Abstract
We recently identified adenosine monophosphate-activated protein kinase (AMPK) as a novel inducer of heme oxygenase-1 (HO-1) and surprisingly found that compound C (6-[4-(2-piperidin-1-yl-ethoxy)-phenyl]3-pyridin-4-yl-pyrazolo[1,5-a] pyrimidine), a cell-permeable inhibitor of AMPK, could also elevate HO-1 suggesting other AMPK-independent actions for this agent. In this study, we investigated the biochemical mechanism by which compound C stimulates HO-1 expression in human endothelial cells (ECs) and determined the biological significance of the induction of HO-1 by compound C in these cells. Compound C stimulated a concentration- and time-dependent increase in HO-1 expression and an increase in HO-1 promoter activity that was abrogated by mutating the antioxidant responsive elements (AREs) in the HO-1 promoter or by overexpressing a dominant negative mutant of NF-E2-related factor-2 (Nrf2). Compound C also stimulated Nrf2 expression and this was associated with an increase in the production of reactive oxygen species and with a decline in intracellular glutathione levels. Interestingly, the glutathione donor N-acetyl-L-cysteine or the NADPH oxidase inhibitor apocynin blocked the induction of HO-1 by compound C. Finally, compound C stimulated EC death and this was potentiated by silencing HO-1 expression and reversed by the administration of CO, biliverdin, or bilirubin. In conclusion, this study demonstrates that compound C stimulates HO-1 gene expression in human vascular endothelium via the activation of the Nrf2/ARE signaling pathway to counteract compound C-mediated cell death. The ability of compound C to induce HO-1 expression may contribute to the pleiotropic actions of this agent and suggest caution when using compound C to probe for AMPK functions.
Keywords: compound C, heme oxygenase-1, oxidative stress, endothelial cells
1. Introduction
Compound C (6-[4-(2-piperidin-1-yl-ethoxy)-phenyl]3-pyridin-4-yl-pyrazolo[1,5-a] pyrimidine) is a cell-permeable pyrrazolopyrimidine derivative that functions as a potent ATP-competitive inhibitor of adenosine monophosphate-activated protein kinase (AMPK) [1]. Although compound C is frequently employed to determine the role for AMPK in various biological processes, several AMPK-independent actions of compound C have been discovered. Recently, compound C has been found to inhibit the activation of hypoxia-inducible factor-1, bone morphogenetic protein type I receptors, and several kinases in an AMPK-independent fashion [2–4]. In addition, compound C exerts potent anti-proliferative and pro-apoptotic effects in various cell lines through AMPK-independent mechanisms [5–8]. Interestingly, we recently identified AMPK as a novel inducer of HO-1 in endothelial cells and surprisingly found that compound C could likewise stimulate the expression of HO-1 in these cells, suggesting another AMPK-independent action of this compound [9].
Heme oxygenase-1 (HO-1) is an inducible enzyme that oxidatively degrades heme to generate equivalent molar amounts of carbon monoxide (CO), biliverdin, and ferrous iron [10]. Subsequently, biliverdin is metabolized to bilirubin by biliverdin reductase while iron is sequestered by ferritin and either secreted by cells or recycled for heme biosynthesis. HO-1 is a highly inducible gene that is activated by a number of transcription factors; however, nuclear factor-erythroid-related 2-related factor 2 (Nrf2) has emerged as the predominant mediator of HO-1 gene transcription [11]. Activation of Nrf2 is regulated by the cytosolic protein Kelch-like erythroid cell-derived protein 1 (Keap1) that negatively modulates the nuclear translocation of Nrf2 and facilitates degradation of Nrf2 via the proteasome. Upon activation, Nrf2 enters the nucleus where it binds to the antioxidant responsive element (ARE) of the HO-1 promoter to trigger gene expression. Nrf2 contributes to the induction of HO-1 in response to various forms of cellular stress, including oxidative, nitrosative, hemodynamic, and endoplasmic reticulum stress [12–15]. The induction of HO-1 in response to stress provides a crucial defense mechanism against cell injury [16–18]. The protective functions of HO-1 are attributable to its ability to degrade the pro-oxidant and pro-inflammatory molecule heme and to its enzymatic reaction products. In particular, the bile pigments biliverdin and bilirubin are potent anti-oxidants that are capable of scavenging various oxidants while CO is a diatomic gas that prevents cell death in response to a large number of inimical stimuli.
In the present study, we investigated the mechanism by which compound C stimulates the expression of HO-1 in human endothelial cells. Furthermore, we determined the functional significance of the induction of HO-1 by compound C in vascular endothelium.
2. Materials and Methods
2.1 Materials
Penicillin, gelatin, uric acid, methyl-L-arginine, streptomycin, Nonidet P40, rotenone, antimycin A, allopurinol, dithiothreitol, sodium dodecyl sulfate (SDS), NaCl, EDTA, ferrous iron, glycerol, ethidium bromide, Triton X-100, heparin, trypan blue, N-acetyl-L-cysteine, Tris, Hepes, glutathione, tricarbonyldichlororuthenium (II) dimer (CORM2), and compound C were from Sigma-Aldrich (St. Louis, MO). Phenylmethylsulfonyl fluoride, aprotinin, leupeptin, and pepstatin A were from Roche Applied Sciences (Indianapolis, IN). M199 medium, L-glutamine, bovine calf serum, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 5-(and-6)-chloromethyl-2’,7’-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2-DCFDA), and MitoSOX red reagent were from Invitrogen Corporation (Carlsbad, CA). Bilirubin and biliverdin were from Frontier Scientific (Logan, UT). Apocynin was from EMD Chemicals (Gibbstown, NJ). Endothelial cell growth factor was from Becton Dickinson Biosciences (Bedford, MA). A polyclonal antibody against HO-1 was from Assay Designs (Ann Arbor, MI). Antibodies against Nrf2 and β-actin were from Santa Cruz Biotechnology (Santa Cruz, CA). α-[32P]dCTP (3000 Ci/mmol) was from Amersham Life Sciences (Arlington Heights, IL). The HO-1 small interference RNA (siRNA; sense:5’-AUGCUGAGUUCAUGAGGA AUU-3’, antisense:5’-PUUCCUCAUGAACUCAGCAUUU-3’) and a non-targeting siRNA (5’-AAUGGAAGACCACUCCCACUC-3’) were purchased from Dharmacon Incorporated (Lafayette, CO).
2.2 Cell culture
Human umbilical vein endothelial cells were purchased from Lonza Incorporated (Allendale, NJ) and serially cultured on gelatin-coated dishes, as we previously described [19]. Cells were grown in M199 medium supplemented with 20% bovine calf serum, 2mM L-glutamine, 50µg/ml endothelial cell growth factor, 90µg/ml heparin, and 100U/ml of penicillin and streptomycin in an atmosphere of 95% air/5% CO2.
2.3 Western blotting
Cells were scrapped in lysis buffer (125mM Tris [pH 6.8], 12.5% glycerol, 2% SDS, and trace bromophenol blue) and proteins separated by SDS-PAGE. Following transfer to nitrocellulose membranes, blots were blocked with PBS containing Triton X-100 (0.25%) and nonfat milk (5%) and then incubated with antibodies against HO-1 (1:1,500), Nrf2 (1:200), and β-actin (1:1000). Membranes were washed in PBS containing Triton X-100 (0.25%), incubated with horseradish peroxidase-conjugated goat anti-rabbit or rabbit anti-goat antibodies (Santa Cruz Biotechnologies, Santa Cruz, CA) and developed with commercial chemoluminescence reagents (Amersham, Arlington Heights, IL). Protein expression was quantified by scanning densitometry and normalized with respect to β-actin.
2.4 Northern blotting
Total RNA was isolated from cells with Trizol (Invitrogen Corporation, Carlsbad, CA) and loaded onto 1.2% agarose gels. RNA was fractionated by electrophoresis, blot transferred to Gene Screen Plus membranes (Perkin Elmer Life Sciences, Waltham, MA), and membranes prehybridized for 4 hours at 68°C in rapid hybridization buffer (Amersham, Arlington Heights, IL). Membranes were then incubated overnight at 68°C in hybridization buffer containing [32P]DNA probes (1 × 108 cpm) for HO-1 or 18S mRNA. DNA probes were generated by RT-PCR and labeled with [32P]dCTP using a random priming kit (Amersham, Arlington Heights, IL) [14,15,19]. Following hybridization, membranes were washed, exposed to X-ray film, and HO-1 expression quantified by scanning densitometry and normalized with respect to 18S rRNA.
2.5 HO-1 promoter analysis
HO-1 promoter activity was determined using HO-1 promoter/firefly luciferase constructs that were generously supplied by Dr. Jawed Alam (Ochsner Clinic Foundation, New Orleans, LA). These constructs consisted of the wild type enhancer (E1) that contains three antioxidant responsive elements (ARE) core sequences coupled to a minimum HO-1 promoter as well as the mutant enhancer (M739) that has mutations in its three ARE sequences [20,21]. In some experiments, a plasmid expressing a dominant-negative Nrf2 mutant (dnNrf2) that was provided by Dr. Jawed Alam (Ochsner Clinic Foundation, New Orleans, LA) was employed. Transfection efficiency was controlled by introducing a plasmid encoding Renilla luciferase (hRluc/TK]-Renilla luciferase; Promega, Madison, WI) into all samples. Cells were transfected with plasmids (1µg/ml) using lipofectamine (Invitrogen Corporation, Carlsbad, CA), incubated for 48 hours, and then exposed to compound C for 8 hours. Cells were then lysed and luciferase activity determined using a Glomax luminometer (Promega, Madison, WI). Firefly luciferase activity was normalized with respect to Renilla luciferase activity, and this ratio was expressed as fold induction over control cells.
2.6 Nrf2 activation
Cells were scraped into ice-cold lysis buffer (10mM HEPES, pH 7.9, 10mM KCl, 0.1mM EDTA, 0.5mm phenylmethylsulfonyl fluoride, 10µg/ml aprotonin, 10µg/ml leupeptin, 10µg/ml pepstatin A, 0.5mM dithiothreitol, and 0.4% Nonidet P-40), incubated for 10 minutes, and centrifuged at 14,000 × g for 3 minutes at 4°C. The resulting nuclear pellet was resuspended in extraction buffer (20mM HEPES, pH 7.9, 0.4M NaCl, 1.0mM EDTA, 1mM dithiothreitol, 10µg/ml aprotonin, 10µg/ml leupeptin, 10µg/ml pepstatin A, and 10% glycerol), kept on ice for 15 minutes, and centrifuged at 14,000 × g for 5 minutes at 4°C. The supernatant containing nuclear protein was collected and stored at −70°C. Nuclear Nrf2 activity was determined by measuring the binding of Nrf2 to the ARE using an ELISA-based TransAM Nrf2 kit (Active Motif, Carlsbad, CA). Nuclear extracts (5µg) were incubated with ARE consensus site oligonucleotides (5’-GTCACAGTGACTCAGCAGAATCTG-3’) immobilized to 96-well plates. Bound protein was detected using an antibody specific to DNA-bound Nrf2, visualized by colorimetric reaction catalyzed by horseradish peroxidase-conjugated secondary antibody, and absorbance measured at 405nm.
2.7 Measurement of intracellular reactive oxygen species (ROS)
The cell permeable probe CM-H2-DCFDA was used to assess ROS production, as we previously reported [19]. The dye (5µM) was preloaded to cells grown on glass coverslips for 30 minutes at 37°C. Cells were then washed with PBS and exposed to various treatment regimens. Cell fluorescence intensity was assessed using a Bio-Rad Radiance 2000 confocal system coupled to an inverted microscope at 200× using excitation and emission wavelengths of 480nm and 520nm, respectively.
2.8 Measurement of mitochondrial superoxide production
Mitochondrial superoxide production was measured in live endothelial cells using MitoSOX Red reagent (514-nm excitation/585-nm emission). This fluorogenic dye selectively enters mitochondria within living cells, where it is oxidized by superoxide anions. This oxidation reaction then emits a red fluorescence when bound to mitochondrial DNA. Endothelial cells were incubated with MitoSOX Red (2.5µM) for 20 minutes, washed with PBS, and images obtained by confocal microscopy (Zeiss LSM 510; Carl Zeiss Incorporated, Thornwood, NY) and overlaid using LSM software. Digital images were collected, corrected for autofluorescence, and background fluorescence excluded from calculations by thresholding. The mean fluorescence intensity per image was calculated using MetaMorph software (Molecular Devices, Sunnyvale, CA).
2.9 Determination of intracellular glutathione levels
Cells were lysed in 5% sulfosalicylic acid and homogenized by passing through a 26 gauge needle. Homogenates were centrifuged at 8,000 × g for 10 minutes and supernatants collected and assayed. Glutathione levels were measured using a commercial gluthathione detection kit from Biovision Research Products (Mountain View, CA). Absorbance was measured at 412nm with a μQuant spectrophotometer (Bio-Tek Instruments, Winooski, VT) and glutathione levels determined relative to a standard curve obtained using known concentrations of glutathione.
2.10 Cell Viability
Cell viability was assessed by measuring the uptake of the membrane impermeable stain trypan blue. Cells were treated with trypsin (0.25%), collected, diluted (1:4) with trypan blue and examined by microscopy. Viability was determined by the percentage of cells that excluded trypan blue, as we previously described [22]. In some cases, cell viability was also determined using the MTT assay that relies upon the cellular reduction of positively charged tetrazolium salts to their intensely colored formazan product by mitochondrial dehydrogenases. Briefly, cells were incubated with MTT (0.5 mg/ml) at 37 °C for 4 hours to allow the formation of formazan crystals. Residual MTT was carefully removed, and crystals dissolved by incubation with dimethyl sulfoxide for 10 minutes. Absorbance was measured by spectrophotometry at 540 nm wavelength using a plate reader (μQuant spectrophotometer, Bio-Tek Instruments, Winooski, VT).
2.11 Statistics
Results are expressed as mean ± SEM. Statistical analyses were performed with the use of a Student’s two-tailed t-test and an analysis of variance with the Tukey post-hoc test when more than two treatment regimens were compared. P values < 0.05 were considered statistically significant.
3. Results
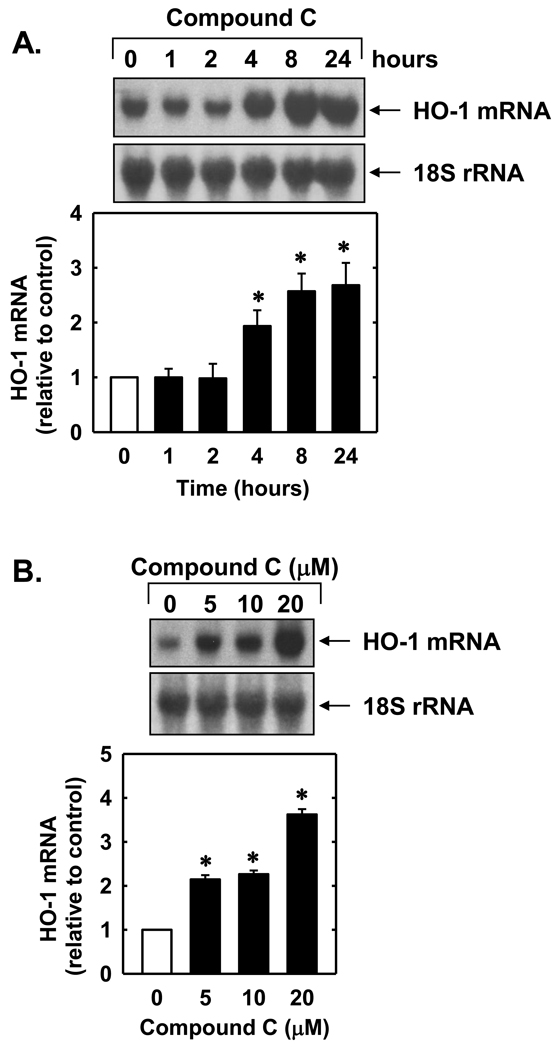
Treatment of endothelial cells with compound C stimulated a concentration- and time-dependent increase in HO-1 protein. The induction of HO-1 protein by compound C was delayed, with a significant increase in HO-1 protein appearing 8 hours after compound C administration, and levels remained elevated following 24 hours of treatment (Figure 1A). An increase in HO-1 protein was detected after 24 hours with 2µM of compound C and higher concentrations of compound C resulted in a progressive increase in HO-1 protein (Figure 1B). The induction of HO-1 protein by compound C was associated with a concentration-dependent rise in HO-1 mRNA expression that preceded the increase in HO-1 protein (Figures 2A and B). A significant and progressive increase in HO-1 mRNA was first detected 4 hours after compound C exposure.
Figure 1.
Compound C stimulates HO-1 protein expression in human endothelial cells. A. Time-course of HO-1 protein expression following the administration of compound C (20µM). B. Concentration-dependent effect of compound C (2–20µM for 24 hours) on HO-1 protein expression. HO-1 protein was quantified by scanning densitometry, normalized with respect to β-actin, and expressed relative to that of control, untreated cells. Results are means ± SEM (n=4). *Statistically significant effect of compound C.
Figure 2.
Compound C stimulates HO-1 mRNA expression in human endothelial cells. A. Time-course of HO-1 mRNA expression following the administration of compound C (20µM). B. Concentration-dependent effect of compound C (5–20µM for 24 hours) on HO-1 mRNA expression. HO-1 mRNA was quantified by scanning densitometry, normalized with respect to 18 S rRNA, and expressed relative to that of control, untreated cells. Results are means ± SEM (n=4). *Statistically significant effect of compound C.
Incubation of endothelial cells with the transcriptional inhibitor, actinomycin D (2µg/ml), suppressed basal and compound C-mediated induction of HO-1 mRNA and protein by compound C (Figures 3A and B). In contrast, the protein synthesis inhibitor, cycloheximide (5µg/ml), had no effect on basal or compound C –mediated increases in HO-1 mRNA (Figure 3B). However, cycloheximide attenuated basal HO-1 protein expression as well as the increase in HO-1 protein expression evoked by compound C (Figure 3A).
Figure 3.
Compound C-mediated HO-1 gene expression requires de novo RNA synthesis. A. Effect of actinomycin D (ActD;2µg/ml) or cycloheximide (CX;5µg/ml) on compound C (20µM for 24 hours)-mediated increase in HO-1 protein. B. Effect of actinomycin D (ActD; 2µg/ml) or cycloheximide (CX; 5µg/ml) on compound C (20µM for 8 hours)-mediated increase in HO-1 mRNA. HO-1 protein and mRNA expression were quantified by scanning densitometry, normalized with respect to β-actin or 18 S rRNA, respectively, and expressed relative to that of control, untreated cells. Results are means ± SEM (n=3). *Statistically significant effect of compound C.
To further examine the molecular mechanism by which compound C induces HO-1 gene expression we investigated whether stabilization of HO-1 mRNA contributes to the induction of HO-1. However, actinomycin D chase experiments revealed that compound C had no effect on the degradation of HO-1 mRNA (Figure 4A). Subsequently, we determined whether increases in HO-1 expression in response to compound C involve transcriptional activation of the gene. Endothelial cells were transiently transfected with a HO-1 reporter, treated with compound C, and promoter activity monitored. Compound C evoked a significant increase in HO-1 promoter activity (Figure 4B). Interestingly mutation of the AREs attenuated basal promoter activity and abrogated the response to compound C, indicating that AREs are required for the activation of HO-1 promoter. In addition, transfection of endothelial cells with a dominant-negative mutant of Nrf2 that had its activation domain deleted blocked the compound C-mediated increase in promoter activity. Furthermore, treatment of endothelial cells with compound C resulted in a marked increase in Nrf2 protein expression beginning 4 hours after compound C exposure and peaked at 8 hours (Figure 4C). Compound C also stimulated the activation of Nrf2, as reflected by the concentration-dependent increase in nuclear Nrf2 binding to the ARE (Figure 4D).
Figure 4.
Compound C stimulates HO-1 promoter activity and Nrf2 activation without affecting HO-1 mRNA stability in human endothelial cells. A. Effect of compound C on HO-1 mRNA stability. Cells were pretreated with compound C (CC;20µM for 24 hours), washed, and then exposed to actinomycin D (2µg/ml) in the presence or absence of compound C (20µM). B. Compound C stimulated HO-1 promoter activity. Cells were transfected with a HO-1 promoter construct (E1) or a mutated HO-1 promoter construct (M739) and a Renilla luciferase construct, treated with compound C (CC; 20µM for 8 hours), and then analyzed for luciferase activity. In some instances, a dominant-negative Nrf2 (dnNrf2) construct was co-transfected into cells. C. Time-course of Nrf2 protein expression after administration of compound C (20µM). D. Concentration-dependent effect of compound C (5–20µM for 8 hours) on Nrf2 activation. Protein expression was quantified by scanning densitometry, normalized with respect to β-actin, and expressed relative to that of control, untreated cells. Results are means ± SEM (n=4–6). *Statistically significant effect of compound C.
In follow-up experiments, we determined the upstream signaling pathway that stimulates Nrf2 and HO-1 expression. Since reactive oxygen species have been implicated in the activation of Nrf2 [11,23,24], the involvement of oxidative stress was examined. Treatment of endothelial cells with compound C resulted in a rise in reactive oxygen species that progressively increased over time (Figures 5A). The induction of oxidative stress by compound C was associated with a time-dependent decline in intracellular glutathione levels (Figure 5B). However, compound C failed to stimulate mitochondrial superoxide by endothelial cells (Figure 5C). Interestingly, the glutathione donor N-acetyl-L-cysteine (10mM) blocked the increase in the formation of reactive oxygen species by compound C and inhibited the induction of HO-1 in response to compound C (Figure 6A and B). N-Acetyl-L-cysteine also attenuated the compound C-mediated increase in Nrf2 protein (Figure 6C). In contrast, inhibitors of nitrosative stress, including the nitric oxide synthase inhibitor methyl-L-arginine (1mM) or the peroxynitrite scavenger uric acid (500µM) failed to block the compound C-mediated induction of HO-1 (Figure 6D).
Figure 5.
Compound C stimulates oxidative stress in human endothelial cells. A. Effect of compound C (20µM) on the production of reactive oxygen species (ROS). B. Effect of compound C (20µM) on intracellular glutathione levels. C. Effect of compound C (20µM) on mitochondrial superoxide production. Results are means ± SEM (n=4–6). *Statistically significant effect of compound C.
Figure 6.
Compound C-mediated HO-1 expression is dependent on oxidative but not nitrosative stress. A. Effect of N-acetyl-L-cysteine (NAC; 10mM) on compound C (CC;20µM for 24 hours)-mediated reactive oxygen species (ROS) production. B. Effect of NAC (10mM) on compound C (CC;20µM for 24 hours)-mediated HO-1 protein expression. C. Effect of NAC (10mM) on compound C (CC;20µM for 8 hours)-mediated Nrf2 protein expression. D. Effect of methyl-L-arginine (L-NMA; 1mM) or uric acid (UA; 500µM) on compound C (CC;20µM for 24 hours)-mediated HO-1 protein expression. Protein expression was quantified by scanning densitometry, normalized with respect to β-actin, and expressed relative to that of control, untreated cells. Results are means ± SEM (n=4). *Statistically significant effect of compound C. †Statistically significant effect of NAC.
In subsequent experiments, we investigated potential enzymatic sources of oxidants responsible for the induction of HO-1 by compound C. The role of the mitochondrial electron transport chain in mediating the induction of HO-1 in endothelial cells was tested by incubating cells with the mitochondrial complex I inhibitor rotenone (2µM) or the mitochondrial complex III inhibitor mitomycin A (10µM). However, neither mitochondrial complex inhibitor affected the induction of HO-1 by compound C (Figure 7A). Similarly, the xanthine oxidase inhibitor allopurinol (100µM) failed to block the compound C-mediated increase in HO-1 expression (Figure 7B). In contrast, the NADPH oxidase inhibitor apocynin (300µM) abolished the induction of HO-1 by compound C (Figure 7C).
Figure 7.
Compound C-mediated HO-1 expression is dependent on NADPH oxidase activity. A. Effect of rotenone (Rot;2µM) or antimycin A (AMA;10µM) on compound C (CC; 20µM for 24 hours)-mediated HO-1 protein expression. B. Effect of allopurinol (Allo;100µM) on compound C (CC; 20µM for 24 hours)-mediated HO-1 protein expression. C. Effect of apocynin (Apo;300µM) on compound C (CC;20µM for 24 hours)-mediated HO-1 protein expression. Results are means ± SEM (n=3). *Statistically significant effect of compound C.
In a final series of experiments, the functional role of HO-1 induction in endothelial cells by compound C was investigated. Treatment of endothelial cells with compound C (20µM) resulted in a significant decline in cell viability, as measured by trypan blue staining (Figure 8A). Interestingly, transfection of endothelial cells with a HO-1 siRNA (0.1µM) potentiated compound C-mediated cell toxicity whereas the non-targeting siRNA had no effect (Figure 8A). Similar results were obtained when cell viability was assessed used the MTT assay (Figure 8B). In the absence of compound C, HO-1 siRNA, or the non-targeting siRNA had no adverse effect on cell survival. In addition, transfection of endothelial cells with the HO-1 siRNA abolished basal and compound C-mediated increases in HO-1 protein (Figure 8C). In contrast, the non-targeting siRNA had no effect on HO-1 protein expression, confirming the efficacy and selectivity of the HO-1 knockdown approach. Finally, we determined which of the HO-1 products mediates this cytoprotective effect. Interestingly, incubation of HUVEC with the CO donor CORM2 (20µM), bilirubin (20µM), or biliverdin (20µM) reversed the cytotoxic effect of compound C (Figure 8D). In contrast, the addition of ferrous iron (20µM) had no effect on cell toxicity.
Figure 8.
Induction of HO-1 by compound C promotes human endothelial cell survival. A. Cell viability assessed by trypan blue exclusion following treatment of cells with compound C (20µM for 24 hours) and transfection of cells with HO-1 small interference RNA (HO-1 siRNA; 1µM) or non-targeting siRNA (NT siRNA;1µM). Results are means ± SEM (n=3). *Statistically significant effect of compound C. †Statistically significant effect of HO-1 siRNA. B. Cell viability assessed by MTT reduction following treatment of cells with compound C (20µM for 24 hours) and transfection of cells with HO-1 siRNA (1µM) or NT siRNA (1µM). Results are means ± SEM (n=3). *Statistically significant effect of compound C. †Statistically significant effect of HO-1 siRNA. C. HO-1 protein expression in cells treated with compound C (20µM for 24 hours) and transfected with HO-1 siRNA (1µM) or NT siRNA (1µM). HO-1 protein was quantified by scanning densitometry, normalized with respect to β-actin, and expressed relative to that of control, untreated cells. Results are means ± SEM (n=3). *Statistically significant effect of compound C. †Statistically significant effect of HO-1 siRNA. D. Cell viability assessed by trypan blue exclusion following treatment of cells with compound C (20µM for 24 hours) in the presence or absence of CORM2 (20µM), biliverdin (BR;20µM), bilirubin (BV;20µM), or iron (Fe;20µM). Results are means ± SEM (n=6). *Statistically significant effect of compound C. †Statistically significant effect of heme metabolites.
4. Discussion
The present study identifies compound C as a potent inducer of HO-1 gene expression in human endothelial cells. The induction of HO-1 is mediated by the Nrf2-ARE signaling pathway and is dependent on the production of reactive oxygen species. In addition, the induction of HO-1 functions to limit the cytotoxic effect of compound C through the generation of biliverdin, bilirubin, and CO. The ability of compound C to stimulate HO-1 expression may contribute to the pleiotropic actions of this agent and suggests caution when using compound C as a pharmacological probe in identifying cellular functions of AMPK.
Treatment of endothelial cells with compound C results in a concentration- and time-dependent increase in HO-1 mRNA and protein. Interestingly, increases in both HO-1 mRNA and protein are noted with concentrations between 5–20µM that are often employed to block AMPK activity in endothelial cells [25–27], demonstrating that pharmacologically relevant concentrations of compound C are able to induce HO-1 gene expression. The induction of HO-1 mRNA expression by compound C is dependent on de novo RNA synthesis since the transcriptional inhibitor actinomycin D blocks the induction of HO-1 message. Interestingly, actinomycin D suppresses basal HO-1 mRNA expression, suggesting that HO-1 gene expression is activated in endothelial cells in culture. In contrast, the increase in HO-1 mRNA does not require de novo protein synthesis as the protein synthesis inhibitor cycloheximide has no effect on the induction of HO-1. In addition, actinomycin D chase experiments revealed that HO-1 mRNA stability is unaffected by compound C. However, compound C stimulates a significant increase in HO-1 promoter activity. The stimulation of HO-1 promoter activity is dependent on the presence of AREs, since mutation of these responsive elements abrogates the increase in promoter activity by compound C. Since Nrf2 plays a dominant role in ARE-dependent HO-1 gene expression [11], we investigated the involvement of this transcription factor in mediating the induction of HO-1. Indeed, we found that compound C elevates Nrf2 expression and activity, and Nrf2 binding to the HO-1 promoter. Moreover, transfection of endothelial cells with a dominant-negative Nrf2 mutant abolishes the increase in HO-1 promoter activity in response to compound C, indicating an essential role for Nrf2 in mediating the induction of HO-1. The ability of compound C to activate Nrf2 provides a novel pathway by which this agent can regulate gene transcription.
The induction of HO-1 expression by compound C is dependent on oxidative stress. We found that compound C evokes a progressive increase in the production of reactive oxygen species that is paralleled by a decline in intracellular glutathione levels. The ability of compound C to evoke oxidative stress in endothelial cells is in agreement with recent studies in mesangial and glioma cells [5,8]. Significantly, administration of the glutathione donor N-acetyl-L-cysteine attenuates the compound C-mediated generation of reactive oxygen and induction of HO-1 expression in endothelial cells. Since nitrosative stress is a well established inducer of HO-1 [28,29] we also investigated its involvement in the induction of HO-1. However, blocking nitric oxide synthesis with the nitric oxide synthase inhibitor methyl-L-arginine fails to inhibit compound C-mediated HO-1 expression. Furthermore, uric acid which is poor scavenger of superoxide but an efficient scavenger peroxynitrite [30], has no effect on the induction of HO-1. Thus, the capacity of compound C to stimulate HO-1 expression is dependent on oxidative but not nitrosative stress. Although the precise mechanism by which compound C activates Nrf2 is not known, it likely involves the oxidation of cysteine residues in Keap1 since several cysteine residues undergo redox-dependent alterations that result in the liberation and/or inhibition Keap1-dependent ubiquitination and degradation of Nrf2 [23,24]. Consistent with this notion, we found that the antioxidant N-acetyl-L-cysteine blocks the induction of Nrf2 by compound C.
Emerging evidence indicate that mitochondria, xanthine oxidase, and NADPH oxidase are major sources of reactive oxygen species that contribute to signaling events in endothelial cells [31]. However, mitochondrial-derived oxidants are not involved in the induction of HO-1 by compound C since compound C fails to stimulate the generation of reactive oxygen by this organelle. Moreover, inhibitors of the mitochondrial electron transport chain have no effect on the induction of HO-1 by compound C. Similarly, a pharmacological inhibitor directed against xanthine oxidase fails to prevent the compound C-mediated increase in HO-1 expression. In contrast, we found that apocynin, an inhibitor of NADPH oxidase [32], abrogates the induction of HO-1 by compound C, suggesting a role for this enzyme in mediating the induction of HO-1. However, results with apocynin should be cautiously interpreted since in certain conditions this compound can exert direct anti-oxidant effects [33].
The induction of HO-1 in endothelial cells following compound C exposure likely represents an important adaptive response to ameliorate the deleterious effect of oxidative stress. Consistent with previous reports in other cells [5,7,8], we found that compound C stimulates endothelial cell death. Interestingly, compound C-mediated endothelial cell death is potentiated following knockdown of HO-1 expression, indicating that the induction of HO-1 by compound C promotes cell survival. This is consistent with studies showing that genetic deletion of HO-1 sensitizes endothelial cells to the detrimental effects of oxidative stress [34,35]. The cytoprotective action of HO-1 is likely mediated via the formation of biliverdin, bilirubin, and CO since the exogenous administration of these heme metabolites reproduces the protection afforded by HO-1. In contrast, the other HO-1 product, iron, does not protect endothelial cells against compound C. The cytoprotection provided by the bile pigments and CO may reflect their ability to restore redox balance within cells. In this regard, biliverdin and bilirubin are established scavengers of oxidants while CO can exert an anti-oxidant effect by stimulating the expression of antioxidant genes and inhibiting the activity of pro-oxidant enzymes [36–38].
In summary, the present study demonstrates that compound C stimulates HO-1 gene expression in endothelial cells via the Nrf2/ARE pathway. The induction of HO-1 by this pathway is dependent on the production of reactive oxygen species and functions to counteract compound C-mediated cell death through the formation of CO, biliverdin, and bilirubin. This study adds to a growing list of AMPK-independent actions by compound C and suggests caution when using this agent as a pharmacological tool in assessing vascular functions of AMPK. Moreover, the ability of compound C to stimulate HO-1 expression may contribute to the pleiotropic actions of this agent.
Acknowledgements
This work was supported by the National Institutes of Health Grants HL74966, HL59976, and HL77288.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMPK-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlin H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emerling BM, Viollet B, Tormos KV, Chandel NS. Compound C inhibits hypoxic activation of HIF-1-independent of AMPK. FEBS Lett. 2007;581:5727–5731. doi: 10.1016/j.febslet.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Block KD, Peterson RT. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vucicevic L, Misirkic M, Janjetovic K, Harhaji-Trajkovic, Prica M, Stevanovic D, Isenovic E, Sudar E, Sumarac-Dumonovic M, Micic D, Trajkovic V. AMPK-activated protein kinase-dependent and –independent mechanisms underlying in vitro antiglioma action of compound C. Biochem Pharmacol. 2009;77:1684–1693. doi: 10.1016/j.bcp.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Nam M, Lee WH, Bae EJ, Kim SG. Compound C inhibits clonal expansion of preadipocytes by increasing p21 level irrespective of AMPK inhibition. Arch Biochem Biophys. 2008;479:74–81. doi: 10.1016/j.abb.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Jin J, Mullen TD, Hou Q, Bielawski J, Bielawski A, Zhang X, Obeid LM, Hannun YA, Hsu Y-T. AMPK inhibitor compound C stimulates ceramide production and promotes Bax redistribution and apoptosis in MCF7 breast carcinoma cells. J Lipid Res. 2009;50:2389–2397. doi: 10.1194/jlr.M900119-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jang JH, Lee TJ, Yang ES, Min DS, Kim YH, Kim SH, Choi YH, Park J-W, Choi KS, Kwon TK. Compound C sensitizes Caki renal cancer cells to TRAIL-induced apoptosis through reactive oxygen species-mediated down-regulation of c-FLIPL and Mcl-1. Exp Cell Res. 2010;316:2194–2203. doi: 10.1016/j.yexcr.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 9.Liu XM, Peyton KJ, Shebib AR, Wang H, Korthuis RJ, Durante W. Activation of AMPK stimulates heme oxygenase-1 gene expression and human endothelial cell survival. Am J Physiol Heart Circ Physiol. 2011;300:H84–H93. doi: 10.1152/ajpheart.00749.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alam J, Cook JL. Transcriptional regulation of the heme oxygenase-1 gene via the stress response pathway. Curr Pharm Des. 2003;9:2499–2511. doi: 10.2174/1381612033453730. [DOI] [PubMed] [Google Scholar]
- 12.Ishii T, Itoh K, Ruiz E, Leake DS, Unoki H, Yamamoto M, Mann GE. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circ Res. 2004;94:609–616. doi: 10.1161/01.RES.0000119171.44657.45. [DOI] [PubMed] [Google Scholar]
- 13.Chen X-L, Varner SE, Rao AS, Grey JY, Thomas S, Cook CK, Wasserman MA, Medford RM, Jaiswal AK, Kunsch C. Laminar flow induction antioxidant response element-mediated genes in endothelial cells; a novel anti-inflammatory mechanism. J Biol Chem. 2003;278:703–711. doi: 10.1074/jbc.M203161200. [DOI] [PubMed] [Google Scholar]
- 14.Liu XM, Peyton KJ, Ensenat D, Wang H, Hannink M, Alam J, Durante W. Nitric oxide simulates heme oxygenase-1 gene transcription via the Nrf2/ARE complex to promote vascular smooth muscle cell survival. Cardiovasc Res. 2007;75:381–389. doi: 10.1016/j.cardiores.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu XM, Peyton KJ, Ensenat D, Wang H, Schafer AI, Alam J, Durante W. Endoplasmic reticulum stress stimulates heme oxygenase-1 gene expression in vascular smooth muscle cells: role in cell survival. J Biol Chem. 2005;280:872–877. doi: 10.1074/jbc.M410413200. [DOI] [PubMed] [Google Scholar]
- 16.Durante W. Targeting heme oxygenase-1 in vascular disease. Curr Drug Targets. 2010;11:1504–1516. doi: 10.2174/1389450111009011504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morse D, Lin L, Choi AM, Ryter SW. Heme oxygenase-1, a critical arbitrator of cell death in lung injury and disease. Free Radic Biol Med. 2009;47:1–12. doi: 10.1016/j.freeradbiomed.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi T, Morita K, Akagi R, Sassa S. Heme oxygenase-1: a novel therapeutic target in oxidative tissue injuries. Curr Med Chem. 2004;11:1546–1561. doi: 10.2174/0929867043365080. [DOI] [PubMed] [Google Scholar]
- 19.Lin CC, Liu XM, Peyton KJ, Wang H, Yang WC, Lin SJ, Durante W. Far infrared therapy inhibits vascular endothelial inflammation via the induction of heme oxygenase-1. Arterioscler Thromb Vasc. Biol. 2008;28:739–745. doi: 10.1161/ATVBAHA.107.160085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alam J. Multiple elements within the 5’ distal enhancer of the mouse heme oxygenase-1 gene mediate induction by heavy metals. J Biol Chem. 1994;269:25049–25056. [PubMed] [Google Scholar]
- 21.Alam J, Wick C, Stewart D, Gong P, Touchard C, Otterbein S, Choi AMK, Burow ME, Tou J. Mechanism of heme oxygenase-1 gene activation by cadmium in MCF-7 mammary epithelial cells: role of p38 kinase and Nrf2 transcription factor. J Biol Chem. 2000;275:27694–27702. doi: 10.1074/jbc.M004729200. [DOI] [PubMed] [Google Scholar]
- 22.Liu XM, Ensenat D, Wang H, Schafer AI, Durante W. Physiologic stretch inhibits apoptosis in vascular endothelium. FEBS Lett. 2003;541:52–56. doi: 10.1016/s0014-5793(03)00285-0. [DOI] [PubMed] [Google Scholar]
- 23.Itoh K, Wakabayashi N, Katoh Y, Ishii T, O’Connor Y, Yamamoto M. Keap1 regulates both cytoplasmic-nuclear shuffling and degradation of Nrf2 in response to electrophiles. Genes Cells. 2003;8:379–391. doi: 10.1046/j.1365-2443.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- 24.Zang DD, Hannink M. Distinct cysteine residues in keap1 are required for keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventative agents and oxidative stress. Mol Cell Biol. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schulz E, Dopheide J, Schuhmacher S, Thomas SR, Chen K, Daiber A, Wenzel P, Munzel T, Keaney JF., Jr Suppression of JNK pathway by induction of a metabolic stress response prevents vascular injury and dysfunction. Circulation. 2008;118:1347–1357. doi: 10.1161/CIRCULATIONAHA.108.784298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Z, Peng I-C, Sun W, Su M-I, Hsu P-H, Fu Y, Zhu Y, DeFea K, Pan S, Tsai M-D, Shyy JY-J. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase ser633. Circ Res. 2009;104:496–505. doi: 10.1161/CIRCRESAHA.108.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young A, Wu W, Sun W, Benjamin Larmin H, Wang N, Li YS, Shyy JY, Chien S, Garcia-Cardena G. Flow activation of AMPK-activated protein kinase in vascular endothelium leads to Kruppel-like factor 2 expression. Arterioscler. Thromb. Vasc Biol. 2009;29:1902–1908. doi: 10.1161/ATVBAHA.109.193540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motterlini R, Foresti R, Intaglietta M, Winslow RM. NO-mediated activation of heme oxygenase: endogenous cytoprotection against oxidative stress to the endothelium. Am J Physiol Heart Circ Physiol. 1996;270:H107–H114. doi: 10.1152/ajpheart.1996.270.1.H107. [DOI] [PubMed] [Google Scholar]
- 29.Durante W, Kroll MH, Christodoulides N, Peyton KJ, Schafer AI. Nitric oxide induces heme oxygenase and carbon monoxide production in vascular smooth muscle cells. Circ Res. 1997;80:557–564. doi: 10.1161/01.res.80.4.557. [DOI] [PubMed] [Google Scholar]
- 30.Santos CX, Anjos EI, Augusto O. Uric acid oxidation by peroxynitrite: multiple reactions, free radical formation, and amplification of lipid oxidation. Arch Biochem Biophys. 1999;372:285–294. doi: 10.1006/abbi.1999.1491. [DOI] [PubMed] [Google Scholar]
- 31.Mueller CF, Laude K, McNally JS, Harrison DG. ATVB in focus: redox mechanisms in blood vessels. Arterioscler Thromb Vasc Biol. 2005;25:274–278. doi: 10.1161/01.ATV.0000149143.04821.eb. [DOI] [PubMed] [Google Scholar]
- 32.Stolk J, Hilterman TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- 33.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HHHW, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 34.Yachie A, Niida Y, Wada T, Igarishi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes endothelial cell injury in human heme oxygenase-1-deficiency. J Clin Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen S, Sega M, Agarwal A. “Lumen digestion” technique for isolation of aortic endothelial cells from heme oxygenase-1 knockout mice. Biotechniques. 2004;37:84–86. doi: 10.2144/04371ST05. [DOI] [PubMed] [Google Scholar]
- 36.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 37.Piantodosi CA, Carraway MS, Suliman HB. Carbon monoxide, oxidative stress, and mitochondrial permeability pore transition. Free Radic Biol Med. 2006;40:1332–1339. doi: 10.1016/j.freeradbiomed.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 38.Taille C, El-Benna J, Lanone S, Boczkowski, Motterlini R. Mitochondrial respiratory chain and NAD(P)H oxidase are targets for the antiproliferative effect of carbon monoxide in human airway smooth muscle. J Biol Chem. 2005;280:25350–25360. doi: 10.1074/jbc.M503512200. [DOI] [PubMed] [Google Scholar]