Introduction
It seems obvious that brain regions must cooperate to accomplish the various tasks in which they are communally required. The systems underlying learning and memory are a particularly useful example of this requirement for cooperation. Diverse forebrain regions are each involved in one or other form of memory; specific forms seem to require interactions between specific regions. To study the interactions that mediate this inter-area cooperation, neurophysiologists and neuroimagers alike have made the same hypothesis: that brain regions that functionally interact will synchronize their neural activity (see Box 1). In particular, interactions between the hippocampus and prefrontal cortex, two brain regions that subserve spatial working memory in rodents, have been the focus of much investigation. This review will focus on these efforts to identify and characterize the nature and function of hippocampal-prefrontal synchrony during behavior.
BOX 1. Useful Terms.
Many different and overlapping terms are used to describe interactions and synchrony within and between brain regions. Here is a basic description of some of the terms used in this review.
| Term | Definition |
|---|---|
| Functional connectivity | Interactions between activity in multiple brain regions, typically assayed by measuring synchrony |
| Synchrony | Activity changes in two or more brain areas that occur together over time |
| Oscillation | Activity that goes up and down repeatedly over time within a defined frequency range, in a more-or-less circular fashion |
| Coherence | A frequency-specific measure of synchrony between two signals, reflects the consistency of their temporal relationship |
| Phase-locking | A measure of synchrony, reflects the degree to which neuronal action potentials occur at a particular phase of an oscillation |
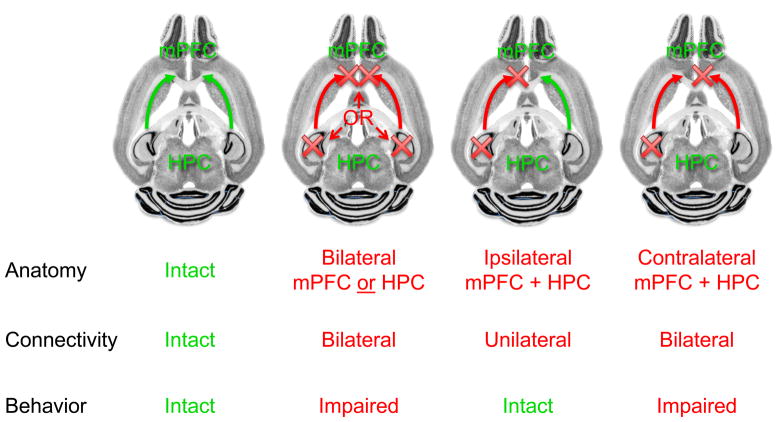
A role for functional connectivity between the hippocampus and prefrontal cortex in spatial working memory was suggested by results from lesion studies in rodents. Bilateral lesions or silencing of either the hippocampus or the medial prefrontal cortex (mPFC) impair spatial working memory, yet unilateral manipulations of either region have minimal or no effect. [1–5]. Meanwhile, the anatomical connectivity between these regions is also unilateral; hippocampal efferents innervate primarily the ipsilateral mPFC [6,7]. Taking advantage of this connectivity, various groups have demonstrated the requirement for interactions between the hippocampus and mPFC. Unilateral hippocampal lesions combined with ipsilateral mPFC lesions do not disrupt spatial working memory performance [5,8], presumably because the intact contralateral hippocampal-prefrontal system is able to direct successful behavior. Combining unilateral hippocampal lesions with lesions of the contralateral mPFC, however, disrupts task performance [5,8], demonstrating that isolated contralateral hippocampal and mPFC regions are insufficient for normal behavior (See Figure 1). Such findings are consistent with the notion that the hippocampal-mPFC projection is crucial for normal spatial working memory function.
Figure 1. Disconnection experiments implicate the connections between the hippocampus and prefrontal cortex in spatial working memory in rodents.
Spatial working memory behavior is disrupted by bilateral lesions of either the hippocampus (HPC) or medial prefrontal cortex (mPFC). Unilateral lesions of either structure, or ipsilateral lesions of both structures, do not impair behavior because they leave intact the contralateral structures and the connection between them. Contralateral lesions of each structure, while preserving one side of each structure, disrupt direct functional connectivity on both sides, impairing behavior.
Harnessing oscillations to measure temporal synchrony
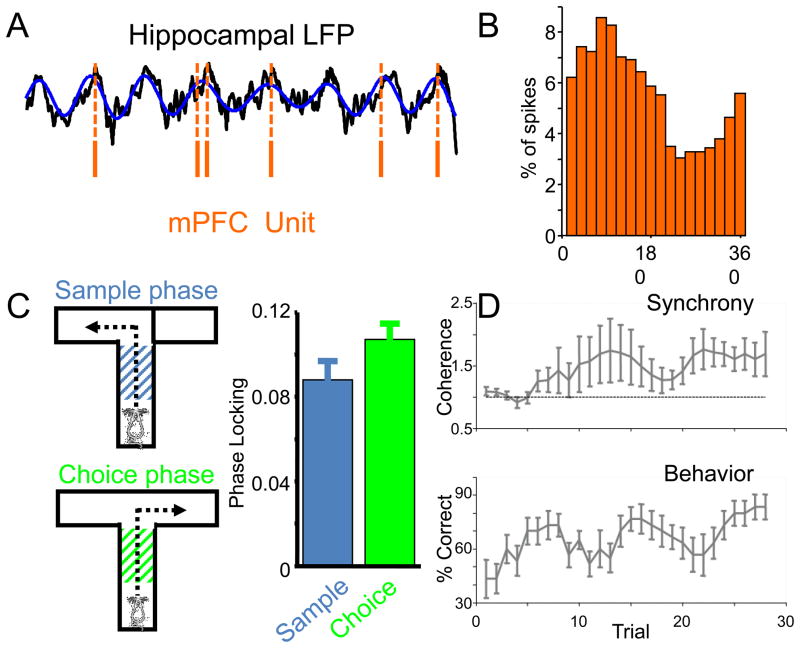
The results of disconnection studies led to a search for the nature of the hippocampal-prefrontal interactions. One common way to explore such interactions between brain regions is to record from multiple single neurons in each region and then hunt for evidence of synchronization of their activity, typically using cross-correlation analysis to measure the effect of one neuron’s activity on another. While such experiments have been conducted in the hippocampal/prefrontal system, they are limited because of the relative scarcity of detectable interactions [9]. Siapas et al. [9] and others [10,11] overcame this obstacle by taking advantage of the hippocampal theta oscillation, an ~8 hz oscillation that is the dominant mode of activity in the local field potential (LFP) recorded from the hippocampus of the exploring rodent [12]. Neurons in the hippocampus tend to fire at particular phases of the theta oscillation [13], suggesting that their efferents should induce downstream neurons to also fire in synchrony with hippocampal theta. Indeed, many single neurons in the mPFC are modulated by the hippocampal theta oscillation, firing at particular phases of the theta cycle [9,10] (See Figure 2). Moreover, by measuring the strength of this “phase-locking” at a range of time lags between the hippocampal LFP and the mPFC spikes, Siapas et al. [10] were able to determine that most mPFC spikes followed hippocampal theta rather than leading it, suggesting a hippocampus-to-mPFC directionality consistent with the known anatomy [6,7].
Figure 2. Phase locking of mPFC neurons to dHPC theta oscillation is altered by working memory behavior.
A, Spikes from a mPFC neuron (orange lines) occur preferentially near the peak of the theta oscillation (blue curve) present in the hippocampal LFP (black curve). B, Histogram of spikes from the mPFC neuron shown in A, plotted as a function of the phase of the hippocampal theta oscillation. The peak of theta is arbitrarily defined as 180°, the trough as 0°/360°. C, Right, Diagram of the T-maze spatial working memory task used in mice, with a control sample phase, and a working-memory requiring choice phase. Left, the strength of phase-locking of mPFC neurons to the hippocampal theta oscillation is increased in the choice phase. D, Theta-frequency coherence (upper panel) between the hippocampal and mPFC LFPs increases along with behavioral performance (lower panel) as mice learn the T-maze task. Data are from ref. 22.
Indeed, theta-frequency synchrony seems to be a generalized mechanism by which the hippocampus interacts with various downstream targets, including the striatum, entorhinal cortex and amygdala [11,14–17]. Importantly, temporal synchrony has also been demonstrated not only with single unit phase locking, but also with LFPs recorded directly from target regions, using coherence and other measures to quantify synchrony between target and hippocampal LFPs [11,14–18]. While LFP recordings require a great deal of care to ensure that the recorded signals reflect truly “local” neuronal activity, they simplify the technical methods necessary to quantify synchrony between multiple brain regions [18,19]. When combined with unit studies, LFP-LFP synchrony measures can serve as independent lines of evidence confirming the strength and directionality of temporal coupling between the hippocampus and its downstream targets [16,20,21].
Behavioral modulation
Using these and related methods, various groups have demonstrated the presence of synchrony between the hippocampus and its downstream targets. Importantly, these interactions are not static, but rather vary with ongoing behavioral demands. A canonical example of a behavioral effect comes again from the Wilson group, who demonstrated that hippocampal-prefrontal synchrony increases during a spatial working memory task similar to those studied with disconnection lesions. Recording single units and LFPs from the mPFC, as well as LFPs from the dorsal hippocampus, Jones and Wilson [21] showed that phase-locking of mPFC units to the hippocampal theta oscillation increases during the working memory-requiring segments of the task in rats. The single unit findings were corroborated by similar increases in theta-frequency coherence between hippocampal and mPFC LFPs. Recently, we have extended these findings in mice, showing that hippocampal-mPFC coherence increases throughout training in parallel with behavioral performance [22] (See Figure 2), in line with similar increases in intrinsic theta synchrony seen within the mPFC itself during trace conditioning acquisition [23]. These correlative physiological data are consistent with the notion that interactions between the hippocampus and mPFC support spatial working memory performance, especially when considered in the context of the earlier data from disconnection lesions.
Synchrony in the theta range between the hippocampus and downstream targets can be modulated by a variety of behaviors. For example, learned fear induces synchronous theta oscillations in the hippocampus and amygdala [17], while innate fear enhances theta-frequency phase locking and coherence between the mPFC and the ventral hippocampus [24]. Procedural learning is accompanied by increases in theta synchrony with the striatum [14]. Even the cerebellum gets into the act, synchronizing with the hippocampus during trace eye blink conditioning in rabbits [25]. A common thread running through these various results is that when the hippocampus works together with a brain region during behavior, theta frequency synchrony is enhanced.
In general each of these studies have analyzed synchrony only during a single task, so it is difficult to know whether behavioral modulation of theta-synchrony is anatomically specific (to a given circuit) or is generally enhanced (between the hippocampus and each of its various downstream targets). Nonetheless, there are some clues to suggest that behavioral modulation might be circuit specific. The strongest evidence for circuit specificity is for hippocampal subregions, which demonstrate remarkable heterogeneity of behavioral modulation of theta strength and synchrony across both the transverse [26] and dorsoventral [24,27] axes. Indeed, we have shown that anxiety-related enhancements in theta-frequency synchrony with the mPFC are specific to the ventral, but not dorsal hippocampus [24], consistent with lesion and silencing data demonstrating functional dissociation across the dorsoventral axis [28]. Whether circuit specificity extends to particular downstream targets of a given hippocampal subregion remains uncertain.
Relevance to function and dysfunction
That synchrony between the hippocampus and mPFC increases with behavioral demands is thus well established. Determining the relevance of such synchrony to behavior is a more challenging task. It is attractive to speculate that the synchronous activation of hippocampal and prefrontal neurons facilitates the transfer of information key to task performance. For example, during spatial working memory tasks, information about spatial location and reward contingency might be transferred from the hippocampus for use by decision-making machinery in the mPFC. Consistent with this hypothesis, mPFC unit phase-locking to hippocampal theta is reduced during error trials in working memory tasks [21,29]. More direct evidence for this hypothesis would be to show that those units with working memory-related firing patterns are preferentially phase-locked to the hippocampal theta oscillation (compared to neurons without such task-related firing). While clear evidence for such a relationship has not yet been described, Hyman et al [10] demonstrated a rough correlation between hippocampal phase-locking and task-related firing in mPFC units recorded during rewarded exploration. Similarly, Benchenane et al [30] have demonstrated that hippocampal-mPFC theta coherence peaks at the choice point in a (non-working memory dependent) Y-maze task, consistent with a role for synchrony in guiding choice behavior.
Relevance to behavior has also been demonstrated by correlating individual differences in behavior and synchrony across animals. For example, the strength of theta-frequency coherence between the hippocampus and mPFC predicted the length of time it took for learning-impaired mutant mice to acquire a spatial working memory task [22]. Such correlations have also been observed during several other behaviors, including innate [24] and learned [16] anxiety; similar behavior-physiology correlations have also been described in the hippocampal-striatal circuit [14]. These findings suggest that individual differences in task performance may be related to differences in the ability of the hippocampus and mPFC to functionally interact.
Such findings raise the intriguing possibility that abnormalities in functional interactions between the hippocampus and mPFC might underlie specific disease states. Indeed, the mutant mice mentioned above carry a microdeletion that models a high-penetrance copy number variant seen in patients with schizophrenia [31–33]. Both the hippocampus and prefrontal cortex have been implicated in schizophrenia, and imaging and electroencephalography studies have documented abnormalities in functional connectivity between the frontal and temporal lobes in patients with the disease [34–37]. Given the well-defined role of these structures in working memory, and the profound working memory deficits seen in schizophrenia patients, it is tempting to speculate that deficits in hippocampal-prefrontal synchrony contribute to the pathophysiology of schizophrenia.
Are oscillations important for functional interactions?
Key questions remain unanswered by the currently available body of evidence. Among these is the causal relationship between oscillations, synchrony, and the functional interactions they mark. Are oscillations in general, or theta oscillations in particular, important for synchrony? Is synchronous activity itself required for functional interactions between brain regions, or simply a result of such interactions? Theoretically, information transfer between neurons should be more efficacious if the neurons are active together, or more precisely, if the downstream neuron lags the upstream neuron by the approximate conduction delay. At least for the ventral hippocampus-mPFC recordings, where both lag and monosynaptic conduction delay have been measured, these measures are in broad agreement [20,24]. However, this is precisely the result one would also expect if the mPFC neurons were simply driven substantially by direct hippocampal input. One wonders whether desynchronizing cortical activity (and thus reducing the strength of the intrinsic oscillations in the mPFC) without affecting hippocampal inputs would be sufficient to disrupt hippocampal-mPFC information transfer and working memory behavior.
Assuming for a moment that synchronous activity is required to support hippocampal-mPFC interactions, it is still unclear as to whether oscillations, or theta-frequency oscillations in particular, are required. Here there is some evidence that raises the possibility that theta-frequency oscillations may be particularly privileged to facilitation hippocampal-mPFC interactions. For example, while phase-locking and coherence between the mPFC and hippocampus can be demonstrated across a broad range of frequencies at baseline, only theta-frequency synchrony is enhanced with working memory [21,22]. Theta-frequency synchrony in specifically altered in error trials [21,29] and correlated with behavioral performance [22]. Synchrony at other frequency ranges, including delta and gamma, do not correlate with behavioral state or task performance. Similar specificity of behavioral modulation for the theta-frequency range has been reported in other behavioral paradigms [16,24] suggesting that there might be something special about theta-frequency oscillations that facilitates sychronization and/or transfer of behaviorally relevant information across distant brain regions. Yet synchrony between far-flung brain regions has been well established in other frequency ranges, raising the possibility that what is special about theta synchrony is that it involves the hippocampus. Perhaps theta-frequency synchrony marks hippocampal interactions simply because theta is the dominant mode of activity in the awake, behaving hippocampus.
Future Directions
The precise role of synchrony and oscillations in support of functional interactions may be quite difficult to dissect, but there are arguably lower hanging fruit amenable to investigation in the near future. For example, most of the work discussed above deals with interactions between the dorsal hippocampus and the medial prefrontal cortex, yet there is no direct monosynaptic connection between these brain regions. Dissecting out the precise circuitry whereby information flows from the dorsal hippocampus to the mPFC will aid both in understanding how the synchrony develops, and in developing new tools (such as opto- or pharmacogenetic manipulations) to study the role of synchrony in behavior. Perhaps even more interesting is trying to understand what how behavioral modulation of synchrony occurs. Is it simply a matter of increased activity in the hippocampus playing a larger role in driving mPFC neurons, or is there an active switch in the circuit that enhances the efficacy of hippocampal inputs? What role do the neuromodulators and cellular constituents of oscillatory behavior play in facilitating synchrony and the behaviors that depend on it?
Answering these questions will build upon the strong foundation laid by the studies described above, in which measurement and quantification of hippocampal-mPFC interactions has been accomplished in real time. Correlation of these interactions with ongoing behavior has implicated them in spatial working memory and a variety of other behaviors. Such findings serve as mechanistic reminders that brain regions work not in isolation but in cooperation, and that understanding the nature and details of this cooperation will be key to understanding how brain systems generate behavior.
Acknowledgments
I would like to thank A. Adhikari, P. O’Neill, and T. Sigurdsson for critical comments on the manuscript. I am grateful for support from the NIMH (R01 MH081968), and a Rising Star Award from the International Mental Health Research Organization.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brito GN, Thomas GJ, Davis BJ, Gingold SI. Prelimbic cortex, mediodorsal thalamus, septum, and delayed alternation in rats. Experimental Brain Research. 1982;46:52–58. doi: 10.1007/BF00238097. [DOI] [PubMed] [Google Scholar]
- 2.Seamans JK, Floresco SB, Phillips AG. Functional differences between the prelimbic and anterior cingulate regions of the rat prefrontal cortex. Behav Neurosci. 1995;109:1063–1073. doi: 10.1037//0735-7044.109.6.1063. [DOI] [PubMed] [Google Scholar]
- 3.Lee I, Kesner RP. Time-dependent relationship between the dorsal hippocampus and the prefrontal cortex in spatial memory. J Neurosci. 2003;23:1517–1523. doi: 10.1523/JNEUROSCI.23-04-01517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, Moore H, Kandel ER. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- **5.Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. A thorough demonstration of the effects of hippocampal, prefrontal and disconnection lesions on working memory tasks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1991;313:574–586. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- 7.Conde F, Maire-Lepoivre E, Audinat E, Crepel F. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. Journal of Comparative Neurology. 1995;352:567–593. doi: 10.1002/cne.903520407. [DOI] [PubMed] [Google Scholar]
- 8.Churchwell JC, Morris AM, Musso ND, Kesner RP. Prefrontal and hippocampal contributions to encoding and retrieval of spatial memory. Neurobiology of Learning and Memory. 2010;93:415–421. doi: 10.1016/j.nlm.2009.12.008. [DOI] [PubMed] [Google Scholar]
- **9.Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46:141–151. doi: 10.1016/j.neuron.2005.02.028. This study demonstrates synchrony of prefrontal spiking activity with hippocampal theta, and cleverly demonstrates that the prefrontal spikes lag, rather than lead, the hippocampal LFP, strongly suggesting information flows from the hippocampus to the mPFC. [DOI] [PubMed] [Google Scholar]
- 10.Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Medial prefrontal cortex cells show dynamic modulation with the hippocampal theta rhythm dependent on behavior. Hippocampus. 2005;15:739–749. doi: 10.1002/hipo.20106. [DOI] [PubMed] [Google Scholar]
- *11.Berke JD, Okatan M, Skurski J, Eichenbaum HB. Oscillatory entrainment of striatal neurons in freely moving rats. Neuron. 2004;43:883–896. doi: 10.1016/j.neuron.2004.08.035. Among the first papers to demonstrate theta entrainment of downstream activity in awake rodents. [DOI] [PubMed] [Google Scholar]
- 12.Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- *13.Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. An outstanding review about the mechansims underlying theta oscillations, still timely. [DOI] [PubMed] [Google Scholar]
- 14.DeCoteau WE, Thorn C, Gibson DJ, Courtemanche R, Mitra P, Kubota Y, Graybiel AM. Learning-related coordination of striatal and hippocampal theta rhythms during acquisition of a procedural maze task. Proc Natl Acad Sci U S A. 2007;104:5644–5649. doi: 10.1073/pnas.0700818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tort AB, Kramer MA, Thorn C, Gibson DJ, Kubota Y, Graybiel AM, Kopell NJ. Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proc Natl Acad Sci U S A. 2008;105:20517–20522. doi: 10.1073/pnas.0810524105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popa D, Duvarci S, Popescu AT, Lena C, Pare D. Coherent amygdalocortical theta promotes fear memory consolidation during paradoxical sleep. Proc Natl Acad Sci U S A. 2010;107:6516–6519. doi: 10.1073/pnas.0913016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **17.Seidenbecher T, Laxmi TR, Stork O, Pape HC. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003;301:846–850. doi: 10.1126/science.1085818. A striking demonstration of fear learning-induced theta-frequency synchrony between the hippocampus and the amgydala. [DOI] [PubMed] [Google Scholar]
- *18.Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsaki G. Entrainment of Neocortical Neurons and Gamma Oscillations by the Hippocampal Theta Rhythm. Neuron. 2008;60:683–697. doi: 10.1016/j.neuron.2008.09.014. Thorough documentation of the degree to which neocortical units and fields synchronize with the hippocampal theta rhythm, demonstrating the need for careful controls of volume conduction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dzirasa K, Fuentes R, Kumar S, Potes JM, Nicolelis MAL. Chronic in vivo multi-circuit neurophysiological recordings in mice. Journal of Neuroscience Methods. 2010 doi: 10.1016/j.jneumeth.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adhikari A, Sigurdsson T, Topiwala MA, Gordon JA. Cross-correlation of instantaneous amplitudes of field potential oscillations: A straightforward method to estimate the directionality and lag between brain areas. Journal of Neuroscience Methods. 2010;191:191–200. doi: 10.1016/j.jneumeth.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **21.Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. Demonstrates that hippocampal-prefrontal synchrony is enhanced during successful working memory performance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **22.Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal–prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–767. doi: 10.1038/nature08855. Further links hippocampal-prefronal synchrony to behavior by showing increased synchrony during learning, and correlating behavioral and synchrony deficits in a mouse model of schizophrenia predisposition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paz R, Bauer EP, Pare D. Theta synchronizes the activity of medial prefrontal neurons during learning. Learning & Memory. 2008;15:524–531. doi: 10.1101/lm.932408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *24.Adhikari A, Topiwala MA, Gordon JA. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron. 2010;65:257–269. doi: 10.1016/j.neuron.2009.12.002. This paper shows that circuit specific-enhancements in theta sychrony with anxiety; and demonstrates stronger synchrony between the mPFC and the dorsal hippocampus compared to the ventral hippocampus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wikgren J, Nokia MS, Penttonen M. Hippocampo–cerebellar theta band phase synchrony in rabbits. Neuroscience. 2010;165:1538–1545. doi: 10.1016/j.neuroscience.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 26.Montgomery SM, Betancur MI, Buzsaki G. Behavior-Dependent Coordination of Multiple Theta Dipoles in the Hippocampus. Journal of Neuroscience. 2009;29:1381–1394. doi: 10.1523/JNEUROSCI.4339-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Royer S, Sirota A, Patel J, Buzsaki G. Distinct Representations and Theta Dynamics in Dorsal and Ventral Hippocampus. Journal of Neuroscience. 2010;30:1777–1787. doi: 10.1523/JNEUROSCI.4681-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- *29.Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Working memory performance correlates with prefrontal-hippocampal theta interactions but not with prefrontal neuron firing rates. Frontiers in Integrative Neuroscience. 2010:4. doi: 10.3389/neuro.07.002.2010. A first attempt to correlate task-related firing patterns and synchrony with the hippocampus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, Wiener SI. Coherent Theta Oscillations and Reorganization of Spike Timing in the Hippocampal- Prefrontal Network upon Learning. Neuron. 2010;66:921–936. doi: 10.1016/j.neuron.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Karayiorgou M, Gogos JA. The molecular genetics of the 22q11-associated schizophrenia. Brain Res Mol Brain Res. 2004;132:95–104. doi: 10.1016/j.molbrainres.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 32.Mukai J, Dhilla A, Drew LJ, Stark KL, Cao L, MacDermott AB, Karayiorgou M, Gogos JA. Palmitoylation-dependent neurodevelopmental deficits in a mouse model of 22q11 microdeletion. Nat Neurosci. 2008;11:1302–1310. doi: 10.1038/nn.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stark KL, Xu B, Bagchi A, Lai WS, Liu H, Hsu R, Wan X, Pavlidis P, Mills AA, Karayiorgou M, et al. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet. 2008 doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- *34.Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, Berman KF. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62:379–386. doi: 10.1001/archpsyc.62.4.379. [DOI] [PubMed] [Google Scholar]
- *35.Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry. 2002;51:1008–1011. doi: 10.1016/s0006-3223(02)01316-1. [DOI] [PubMed] [Google Scholar]
- *36.Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol Psychiatry. 2002;51:485–492. doi: 10.1016/s0006-3223(01)01335-x. [DOI] [PubMed] [Google Scholar]
- *37.Uhlhaas PJ, Haenschel C, Nikolic D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr Bull. 2008;34:927–943. doi: 10.1093/schbul/sbn062. This group of reference (34–37) are among several to detail attempts to identify functional connectivity deficits in patients with schizophrenia. [DOI] [PMC free article] [PubMed] [Google Scholar]