Abstract
Previous attempts to review the literature on magnetic nanomaterials for hyperthermia-based therapy focused primarily on magnetic fluid hyperthermia (MFH) using mono metallic/metal oxide nanoparticles. The term “Hyperthermia” in the literature was also confined only to include use of heat for therapeutic applications. Recently, there have been a number of publications demonstrating magnetic nanoparticle-based hyperthermia to generate local heat resulting in the release of drugs either bound to the magnetic nanoparticle or encapsulated within polymeric matrices. In this review article, we present a case for broadening the meaning of the term “hyperthermia” by including thermotherapy as well as magnetically modulated controlled drug delivery. We provide a classification for controlled drug delivery using hyperthermia: Hyperthermia-based controlled Drug delivery through Bond Breaking (DBB) and Hyperthermia-based controlled Drug delivery through Enhanced Permeability (DEP). The review also covers, for the first time, core-shell type magnetic nanomaterials, especially nanoshells prepared using layer-by-layer self-assembly, for the application of hyperthermia-based therapy and controlled drug delivery. The highlight of the review article is to portray potential opportunities in the combination of hyperthermia-based therapy and controlled drug release paradigms for successful application in personalized medicine.
Keywords: Hyperthermia, hyperthermia-based therapy, hyperthermia-based controlled drug delivery, core-shell magnetic nanoparticles, theranostics
1. INTRODUCTION
The etymological meaning of the word “Hyperthermia” is generation of heat and with respect to cancer therapy, the term is used to imply treatment based on generation of heat at the tumor site.[1] The approach involves raising the temperature of local environment of a tumor resulting in changing the physiology of diseased cells finally leading to apoptosis.[2] This treatment modality complements currently available treatments including chemotherapy, radiation therapy, surgery, gene therapy, and immunotherapy for cancer. Depending on the degree of temperature raise, hyperthermia treatment can be classified into different types. In thermo ablation, a tumor is subjected to high temperatures of heat >46 °C (up to 56°C) causing cells to undergo direct tissue necrosis, coagulation or carbonization. Moderate hyperthermia (41°C<T<46°C) has various effects both at the cellular and tissue levels. Diathermia uses lower temperatures (T<41°C) for the treatment of rheumatic diseases in physiotherapy. During moderate hyperthermia, which is traditionally termed as hyperthermia treatment, cells undergo heat stress in the temperature range of 41–46°C resulting in activation and/or initiation of many intra and extracellular degradation mechanisms like protein denaturation, protein folding, aggregation and DNA cross linking. With a single heat treatment, permanent irreversible protein damage can occur resulting in protein aggregation and/or inhibition of many cellular functions.[3] The other cellular effects of moderate hyperthermia include induction and regulation of apoptosis, signal transduction, multidrug resistance and heat shock protein (HSP) expression. The tissue level effects include pH changes, perfusion and oxygenation of tumor micro environment.[4, 5, 6] The effectiveness of any hyperthermia treatment greatly depends on the temperatures generated at the targeted sites of action, duration of exposure and characteristics of particular cancer cells.[7]
Hyperthermia is also categorized into local, regional and whole body hyperthermia depending on the location of disease. Local hyperthermia involves subjection of heat only to a small area of interest such as a tumor. Regional hyperthermia involves heat subjection to larger areas such as whole tissue and organ. Whole body hyperthermia is applied only to treat metastatic cancer cells when spread throughout the body. The challenge here is to heat only the tumor cells without damaging the healthy tissues. Of these, local hyperthermia is gaining much more attention due to involvement of intracellular heat subjection to a specified region of interest. [8, 9]
Traditionally, hyperthermia treatment was administered by using external devices to transfer energy to tissues by either irradiation with light or electromagnetic waves. Currently available techniques for induction of hyperthermia are ultrasound, radiofrequency, microwaves, infrared radiation, magnetically excitable thermoseeds, and tubes with hot water. However, each of these methods suffers from its own limitations. [10] Oncologists often use the heat treatment in combination with radiotherapy or chemotherapy or both. The combined approach results in eliminating many cancer cells in addition to making the resistant cancer cells more vulnerable to other treatments. Some of the challenges in traditional hyperthermia treatment are [11]: 1) unavoidable heating of healthy tissue resulting in burns, blisters and discomfort 2) limited penetration of heat into body tissues by microwave, laser and ultrasound energy and 3) thermal under- dosage in the target region, a nearly unsolved problem in the case of bone of pelvis or scull which shield deep tissues; often yielding recurrent tumor growth.
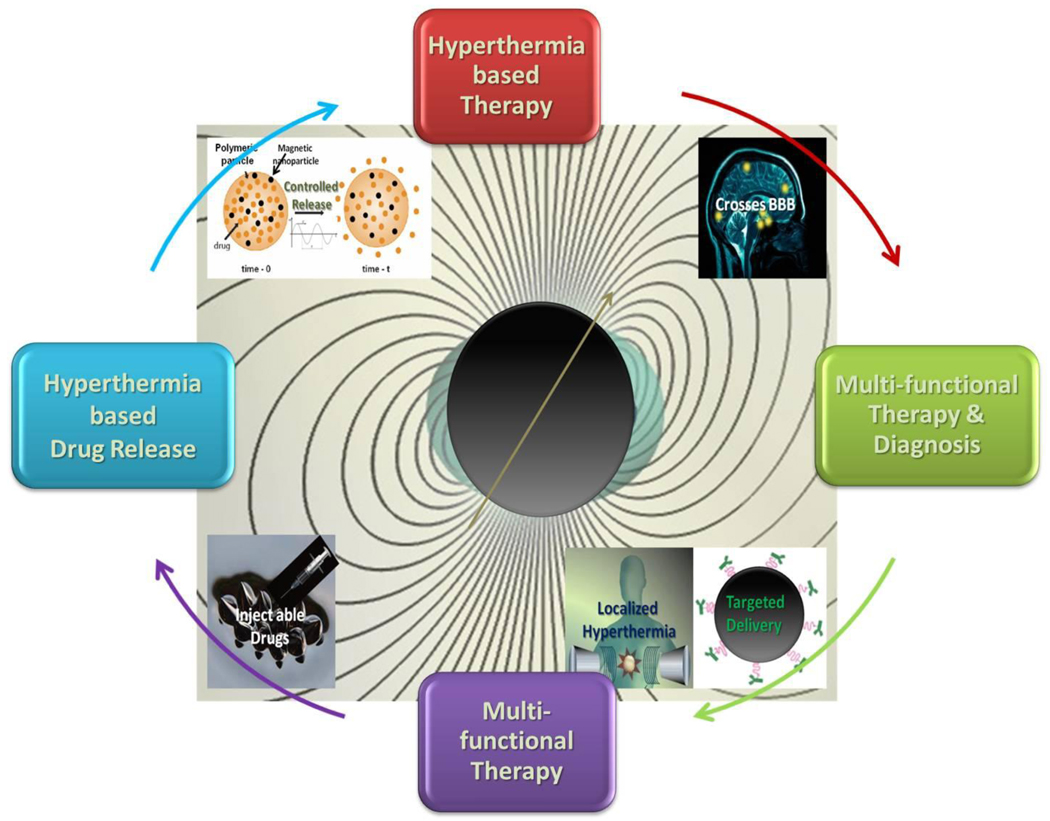
With the possibility to convert dissipated magnetic energy into thermal energy, the application of magnetic materials for hyperthermia treatment of cancer was first proposed in 1957. [12] Since then the approach evolved into a well-researched field due to the introduction of magnetic nanoparticles (MNPs). MNP-based hyperthermia treatment has a number of advantages compared to conventional hyperthermia treatment. These, schematically highlighted in Figure-1, are: 1) cancer cells absorb MNPs thereby increasing the effectiveness of hyperthermia by delivering therapeutic heat directly to them, 2) MNPs can be targeted through cancer-specific binding agents making the treatment much more selective and effective, 3) the frequencies of oscillating magnetic fields generally utilized pass harmlessly through the body and generate heat only in tissues containing MNPs, [13] 4) MNPs can also effectively cross blood-brain barrier (BBB) and hence can be used for treating brain tumors, 5) effective and externally stimulated heating can be delivered at cellular levels through alternating magnetic field (AMF), [14] 6) with the possibility to obtain stable colloids using MNPs, they can be administered through a number of drug delivery routes, [15] 7) MNPs used for hyperthermia are only few tens of nanometer in size and therefore, allows easy passage into several tumors whose pore sizes are in 380–780 nm range, [16] 8) compared to macroscopic implants, MNP based heat generation is much more efficient and homogeneous,[17] 9) MNP-based hyperthermia treatment may induce antitumoral immunity,[18] 10) last but most important aspect is that MNP-based hyperthermia can also be utilized for controlled delivery of drugs (Section 4.0–6.0) and first such nano construct for this purpose was made using layer-by-layer self-assembly approach.[19] This additional feature opens up possibilities for the development of multifunctional and multi-therapeutic approaches for treating a number of diseases.
Figure-1.
A schematic representation of some of the unique advantages of magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery
There have been an increasing number of investigations, in the past two decades, into various aspects of magnetic nanomaterials for hyperthermia-based therapy. While there are few review articles in the literature, their focus has been primarily on either iron oxide nanoparticle or monometallic/oxide MNP-based hyperthermia. For example, in the most recent review [20] an attempt was made to present the status and prospects of magnetite nanoparticle-based hyperthermia covering only theoretical and experimental investigations related to heat dissipation by Néel relaxation. There is a review that covers clinical applications of MNPs for hyperthermia with focus on recent clinical trials conducted by MagForce Nanotechnologies AG, Berlin, Germany.[21] In yet another mini review, Hergt et al discuss how MNP properties and electromagnetic field parameters may be optimized for cancer therapy based on magnetic fluid hyperthermia (MFH).[22] On the similar lines, fundamental aspects related to the design of MNPs for cancer-localized hyperthermia was reviewed; once again focusing on iron oxide-based MNPs.[23] While single domain MNPs absorb much more power at tolerable magnetic fields and frequencies than those by multidomain particles, optimization of MNPs for MFH is not yet well established.[24] There is a strong need for the design of MNPs with enhanced magnetic properties specifically tailored for hyperthermia related applications. There is also an unmet technical challenge to design new MNPs which possess not only high thermal efficiency as heating elements but are also biocompatible (nontoxic), and stable in aqueous solution. In addition, the expectations are that they should have high capacity for accumulation inside the tumor cells and with right Curie temperature (Tc) to provide local temperature control system. [25]
Nanoparticles (NPs) offer an opportunity to create multifunctionality with potential for innovative diagnostic and therapeutic modalities for a number of diseases.[26] Controlled release of drugs with spatiotemporal control is the key for meeting a number of challenges in drug delivery applications.[27] Controlled release of drugs from NP based drug delivery systems, triggered by a number of external stimuli, has been extensively studied. [28] While the term “Hyperthermia” in the literature, so far has been confined only to include the use of heat for therapy, it was found from the recent literature studies that MNP-based hyperthermia can also be utilized to generate intense local heating within polymeric matrices there by creating voids for the release of encapsulated drugs (Figure-1). The use of magnetic fields to control drug release from large polymeric matrices was first reported nearly three decades back. [29–31] These investigations conceptually demonstrated the utility of magnetic field hyperthermia for controlled drug release albeit using millimeter size magnetic materials. The very first report of potential opportunity to utilize MNPs as hyperthermic agents for controlled drug release appeared in 2005. [19] The drug delivery system in this case was prepared by using layer-by-layer self-assembly technique. Since then a number of investigations have been carried out to demonstrate this concept in a number of materials and for a number of application types. A broad range of frequencies of oscillating magnetic fields, from 50 kHz to 10 MHz, were utilized to demonstrate the concept of magnetically modulated release of drugs using MNP encapsulated delivery systems.[19, 32] A possible mechanism based on the creation of nanopores within a polymeric matrix through generation of heat by switching on/off of magnetic moments was proposed. The effect was also found to be physically reversible upon short-term field exposure and irreversible under long term exposure. [19, 33] Similar to MNPs investigated for hyperthermia treatment, majority of the investigations related to use of hyperthermia for controlled release have been utilizing iron oxide NPs. In a recent review by Brazel, [34] an attempt was made to analyze magnetothermally-triggered drug delivery systems. However, the scope of the article was limited to MNP encapsulated polymeric systems.
In this review, we present a case for broadening the meaning of the term “hyperthermia” by including therapy as well as magnetically modulated controlled drug delivery through heating. We review current literature on MNP-based hyperthermia for both thermotherapy as well as controlled drug release in order to provide a better understanding on the present status as well as future perspectives. The review also focuses for the first time on the application of core-shell type magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. For the purpose of this review, core-shell MNPs are those that have a core and a shell made up of different metals or oxides. Polymers or surfactant stabilized MNPs are not considered truly as core-shell MNPs under this definition. Since there are a large number of review articles covering synthesis of core-shell MNPs, especially those using layer-by-layer self-assembly approach, [35] we will only make references to their synthesis where the materials have been synthesized specifically for the purpose of hyperthermia-based treatment or controlled drug release. We will dwell on targeted hyperthermia and spatiotemporal drug delivery with potential opportunities for cancer treatment. We will also discuss how the core-shell MFH offers a unique possibility to create materials that combine the potential of magnetic properties with required hyperthermic and biological characteristics facilitating a safe and effective treatment. Finally, we hope to bring out a message that a combination of hyperthermia-based therapy and controlled drug release paradigms has potential to succeed as “intelligent theranostics” for successful application in personalized medicine.
2.0 MECHANISM OF MAGNETIC NANOMATERIALS-BASED HYPERTHERMIA: IMPLICATIONS FOR THERAPY AND CONTROLLED DRUG DELIVERY
The absorption efficiency of any material to generate heat due to AMF is measured in terms of specific absorption rate (SAR) or specific loss power (SLP). These terms are generally used to define the transformation of magnetic energy into heat. [36] For a majority of applications, it is desirable to have higher temperature enhancement rates and MNPs are proving to be far superior to micrometric particles due to their efficiency in conversion of magnetic energy into heat even at low concentrations.[37]
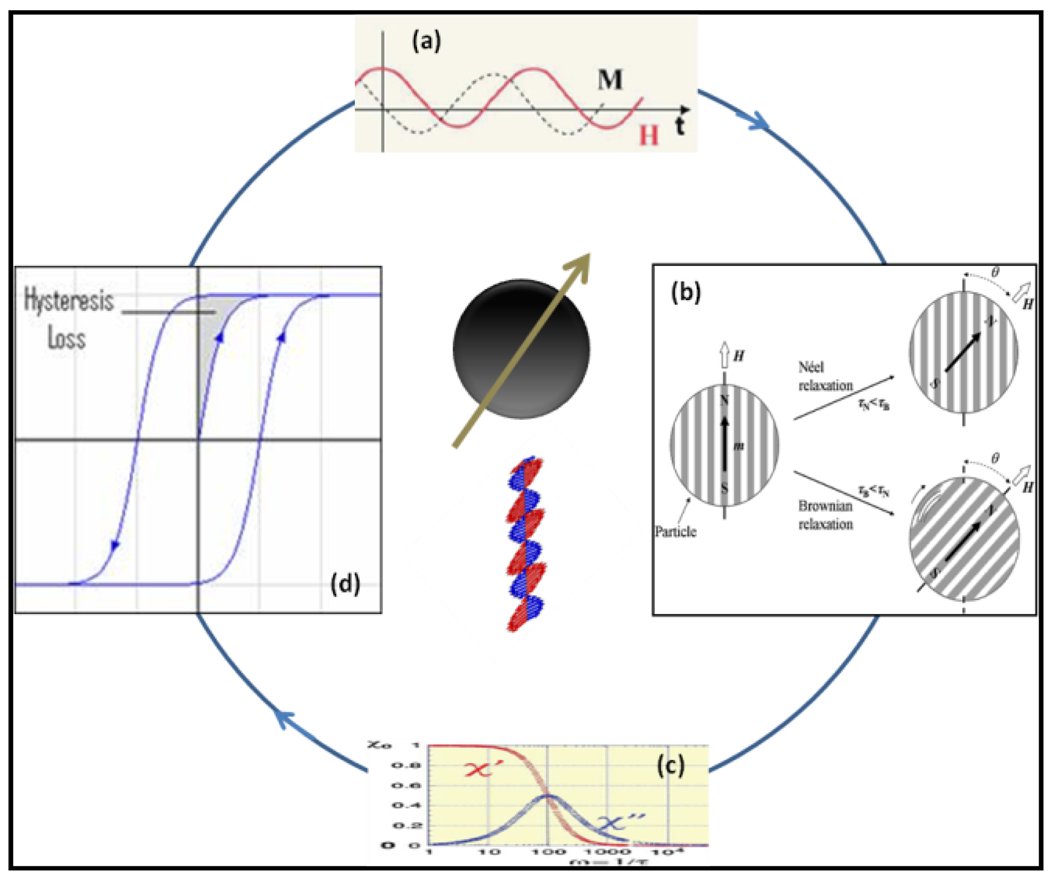
For MNP based hyperthermia, a general procedure involves distribution of particles throughout the targeted tumor site, followed by generation of heat to the tumor using an external AMF. The dynamic response of a dipole with its magnetic moment in a single direction due to an external AMF during the transformation of magnetic energy into heat is governed mainly by thermal fluctuations that occur in a particle. While there are a number of effects occurring in MNPs, the heat generation mechanism can be attributed to two different phenomena: relaxation and hysteresis loss (Figure-2). The relaxation is of two types: Néel and Brownian relaxation. Heat generation through Néel relaxation is due to rapidly occurring changes in the direction of magnetic moments relative to crystal lattice (internal dynamics). This is hindered by energy of anisotropy that tends to orient magnetic domain in a given direction relative to crystal lattice. Brownian relaxation is due to physical rotation of particles within a medium in which they are placed (external dynamics) and is hindered by the viscosity that tend to counter the movement of particles in the medium.[20]
Figure-2.
A schematic representation of possible mechanisms for conversion of magnetic energy into heat (Modified based on figure-13 from Ref 57)
The internal (Néel) and external (Brownian) sources of friction that lead to a phase lag between applied magnetic field and the direction of the magnetic moments tends to generate thermal losses. By using linear response models with known Néel and Brownian relaxation times, one can easily predict SPL values for MNPs. [38] One predominant heating mechanism that governs heating capacity of a given MNP is based on the induction of rapid variation of magnetic moments. In general, the SPL values increase with frequency of applied magnetic field and are proportional to the square of magnetic field intensity. SPL values are measured in terms of rise in temperature per unit time and per gram of magnetic material, multiplied by calorific capacity of a sample. Figure-2 shows that magnetization, M lags in phase behind applied field, H (a) and relaxation happens through two major pathways (b & d). One can also see that the real part, χ′ or the in-phase component and the imaginary part, χ″ or the loss component of the susceptibility is a function of frequency, v (c) and χ″ is maximum when the angular frequency ω(=2πv) = 1/τ where τ is the relaxation time. [57]
Generally SPL values depend on parameters such as MNPs structure (size), magnetic properties (magnetic anisotropy and temperature dependence of magnetizations) and amplitude (H) and frequency (f) of AMF. [39, 40, 41] Reduction in SPL values with polydispersity of MNPs is observed and this can be due to decrease in the proportion of particles contributing to total heat generation. The heating power of many MNPs also change with surrounding environment e.g. for those particles internalized within the cells. In such cases, the nuclear endosomes and/or other intracellular components generally hinder the movement of the particles resulting in total heat contribution largely coming only from Néel relaxation. [42] Therefore, for intracellular MFH, Néel relaxation is the major contributor for heat release. The most effective AMF parameters reported to date for hyperthermia applications, where the highest SLP was obtained, are a frequency of 500 kHz and at the field amplitude of 10 kA/m. [43]
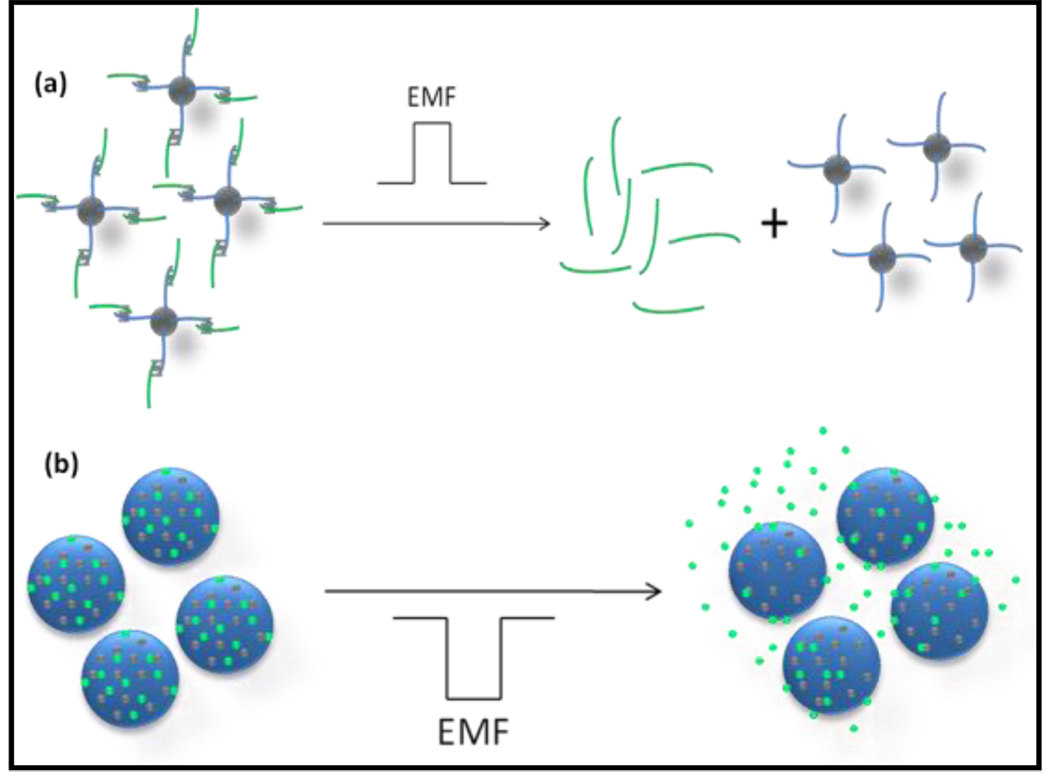
In the case of MNPs for hyperthermia–based controlled drug delivery, one can postulate two distinct types of mechanisms (Figure-3). In the first type, a drug molecule is attached to MNP through a linker and on application of an AMF, the drug molecule is released due to heating of the linker molecule attached to the NP’s surface.[44] By varying the power of electromagnetic field (EMF) pulses, it is possible to arrive at complex release profiles to release multiple drugs in series, or in combinations. This approach, represented schematically in figure-3a, can be termed as hyperthermia-based controlled drug delivery through bond breaking (DBB). In the second type, release of drugs takes place from within a polymeric matrix (nano/micro particles/thin films) encapsulated with MNPs on application of AMF/EMF (figure-3b).[19, 29] A possible mechanism for this type of hyperthermia-based drug delivery is formation of crevices or cracks of nanometer scale within a polymeric matrix due to local heat generated by the MNPs, thereby releasing encapsulated drugs. The second type of controlled drug release can be termed as hyperthermia-based controlled drug delivery through enhanced permeability (DEP). Depending on the nature of the polymer, hyperthermia-based drug release can be due to creation of a mechanically force openings or thermally responsive openings in the case of thermo responsive polymers. [34] The change in dimension of nanocrevices to a certain degree is physically reversible upon short-term field exposure. However, following long-term exposure, the nanocrevices further enlarge to nanometer-scale cracks that propagate along the spherical shell structure and ultimately form irreversible deformations. Having absorbed sufficient amounts of magnetic energy, the shell ruptures resulting in increase of both pore volume and surface area. As for the frequency dependency of permeability, the aggregates rotate or oscillate under the action of magnetic field with elastic deformation of capsule walls. At high frequencies, they will not follow variation of magnetic fields and the deformation will be minimal leading to a decrease in permeability. This is similar to the case of forced oscillation of a pendulum at frequencies above the resonance frequency.
Figure-3.
Schematic representation of the two types of controlled drug delivery using magnetic nanoparticle-based hyperthermia.
3.0 MAGNETIC NANOMATERIALS FOR HYPERTHERMIA-BASED THERAPY
A number of types of magnetic nanomaterials, ranging from well known and well investigated iron oxide-based nanomaterials to metallic NPs such as Mn, Fe, Co, Ni, Zn, Gd, Mg, and their oxides have been investigated for their hyperthermic potential. Some of the well known hyperthermic agents based on iron oxide are magnetite NPs (Fe3O4) stabilized by a variety of ligands such as dextran, [45] cationic liposomes, [46] polyvinyl alcohol, hydro-gel, [47] lauric acid [25, 48] and maghemite NPs (γ-Fe2O3) [47] stabilized by ligands such as dextran. [45] Yet another category is based on ferrites such as cobalt ferrites (CoFe2O4), manganese ferrite (MnFe2O4), nickel ferrite (NiFe2O4), lithium ferrite (Li0.5Fe2.5O4), mixed ferrites of nickel-zinc-copper (Ni0.65Zn0.35Cu0.1Fe1.9O4) and cobalt-nickel ferrite (CoxNi(1-x)Fe2O4). [25, 48, 49] There are also ferromagnetic NPs such as Fe doped Au, Zn-Mn doped iron oxides (ZnxMn(1-x)Fe3O4) and Mn-Zn-Gd doped iron oxide (MnxZnxGdxFe(2-x)O4) composites. [50] Very recently, extremely high heating performance of 1300–1600 W/g was reported based on FeCo metallic NPs. [51] However, iron oxide-based MNPs continue to attract attention due to their lack of toxicity, excellent biocompatibility, and in addition to the fact that they can be metabolized by heme oxygenase-1 to form blood hemoglobin and hence maintain iron cell homeostasis by cells. [52, 53] In addition, magnetite was found to be superior to cobalt NPs with respect to its high Curie temperature, saturation magnetization (Ms) (90–98 emu/g, or ~ 450–500 emu/cm3) and lower toxicity in preclinical tests.[25, 48] Deger et al tested a combination of hyperthermia with 3D conformal radiotherapy using Co-Pd thermo seeds for the treatment of prostate cancer and the intra-prostatic temperatures achieved were about 42–46°C with no side effects. [54] There are theoretical studies predicting that hyperthermia is dependent on particle size, size distribution, magnetic properties such as magnetic moment and magnetic anisotropy, and viscosity of fluid in which they are dispersed. [55] It is instructive to see how the influence of these features has been validated experimentally as well. A number of factors influencing the design of MNPs for optimizing their hyperthermic potential have been reported and these are reviewed as described below.
Size and size-distribution
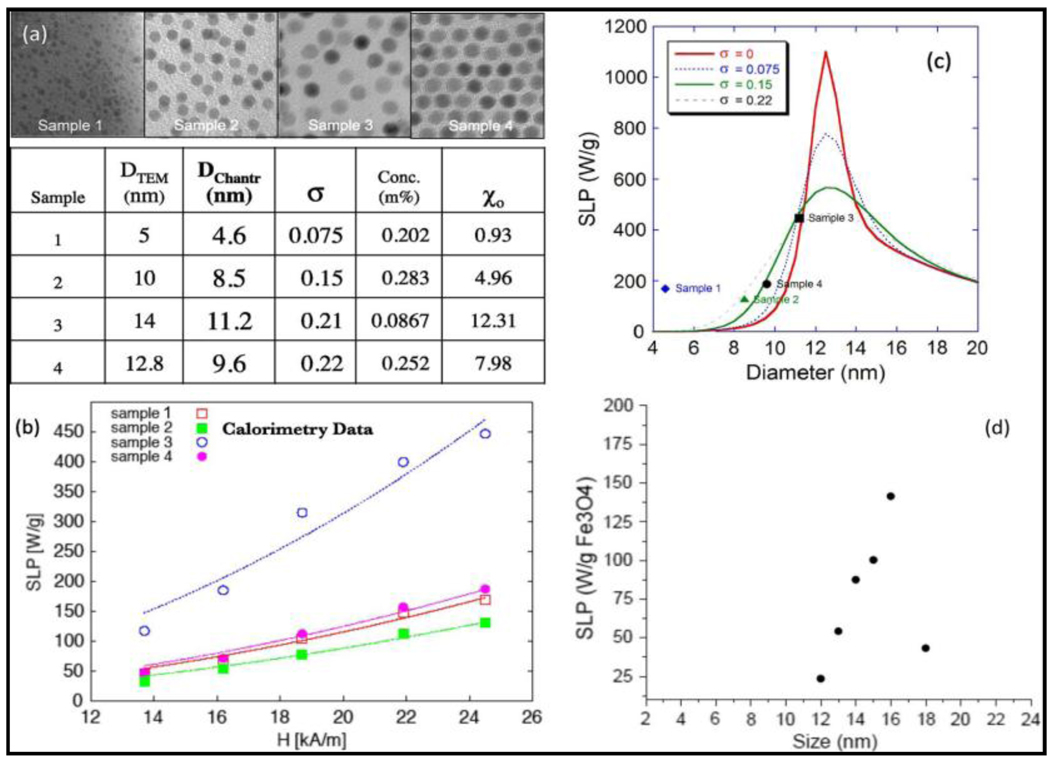
The heating power of MNPs is governed by magnetic energy dissipation, and therefore, is dependent on the size of MNPs. Size-dependent hyperthermia of highly crystalline and monodisperse iron oxide NPs showed that the heating rates with highest SLP of 447 W/g was achieved for 14 nm particle when particles in size range 5–15 nm were screened with a field amplitude of 24.5 kA/m and a frequency of 400 kHz. [56] Figure-4 shows the effect of size and size-distribution in magnetite nanocrystals of four different sizes. The size and size-distributions, shown in figure-4(a), were obtained based on TEM and from fittings of the magnetization curves. The variation in their SPL values on application of oscillating magnetic field of 400 kHz is shown in figure-4(b). It is interesting to see an agreement between experimental and theoretical values of SLP as a function of particle size and polydispersity indices (figure-4(c) and (d)).[57] On the other hand, at the same field amplitude of 24.5 kA/m but at a higher frequency of 700 kHz, maghemite crystals showed size-dependent SLP with the particle size of 16.5 nm having the highest SLP of 1650 W/g. In comparison, cobalt ferrite of 12 nm showed highest SLP of 420 W/g when sizes 6–15 nm were compared for their SLPs. Extending the size-dependent studies to larger particles (with sizes in the range 15–50 nm), the heating efficiency shows a maximum of 950 W/g for a 15 nm particle.[47] These results are once again in consistent agreement with both theoretical predictions as well as experimentally generated size-dependent magnetization values for highly crystalline and monodispersed iron oxide nanocrystals.[58]
Figure-4.
Size-dependent hyperthermia of MNPs (Reproduced from figure-14 in ref 57).
Overall, the size-dependent investigations reported to date clearly demonstrate that single domain iron oxide MNPs with monodispersed size of 14–16 nm are the most superior. However, what is surprising is the finding that bacterial magnetosomes consisting of ~30 nm γ-Fe2O3, unlike those synthesized in the laboratory, have the highest SLP value of 960 W/g (at 410 kHz and 10 kA/m) reported to date. [59] Yet another recent study [60] also showed high magnetic field induction heating of aqueous dispersions of meso-2,3-dimercaptosuccinic acid stabilized single-crystalline iron oxide NPs. In this case, SAR increased with increasing particle size until it peaked for particles with an average diameter of 10.5 nm and then decreased for particles with an average diameter of 12.1 nm. Theoretical calculations of the effect of particle size on the heat generation were in agreement with this trend as well. These studies also showed that there was a decrease in the contribution of Brownian relaxation to the heating, leading to a decrease in the SAR with larger particles. Extending size-dependent investigations to alloy NPs, one can predict that the heating potential of FePt and NiPd NPs is likely to change with their size. [38]
Carrier Viscosity
The heating rate in MFH is also dependent on carrier viscosity.[20] Surprisingly, not much work is reported on the influence of viscosity. In a recent publication, SLP of monodispersed particles of maghemite and cobalt ferrite in two different solvents, water and glycerol, was investigated. With field amplitude of 24.8 kA/m, it was demonstrated that SLP decreased with increase in viscosity with the effect being more pronounced with cobalt ferrite than with maghemite NPs.[40]
Magnetic Anisotropy
Yet another factor that is important for MNP-based hyperthermia is to increase the anisotropy of NPs (shape or magnetocrystalline) or increasing the applied magnetic field strength used for treatment. Even though high-quality MNPs with much higher anisotropies, such as Co and Co-based alloys are readily synthesized, their known toxicity prevents their utility in in vivo applications. [25, 61]
Stabilizing Ligand
In order to study the influence of stabilizing ligand on biocompatibility and cellular uptake in vitro, Pradhan et al used different coatings of dextran and lauric acid on Fe3O4 NPs. In their observation, there is a lesser biocompatibility and higher uptake of lauric acid-coated magnetite NPs in comparison to that of dextran-coated NPs in L929 mouse fibroblast cells. This is likely due to different cellular interactions caused by the coating material.[53] In a similar study by Jordan et al with coatings of dextran and silane on magnetite NPs, differential endocytosis occurred in terms of uptake due to the coating material. The study once again confirms that biocompatibility is dependent on the nature of the coating material and NP-cell interaction.[62]
Ability for tumor size reduction
While optimization of physical properties of MNPs to arrive at higher SLP values is important, it is also critical to evaluate their performance with respect to tumor size reduction. Recently, a number of in vivo studies using animal models were undertaken where MNPs were injected into a tumor followed by AMF exposure and resulting changes in the tissue and organs were investigated. These include C3H mouse mammary carcinoma [63], mouse EL4 T-lymphoma [11], MX11 mouse sarcoma [64], human prostate cancerous and bone marrow cells in transgenic mice [46], EL4 mouse T-lymphoma [65], T-9 rat glioma cells [66], B16 mouse melanoma [67], MM46 mammary and skin carcinoma [68], murine B16-F10 mouse melanoma [69], cat mammary tumor gland [70], Os515 hamster osteosarcoma [71], VX-7 squamous cell carcinoma in rabbit tongue [72], DMBA induced rat mammary carcinoma [73], and human glioma cells. [74] Most of the studies though provided positive evidence for decrease in tumor size due to hyperthermia, there is a clear lack of complete information on host and material response. Host response corresponds to influence of living cells on the magnetic nanomaterial and vice versa for magnetic nanomaterial’s response on cells. [75] Also, the effect of MNP uptake by healthy cells and their fate before and after the application of oscillating magnetic field is not clear. A better understanding of MNPs and their interactions with tissue, and organs are of greatest importance for optimizing hyperthermia treatment in clinical setting.
Self-regulated hyperthermia
A highly desirable form of hyperthermia is one that is self-regulated. Self-regulation is possible with MNPs that have Curie temperature at around therapeutic temperature range. With such particles, similar to traditional hyperthermia, they are heated when AC field is applied. However, once they reach the Curie temperature, the Ms of particles drops to zero, and heating stops. Thus, if the Curie temperature can be fixed by a judicious selection of particle composition and size, hyperthermia can be applied and carefully controlled; preventing from reaching excessive temperatures. Some of the obvious choice of materials for such a self-regulated therapy includes Ni-Cu alloys, Mn-Cu ferrites, Ni-Pd alloys and Co-Pd alloys. Recent studies on polymer encapsulated Cu-Ni NPs, [76] Gd-substituted Mn-Zn ferrite NPs, [77] Fe1-xMnxFe2O4 NPs, [78] and FePt, NiPt, NiPd [79] NPs are very promising in this direction. The Curie temperature of these alloy systems can be tuned by changing their composition especially since Fe27Pt73 and Ni28Pd72 alloys have Curie temperature near 45 °C.[79] The Curie temperature is also expected to change with size and certainly with composition of MNPs. The methods to make FePt and NiPd NPs of different sizes (2–9nm) are already available. [79–82] Therefore, by extending the known compositional dependence of Curie temperature (TC) for the face centered cube (fcc)-phase at high Ni concentrations, one can expect metastable FeNi alloys to have low TC’s in the Fe-rich region of the phase diagram. Thus making them suitable for self-regulated radio frequency (Rf) heating in cancer hyperthermia. [83]
Multi-functionality
Demonstrating potential opportunities that exist for combining hyperthermia with diagnosis, a multifunctional NP (< 50 nm) with a Fe2O3 core and labeled with a targeting agent luteinizing hormone releasing hormone (LHRH) was designed. The multi-functionality of the nanoparticle ensured specific targeting to cancer cells of breast and prostate in addition to having a two-photon fluorescent probe to aid in optical tracking.[84] Such a multifunctional NP, termed as “nanoclinics” was used for magnetocytolysis of MCF-7 and UCI cancer cells using a DC magnetic field in addition to providing optical imaging capability. Since the magnetocytolosys was performed using a 7 Tesla magnetic field NMR instrument, it is not clear what the frequencies of oscillating magnetic field are. In any case, this investigation is a step in the right direction to bring multifunctionality to traditional MFH.
4.0 MAGNETIC NANOMATERIALS FOR HYPERTHERMIA-BASED CONTROLLED DRUG DELIVERY
Drug release from the surface of NPs was previously accomplished by external stimuli such as electric current, magnetic fields, temperature, light, and ultrasound and so on. In particular, the concept of using external magnetic fields to achieve pulsatile release from polymer composites was first demonstrated using millimeter size magnetic particles. In this investigation, an externally controlled on-demand insulin release was demonstrated from a magnetic composite of ethylene vinylacetate by application of low frequency oscillating magnetic field. [29] This concept did not gain attention till recently where modulation of permeability of polyelectrolyte microcapsule embedded with MNPs, fabricated using layer-by-layer self-assembly, was first demonstrated. [19] Since then there have been a number of reports in the literature related to application of magnetic nanomaterials for hyperthermia-based controlled drug delivery. These can be classified into two major types.
Hyperthermia-based controlled drug delivery through Bond Breaking (DBB)
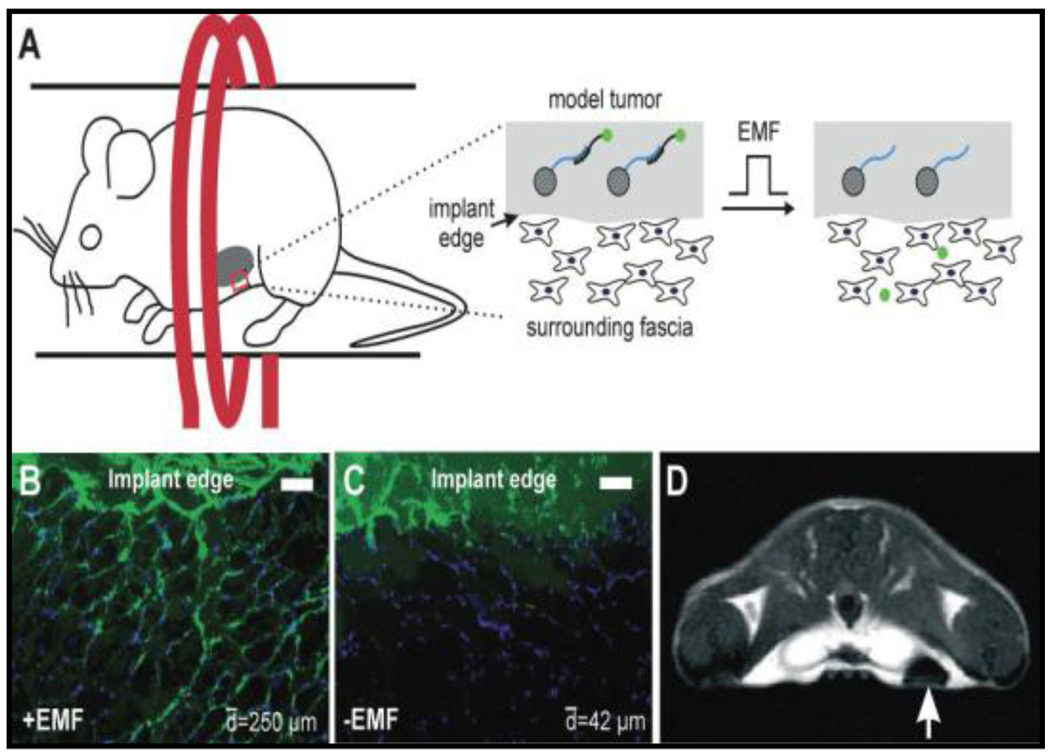
The first successful demonstration of this concept was reported using radiofrequency EMF activation of release of fluorescein-labeled 18 bp in a model tumor near the posterior mammary fat pad of mice (Figure-5). [44] As shown in the figure, first MNP bound biomolecules through a heat labile linker are mixed with matrigel and injected subcutaneously near the posterior mammary fat pad of mice, forming a model tumor (A). On application of EMF, the fluorescent biomolecules were released into surrounding tissue (B) when compared to unexposed controls (C, scale bar = 100 micrometers). On further examination of the mice using a 7 T MRI scanner, one can see an image contrast due to the presence of NPs (arrow). This magnetically stimulated platform for controlled drug release demonstrates ability to remotely trigger release of a biomolecule, if necessary in a sequence, from the surface of MNPs. Yet another report [85] confirms similar concept by triggering release of fluorophore bimane amine from the surface of superparamagnetic iron oxide nanoparticles (SPIONs) in presence of oscillating magnetic fields.
Figure-5.
Demonstration of the concept of DBB in vivo (Reproduced from reference 44).
Hyperthermia-based controlled Drug delivery through Enhanced Permeability (DEP)
Unlike the concept of DBB, hyperthermia-based controlled drug delivery through enhanced permeability (DEP) involves release of drug from within a polymeric nanoparticle (PNP) wherein MNPs and drug are encapsulated. DEP has been found to be reversible under certain conditions and is dependent on frequency of the oscillating magnetic field and the applied magnetic field strength. Such a release was demonstrated from polymers, hydro gels and thermo responsive polymers.
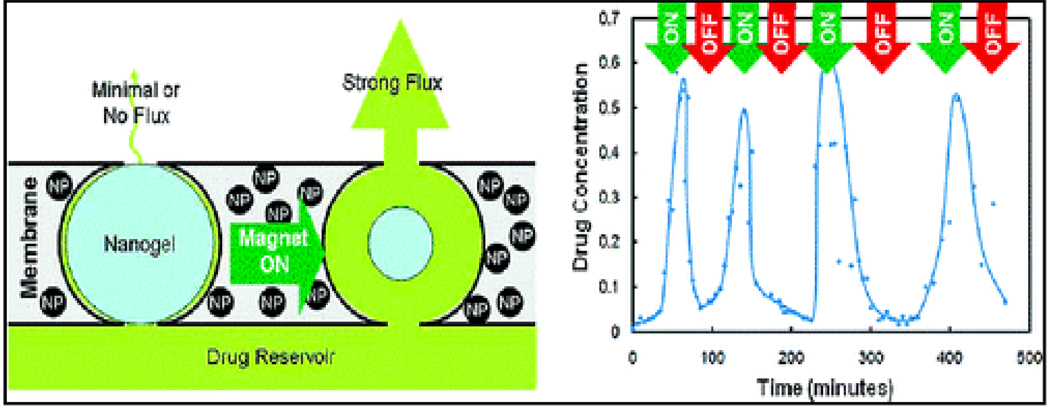
Remotely controlled pulsatile drug release for a number of different drugs as well as for different “on– off” durations of oscillating magnetic fields was demonstrated from temperature sensitive poly (N-isopropylacrylamide) hydrogels incorporated with SPIONs. [86] Other hydrogel-based DEP systems are poly(N-isopropylacrylamide) (PNIPAM) microgel containing MNPs with the ability to tune magnetic and thermoresponsive properties of individual components (NPs and microgels). [87] Extending this concept further, an approach to the development of artificial membranes with stimulus-responsive opening was recently demonstrated.[88] On-demand “on-off” release of sodium fluorescein over multiple magnetic cycles was successfully investigated using prototype nanocomposite membranes based on thermosensitive, poly(N-isopropylacrylamide)-based nanogels and magnetite NPs upon the application of an oscillating magnetic field. The concept is schematically represented in Figure-6. The figure shows magnetic triggering and differential flux of sodium fluorescein out of membrane-capped devices as a function of time over successive on/off cycles of the external magnetic field. Similarly, a novel magneto-active gel based on iron oxide NPs within a temperature sensitive PNIPAM was used as an externally tunable flow controller inside a micro fluidic channel. [89] This opened up avenues for the development of devices such as magnetic micro/nano pumps, magnetic field controlled drug delivery devices and magnetic switches. Such controlled release was also reported from magnetic lipid NPs. [90]
Figure-6.
Stimulus-responsive membrane triggering in vitro (Reproduced from reference 88)
In addition to relatively fluidic polymeric gels, the concept of DEP was also demonstrated in high density organic and inorganic polymeric particles incorporated with MNPs. Examples using organic polymer particles include those made from poly-lactic acid (PLA)[91], poly(ethylene glycol) ethyl ether methacrylate-copoly(ethylene glycol) methyl ether methacrylate[92], Pluronic F127 (F127)[93], poly(methylmethacrylate) (PMMA)[94], poly-n-isopropylacrylamide (PNIPAM)[95, 96], poly(ethyleneimine)-modified poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) (PEO-PPO-PEO) block copolymer[97] and nanoparticle-assembled capsules (NACs) using Poly(allylamine hydrochloride), disodium phosphate, and citrate bound MNPs.[98] An example where the behavior of drug release is found to be significantly different depending on whether the applied field varies sinusoidally or in a step-function manner with time was reported using iron oxide core-chitosan shell microspheres. [99] In yet another example, inorganic polymer NPs such as those made from silica are utilized. In this case, zinc-doped iron oxide nanocrystals are encapsulated within a mesoporous silica framework that was surface-modified with pseudorotaxanes.[100] In addition to magnetic-polymer particles, magnetic liposomes [101, 102] are also suitable as DEP-type drug delivery systems.
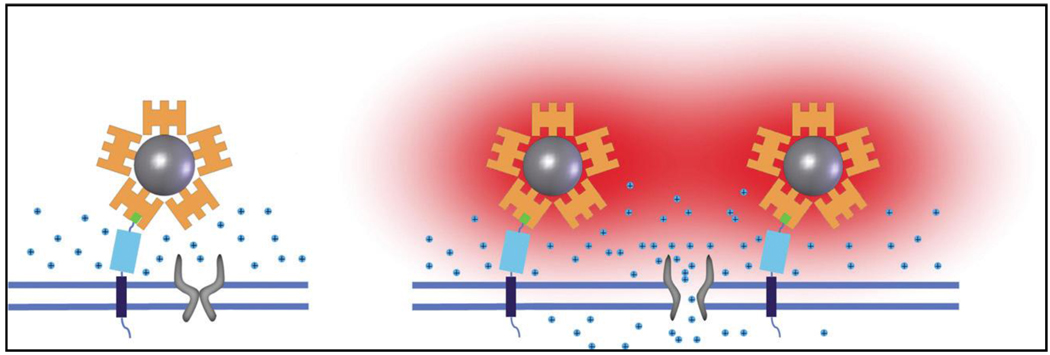
Extending the concept of DEP, radio-frequency magnetic-field heating of NPs was used to remotely activate temperature-sensitive cation channels within cells. [103] In this example, superparamagnetic ferrite NPs were targeted to specific proteins on the plasma membrane of cells expressing TRPV1 (also known as Capsaicin receptor), and heated by a radiofrequency magnetic field. High localized induction of temperature increase was noted using fluorophores as molecular thermometers leading to the activation of opening of TRPV1. As shown in Figure-7, streptavidin–DyLight549 (orange)-coated superparamagnetic nanoparticles (grey) were prepared and the AP-CFP-TM protein was bound to the NPs through biotinylated AP domain (green box), which is anchored to the membrane by the TM (blue box) and CFP (cyan box) domains. On the application of oscillating magnetic field, the NPs are locally heated resulting in heat (red)-induced opening of TRPV1. Novelty of this approach is that it can be generalized to stimulate other cell types in addition to the possibility to remotely manipulate other cellular machinery for novel therapeutics. It is noteworthy that use of similar inductive heating effects were also utilized for controlled release of drugs by switching the high frequency magnetic fields (HFMF) on and off from folic acid (FA) and β-cyclodextrin (CD)-functionalized SPIONs.[104] Yet another variation of the concept is reported where micro containers with embedded superparamagnetic nanoparticles can be changed into bubble micro reactors upon exposure to AMF, which acts as a remote trigger for release of encapsulated material.[105]
Figure-7.
A schematic representation of the principles of ion channel stimulation using nanoparticle heating and local temperature sensing (Reproduced from reference 103).
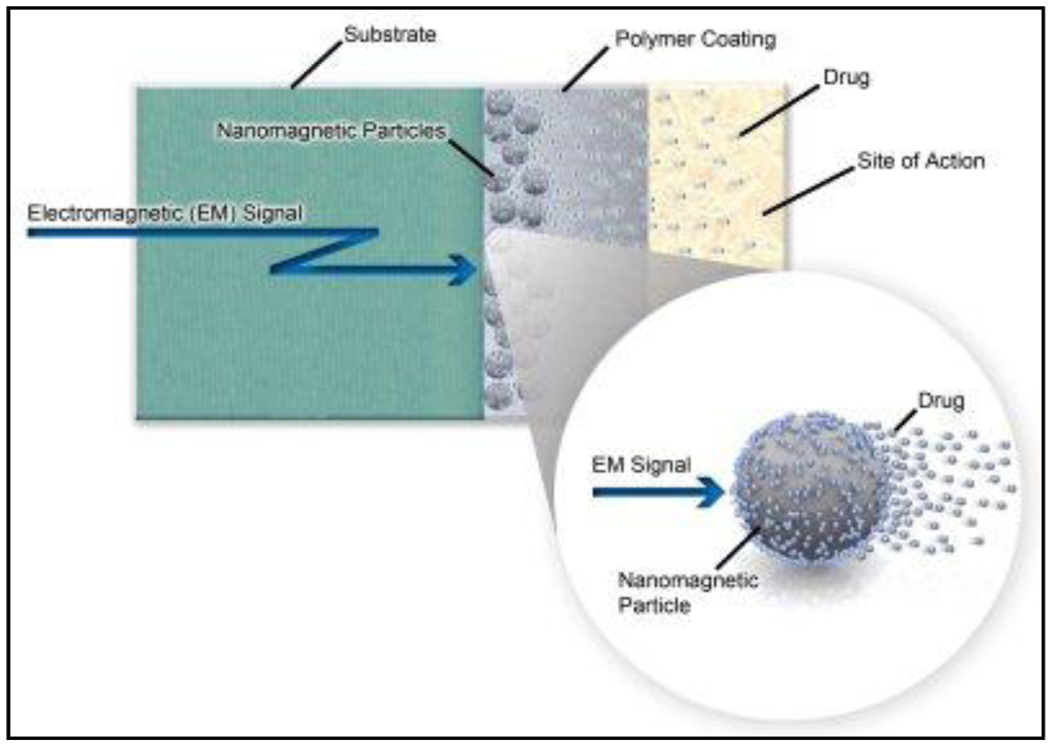
Finally, an important question is if there are potential opportunities for translational research leading to commercialization of products based on the concept of hyperthermia-based controlled drug delivery. In this context, it is worth mentioning the efforts from the company, Biophan technologies Inc (http://www.biophan.com/). The company is developing “active drug-elution technology” where drug loaded MNPs are encapsulated in a polymer coating on a stent or other medical devices from which the drugs can later be selectively released by applying a controlled EMF at a specific frequency (Figure-8). Interestingly, by fine tuning the magnetic properties of MNPs, there is an opportunity for using different MNPs to respond selectively to a wide range of frequencies of oscillating magnetic fields in order to release multiple drugs temporally to treat various complications.
Figure-8.
A schematic representation of “Active drug-elution technology.” (Reproduced from the web site http://www.biophan.com)
5.0 CORE-SHELL MAGNETIC NANOMATERIALS FOR HYPERTHERMIA-BASED THERAPY AND CONTROLLED DRUG DELIVERY
5.1 Why core-shell magnetic nanoparticles?
As the properties of nanomaterials are surface-dependent, there are a number of ways one can bring about surface changes leading to concomitant changes in the properties. With the unraveling of their chemical, physical and biological properties, nanomaterials of different sizes and shapes are being investigated and recent review articles and book chapters cover various aspects of these nanomaterials. [106–115] In addition to the size and shape effects, newer design of nanomaterials, most often synthesized using layer-by-layer approach, have also begun gaining prominence providing further opportunities to tailor properties of nanomaterials and investigate their fundamental behavior. Some of these new designs are core-shell, onion-type, multilayered, alloy, multimetallic and multifunctional nanomaterials (Figure-9). Of these, the layer-by-layer approach for the synthesis of core-shell architecture offers a great opportunity to modulate properties of both cores as well as shells. Not surprisingly, this approach has lead to the development of a variety of core-shell nanomaterials and continues to be the focus of scientific efforts.
Figure-9.
Different architectures of nanomaterials.
Core-shell nanomaterials are extremely important as they can have a combination of different properties and offer multifunctionality because of core and shell can have different material compositions in a single particle. They represent a novel class of hybrid materials, where composition and microstructure varies in the radial direction. [116] A number of possibilities and opportunities can be envisaged using this architecture. A shell can be utilized as a protective shield for sensitive core material, for example, in the case of air sensitive magnetic nanomaterials like Cobalt. [117, 118] The dimensions and composition of shell can be modulated in order to influence the properties of a core and similarly the core dimensions can be modulated to influence the properties of a shell. [119] The shell surface can also be utilized for bio-functionalization; which otherwise either difficult or not possible on a core surface, for example, gold or silica coated ferromagnetic nanomaterials. [117, 118, 120] Having a biocompatible shell around toxic core material enables reduction in their toxicity, for example, coated quantum dots[121] or cobalt NPs.[122] Expensive nanomaterials can be formed as shells around inexpensive core nanomaterials. This is particularly useful in catalysis. [123] Finally, multiple functions such as detection and treatment modalities or multiple treatment options can be incorporated in a single core-shell particle.[124] All these different possibilities are reflected in enhancing the properties of core-shell nanomaterials in a number of applications and especially in the case of MNPs and more so for utilization of MFH for therapy and controlled drug release. MagForce Nanotechnologies AG, Germany is the only company that is utilizing iron oxide NPs coated with aminosilane for hyperthermia treatment of tumors and the technology is at an advanced stage of clinical trials.[125] On the other hand utilization of MNP-based hyperthermia for controlled release of drugs is relatively new and to the best of our knowledge no clinical trials have been started. Thus, there is a need for designing better and more effective hyperthermic agents and core-shell architecture presents one such opportunity. Similarly, application of core-shell MFH is relatively new and here is the first attempt to review the investigations carried out to date.
5.2 Core-shell magnetic nanomaterials for hyperthermia-based therapy
Core-shell MNPs offer a number of advantages over mono metallic/metal oxide NPs for hyperthermia-based therapy. Some of the examples from literature demonstrating their potential advantages are given below.
Prevent anisotropic magnetic dipolar attraction
Anisotropic magnetic dipolar attraction prevents stability of magnetic fluids even in the absence of an external magnetic field. This continues to be one of the main problems when dealing with magnetic fluids. One approach to successfully prevent this attraction is by providing a coating around magnetic particles so that they behave as isotropic dispersions in a zero magnetic field and reversibly form anisotropic structures in the presence of an external magnetic field. [126] This approach was demonstrated recently in silica coated maghemite NPs; resulting in a more stable magnetic fluid. [127]
Provide Oxidative stability
A shell around magnetic core offers oxidative stability for air sensitive magnetic nanomaterials. This is especially important for high magnetic moment particles which can provide high values of SLP or SPL (Specific power loss). For example, body centered cubic (bcc) CoFe alloys have the highest Ms (~240 emu/g) in this class of materials and could be an ideal magnetic material for hyperthermia applications. However, their chemical instability makes their synthesis very challenging. [128] A graphite shell around these particles led to their oxidative stability without loss of their original high magnetic moment. [129]
Enhanced hyperthermia
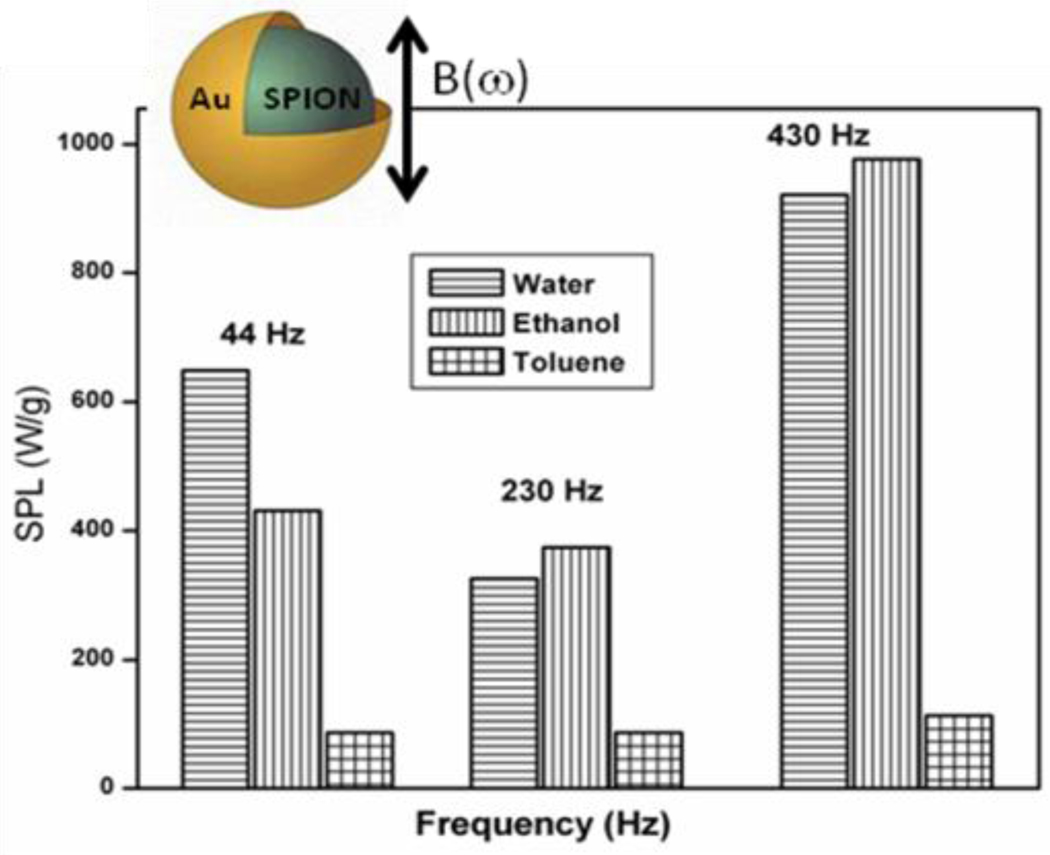
Kim et al achieved close to 90% cancer cell destruction in vitro using FeNi magnetic-vortex microdiscs (MDs) of 60 nm size coated with a 5 nm thick gold layer each side by the application of only a few tens of hertz AMF for just ten minutes. [130] This confirms that operation of MFH at lower frequencies for effective heat generation can be achieved using core-shell type of structures. Likewise, in yet another demonstration, a gold coating of approximately 0.4 to 0.5 nm thickness around SPIONs resulted in a four- to five-fold increase in the amount of heat released (the highest value of 976 W/g in ethanol at 430 Hz frequency) in comparison with SPIONs on application of low frequency oscillating magnetic fields (44–430 Hz) (Figure-10). [131] In addition, the SPIONs@Au were found to be not particularly cytotoxic to mammalian cells (MCF-7 breast carcinoma cells and H9c2 cardiomyoblasts) in in vitro studies. When similar heating experiments were carried out using stable water suspensions of La0.75Sr0.25MnO3 cores covered by silica (Conc. of Mn=3.39 mg/ml), highest SAR of 130 W/g Mn at 37 °C was reached for the applied amplitude and frequency of 8.7 kAm−1, 480 kHz respectively. [132]
Figure-10.
Solvent dependent SPL values for SPIONs@Au (Reproduced from the reference 131)
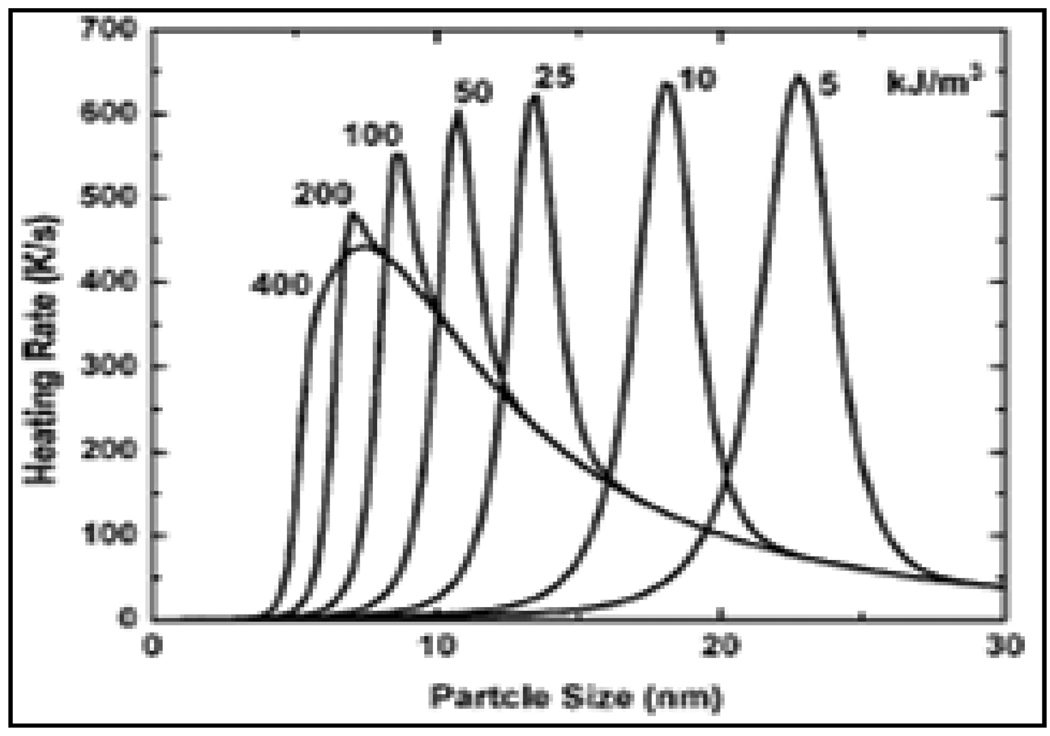
There are potential opportunities to extend these investigations further in order to enhance hyperthermia through manipulation of magnetic properties. For example, a tunable shell of 0.5 to 3 nm of Fe3O4 on FePt NPs on annealing resulted in enhancing the core Ms values to 1040 emu/cc. [133] Novel core-shell anisotropic structures such as Ni@Co nanorods with Ni nanowire as core and Co nanotube as shell were recently synthesized demonstrating the potential to fine tune the magnetic anisotropy to further optimize hyperthermic potential. [134] Finally, here is an interesting example [135] where the heating rates for dispersion of FeCo MNPs with different anisotropy values are shown in Figure-11. For increasing anisotropy, the particle size at which the maximum heating rate occurs decreases, while the peak heating rate itself is not significantly affected. Above a certain value, increase in anisotropy gives no significant change in the heating rate dependency on particle size since Néel relaxation ceases to occur. The remaining Brownian relaxation is independent of material’s parameters and only depends on the particle size. Hence, one can expect to achieve high heating rate at desirable sizes for core-shell MNPs.
Figure-11.
Variation in heating rates for FeCo MNPs with change in anisotropy constant (Reproduced from the reference 135).
Reduce toxicity and increase biocompatibility
With an aim to evaluate potential cellular perturbations and explore the relationship between biocompatibility and surface chemistry of carbon coated iron NPs (Fe@CNPs), Mu et al investigated their dynamic cellular responses, uptake, oxidative stress and effects on cellular apoptosis, and cell cycle. Results indicate that biocompatibility of Fe@CNPs is dependent on both cell type and NP’s surface chemistry. [136] Similarly, magnetic manganese oxide NPs when coated with a silica shell resulted in achieving better water stability at high concentrations and superior biocompatibility. The application of AMF of 15 mT and 100 kHz for 30 min. produced cellular damage that finally lead to apoptotic cell death; even though the temperature increase in the cell culture was lower than 0.5 °C. [137]
5.3 Core-shell magnetic nanomaterials for hyperthermia-based controlled drug delivery
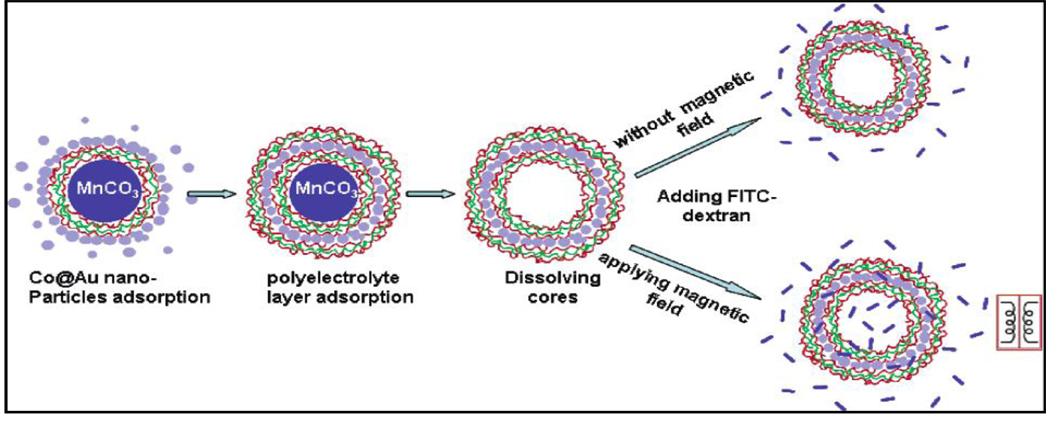
The approach of hyperthermia-based controlled drug delivery using nanomaterials is relatively new and more so with core-shell magnetic nanomaterials. However, there are recent examples from the literature which demonstrate unique opportunities for core-shell MNPs to play a significant role. The very first example was reported in 2005 where investigations using a magnetic field to modulate the permeability of polyelectrolyte microcapsules containing core-shell MNPs were initiated.[19] In these investigations, ferromagnetic gold-coated cobalt (Co@Au) NPs (3 nm) were embedded inside the polymeric capsule walls using layer-by-layer self-assembly technique. The final 5 µm diameter microcapsules had wall structures consisting of 4 bilayers of poly (sodium styrene sulfonate)/poly(allylamine hydrochloride) (PSS/PAH), 1 layer of Co@Au, and 5 bilayers of PSS/PAH. Oscillating magnetic fields of 100–300 Hz at field strength of 1200 Oe were applied resulting in distortion of the capsule wall and drastically increased its permeability to macromolecules like FITC-labeled dextran (Figure-12). The capsule permeability change was estimated by taking the capsule interior and exterior fluorescent intensity ratio using confocal laser scanning microscopy. Capsules with 1 layer of Co@Au NPs and 10 polyelectrolyte bilayers were found to be optimal for magnetically controlling permeability. This example not only demonstrate the importance of layer-by-layer self-assembly approach to fabrication of required core-shell nanomaterials for hyperthermia-based drug delivery, but also strongly supports the unique feature this self-assembly process provides in controlling core and shell dimensions.
Figure-12.
Scheme of the layer-by-layer sel-assembly and permeability test for microcapsules embedded with Co@Au NPs under an oscillating magnetic field (Reproduced from the reference 19).
Other examples from the literature are as follows. Iron core silica shell (Fe@SiO2) NPs of about 50 nm were recently synthesized by an in situ process, and on a short exposure to a high frequency magnetic fields (HFMF) they were able to release specific amounts of drug in a burst manner. [32] The HFMF accelerated the rotation of MNPs deposited in the silica matrix; subsequently enlarging the nanostructure of the silica matrix to produce porous channels thereby releasing the drug easily. Learning from such magnetically responsive controllable drug release systems, one can also design similar magnetic silica nanospheres for controlled burst release of therapeutic agents especially for urgent physiological needs. Interestingly a novel core-shell structure where the drug is encapsulated within a silica core surrounded by single-crystalline iron oxide shell was also reported. [33] This design is conceptually interesting as it can protect biomolecules encapsulated in the core from damage from harsh environments. It can also eliminate uncontrollable release resulting from natural diffusion of molecules upon delivery, for example, in a patient’s body. In addition, the magnetic nanoshell displays an ultrafast response and sensitivity upon exposure to HFMF. What is important is that the high magnetic-sensitivity of the nanometer-scale shell allows a controlled burst release of drug in a quantitative manner. Yet another core-shell structure, magnetic organic-inorganic nanohybrid, involving drug-intercalated layered double hydroxides coated on a magnesium ferrite core, was reported for magnetically controlled drug release. [138]
With ever increasing ability to fabricate fascinating types of core-shell NPs, the exploration of hyperthermia-based controlled drug delivery systems using core-shell magnetic nanomaterials is poised to take off. For example, the so-called “yolk-shell” structured materials with movable cores and porous shells [139] could exhibit unique releasing properties for drug/gene delivery because of their hierarchical porous structures. Further, there is also a possibility to expand the field into the arena of implantable microchips as demonstrated recently. In this particular study, drug-carrying magnetic core-shell SPION@SiO2 NPs were deposited onto an electrically conductive flexible PET substrate. A flexible drug delivery chip was made using such substrates for magnetically-controlled release of anti-epileptic drugs such as ethosuximide (ESM). [96]
6.0 CURRENT CHALLENGES AND OPPORTUNITIES IN MNP-BASED HYPERTHERMIA FOR THERAPY AND CONTROLLED DRUG DELIVERY
Taking into consideration more recent experimental results, a critical examination reveals some of the major challenges and opportunities that MNPs offer for thermotherapy and controlled drug delivery. These are given below.
Maximizing Specific Absorption Rate (SAR)
The most challenging task is to maximize the SAR in hyperthermia, which allows reduction of ferrofluid dose in vivo. The dependence of SAR on parameters like magnetization, size, and size-distribution of particles, magnetic-field strength, and frequency of the AC field were previously discussed. [78] However, what is clearly needed is a concerted effort to examine all the variables together and come to a comprehensive and conclusive understanding leading to maximization of SAR. This is also critical to ensure that maximum SAR is obtained intracellular and at relatively lower dosages.
Tailoring properties of magnetic nanomaterials
Numerous MNP-based alloys of Fe and Co can be readily synthesized with superior magnetic properties. However, most of these are not biocompatible and, typically, they are not suitable for in vivo applications. One strategy would be to take advantage of their superior magnetic properties and enhanced hyperthermia concentrations low enough to be non toxic. Alternatively, a core shell strategy to mitigate their toxicity needs to be explored. Yet another option could be to take a well-known magnetic material, e.g., magnetite, which has already been approved for human use and optimize its size, composition and shape-dependent magnetic characteristics. After suitably functionalizing its surface and, following appropriate cytotoxicity and pharmacokinetics studies, it stands a very high chance of being readily used in vivo. However, even though shape anisotropy is known to play an important role in magnetism, very little work has been done in utilizing this approach to tailor magnetic nanomaterials for hyperthermia. In the case of hyperthermia-based controlled drug delivery, not many types of MNPs have been investigated to date. The field is relatively new and there are a number of opportunities to fine tune the properties of MNPs in order to optimize controlled drug delivery through both DBB and DEP mechanisms.
Eliminating or minimizing toxicity from NPs
Though most of hyperthermia studies report cell death due to heat release, there is clearly a lack of sophisticated tools to determine the information about the fate of cells before and after MNPs are used for hyperthermia. This is mainly because most of the currently used molecular biology assays measure only the changes occurring in short intervals of time and not much information is available about the NPs after their use. The change due to hysteresis and relaxation losses in the biological fluids, concentration of material, frequencies applied and cell death are poorly explained. A better understanding between NP’s physical properties and their influence on cell’s integrity needs further optimization. What is clearly missing in the literature is the confirmation that MNP based hyperthermia results cell death only due to hyperthermia and not due to inherent toxicity of some of the MNPs. [75] Since a number of magnetic nanomaterials investigated for hyperthermia-based therapy are extremely large in comparison with those investigated for hyperthermia-based drug delivery, there is a great opportunity to design better magnetic nanomaterials with minimum or no toxicity for drug delivery applications.
Biocompatibility, Catabolism and Clearance issues
The unusual properties of a number of MNPs, including their multivalency and multifunctionality, pose challenge for understanding their pharmacokinetics because different components will have different features that affect their distribution, clearance and catabolism. A careful examination of each of the components as well as combination of them is required in order to arrive at reliable pharmacokinetics. Such studies are crucial, especially when designing materials that have sophisticated control systems, irrespective of their application either for heat treatment or drug delivery.
Induce and sustain therapeutic temperatures
In practice, it is technically challenging to induce and sustain temperatures clearly above the systemic temperature of 37.5 °C in a defined target volume. Perfusion counteracts the temperature rise and perfusion rates vary widely in tumors with leaky vasculatures. Therefore, reaching therapeutic temperatures of 42–44 °C in the critical parts of tumors requires a specific heating power (SHP) in local target regions. [140] The cooling action of flowing blood must also be taken into consideration, and furthermore, blood flow rates will vary during hyperthermia treatment. Taken together, these effects invariably result in non-uniform temperature distributions. Adding to this complexity is the well known fact that in some tumors, the blood flow may altogether stop completely during extended periods of hyperthermia treatment.[141] Thermal convection, caused by strong blood perfusion, decreases the specific energy absorption and hence reduces the temperature of the tumor. Therefore, tumors located in regions with high perfusion such as those within liver, lung, and kidney receive lower heat dose, and hence decrease in hyperthermia efficiency. In such cases, it is not possible to maintain therapeutic temperatures for long periods of time and hence, they are not treated by the methods of regional hyperthermia alone. In these cases, a combination of regional hyperthermia with X-ray therapy and chemotherapy strengthens the anti-tumor effect and frequently results in desired remission. Therefore, regional hyperthermia is widely used in combination with X-ray therapy and chemotherapy. On the other hand it is interesting to note that interface effects are the reason why cancers of the brain are treated now only with application of regional hyperthermia, including cranial trepanation.
Finally, the principal remaining problems with MFH are invasiveness, targeting (restricting the hyperthermia effect to a specific area of interest) and achieving homogenous heat distributions within the target organ.[4, 8, 54, 142] Failure to solve these problems may lead to either insufficient treatment effects or, worse, lethal exposure to neighboring healthy cells.
Monitoring temperature distribution during heating
This is essential but remains an ongoing challenge. There is a lack of systematic studies on dose and thermal response relationship especially at targeted sites. Currently, in regional hyperthermia systems, the temperature is monitored with the use of invasive thermometry where thermo sensors are delivered through catheters implanted surgically or hypodermically. A specific challenge is to develop noninvasive methods for monitoring of local temperatures. To accomplish this, a two pronged approach that is based on modeling studies and on designing appropriate thermo sensitive materials is essential. In addition to optimizing the physics of heating, further developments in realistic heating models taking into consideration perfusion as well as including systematic studies with phantoms are required. Estimates of the amount of heat that will be required and knowledge of the temperature distribution within the tumor and the healthy tissue will be important. There are already some efforts in this direction. Recently, a model was developed based on two finite concentric spherical regions; the inner sphere of cancerous tissue with magnetic particles, and the outer sphere containing healthy tissue. [143] Penne’s bioheat transfer equation [144] describes the temperature both in the diseased as well as healthy tissue as three different functions of heat; heat generation by MNPs, the heat conducted through the tissue, and the heat removed by blood perfusion. The model, applied successfully to self-regulated hyperthermia, showed that proper distribution of magnetic particles throughout the tumor is the key to minimize any damage to the surrounding healthy tissue while still maintaining a therapeutic temperature within the tumor. [145] The model can also be used to find ways in applying the magnetic field to minimize damage to healthy tissues and the patient’s exposure to the field. Future computer simulations could assist doctors in determining how, when and where to place the magnetic fluids. Precise treatments would increase the likelihood of eliminating the tumor while decreasing the side effects of heating healthy tissue.
In terms of designing thermo sensitive materials for monitoring heat distribution, there are some recent efforts worth mentioning. For example, Herrera et al [146] coated MNPs with a temperature-responsive fluorescent polymer built from N-isopropylacrylamide (NIPAM) and a modified acrylamide. By observing the changes in the fluorescence of the polymer using a spectrofluorometer, temperature of the medium surrounding the NPs can be monitored non-invasively. Another approach is to use infrared thermacam, which is insensitive to AC magnetic fields but sensitive to measure thermal gradients during magnetic heating. [147]
In the case of hyperthermia-based drug delivery it is important to know, both from computational modeling studies as well as experimental determinations, the relationship between local heating and its influence on bond breaking in the case of DBB and permeability through polymer in the case of DEP. These studies, hitherto never undertaken, are critical to design controlled drug delivery systems with required release profiles.
Visualization
In addition to monitoring the temperature distribution, ability to visualize accumulation of drug carrying NPs at the target site, the release of drug and its distribution is critical in designing hyperthermia-based drug delivery systems. While some progress has been made in this direction, [148] there is clearly a need for focusing future efforts to enable noninvasive assessment of accumulation of particles, drug release and distribution. This requirement gains added importance with possibility for extending imaging capabilities to investigate kinetics of drug release in vivo as one can correlate the in vitro characteristics of drug carriers to their in vivo capabilities.
Theranostics
Ability to simultaneously carry out diagnosis and therapy, termed as Theranostics, is now gaining popularity thanks to unique features provided by nanosystems for both diagnosis and therapy. This is relevant for both hyperthermia-based treatment as well as controlled drug delivery. Diagnosis- guided therapy will result in establishing treatment regimens in real time and thereby increasing the therapeutic index. Even though there are already investigations reported where diagnosis is combined with hyperthermia-based therapy or drug delivery, [44, 84] it is clear that there exists a great opportunity for this type of application in future personalized medicine.
Thermo tolerance
Variation in thermo tolerance of cells is a major concern. For example, this variation in cells ranges from those with increased heat sensitivity to high heat resistance depending on the temperature programming. Therefore, determining ideal temperature programming is a challenge. One factor that appears to be consistent in developing such ideal temperature programming is the fact that it is critical to ensure rapid initial heating. Scientific advances are needed towards designing heat mediators by improving material magnetization and size-dispersion. Similarly, development of new strategies to differentiate intracellular heating from extracellular based on in vivo studies is needed.[149] While local hyperthermia is crucial for treatment, it is the response of normal tissues that determines what “dose” of heat can be applied. Based on the available data it appears that the dose-response curves for hyperthermia look similar to those for radiation or drug dose. However, the challenge is to understand critical cellular targets of thermal inactivation both in cancer cells as well as in the surrounding healthy cells. [150] Meeting this challenge is critical for mapping thermo tolerance in the critical regions of a tissue.
Tolerance of magnetic field and frequency of oscillation
It is known that electrical conductivity of biological tissue is sufficiently higher than that of AMF. This may, therefore, generate eddy currents and cause non-selective heating of both cancer as well as normal tissue. The heat generated by such induced eddy currents needs to be considered in determining the conditions for optimizing the specific heating power for a given NP-application. The applied frequency is also important, as researchers have generally focused on pulsed frequencies in the range of 50 kHz to 10 MHz to achieve heating. [132] There is also a limit on the amplitude of applied field; which is about 8–16 kAm−1
Since it is well established that the required reversible profiles are obtained only under certain magnetic field strengths and frequencies,[19] determining these values for each type of system is required for hyperthermia-based drug delivery systems. Especially investigation of kinetics of structural changes is crucial.
Self-regulation of heating
Achieving self-controlled and self regulated heating is important for minimizing some of the deleterious effects of hyperthermia. One way to achieve this self-control is by focusing on two important magnetic properties of the NPs- magnetization and coercivity. These two can control the heating efficiency and Curie temperature so that they can be adjusted slightly above the therapeutic temperature (approx. 45 °C) in order to achieve a self-controlled heating mechanism. The route towards self-regulating heating mediators is open and requires further improvement to permit sufficient heating. Low-Curie temperature NPs such as iron-platinum (FePt) and nickel-palladium (NiPd) NPs with ideal sizes and magnetic properties would heat efficiently and maintain therapeutic temperatures. Another possible approach is to use complex magnetic oxides as core materials (for example La1-xSrxMnO3 (LSMO) perovskites),[151] whose magnetic properties can be properly tailored in various ways, in order to control heating efficiencies with self regulation.
Practical targeted hyperthermia
There is a need for development of new targeting strategies which can effectively drive the intravenously injected particles to targeted sites and complete excretion of the injected particles after the treatment. This is relevant for both hyperthermia-treatment and drug delivery. It is important that MNP formulations have the ability to overcome one of the main biological barriers such as exclusion by BBB, the vascular endothelium, the typical higher osmotic pressure, cancer lesions causing the outward flow of any therapeutic agents, phagocytosis and clearance from circulation by the reticulo-endothelial system (RES) that prevent them from reaching their targets. [57, 152] It is interesting to note that macrophages significantly took up oligomannose-coated liposomes (OMLs) when injected into the peritoneal cavity, and then gradually accumulated in the omentum and other lymphoid tissues within 24 hours. [153] When MNPs were encased in the OMLs to achieve in vivo hyperthermia at the site in a mouse i.p. metastasis model, it successfully controlled tumor development. What is truly encouraging is the accumulation of high concentrations of magnetite NPs, even up to 160 mg, in the momentum. Similarly, based on our recent results, [154] where targeting through luteinizing hormone and releasing hormone (LHRH) resulted in accumulation of large concentration of magnetite NPs (upto 72 pg/cell) in lung metastases bode well for successful practical targeted hyperthermia and controlled drug delivery. With potential avenues to tailor make MNPs to obtain high SAR; it is possible to have successful targeted hyperthermia-based treatment and drug delivery at relatively lower dosages.
Antitumor immunity after hyperthermia
A number of literature reports suggest potential of developing antitumor immunity after hyperthermia based treatment. For example, Siva Sai et al showed that ligand protected Fe/Fe3O4 inorganic core-shell MNPs caused the development of anti-tumor effect on murine B16-F10 melanoma in mice. Only after a three short 10-minute AMF activation (366 kHz), an appreciable decrease in the amount of tumor size was observed. [69] Similar mice studies by Suzuki et al, after magnetite cationic liposomes (MCLs) injection into melanoma nodules followed by AMF subjection, showed complete tumor regression followed by rejection of melanoma cells by cured mice; confirming the generation of antitumor immunity. [67] These studies are supported by the observations of Ito et al that there is development of antitumor immunity after hyperthermia treatment with MCLs in rat models as well.[68] Similar antitumor immunity was also observed in addition to tumor size reduction in cat mammary carcinoma.[70] Therefore, there appears to be an opportunity not only for tumor reduction due to hyperthermia but also protection from future tumor development through the development of antitumor immunity. In principle, this approach can be utilized for the development of in situ vaccinations leading to advancement of novel heat-immuno based therapies. [65, 68]
Combination of treatments
Literature reports show that hyperthermia cancer treatment is much more effective when applied in combination with other treatment methods like radiation, chemotherapies etc than applying alone. When synergistically applied with radiation, the heat released first will bring changes in cell cycle causing faster denaturation of malignant cells through aggregation of nuclear proteins; thereby enhancing the sensitivity of already denatured cells to death by radiation.[63, 155] The thermal enhancement ratio (TER) is defined as the ratio of radiation sensitivity at 37.5 °C to the sensitivity at an elevated temperature. [64] Highest TER is obtained with a combination treatment, since the inner portions of tumor bed are in an oxygen deprived state (hypoxia) and radiation cannot easily pass through it during radiation therapy. A combination therapy allows denaturation of tumor cells from inside by heat induced MNPs and outside by radiation since the tumor beds from inside are radiation resistant and thermo sensitive.[67] Further, adjuvant therapy combining MFH with chemo-radiation strategies also appear to hold great promise in oncology. Even though synergy between heat and radiation dose has been validated by a number of preclinical studies, [156, 157] the time difference between the treatments and their sequence of application is critical.[158] For example, best results are obtained with combined treatments through simultaneous application rather by applying heat therapy alone. However, this may be difficult to realize in clinical practice. Overall, it can be safely concluded that higher levels of survival of experimental animals were achieved when hyperthermia was performed in combination with chemotherapy and sensitization of tumor tissues.[159]
Multi-therapeutic and multi-functional modalities
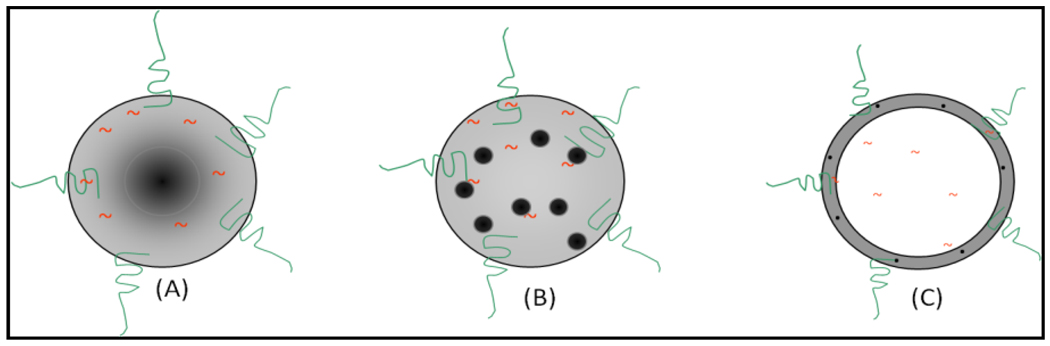
Potential opportunities exist for multi-therapeutic modalities-combined hyperthermia treatment and drug delivery. For example, different types of nano constructs can be envisaged. Three examples are: 1) magnetic core polymer shell nanoparticles with encapsulated drug within a polymeric shell and a targeting agent on its surface (Figure-13A). 2) MNP, drug and polymer nanocomposite with a targeting agent on its surface (Figure-13B). 3) A core containing drug with a polymer shell encapsulated with MNPs and a targeting agent on its surface (Figure-13C). However, there is a need to experimental validate the potential of these type of constructs for hyperthermia-based therapy and drug release. It is critical to optimize the influence of nature of polymer and MNPs on controlled drug release, as it was recently shown that [94] the release of FITC from magnetite-PMMA particles can be induced thermally but not magnetically. On the other hand there was no release of FITC from cobalt-PMMA composites either through thermal or magnetic induction. This type of nano constructs can also meet some of the current challenges in spatiotemporal control delivery of therapeutic agents. In addition, further fine tuning of drug delivery is possible with potential for remotely triggered drug release profile-controllable and switchable-between sustainable and pulsatile pattern - to precisely control under a variety of clinical settings.
Figure 13.
A sechematic representation of three different types of nanoconstructs for hyperthermia-based drug delivery type DEP.
Drug loading and stability
It is well established that traditional approach for controlled drug delivery using polymeric NPs for encapsulation of high quantities of drugs has several problems. If the particles are small, they tend to reduce their overall stability leading to an undesired burst release effect and reduced efficacy.[160] On the other hand, larger particles tend to have slower in vitro release profiles, but when systemically delivered may be more readily detected and cleared from circulation, resulting in lack of efficacy. [161] While magnetically controlled drug release using traditional polymeric NPs may have similar problems, a core shell structure containing drug within a core and a shell composed of MNPs can overcome this problem. In addition to drug loading, stability of the drug within the nanoparticle needs to be evaluated. The drug-nanoparticle interaction studies with focus on drug stability are critically important for both types of hyperthermia-based controlled drug delivery. Preliminary investigations reported to date have only been focused on not very sensitive drug molecules.[44] However, what is required is detailed drug-excipient compatibility studies, particularly for drug molecules which are thermo-sensitive and prone to redox reactions.
Influence of nature and size of the shell
This is relevant in the case of hyperthermia-based drug delivery where drug molecules and MNPs are encapsulated. To date, there are no experimental studies that provide a clear understanding of the influence of nature of polymeric shell and its thickness in providing controlled release profiles. While few standard computational models available for drug release from polymeric materials, what is truly needed is a model system that takes into account multi component systems and stimuli responsive polymers.
7.0 CONCLUSIONS
At the very outset, it should be pointed out that MNPs are classified as medical devices for regulatory purposes and as per the US-FDA should conform to ISO 10993 guidelines. This classification is very important as it will enable faster translation of innovative research using nanomaterials for hyperthermia –based treatment and drug release. For the sake of clarity, we have summarized (Table-1) a list of different types of magnetic nanomaterials that are currently in development for these two applications. The table provides an overview of the possibilities for these different types of magnetic nanomaterials. In our view and as described in this review, classification of heat treatment and drug delivery under the category of hyperthermia is extremely important in order to unleash the advantages of the combination. The possibilities to create core-shell magnetic nanomaterials and nanoshells, especially the opportunities to utilize layer-by-layer self-assembly approaches, [19, 35} will lead to innovative designs for different types of hyperthermia-based treatments. Such combination offers multi-therapeutic modalities in a single treatment. As the field progresses, new paradigms such as magnetomechanical stimuli induced magnetic discs for treatment can also be added under this umbrella. In this approach magnetic discs are targeted to tumors and cancer cell destruction takes place through disruption of the cell membrane initiated by spinning them on cue. [162] Unlike in traditional hyperthermia, biologically relevant effect can be achieved through application of weak magnetic fields (<100 Oe) with frequency of a few tens of Hz, applied for a duration of only 10 min. Ultimately, we envision a future in which nanostructured materials are created with multifunctionalities with optimized pharmacokinetics and pharmacodynamics offering most effective and truly intelligent therapies.[163] In addition to intelligent therapies, we see a great opportunity for combining in real time diagnostic modalities. Such a powerful combination is especially attractive for hyperthermia-based treatment and drug delivery using MNPs wherein appropriate imaging tools such as MRI and fluorescence imaging can be added to create a truly intelligent “theranostics”. Future investigations will lead to creation of “intelligent theranostics” for not only noninvasive assessment of pharmacokinetics and pharmacodynamics of drug but also for real-time monitoring of therapeutic responses. It is our belief that biomedical nanotechnology in general and hyperthermia-based treatment and drug delivery approaches based on magnetic nanomaterials in particular will move pharmaceutical industry from ‘blockbuster drug’ model to ‘personalized medicine’.[164]
Table-1.
| S. No | Type of magnetic nanoparticle | Application | Reference |
|---|---|---|---|
| 1. | Fe doped Au NPs | Hyperthermia-based therapy | 50(a) |
| 2. | y-Fe2O3 Cobalt ferrite | Hyperthermia-based therapy | 40(b), 47 |
| 3. | Fe3O4 Fe3O4-poly vinyl alcohol | Hyperthermia-based therapy | 47(a) |
| 4. | NiFe2O4 | Hyperthermia-based therapy | 48 |
| 5. | γ-Fe2O3 | Hyperthermia-based therapy | 22, 47(b) |
| 7. | Fe3O4@Chitosan | Hyperthermia-based therapy | 42(a) |
| 8. | Fe3O4@Block copolymers | Hyperthermia-based therapy | 62(b) |
| 9. | Fe3O4-Dextran stabilized Fe3O4@Aminosilan | Hyperthermia-based therapy | 62 |
| 10. | Ferrite-Dextran stabilized | Hyperthermia-based therapy | 62, 63, 64 |
| 11. | Fe3O4-dextran (mono & bilayer) stabilized | Hyperthermia-based therapy | 45 |
| 12. | Fe3O4-Lauric acid stabilized | Hyperthermia-based therapy | 70 |
| 13. | Fe3O4-Lauric acid stabilized MnFe2O4-Lauric acid stabilized CoFe2O4-Lauric acid stabilized | Hyperthermia-based therapy | 25(a) |
| 14. | Fe@ biscarboxyl- terminated poly(ethylene glycol) (cPEG) | Hyperthermia-based therapy | 82(a) |
| 15. | γ-MnxFe2-xO3 coated Acrypol 934 polymer | Hyperthermia-based therapy | 142 |
| 16. | FeCo@Au | Hyperthermia-based therapy | 47(a), 56, 61 |
| 17. | Fe@MgO | Hyperthermia-based therapy | 165 |
| 18. | Fe3O4@Si | Hyperthermia-based therapy | 169 |
| 19. | Fe2O3@SiO2 | Hyperthermia-based therapy | 84 |
| 20. | FeNi@Au microdiscs | Hyperthermia-based therapy | 130 |
| 21. | Fe@Fe3O4 | Hyperthermia-based therapy | 69 |
| 22. | La0 56(CaSr)0 22MnO3 @ SiO2 | Hyperthermia-based therapy | 168 |
| 23. | Fe3O4@Au | Hyperthermia-based therapy | 131 |
| 24. | Magnetite cationic liposomes (MCL) | Hyperthermia-based therapy | 11, 66, 67, 68, 71, 72, 78. |
| 25. | Fe3O4-Lauric acid stabilized | Hyperthermia-based therapy | 59,70 |
| 26. | Fe2O3@SiO2 bound LHRH | Hyperthermia-based therapy | 84 |
| 27. | SPIONs bound fluorophore bimane | Hyperthermia-based Controlled drug delivery | 85 |
| 28. | Porous Fe3/Fe/SiO2 | Hyperthermia-based Controlled drug delivery | 32,33 |
| 29. | Fe@SiO2 | Hyperthermia-based Controlled drug delivery | 93 |
| 30. | poly-(N-vinyl- 2- pyrrolidone) (PVP)- modified silica core @ iron oxide shell | Hyperthermia-based Controlled drug delivery | 33 |
| 31. | Mg-Al layered double hydroxides (LDH) coated magnesium ferrite NPs | Hyperthermia-based Controlled drug delivery | 138 |
| 32. | Yolk-shell type nanospheres with movable cores of Au, SiO2, Fe3O4. | Hyperthermia-based Controlled drug delivery | 139 |
| 33. | γ-MnxFe2 xO3 coated Acrypol 934 polymer | Hyperthermia-based therapy and controlled drug delivery | 142 |
| 34. | Fe3O4@lipid membrane (MCL, magnetite cationic liposome) | Hyperthermia-based therapy and controlled drug delivery | 71 |
| 35. | Fe@Carbon nanoparticles bound polymer | Hyperthermia-based therapy and controlled drug delivery | 136 |
| 36. | Co@Au@ poly(sodium styrene sulfonate)/poly(allylamine hydrochloride) | Hyperthermia-based therapy and controlled drug delivery | 19 |
| 37. | SPIONs@ sensitive poly (N-isopropylacrylamide) hydrogels | Hyperthermia-based therapy and controlled drug delivery | 86 |
| 38. | Fe@Fe3O4 loaded 4- tetracarboxy phenyl porphyrin | Hyperthermia-based therapy and controlled drug delivery | 69 |
| 39. | Carboplatin-Fe@C-loaded chitosan | Hyperthermia-based therapy and controlled drug delivery | 166 |
| 40. | Zinc doped iron oxide nanocrystals encapsulated mesoporous silica | Hyperthermia-based therapy and controlled drug delivery | 100 |
| 41. | MCL loaded 4-S- Cysteaminylphenol | Hyperthermia-based therapy and controlled drug delivery | 167 |
ACKNOWLEDGEMENT
We thank Louisiana Board of Regents for an equipment grant to purchase SQUID magnetometer (LEQSF (2008-10)-ENH-TR-07); which was used to generate magnetic data on some of our MNPs. Funding from NIH (1RO1CA142-01A1) is gratefully acknowledged. FM acknowledges NIH-supported INBRE program of NCRR (P20RR016456) for the summer-2010 funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.(a) Nagarajan S, Yong Z. Use of Core/Shell structured nanoparticles for biomedical applications. Recent Pat. Biomed. Eng. 2008;1:34–42. [Google Scholar]; (b) http://www.medicinenet.com/hyperthermia/article.html.
- 2.Loo GV, Saelens X, Gurp MV, MacFarlane M, Martin SJ, Vandenabeele P. The role of mitochondrial factors in apoptosis: a Russian roulette with more than one bullet. Cell Death Differ. 2002;9:1031–1042. doi: 10.1038/sj.cdd.4401088. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein LS, Dewhirst MW, Repacholi M, Kheifets L. Summary, conclusions and recommendations: adverse temperature levels in the human body. Int. J. Hyperther. 2003;19:373–384. doi: 10.1080/0265673031000090701. [DOI] [PubMed] [Google Scholar]
- 4.Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, Felix R, Riess H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. Hematol. 2002;43:33–56. doi: 10.1016/s1040-8428(01)00179-2. [DOI] [PubMed] [Google Scholar]
- 5.(a) Suto R, Srivastava PK. A mechanism for specific immunogenicity of heat shock protein - chaperoned peptides. Science. 1995;269:1585–1588. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]; (b) Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag PM. Hyperthermia in combined treatment of cancer. The Lancet Oncol. 2002;3:487–497. doi: 10.1016/s1470-2045(02)00818-5. [DOI] [PubMed] [Google Scholar]
- 6.Santos-Marques MJ, Carvalho F, Sousa C, Remiao F, Vitorino R, Amado F, Ferreira R, Duarte JA, Bastos ML. Cytotoxicity and cell signalling induced by continuous mild hyperthermia in freshly isolated mouse hepatocytes. Toxicology. 2006;224:210–218. doi: 10.1016/j.tox.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 7.Raaphorst GP, Freeman ML, Dewey WC. Radiosensitivity and recovery from radiation damage in cultured CHO cells exposed to hyperthermia at 42.5 or 45.0°C. Radiat. Res. 1979;79:390–402. [PubMed] [Google Scholar]
- 8.Habash RWY, Bansal R, Krewski D, Alhafid H, Thermal therapy. Part 2: Hyperthermia techniques. Critical Reviews ™ in Biomed. Eng. 2006;34:491–542. doi: 10.1615/critrevbiomedeng.v34.i6.30. [DOI] [PubMed] [Google Scholar]
- 9.(a) http://www.cancer.gov/cancertopics/factsheet/Therapy/hyperthermia.; (b) Falk MH, Issels RD. Hyperthermia in oncology. Int. J. Hyperther. 2001;17:1–18. doi: 10.1080/02656730150201552. [DOI] [PubMed] [Google Scholar]
- 10.Jordan A. Euronanoforum proceddings. 2005:76–80. [Google Scholar]
- 11.Tanaka K, Ito T, Kobayashi T, Kawamura T, Shimada S, Matsumoto K, Saida T, Honda H. Heat immunotherapy using magnetic nanoparticles and dendritic cells for T-lymphoma. J. Biosci. Bioeng. 2005;100:112–115. doi: 10.1263/jbb.100.112. [DOI] [PubMed] [Google Scholar]
- 12.Gilchrist RK, Medal R, Shorey WD, Hanselman RC, Parrott JC, Taylor CB. Selective inductive heating of lymph nodes. Ann. Surg. 1957;146:596–606. doi: 10.1097/00000658-195710000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rand RW, Snow HD, Elliott DG, Haskins GM. Induction heating method for use in causing necrosis of neoplasm. US Patent Specification. 1985;4:545. 368. [Google Scholar]
- 14.Ito A, Honda H, Kobayashi T. Cancer immnotherapy based on intracellular hyperthermia using magnetite nanoparticles: a novel concept of "heat-controlled necrosis" with heat shock protein expression. Cancer Immunol. Immunother. 2006;55:320–328. doi: 10.1007/s00262-005-0049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) Faraji M, Yamini Y, Rezaee M. Magnetic Nanoparticles: Synthesis, Stabilization, Functionalization, Characterization, and Applications. J. Iran. Chem. Soc. 2010;7:1–37. [Google Scholar]; (b) Whitesides GM. The 'right' size in nanobiotechnology. Nat. Biotechnol. 2003;21:1161–1165. doi: 10.1038/nbt872. [DOI] [PubMed] [Google Scholar]
- 16.Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2002;54:631–651. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 17.Bahadur D, Giri J. Biomaterials and magnetism. Sadhana. 2003;28:639–656. [Google Scholar]
- 18.Ito A, Shinkai M, Honda H, Kobayashi T. Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng. 2005;100:1–11. doi: 10.1263/jbb.100.1. [DOI] [PubMed] [Google Scholar]
- 19.Zonghuan L, Malcolm PD, Zhanhu G, Vladimir GO, Kumar CSSR, Yuri LM. Magnetic Switch of Permeability for Polyelectrolyte Microcapsules Embedded with Co@Au Nanoparticles. Langmuir. 2005;21:2042–2050. doi: 10.1021/la047629q. [DOI] [PubMed] [Google Scholar]
- 20.Jeyadevan B. Present status and prospects of magnetite nanoparticles-based hyperthermia. J. ceram. Soc. Jpn. 2010;118:391–401. [Google Scholar]
- 21.Thiesen B, Jordan A. Clinical applications of magnetic nanoparticles for hyperthermia. Int. J. Hyperther. 2008;24:467–474. doi: 10.1080/02656730802104757. [DOI] [PubMed] [Google Scholar]
- 22.Hergt R, Dutz S, Müller R, Zeisberger M. Magnetic particle hyperthermia: nanoparticle magnetism and materials development for cancer therapy. J. Phys.: Condens. Matter. 2006;18:S2919–S2934. [Google Scholar]
- 23.Gazeau F, Lévy M, Wilhelm C. Optimizing magnetic nanoparticle design for nanothermotherapy. Nanomedicine: Future Medicine. 2008;3:831–844. doi: 10.2217/17435889.3.6.831. [DOI] [PubMed] [Google Scholar]
- 24.Müller S. Magnetic fluid hyperthermia therapy for malignant brain tumors-an ethical discussion. Nanomedicine. 2009;5:387–393. doi: 10.1016/j.nano.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 25.(a) Pradhan P, Giri J, Samanta G, Sarma HD, Mishra KP, Bellare J, Banerjee R, Bahadur D. Comparative evaluation of heating ability and biocompatibility of different ferrite-based magnetic fluids for hyperthermia application. J. Biomed. Mater. Res. B: Appl. Biomater. 2007;81B:12–22. doi: 10.1002/jbm.b.30630. [DOI] [PubMed] [Google Scholar]; (b) Kim DH, Thai YT, Nikles DE, Brazel CS. Heating of aqueous dispersions containing MnFe2O4 nanoparticles by radio-frequency magnetic field induction. IEEE T. Magn. 2009;45:64–70. [Google Scholar]; (c) Kaman O, Pollert E, Veverka P, Veverka M, Hadova E, Kny′zek K, Marysko M, Kaspar P, Klementova M, Grunwaldova V, Vasseur S, Epherre R, Mornet S, Goglio G, Duguet E. Silica encapsulated manganese perovskite nanoparticles for magnetically induced hyperthermia without the risk of overheating. Nanotechnology. 2009;20:275610. doi: 10.1088/0957-4484/20/27/275610. [DOI] [PubMed] [Google Scholar]; (d) Atsarkin VA, Levkin LV, Posvyanskiy VS, Melnikov OV, Markelova MN, Gorbenko OY, Kaul AR. Solution to the bioheat equation for hyperthermia with La1-xAgyMnO3-δ nanoparticles: The effect of temperature autostabilization. Int. J. Hyperther. 2009;25:240–247. doi: 10.1080/02656730802713565. [DOI] [PubMed] [Google Scholar]; (e) Kong G, Braun RD, Dewhirst MW. Characterization of the effect of hyperthermia on nanoparticle extravasation from tumour vasculature. Cancer Res. 2001;61:3027–3032. [PubMed] [Google Scholar]; (f) Martina MS, Wilhelm C, Lesieur S. The effect of magnetic targeting on the uptake of magnetic-fluid-loaded liposomes by human prostatic adenocarcinoma cells. Biomaterials. 2008;29:4137–4145. doi: 10.1016/j.biomaterials.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Kumar Challa., editor. Nanotechnologies for the life sciences. Vol. 6–10. Wiley-VCH; [Google Scholar]
- 27.Chan JM, L Zhang, Tong R, Ghosh D, Gao W, Liao G, Yuet KP, Gray D, Rhee J-W, Cheng J, Golomb G, Libby P, Langer R, Farokhzad OC. Spatiotemporal controlled delivery of nanoparticles to injured vasculature. PNAS. 2010;107:2213–2218. doi: 10.1073/pnas.0914585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.(a) Zhao Y, L Vivero-Escoto J, Slowing II, Trewyn BG, Lin VS-Y. Capped mesoporous silica nanoparticles as stimuli-responsive controlled release systems for intracellular drug/gene delivery. Expert Opin. Drug Deliv. 2010;7:1013–1029. doi: 10.1517/17425247.2010.498816. [DOI] [PubMed] [Google Scholar]; (b) Timko BP, Dvir T, Kohane DS. Remotely Triggerable Drug Delivery Systems. Adv. Mater. 2010 doi: 10.1002/adma.201002072. [DOI] [PubMed] [Google Scholar]
- 29.Kost J, Wolfrum J, Langer R. Magnetically enhanced insulin release in diabetic rats. J. Biomed. Mater. Res. 1987;21:1367–1373. doi: 10.1002/jbm.820211202. [DOI] [PubMed] [Google Scholar]
- 30.Edelman ER, Kost J, Bobeck H, Langer R. Regulation of drug release from polymer matrices by oscillating magnetic fields. J. Biomed. Mater. Res. 1985;19:67–83. doi: 10.1002/jbm.820190107. [DOI] [PubMed] [Google Scholar]
- 31.Kost J, Noecker R, Kunica E, Langer R. Magnetically controlled release systems: Effect of polymer composition. J. Biomed. Mater. Res. 1985;19:935–940. doi: 10.1002/jbm.820190805. [DOI] [PubMed] [Google Scholar]
- 32.Hu SH, Liu TY, Huang HY, Liu DM, Chen SY. Magnetic-Sensitive Silica Nanospheres for Controlled Drug Release. Langmuir. 2008;24:239–244. doi: 10.1021/la701570z. [DOI] [PubMed] [Google Scholar]
- 33.Hu SH, Chen SY, Liu DM, Hsiao CS. Core/Single-Crystal-Shell Nanospheres for Controlled Drug Release via a Magnetically Triggered Rupturing Mechanism. Adv. Mater. 2008;20:2690–2695. doi: 10.1002/adma.200800193. [DOI] [PubMed] [Google Scholar]
- 34.Brazel SC. Magnetothermally-responsive nanomaterials: Combining magnetic nanostructures and thermally-sensitive polymers for triggered drug release. Pharm. Res. 2009;26:644–656. doi: 10.1007/s11095-008-9773-2. [DOI] [PubMed] [Google Scholar]
- 35.(a) Schärtl W. Current directions in core-shell nanoparticle design. Nanoscale. 2010;2:829–843. doi: 10.1039/c0nr00028k. [DOI] [PubMed] [Google Scholar]; (b) De Villiers MM, Lvov Y. In: Nanoshells for Drug Delivery, Vol. 10 (Nanomaterials for Medical Diagnosis and Therapy) of the book series on Nanotechnologies for Life Sciences. Kumar Challa SSR., editor. Wiley VCH; 2007. [Google Scholar]
- 36.Bornstein BA, Zouranjian PS, Hansen JL, Fraser SM, Gelwan LA, Teicher BA, Svensson GK. Local hyperthermia, radiation therapy, and chemotherapy in patients with local-regional recurrence of breast carcinoma. J. Radiat. Oncol. Biol. Phys. 1993;25:79–85. doi: 10.1016/0360-3016(93)90148-o. [DOI] [PubMed] [Google Scholar]
- 37.Jordan A, Wust P, Fahling H, John W, Hinz A, Felix R. Inductive heating of ferrimagnetic particles and magnetic fluids: Physical evaluation of their potential for hyperthermia. Int. J. Hyperther. 1993;9:51–68. doi: 10.3109/02656739309061478. [DOI] [PubMed] [Google Scholar]
- 38.Rosensweig RE. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002;252:370–374. [Google Scholar]
- 39.Hergt R, Andrä W, d’Ambly CG, Hilger I, Kaiser WA, Richter U, Schmidt HG. Physical limits of hyperthermia using fine magnetite particles. IEEE T. Magn. 1998;34:3745–3754. [Google Scholar]
- 40.(a) Fortin JP, Wilhelm C, Servais J, Menager C, Bacri JC, Gazeau F. Size-sorted anionic iron oxide nanomagnets as colloidal mediators for magnetic hyperthermia. J. Am. Chem. Soc. 2007;129:2628–2635. doi: 10.1021/ja067457e. [DOI] [PubMed] [Google Scholar]; (b) Fortin JP, Gazeau F, Wilhelm C. Internal heating of living cells with magnetic nanomediators. Eur. Biophys. J. 2008;37:223–228. doi: 10.1007/s00249-007-0197-4. [DOI] [PubMed] [Google Scholar]
- 41.(a) Panhurst QA, Connolly J, Jones SK, Dobson J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D: Appl. Phys. 2003;36:R167–R181. [Google Scholar]; (b) Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021. doi: 10.1016/j.biomaterials.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 42.(a) Zhao DL, Wang XX, Zeng XW, Xia QS, Tang JT. Preparation and inductive heating property of Fe3O4-chitosan composite nanoparticles in an AC magnetic field for localized hyperthermia. J. Alloys Compd. 2009;477:739–743. [Google Scholar]; (b) Riviere C, Wilhelm C, Cousin F, Dupuis V, Gazeau F, Perzynski R. Internal structure of magnetic endosomes. Eur. Phys. J. E Soft Matter. 2007;22:1–10. doi: 10.1140/epje/e2007-00014-1. [DOI] [PubMed] [Google Scholar]
- 43.Hergt R, Dutz S. Magnetic particle hyperthermia—biophysical limitations of a visionary tumour therapy. J. Magn. Magn. Mater. 2007;311:187–192. [Google Scholar]
- 44.Derfus AM, Maltzahn GV, Harris TJ, Duza T, Vecchio KS, Ruoslahti E, Bhatia SN. Remotely Triggered Release from Magnetic Nanoparticles. Adv. Mater. 2007;19:3932–3936. [Google Scholar]
- 45.Dennis CL, Jackson AJ, Borchers JA, Ivkov R, Foreman AR, Hoopes PJ, Strawbridge R, Pierce Z, Goerntiz E, Lau JW, Gruettner C. The influence of magnetic and physiological behaviour on the effectiveness of iron oxide nanoparticles for hyperthermia. J. Phys. D: Appl. Phys. 2008;41:134020. [Google Scholar]
- 46.Kawai N, Futakuchi M, Yoshida T, Ito A, Sato S, Naiki T, Honda H, Shirai T, Kohri K. Effect of heat therapy using magnetic nanoparticles conjugated with cationic liposomes on prostate tumor in bone. The Prostate. 2008;68:784–792. doi: 10.1002/pros.20740. [DOI] [PubMed] [Google Scholar]
- 47.(a) Suto M, Hirota Y, Mamiya H, Fujita A, Kasuya R, Tohji K, Jeyadevan B. Heat dissipation mechanism of magnetite nanoparticles in magnetic fluid hyperthermia. J. Magn. Magn. Mater. 2009;321:1493–1496. [Google Scholar]; (b) Lévy M, Wilhelm C, Siaugue J-M, Horner O, Bacri J-C, Gazeau F. Magnetically induced hyperthermia: size-dependent heating power of γ-Fe2O3 nanoparticles. J. Phys: Condens. Matter. 2008;20:204133. doi: 10.1088/0953-8984/20/20/204133. [DOI] [PubMed] [Google Scholar]
- 48.Bae S, Lee SW, Takemura Y, Yamashita E, Kunisaki J, Zurn S, Kim CS. Dependence of frequency and magnetic field on self-heating characteristics of NiFe2O4 nanoparticles for hyperthermia,". IEEE T. Magn. 2006;42:3566–3568. [Google Scholar]
- 49.Kim DH, Lee SH, Kim KN, Kim KN, Kim KM, Shim IB, Lee YK. Temperature change of various ferrite particles with alternating magnetic field for hyperthermic application. J. Magn. Magn. Mater. 2005;293:320–327. [Google Scholar]
- 50.(a) Wijaya A, Brown KA, Alper JD, Schifferli KH. Magnetic field heating study of Fe-doped Au nanoparticles. J. Magn. Magn. Mater. 2007;309:15–19. [Google Scholar]; (b) Sharma R, Chen CJ. Newer nanoparticles in hyperthermia treatment and thermometry. J. Nanopart. Res. 2009;11:671–689. [Google Scholar]
- 51.Nojima H, Ge S, Katayama Y, Ueno S, Iramina K. Effect of the stimulus frequency and pulse number of repetitive transcranial magnetic stimulation on the inter-reversal time of perceptual reversal on the right superior parietal lobule. J. Appl. Phys. 2010;107 09B320-09B320-3. [Google Scholar]
- 52.(a) L Huber D, Synthesis Properties. and Applications of Iron Nanoparticles. Small. 2005;1:482–501. doi: 10.1002/smll.200500006. [DOI] [PubMed] [Google Scholar]; (b) Gould P. Nanoparticles probe biosystems. Mater. Today. 2004;7:36–43. [Google Scholar]
- 53.(a) Pradhan P, Giri J, Banerjee R, Bellare J, Bahadur D. Cellular interactions of lauric acid and dextran-coated magnetite nanoparticles. J. Magn. Magn. Mater. 2007;311:282–287. [Google Scholar]; (b) Corchero JL, Villaverde A. Biomedical applications of distally controlled magnetic nanoparticles. Trends Biotechnol. 2009;27:468–476. doi: 10.1016/j.tibtech.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Deger S, Boehmer D, Türk I, Roigas J, Budach V, Loening SA. Interstitial Hyperthermia using Self-Regulating Thermoseeds Combined with Conformal Radiation Therapy. Eur. Urol. 2002;42:147–153. doi: 10.1016/s0302-2838(02)00277-4. [DOI] [PubMed] [Google Scholar]
- 55.Ondeck CL, Habib AH, Ohodnicki P, Miller K, Sawyer CA, Chaudhary P, McHenry ME. Theory of magnetic fluid heating with an alternating magnetic field with temperature dependent materials properties for self-regulated heating. J. Appl. Phys. 2009;105 07B324 (3pages) [Google Scholar]
- 56.Weimuller MG, Zeisberger M, Krishnan KM. Size-dependant heating rates of iron oxide nanoparticles for magnetic fluid hyperthermia. J. Magn. Magn. Mater. 2009;321:1947–1950. doi: 10.1016/j.jmmm.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krishnan KM. Biomedical Nanomagnetics: A spin through possibilities in imaging, diagnostics, and therapy. IEEE T Magn. 2010;46:2523–2558. doi: 10.1109/TMAG.2010.2046907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.(a) Jun YW, Huh YM, Choi JS, Lee JH, Song HT, Kim S, Yoon S, Kim KS, Shin JS, Suh JS, Cheon J. Nanoscale size effect of magnetic nanocrystals and their utilization for cancer diagnosis via magnetic resonance imaging. J. Am. Chem. Soc. 2005;127:5732–5733. doi: 10.1021/ja0422155. [DOI] [PubMed] [Google Scholar]; (b) Purushotham S, Ramanujan RV. Modeling the performance of magnetic nanoparticles in multimodal cancer therapy. J. Appl. Phys. 2010;107:114701. [Google Scholar]
- 59.Hergt R, Hiergeist R, Zeisberger M, Schüler D, Heyen U, Hilger I, Kaiser WA. Magnetic properties of bacterial magnetosomes as potential diagnostic and therapeutic tools. J. Magn. Magn. Mater. 2005;293:80–86. [Google Scholar]
- 60.Kim D-H, Thai TY, Nikles DE, Brazel CS. Heating of aqueous dispersions containing MnFe2O4 nanoparticles by radio-frequency magnetic field induction. IEEE T. Magn. 2009;45:64–70. [Google Scholar]
- 61.Marcela GW, Matthias Z, Kannan MK. Size-dependant heating rates of iron oxide nanoparticles for magnetic fluid hyperthermia. J. Magn. Magn. Mater. 2009;321:1947–1950. doi: 10.1016/j.jmmm.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.(a) Jordan A, Scholz R, Wust P, Schirra H, Schiestel T, Schmidt H, Felix R. Endocytosis of dextran and silan-coated magnetite nanoparticles and the effect of intracellular hyperthermia on human mammary carcinoma cells in vitro. J. Magn. Magn. Mater. 1999;185:185–196. [Google Scholar]; (b) Aqil A, Vasseur S, Duguet E, Passirani C, Benoît JP, Jérôme R, Jérôme C. Magnetic nanoparticles coated by temperature responsive copolymers for hyperthermia. J. Mater. Chem. 2008;18:3352–3360. [Google Scholar]
- 63.Jordan A, Scholz R, Wust P, Fahling H, Felix R. Magnetic fluid hyperthermia (MFH): cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J. Magn. Magn. Mater. 1999;201:413–419. [Google Scholar]
- 64.Brusentsov NA, Nikitin LV, Brusentsova TN, Kuznetsov AA, Bayburtskiy FS, Shumakov LI, Jurchenko NY. Magnetic fluid hyperthermia of the mouse experimental tumor. J. Magn. Magn. Mater. 2002;252:378–380. [Google Scholar]
- 65.Tanaka K, Ito A, Kobayashi K, Kawamura T, Shimada S, Matsumoto K, Saida T, Honda H. Intratumoral injection of immature dendritic cells enhances antitumor effect of hyperthermia using magnetic nanoparticles. Int. J. Cancer. 2005;116:624–633. doi: 10.1002/ijc.21061. [DOI] [PubMed] [Google Scholar]
- 66.Yanase M, Shinkai M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Intracellular hyperthermia for cancer using magnetite cationic liposomes: an in vivo study. Jpn. J. Cancer Res. 1998;89:463–469. doi: 10.1111/j.1349-7006.1998.tb00586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suzuki M, Shinkai M, Honda H, Kobayashi T. Anti-cancer effect and immune induction by hyperthermia of malignant melanoma using magnetite cationic liposomes. Melanoma Res. 2003;13:129–135. doi: 10.1097/00008390-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 68.(a) Ito A, Shinkai M, Honda H, Yoshikawa K, Saga S, Wakabayashi T, Yoshida J, Kobayashi T. Heat shock protein 70 expression induces antitumor immunity during intracellular hyperthermia using magnetite nanoparticles. Cancer Immunol. Immun. 2003;52:80–88. doi: 10.1007/s00262-002-0335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ito A, Nakahara Y, Fujioka M, Kobayashi T, Takeda T, Nakashima I, Honda H. Complete regression of hereditary melanoma in a mouse model by repeated hyperthermia using magnetite cationic liposomes. Jpn. J. Hyperthermic Oncol. 2005;11:139–149. [Google Scholar]
- 69.Sivasai B, Rajashekar R, Hongwang W, Thilani SN, RajKumar D, Marla P, Franklin KO, Brandon W, Xiaoxuan L, Olga KB, Masaaki T, Viktor C, Stefan BH, Deryl TL. A/C magnetic hyperthermia of melanoma mediated by iron(0)/iron oxide core/shell magnetic nanoparticles: a mouse study. BMC Cancer. 2010;10:1–9. doi: 10.1186/1471-2407-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sincai M, Ganga D, Ganga M, Argherie D, Bica D. Antitumor effect of magnetite nanoparticles in cat mammary adenocarcinoma. J. Magn. Magn. Mater. 2005;293:438–441. [Google Scholar]
- 71.Matsuoka F, Shinkai M, Honda H, Kubo T, Sugita T. Kobyashi T. Hyperthermia using magnetite cationic liposomes for hamster osteosarcoma. BioMagn. Res. Techn. 2004;2:1–6. doi: 10.1186/1477-044X-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matsuno H, Tohnai I, Mitsudo K, Hayashi K, Ito M, Shinkai M, Kobayashi T, Yoshida J, Ueda M. Interstitial hyperthermia using magnetite cationic liposomes inhibit to tumor growth of VX-7 transplanted tumor in rabbit tongue. Jpn. J. Hyperthermic Oncol. 2001;17:141–150. [Google Scholar]
- 73.Motoyama J, Yamashita N, Morino T, Tanaka M, Kobayashi T, Honda H. Hyperthermic treatment of DMBA-induced rat mammary cancer using magnetic nanoparticles. Biomagn. Res. Technol. 2008;6:1–6. doi: 10.1186/1477-044X-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Le B, Shinkai M, Kitade T, Honda H, Yoshida J, Wakabayashi T, Kobyashi T. Preparation of Tumor-Specific Magnetoliposomes and Their Application for Hyperthermia. J. Chem. Eng. Jpn. 2001;34:66–72. [Google Scholar]
- 75.Black J. Biological Performance of Materials: Fundamentals of Biocompatibility. 3rd ed. New York: Marcel Dekker, Inc; 1999. [Google Scholar]
- 76.Chatterjee J, Bettge M, Haik Y, Chen CJ. Synthesis and characterization of polymer encapsulated Cu-Ni magnetic nanoparticles for hyperthermia applications. J. Magn. Magn. Mater. 2005;293:303–309. [Google Scholar]
- 77.Brusentsova TN, Brusentsov NA, Kuznetsov VD, Nikiforov VN. Synthesis and investigation of magnetic properties of Gd-substituted Mn-Zn ferrite nanoparticles as a potential low-TC agent for magnetic fluid hyperthermia. J. Magn. Magn. Mater. 2005;293:298–302. [Google Scholar]
- 78.Giri J, Pradhan P, Sriharsha T, Bahadur D. Preparation and investigation of potentiality of different soft ferrites for hyperthermia applications. J. Appl. Phys. 2005;97:10Q916. [Google Scholar]
- 79.Bagaria HG, Phillips JL, Nikles DE, Johnson DT. Self-regulated magnetic fluid hyperthermia. AIChE Annual Proceedings. 2005:14336–14340. [Google Scholar]
- 80.Sun S, Murray CB, Weller D, Folks L, Moser A. Monodisperse FePt Nanoparticles and Ferromagnetic FePt Nanocrystal Superlattices. Science. 2000;287:1989–1992. doi: 10.1126/science.287.5460.1989. [DOI] [PubMed] [Google Scholar]
- 81.Chen M, Liu JP, Sun S. One-step synthesis of FePt nanoparticles with tunable size. J. Am. Chem. Soc. 2004;126:8394–8395. doi: 10.1021/ja047648m. [DOI] [PubMed] [Google Scholar]
- 82.Son SU, Jang Y, Park J, Na HB, Park HM, Yun HJ, Lee J, Hyeon T. Designed synthesis of atom-economical Pd/Ni bimetallic nanoparticle-based catalysts for sonogashira coupling reactions. J. Am. Chem. Soc. 2004;126:5026–5027. doi: 10.1021/ja039757r. [DOI] [PubMed] [Google Scholar]
- 83.McNerny KL, Kim Y, Laughlin DE, McHenry ME. Chemical synthesis of monodisperse y-Fe–Ni magnetic nanoparticles with tunable Curie temperatures for self-regulated hyperthermia. J. Appl. Phys. 2010;107:09A312. [Google Scholar]
- 84.(a) Hadjipanayis CG, Bonder MJ, Balakrishnan S, Wang X, Mao H, Hadjipanayis GC. Metallic iron nanoparticles for MRI contrast enhancement and local hyperthermia. Small. 2008;4:1925–1929. doi: 10.1002/smll.200800261. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bergey EJ, Levy L, Wang X, Krebs LJ, Lai M, Kim K-S, Pakatchi S, Liebow C, Prasad PN. DC Magnetic Field Induced Magnetocytolysis of Cancer Cells Targeted by LH-RH Magnetic Nanoparticles in vitro. Biomed. Microdevices. 2002;4:293–299. [Google Scholar]
- 85.McGill SL, Cuylear CL, Adolphi NL, Osiński M, Smyth HD. Magnetically responsive nanoparticles for drug delivery applications using low magnetic field strengths. IEEE T. Nanobioscience. 2009;8:33–42. doi: 10.1109/TNB.2009.2017292. [DOI] [PubMed] [Google Scholar]
- 86.Satarkar NS, Hilt JZ. Magnetic hydrogel nanocomposite for remote controlled pulsatile drug release. J. Controlled Release. 2008;130:246–251. doi: 10.1016/j.jconrel.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 87.Wong JE, Gaharwar AK, Schulte DM, Bahadur D, Richtering W. Layer-by-layer assembly of a magnetic nanoparticle shell on a thermoresponsive microgel core. J. Magn. Magn. Mater. 2007;311:219–223. [Google Scholar]
- 88.Hoare T, Santamaria J, Goya GF, Irusta S, Lin D, Lau S, Padera R, Langer R, Kohane DS. A magnetically triggered composite membrane for on-demand drug delivery. Nano Lett. 2009;9:3651–3657. doi: 10.1021/nl9018935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ghosh S, Neogi A, Yang C, Cai T, Mitra SG, Diercks D, Hu Z. Thermoresponsive hydrogel microvalve based on magnetic nanoheaters for microfluidics. Mater. Res. Soc. Symp. Proc. 2008;1095 1095-EE03-20. [Google Scholar]
- 90.Hsu MH, Su YC. Iron-oxide embedded solid lipid nanoparticles for magnetically controlled heating and drug delivery. Biomed. Microdevices. 2008;10:785–793. doi: 10.1007/s10544-008-9192-5. [DOI] [PubMed] [Google Scholar]
- 91.Yin H, Yu S, Casey PS, Chow GM. Synthesis and properties of poly(d,l-lactide) drug carrier with maghemite nanoparticles. Mater. Sci. Eng. C. 2010;30:618–623. [Google Scholar]
- 92.Ghosh S, Mitra SG, Cai T, Diercks DR, Mills NC, L Hynds D. Alternating Magnetic Field Controlled, Multifunctional Nano-Reservoirs: Intracellular Uptake and Improved Biocompatibility. Nanoscale Res. Lett. 2010;5:195–204. doi: 10.1007/s11671-009-9465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu TY, Hu SH, Liu KH, Shaiu RS, Liu DM, Chen SY. Instantaneous Drug Delivery of Magnetic/Thermally Sensitive Nanospheres by a High-Frequency Magnetic Field. Langmuir. 2008;24:13306–13311. doi: 10.1021/la801451v. [DOI] [PubMed] [Google Scholar]
- 94.Urbina MC, Zinoveva S, Miller T, Sabliov CM, Monroe WT, Kumar CSSR. Investigation of magnetic nanoparticle-polymer composites for multiple-controlled drug delivery. J. Phys. Chem. C. 2008;112:11102–11108. [Google Scholar]
- 95.Purushotham S, Chang PEJ, Rumpel H, Kee IHC, Ng RTH, Chow PKH, Tan CK, Ramanujan RV. Thermoresponsive core-shell magnetic nanoparticles for combined modalities of cancer therapy. Nanotechnology. 2009;20:305101. doi: 10.1088/0957-4484/20/30/305101. (11 pages) [DOI] [PubMed] [Google Scholar]
- 96.Regmi R, Bhattarai SR, Sudakar C, Wani AS, Cunningham R, Vaishnava PP, Naik R, Oupicky D, Lawes G. Hyperthermia controlled rapid drug release from thermosensitive magnetic microgels. J. Mater. Chem. 2010;20:6158–6163. [Google Scholar]
- 97.Chen S, Li Y, Guo C, Wang J, Ma J, Liang X, Yang LR, Liu HZ. Temperature-responsive magnetite/PEO-PPO-PEO block copolymer nanoparticles for controlled drug targeting delivery. Langmuir. 2007;23:12669–12676. doi: 10.1021/la702049d. [DOI] [PubMed] [Google Scholar]
- 98.Sheng K, Wang L, Read P, Larner J, Yang W. Geometrically Targeted Radiation Enhancer Using Semiconductive Nanoparticles. Med. Phys. 2010;37:3172. [Google Scholar]
- 99.Gong X, Peng S, Wen W, Sheng P, Li W. Design and Fabrication of Magnetically Functionalized Core/Shell Microspheres for Smart Drug Delivery. Adv. Funct. Mater. 2009;19:292–297. [Google Scholar]
- 100.Thomas CR, Ferris DP, Lee JH, Choi E, Choo MH, Kim ES, Shin JS, Stoddart JF, Cheon J, Zink JI. Noninvasive remote-controlled release of drug molecules in vitro using magnetic actuation with silica-encapsulated iron oxide. J. Am. Chem. Soc. 2010;132:10623–10625. doi: 10.1021/ja1022267. [DOI] [PubMed] [Google Scholar]
- 101.Nappini S, Bombelli FB, Bonini M, Nordèn B, Baglioni P. Magnetoliposomes for controlled drug release in the presence of low-frequency magnetic field. Soft Matter. 2010;6:154–162. [Google Scholar]
- 102.Tai LA, Tsai PJ, Wang YC, Wang YJ, Lo LW, Yang CS. Thermosensitive liposomes entrapping iron oxide nanoparticles for controllable drug release. Nanotechnology. 2009;20:135101/1–135101/9. doi: 10.1088/0957-4484/20/13/135101. [DOI] [PubMed] [Google Scholar]
- 103.Huang H, Delikanli S, Zeng H, Ferkey DM, Pralle A. Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nat. Nanotechnol. 2010;5:602–606. doi: 10.1038/nnano.2010.125. [DOI] [PubMed] [Google Scholar]
- 104.Hayashi K, Ono K, Suzuki H, Sawada M, Moriya M, Sakamoto W, Yogo T. High-frequency, magnetic-field-responsive drug release from magnetic nanoparticle/organic hybrid based on hyperthermic effect. ACS Appl. Mater. Interfaces. 2010;2:1903–1911. doi: 10.1021/am100237p. [DOI] [PubMed] [Google Scholar]
- 105.Yang F, Chen P, He W, Gu N, Zhang X, Fang K, Zhang Y, Sun J, Tong J. Bubble microreactors triggered by an alternating magnetic field as diagnostic and therapeutic delivery devices. Small. 2010;6:1300–1305. doi: 10.1002/smll.201000173. [DOI] [PubMed] [Google Scholar]
- 106.Roduner E. Nanoscopic Materials: Size Dependent Phenomena. Cambridge, UK: RSC Publishing; 2006. [Google Scholar]
- 107.Daniel MC, Astruc D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, applications, toward biology, catalysis, and nanotechnology. Chem. Rev. 2004;104:293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 108.Jun YW, Seo JW, Cheon J. Nanoscaling Laws of Magnetic Nanoparticles and Their Applicabilities in Biomedical Sciences. Acc. Chem. Res. 2008;41:179–189. doi: 10.1021/ar700121f. [DOI] [PubMed] [Google Scholar]
- 109.Burda C, Chen X, Narayanan R, El-Sayed MA. Chemistry and Properties of Nanocrystals of Different Shapes. Chem. Rev. 2005;105:1025–1102. doi: 10.1021/cr030063a. [DOI] [PubMed] [Google Scholar]
- 110.Saini S, Goundla S, Bagchi B. Distance and Orientation Dependence of Excitation Energy Transfer: From Molecular Systems to Metal Nanoparticles. J. Phys. Chem. B. 2009;113:1817–1832. doi: 10.1021/jp806536w. [DOI] [PubMed] [Google Scholar]
- 111.Prasad BLV, Sorensen CM, Klabunde KJ. Gold nanoparticle superlattices. Chem. Soc. Rev. 2008;37:1871–1883. doi: 10.1039/b712175j. [DOI] [PubMed] [Google Scholar]
- 112.Narayanan R, Tabor C, El-Sayed MA. Can the observed changes in the size or shape of a colloidal nanocatalyst reveal the nanocatalysis mechanism type:Homogeneous or Heterogeneous? Topics in Catalysis. 2008;48:60–74. [Google Scholar]
- 113.Grzelczak M, Perez-Juste J, Mulvaney P, Liz-Marzan LM. Chemlnform Abstract: Shape Control in Gold Nanoparticle Synthesis. Chemlnform. Chem. Soc. Rev. 2008;37:1783–1791. doi: 10.1039/b711490g. [DOI] [PubMed] [Google Scholar]
- 114.L Nehl C, Hafner JH. Shape-dependent plasmon resonances of gold nanoparticles. J. Mater. Chem. 2008;18:2415–2419. [Google Scholar]
- 115.Lisiecki I, Size Shape. and Structural Control of Metallic Nanocrystals. J. Phys. Chem. B. 2005;109:12231–12244. doi: 10.1021/jp058018p. [DOI] [PubMed] [Google Scholar]
- 116.Liu Z, Elbert D, Chien CL, Searson PC. FIB/TEM Characterization of the Composition and Structure of Core/Shell Cu-Ni Nanowires. Nano Lett. 2008;8:2166–2170. doi: 10.1021/nl080492u. [DOI] [PubMed] [Google Scholar]
- 117.(a) Wang L, Park H-Y, Lim SI, Schadt MJ, Mott D, Luo J, Wang X, Zhong C-J. Core@shell nanomaterials: gold-coated magnetic oxide nanoparticles. J. Mater. Chem. 2008;18:2629–2635. [Google Scholar]; (b) Latham AH, Williams ME. Controlling transport and chemical functionality of magnetic nanoparticles. Acc. Chem. Res. 2008;41:411–420. doi: 10.1021/ar700183b. [DOI] [PubMed] [Google Scholar]
- 118.Silke B, Helmut B, Matoussevitch N, Eckhard D, Modrow H, Palina N, Frerichs M, Kempter V, Wolfgang M-F, Heinemann A, Kammel M, Wiedenmann A, Pop L, Odenbach S, Uhlmann E, Bayat N, Hesselbach J, Guldbakke JM. Air-stable Co-, Fe-, and Fe/Co-nanoparticles and ferrofluids. Z. Phys. Chem. 2006;220:3–40. [Google Scholar]
- 119.(a) Zhai J, Huang M, Dong S. Electrochemical designing of Au/Pt core shell nanoparticles as nanostructured catalyst with tunable activity for oxygen reduction. Electroanalysis. 2007;19:506–509. [Google Scholar]; (b) Hormes J, Modrow H, Bonnemann H, Kumar CSSR. The influence of various coatings on the electronic, magnetic,and geometric properties of cobalt nanoparticles. J. Appl. Phys. Lett. 2005;97 10R102-6. [Google Scholar]; (c) Sweeney CM, Hasan W, Nehl CL, Odom TW. Optical properties of anisotropic core-shell pyramidal particles. J. Phys. Chem. A. 2009;113:4265–4268. doi: 10.1021/jp810837u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Reiss P, Protiere M, Li L. Core/shell semiconductor nanocrystals. Small. 2009;5:154–168. doi: 10.1002/smll.200800841. [DOI] [PubMed] [Google Scholar]
- 120.(a) Lellouche JP. In: Biofunctionalization of nanomaterials. Kumar Challa SSR., editor. Wiley-VCH; 2005. pp. 299–329. [Google Scholar]; (b) Han SK, Kim RS, Lee JH, Tae G, Cho SH, Yuk SH. In: Nanomaterials for Medical Diagnosis and Therapy. Kumar Challa SSR., editor. Wiley-VCH; 2007. pp. 143–188. [Google Scholar]; (c) Yamaguchi Y, Igarashi R. In: Nanomaterials for Medical Diagnosis and Therapy. Kumar Challa SSR., editor. Wiley-VCH; 2007. pp. 310–341. [Google Scholar]; (d) De Villiers M, Lvov Y. In: Nanomaterials for Medical Diagnosis and Therapy. Kumar Challa SSR., editor. Wiley-VCH; 2007. pp. 527–556. [Google Scholar]; (e) He X, Lin X, Wang K, Chen L, Wu P, Yuan Y, Tan W. In: Encyclopedia of nanoscience nanotechnology. Nalwa HS, editor. Vol. 1. American Scientific Publishers; 2004. pp. 235–253. [Google Scholar]; (f) Kumar Challa SSR., editor. Mixed metal nanomaterials. Wiley-VCH; 2008. [Google Scholar]
- 121.Zhang T, L Stilwell J, Gerion D, Ding L, Elboudwarej O, Cooke PA, Gray JW, Alivisatos AP, Chen FF. Cellular Effect of High Doses of Silica-Coated Quantum Dot Profiled with High Throughput Gene Expression Analysis and High Content Cellomics Measurements. Nano Lett. 2006;6:800–808. doi: 10.1021/nl0603350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Matoussevitch N, Gorschinski A, Habicht W, Bolle J, Dinjus E, Boennemann H, Behrens S. Surface modification of metallic Co nanoparticles. J. Mag. Mag. Mater. 2007;311:92–96. [Google Scholar]
- 123.(a) Zhang X-B, Yan J-M, Han S, Shioyama H, Xu Q. Magnetically recyclable Fe@Pt Core-Shell nanoparticles and their use as electrocatalysts for ammonia borane oxidation: The role of crystallinity of the core. J. Am. Chem. Soc. 2009;131:2778–2779. doi: 10.1021/ja808830a. [DOI] [PubMed] [Google Scholar]; (b) Joo SH, Park JY, Tsung C-K, Yamada Y, Yang P, Somorjai GA. Thermally stable Pt/mesoporous silica core-shell nanocatalysts for high-temperature reactions. Nat. Mater. 2009;8:126–131. doi: 10.1038/nmat2329. [DOI] [PubMed] [Google Scholar]
- 124.(a) Schneider GF, Subr V, Ulbrich K, Decher G. Multifunctional Cytotoxic Stealth Nanoparticles. A Model Approach with Potential for Cancer Therapy. Nano Lett. 2009;9:636–642. doi: 10.1021/nl802990w. [DOI] [PubMed] [Google Scholar]; (b) Sengupta S, Eavarone D, Capila I, Zhao G, Watson N, Kiziltepe T, Sasisekharan R. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature. 2005;436:568–572. doi: 10.1038/nature03794. [DOI] [PubMed] [Google Scholar]
- 125.Jordan A. Size matters. How the tiny world of nanotechnology is having a big impact on the pharma industry. Next Generation Pharma. 2007 Available from: http://www.ngpharma.com/article/lssue-4/Drug-Discovery-AND-Development/Size-matters/ [Google Scholar]
- 126.Hayter JB, Pynn R. Structure Factor of a Magnetically Saturated Ferrofluid. Phys. Rev. Lett. 1982;49:1103–1106. [Google Scholar]
- 127.Tartaj P, Gonzalez-Carreno T, Serna CJ. Single-step nanoengineering of silica coated maghemite hollow spheres with tunable magnetic properties. Adv. Mater. 2001;13:1620–1624. [Google Scholar]
- 128.Wang C, Peng S, Lacroix LM, Sun S. Synthesis of high magnetic moment CoFe nanoparticles via interfacial diffusion in core/shell structured Co/Fe nanoparticles. Nano Res. 2009;2:380–385. [Google Scholar]
- 129.Seo WS, Lee JH, Sun X, Suzuki Y, Mann D, Liu Z, Terashima M, Yang PC, McConnell MV, Nishimura DG, Dai H. FeCo/graphitic-shell nanocrystals as advanced magnetic-resonance-imaging and near-infrared agents. Nat. Mater. 2006;5:971–976. doi: 10.1038/nmat1775. [DOI] [PubMed] [Google Scholar]
- 130.Kim DH, Rozhkova EA, Ulasov IV, Bader SD, Rajh T, Lesniak MS, Novosad V. Biofunctionalized magnetic-vortex microdiscs for targeted cancer-cell destruction. Nat. Mater. 2009;9:165–171. doi: 10.1038/nmat2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mohammad F, Balaji G, Weber A, Uppu RM, Kumar CSSR. Influence of gold nanoshell on Hyperthermia of superparamagnetic iron oxide nanoparticles. J. Phys. Chem. C. 2010;114:19194–19201. doi: 10.1021/jp105807r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pollert E, Kaman O, Veverka P, Veverka M, Marysko M, Zaveta K, Kácenka M, Lukes I, Jendelová P, Kaspar P, Burian M, Herynek V. Core-shell La1-xSrxMnO3 nanoparticles as colloidal mediators for magnetic fluid hyperthermia. Phil. Trans. R. Soc. A. 2010;368:4389–4405. doi: 10.1098/rsta.2010.0123. [DOI] [PubMed] [Google Scholar]
- 133.Zeng H, Li J, L Wang Z, Liu JP, Sun S. Bimagnetic Core/Shell FePt/Fe3O4 Nanoparticles. Nano Lett. 2004;4:187–190. [Google Scholar]
- 134.Narayanan TN, Shaijumon MM, Ajayan PM, Anantharaman MR. Synthesis of high coercivity core-shell nanorods based on Nickel and Cobalt and their magnetic properties. Nanoscale Res. Lett. 2010;5:164–168. doi: 10.1007/s11671-009-9459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Habib AH, L Ondeck C, Chaudhary P, Bockstaller MR, McHenry ME. Evaluation of Iron-Cobalt/Ferrite core-shell nanoparticles for cancer thermotherapy. J. Appl. Phys. 2008;103:07A307. [Google Scholar]
- 136.Mu Q, Yang L, Davis JC, Vankayala R, Hwang KC, Zhao J, Yan B. Biocompatibility of polymer grafted core/shell iron/carbon nanoparticles. Biomaterials. 2010;31:5083–5090. doi: 10.1016/j.biomaterials.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Villanueva A, de la Presa P, Alonso JM, Rueda T, Martinez A, Crespo P, Morales MP, Gonzalez-Fernandez MA, Valdes J, Rivero G. Hyperthermia HeLa Cell Treatment with Silica-Coated Manganese Oxide Nanoparticles. J. Phys. Chem. C. 2010;114:1976–1981. [Google Scholar]
- 138.Zhang H, Pan D, Zou K, He J, Duan X. A novel core-shell structured magnetic organic-inorganic nanohybrid involving drug-intercalated layered double hydroxides coated on a magnesium ferrite core for magnetically controlled drug release. J. Mater. Chem. 2009;19:3069–3077. [Google Scholar]
- 139.Liu J, Qiao S, Hartono SB, Lu G. Monodisperse Yolk-Shell Nanoparticles with a Hierarchical Porous Structure for Delivery Vehicles and Nanoreactors. Angew. Chem. Int. Ed. 2010;49:4981–4985. doi: 10.1002/anie.201001252. [DOI] [PubMed] [Google Scholar]
- 140.Tilly W, Wust P, Rau B, Harder C, Gellermann J, Schlag P, Budach V, Felix R. Temperature data and specific absorption rates in pelvic tumours: Predictive factors and correlations. Intl. J. Hyperther. 2001;17:172–188. doi: 10.1080/02656730150502323. [DOI] [PubMed] [Google Scholar]
- 141.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, metabolic and microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 142.Prasad KN, Rathinasamy K, Panda D, Bahadur D. Mechanism of cell death induced by magnetic hyperthermia with nanoparticles of γ-MnxFe2-xO3 synthesized by a single step process. J. Mater. Chem. 2007;17:5042–5051. [Google Scholar]
- 143.Bagaria HG, Johnson DT. Transient solution to the bioheat equation and optimization for magnetic fluid hyperthermia treatment. Int. J. Hyperther. 2005;21:57–75. doi: 10.1080/02656730410001726956. [DOI] [PubMed] [Google Scholar]
- 144.Wissler EH. Pennes' 1948 paper revisited. J. Appl. Physiol. 1998;85:35–41. doi: 10.1152/jappl.1998.85.1.35. [DOI] [PubMed] [Google Scholar]
- 145.Phillips JL, Bagaria HG, Johnson DT. AlChE annual conference. Austin, TX: 2004. Nov, Simulations of the Heat Generation in Magnetic Fluid Hyperthermia Cancer Treatment. [Google Scholar]
- 146.Herrera AP, Rodríguez M, Torres-Lugo M, Rinaldi C. Multifunctional magnetite nanoparticles coated with fluorescent thermo-responsice polymeric shells. J. Mater. Chem. 2008;18:855–858. [Google Scholar]
- 147.Kim DH, Nikles DE, Johnson DT, Brazel CS. Heat generation of aqueously dispersed CoFe2O4 nanoparticles as heating agents for magnetically activated drug delivery and hyperthermia. J. Magn. Magn. Mater. 2008;320:2390–2396. [Google Scholar]
- 148.Lammers T, Kiessling F, Hennink WE, Storm G. Nanotheranostics and Image-Guided Drug Delivery: Current Concepts and Future Directions. Mol. Pharmaceutics. 2010 doi: 10.1021/mp100228v. [DOI] [PubMed] [Google Scholar]
- 149.Gordon RT, Hines JR, Gordon D. Intracellular hyperthermia a biophysical approach to cancer treatment via intracellular temperature and biophysical alterations. Med. Hypothesis. 1979;5:83–102. doi: 10.1016/0306-9877(79)90063-x. [DOI] [PubMed] [Google Scholar]
- 150.Jordan A, Scholz R, Schtiler J, Wust P, Felix R. Arrhenius analysis of the thermal response of human colonic adenocarcinoma cells in vitro using the multi-target, single-hit and the linear-quadratic model. Int. J. Hyperther. 1997;l3:83–88. doi: 10.3109/02656739709056432. [DOI] [PubMed] [Google Scholar]
- 151.Pollert E, Veverka P, Veverka M, Kaman O, Záv̌eta K, Vasseur S, Epherre R, Goglio G, Duguet E. Search of new core materials for magnetic fluid hyperthermia: preliminary chemical and physical issues. Prog. Solid State Chem. 2009;37:1–14. [Google Scholar]
- 152.Jain RK. The next frontier of molecular medicine: Delivery of therapeutics. Nat. Med. 1998;4:655–657. doi: 10.1038/nm0698-655. [DOI] [PubMed] [Google Scholar]
- 153.Yuzuru I, Toru N, Le B, Kabata IS, Norifumi O, Takeshi K, Yoshitaka S, Naoya K, Hayao N. A carbohydrate recognition-based drug delivery and controlled release system using intraperitoneal macrophages as a cellular vehicle. Cancer Res. 2006;66:8740–8748. doi: 10.1158/0008-5472.CAN-06-0470. [DOI] [PubMed] [Google Scholar]
- 154.Branca T, Cleveland ZI, Leuschner C, Kumar C, Fubara B, Maronpot RR, Warren W, Driehuys B. Hyperpolarized 3He MRI to Detect Lung Metastases Targeted by Magnetic Nanoparticles. Proc. Natl. Acad. Sci. 2010 doi: 10.1073/pnas.1000386107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Park BH, Koo BS, Kim YK, Kim MK. The Induction of Hyperthermia in Rabbit Liver by means of Duplex Stainless Steel Thermoseeds. Korean J. Radiol. 2002;3:98–104. doi: 10.3348/kjr.2002.3.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bull JMC. An update on the anticancer effects of a combination of chemotherapy and hyperthermia. Cancer Res. 1984;44:4853s–4856s. [PubMed] [Google Scholar]
- 157.Dahl O. Interaction of hyperthermia and chemotherapy. Recent Results Cancer Res. 1988;107:157–169. doi: 10.1007/978-3-642-83260-4_23. [DOI] [PubMed] [Google Scholar]
- 158.Overgaard J, Suit HD. Time-temperature relationship in hyperthermic treatment of malignant and normal tissue in vivo. Cancer Res. 1979;39:3248–3253. [PubMed] [Google Scholar]
- 159.Nikiforov VN. Magnetic induction hyperthermia. Russian Phys. J. 2007;50:913–924. [Google Scholar]
- 160.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable, polymeric nanoparticles as drug delivery devices. J. Control Release. 2001;70:1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 161.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008;5:505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bagaria HG, Johnson DT, Nikles DE. AlChE Annual Proceedings. 2004 [Google Scholar]
- 163.(a) Haberzettl CA. Nanomedicine: destination or journey? Nanotechnology. 2002;13:R9–R13. [Google Scholar]; (b) Whitesides GM. The once and future nanomachine. Sci. Am. 2001;285:78–83. doi: 10.1038/scientificamerican0901-78. [DOI] [PubMed] [Google Scholar]
- 164.Kumar CSSR. Nanotechnology Tools in Pharmaceutical R&D. Mater. Today. 2010;12:24–30. [Google Scholar]
- 165.(a) Chalkidou A, Simeonidis K, Angelakeris M, Samaras T, Martinez-Boubeta C, Balcells L, Papazisis K, Dendrinou-Samara C, Kalogirou O. O. In vitro application of Fe/MgO nanoparticles as magnetically mediated hyperthermia agents for cancer treatment. Journal of Magnetism and Magnetic Materials. 2011;323:775–780. [Google Scholar]; (b) Martinez-Boubeta C, Balcells L, Cristofol R, Sanfeliu C, Rodriguez E, Weissleder R, Lope-Piedrafita S, Simeonidis K, Angelakeris M, Sandiumenge F. Self-assembled multifunctional Fe/MgO nanospheres for magnetic resonance imaging and hyperthermia. Nanomedicine. 2010;6:362–370. doi: 10.1016/j.nano.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 166.Li F, Yan W, Guo Y, Qi H, Zhou H. Preparation of carboplatin-Fe@C-loaded chitosan nanoparticles and study on hyperthermia combined with pharmacotherapy for liver cancer. International Journal of Hyperthermia. 2009;25:383–391. doi: 10.1080/02656730902834949. [DOI] [PubMed] [Google Scholar]
- 167.Ito A, Fujioka M, Yoshida T, Wakamatsu K, Ito S, Yamashita T, Jimbow K, Honda H. 4-S-cysteaminylphenol-loaded magnetite cationic liposomes for combination therapy of hyperthermia with chemotherapy against malignant melanoma. Cancer Science. 2007;98:424–430. doi: 10.1111/j.1349-7006.2006.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Villanueva A, de la Presa P, Alonso JM, Rueda T, Martinez A, Crespo P, Morales MP, Gonzalez-Fernandez MA, Valdes J, Rivero G. Hyperthermia HeLa Cell Treatment with Silica-Coated Manganese Oxide Nanoparticles. Journal of Physical Chemistry C. 2010;114:1976–1981. [Google Scholar]
- 169.Gonzalez-Fernandez MA, Torres TE, Andrés-Vergés M, Costo R, de la Presa P, Serna CJ, Morales MP, Marquina C, Ibarra MR, Goya GF. Magnetic nanoparticles for power absorption: Optimizing size, shape and magnetic properties. Journal of Solid State Chemistry. 2009;182:2779–2784. [Google Scholar]