HT6 receptor antagonism and cholinesterase inhibition in models of cognitive impairment in the rat. Br J Pharmacol. 2008;155:434–440. doi: 10.1038/bjp.2008.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell ES, Hoplight BJ, Lear SP, Neumaier JF. BGC20-761, a novel tryptamine analog, enhances memory consolidation and reverses scopolamine-induced memory deficit in social and visuospatial memory tasks through a 5-HT6 receptor-mediated mechanism. Neuropharmacology. 2006;50:412–420. doi: 10.1016/j.neuropharm.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Mitchell ES, Neumaier JF. 5-HT6 receptors: a novel target for cognitive enhancement. Pharmacol Ther. 2005;108:320–333. doi: 10.1016/j.pharmthera.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Mitchell ES, Neumaier JF. 5-HT6 receptor antagonist reversal of emotional learning and prepulse inhibition deficits induced by apomorphine or scopolamine. Pharmacol Biochem Behav. 2008;88:291–298. doi: 10.1016/j.pbb.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell ES, Sexton T, Neumaier JF. Increased expression of 5-HT6 receptors in the rat dorsomedial striatum impairs instrumental learning. Neuropsychopharmacology. 2007;32:1520–1530. doi: 10.1038/sj.npp.1301284. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Miyazaki KW, Doya K. Activation of dorsal raphe serotonin neurons underlies waiting for delayed rewards. J Neurosci. 2011a;31:469–479. doi: 10.1523/JNEUROSCI.3714-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki KW, Miyazaki K, Doya K. Activation of the central serotonergic system in response to delayed but not omitted rewards. Eur J Neurosci. 2011b;33:153–160. doi: 10.1111/j.1460-9568.2010.07480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsma FJ, Jr, Shen Y, Ward RP, Hamblin MW, Sibley DR. Cloning and expression of a novel serotonin receptor with high affinity for tricyclic psychotropic drugs. Mol Pharmacol. 1993;43:320–327. [PubMed] [Google Scholar]
- Neumaier JF, Vincow ES, Arvanitogiannis A, Wise RA, Carlezon WA., Jr Elevated expression of 5-HT1B receptors in nucleus accumbens efferents sensitizes animals to cocaine. J Neurosci. 2002;22:10856–10863. doi: 10.1523/JNEUROSCI.22-24-10856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Platt B, Beyer CE, Schechter LE, Rosenzweig-Lipson S. Schedule-induced polydipsia: a rat model of obsessive-compulsive disorder. Curr Protoc Neurosci. 2008;Chapter 9 doi: 10.1002/0471142301.ns0927s43. Unit 9 27. [DOI] [PubMed] [Google Scholar]
- Roberts JC, Reavill C, East SZ, Harrison PJ, Patel S, Routledge C, Leslie RA. The distribution of 5-HT(6) receptors in rat brain: an autoradiographic binding study using the radiolabelled 5-HT(6) receptor antagonist [(125)I]SB-258585. Brain Res. 2002;934:49–57. doi: 10.1016/s0006-8993(02)02360-0. [DOI] [PubMed] [Google Scholar]
- Roth BL, Craigo SC, Choudhary MS, Uluer A, Monsma FJ, Jr, Shen Y, Meltzer HY, Sibley DR. Binding of typical and atypical antipsychotic agents to 5-hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors. J Pharmacol Exp Ther. 1994;268:1403–1410. [PubMed] [Google Scholar]
- Schechter LE, Lin Q, Smith DL, Zhang G, Shan Q, Platt B, Brandt MR, Dawson LA, Cole D, Bernotas R, Robichaud A, Rosenzweig-Lipson S, Beyer CE. Neuropharmacological profile of novel and selective 5-HT6 receptor agonists: WAY-181187 and WAY-208466. Neuropsychopharmacology. 2008;33:1323–1335. doi: 10.1038/sj.npp.1301503. [DOI] [PubMed] [Google Scholar]
- Schweighofer N, Bertin M, Shishida K, Okamoto Y, Tanaka SC, Yamawaki S, Doya K. Low-serotonin levels increase delayed reward discounting in humans. J Neurosci. 2008;28:4528–4532. doi: 10.1523/JNEUROSCI.4982-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soomro GM, Altman D, Rajagopal S, Oakley-Browne M. Selective serotonin re-uptake inhibitors (SSRIs) versus placebo for obsessive compulsive disorder (OCD) Cochrane Database Syst Rev. 2008:CD001765. doi: 10.1002/14651858.CD001765.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel OA, der Werf YD, Verhoef KM, de Wit S, Berendse HW, Wolters E, Veltman DJ, Groenewegen HJ. Frontal-striatal abnormalities underlying behaviours in the compulsive-impulsive spectrum. J Neurol Sci. 2010;289:55–59. doi: 10.1016/j.jns.2009.08.043. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, Schetters D, Schoffelmeer AN, De Vries TJ. 5-HT6 antagonism attenuates cue-induced relapse to cocaine seeking without affecting cocaine reinforcement. Int J Neuropsychopharmacol. 2010;13:961–965. doi: 10.1017/S1461145710000428. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Behavioral and neural mechanisms of compulsive drug seeking. Eur J Pharmacol. 2005;526:77–88. doi: 10.1016/j.ejphar.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Ward RP, Dorsa DM. Colocalization of serotonin receptor subtypes 5-HT2A, 5-HT2C, and 5-HT6 with neuropeptides in rat striatum. J Comp Neurol. 1996;370:405–414. doi: 10.1002/(SICI)1096-9861(19960701)370:3<405::AID-CNE10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Wickens JR, Horvitz JC, Costa RM, Killcross S. Dopaminergic mechanisms in actions and habits. J Neurosci. 2007;27:8181–8183. doi: 10.1523/JNEUROSCI.1671-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology. 2004;29:1331–1343. doi: 10.1038/sj.npp.1300434. [DOI] [PubMed] [Google Scholar]
- Woolley ML, Marsden CA, Fone KC. 5-ht6 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:59–79. doi: 10.2174/1568007043482561. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Blockade of NMDA receptors in the dorsomedial striatum prevents action-outcome learning in instrumental conditioning. Eur J Neurosci. 2005a;22:505–512. doi: 10.1111/j.1460-9568.2005.04219.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Inactivation of dorsolateral striatum enhances sensitivity to changes in the action-outcome contingency in instrumental conditioning. Behav Brain Res. 2006;166:189–196. doi: 10.1016/j.bbr.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci. 2005b;22:513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]