Abstract
Acetylation of lysine residues is conserved in all three kingdoms; however, its role in prokaryotes is unknown. Here we demonstrate that acetylation enables the reference bacterium Escherichia coli to withstand environmental stress. Specifically, the bacterium reaches higher cell densities and becomes more resistant to heat and oxidative stress when its proteins are acetylated as shown by deletion of the gene encoding acetyltransferase YfiQ and the gene encoding deacetylase CobB as well as by overproducing YfiQ and CobB. Furthermore, we show that the increase in oxidative stress resistance with acetylation is due to the induction of catalase activity through enhanced katG expression. We also found that two-component system proteins CpxA, PhoP, UvrY, and BasR are associated with cell catalase activity and may be responsible as the connection between bacterial acetylation and the stress response. This is the first demonstration of a specific environmental role of acetylation in prokaryotes.
Keywords: lysine acetylation, stress resistance, protein acetylation, stress response, CobB, YfiQ
INTRODUCTION
The post-translational modification of acetylation occurs in all three domains of life [1] and regulates diverse aspects of metabolism in that 2700 proteins in mammals are acetylated related to central metabolism, mRNA splicing, protein synthesis, cell morphology, and cell cycle [2]. Although identified in 1963 for eukaryotes [2], in bacteria, the role of acetylation has not been well characterized even though this modification is relatively common in that at least 91 proteins are acetylated in the best-studied strain, Escherichia coli, including the stress related heat shock proteins like DnaK and superoxide dismutase [3,4].
In Salmonella enterica, there is only one major bacterial protein acetyltransferase, Pat, and one nicotinamide adenine dinucleotide-dependent deacetylase, CobB. These two enzymes control the status of lysine acetylation for acetyl-CoA synthetase as well as the acetylation of a number of central metabolic enzymes in S. enterica [5]. In E. coli, there are 23 putative lysine acetyltransferases that add acetyl groups to the epsilon amine of lysine using acetyl-coenzyme A as a substrate [1]. Ten Gcn-5 acetyltransferases in E. coli are confirmed for their function while the other thirteen remain enigmatic, including YfiQ which is the homolog of the single acetyltransferase in S. enteric [1]. Hence we chose to study YfiQ as the acetyltransferase because we expect that it also plays an important role in E. coli similar to Pat in S. enteric [1] and chose to study CobB as the deacetylase since it is the only confirmed deacetylase activity in E. coli [6]. Our goal was to determine the role of YfiQ and CobB in bacterial physiology by changing acetylation in E. coli.
Bacteria respond to various stresses by producing global regulators [7]. The universal stress proteins UspA and UspD are required for resistance to superoxide-generating agents [8]. Similarly, OxyR regulates the peroxide-mediated stress response in which at least 30 proteins are elevated over the basal levels upon the addition of peroxide stress [7]. In addition, the sigma factor RpoS regulates katG which encodes catalase in an OxyR-dependent way [9]. RpoS is also required for acid, heat, and salt resistance in E. coli O157:H7 [10].
Since the ability of bacteria to respond rapidly to stress is a hallmark of their success and since protein modifications allow the most rapid response, we hypothesized that conserved protein acetylation may be related to the ability of the cell to withstand stress. Here we demonstrate that cells with decreased acetylation, through enhanced deacetylase CobB activity, are less resistant to heat and oxidative stress. Both a whole-transcriptome analysis and quantitative, reverse-transcription polymerase chain reaction (qRT-PCR) showed repression of katG under oxidative stress conditions when CobB is produced. Furthermore, we found that the two component regulator proteins CpxA, UvrY, PhoP, and BasR are related to catalase activity and may work as the acetylation targets, which can thus control stress response of the cell. Hence, we propose that the activity of some regulators that control stress gene expression, especially katG expression, is altered by acetylation.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions
The bacterial strains and plasmids used in this study are listed in Table 1. E. coli K-12 BW25113 and its isogenic mutants [11] were obtained from the Genome Analysis Project in Japan. Plasmids pCA24N_cobB and pCA24N_yfiQ, carrying cobB and yfiQ under control of the PT5-lac promoter with tight regulation via the lacIq repressor, and the empty plasmid pCA24N were also obtained from the Genomic Analysis Project in Japan [12]. Expression of cobB and yfiQ was induced by 0.1 mM isopropyl-β-D-thiogalactopyranoside (IPTG) (Sigma, St. Louis, MO). All experiments were conducted in Luria-Bertani medium (LB) medium [13] at 37°C unless indicated otherwise. Kanamycin (50 µg/mL) was used for pre-culturing the isogenic knock-outs. Chloramphenicol (30 μg/mL) was used for maintaining the pCA24N-based plasmids.
Table 1.
E. coli strains and plasmids used in this study. Kmr and Cmr denote kanamycin and chloramphenicol resistance, respectively.
| Strain/Plasmid | Genotype | Source |
|---|---|---|
| Strain | ||
| BW25113 | lacIq rrnBT14 ΔlacZWJ16 hsdR514 ΔaraBADAH33 ΔrhaBADLD78 | [41] |
| BW25113 cobB | BW25113 ΔcobB Ω Kmr | [11] |
| BW25113 yfiQ | BW25113 ΔyfiQ Δ Kmr | [11] |
| BW25113 katE | BW25113 ΔkatE Ω Kmr | [11] |
| BW25113 cpxA | BW25113 Δ cpxA Ω Kmr | [11] |
| BW25113 cpxR | BW25113 ΔcpxR Ω Kmr | [11] |
| BW25113 barA | BW25113 ΔbarA Ω Kmr | [11] |
| BW25113 uvrY | BW25113 ΔuvrY Ω Kmr | [11] |
| BW25113 basS | BW25113 ΔbasS Ω Kmr | [11] |
| BW25113 basR | BW25113 ΔbasR Ω Kmr | [11] |
| BW25113 phoQ | BW25113 ΔphoQ Ω Kmr | [11] |
| BW25113 phoP | BW25113 ΔphoP Ω Kmr | [11] |
| BW25113 rcsC | BW25113 ΔrcsC Ω Kmr | [11] |
| BW25113 rcsB | BW25113 ΔrcsB Ω Kmr | [11] |
| BW25113 arcB | BW25113 ΔarcB Ω Kmr | [11] |
| BW25113 arcA | BW25113 ΔarcA Ω Kmr | [11] |
| BW25113 evgS | BW25113 ΔevgS Ω Kmr | [11] |
| BW25113 evgA | BW25113 ΔevgA Ω Kmr | [11] |
| Plasmid | ||
| pCA24N | Cmr; lacIq, pCA24N | [12] |
| pCA24N_cobB | Cmr; lacIq, pCA24N pT5-lac::cobB | [12] |
| pCA24N_yftQ | Cmr; lacIq, pCA24N pT5-lac::yfiQ | [12] |
| pCA24N_rpoS | Cmr; lacIq, pCA24N pT5-lac::rpoS | [12] |
| pCA24N_katG | Cmr; lacIq, pCA24N pT5-lac::katG | [12] |
| pCA24N_katE | Cmr; lacIq, pCA24N pT5-lac::katE | [12] |
For the growth tests, overnight cultures were diluted to a turbidity of 0.05 at 600 nm and grown for 2 h, then 0.1 mM IPTG was used to induce cobB and yfiQ expression. One mL culture was taken out at each time point, and cell turbidity at 600 nm was measured.
Stress assays
Overnight cultures were diluted to a turbidity of 0.05 at 600 nm and grown for 2 h. Then 0.1 mM IPTG was used to induce cobB and yfiQ expression for 10 to 12 h. Cells were centrifuged and resuspended in phosphate buffered saline (PBS) to a turbidity of 1.0. For the heat resistance assay, samples were treated at 65°C for 20 min [14]. For the H2O2 resistance assay, samples were mixed with 20 mM H2O2 for 20 min [15].
Catalase assays
Overnight cultures were diluted to a turbidity of 0.05 at 600 nm and grown for 2 h. IPTG (0.1 mM) was added, and the cultures were grown for another 4 h. Catalase activity was then measured two different ways in this study. For the spectrophotometric assay [16], one mL culture for each sample was washed and resuspended into PBS buffer for a turbidity at 600 nm of 0.5. For each sample, catalase activity was measured by checking the rate of H2O2 decrease which is reflected by the rate of change of absorbance at 240 nm value at 37°C. Two tubes were used for each sample, and 5 mM H2O2 was added for one while the other one was used as a control. The conversion between H2O2 concentration and absorbance was that 10 mM H2O2 is equal an optical density (OD) of 0.36 at 240 nm [16]. For the catalase activity of the two-component system mutants, overnight cultures for each strain were diluted to a turbidity of 0.05 at 600 nm and grown until a turbidity at 600 nm as 1.0. One mL culture for each sample was taken and catalase activity was measured as described above.
The second method for catalase activity used a colorimetric assay with dicarboxidine/lactoperoxidase [17]. The cultures were washed and resuspended in M9 glucose medium for a turbidity of 1.0 at 600 nm. IPTG (0.1 mM) was added to each sample to induce the expression of cobB and yfiQ. The cultures were then incubated for 4 h. Before the test, a solution of 50 μg/mL of lactoperoxidase was mixed with an equal volume of 1 mM dicarboxidine solution in water (dicarboxidine is converted into a yellow product in a reaction catalyzed by the activity of lactoperoxidase, and the amount of color developed is directly proportional to the amount of H2O2 present in the medium). To start the reaction, 10 mM H2O2 was added to the culture, and samples (10 μL) were taken after every 2 minutes at the beginning and 5 to 10 minutes later. The samples were added to a 200 μL reaction mixture, and the absorbance (OD 450 nm) was measured immediately. Higher catalase activity causes a faster decrease in the OD 450 nm.
qRT-PCR
qRT-PCR was performed using the StepOne™ Real-Time PCR System (Applied Biosystems, Foster City, CA). After isolating RNA [18] using RNAlater™ (Ambion, Austin, TX), 50 ng of total RNA was used for the qRT-PCR reaction using the Power SYBR® Green RNA-to-CT™ 1-Step Kit (Applied Biosystems). The primers are listed in Table 2. The housekeeping gene rrsG was used to normalize gene expression data [19]. The annealing temperature was 60°C for all the genes in this study. To investigate the transcription level of rpoS, katG, and katE under oxidative stress conditions, overnight cultures were grown to a turbidity of 0.05 at 600 nm, grown for 2 h, 0.1 mM IPTG was added for another 4 h to induce cobB and yfiQ expression, and the cells were exposed to 20 mM H2O2 for 10 min.
Table 2.
Primers used for qRT-PCR in this study.
| Primer name | Sequence |
|---|---|
| rpoS-f | 5’-AGAGTAACTTGCGTCTGGTGGTAAA-3’ |
| rpoS-r | 5’-ATAGTACGGGTTTGGTTCATAATCG-3’ |
| katG-f | 5’-CTGGTGTGGTTGGTGTTGAG-3’ |
| katG-r | 5’-AGTGACTCGGTGGTGGAAAC-3' |
| katE-f | 5’- GATCTTCTCGATCCAACCAAAC-3’ |
| katE-r | 5’-CACCAAGACGACTGATTTGTGT-3’ |
| rrsG-f | 5’-TATTGCACAATGGGCGCAAG-3’ |
| rrsG-r | 5’-ACTTAACAAACCGCTGCGT-3’ |
Whole-transcriptome analysis
Overnight cultures of cobB/pCA24N_cobB and cobB/pCA24N were cultured to a turbidity of 0.05 at 600 nm, grown for 2 h, 0.1 mM IPTG was added for another 4 h to induce cobB expression, and the cells were exposed to 20 mM H2O2 for 10 min. Cell pellets were collected and resuspended in RNAlater (Ambion Inc., Austin, TX), and total RNA was isolated using the RNeasy Mini Kit (Qiagen Inc., Valencia, CA) [18]. The E. coli GeneChip Genome 2.0 array (Affymetrix, P/N 900551) was used, and cDNA synthesis, fragmentation, and hybridizations were performed as described previously [20]. If the gene with the larger transcription rate did not have a consistent transcription rate based on the 11–15 probe pairs (P-value less than 0.05), these genes were discarded. A gene was considered differentially expressed when the P-value for comparing two chips was lower than 0.05 (to assure that the change in gene expression was statistically significant and that false positives arise less than 5%) and if their fold change is higher than standard deviation for the whole genome [21]. The data have been deposited at the NCBI Gene Expression Omnibus (GEO ID: GSE29803).
Mass spectrometry (MS)
His-tagged RpoS, KatG, and KatE were purified from pCA24N-based plasmids in the cobB mutant (to purify potential acetylated proteins) and in the yfiQ mutant (to purify the potential unacetylated proteins). Overnight cultures were diluted in 1 L to a turbidity as 0.05 at 600 nm. Each sample was grown until the cell turbidity at 600 nm reaches 0.6 to 1.0. Cultures were kept at 4°C for 45 min and then induced with 0.1 mM IPTG overnight at room temperature. Cell pellets were collected and lysed with a French Press. Supernatants were treated with 1 mL Ni-NTA agarose resin for 2 h. Purified His-tag proteins were digested with trypsin and cleaned with a zip-tip cleaning method. Samples were eluted with 4 μL 0.1% formic acid plus 25% and 50% acetonitrile. The MS results were obtained using 4800 MALDI TOF/TOF Analyzer (Applied Biosystems/MDS Sciex, Carlsbad, CA) [22].
RESULTS
YfiQ increases stress resistance and CobB decreases stress resistance
To test our hypothesis that lysine acetylation is related to stress resistance, we subjected E. coli yfiQ and cobB mutants to oxidative and heat stress (Fig. 1); the rationale was that inactivation of acetyltransferase YfiQ should decrease acetylation and inactivation of deacetylase CobB should increase acetylation of all the cell proteins. We found that decreasing acetylation by deleting yfiQ decreased oxidative and heat stress by 4 fold and 10 fold, respectively, while increasing acetylation by deleting cobB increased oxidative and heat stress resistance by 100 fold and 3 fold, respectively. These results were complemented by increasing acetylation by producing YfiQ via plasmid pCA24N_yfiQ (110-fold increase in oxidative stress resistance and 370-fold increase in heat resistance) and by decreasing acetylation by producing CobB via plasmid pCA24N_cobB (10-fold reduction in oxidative stress resistance and 27-fold reduction in heat resistance). Therefore, acetylation by YfiQ increases resistance to heat and oxidative stress while deacetylation by CobB decreases it. Hence, protein acetylation helps the cell respond to environmental stress.
Figure 1. Resistance to oxidative and heat stress.
E. coli BW25113 wild-type/pCA24N, cobB/pCA24N, cobB/pCA24N_cobB, yfiQ/pCA24N, and yfiQ/pCA24N_yfiQ were tested for H2O2 resistance (A) and heat resistance (B) in LB medium at 37°C.
CobB decreases catalase activity
Since producing CobB decreased resistance to hydrogen peroxide (Fig. 1A), we investigated whether this phenotype was related to RpoS since RpoS is a positive regulator of catalase activity via katG and katE [23]. Catalase deactivates H2O2 by converting it to H2O and O2 [24]. Using two independent assays, we found that removing acetylation by producing CobB abolished catalase activity and increasing acetylation by deleting cobB increased catalase activity by 4.4 fold (Fig. 2). Therefore, acetylation increases catalase activity. However, the catalase activity was not changed much by producing YfiQ (Fig. 2). This is probably because of the contribution of the other nine functional acetyltransferases in E. coli [1].
Figure 2. Catalase activity.
E. coli BW25113 wild-type/pCA24N, cobB/pCA24N, cobB/pCA24N_cobB, yfiQ/pCA24N, and yfiQ/pCA24N_yfiQ were compared for their catalase activity using the a spectrophotometric method (A) and a colorimetric method with dicarboxidine/lactoperoxidase (B). Wild-type/pCA24N data are indicated by filled circles, cobB/pCA24N by empty circles, cobB/pCA24N_cobB by filled triangles, yfiQ/pCA24N by empty triangles, and yfiQ/pCA24N_yfiQ by solid squares.
Catalase related proteins KatG, KatE, and RpoS are not acetylated
Since catalase activity was affected by acetylation (Fig. 2), we investigated whether catalases KatG and KatE, as well as the regulator that controls catalase gene expression, RpoS, are acetylated. After analyzing around 100 peptide sequences, the MS results indicated that none of these proteins are directly acetylated on lysine residues. Hence, the KatG, KatE, and RpoS are all not acetylated, and the increased catalase activity by acetylation is not because of direct modification of these three proteins.
Catalase genes are induced by acetylation
To check if there are any differences in transcription of the catalase genes caused by acetylation, we measured gene expression via qRT-PCR for rpoS, katG, and katE (Table 3). katG was repressed 3.8 ± 0.3 fold in the cobB/pCA24N_cobB strain compared to the cobB/pCA24N strain without H2O2 addition. With the addition of 10 mM H2O2 for 10 min, katG was repressed even more (25 ± 2 fold) for cobB/pCA24N_cobB vs. cobB/pCA24N. Hence, catalase genes are induced due to acetylation of some unknown cellular proteins. Probably stress activates some regulator via a post-translational modification which leads to induction of katG.
Table 3.
qRT-PCR results for catalase-related genes rpoS, katG, and katE. ΔCt is the threshold difference between each gene and the housekeeping gene rrsG. Fold change indicates the gene transcription difference between the cobB/pCA24N_cobB strain vs. the cobB/pCA24N strain in LB medium at 37°C with 20 mM H2O2 for 10 min. Fold changes are relative to the cobB/pCA24N sample.
| Strain name | rpoS | katG | katE | |||
|---|---|---|---|---|---|---|
| ΔCt | Fold change | ΔCt | Fold change | ΔCt | Fold change | |
| No stress | ||||||
| cobB/pCA24N | 12.36 ± 0.07 | 1.00 | 8.96 ± 0.07 | 1.00 | 11.8 ± 0.1 | 1.00 |
| cobB/pCA24N_cobB | 11.8 ±0.1 | 1.47 | 10.90 ± 0.07 | −3.84 | 12.48 ± 0.04 | −1.60 |
| 1 min H2O2 treatment | ||||||
| cobB/pCA24N | 11.0 ± 0.3 | 1.00 | 7.7 ± 0.3 | 1.00 | 10.1 ± 0.3 | 1.00 |
| cobB/pCA24N_cobB | 13.5 ± 0.1 | −5.66 | 11.87 ± 0.07 | −18.00 | 13.03 ± 0.08 | −7.62 |
| 10 min H2O2 treatment | ||||||
| cobB/pCA24N | 11.7 ± 0.1 | 1.00 | 7.4 ± 0.3 | 1.00 | 10.9 ± 0.3 | 1.00 |
| cobB/pCA24N_cobB | 13.2 ± 0.2 | −2.83 | 12.05 ± 0.09 | −25.11 | 13.1 ± 0.1 | −4.59 |
Acetylation induces the transcription of genes involved for various stresses
To analyze the global effect of acetylation on gene transcription, a whole-transcriptome analysis was performed with the cobB/pCA24N_cobB strain vs. the cobB/pCA24N strain with the rationale that production of CobB should remove the acetyl groups on all the cell proteins. We found that in addition to katG and katE, various stress-related genes are repressed by deacetylation, including the heat shock genes dnaK [25], osmotic stress genes osmB [26] and osmY [27], acid resistance genes gadABCE and hdeABD [28], cold shock genes cspAB [29], carbon starvation gene csiD and slp [30], and general stress gene yhbO [31] (Table 4). Hence, protein acetylation is involved in various bacterial stress response systems.
Table 4.
Summary of the whole-transcriptome results showing the stress genes that are repressed by production of CobB. Fold change indicates the gene transcription difference between the cobB/pCA24N_cobB strain vs. the cobB/pCA24N strain when cultured in LB medium at 37°C with 20 mM H2O2 for 10 min and with 0.1 mM IPTG to induce production of CobB.
| Gene | Fold change |
Gene function |
|---|---|---|
| dnaK | −4.0 | chaperone Hsp70; DNA biosynthesis; autoregulated heat shock proteins |
| osmB | −3.3 | osmotically inducible lipoprotein |
| osmY | −3.0 | hyperosmotically inducible periplasmic protein |
| gadC | −12.1 | predicted glutamate-GABA antiporter; glutamate-dependent enzyme, may function in protection against cytoplasmic acidification |
| gadB | −18.4 | glutamate decarboxylase isozyme |
| gadE | −6.5 | transcriptional regulator of the gadABC operon |
| gadA | −14.9 | glutamate decarboxylase A; RpoS regulon. EvgAS regulon. H-NS repressed. Induced by acid shock and salt stress. |
| cspB | −3.2 | cold shock protein |
| cspA | −3.0 | cold shock protein |
| katE | −3.7 | catalase; hydroperoxidase HPII(III) |
| katG | −4.9 | catalase; hydroperoxidase HPI(I) |
| csiD | −3.2 | carbon starvation induced gene |
| yhbO | −3.5 | stress-resistance protein, protease homolog |
| slp | −11.3 | outer membrane protein induced after carbon starvation |
| hdeB | −16.0 | periplasmic chaperone of acid-denatured proteins; H-NS repressed |
| hdeA | −14.9 | periplasmic chaperone of acid-denatured proteins; H-NS repressed |
| hdeD | −7.5 | putative membrane transporter, H-NS repressed |
Two-component systems may work as the targets for acetylation
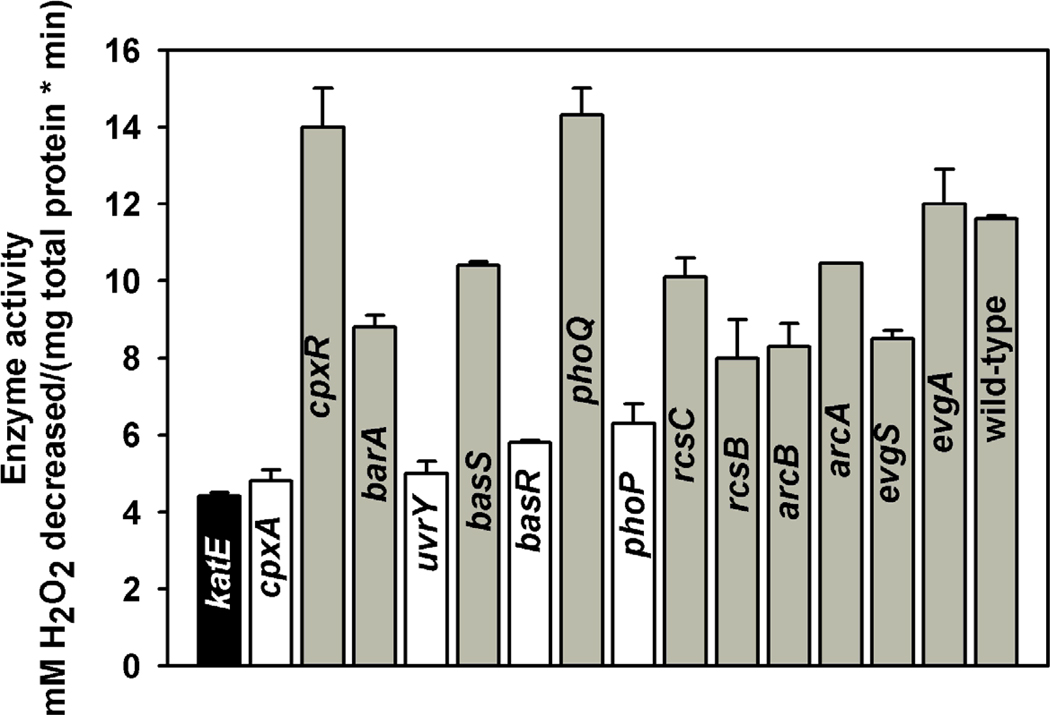
Since various stress genes changed expression upon acetylation (Table 4), we tested 14 mutants from all major two-component systems in E. coli for their catalase activity to see if any of these two-component system proteins can directly change catalase activity and can work as the targets for acetylation. The two-component systems tested included CpxA/CpxR (heat shock regulon [32]), BarA/UvrY (related to oxidative stress [33]), BasS/BasR (related to iron response [34]), PhoQ/PhoP (related to acid resistance response [35]), RcsC/RcsB (regulate cell division genes [36]), ArcB/ArcA (related to resistance to reactive oxygen stress [37]), and EvgS/EvgA (related to acid resistance and multidrug resistance [38]). Of these, we found that the cpxA, uvrY, phoP, and basR mutants showed less catalase activity than the other mutants (Fig. 3). Hence we hypothesize that proteins CpxA, UvrY, PhoP, and BasR may work as the targets for acetylation by YfiQ and control bacterial stress response depending on their acetylation state.
Figure 3. Catalase activity for mutants of two-component systems.
Single mutants from seven sets of two-component systems were compared for their catalase activity using the spectrophotometric method. The katE mutant was used as a negative control.
YfiQ increases growth yield and CobB decreases it
We also found that cells with acetylation from producing YfiQ had a dramatically increased growth yield in rich medium while cells that lacked acetylation had reduced yield (Fig. 4). Therefore, acetylation helps cells cope with the stress associated with stationary phase growth.
Figure 4. Growth curves.
The strains were grown in LB medium with 30 μg/mL chloramphenicol to retain the plasmids at 37°C, and 0.1 mM IPTG was added to induce cobB and yfiQ expression after 2 h. Symbols as defined in Fig. 2B.
DISCUSSION
Previously, the stress proteins heat shock protein DnaK [4], heat shock chaperone HtpG, superoxide dismutase SodA [4], SodB [3], alkylhydroperoxide reductase AhpC [3], and thioredoxin TrxA [3] were found to be acetylated in E. coli. However, there has been no prior report connecting the post-translational modification of acetylation and resistance to any environmental stress, and there has been no connection made between acetylation and a specific stress pathway. We discovered here that acetylation plays a significant role in the resistance to both oxidative stress and heat resistance; therefore, we connected protein acetylation and these two environmental stresses. We also found through a whole-transcriptome approach that acetylation controls an even broader range of stresses by altering expression of genes related to stress resistance (including osmotic, acid, cold, and carbon starvation); this is the first whole-transcriptome study for acetylation. Hence, here we connected protein acetylation to the resistance of specific stresses (i.e., showed acetylation is involved in both the response to oxidative and heat stress as well as linked acetylation to osmotic, acid, cold, and carbon starvation) and showed that for oxidative stress, the resistance stems from changes in transcription of the katG gene which encodes catalase.
Bacteria sense some stresses via two-component systems and then regulate the expression of various genes. The survey of seven sets of two-component system proteins showed that CpxA, UvrY, PhoP, and BasR are involved in the cell stress response to oxidative stress (Fig. 2); therefore, these proteins are likely to be involved in the acetylation response required for catalase activity. Since stress resistance genes are activated less in the CobB overproduction strain (due to deacetylation), we predict that one or more of these two-component proteins (a sensor or regulator) or the essential genes that the regulator directly controls, needs acetylation to respond to oxidative stress. Previously the two-component system protein RcsB was found to be acetylated in E. coli [39]. However, in our results we did not see a large effect of RcsB on catalase activity (Fig. 3). Since the RcsB/RcsC two-component system is required for acid resistance [40], it is also possible that RcsB may work with other two-component system proteins after acetylation and thus change the cell physiology.
RESEARCH HIGHLIGHTS.
Determine the role of protein acetylation in bacteria is for the stress response.
Provide the first transcriptome analysis for the affect of protein acetylation.
Connect protein acetylation to a specific stress pathway (katG and catalase).
ACKNOWLEDGEMENTS
This work was supported by the NIH (R01 GM089999). We are grateful for the Keio and ASKA strains provided by the Genome Analysis Project in Japan. T.W. is the T. Michael O’Connor Endowed Professor at Texas A & M University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Escalante-Semerena JC. Nepsilon-Lysine Acetylation Control Conserved in All Three Life Domains. Microbe. 2010;5:340–344. doi: 10.1128/microbe.5.340.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.H. Linda I, L. Bruno P, W. Alan J. Bacterial protein acetylation: the dawning of a new age. Molecular Microbiology. 2010;77:15–21. doi: 10.1111/j.1365-2958.2010.07204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J, Sprung R, Pei J, Tan X, Kim S, Zhu H, Liu CF, Grishin NV, Zhao Y. Lysine acetylation is a highly abundant and evolutionarily conserved modification in Escherichia coli. Mol Cell Proteomics. 2009;8:215–225. doi: 10.1074/mcp.M800187-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu BJ, Kim JA, Moon JH, Ryu SE, Pan JG. The diversity of lysine-acetylated proteins in Escherichia coli. J Microbiol Biotechnol. 2008;18:1529–1536. [PubMed] [Google Scholar]
- 5.Wang Q, Zhang Y, Yang C, Xiong H, Lin Y, Yao J, Li H, Xie L, Zhao W, Yao Y, Ning ZB, Zeng R, Xiong Y, Guan KL, Zhao S, Zhao GP. Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science. 2010;327:1004–1007. doi: 10.1126/science.1179687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li R, Gu J, Chen YY, Xiao CL, Wang LW, Zhang ZP, Bi LJ, Wei HP, Wang XD, Deng JY, Zhang XE. CobB regulates Escherichia coli chemotaxis by deacetylating the response regulator CheY. Mol Microbiol. 2010;76:1162–1174. doi: 10.1111/j.1365-2958.2010.07125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farr SB, Kogoma T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol Rev. 1991;55:561–585. doi: 10.1128/mr.55.4.561-585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nachin L, Nannmark U, NystrÖm T. Differential roles of the universal stress proteins of Escherichia coli in oxidative stress resistance, adhesion, and motility. J Bacteriol. 2005;187:6265–6272. doi: 10.1128/JB.187.18.6265-6272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivanova A, Miller C, Glinsky G, Eisenstark A. Role of rpoS (katF) in oxyR-independent regulation of hydroperoxidase I in Escherichia coli. Mol Microbiol. 1994;12:571–578. doi: 10.1111/j.1365-2958.1994.tb01043.x. [DOI] [PubMed] [Google Scholar]
- 10.Cheville AM, Arnold KW, Buchrieser C, Cheng CM, Kaspar CW. rpoS regulation of acid, heat, and salt tolerance in Escherichia coli O157:H7. Appl Environ Microbiol. 1996;62:1822–1824. doi: 10.1128/aem.62.5.1822-1824.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. 2006;2 doi: 10.1038/msb4100050. 2006 0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitagawa M, Ara T, Arifuzzaman M, Ioka-Nakamichi T, Inamoto E, Toyonaga H, Mori H. Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res. 2005;12:291–299. doi: 10.1093/dnares/dsi012. [DOI] [PubMed] [Google Scholar]
- 13.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. N.Y.: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 14.Zhang XS, García-Contreras R, Wood TK. YcfR (BhsA) influences Escherichia coli biofilm formation through stress response and surface hydrophobicity. J Bacteriol. 2007;189:3051–3062. doi: 10.1128/JB.01832-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Kim Y, Hong SH, Ma Q, Brown BL, Pu M, Tarone AM, Benedik MJ, Peti W, Page R, Wood TK. Antitoxin MqsA helps mediate the bacterial general stress response. Nat. Chem. Biol. 2011;7:359–366. doi: 10.1038/nchembio.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gusarov I, Nudler E. NO-mediated cytoprotection: instant adaptation to oxidative stress in bacteria. Proc Natl Acad Sci U S A. 2005;102:13855–13860. doi: 10.1073/pnas.0504307102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macvanin M, Hughes D. Assays of sensitivity of antibiotic-resistant bacteria to hydrogen peroxide and measurement of catalase activity. Methods Mol Biol. 2010;642:95–103. doi: 10.1007/978-1-60327-279-7_7. [DOI] [PubMed] [Google Scholar]
- 18.Ren D, Bedzyk LA, Thomas SM, Ye RW, Wood TK. Gene expression in Escherichia coli biofilms. Appl Microbiol Biotechnol. 2004;64:515–524. doi: 10.1007/s00253-003-1517-y. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, Maeda T, Hong SH, Wood TK. Reconfiguring the quorum-sensing regulator SdiA of Escherichia coli to control biofilm formation via indole and N-acylhomoserine lactones. Appl Environ Microbiol. 2009;75:1703–1716. doi: 10.1128/AEM.02081-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.González Barrios AF, Zuo R, Hashimoto Y, Yang L, Bentley WE, Wood TK. Autoinducer 2 controls biofilm formation in Escherichia coli through a novel motility quorum-sensing regulator (MqsR, B3022) J Bacteriol. 2006;188:305–316. doi: 10.1128/JB.188.1.305-316.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren D, Bedzyk LA, Ye RW, Thomas SM, Wood TK. Differential gene expression shows natural brominated furanones interfere with the autoinducer-2 bacterial signaling system of Escherichia coli. Biotechnol Bioeng. 2004;88:630–642. doi: 10.1002/bit.20259. [DOI] [PubMed] [Google Scholar]
- 22.Sherrod SD, Diaz AJ, Russell WK, Cremer PS, Russell DH. Silver nanoparticles as selective ionization probes for analysis of olefins by mass spectrometry. Anal Chem. 2008;80:6796–6799. doi: 10.1021/ac800904g. [DOI] [PubMed] [Google Scholar]
- 23.Lacour S, Landini P. SigmaS-dependent gene expression at the onset of stationary phase in Escherichia coli: function of SigmaS-dependent genes and identification of their promoter sequences. J. Bacteriol. 2004;186:7186–7195. doi: 10.1128/JB.186.21.7186-7195.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robbe-Saule V, Coynault C, Ibanez-Ruiz M, Hermant D, Norel F. Identification of a non-haem catalase in Salmonella and its regulation by RpoS (σS) Mol Microbiol. 2001;39:1533–1545. doi: 10.1046/j.1365-2958.2001.02340.x. [DOI] [PubMed] [Google Scholar]
- 25.Seyer K, Lessard M, Piette G, Lacroix M, Saucier L. Escherichia coli heat shock protein DnaK: production and consequences in terms of monitoring cooking. Appl Environ Microbiol. 2003;69:3231–3237. doi: 10.1128/AEM.69.6.3231-3237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung JU, Gutierrez C, Martin F, Ardourel M, Villarejo M. Transcription of osmB, a gene encoding an Escherichia coli lipoprotein, is regulated by dual signals. Osmotic stress and stationary phase. J Biol Chem. 1990;265:10574–10581. [PubMed] [Google Scholar]
- 27.Hengge-Aronis R, Lange R, Henneberg N, Fischer D. Osmotic regulation of rpoS-dependent genes in Escherichia coli. J Bacteriol. 1993;175:259–265. doi: 10.1128/jb.175.1.259-265.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J, Page R, Garcia-Contreras R, Palermino JM, Zhang XS, Doshi O, Wood TK, Peti W. Structure and function of the Escherichia coli protein YmgB: a protein critical for biofilm formation and acid-resistance. J Mol Biol. 2007;373:11–26. doi: 10.1016/j.jmb.2007.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ulusu NN, Tezcan EF. Cold shock proteins. Turk J. Med. Sci. 2001;31:283–290. [Google Scholar]
- 30.Alexander DM, St John AC. Characterization of the carbon starvation-inducible and stationary phase-inducible gene slp encoding an outer membrane lipoprotein in Escherichia coli. Mol Microbiol. 1994;11:1059–1071. doi: 10.1111/j.1365-2958.1994.tb00383.x. [DOI] [PubMed] [Google Scholar]
- 31.Abdallah J, Kern R, Malki A, Eckey V, Richarme G. Cloning, expression, and purification of the general stress protein YhbO from Escherichia coli. Protein Expr Purif. 2006;47:455–460. doi: 10.1016/j.pep.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Cuny C, Lesbats M, Dukan S. Induction of a global stress response during the first step of Escherichia coli plate growth. Appl Environ Microbiol. 2007;73:885–889. doi: 10.1128/AEM.01874-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herren CD, Mitra A, Palaniyandi SK, Coleman A, Elankumaran S, Mukhopadhyay S. The BarA-UvrY two-component system regulates virulence in avian pathogenic Escherichia coli O78:K80:H9. Infect Immun. 2006;74:4900–4909. doi: 10.1128/IAI.00412-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hagiwara D, Yamashino T, Mizuno T. A genome-wide view of the Escherichia coli BasS-BasR two-component system implicated in iron-responses. Biosci Biotechnol Biochem. 2004;68:1758–1767. doi: 10.1271/bbb.68.1758. [DOI] [PubMed] [Google Scholar]
- 35.Bearson BL, Wilson L, Foster JW. A low pH-inducible, PhoPQ-dependent acid tolerance response protects Salmonella typhimurium against inorganic acid stress. J Bacteriol. 1998;180:2409–2417. doi: 10.1128/jb.180.9.2409-2417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carballes F, Bertrand C, Bouche JP, Cam K. Regulation of Escherichia coli cell division genes ftsA and ftsZ by the two-component system rcsC-rcsB. Mol Microbiol. 1999;34:442–450. doi: 10.1046/j.1365-2958.1999.01605.x. [DOI] [PubMed] [Google Scholar]
- 37.Loui C, Chang AC, Lu S. Role of the ArcAB two-component system in the resistance of Escherichia coli to reactive oxygen stress. BMC Microbiol. 2009;9:183. doi: 10.1186/1471-2180-9-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eguchi Y, Itou J, Yamane M, Demizu R, Yamato F, Okada A, Mori H, Kato A, Utsumi R. B1500, a small membrane protein, connects the two-component systems EvgS/EvgA and PhoQ/PhoP in Escherichia coli. Proc Natl Acad Sci U S A. 2007;104:18712–18717. doi: 10.1073/pnas.0705768104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thao S, Chen CS, Zhu H, Escalante-Semerena JC. Nε-lysine acetylation of a bacterial transcription factor inhibits Its DNA-binding activity. PLoS ONE. 2010;5:e15123. doi: 10.1371/journal.pone.0015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson MD, Burton NA, Gutierrez B, Painter K, Lund PA. RcsB is required for inducible acid resistance in E. coli and acts at gadE dependent and independent promoters. J Bacteriol online. 2011 doi: 10.1128/JB.05040-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]