Abstract
Objective
Pleuropulmonary blastoma (PPB) is a childhood cancer arising from pleuropulmonary mesenchyme. This neoplasm is a sentinel disease in a familial tumor syndrome recently found to be associated with germline mutations in DICER1. Observations of ovarian sex cord-stromal tumors (OSCST) in PPB kindreds led to further study. We sought to characterize ovarian tumors seen in probands and families with PPB and PPB-related conditions and define germline DICER1 status.
Methods
Patient and family records of pathology-reviewed PPB cases enrolled in the International PPB Registry (IPPBR) were searched for ovarian tumors. Ovarian tumor pathology specimens were obtained and centrally-reviewed. Germline DNA from patients with ovarian tumors was tested for DICER1 mutations. Three additional OSCST patients registered in the IPPBR were also tested for mutations in DICER1.
Results
Among 296 kindreds including 325 children with PPB, we observed 3 children with both PPB and Sertoli-Leydig cell tumors (SLCT)/Sertoli cell tumors. Among family members of PPB patients, we identified 6 OSCST (3 SLCT, 1 Sertoli cell tumor, 1 juvenile granulosa cell tumor, 1 gynandroblastoma). Age at ovarian tumor diagnosis was youngest in PPB probands and younger in family members than in OSCST in general. Germline DICER1 mutations were identified in 4 of 6 patients with OSCST from PPB kindreds and in 2 of 3 children with OSCST and no personal or family history of PPB.
Conclusions
Primary ovarian neoplasms, particularly OSCST, are a manifestation of the familial PPB syndrome and may be the initial clinical presentation of DICER1 mutations within a family.
Keywords: sex cord-stromal tumors, ovary, cancer, DICER1
BACKGROUND
Pleuropulmonary blastoma (PPB) is the most common primary lung cancer of childhood.[1] It is the pulmonary analog of other dysontogenetic or embryonal neoplasms in this age group such as Wilms' tumor, neuroblastoma, retinoblastoma and others. PPB is genetically determined in approximately 70% of cases [2, 3] and is associated with a distinctive inherited tumor predisposition syndrome. Approximately 30% – 35% of kindreds in which a child has been diagnosed with PPB have findings consistent with the PPB Family Tumor and Dysplasia Syndrome (OMIM #601200) which includes PPB, pulmonary cysts with pathologic features of involuted or regressed PPBs, cystic nephroma, other soft tissue sarcomas, nasal chondromesenchymal hamartoma, nodular hyperplasia and carcinoma of the thyroid gland, ciliary body medulloepithelioma and embryonal rhabdomyosarcoma of the uterine cervix [4–8].
Familial PPB is associated with germline mutations in DICER1 [9]. DICER1 encodes an enzyme required for the production of mature microRNAs (miRNAs) [10]. miRNAs are important regulators of gene expression and are critical in normal organ development [11].
The current study is based upon the identification of several cases of ovarian sex cord-stromal tumors (OSCST) in PPB kindreds. OSCST arise from the mesenchymal or interstitial stroma whose differentiated elements include granulosa cells and other specialized supporting elements. The specific tumor types in the OSCST category are Sertoli-Leydig cell tumors (SLCT), granulosa cell tumors (juvenile and adult), Sertoli cell tumors, thecoma-fibroma and gynandroblastoma. Gynandroblastoma is a unique tumor, having both SLCT and juvenile granulosa tumor components [12]. Both PPB and OSCST are rare neoplasms and their precise incidence is difficult to determine from the literature.
In this study we present our investigation of the associations between PPB, OSCST and germline DICER1 mutations.
METHODS
Human subjects sample collection
The records of families enrolled in the International PPB Registry (IPPBR, www.ppbregistry.org) were reviewed for proband and/or family member history of ovarian neoplasms. Central review of PPB pathology (LPD, DAH) was required for IPPBR enrollment. Ovarian tumor histopathology was centrally-reviewed (MCP, LPD, DAH). All study protocols were approved by participating institutional human subjects committees. All participants providing germline samples for DICER1 sequencing provided written consent for the molecular and family history studies as approved by the Human Research Protection Offices at Washington University in St. Louis, MO and Children’s National Medical Center in Washington, DC. Medical records, blood, saliva and tumor specimens were collected from PPB probands, affected family members and other relatives. Genomic DNA was extracted from peripheral blood lymphocytes or saliva using standard protocols. Family histories were obtained by treating physicians and IPPBR staff. Three children with OSCST from two families without a history of PPB were also included in this report as they were referred to the IPPBR based on clinical suspicion for genetically-determined OSCST.
Sequence analysis of germline or tumor DNA
DICER1 sequences were extracted from the public draft human genome database (NM_177438) and used as a reference sequence for assembly and primer construction. Primers were designed to amplify all of the coding exons including intron-exon boundaries (Supplemental Table 1). PCR reactions were performed using genomic DNA. The resultant products were purified and directly sequenced using BigDye Terminator chemistry (v3.1 Applied Biosystems, Valencia CA) and the ABI3730 sequencer (Applied Biosystems). For a subset of cases, we used high throughput sequencing services (Agencourt, BeckmanCoulter Genomics, Danvers MA). The sequence traces were assembled and scanned for variations using Sequencher version 4.8 (Gene Codes, Ann Arbor, MI) and visual inspection of chromatograms. Variants were queried against the SNP database (http://www.ncbi.nlm.nih.gov/projects/SNP/). SIFT and Polyphen algorithms were used to assess the potential significance of predicted novel amino acid substitutions (http://blocks.fhcrc.org/sift/SIFT.html) [13].
RESULTS
Clinical Characteristics
Among 325 patients from 296 families with PPB enrolled in the IPPBR, we identified 9 patients or close relatives with an OSCST(Table 1). These included seven Sertoli-Leydig tumors, one juvenile granulosa cell tumor and one gynandroblastoma. In three families, the proband with PPB was also diagnosed with an ovarian Sertoli-Leydig tumor (ages at diagnosis 6, 8 and 13 years). These three children had received chemotherapy and surgery for PPB 5, 6 and 11 years prior to ovarian tumor diagnosis respectively. Six OSCST were diagnosed in first or second-degree relatives of a child with PPB. Ages at diagnosis for OSCST in these family members were 2, 4, 7, 15, 18 and 32 years (median, 11 years) (Table 1). An ovarian dysgerminoma was seen in a third-degree relative of a PPB patient. No other ovarian tumors were confirmed.
Table 1.
Ovarian tumors in PPB probands and family members.
| Case Relationship to PPB |
Ovarian Diagnosis (age at diagnosis, years) |
Other Diagnoses in This Patient (age at diagnosis, years) |
Ovarian Tumor Treatment |
Outcome since Ovarian Diagnosis (duration, years) |
|---|---|---|---|---|
| PPB Patients with Ovarian Tumors | ||||
|
1 PPB patient |
SLCT - poorly differentiated, with heterologous elements (13) |
Congenital phthisis bulbi (0) PPB (2) NCMH (12) |
surgery | NED, 6.5 |
|
2 PPB patient |
Sertoli cell - SLCT (6) |
PPB (0.5) CN (1) |
surgery | NED, 2 |
|
3 PPB patient |
SLCT - retiform (8) | PPB (2) | surgery, chemotherapy | NED, 1.5 |
| Relatives of PPB Patients with Ovarian Tumors | ||||
|
4 second degree |
SLCT (15) | none | surgery × 3 | NED*, 25 |
|
5 second degree |
SLCT - retiform (7) | NTH (18) | surgery | NED, 16 |
|
6 second degree |
SLCT (32) |
NTH (12) Pituitary adenoma (25) |
surgery | NED, 16 |
|
7 first degree |
Sertoli cell tumor with torsion (likely SLCT) (4) | none | surgery | NED, 45 |
|
8 second degree |
jGCT (2) | none | surgery | NED, 33 |
|
9 first degree |
Gynandroblastoma (18) | none | surgery | NED, 13 |
| Patients with diseases associated with PPB without PPB in the family | ||||
| 10 | SLCT (10) | Ovarian embyonal rhabdomyosarcoma with anaplasia (14) |
surgery, chemotherapy | NED, 11 |
| 11 | jGCT (16) | NTH (16) Lung cyst (16) |
surgery, chemotherapy | NED, 1.5 |
| 12 | Gynandroblastoma (16) | NTH (15) | surgery | NED, 5 |
|
Evidence of hormone production: Case 9: elevated serum testosterone & serum CA-125; Case 6: virilization | ||||
Abbreviations: Dx: diagnosis; NED: no evidence of disease; PPB: pleuropulmonary blastoma; NCMH: nasal chondromesenchymal hamartoma; CN: cystic nephroma; SLCT: Sertoli-Leydig cell tumor; jGCT: juvenile granulosa cell tumor; NTH nodular thyroid hyperplasia
Recurred 1 year after SLCT diagnosis: nodes, bowel, uterus
For the children with no personal or family history of PPB, two were sisters, one diagnosed with juvenile granulosa cell tumor and the other with gynandroblastoma (Table 1). Interestingly, a lung cyst was identified in the sister with juvenile granulosa cell tumor (Fig. 1). The third child developed bilateral ovarian tumors: a poorly differentiated sarcoma with limited myogenic differentiation (age 10 years) and subsequently a Sertoli-Leydig tumor of the contralateral ovary without heterologous elements (age 14 years).
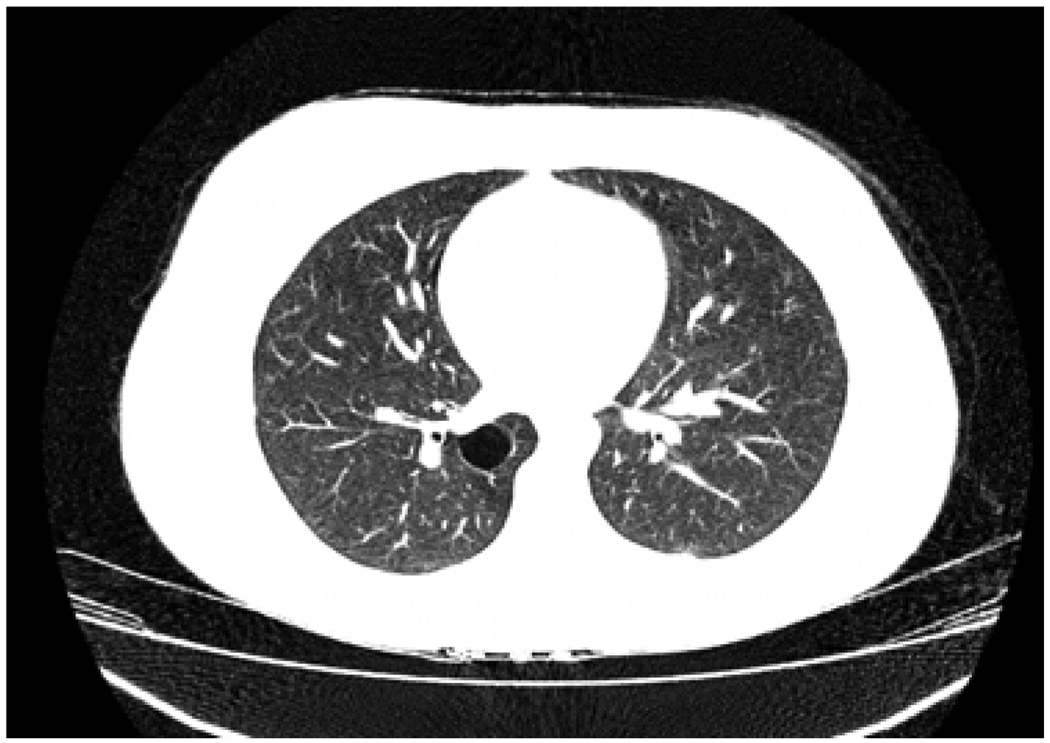
Fig. 1.
Lung cyst discovered at age 16 years in child with juvenile granulosa cell tumor (Case 11). Such cysts discovered after age 8 years in patients with PPB-associated conditions are very likely benign cystic variants of PPB [3].
All three children with PPB and OSCST underwent complete surgical resection of SLCT; one child also received chemotherapy. All are alive and without evidence of PPB or SLCT at 1.5 to 6.5 years following the SLCT diagnosis (Table 1). Fewer clinical records are available for family members, however, all underwent surgical excision and at least one received chemotherapy. One of these individuals had recurrence of SLCT in lymph nodes and peritoneal surfaces one year after diagnosis, underwent surgery alone and was alive at last contact, 25 years after the diagnosis of SLCT. All family members with OSCST are alive without evidence of disease at a median of 20.5 years (range, 13 – 45 years) following the ovarian tumor diagnosis.
Pathology and Genetics
The nine OSCST in individuals with PPB (3 cases) or in relatives of those with PPB (6 cases) were all unilateral neoplasms without a predilection for either the right or left ovary. The tumors included five SLCT, two Sertoli cell tumors, one juvenile granulosa cell tumor and one gynandroblastoma (Fig. 2). The SLCT were intermediate to poorly-differentiated. One of the SLCT had heterologous elements with enteric-like glands and sarcomatous stroma. One of the two Sertoli cell tumors presented at the age of six years with abdominal pain secondary to torsion of a 8 × 4 × 2 cm hemorrhagic mass in the left ovary (Fig. 3). This child was diagnosed with multiple Type I PPBs at five months of age and a cystic nephroma of the left kidney at eleven months of age. A juvenile granulosa cell tumor was diagnosed at the age of two years in a second degree relative.
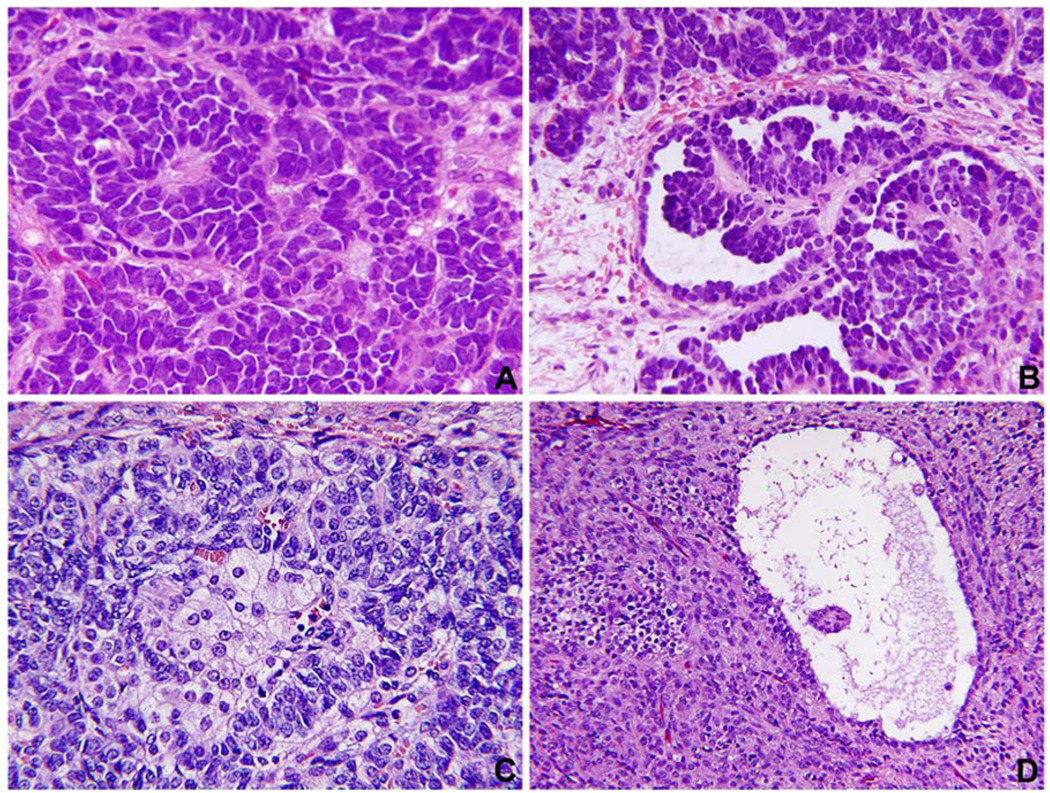
Fig. 2.
(4 panel photomicrographs). A. Sertoli cell tumor of the ovary (case 2) shows the pattern of solid cords of uniform basophilic cells. B. Other patterns in the same tumor (case 2) demonstrate small tubules adjacent to retiform or papillary profiles. C. Sertoli-Leydig cell tumor (case 4) was largely necrotic and hemorrhagic but the viable areas show the presence of small basophilic cells in cord-like structures with intermixed clusters of larger, pale staining cells representing Leydig cells. D. Juvenile granulosa cell tumor (case 8) with a cystic structure resembling a graffian follicle is surrounded by a densely cellular stroma.
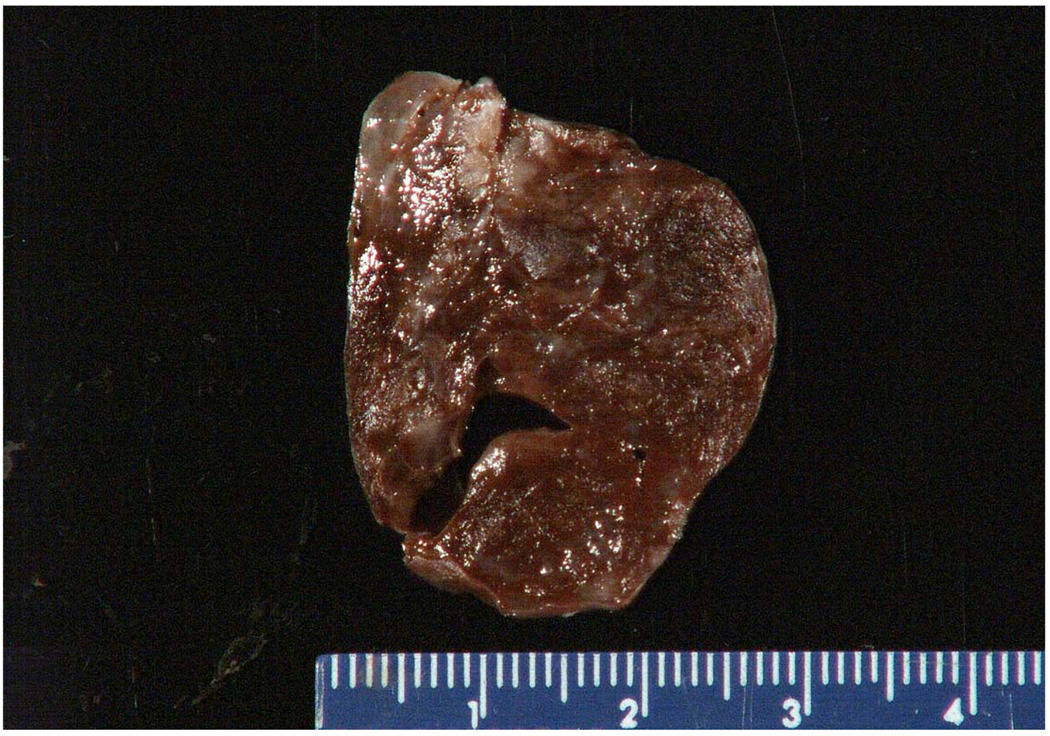
Fig. 3.
(gross). Sertoli cell tumor of the ovary (case 2) presented with persistent abdominal pain in a 6-year-old female with the previous history of multiple PPBs and a cystic nephroma of the kidney. The hemorrhagic appearance of this 8 cm ovarian mass reflects the torsion with hemorrhagic infarction.
There were three additional cases of OSCST: a SLCT, a juvenile granulosa cell tumor and a gynandroblastoma, in patients who had no history of a PPB in their family, but had clinical findings suggestive of familial disease; two of these patients had germline DICER1 mutations.
Germline DNA samples were available for 8 of 12 patients described in this report. Six of these eight were found to have a DICER1 mutation. One additional patient with SLCT was found to have an intronic variant of uncertain clinical significance. Because these cases are unusual and clinically distinct and some genetic results were obtained in the research setting, results have been aggregated where appropriate to prevent unintentional specific disclosure of results. Mutation analysis results are detailed in Table 2. Importantly, germline DICER1 mutations were seen in patients with SLCT, juvenile granulosa cell tumors and gynandoblastoma.
Table 2.
DICER1 mutations in patients described in this report.
| Ovarian Diagnosis | Mutation |
|---|---|
| SLCT | c.1507G>T; p.E503X |
| SLCT | c.2243_2244insCTAA; p.C748fs |
| SLCT | c.1376+1G>T; splice site |
| SLCT | * |
| juvenile granulosa cell tumor | c.3073G>T; p.Glu1025X** |
| Gynandroblastoma | c.3777dupC; p.V1260fs |
| Gynandroblastoma | c.3073G>T; p.Glu1025X** |
Abbreviation: SLCT: Sertoli-Leydig cell tumor
intronic variant of uncertain clinical significance;
sisters
DISCUSSION
We identified nine individuals with OSCST among PPB probands and their relatives in the IPPBR. Germline DICER1 mutations were seen in 6 of 8 patients in this report.
Because the precise incidence of OSCST and PPB are difficult to ascertain, we cannot determine the expected incidence of these conditions together in a single patient or in a family kindred. Both conditions are inadequately tabulated in national registries such as Surveillance, Epidemiology and End Results (SEER) [14]. Between 25 and 50 children are diagnosed with PPB annually in the United States. SLCT comprise less than 1% of ovarian tumors seen in children and adults. It is a reasonable presumption that these two rare cancers occurring in individuals or relatives have a related pathogenesis. Also, the ovarian tumors seen are predominantly limited to the otherwise rare specific histologic subtype of OSCST with a predominance of SLCT. The ages at diagnosis of SLCT/Sertoli cell tumors for PPB patients (6, 8 and 13 years) and family members (4, 7, 15 and 32 years) are generally younger than the age of diagnosis of “sporadic” SLCT (mean 25 years) [15]. Although our numbers are small, these data suggest that SLCTs associated with PPB occur at a younger age than sporadic examples, as is typical of hereditary tumor predisposition. All of the patients described are alive without evidence of disease, including one patient who relapsed and underwent surgery, although the numbers are too small to draw conclusions regarding prognosis.
In addition to the PPB-associated cases, we identified three children with OSCST for whom there was no personal or family history of PPB, however clinical findings were suggestive of a genetic tumor predisposition. Two were sisters with OSCST (one with a lung cyst); the third patient had ovarian sarcoma with contralateral OSCST. DICER1 mutations were also seen in this group.
In general, the pathogenesis of OSCST is unknown, in part because of the tumor’s rarity. Genetic analyses of tumor tissue have generally shown balanced karyotypes, with chromosomal imbalances in up to 25%, occasionally gain of chromosome 12 [16]. Trisomy 8 was seen in one case of metastasizing SLCT [17]. Sex cord-stromal tumors have been described in other genetic syndromes, suggesting that genetic predisposition is important. Sex cord-stromal tumor with annular tubules are associated with Peutz Jeghers syndrome characterized by the STK11 gene mutation but STK11 gene mutations have not been observed in sporadic OSCST [18]. Juvenile granulosa cell tumors have been described in association with Ollier enchondromatosis and Maffucci syndrome [19].
Four OSCST patients we report had nodular thyroid hyperplasia (NTH) (Table 1). The association of NTH with SLCT has recently been shown to be related to germline mutations in DICER1 [20, 21] lending further support to the association described here. Our research expands these findings to include cases of juvenile granulosa cell tumor and gynandroblastoma and defines a link between PPB, OSCST and germline DICER1 mutations.
Germ-cell tumors are a separate entity from OSCST. We observed one case of ovarian dysgerminoma in a third-degree relative of a PPB patient. Three seminomas have also been observed in relatives of PPB patients. Germline mutations results were available for one patient with seminoma; we did not find DICER1 mutation. Of 71 patients with seminoma, Slade et al. identified one DICER1 mutation.[22] It is currently unclear whether the dysgerminoma/seminoma group of gonadal tumors is part of the PPB-related spectrum of diseases.
Our sequence analysis identified heterozygous germline loss of function DICER1 mutations in 4 of 6 patients with PPB-associated OSCST. We also identified a DICER1 mutation in two young sisters with OSCST and no known family or personal history of PPB. On staging chest CT one sister was also found to have a lung cyst (Figure 1), likely an occult manifestation of the cystic variant of PPB. [23]. The finding of a lung cyst or one of the PPB-related tumors in a patient or close family member with OSCST should suggest the possibility of germline DICER1 mutation. DICER1 mutations were first identified in PPB patients [9] and have subsequently been seen in other conditions seen in familial PPB cases [3, 8, 21, 24]. Clinical testing for DICER1 mutation status is available.
Mutations in DICER1 are hypothesized to alter production of mature miRNAs leading to dysregulated gene expression [9, 11]. Because miRNAs are important in controlling many biological functions, the effects of reduced DICER1 expression in different tissues at different stages of growth, development or homeostasis may be expected to lead to pleiotropic effects. DICER1 may function as a haploinsufficient tumor suppressor [25]. Decreased DICER1 expression in tumor tissue has recently been shown to be associated with poorer prognosis in adults with epithelial ovarian cancer [13]. The role of germline DICER1 mutations and downstream implications for miRNA expression in presumed sporadic OSCST is not yet clear. DICER1 mutations are not found in all cases of PPB or OSCST and many carriers of DICER1 mutations are phenotypically normal, thus other genetic factors are also likely to be important in these conditions.
The PPB Family Tumor and Dysplasia Syndrome phenotype includes PPB, lung cysts, cystic nephroma, OSCST (especially SLCT) as described here, thyroid nodular hyperplasia (NTH) and differentiated carcinomas, nasal chondomesenchymal hamartoma, ciliary body medulloepitheioma and sarcoma botryoides of the uterine cervix as well as more familiar childhood cancers and dysplasias [3]. The importance of detailed family histories is thus apparent, and co-occurrence in a child or family of any of the PPB-associated illnesses raises the possibility of genetically-determined disease. DICER1 sequencing may be indicated and if such a genetic marker is found, the at-risk family members can be identified. Because PPB-related diseases affect many organs over the first 20–30 years of life and because disease penetrance is low, disease screening must be very carefully considered. On the other hand, families and medical professionals should be aware of familial diseases associated with OSCST. Obtaining detailed family histories from patients with OSCST may lead to timely diagnosis of a familial cancer syndrome with important implications for young children within the family. For very young children who carry DICER1 gene mutations, screening with chest CT scan may be most helpful in early identification of PPB, which is by far the most lethal tumor seen within this familial tumor syndrome and a tumor in which early detection provides the best opportunity for cure. [26] Clinicians and family members should be alert for signs of an abdominal mass, pain or signs of hormone production such as precocity or virilization that may herald the presence of an ovarian sex cord-stromal tumor.
From this group of patients we cannot determine the prevalence of germline DICER1 mutations in patients with OSCST in general. Medical or family history of lung cysts, lung tumors diagnosed at a young age (especially PPB), hyperplastic thyroid disease, gonadal tumors, cystic nephroma, intestinal hamartomas, childhood benign or malignant tumors or SLCT/OSCST diagnosed at a younger than expected age strongly suggest the need for genetic counseling and consideration of DICER1 testing. Studies underway and in development will offer further insights into these important questions.
CONCLUSIONS
We have shown that germline DICER1 mutations may be found in PPB-associated OSCST cases. DICER1 mutations may also be found in other OSCST patients, including those with SLCT, juvenile granulosa cell tumor and gynandroblastoma whose clinical presentation suggests genetic predisposition. With this insight, we seek to gain a better understanding of tumor pathogenesis; thus leading to improved treatments and outcome for women with sporadic OSCST, which continue to have significant morbidity. Although we have no specific recommendations for screening at this time, recognition of this clinical association may promote early recognition of ovarian tumor symptoms in a predisposed child or family member.
Supplementary Material
Acknowledgements
The authors thank the physicians and data managers who supply information and thank the many patients and families who participate in the International PPB Registry. We thank Leo Twiggs, MD for critical reading of the manuscript. The Pine Tree Apple Tennis Classic and the Theodora H Lang Charitable Trust support the International PPB Registry and its staff. Additional support is also provided by NIH 1R01CA143167-01 (Hill P.I.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: All authors report that they have no conflict of interest.
REFERENCES
- 1.Manivel JC, Priest JR, Watterson J, Steiner M, Woods WG, Wick MR, Dehner LP. Pleuropulmonary blastoma. The so-called pulmonary blastoma of childhood. Cancer. 1988;62:1516–1526. doi: 10.1002/1097-0142(19881015)62:8<1516::aid-cncr2820620812>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 2.Hill DA, Wang JD, Schoettler P, Dehner L, Ivanovich J, Bracamontes D, Williams G, Messinger Y, Goodfellow PJ, Priest J. Germline DICER1 mutations are common in both hereditary and presumed sporadic pleuropulmonary blastoma. Lab Invest. 2010;90:311. [Abstract] [Google Scholar]
- 3.Slade I, Bacchelli C, Davies H, Murray A, Abbaszadeh F, Hanks S, Barfoot R, Burke A, Chisholm J, Hewitt M, Jenkinson H, King D, Morland B, Pizer B, Prescott K, Saggar A, Side L, Traunecker H, Vaidya S, Ward P, Futreal PA, Vujanic G, Nicholson AG, Sebire N, Turnbull C, Priest JR, Pritchard-Jones K, Houlston R, Stiller C, Stratton MR, Douglas J, Rahman N. DICER1 syndrome: clarifying the diagnosis, clinical features and management implications of a pleiotropic tumour predisposition syndrome. J Med Genet. doi: 10.1136/jmg.2010.083790. [DOI] [PubMed] [Google Scholar]
- 4.Priest JR, Williams GM, Hill DA, Dehner LP, Jaffe A. Pulmonary cysts in early childhood and the risk of malignancy. Pediatr Pulmonol. 2009;44:14–30. doi: 10.1002/ppul.20917. [DOI] [PubMed] [Google Scholar]
- 5.Boman F, Hill DA, Williams GM, Chauvenet A, Fournet JC, Soglio DB, Messinger Y, Priest JR. Familial association of pleuropulmonary blastoma with cystic nephroma and other renal tumors: a report from the International Pleuropulmonary Blastoma Registry. J Pediatr. 2006;149:850–854. doi: 10.1016/j.jpeds.2006.08.068. [DOI] [PubMed] [Google Scholar]
- 6.Priest JR, Watterson J, Strong L, Huff V, Woods WG, Byrd RL, Friend SH, Newsham I, Amylon MD, Pappo A, Mahoney DH, Langston C, Heyn R, Kohut G, Freyer DR, Bostrom B, Richardson MS, Barredo J, Dehner LP. Pleuropulmonary blastoma: a marker for familial disease. J Pediatr. 1996;128:220–224. doi: 10.1016/s0022-3476(96)70393-1. [DOI] [PubMed] [Google Scholar]
- 7.Priest JR, Williams GM, Mize WA, Dehner LP, McDermott MB. Nasal chondromesenchymal hamartoma in children with pleuropulmonary blastoma--A report from the International Pleuropulmonary Blastoma Registry registry. Int J Pediatr Otorhinolaryngol. 2010;74:1240–1244. doi: 10.1016/j.ijporl.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Priest JR, Williams GM, Manera R, Jenkinson H, Brundler MA, Davis S, Murray TG, Galliani CA, Dehner LP. Ciliary body medulloepithelioma: four cases associated with pleuropulmonary blastoma--a report from the International Pleuropulmonary Blastoma Registry. Br J Ophthalmol. 2010 doi: 10.1136/bjo.2010.189779. [DOI] [PubMed] [Google Scholar]
- 9.Hill DA, Ivanovich J, Priest JR, Gurnett CA, Dehner LP, Desruisseau D, Jarzembowski JA, Wikenheiser-Brokamp KA, Suarez BK, Whelan AJ, Williams G, Bracamontes D, Messinger Y, Goodfellow PJ. DICER1 mutations in familial pleuropulmonary blastoma. Science. 2009;325:965. doi: 10.1126/science.1174334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 11.Asli NS, Pitulescu ME, Kessel M. MicroRNAs in organogenesis and disease. Curr Mol Med. 2008;8:698–710. doi: 10.2174/156652408786733739. [DOI] [PubMed] [Google Scholar]
- 12.Schneider DT, Calaminus G, Wessalowski R, Pathmanathan R, Selle B, Sternschulte W, Harms D, Gobel U. Ovarian sex cord-stromal tumors in children and adolescents. J Clin Oncol. 2003;21:2357–2363. doi: 10.1200/JCO.2003.05.038. [DOI] [PubMed] [Google Scholar]
- 13.Merritt WM, Lin YG, Han LY, Kamat AA, Spannuth WA, Schmandt R, Urbauer D, Pennacchio LA, Cheng JF, Nick AM, Deavers MT, Mourad-Zeidan A, Wang H, Mueller P, Lenburg ME, Gray JW, Mok S, Birrer MJ, Lopez-Berestein G, Coleman RL, Bar-Eli M, Sood AK. Dicer, Drosha, and outcomes in patients with ovarian cancer. N Engl J Med. 2008;359:2641–2650. doi: 10.1056/NEJMoa0803785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altekruse SFKC, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG, Edwards BK, editors. Bethesda, MD: National Cancer Institute; 1975–2007. SEER Cancer Statistics Review. http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site, 2010. [Google Scholar]
- 15.Young RH, Scully RE. Ovarian Sertoli-Leydig cell tumors. A clinicopathological analysis of 207 cases. Am J Surg Pathol. 1985;9:543–569. doi: 10.1097/00000478-198508000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Schneider DT, Calaminus G, Harms D, Gobel U. Ovarian sex cord-stromal tumors in children and adolescents. J Reprod Med. 2005;50:439–446. [PubMed] [Google Scholar]
- 17.Manegold E, Tietze L, Gunther K, Fleischer A, Amo-Takyi BK, Schroder W, Handt S. Trisomy 8 as sole karyotypic aberration in an ovarian metastasizing Sertoli-Leydig cell tumor. Hum Pathol. 2001;32:559–562. doi: 10.1053/hupa.2001.24316. [DOI] [PubMed] [Google Scholar]
- 18.Kato N, Romero M, Catasus L, Prat J. The STK11/LKB1 Peutz-Jegher gene is not involved in the pathogenesis of sporadic sex cord-stromal tumors, although loss of heterozygosity at 19p13.3 indicates other gene alteration in these tumors. Hum Pathol. 2004;35:1101–1104. doi: 10.1016/j.humpath.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Vaz RM, Turner C. Ollier disease (enchondromatosis) associated with ovarian juvenile granulosa cell tumor and precocious pseudopuberty. J Pediatr. 1986;108:945–947. doi: 10.1016/s0022-3476(86)80936-2. [DOI] [PubMed] [Google Scholar]
- 20.Clement PB, Young RH, Scully RE. Clinical syndromes associated with tumors of the female genital tract. Semin Diagn Pathol. 1991;8:204–233. [PubMed] [Google Scholar]
- 21.Rio Frio T, Bahubeshi A, Kanellopoulou C, Hamel N, Niedziela M, Sabbaghian N, Pouchet C, Gilbert L, O'Brien PK, Serfas K, Broderick P, Houlston RS, Lesueur F, Bonora E, Muljo S, Schimke RN, Bouron-Dal Soglio D, Arseneau J, Schultz KA, Priest JR, Nguyen VH, Harach HR, Livingston DM, Foulkes WD, Tischkowitz M. DICER1 mutations in familial multinodular goiter with and without ovarian Sertoli-Leydig cell tumors. JAMA. 2011;305:68–77. doi: 10.1001/jama.2010.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slade I, Bacchelli C, Davies H, Murray A, Abbaszadeh F, Hanks S, Barfoot R, Burke A, Chisholm J, Hewitt M, Jenkinson H, King D, Morland B, Pizer B, Prescott K, Saggar A, Side L, Traunecker H, Vaidya S, Ward P, Futreal PA, Vujanic G, Nicholson AG, Sebire N, Turnbull C, Priest JR, Pritchard-Jones K, Houlston R, Stiller C, Stratton MR, Douglas J, Rahman N. DICER1 syndrome: clarifying the diagnosis, clinical features and management implications of a pleiotropic tumour predisposition syndrome. J Med Genet. 2011 doi: 10.1136/jmg.2010.083790. [DOI] [PubMed] [Google Scholar]
- 23.Hill DA, Jarzembowski JA, Priest JR, Williams G, Schoettler P, Dehner LP. Type I pleuropulmonary blastoma: pathology and biology study of 51 cases from the international pleuropulmonary blastoma registry. Am J Surg Pathol. 2008;32:282–295. doi: 10.1097/PAS.0b013e3181484165. [DOI] [PubMed] [Google Scholar]
- 24.Bahubeshi A, Bal N, Frio TR, Hamel N, Pouchet C, Yilmaz A, Bouron-Dal Soglio D, Williams GM, Tischkowitz M, Priest JR, Foulkes WD. Germline DICER1 mutations and familial cystic nephroma. J Med Genet. 2010;47:863–866. doi: 10.1136/jmg.2010.081216. [DOI] [PubMed] [Google Scholar]
- 25.Lambertz I, Nittner D, Mestdagh P, Denecker G, Vandesompele J, Dyer MA, Marine JC. Monoallelic but not biallelic loss of Dicer1 promotes tumorigenesis in vivo. Cell Death Differ. 2010;17:633–641. doi: 10.1038/cdd.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Priest JR, McDermott MB, Bhatia S, Watterson J, Manivel JC, Dehner LP. Pleuropulmonary blastoma: a clinicopathologic study of 50 cases. Cancer. 1997;80:147–161. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.