Abstract
5-Fluorouracil (5-FU) and 5-fluorodeoxyuridine (FdUrd, floxuridine) have activity in multiple tumors, and both agents undergo intracellular processing to active metabolites that disrupt RNA and DNA metabolism. These agents cause imbalances in dNTP levels and the accumulation of uracil and 5-FU in the genome, events that activate the ATR-and ATM-dependent checkpoint signaling pathways as well as the base excision repair (BER) pathway. Here, we assessed which DNA damage response and repair processes influence 5-FU and FdUrd toxicity in ovarian cancer cells. These studies revealed that disabling the ATM, ATR or the BER pathways using small inhibitory RNAs did not affect 5-FU cytotoxicity. In stark contrast, ATR and a functional BER pathway protected FdUrd-treated cells. Consistent with a role for the BER pathway, the poly(ADP-ribose) polymerase (PARP) inhibitors ABT-888 (veliparib) and AZD2281 (olaparib) markedly synergized with FdUrd but not with 5-FU in ovarian cancer cell lines. Furthermore, ABT-888 synergized with FdUrd far more effectively than to other agents commonly used to treat ovarian cancer. These findings underscore differences in the cytotoxic mechanisms of 5-FU and FdUrd and suggest that combining FdUrd and PARP inhibitors may be an innovative therapeutic strategy for ovarian tumors.
Keywords: Base excision repair, checkpoints, ovarian cancer, 5-fluorouracil, floxuridine, poly(ADP-ribose) polymerase
INTRODUCTION
5-Fluorouracil (5-FU) has activity in multiple neoplastic diseases and is one of the mostly widely used chemotherapy agents. 5-FU enters cells by facilitated transport and undergoes extensive metabolism to multiple active metabolites [Fig. 1A, Rev. in (1)]. On one hand, 5-FU can be converted to the ribonucleotide FUTP (5-fluorouridine triphosphate), which exerts cytotoxic activity when it is incorporated into RNAs by RNA polymerases. On the other hand, 5-FU also has complex effects on DNA replication following its conversion to the active metabolites FdUMP (5-fluorodeoxyuridine monophosphate) and FdUTP (5-fluorodeoxyuridine triphosphate). Whereas FdUTP is incorporated directly into DNA, FdUMP inhibits thymidylate synthase, resulting in depletion of dTTP, accumulation of dUTP and its subsequent incorporation into DNA, and disruption of dNTP ratios.
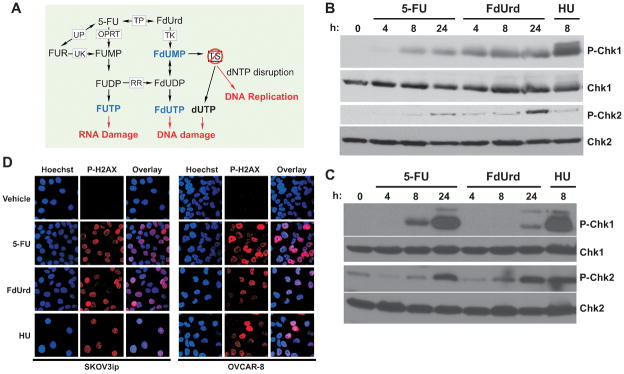
Figure 1.
5-FU and FdUrd activate ATM and ATR checkpoints and induce DNA damage in ovarian cancer cells. (A) Intracellular metabolism of 5-FU and FdUrd. (B,C) OVCAR-8 (B) and SKOV3ip (C) cells were treated with 300 μM 5-FU, 300 μM FdUrd, or 10 mM hydroxyurea (HU) for the indicated times. Cell extracts were blotted for phospho-Ser317-Chk1 (P-Chk1), P-Thr68-Chk2 (P-Chk2), Chk1, and Chk2. (D) SKOV3ip and OVCAR-8 cells were treated with 300 μM 5-FU, 300 μM FdUrd for 24 h or 10 mM hydroxyurea (HU) for 8 h, fixed, and stained with anti-phospho-Ser139-histone H2AX (red) and with Hoechst 33342 to detect DNA (blue). TS, thymidylate synthase; TP, thymidine phosphorylase; UP, uridine phosphorylase; UK, uridine kinase; OPRT, orotate phosphoribosyltransferase; RR, ribonucleotide reductase; FUR, 5-fluorouridine; FUMP, 5-fluorouridine monophosphate; FUDP, 5-fluorouridine diphosphate; FUTP, 5-fluorouridine triphosphate; FdUMP, 5-fluorodeoxyuridine monophosphate; FdUDP, 5-fluorodeoxyuridine diphosphate; FdUTP, 5-fluorodeoxyuridine triphosphate.
In addition to being a metabolite of 5-FU, FdUrd (also known as floxuridine) is an FDA-approved drug for the treatment of hepatic colon metastases (2). Moreover, the drug has activity in multiple cancers, including ovarian cancer (3–11). Unlike 5-FU, however, FdUrd is generally believed to exert its antiproliferative effects primarily through the disruption of DNA replication (i.e., by inhibiting thymidylate synthase and/or causing the incorporation of 5-FU into genomic DNA)(12). Thus, in addition to being a useful clinical agent, FdUrd is also frequently used by basic researchers as a means to specifically focus on 5-FU’s DNA-directed effects.
Nucleoside analogs, including 5-FU and FdUrd, disrupt dNTP levels and are incorporated into DNA, two events that stall DNA replication and activate ATR (13–22), an apical kinase in the ATR checkpoint signaling pathway. Activated ATR phosphorylates multiple substrates, including the kinase Chk1. Collectively, ATR and Chk1 phosphorylate substrates that promote cell survival by impeding cell cycle progression, orchestrating DNA repair, and stabilizing stalled replication forks (23). Notably, however, FdUrd and 5-FU also induce double-stranded DNA breaks (24, 25), which activate the ATM signaling pathway (26), including the ATM substrate checkpoint kinase 2 (Chk2). Like the ATR pathway, the ATM pathway promotes survival of cells with double-stranded DNA breaks by blocking cell cycle progression and mobilizing DNA repair machinery. Although both the ATR and ATM signaling pathways are activated by 5-FU and FdUrd, the roles these pathways play in regulating the survival of human tumors treated with these agents have not been explored fully.
The genomically incorporated uracil (U) and 5-FU are also targets of the base excision repair (BER) machinery (12). In this repair pathway, non-bulky DNA lesions are first recognized and cleaved by a DNA glycosylase, producing an abasic site, which is further processed to a single-stranded DNA break by an endonuclease activity such as apurinic/apyrimidinic endonuclease 1 (27). The single-stranded DNA break attracts poly(ADP-ribose) polymerase 1 or 2 (collectively referred to as PARP), which subsequently poly(ADPribosyl)ates itself and other proteins, leading to the binding of the scaffolding protein XRCC1 and additional proteins required for completion of BER (28).
Despite our in-depth understanding of the BER machinery, surprisingly little is known about how U and 5-FU DNA lesions are processed in tumor cells treated with 5-FU or FdUrd. Although there are four known uracil glycosylases—UNG, SMUG1, TDG, and MBD4—that can excise these lesions in vitro, it remains unclear what roles these glycosylases play in human tumor cells (12). Similarly, the roles of the downstream repair proteins remain poorly explored in human cells, particularly the role of PARP. Given that small molecule PARP inhibitors are now in clinical development as single agents for the treatment of tumors with defects in BRCA1/BRCA2-dependent repair or as sensitizers to other chemotherapy agents (28), this question may be relevant for the development of novel therapies that include PARP inhibitors.
Here we have systematically explored the checkpoint and DNA repair processes that are important in ovarian cancer cells treated with 5-FU and FdUrd. Our studies demonstrate that 5-FU and FdUrd have distinct mechanisms of action in these tumor cells. Based on these findings, we have discovered that small molecule PARP inhibitors synergize with FdUrd but not with 5-FU, raising the possibility that a combination of FdUrd and a PARP inhibitor may have activity in ovarian cancer.
MATERIALS AND METHODS
Cell lines and culture
A2780, OVCAR-3, OVCAR-5, OVCAR-8, and SKOV3ip cells were cultured at 37° C in 5% CO2 with 10% fetal bovine serum (Atlanta Biologicals) in the following media: A2780 and OVCAR-3, RPMI-1640 supplemented with 10 μg/mL insulin (Gibco); OVCAR-5 and OVCAR-8, RPMI-1640 (Mediatech); SKOV3ip, DMEM (Mediatech); and WS1, MEM (Mediatech). OSEtsT/hTERT cells (29) were cultured in 5% CO2 at 34° C in a 1:1 mix of M199:MCDB105 (Sigma) supplemented with 10% fetal bovine serum and 20 μg/ml hygromycin B. For clonogenic assays, the above media were supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin (Mediatech). OVCAR-3 and WS1 cell lines were obtained from ATCC, which authenticated the lines by short tandem repeat profiling. OVCAR-5 and OVCAR-8 were a gift from Dominic Scudierio (National Cancer Institute). SKOV3ip, A2780, and OSEtsT/hTERT cells were gifts from Viji Shridhar (Mayo Clinic) and were genotyped shortly before acquisition. Every 3 months, all cell lines were re-initiated from cryopreserved stocks prepared immediately after receipt from the indicated sources.
Materials
Reagents were from the following suppliers: 5-fluorouracil (APP Pharmaceuticals), FdUrd (Bedford Laboratories), ABT-888 (Selleck Chemicals and ChemieTek), AZD2281 (ChemieTek), cisplatin (Teva Pharmaceuticals), gemcitabine (Eli Lilly), oxaliplatin (Tocris), carboplatin (NovaPlus), temozolomide (Cancer Therapy Evaluation Program, NCI), SuperSignal Pico West (Thermo Scientific), annexin V-FITC and Annexin Binding Buffer (BD Pharmingen). Reagents for MTS assays were obtained from Promega. All other materials were from Sigma-Aldrich.
Antibodies to the following antigens were as follows: phospho-Ser317-Chk1 (R&D Systems); phospho-Thr68-Chk2, ATR, thymidylate synthase, horseradish peroxidase-linked rabbit IgG, and horseradish peroxidase-linked mouse IgG (Cell Signaling); Chk1 (Santa Cruz Biotechnology); Chk2 and ATM (Epitomics); phospho-Ser139-H2AX (Millipore); XRCC1 (Bethyl Laboratories); fluorescein-conjugated goat anti-mouse IgG (Invitrogen); PARP1, G.G. Poirier (Université Laval, Quebec, Canada); β-actin, Sigma-Aldrich; and HSP90, D. Toft (Mayo Clinic, Rochester, MN).
Cell transfections and small interfering (si)RNAs
siRNAs (400 nmol/transfection) were mixed with 5×106 - 1×107 cells in 0.2 mL RPMI-1640 containing 10% fetal bovine serum in a 0.4-cm electroporation cuvette and electroporated with two 10-mS, 280-V pulses in a BTX T820 square wave electroporator (Harvard Apparatus, Holliston, MA). The transfected cells were cultured for 48 h before use. Sequences of siRNAs were: ATM-1, 5′-AAGCACCAGTCCAGTATTGGC-3′ (30); ATR-2, 5′-CCTCCGTGATGTTGCTTGA-3′ (31); XRCC1-2, 5′-CUCGACUCACUGUGCAGAAUU-3′ (32); XRCC1-3, 5′-CCAGGAAGATATAGACATT-3′; PARP1-1, 5′-AAGCCUCCGCUCCUGAACAAU-3′ and PARP1-2, 5′-AAGAUAGAGCGUGAAGGCGAA-3′ (33); luciferase, 5′-CTTACGCUGAGUACUUCGA-3′ (34).
Cell cycle analyses, clonogenic assays, cell lysis, immunostaining, annexin V staining, MTS assays, and cell irradiation
Cell cycle analyses, clonogenic assays, cell lysis, immunoblotting and immunostaining were performed as described (35, 36). For clonogenic assays using non-transfected cells, percent survivals of all individual and combination treatments were normalized to cells treated with vehicle only. For clonogenic assays using cells transfected with siRNA, percent survivals at each drug concentration were normalized to the vehicle-treated control for the given siRNA. For MTS assays, 2000–3000 log-phase OSEtsT/hTERT or WS1 cells were plated in 96-well plates, and after 4 h the indicated concentrations of ABT-888 and FdUrd were added. The plates were incubated for 4 d, reacted with MTS and phenazine methosulfate as instructed by the supplier for 2 to 3 h, and absorbances at 490 nm were determined. All percent cell viabilities were normalized to controls treated with vehicle only. Annexin V staining was performed according to supplier’s protocols. Cells were irradiated with a RS-2000 Biological Irradiator, Rad Source (Suwanee, GA) 4–6 h after plating.
RESULTS
5-FU and FdUrd activate checkpoint kinases
Previously published results have shown that antimetabolites, including 5-FU and FdUrd, activate checkpoint signaling pathways (15–22, 37). Accordingly, we found that 5-FU and FdUrd induced phosphorylation of Chk1 and Chk2 in two ovarian cancer cell lines, OVCAR-8 (Fig. 1B) and SKOV3ip (Fig. 1C). In the SKOV3ip cells, 5-FU induced Chk1 and Chk2 activation at 8 h and 24 h, with levels similar to those seen with the ribonucleotide reductase inhibitor hydroxyurea, which was used as a positive control. In contrast, FdUrd triggered modest and delayed Chk1 phosphorylation in these cells. In OVCAR-8 cells, 5-FU induced delayed Chk1 and Chk2 activation, whereas FdUrd caused rapid Chk1 phosphorylation. Consistent with the observed effects on checkpoint signaling, 5-FU and FdUrd induced phosphorylation of histone H2AX (Fig. 1D), a marker of DNA damage (38). Taken together, these results demonstrate that both fluoropyrimidines induce DNA damage and activate the ATM and ATR checkpoint signaling pathways.
ATR but not ATM is important for FdUrd toxicity
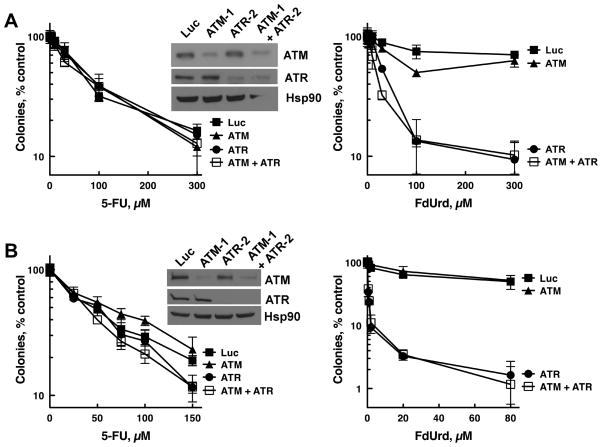
Activation of Chk1 and Chk2 suggests that signaling through ATM and/or ATR, both of which affect the survival of cells treated with multiple distinct genotoxins, may influence the toxicity of these agents. To assess how these kinases impact 5-FU and FdUrd cytotoxicity, OVCAR-8 cells were transfected with siRNAs that deplete ATM (ATM-1) and ATR (ATR-2). These siRNAs showed no cytotoxicity on their own (ATM siRNA-transfected cell plating efficiency = 106.7 ± 4.3%, mean ± SEM, n = 4; ATR-siRNA transfected cell plating efficiency = 100.3 ± 11.8%, n = 5, compared to luciferase siRNA-transfected cells) and did not affect formation of the FdUMP-thymidylate synthase complex, indicating that they did not alter uptake and/or metabolism of 5-FU and FdUrd (Fig. S1A). Surprisingly, neither ATM nor ATR depletion sensitized either cell line to 5-FU (Fig. 2A-B, left panels), demonstrating that even though the ATM and ATR pathways are activated, they do not protect these cell lines from 5-FU. Far different results were seen with FdUrd. Whereas ATM depletion (which sensitized to ionizing radiation, Fig. S1B) had no effect on FdUrd cytotoxicity, ATR depletion profoundly sensitized the cells to FdUrd (Fig. 2A-B, right panels; see Fig. S2A for re-plotted data highlighting the effects of low concentrations of FdUrd). Furthermore, cells simultaneously depleted of ATM and ATR were not more sensitive to FdUrd than cells depleted only of ATR, indicating that even when ATR levels are severely reduced, ATM does not affect FdUrd toxicity.
Figure 2.
Effects of ATM and ATR depletion on 5-FU- and FdUrd-induced cytotoxicity. (A,B) SKOV3ip (A) and OVCAR-8 (B) cells were transfected with control (Luc), ATM (siRNA ATM-1), and ATR (siRNA ATR-2) siRNAs. 48 h later cells were immunoblotted for the indicated antigens (insets) or re-plated and exposed to the indicated concentrations of 5-FU and FdUrd for 24 h, washed, and incubated for 7–10 d to allow colony formation. Data shown are a representative experiment from three independent replicates. n = 3 ± SD.
Depletion of XRCC1 and PARP1 enhances sensitivity to FdUrd but not 5-FU
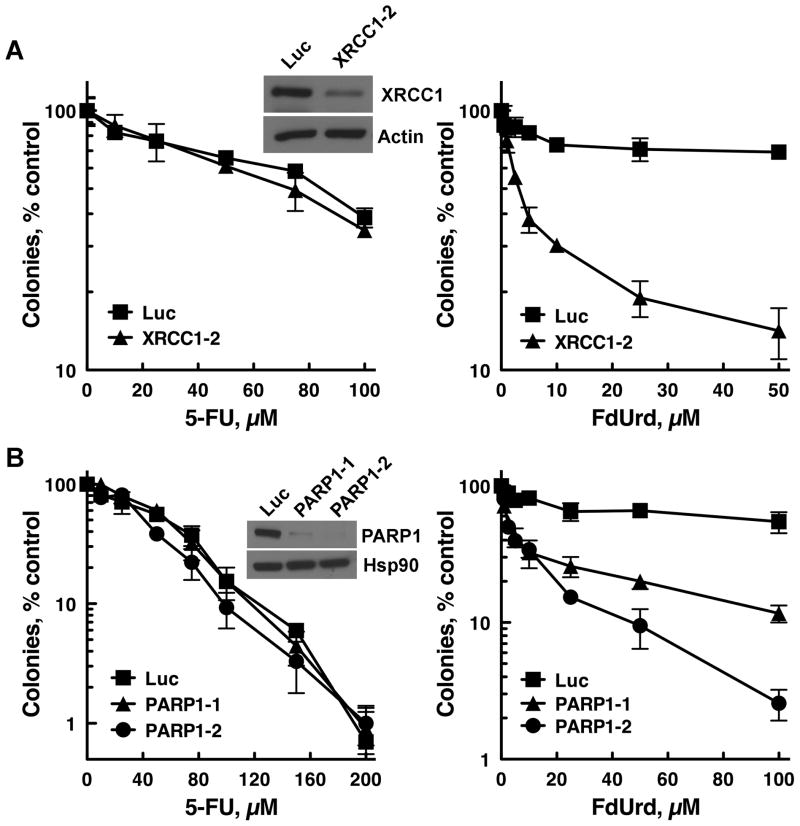
The results presented in Fig. 2 indicated that disabling checkpoint signaling affected the cytotoxicity of FdUrd but not 5-FU, raising the possibility that these agents also may differ in their requirements for DNA repair. Because genomically incorporated U and 5-FU are substrates for the BER pathway, we investigated the role of BER in the cytotoxicity of these agents by depleting OVCAR-8 cells of XRCC1, a BER scaffolding protein. siRNA-mediated reduction of XRCC1 moderately reduced plating efficiency (XRCC1-2 = 71.2 ± 5.3, mean ± SEM, n = 4; XRCC1-3 = 81.2 ± 10.3, n = 3 compared to luciferase siRNA-transfected cells). Notably, however, even after adjusting for the reduced plating efficiency, XRCC1 depletion with two different siRNAs sensitized to FdUrd, whereas it did not affect 5-FU cytotoxicity (Fig. 3A; see Fig. S2B for alternate X-axis scale of Fig. 3A; and Fig. S2C for additional XRCC1 siRNA). Similarly, depletion of PARP1 (which did not affect plating efficiency; PARP1-1 = 102.7 ± 2.5, mean ± SEM, n = 3; PARP1-2 = 101.6 ± 9.2, n = 3, compared to luciferase-transfected cells) did not sensitize to 5-FU but did sensitize to FdUrd (Fig. 3B; see Fig S2B for alternate X-axis scale of Fig. 3B). These data indicate that BER is important in ovarian cancer cells treated with FdUrd but not 5-FU. Furthermore, taken in conjunction with our checkpoint signaling studies (Fig. 2), they are consistent with the idea that 5-FU does not kill these cells by inflicting DNA damage.
Figure 3.
XRCC1 and PARP1 depletions sensitize to FdUrd but not 5-FU. (A,B) OVCAR-8 cells were transfected with control (Luc), XRCC1 (XRCC1-2) (A), or PARP1 (PARP1-1 and PARP1-2) (B) siRNAs. 48 h later cells were trypsinized and immunoblotted for the indicated antigens (insets) or re-plated and exposed to the indicated concentrations of 5-FU and FdUrd for 24 h, washed, and incubated for 7–10 d to allow colony formation. Data shown are a representative experiment from two (PARP1) or three (XRCC1) independent replicates. n = 3 ± SD.
Small molecule PARP inhibitors potentiate the antiproliferative activity of FdUrd but not 5-FU
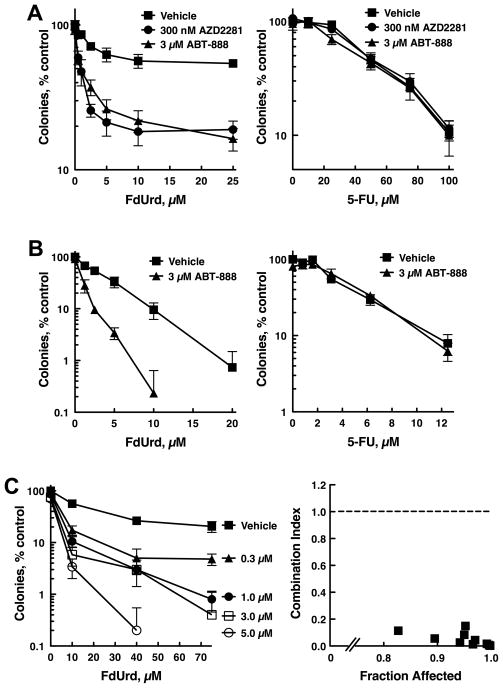
Given that PARP1 depletion increased the cytotoxicity of FdUrd, we reasoned that small molecule inhibitors of PARP would affect the sensitivity of ovarian cancer cells to this agent. Continuous exposure to either AZD2281 (olaparib) or ABT-888 (veliparib), two PARP inhibitors currently in clinical trials, had minimal effects on the cloning efficiency as single agents, with 3 μM ABT-888 reducing the surviving fraction to 90.3 ± 2.4% (mean ± SEM, n = 12). In contrast, both PARP inhibitors markedly increased killing when cells were co-exposed to FdUrd and the PARP inhibitor for 24 h (Fig. 4A, left panel), followed by continuous treatment with the PARP inhibitor. No such increase in cytotoxicity was seen with 5-FU and PARP inhibitor co-exposure (Fig. 4A, right panel).
Figure 4.
PARP inhibitors sensitize OVCAR-8 cells to FdUrd but not 5-FU. (A) OVCAR-8 cells were exposed to the indicated concentrations of FdUrd (left) or 5-FU (right) along with vehicle, 3 μM ABT-888 or 300 nM AZD2281 for 24 h. Following washing, ABT-888 and AZD2281 were re-added to the plates initially exposed to these agents, and cells were cultured in the continued presence of ABT-888 and AZD2281 for 8 d until colonies formed. (B) OVCAR-8 cells were exposed continuously to the indicated agents for 8 d. (C, left panel) OVCAR-8 cells treated as in (A) except that the indicated concentrations of ABT-888 were used. Data shown are a representative experiment from three independent replicates. n = 3 ± SD. (C, right panel) Synergy between FdUrd and ABT-888 was calculated from the data in (C, left panel) using the median effect method and assuming that the agents are mutually exclusive. Combination index values less than 1 indicate synergy.
To explore the effects of ABT-888 when cells are exposed to lower concentrations of FdUrd and 5-FU for extended periods, OVCAR-8 cells were treated with FdUrd or 5-FU plus ABT-888 for the duration of the clonogenic assay (8 d). As shown in Fig. 4B, the concentrations of 5-FU and FdUrd that inhibited proliferation by 50% (IC50) were reduced when cells were continuously exposed to these agents, and were similar to the IC50s of colon cancer cell lines that have been extensively studied with these fluoropyrmidines (Fig. S3). Importantly, using this exposure paradigm, ABT-888 also potentiated the effects of FdUrd but not 5-FU in OVCAR-8 (Fig. 4B) and SKOV3ip cells (Fig. S4A). Further experiments showed that even when the clonogenic assays were performed with dialyzed fetal bovine serum, which lacks thymidine and therefore enhances 5-FU’s DNA-directed cytotoxicity (39), ABT-888 still did not increase cell killing by 5-FU (Fig. S4B), further demonstrating that 5-FU does not exert its antiproliferative effects by causing DNA damage in these cells. Additionally, as was seen with the siRNAs employed earlier, treatment with the PARP inhibitor ABT-888 did not alter formation of the FdUMP-thymidylate synthase complex in response to treatment with 5-FU and FdUrd (Fig. S4C).
Given that cells treated continuously with 3 μM ABT-888 had modestly reduced survival compared to vehicle-treated control cells, we next asked whether the cytotoxicity of ABT-888 and FdUrd was synergistic. OVCAR-8 cells were treated with increasing concentrations of FdUrd plus the indicated concentrations of ABT-888 for 24 h (Fig. 4C, left panel). After washing, the initial concentrations of ABT-888 were then re-added to the cultures, which were incubated until colonies formed. From these data, we conducted a formal analysis of synergy using the median effect method of Chou and Talalay (40), assuming that the agents were mutually exclusive. This analysis revealed that the combination indices for all the data points were far below 1 (Fig. 4C, right panel), thus indicating strong synergistic killing over a wide range of concentrations.
Of final note, these studies showed that ABT-888 concentrations as low as 0.3 μM synergized with FdUrd, with higher ABT-888 concentrations even more effectively enhancing FdUrd-induced cytotoxicity. Notably, in human Phase 0 clinical trials, peak plasma concentrations of ABT-888 were 0.6 μM and 0.9 μM for patients treated with a single oral dose of 25 mg or 50 mg ABT-888, respectively (41), thus demonstrating that concentrations of ABT-888 achieved in human serum following a single dose of ABT-888 synergize with FdUrd.
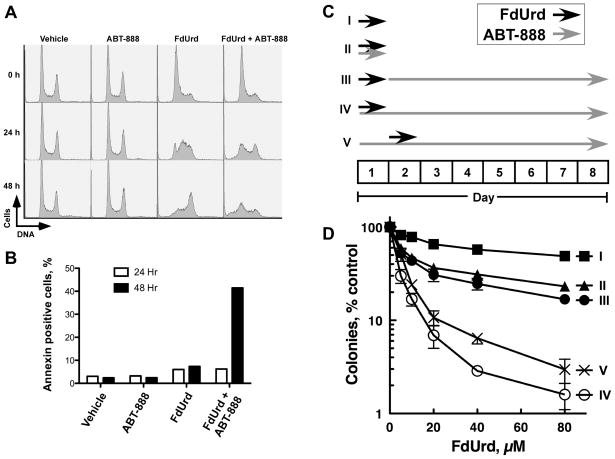
ABT-888 prevents recovery from FdUrd-induced cell cycle arrest and promotes FdUrd-induced apoptosis
To further understand the effects of these agents on cells, we examined how ABT-888 alone, FdUrd alone, and the combination of these two agents (FdUrd+ABT-888) influenced the cell cycle of OVCAR-8 cells. Identical culture plates were exposed for 24 h to ABT-888 alone, FdUrd alone, or the combination (FdUrd+ABT-888), washed, and re-fed with medium or with medium containing ABT-888 (for cells that were initially exposed to ABT-888). Plates were then harvested immediately (0 h) or after incubation for an additional 24 h or 48 h. ABT-888 alone had no effect on the cell cycle at any time point (Fig. 5A). In contrast, 24-h exposure to FdUrd alone caused a G1/S-phase arrest. Following removal of the FdUrd, the G1/S-phase-arrested cells moved synchronously through S phase and into G2/M. Similarly, 24-h exposure to FdUrd+ABT-888 caused a G1/S-phase arrest. However, following removal of the FdUrd (and in the continued presence of ABT-888) the cells accumulated in early S phase and in G2/M. Additionally, at the 48-h time point cells with sub-G1 levels of DNA appeared, suggesting that the cells were undergoing apoptosis. Indeed, over 40% of the cells treated with FdUrd+ABT-888 were annexin V-positive, another marker for apoptotic cells, at the 48 h time point (Fig. 5B), with near-background numbers of annexin V-positive cells in all other treated samples. Taken together, these results demonstrate that although ABT-888 does not affect cell cycle progression in untreated cells, this PARP inhibitor dramatically slows the progression of cells with FdUrd-induced lesions and promotes apoptosis.
Figure 5.
ABT-888 blocks recovery from FdUrd-induced cell cycle arrest, enhances FdUrd-induced apoptosis, and maximally increases killing when present during and after FdUrd exposure. (A) OVCAR-8 cells were incubated with vehicle, 3 μM ABT-888, 25 μM FdUrd, or 3 μM ABT-888 and 25 μM FdUrd for 24 h. One set of cells was immediately stained with propidium iodide (0 h). The remaining samples were washed, ABT-888 re-added (to the samples initially exposed to ABT-888), and stained 24 h and 48 h later. (B) Cells were treated as in (A) and stained with annexin V-FITC 24 h and 48 hr after removal of FdUrd (and re-addition of ABT-888 to samples initially exposed to ABT-888). Apoptosis was measured as the percentage of annexin V-positive cells. (C, D) OVCAR-8 cells were plated, treated with indicated concentrations of FdUrd and 3 μM ABT-888 using the exposure schemes depicted in (C) and assayed for clonogenicity (D).
ABT-888 is most effective when present during and after the FdUrd exposure
For the experiments shown in Fig. 4, ABT-888 was present during and after the FdUrd exposure period. However, it was unclear when ABT-888 exposure would most effectively synergize with FdUrd. We therefore compared a series of FdUrd and ABT-888 exposure schemes (Fig. 5C). Modestly increased cytotoxicity was observed when OVCAR-8 cells were exposed to FdUrd and ABT-888 simultaneously for 24 h (Sequence II), compared to FdUrd alone (Sequence I)(Fig. 5D). Similarly, exposure to FdUrd alone for 24 h followed by continuous incubation with ABT-888 modestly increased cytotoxicity over FdUrd alone (Sequence III). In contrast, the most robust killing was seen with Sequences IV and V, in which cells were simultaneously exposed to FdUrd and ABT-888, followed by continuous ABT-888 treatment after FdUrd removal.
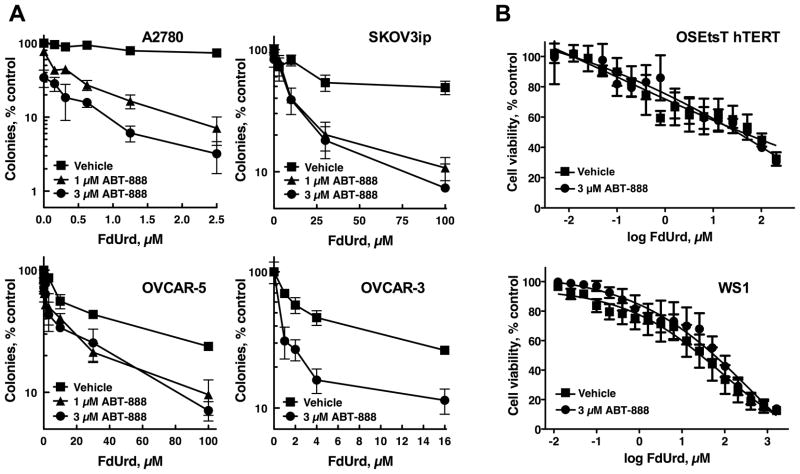
PARP inhibition synergizes with FdUrd in multiple ovarian cancer cell lines but not in normal cells
To determine whether FdUrd and ABT-888 synergized in other ovarian cancer cell lines, we assessed these agents in the ovarian cancer cell lines A2780, OVCAR-3, OVCAR-5, and SKOV3ip. ABT-888 robustly potentiated the activity of FdUrd in A2780, OVCAR-3, and SKOV3ip cells, with modest effects seen in OVCAR-5 cells (Fig. 6A). Formal analyses of synergy showed that this killing was synergistic across a wide range of concentrations in the A2780, OVCAR3, and SKOV3ip cells (Fig. S5A). In contrast, ABT-888 did not alter the cytotoxicity of FdUrd in OSEtsT/hTERT (29), which are immortalized, non-transformed ovarian surface epithelial cells, or in WS1 cells (Fig. 6B), normal human fibroblasts that undergo a limited number of replications (42).
Figure 6.
ABT-888 sensitizes multiple ovarian cancer cell lines but not normal cells to FdUrd. (A) A2780, SKOV3ip, OVCAR-5, and OVCAR-3 ovarian cancer cells were treated with FdUrd for 24 h in combination with vehicle or the indicated concentrations of ABT-888 for 24 h. Following washing, ABT-888 was re-added to samples initially exposed to ABT-888, and cells were cultured until colonies formed. Data shown are a representative experiment from three independent replicates. n = 3 ± SD. (B) OSEtsT/hTERT immortalized ovarian surface epithelial cells and WS1 human fibroblasts were treated FdUrd with and without 3 μM ABT-888. Cell viability was assessed via MTS assay. Data shown are the averages of three independent experiments. n = 3 ± SEM.
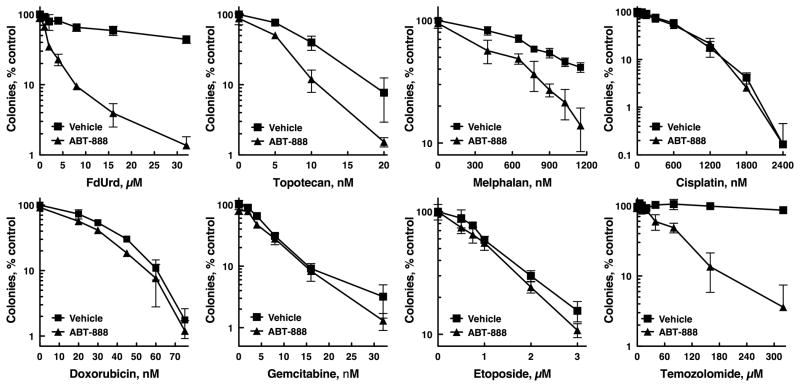
Comparison of FdUrd plus ABT-888 to other chemotherapy plus ABT-888 combinations
PARP inhibition has been reported to sensitize tumor cells to multiple chemotherapy agents (28). We therefore evaluated the relative ability of ABT-888 to sensitize to various therapies that are used in the treatment of ovarian cancer. Consistent with published results, ABT-888 sensitized OVCAR-8 cells to the topoisomerase I poison, topotecan (Fig. 7)(28). Similarly, ABT-888 modestly increased the antiproliferative effect of the nitrogen mustard melphalan. In contrast, ABT-888 did not sensitize to the platinating agents, cisplatin, oxaliplatin, carboplatin; the anthracycline antibiotic doxorubicin; the nucleoside analog gemcitabine; the topoisomerase II poison etoposide; or the antimitotic agent vinorelbine (Fig. 7 and Fig. S5B). As a control we also assessed the effects of ABT-888 on temozolomide, an alkylating agent that induces lesions repaired by BER (43). Notably, due to the profound sensitizing effect of ABT-888 to temozolomide (28), multiple clinical trials combining ABT-888 with temozolomide are now underway (44). In this head-to-head comparison, ABT-888 sensitized these cells to FdUrd as effectively as it sensitized to temozolomide (Fig. 7, compare upper left panel to lower right panel).
Figure 7.
ABT-888 sensitizes to FdUrd and temozolomide more effectively than to other chemotherapy agents. OVCAR-8 cells were treated with indicated concentrations of FdUrd, topotecan, melphalan, cisplatin, doxorubicin, gemcitabine, etoposide, and temozolomide in the presence or absence of 3 μM ABT-888 for 24 h. Following washing, ABT-888 was re-added to samples initially exposed to ABT-888, and cells were cultured until colonies formed. Data shown are a representative experiment from two independent replicates. n = 3 ± SD. Experiments with FdUrd, topotecan, melphalan, and temozolomide were independently replicated three times.
DISCUSSION
Despite intense study for over five decades, it remains unclear whether 5-FU exerts cytotoxicity primarily by disrupting DNA replication (via inhibition of thymidylate synthase and/or nucleotide mis-incorporation) or by incorporation into RNA in human tumor cell lines. In the present report, we have systematically assessed the roles of the ATR and ATM checkpoint signaling pathways and BER to understand how 5-FU kills ovarian cancer cells with the long-term goal of identifying novel therapies for this disease. Our results demonstrate that although 5-FU activates both the ATR and ATM checkpoint signaling pathways and causes DNA damage (as indicated by phosphorylation of H2AX), these pathways do not affect the survival of cells treated with 5-FU. Similarly, our studies of the BER pathway demonstrated that disrupting this repair pathway by depleting XRCC1 or disabling PARP1 (with siRNA depletion or small molecule inhibition) did not sensitize these cell lines to 5-FU. Taken together, these results suggest that even though 5-FU is causing DNA damage, its primary cytotoxic effect is due to the disruption of a different cellular process, which most likely depends on the incorporation of 5-FU into RNA.
Our results on the roles of the checkpoint signaling pathways in 5-FU-treated ovarian cancer differ from findings reported in cell lines derived from other types of cancer. In these previously published studies, disruption of ATR or Chk1 sensitized RKO, SW620, HCT116 parental, and p53−/− HCT116 cells (derived from colon cancer), Panc-1 (derived from pancreatic cancer), and HeLa cells (derived from endometrial cancer) to 5-FU (17, 21, 22, 37, 45). In contrast, we found that depletion of ATR alone, ATM alone, or even simultaneous ATM and ATR depletion did not sensitize either cell line to 5-FU. Given that in the same experiments the ATM depletion effectively sensitized to ionizing radiation (Fig. S1B) and that ATR depletion dramatically sensitized to FdUrd (Fig. 2A-B), it is unlikely that our results are explained by insufficient depletion of these checkpoint kinases. Instead, our findings suggest that unknown molecular differences among these cell lines may underlie these divergent findings.
In contrast to our findings with 5-FU, our studies with FdUrd found that depletion of ATR, XRCC1, and PARP1 sensitized OVCAR-8 cells to FdUrd, consistent with observations that FdUrd primarily exerts its antiproliferative effects in human cells by disrupting DNA replication (12). Nonetheless, our results differ from some of the published findings in rodent cells treated with FdUrd. Mouse embryo fibroblasts lacking DNA polymerase β (Polb−/− cells), a polymerase that participates in the final steps of short-patch BER, and XRCC1-deficient Chinese hamster ovary cells were not more sensitive to FdUrd (12), suggesting that BER was not important for repair of the lesions induced by FdUrd. In contrast, McNiell et al, found that expression of a catalytically inactive, dominant negative APE1 mutant sensitized Chinese hamster ovary cells to FdUrd (46). Consistent with the latter result, we found that BER plays a critical role in the repair of FdUrd-inflicted lesions in human ovarian cancer cells.
Our finding that XRCC1 and PARP1 depletion sensitized ovarian cancer cells to FdUrd immediately suggested that small molecule PARP inhibitors might also sensitize these cell lines to FdUrd. Indeed, both ABT-888 and AZD2281 robustly potentiated the antiproliferative activity of FdUrd in multiple ovarian cancer cell lines. Because ovarian cancer afflicts over 22,000 women and kills over 16,000 women yearly in the United States (47), new therapeutic options are needed. Given that 1) FdUrd has activity against ovarian cancer in clinical trials as a single agent, 2) ABT-888 sensitizes to FdUrd more effectively than to other drugs often used to treat ovarian cancer, and 3) the FdUrd plus ABT-888 combination effectively kills multiple ovarian cancer cell lines, our findings suggest that further pre-clinical studies that combine these agents are warranted.
Supplementary Material
Acknowledgments
We thank Thomas Fischer for laboratory assistance; Dominic Scuderio for OVCAR-5 and OVCAR-8 cells; Viji Shridhar for OSEtsT/hTERT, A2780, and SKOV3ip cells; Guy Poirier and David Toft for antibodies; Pam Becker for manuscript preparation; and the Mayo Flow Cytometry/Optical Morphology Core facility.
This work was supported by R01-CA084321 (LMK), P50-CA136393 (LMK), GT32-M072474 (AMH, AGP), a Mayo Clinic Eagles Pilot Project Award, and Mayo Clinic.
Footnotes
The authors have no conflicts of interest.
References
- 1.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–8. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 2.Power DG, Kemeny NE. The role of floxuridine in metastatic liver disease. Mol Cancer Ther. 2009;8:1015–25. doi: 10.1158/1535-7163.MCT-08-0709. [DOI] [PubMed] [Google Scholar]
- 3.Ardalan B, Lima M. A phase II trial of FUdR in patients with advanced pancreatic cancer. Journal of cancer research and clinical oncology. 2004;130:561–6. doi: 10.1007/s00432-004-0584-5. [DOI] [PubMed] [Google Scholar]
- 4.Vokes EE, Raschko JW, Vogelzang NJ, Warfield EE, Ratain MJ, Doroshow JH, et al. Five-day infusion of fluorodeoxyuridine with high-dose oral leucovorin: a phase I study. Cancer Chemother Pharmacol. 1991;28:69–73. doi: 10.1007/BF00684960. [DOI] [PubMed] [Google Scholar]
- 5.Damascelli B, Marchiano A, Frigerio LF, Salvetti M, Spreafico C, Garbagnati F, et al. Flexibility and efficacy of automatic continuous fluorodeoxyuridine infusion in metastases from a renal cell carcinoma. Cancer. 1991;68:995–8. doi: 10.1002/1097-0142(19910901)68:5<995::aid-cncr2820680514>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 6.Leichman L, Silberman H, Leichman CG, Spears CP, Ray M, Muggia FM, et al. Preoperative systemic chemotherapy followed by adjuvant postoperative intraperitoneal therapy for gastric cancer: a University of Southern California pilot program. J Clin Oncol. 1992;10:1933–42. doi: 10.1200/JCO.1992.10.12.1933. [DOI] [PubMed] [Google Scholar]
- 7.Newman E, Potmesil M, Ryan T, Marcus S, Hiotis S, Yee H, et al. Neoadjuvant chemotherapy, surgery, and adjuvant intraperitoneal chemotherapy in patients with locally advanced gastric or gastroesophageal junction carcinoma: a phase II study. Semin Oncol. 2005;32:S97–100. doi: 10.1053/j.seminoncol.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Brenner B, Shah MA, Karpeh MS, Gonen M, Brennan MF, Coit DG, et al. A phase II trial of neoadjuvant cisplatin-fluorouracil followed by postoperative intraperitoneal floxuridine-leucovorin in patients with locally advanced gastric cancer. Ann Oncol. 2006;17:1404–11. doi: 10.1093/annonc/mdl133. [DOI] [PubMed] [Google Scholar]
- 9.Muggia FM, Liu PY, Alberts DS, Wallace DL, O’Toole RV, Terada KY, et al. Intraperitoneal mitoxantrone or floxuridine: effects on time-to-failure and survival in patients with minimal residual ovarian cancer after second-look laparotomy--a randomized phase II study by the Southwest Oncology Group. Gynecologic oncology. 1996;61:395–402. doi: 10.1006/gyno.1996.0163. [DOI] [PubMed] [Google Scholar]
- 10.Israel VK, Jiang C, Muggia FM, Tulpule A, Jeffers S, Leichman L, et al. Intraperitoneal 5-fluoro-2′-deoxyuridine (FUDR) and (S)-leucovorin for disease predominantly confined to the peritoneal cavity: a pharmacokinetic and toxicity study. Cancer Chemother Pharmacol. 1995;37:32–8. doi: 10.1007/BF00685626. [DOI] [PubMed] [Google Scholar]
- 11.Muggia FM, Chan KK, Russell C, Colombo N, Speyer JL, Sehgal K, et al. Phase I and pharmacologic evaluation of intraperitoneal 5-fluoro-2′-deoxyuridine. Cancer Chemother Pharmacol. 1991;28:241–50. doi: 10.1007/BF00685529. [DOI] [PubMed] [Google Scholar]
- 12.Wyatt MD, Wilson DM., 3rd Participation of DNA repair in the response to 5- fluorouracil. Cell Mol Life Sci. 2009;66:788–99. doi: 10.1007/s00018-008-8557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ewald B, Sampath D, Plunkett W. Nucleoside analogs: molecular mechanisms signaling cell death. Oncogene. 2008;27:6522–37. doi: 10.1038/onc.2008.316. [DOI] [PubMed] [Google Scholar]
- 14.Sampath D, Rao VA, Plunkett W. Mechanisms of apoptosis induction by nucleoside analogs. Oncogene. 2003;22:9063–74. doi: 10.1038/sj.onc.1207229. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Matsuda A, Plunkett W. Ataxia-telangiectasia and Rad3-related and DNA- dependent protein kinase cooperate in G2 checkpoint activation by the DNA strand-breaking nucleoside analogue 2′-C-cyano-2′-deoxy-1-beta-D-arabino- pentofuranosylcytosine. Mol Cancer Ther. 2008;7:133–42. doi: 10.1158/1535-7163.MCT-07-0416. [DOI] [PubMed] [Google Scholar]
- 16.Liu A, Yoshioka K, Salerno V, Hsieh P. The mismatch repair-mediated cell cycle checkpoint response to fluorodeoxyuridine. J Cell Biochem. 2008;105:245–54. doi: 10.1002/jcb.21824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jardim MJ, Wang Q, Furumai R, Wakeman T, Goodman BK, Wang XF. Reduced ATR or Chk1 expression leads to chromosome instability and chemosensitization of mismatch repair-deficient colorectal cancer cells. Mol Biol Cell. 2009;20:3801–9. doi: 10.1091/mbc.E09-04-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilsker D, Bunz F. Loss of ataxia telangiectasia mutated- and Rad3-related function potentiates the effects of chemotherapeutic drugs on cancer cell survival. Mol Cancer Ther. 2007;6:1406–13. doi: 10.1158/1535-7163.MCT-06-0679. [DOI] [PubMed] [Google Scholar]
- 19.Parsels LA, Parsels JD, Tai DC, Coughlin DJ, Maybaum J. 5-fluoro-2′-deoxyuridine-induced cdc25A accumulation correlates with premature mitotic entry and clonogenic death in human colon cancer cells. Cancer Res. 2004;64:6588–94. doi: 10.1158/0008-5472.CAN-03-3040. [DOI] [PubMed] [Google Scholar]
- 20.Robinson HM, Jones R, Walker M, Zachos G, Brown R, Cassidy J, et al. Chk1-dependent slowing of S-phase progression protects DT40 B-lymphoma cells against killing by the nucleoside analogue 5-fluorouracil. Oncogene. 2006;25:5359–69. doi: 10.1038/sj.onc.1209532. [DOI] [PubMed] [Google Scholar]
- 21.Xiao Z, Xue J, Sowin TJ, Zhang H. Differential roles of checkpoint kinase 1, checkpoint kinase 2, and mitogen-activated protein kinase-activated protein kinase 2 in mediating DNA damage-induced cell cycle arrest: implications for cancer therapy. Mol Cancer Ther. 2006;5:1935–43. doi: 10.1158/1535-7163.MCT-06-0077. [DOI] [PubMed] [Google Scholar]
- 22.Xiao Z, Xue J, Sowin TJ, Rosenberg SH, Zhang H. A novel mechanism of checkpoint abrogation conferred by Chk1 downregulation. Oncogene. 2004;24:1403–11. doi: 10.1038/sj.onc.1208309. [DOI] [PubMed] [Google Scholar]
- 23.Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008;9:616–27. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matuo R, Sousa FG, Escargueil AE, Grivicich I, Garcia-Santos D, Chies JA, et al. 5-Fluorouracil and its active metabolite FdUMP cause DNA damage in human SW620 colon adenocarcinoma cell line. J Appl Toxicol. 2009;29:308–16. doi: 10.1002/jat.1411. [DOI] [PubMed] [Google Scholar]
- 25.El-Awady RA, Saleh EM, Dahm-Daphi J. Targeting DNA double-strand break repair: is it the right way for sensitizing cells to 5-fluorouracil? Anticancer Drugs. 2010;21:277–87. doi: 10.1097/CAD.0b013e328334b0ae. [DOI] [PubMed] [Google Scholar]
- 26.Kurz EU, Lees-Miller SP. DNA damage-induced activation of ATM and ATM- dependent signaling pathways. DNA Repair (Amst) 2004;3:889–900. doi: 10.1016/j.dnarep.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 27.Robertson AB, Klungland A, Rognes T, Leiros I. DNA repair in mammalian cells: Base excision repair: the long and short of it. Cell Mol Life Sci. 2009;66:981–93. doi: 10.1007/s00018-009-8736-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nat Rev Cancer. 2010;10:293–301. doi: 10.1038/nrc2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chien J, Narita K, Rattan R, Giri S, Shridhar R, Staub J, et al. A role for candidate tumor-suppressor gene TCEAL7 in the regulation of c-Myc activity, cyclin D1 levels and cellular transformation. Oncogene. 2008;27:7223–34. doi: 10.1038/onc.2008.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Qin J. MSH2 and ATR form a signaling module and regulate two branches of the damage response to DNA methylation. Proc Natl Acad Sci USA. 2003;100:15387–92. doi: 10.1073/pnas.2536810100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casper AM, Durkin SG, Arlt MF, Glover TW. Chromosomal instability at common fragile sites in Seckel syndrome. Am J Hum Genet. 2004;75:654–60. doi: 10.1086/422701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo H, Chan DW, Yang T, Rodriguez M, Chen BP, Leng M, et al. A new XRCC1-containing complex and its role in cellular survival of methyl methanesulfonate treatment. Mol Cell Biol. 2004;24:8356–65. doi: 10.1128/MCB.24.19.8356-8365.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kameoka M, Nukuzuma S, Itaya A, Tanaka Y, Ota K, Ikuta K, et al. RNA interference directed against Poly(ADP-Ribose) polymerase 1 efficiently suppresses human immunodeficiency virus type 1 replication in human cells. J Virol. 2004;78:8931–4. doi: 10.1128/JVI.78.16.8931-8934.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–8. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 35.Huntoon CJ, Nye MD, Geng L, Peterson KL, Flatten KS, Haluska P, et al. Heat shock protein 90 inhibition depletes LATS1 and LATS2, two regulators of the mammalian hippo tumor suppressor pathway. Cancer Res. 2010;70:8642–50. doi: 10.1158/0008-5472.CAN-10-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagner JM, Karnitz LM. Cisplatin-induced DNA damage activates replication checkpoint signaling components that differentially affect tumor cell survival. Mol Pharmacol. 2009;76:208–14. doi: 10.1124/mol.109.055178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgan MA, Parsels LA, Parsels JD, Lawrence TS, Maybaum J. The relationship of premature mitosis to cytotoxicity in response to checkpoint abrogation and antimetabolite treatment. Cell Cycle. 2006;5:1983–8. doi: 10.4161/cc.5.17.3184. [DOI] [PubMed] [Google Scholar]
- 38.Kinner A, Wu W, Staudt C, Iliakis G. Gamma-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 2008;36:5678–94. doi: 10.1093/nar/gkn550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madoc-Jones H, Bruce WR. On the mechanism of the lethal action of 5- fluorouracil on mouse L cells. Cancer Res. 1968;28:1976–81. [PubMed] [Google Scholar]
- 40.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 41.Kummar S, Kinders R, Gutierrez ME, Rubinstein L, Parchment RE, Phillips LR, et al. Phase 0 clinical trial of the poly (ADP-ribose) polymerase inhibitor ABT-888 in patients with advanced malignancies. J Clin Oncol. 2009;27:2705–11. doi: 10.1200/JCO.2008.19.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corfield VA, Hay RJ. Effects of cystine or glutamine restriction on human diploid fibroblasts in culture. In vitro. 1978;14:787–94. doi: 10.1007/BF02617973. [DOI] [PubMed] [Google Scholar]
- 43.Trivedi RN, Almeida KH, Fornsaglio JL, Schamus S, Sobol RW. The role of base excision repair in the sensitivity and resistance to temozolomide-mediated cell death. Cancer Res. 2005;65:6394–400. doi: 10.1158/0008-5472.CAN-05-0715. [DOI] [PubMed] [Google Scholar]
- 44.http://clinicaltrials.gov/ct2/results?term=abt-888+temozolomide.
- 45.Ganzinelli M, Carrassa L, Crippa F, Tavecchio M, Broggini M, Damia G. Checkpoint kinase 1 down-regulation by an inducible small interfering RNA expression system sensitized in vivo tumors to treatment with 5-fluorouracil. Clin Cancer Res. 2008;14:5131–41. doi: 10.1158/1078-0432.CCR-08-0304. [DOI] [PubMed] [Google Scholar]
- 46.McNeill DR, Lam W, DeWeese TL, Cheng YC, Wilson DM., 3rd Impairment of APE1 function enhances cellular sensitivity to clinically relevant alkylators and antimetabolites. Mol Cancer Res. 2009;7:897–906. doi: 10.1158/1541-7786.MCR-08-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics, 2009. CA: a cancer journal for clinicians. 2009;60:277–300. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.