Abstract
Newcastle disease virus (NDV) is an avian virus that is attenuated in primates and is a potential vaccine vector for human use. We evaluated NDV as a vector for expressing selected antigens of the Lyme disease pathogen Borrelia burgdorferi. A series of recombinant NDVs were generated that expressed intracellular or extracellular forms of two B. burgdorferi antigens: namely, the basic membrane protein A (BmpA) and the outer surface protein C (OspC). Expression of the intracellular and extracellular forms of these antigens was confirmed in cultured chicken cells. C3H or Balb/C mice that were immunized intranasally with the NDV vectors mounted vigorous serum antibody responses against the NDV vector, but failed to mount a robust response against either the intracellular or extracellular forms of BmpA or OspC. By contrast, a single immunization of hamsters with the NDV vectors via the intranasal, intramuscular, or intraperitoneal route resulted in rapid and rigorous antibody responses against the intracellular or extracellular forms of BmpA and OspC. When groups of hamsters were separately inoculated with various NDV vectors and challenged with B. burgdorferi (108 cells/animal), immunization with vector expressing either intracellular or extracellular BmpA was associated with a significant reduction of the pathogen load in the joints. Taken together, our studies highlighted the importance of NDV as vaccine vector that can be used for simple yet effective immunization of hosts against bacterial infections including Lyme disease.
Keywords: Borrelia burgdorferi, Newcastle disease virus, Lyme disease, Immunization, Vaccine
1. Introduction
Newcastle disease virus (NDV) is an important pathogen of avian species that causes global economic loss in the poultry industry. NDV is a member of the Paramyxovirdae family under the genus Avulavirus. The genome of NDV consists of a single-stranded negative-sense RNA of 15 kb that encode 6–8 viral proteins including the nucleoprotein (N), phosphoprotein (P), matrix protein (M), fusion protein (F), hemagglutinin-neuraminidase protein (HN) and the large RNA dependent RNA polymerase (L). The production of frame-shifted transcripts of the P gene by RNA editing yields two additional viral proteins, called V and W [1]. The NDV genes are arranged in the order 3′-N-P/V/W-M-F-HN-L-5′, and are transcribed from a single promoter at the 3′ end [2]. Infectious NDV can be produced from transfected cDNAs by reverse genetics, a method that has been instrumental for studying basic biology and pathogenesis of NDV. These technologies are also adopted for studies with translational goals, such as engineering NDV vectors against variety of animal and human diseases [3].
Recently NDV has gained particular attention as a candidate vaccine vector for a number of reasons. This cytoplasmic RNA virus does not integrate into the host genome, and events of genetic recombination are extremely rare. NDV can stably express one or more foreign genes [4] and NDV immunization via the natural intranasal route of infection can induce both local and systemic immune responses [5]. Depending on the strain, NDV can be pathogenic to birds. However, it causes limited infection in non-avian species and natural infection in humans is rare. It is primarily restricted to bird handlers as reflected by transient conjunctivitis or flu-like symptoms [3], [6]. Furthermore, NDV has currently been used for human cancer therapy as an oncolytic agent in multiple preclinical and clinical trials in the United States and elsewhere [7], [8]. The efficacy of experimental NDV-vectored vaccines has been reported in preclinical studies against a number of animal and human pathogens including influenza virus [9], human parainfluenza virus type 3 [10], human respiratory syncytial virus [11], simian immunodeficiency virus [12], human immunodeficiency virus [13], SARS-Coronavirus [14], infectious bursal disease virus [15], bovine herpes virus-1 [16] and Rift valley fever virus [17]. As these immunization studies involve assessment of host protection primarily against viral pathogens, the efficacy of NDV-derived vectors for generation of immunity against bacterial pathogens currently remained unknown.
Lyme borreliosis is a prevalent vector-borne disease in the United States, Europe and parts of Asia. The infection is caused by the bacterial pathogen Borrelia burgdorferi sensu lato [18]. The bacterium persists in a natural cycle involving mammalian hosts and Ixodes scapularis ticks. In addition to the reservoir host, such as white-footed mice in North America, ticks can engorge and transmit the infection on a wide range of incidental hosts including humans [19]. Within a few weeks of tick bite, most of the infected individuals exhibit a characteristic skin lesion called erythema migran, with additional non-specific symptoms such as malaise, arthralgias and myalgias. The pathogen can disseminate and colonize in a diverse range of internal organs. This may lead to severe complications of arthritis, carditis and a variety of neurological disorders. Due to shared clinical manifestations with other diseases and individual patient variations of immune responses, proper and timely diagnosis of Lyme disease remains a challenging task [20]. Antibiotic treatment is available but is not always successful. Earlier studies on Lyme disease led to the development of a FDA-approved vaccine, the recombinant form of B. burgdorferi outer surface protein A [21], [22], [23]. OspA conferred significant host protection by multiple immunization strategies including the use of adjuvants [21], [22], [23], DNA [24] or viral vectors including Venezuelan Equine Encephalitis [25] or Vaccinia virus-based vectors [26]. However, as the effectiveness of the OspA vaccine is dependent on the levels of circulating antibodies, frequent booster immunizations are necessary to sustain the protective efficacy of the vaccine. Incidentally, OspA vaccine was withdrawn from the consumer market within two years of its commercialization due to sales and other patient-related complications [27]. Thus, a vaccine to prevent Lyme disease in humans is no longer available. Continued research into the development of effective therapeutic measures remains an important focus of Lyme disease research.
A number of animal models including mice, rats, hamsters, guinea pigs, rabbits and primates have been developed and extensively used to study the pathogenesis and prevention of Lyme disease [28]. The most popular animal model involves the laboratory mouse, since wild rodents are the primary reservoir host of spirochetes. Certain inbreed mice (i.e. C3H or Balb/c mice) can be infected by intradermal syringe inoculation with B. burgdorferi [29]. Within weeks, spirochetes disseminate throughout the skin and also migrate to distant organs including the heart, joints and urinary bladder. Signs of arthritis and carditis become apparent at 2–3 weeks and then resolve after 7–8 weeks although mice remain infected for months. Similar to mice, hamsters of both sexes and all ages are susceptible to disseminated infection [30], [31]. The use of hamsters as a rodent model of Lyme borreliosis gained immediate attention following discovery of the disease. However, in recent years its use has declined due to the popularity of different strains of mice as a more convenient and efficient animal model [28]. Nevertheless, some of the existing concepts of borrelial pathogenesis, such as loss of infectivity following serial in vitro passage of spirochetes or generation of host protective immunity via active or passive immunization efforts, were initially documented with the hamster models [28].
B. burgdorferi infection elicits a strong immune response in the host [32]. Host protection against the infection primarily depends on the development of neutralizing antibodies [33]. The antibody response is directed against a repertoire of borrelial proteins, most notably against membrane proteins. BmpA and OspC are examples of outer membrane antigens that are produced by the pathogens during infection [34], [35], [36], [37]. Robust antibody responses against both antigens are detectable in infected hosts including humans that are useful for serodiagnosis of Lyme disease [38], [39]. In addition, while OspC immunization results in generation of strain-specific immunity [40], [41], [42], BmpA immunization interferes with B. burgdorferi persistence in a tissue-specific manner, only in the joints [36]. Although the vaccine potential of either antigen is somewhat limited, OspC and BmpA were selected in our study due to their well-known immunogenicity. We sought to test whether NDV-vectored borrelial antigens elicit efficient antibody responses that modulate spirochete infection in the rodent model of Lyme disease.
2. Materials and methods
2.1. Cells, viruses, and bacteria
Chicken embryonic fibroblast (DF1), human epidermoid carcinoma (HEp-2) and mouse embryonic fibroblast (NIH 3T3) cells were obtained from the American Type Culture Collection (ATCC). Cells were grown in Dulbecco's minimal essential medium (DMEM) with 5–10% fetal bovine serum. The viruses were also propagated in 9-day-old specific pathogen free embryonated chicken eggs. Titers of NDVs were determined by a plaque assay using DF-1 cell monolayer, as detailed [43]. Briefly, infected cells were overlaid with methylcellulose, incubated at 37 °C for 5–6 days and stained with a crystal violet solution. A fully infectious isolate of B. burgdorferi B31, clone A3 [44] was used throughout the study.
2.2. Mice, hamsters and chickens
Four- to six-week old C3H/HeN and Balb/c mice were purchased from the National Cancer Institute. Syrian golden hamsters (4–6 weeks old) and domesticated chickens Gallus gallus (2 weeks old) were purchased from Charles River Laboratories. All animal procedures were in compliance with the guidelines set by the Institutional Animal Care and Use Committee of the University of Maryland, College Park.
2.3. Generation of recombinant NDVs expressing B. burgdorferi OspC or BmpA with or without eukaryotic signal sequences
The parent virus for the vector constructs was the NDV LaSota/VF strain [14], [45], which is a version of the highly attenuated vaccine strain LaSota in which the cleavage site of the F protein was modified so that exogenous protease is not necessary for replication in cell culture. Generation of NDVs carrying borrelial antigens was conducted in our Biosafety Level 3 facility. Genes coding B. burgdorferi antigens, BmpA or OspC (without respective amino-terminal signal peptides) were amplified from genomic DNA using following set of primers (start codon underlined): bmpA sense – GCT CTA GAT TAG AAA AAA CAC GGG TAG AAC GCC ACC ATG AAA GGT AGT CTT GGG AGC GAA ATT C; bmpA antisense – GCT CTA GAT ATG ATT AAA TAA ATT CTT TAA GAA ACTT CTC and ospC sense – GCT CTA GAT TAG AAA AAA CAC GGG TAG AAC GCC ACC ATG GAT GGG AAT ACA TCT GCA AAT TCT G; ospC antisense – GCT CTA GAG ATT AAG GTT TTT TTG GAC TTT CTG C. The expression cassette was introduced into a plasmid containing NDV genome that includes the gene-end (GE), intergenic sequence (IGS) and gene-start (GS) transcriptional signals as detailed in Fig. 1 . In order to maintain the genome length as a multiple of six – a requirement for efficient NDV replication [46] – additional nucleotides were introduced at the GE region. A seven-nucleotide Kozak sequence [47] for efficient translation on the upstream of ATG, two XbaI restriction enzyme sites on the upstream and downstream of gene were also introduced (Fig. 1). To enable secretion of virally produced borrelial antigens, additional constructs were produced with insertion of mammalian tissue plasminogen activator (tPA) secretory signal at the amino-terminal of mature BmpA or OspC protein using following forward primers (tPA sequence italicized): tPA-bmpA sense: GGA TGG ATG CAA TGA AGA GAG GGC TCT GCT GTG TGC TGC TGC TGT GTG GAG CAG TCT TCG TTT CGA AAG GTA GTC TTG GGA GCG AAA TTC and tPA-ospC sense: ATG GAT GCA ATG AAG AGA GGG CTC TGC TGT GTG CTG CTG CTG TGT GGA GCA GTC TTC GTT TCG GAT GGG AAT ACA TCT GCA AAT TCT G. In order to introduce the tPA-BmpA or tPA-OspC insert into the NDV genome, the genes were subsequently amplified with following forward primers: tPA-bmpA-NDV sense – GCT CTA GAT TAG AAA AAA CAC GGG TAG AAC GCC ACC ATG GAT GCA ATG AAG AGA GGG and tPA-ospC-NDV sense – GCT CTA GAT TAG AAA AAA CAC GGG TAG AAC GCC ACC ATG GAT GCA ATG AAG AGA GGG. The amplified products were sequenced to confirmed identity and sub-cloned into the plasmid carrying NDV genome using XbaI sites and was transfected into the HEp-2 cells to rescue the recombinant viruses as detailed earlier [48].
Fig. 1.
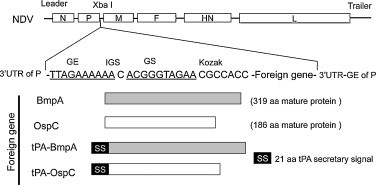
Schematic representation of recombinant NDV constructs expressing B. burgdorferi antigens. The coding sequences of the bmpA and ospC genes were engineered and inserted in the XbaI restriction site of the NDV genome to create an added gene between the NDV P and M genes. The borrelial genes encoding mature proteins without the signal peptide sequences (BmpA and OspC) or fused at the N-terminus with a human tissue plasminogen activator (tPA) secretary signal (tPA-OspC and tPA-BmpA) are indicated. GS, gene start (underlined); GE, gene end (underlined); IGS, intergenic sequence; SS, secretion signal; aa, amino acid; UTR, untranslated region. The GS, GE, and IGS are NDV-specific transcription signals.
2.4. In vitro characterization of recombinant NDVs
DF-1 cells were infected with recombinant NDV producing either BmpA or OspC at a multiplicity of infection (MOI) of 1. Cells were harvested at 24 h following infection and analyzed by Western blot using rabbit polyclonal antibodies made against BmpA or OspC. To examine secretory antigen produced by tPA-BmpA or tPA-OspC virus, the DF-1 cells were infected and, after 24 h, the cells and culture media were separated by centrifugation at 5000 rpm for 10 min. The supernatant was further concentrated using spin filter devices (Millipore). Both cell lysates and concentrated supernatants were analyzed by Western blot using BmpA and OspC antibodies, as detailed [36].
To assess the growth kinetics of NDVs, DF-1 cells were infected with viruses at an MOI of 0.01 in 6-well plates and incubated at 37 °C for 1 h for virus adsorption. Then, the cells were washed with phosphate buffered saline (PBS) for three times to remove unbound virus. DMEM media (2 ml/well) was added and incubated at 37 °C in 5% CO2 incubator. One hundred microliter aliquots of the supernatants were harvested from the culture (which was immediately replenished by the addition of equal volume of fresh media) for 5 days at 24 h intervals and titrated by plaque assay, as detailed [43].
2.5. Virus isolation from immunized mice
Groups of 6- to 8-week old C3H and BALB/c mice (3 animals/group) were intranasally infected with 106 PFU of following wild type or recombinant NDVs: parental NDV, V-BmpA, V-tPA-BmpA, V-OspC and V-tPA-OspC or using PBS as a control group. Three days following inoculum, mice were sacrificed and the lung tissues were collected. The tissues were homogenized in 1 ml DMEM with gentamicin (40 μg/ml) on ice, centrifuged at 5000 rpm for 15 min, and the supernatant fluid was titrated by plaque assay using DF-1 cells to detect the virus.
2.6. Histological analysis
The mice were immunized intranasally with a single dose of NDV (106 PFU/animal) and lung tissues were collected three days post-immunization. The tissues were fixed in 10% formalin, sectioned and stained with anti-NDV HN monoclonal antibody [49] and anti-OspC antibody [50].
2.7. ELISA and Western blots
Serum antibody development in immunized mice and hamsters were assessed by ELISA and Western blots, as detailed [50]. Microtiter plates were coated with control (PBS) or recombinant proteins (0.1 μg/well) and probed with serum collected from infected animals at various dilutions ranging from 1:100 to 10,000 and detected using HRP-conjugated secondary antibodies. For immunoblot assay, recombinant proteins or cell lysates (0.1–1 μg/lane) were probed with 1:1000–2000 dilutions of immune sera and detected using HRP-conjugated secondary antibodies and chemiluminescence substrates, as detailed [50].
2.8. Enzymatic deglycosylation of proteins
Lysates of DF1 cells (20 μg of total protein), which were infected with the various NDVs and prepared 24 h post-infection as described above, were solubilized in glycoprotein denaturing buffer (NEB), denatured by heating at 100 °C for 10 min and incubated with 2 μl PNGaseF in 1% NP40 at 37 °C for 1 h. Parallel batches of infected DF1 cells were processed in the same way except that PNGaseF was not included in the reactions and thus served as controls. The respective cell lysates with or without PNGaseF treatment were resolved on an SDS-PAGE gel and processed for Western blot analysis using either BmpA or OspC antibodies.
2.9. Quantitative PCR
Pathogen levels in infected tissues were measured by quantitative PCR (qPCR), as detailed [51]. Briefly, mice and hamsters were sacrificed 12 days following challenge with B. burgdorferi, and heart, joint, and bladder tissues were isolated. Total tissue RNA were isolated using TRIzol reagent (Invitrogen), converted to cDNA using reverse transcriptase, as described [51]. qPCR analyses were performed using a borrelial house-keeping primer pair targeting flaB gene (flaB-sense: TTG CTG ATC AAG CTC AAT ATA ACC A and flaB anti-sense: TTG AGA CCC TGA AAG TGA TGC, which were further normalized by measuring levels of rodent β-actin using following primers: (mouse β-actin sense: TTG CTG ATC AAG CTC AAT ATA ACC A and mouse β-actin anti-sense: TTG AGA CCC TGA AAG TGA TGC) or (hamster β-actin sense: GCT CTT TTC CAG CCT TCC TT and hamster β-actin anti-sense: GAG CCA GAG CAG TGA TCT CC).
2.10. Immunization and challenge studies
Groups of 6- to 8-week old female mice or 4- to 6-week old female hamsters (6 animals/group) were immunized by intranasal, intramuscular or intraperitoneal inoculation as detailed in Section 3 and figure legends. In one experiment, 2-week-old chickens (3 birds/group) were also immunized by the combined intranasal and intraocular routes, as described in the text. Inoculations consisted of PBS or 106 PFU in 100 μl of parental NDV, V-BmpA, V- tPA-BmpA, V-OspC or V-tPA-OspC. Booster immunizations with the respective viruses were performed as further described in Section 3 and figure legend. In another experiment, as noted in Section 3, some mice were immunized with increased viral doses (107–8 PFU/mouse). Serum samples were collected as described in the text. For the challenge study, hamsters were infected with spirochetes after one week of final immunization. To introduce equal pathogen burden, each animal was infected with 108 B. burgdorferi via a single intraperitoneal injection as described [52]. The heart, joints and bladder samples were collected at 12 days post-challenge and processed for pathogen load by qPCR, as detailed [51].
2.11. Hemagglutination inhibition assay
The NDV antibody levels in the collected serum were evaluated by hemagglutination inhibition (HI) assay using 1% chicken erythrocytes, as described earlier [53]. Serum samples were heat inactivated at 56 °C for 30 min, serially diluted two-fold and used to inhibit the virus.
2.12. Statistical analysis
Results are expressed as the mean ± standard error (SEM). The significance of the difference between the mean values of the groups was evaluated by Student's t test.
3. Results
3.1. Construction of recombinant NDVs expressing B. burgdorferi antigens
A derivative of an attenuated NDV vaccine strain, LaSota/VF, was used as the NDV vector for the expression of B. burgdorferi antigens and immunization of hosts. Two borrelial gene products, the outer membrane antigens BmpA and OspC, were chosen as candidate immunogens for the study. These proteins are immunogenic in diverse species including humans [34], [38] and are known to confer strain-specific immunity against B. burgdorferi infection in mice [40]. Both antigens are lipoproteins that contain type II signal peptides absent in the mature proteins. Therefore, the open reading frame (ORF) of the ospC and bmpA gene encoding respective mature proteins (without the signal peptide encoding sequence) was amplified and inserted at the unique XbaI site between the P and M genes under the control of NDV transcriptional signals (Fig. 1). Thus, the inserted gene would be expressed as a separate mRNA. Extra nucleotides were inserted at the end of the ospC or bmpA ORF transcription cassette to maintain the rule of 6 [46]. For efficient translation, a Kozak sequence [47] was also inserted before the start codon. We anticipated that, in the absence of a secretion signal, NDV-produced borrelial antigens should accumulate intracellularly. Therefore, to examine if extracellular secretion of borrelial antigens elicit a greater humoral immune response than corresponding intracellular forms, additional constructs were generated where amino-terminus of OspC or BmpA was fused to a conserved eukaryotic secretion signal, the 21-amino acid signal sequence of tissue plasminogen activator (tPA) (Fig. 1). All inserts were separately cloned into NDV antigenomic cDNA and resulting plasmids were sequenced to confirm the integrity. The recombinant NDVs, designated as V-BmpA, V-tPA-BmpA, V-OspC and V-tPA-OspC were generated using established reverse genetics technologies, as detailed [48]. Assessment of replication kinetics of rescued NDVs in chicken fibroblast (DF1) cells (Fig. S1A) suggests that insertion of foreign (borrelial) DNA did not affect the growth kinetics of recombinant NDVs. In addition, the recombinant NDV viruses formed similar sized plaques when compared to parental NDV (Fig. S1B).
3.2. Expression of B. burgdorferi antigens by recombinant NDVs in vitro
The ability of V-BmpA, V-tPA-BmpA, V-OspC and V-tPA-OspC to express corresponding forms of BmpA or OspC antigens in DF-1 cells was assessed by immunoblot analysis using specific antibodies. While BmpA produced by the V-BmpA virus displayed an electrophoretic mobility similar to that of the native protein, OspC produced by V-OspC migrated with a higher apparent molecular weight than the corresponding native protein (Fig. 2A). Additionally, OspC produced by tPA-OspC replicon (Fig. 2B) migrated with an even greater apparent molecular weight, and with multiple bands suggestive of possible posttranslational modification of the antigen. As expected, the extracellular forms of borrelial antigens, such as secretory BmpA or OspC, were detectable in the culture medium of cells infected with respective tPA constructs (Fig. 2B). Treatment of cultured cell lysates with a N-glycosidase, such as PNGaseF, resulted in faster migrating borrelial proteins corresponding to the size of the native antigens or size of the concerned NDVs lacking tPA construct (Fig. 2C). These data confirm that the borrelial antigens, when expressed with tPA secretion sequence, are glycosylated in the cells.
Fig. 2.
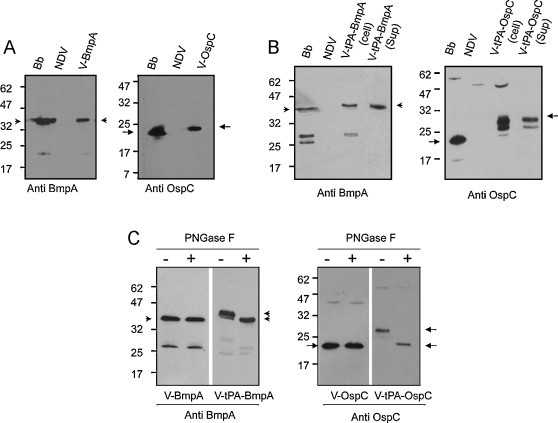
Production of intracellular and secreted forms of OspC and BmpA by recombinant NDV vectors in cultured cells. (A) Detection of BmpA and OspC in NDV-infected DF-1 cells by immunoblot analysis. Anti-BmpA (left panel) or anti-OspC (right panel) antisera were used to probe lysates of B. burgdorferi (Bb) or cultured DF1 cells infected with either parental NDV or recombinant NDVs (V-BmpA or V-OspC) producing BmpA (arrowhead) or OspC (arrow). (B) Production of secretory antigens by NDV expressed using the tissue plasminogen activator signal sequence. DF-1 cells were infected with respective NDVs (parental NDV, V-tPA-BmpA, and V-tPA-OspC), and cell lysates (cell) and culture supernatants (sup) were subjected to immunoblot analysis using BmpA (left panel) or OspC (right panel) antibodies, as detailed in (A). (C) Demonstration that BmpA and OspC produced with the tPA secretion signal contain N-linked carbohydrate, as evidenced by sensitivity to cleavage by the PNGase F endoglycanase. Cell lysates were incubated in the absence (−) or presence (+) of PNGase F for one h at 37 °C prior to being assessed by immunoblot analysis. A downward shift in electrophoretic mobility was observed for the V-tpA-BmpA and V-tpA-OspC forms (arrowheads and arrows, respectively), indicative of cleavage of N-linked carbohydrate, but not for the non-tPA-linked forms (V-BmpA and V-OspC).
3.3. NDV-produced BmpA and OspC failed to induce a robust antibody response in mice
We next assessed the immunogenicity of NDV-produced BmpA and OspC antigens in murine hosts. To accomplish this, groups of C3H mice (3 animals/group) were separately immunized with, V-BmpA, V-tPA-BmpA, V-OspC and V-tPA-OspC viruses. As NDV is a respiratory tract virus, the animals were immunized by a single intranasal inoculum (106 PFU/animal). As control, parallel groups of mice were also infected with the same dose of the parental NDV strain. Our initial studies established that the mice failed to generate antibodies against either of the borrelial antigens. The mice were subsequently boosted two additional times at 7-day intervals following the initial inoculum. Sera were collected at day 14 and 28 following the final inoculum and antibody levels were determined by ELISA. The results indicated a poor antibody response against borrelial antigens produced by V-BmpA or V-OspC (Fig. 3A) or their extracellular counterparts, V-tPA-BmpA or V-tPA-OspC (data not shown). The lack of a detectable antibody response was also observed when mice were immunized via additional routes, such as intramuscularly or intraperitoneally, singly or in combination of all three routes, or even with increased doses of NDV (107–8 PFU/animal) (data not shown). However, unlike borrelial antigens, robust humoral immune responses against NDV proteins were readily evidenced either by ELISA (Fig. 3B) or immunoblot analysis (data not shown). The development of NDV antibodies was also confirmed by the assessment of hemagglutination-inhibition (HI) titer of immunized mice sera (Fig. S2). Collectively, these results suggested that, irrespective of intra- or extracellular production of NDV-produced borrelial antigens, C3H mice were incapable of mounting a vigorous antibody response against NDV-expressed BmpA and OspC. Similar to C3H, immunization of other mice used as murine model of borreliosis also failed to induce borrelial antigen-specific antibody responses. When groups of Balb/C mice (3 animals/group) were separately immunized with either V-tPA-BmpA or V-tPA-OspC viruses, antibodies against BmpA or OspC remained undetectable (data not shown).
Fig. 3.
ELISA of serum antibodies induced in recombinant NDV vector-immunized C3H mice. (A) Absence of BmpA- and OspC-specific antibody development in immunized mice. Groups of mice (3 animals/group) were infected intranasally 3 times at 7-day intervals with 106 PFU per dose of NDV producing BmpA (V-BmpA) or OspC (V-OspC) and serum was collected at 14 (14D) or 28 (28D) days following the last immunization and subjected to ELISA. Differences between the OD values of wells coated with recombinant BmpA or OspC with respective control wells coated with phosphate buffered saline (PBS) were insignificant (P > 0.05). (B) Robust NDV-specific antibody development in immunized mice from part (A). Microtiter wells were coated with NDV lysates or PBS (control) and anti-NDV antibody development was assessed by ELISA. Normal sera denote preimmune sera collected from respective animals before immunization. Differences between the OD values of wells coated with recombinant NDV lysates and respective PBS coated wells were significant (P < 0.01).
3.4. Failure of OspC and BmpA antibody development in mice is not due to the inability of antigen expression by NDV
We next explored whether the failure of antibody development in mice against BmpA and OspC is associated with a lack of antigen expression in the host. To accomplish this, RNA from lung tissues of mice were isolated 3 days following a single intranasal inoculation and converted to cDNA using oligo-dT primers. This would ensure selective priming of transcripts of the Borrelia-specific inserts present in the NDV vectors, since the virally produced mRNA would undergo polyadenylation at the 3′ end. The levels of bmpA and ospC transcripts were measured by quantitative PCR and normalized against NDV HN gene transcripts. The results indicated that both bmpA and ospC transcripts were readily detectable in NDV-infected mice (Fig. 4A), in levels similar to that produced by the respective NDVs in cultured cells (data not shown). Although we were unable to detect BmpA or OspC in isolated murine tissues by Western blotting (data not shown), immunohistochemical analysis of lung tissue sections indicated production of NDV and borrelial antigens, such as OspC in infected lung cells (Fig. 4B). When viruses were re-isolated from V-tPA-BmpA or V-tPA-OspC-infected mice and allowed to infect DF-1 cells, production of both OspC and BmpA antigens was readily observed by Western blot analysis, confirming a stable expression of borrelial antigens by NDV (Fig. 4C). The lack of antibody response against NDV-produced borrelial antigens, as seen in C3H and Balb/c mice, however, was not observed for other hosts. When chickens were immunized with V-tPA-OspC by three intranasal inoculations of 106 PFU/dose at 7-day intervals, development of OspC antibody was readily obvious (Fig. S3). Together, the above series of studies indicated that the failure of antibody development in C3H or Balb/c mice was not due to a lack of antigen expression by NDV but rather appeared to be specific to the murine host.
Fig. 4.
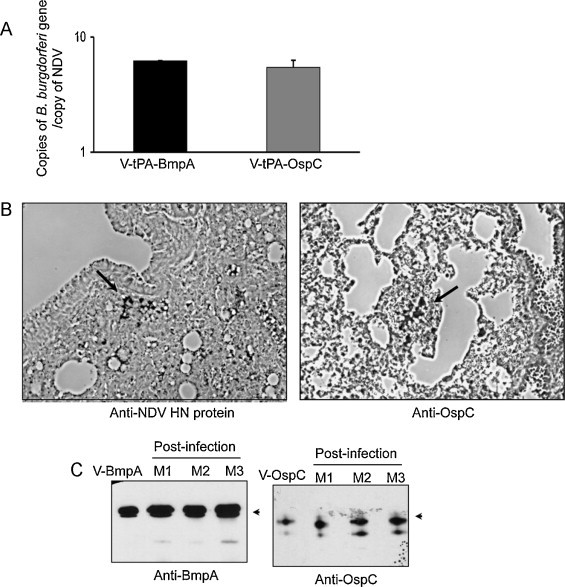
Expression of BmpA and OspC mRNA and protein by recombinant NDV in C3H mice. (A) Detection of NDV vector-encoded bmpA and ospC mRNAs in immunized mice. Groups of mice (3 animals/group) were immunized with a single intranasal inoculation of 106 PFU of the indicated NDV and, 3 days following immunization, lung tissues were isolated and processed for measurement of bmpA, ospC and NDV HN mRNA by qRT-PCR. The levels of bmpA and ospC mRNA were normalized to that of the NDV HN mRNA. (B) Detection of OspC protein in lung tissues of NDV-immunized mice. Lung tissues were isolated from NDV-immunized mice, as detailed in part (A), and processed for immunohistochemical analysis using antibody against the NDV HN and OspC proteins. Arrows indicate specific staining of NDV (left panel) and OspC (right panel) antigen. (C) NDV recovered from infected mice maintained stable expression of BmpA and OspC. Mice (M1, M2 and M3 denote individual animals) were immunized with NDVs, as in part (A). Lung tissues were isolated 3 days post-immunization, virus was recovered, and allowed to infect DF-1 cells. The cells were incubated for 3 days and processed for Western blot analysis using BmpA and OspC antibodies as described in Fig. 2.
3.5. Induction of robust humoral immune response against OspC and BmpA in immunized hamsters
As wild rodents are primary reservoir hosts of B. burgdorferi, hamsters are also used as a reliable animal model of Lyme disease [28]. Therefore, we sought to assess whether hamsters, unlike mice, are able to mount antibody responses against NDV vectored borrelial antigens. To accomplish this, Syrian golden hamsters (3 animals/group) were intranasally immunized with a single inoculation of 106 PFU/animal of parental NDV or recombinant viruses V-BmpA, V-tPA-BmpA, V-OspC or V-tPA-OspC. Fourteen days after immunization, serum was collected from hamsters and assessed for antibody development by immunoblot analysis using recombinant BmpA, OspC and borrelial lysates. Results show that a single inoculum is enough to elicit antibody responses against either intracellular (data not shown) or extracellular forms of BmpA and OspC, as detected by Western blot against respective recombinant proteins (Fig. 5A). To assess the kinetics of antibody development during immunization via multiple routes, separate groups of hamsters (3 animals/group) were inoculated with the V-tPA-BmpA virus by the intranasal, intramuscular, or intraperitoneal route. Serum samples were collected 14 days following inoculation and assessed for BmpA antibody development by ELISA. Results showed that the development of antibody was pronounced in the case of intranasal or intramuscular immunization (Fig. 5B, upper panel). However, when the hamsters were provided with a booster immunization at day 14, the antibody titers in sera collected 7 days later were significantly elevated in all cases (Fig. 5B, lower panel). Assessment of the HI titer of immunized hamster sera also indicated the development of antibodies against the NDV vector (Fig. S4). These results suggest that, unlike certain mouse strains, hamsters are highly immunogenic against NDV-produced borrelial antigens.
Fig. 5.
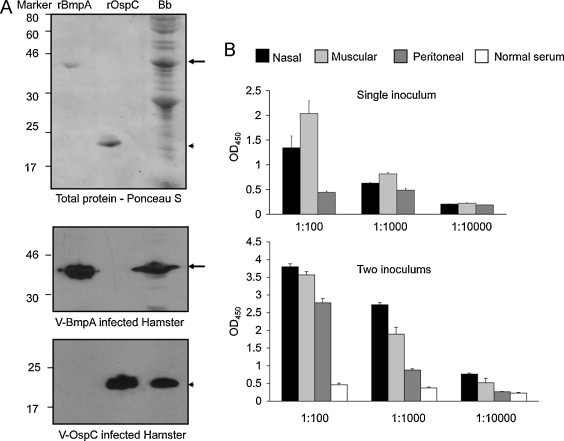
Induction of OspC- and BmpA-specific serum antibody responses in recombinant NDV vector-immunized hamsters. (A) Immunoblot analysis using immunized hamster serum. Groups of hamsters (3 animals/group) were immunized with a single intranasal inoculation of 106 PFU of the indicated recombinant NDV vector (V-BmpA or V-OspC). Serum samples were collected 14 days following immunization and used to probe Western blots of rBmpA and rOspC. Top panel shows separation of purified recombinant borrelial proteins (rBmpA and rOspC) and borrelial lysates (Bb), as revealed by Ponceau S staining. Western blots of replicate gels were probed with serum collected from a representative hamster that had been immunized with V-BmpA (middle panel) or V-OspC (lower panel). (B) Titer and kinetics of antibody development in hamsters immunized via several routes. Hamsters were inoculated with 106 PFU/animal of V-tPA-BmpA by the intranasal, intramuscular, or peritoneal route, as indicated, and serum samples were collected 14 days following immunization (single inoculum). Immediately after serum collection, hamsters were provided with a booster immunization and serum samples were collected 7 days later (two inoculums). Serum samples were prepared in 10-fold serial dilutions and used to assess the titer of BmpA antibodies by ELISA. The normal (pre-immune) serum control was collected from respective animals. The bars represent the mean ± SEM from three independent experiments.
3.6. Immunization of hamsters with NDV-vectored BmpA interferes with B. burgdorferi persistence in a tissue-specific manner
As hamsters were able to mount a serum antibody response against NDV-vectored BmpA and OspC, we next assessed whether these immunizations interfere with subsequent B. burgdorferi infection. To achieve this, separate groups of hamsters (6 animals/group) were intranasally immunized with 106 PFU/animal of parental NDV or recombinant NDV V-BmpA, V-tPA-BmpA, V-OspC, or V-tPA-OspC. Fourteen days following inoculum, the hamsters were boosted once with the respective viruses. Sera were collected one week later, and the development of antibody against the corresponding borrelial antigen was confirmed by ELISA and immunoblot analysis (not shown). Each group of hamsters was then challenged with a single intraperitoneal injection of B. burgdorferi (108 cells/animal), as previously described [30]. Twelve days post challenge, the hamsters were sacrificed and spirochete levels were measured in the joint, heart and bladder samples by quantitative PCR. The results showed that the pathogen levels were not reduced in any of the tested tissues in hamsters that had been immunized with the parental NDV negative control, or with either of the two vectored vaccines V-OspC or V-tPA-OspC (data not shown). However, immunization of hamsters with either of the vectored vaccines V-BmpA or V-tPA-BmpA resulted in significant reductions of B. burgdorferi levels in the joint tissues when compared to parental NDV-immunized animals (Fig. 6 ). Other than the joints, BmpA immunization did not influence pathogen persistence in other tissues, such as heart and bladder tissues (data not shown). Of note, a selective joint tissue-specific effect of BmpA immunization on spirochete persistence was also documented in a previous study in mice [36].
Fig. 6.
Immunization of hamsters with recombinant NDV-BmpA reduces B. burgdorferi levels in joints. Separate groups of 3 hamsters were immunized intranasally with 106 PFU of the indicated recombinant parental NDV (NDV), or recombinant NDV producing the intracellular BmpA (V-BmpA) or secretory form of BmpA (V-tPA-BmpA). Fourteen days later, the animals were boosted once with the respective virus, and 7 days later were challenged with a single intraperitoneal injection of B. burgdorferi (108 cells/animal). Twelve days following pathogen challenge, animals were sacrificed and joints, heart and bladder tissues were harvested. Total RNA was isolated from the tissues and converted to cDNA. Spirochete burden was determined by quantitative PCR assays using flaB and normalized against corresponding levels of hamster β-actin. The B. burgdorferi load in hamster joints receiving V-BmpA or V-tPA-BmpB immunization was significantly lower than in animals receiving control NDV immunization (P < 0.05). Spirochete load of the control hamster in joint tissue was considered as the 100% value and the bars represent the mean ± SEM from three independent experiments.
4. Discussion
Attenuated strains of NDV have long been used as live vaccines in the poultry industry. There also have been reports of accidental human infections from contact with birds, resulting in transient conjunctivitis or flu-like symptoms[54]. More recently, NDV has been confirmed to be highly attenuated in non-human primates, and studies have used recombinant NDV to express a variety of viral antigen, resulting in moderate-to-strong immune responses and protection against challenge [3]. In addition, NDV has gained considerable interest for its potential use as oncolytic agents in humans [7], [8]. In the present study, we showed that NDV-vectored BmpA and OspC antigens induced robust serum antibody responses in immunized hamsters, but not in the two strains of inbred mice that were tested. One of the antigens, namely OspC, failed to evoke protective immunity in hamsters against challenge with B. burgdorferi. This was true for NDV vectors expressing either the intracellular or extracellular form of the antigen. On the other hand, immunization with NDV expressing the intracellular or extracellular form of BmpA resulted in significant reduction of B. burgdorferi in the joints, but not the heart or bladder, following challenge with a high dose of the pathogen. Although exhaustive future studies are warranted, the current results provide a first indication that NDV may have utility as a highly attenuated vaccine vector for delivery of B. burgdorferi antigens in mammalian hosts. Our study also underscores the superior utility of hamster models, compared to mice, for vaccination studies involving NDV or related viral vectors.
Compared to routine adjuvant-based vaccination strategies, the use of NDV to deliver vaccines against bacterial diseases including the Lyme disease has certain advantages. First, immunization of rodents with NDV vectors could be accomplished via multiple routes including intranasal inoculum, although it is not known if this will be the case in primates. Our study shows that, while one inoculum is sufficient to induce antibody responses, a second inoculum leads to further development of high-titer (>10,000) serum antibody levels. Antibody response was detected after fourteen days following first dose of the antigen. It is possible that this reflects potent stimulation of innate and adaptive immunity due to the infectious and replicating nature of the vaccine. Protection from B. burgdorferi infection requires optimal and consistent level of neutralizing antibodies, such as for OspA [22], [23]. Therefore, if frequent booster immunizations are warranted to sustain effective serum antibody levels of against candidate vaccines, NDV vectors could be an ideal choice due to its ability to induce rapid and efficient antibody responses by simple immunization strategies. Secondly, genetic manipulation of NDV allows insertion of large (∼4 kb) of foreign inserts, enabling potential delivery of multivalent or combinatorial subunit vaccines. Infectious B. burgdorferi isolates display tremendous genetic and antigenic diversity [20]. Therefore, use of NDV-derived vectors would allow insertion of multiple inserts encoding multivalent vaccines, potentially targeting multiple antigens from diverse infectious agents. Additionally, genetic exchange, which is common with a number of viral vectors, appears to be very rare with NDV. Cost-effectiveness of vaccination is also an important issue for the development of prevention against Lyme disease. NDV grows in high titers in diverse cell lines, and more efficiently in embryonated chicken eggs. This property would potentially contribute to easy and cost-effective manufacturing of the vaccine [15]. Finally, NDV is highly attenuated in non-human primates [14], [45] and has been found to be safe in humans as an experimental oncolytic agent even when given in large intranasal or parenteral doses [7], [8].
A previous study reported that a different borrelial antigen, namely OspA, linked to the tPA secretion signal and vectored by Venezuelan Equine Encephalitis virus, was glycosylated and expressed extracellularly [25]. In our study, while we are unable to detect appreciable glycosylation of BmpA, OspC was substantially glycosylated when produced with the tPA secretion signal. Interestingly, while only the extracellular form of OspA-VEE replicon particles induced an antibody response in mice [25], both extra- and intracellular forms of NDV-produced BmpA and OspC induced serum and antibody responses in hamsters. OspC can elicit protective immunity in certain mouse models but the degree of host protection are influenced by many factors including immunizing host species [55], [56], challenge strains [40] and antigen expression in specific host environments [57]. In the present study, the lack of protection of hamsters immunized with NDV-vectored OspC is likely due to the inability of hamsters to generate sufficient borreliacidal OspC antibodies, as reported in earlier studies [56]. Although hamsters induced robust antibody responses against NDV-driven OspC, aberrant glycosylation of the antigen could potentially mask epitope(s) that are targets of neutralizing antibodies or affect peptide presentation by MHC II molecules. By contrast, NDV-produced BmpA lacked detectable post-translational modification and induced a serum antibody response in immunized hamsters. In addition, this conferred a tissue-specific interference with B. burgdorferi persistence in host, as previously reported in C3H mice [36]. Evaluation of additional antigens may provide increased protection against B. burgdorferi infection, and NDV will be a useful platform for their evaluation.
C3H and Balb/C mice constitute most popular animal models to study the pathogenesis and prevention of Lyme disease [28]. In our current study, despite a rigorous humoral immune response against NDV, these mice failed to develop a robust serum antibody response against tested NDV-expressed borrelial antigens. The fact that bacterially expressed forms of OspC and BmpA are immunogenic in C3H mice [36], [40], [42], it raises the possibility that the near absence of a murine humoral immune response to borrelial proteins expressed by NDV vectors may be influenced by the production of glycosylated forms of these proteins by NDV. It is known that glycans can protect some viral glycopeptides from degradation in the endocytic compartments of antigen presenting cells [58]. It is also possible that glycosylated forms of borrelial proteins may mimic host sugar moieties leading to tolerance in murine immune system. Indeed, the α(2–8)N-acetyl neuraminic acid capsular structures of Escherichia coli K1 and the type B meningococcal bacteria are poor immunogens because they are autoantigens in most mammals [59]. Other possibilities for the observed failure of antibody responses against OspC or BmpA also exist, such as the deficiency in the ability of antigen presenting cells for proper presentation of the antigens in correct forms besides the inability of T cells to respond to glycosylated forms of the proteins. Furthermore, the potential strong induction of CD8 responses could also affect the CD4-B cell help required for the development of strong humoral responses. Notably unlike NDV, VEE [25] or Vaccinia virus replicons [26] expressing another borrelial antigen (OspA) induced strong humoral immune responses in C3H mice. Thus, the lack of an antibody response in mice against the NDV-expressed borrelial antigens in the present study might be linked to an intrinsic property of the murine species, or the specific borrelial antigens, or post-translational processing, or some unknown deficiency of the NDV vector, or a combination of multiple factors. By contrast, irrespective of the cellular localization of antigens, hamsters were able to mount rapid, specific and efficient antibody responses against both borrelial antigens. The observed humoral immune response in immunized hamsters temporally coincides with the reported development of B. burgdorferi antibodies during tick-borne infection [60]. Therefore, our present study points to variations in immune response to virally produced borrelial vaccine targets in different mammals used as models of Lyme disease. Although immunization of non-human primates with NDV vectors shown to elicit protective immunity against emerging pathogens [14], further evaluation of immune response to virally produced borrelial vaccines in primate models will reveal the suitability of NDV vectors against human Lyme disease.
In conclusion, we have used a hamster model to demonstrate that NDV vectors elicit a robust immune response against borrelial antigens. We also show that immunization with NDV-expressed BmpA can restrict subsequent B. burgdorferi infection in the host. As a single immunization is sufficient to generate antibody response that is quickly and significantly enhanced following booster injections, our current results suggest that NDV vectors could potentially be used for routine vaccination of individuals against diverse infectious agents including B. burgdorferi, especially in cases where multiple booster immunizations are required to maintain effective neutralizing antibody concentration in the blood. Diverse birds are considered as reservoir hosts of Lyme disease pathogens and play important role in borrelial geographic dissemination [61], [62]. Since birds are natural hosts for NDV and live attenuated NDV strains are used as vaccines [61], [62], [63], [64], [65], hypothetically NDV vectors could be important for vaccination of wild birds to prevent incidence of Lyme disease.
Acknowledgements
This research was supported in part by NIH/NIAID Awards (AI080615 and AR055323), NIH/NIAID Contract N01A060009 and NIH Intramural Research Program. We sincerely thank Alexis Smith for her excellent assistance with this study.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.vaccine.2011.05.010.
Appendix A. Supplementary data
Fig. S1.
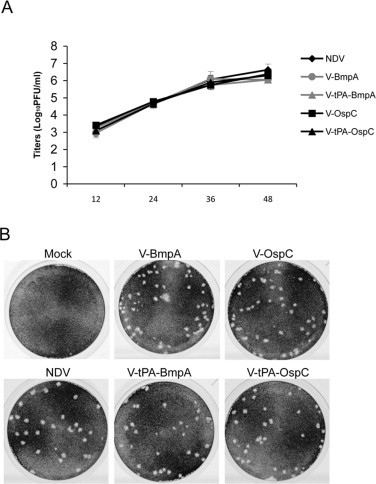
Growth kinetics of parental and recombinant NDVs and plaque morphology of infected cells. DF-1 cells were infected with parental NDV (NDV) or one of the recombinant NDV isolates (V-BmpA, V-tPA-BmpA, V-OspC or V-tPA-OspC) at a multiplicity of infection of 0.01 PFU/cell. (A) Multi-cycle growth kinetics of viruses, shown in h following infection. (B) Morphology the plaques formed in DF-1 cells by the indicated recombinant NDV.
Fig. S2.
Assessment of NDV-specific serum antibody responses in immunized C3H mice by hemagglutination inhibition (HI) assay. Mice were immunized with recombinant NDV as described in Fig. 3 and sera were collected 21 days following immunization.
Fig. S3.
Development of OspC antibody in NDV-immunized chickens. Two-week-old chickens (3 animals/group) were immunized by the oculonasal route with 106 PFU of by recombinant NDV producing OspC (V-OspC). The birds were boosted with the respective viruses on day 14 and sera were collected one week following the last immunization. Antibody development against OspC was assessed by immunoblot analysis using recombinant OspC or another borrelial protein BBA52 (control).
Fig. S4.
Hemagglutination inhibition assay indicates development of NDV-specific humoral immune responses in hamsters. Animals were immunized with recombinant NDV, as described in Fig. 5A and serum was collected 21 days following immunization to determine occurrence of anti-NDV antibodies in hamsters using HI assay, as detailed in the text.
References
- 1.Steward M., Vipond I.B., Millar N.S., Emmerson P.T. RNA editing in Newcastle disease virus. J Gen Virol. 1993;74(December (Pt 12)):2539–2547. doi: 10.1099/0022-1317-74-12-2539. [DOI] [PubMed] [Google Scholar]
- 2.Lamb R.A., Parks G.D. Paramyxoviridae: the viruses and their replication. In: Knipe D.M., Howley P.M., editors. Fields virology. 5th ed. Lippincott Williams and Wilkins; Philadelphia: 2007. pp. 1449–1496. [Google Scholar]
- 3.Bukreyev A., Collins P.L. Newcastle disease virus as a vaccine vector for humans. Curr Opin Mol Ther. 2008;10(February (1)):46–55. [PubMed] [Google Scholar]
- 4.Krishnamurthy S., Huang Z., Samal S.K. Recovery of a virulent strain of newcastle disease virus from cloned cDNA: expression of a foreign gene results in growth retardation and attenuation. Virology. 2000;278(December (1)):168–182. doi: 10.1006/viro.2000.0618. [DOI] [PubMed] [Google Scholar]
- 5.DiNapoli J.M., Yang L., Suguitan A., Jr., Elankumaran S., Dorward D.W., Murphy B.R. Immunization of primates with a Newcastle disease virus-vectored vaccine via the respiratory tract induces a high titer of serum neutralizing antibodies against highly pathogenic avian influenza virus. J Virol. 2007;81(November (21)):11560–11568. doi: 10.1128/JVI.00713-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Z., Elankumaran S., Panda A., Samal S.K. Recombinant Newcastle disease virus as a vaccine vector. Poult Sci. 2003;82(June (6)):899–906. doi: 10.1093/ps/82.6.899. [DOI] [PubMed] [Google Scholar]
- 7.Schirrmacher V., Griesbach A., Ahlert T. Antitumor effects of Newcastle Disease Virus in vivo: local versus systemic effects. Int J Oncol. 2001;18(May (5)):945–952. doi: 10.3892/ijo.18.5.945. [DOI] [PubMed] [Google Scholar]
- 8.Vigil A., Martinez O., Chua M.A., Garcia-Sastre A. Recombinant Newcastle disease virus as a vaccine vector for cancer therapy. Mol Ther. 2008;16(November (11)):1883–1890. doi: 10.1038/mt.2008.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiNapoli J.M., Nayak B., Yang L., Finneyfrock B.W., Cook A., Andersen H. Newcastle disease virus-vectored vaccines expressing the hemagglutinin or neuraminidase protein of H5N1 highly pathogenic avian influenza virus protect against virus challenge in monkeys. J Virol. 2010;84(February (3)):1489–1503. doi: 10.1128/JVI.01946-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bukreyev A., Huang Z., Yang L., Elankumaran S., St Claire M., Murphy B.R. Recombinant newcastle disease virus expressing a foreign viral antigen is attenuated and highly immunogenic in primates. J Virol. 2005;79(November (21)):13275–13284. doi: 10.1128/JVI.79.21.13275-13284.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez-Sobrido L., Gitiban N., Fernandez-Sesma A., Cros J., Mertz S.E., Jewell N.A. Protection against respiratory syncytial virus by a recombinant Newcastle disease virus vector. J Virol. 2006;80(February (3)):1130–1139. doi: 10.1128/JVI.80.3.1130-1139.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakaya Y., Nakaya T., Park M.S., Cros J., Imanishi J., Palese P. Induction of cellular immune responses to simian immunodeficiency virus gag by two recombinant negative-strand RNA virus vectors. J Virol. 2004;78(September (17)):9366–9375. doi: 10.1128/JVI.78.17.9366-9375.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carnero E., Li W., Borderia A.V., Moltedo B., Moran T., Garcia-Sastre A. Optimization of human immunodeficiency virus gag expression by newcastle disease virus vectors for the induction of potent immune responses. J Virol. 2009;83(January (2)):584–597. doi: 10.1128/JVI.01443-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiNapoli J.M., Kotelkin A., Yang L., Elankumaran S., Murphy B.R., Samal S.K. Newcastle disease virus, a host range-restricted virus, as a vaccine vector for intranasal immunization against emerging pathogens. Proc Natl Acad Sci U S A. 2007;104(June (23)):9788–9793. doi: 10.1073/pnas.0703584104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Z., Elankumaran S., Yunus A.S., Samal S.K. A recombinant Newcastle disease virus (NDV) expressing VP2 protein of infectious bursal disease virus (IBDV) protects against NDV and IBDV. J Virol. 2004;78(September (18)):10054–10063. doi: 10.1128/JVI.78.18.10054-10063.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khattar S.K., Collins P.L., Samal S.K. Immunization of cattle with recombinant Newcastle disease virus expressing bovine herpesvirus-1 (BHV-1) glycoprotein D induces mucosal and serum antibody responses and provides partial protection against BHV-1. Vaccine. 2010;28(April (18)):3159–3170. doi: 10.1016/j.vaccine.2010.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kortekaas J., de Boer S.M., Kant J., Vloet R.P., Antonis A.F., Moormann R.J. Rift Valley fever virus immunity provided by a paramyxovirus vaccine vector. Vaccine. 2010;28(June (27)):4394–4401. doi: 10.1016/j.vaccine.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 18.Samuels D.S., Radolf J.D., editors. Borrelia, molecular biology, host interaction and pathogenesis. Caister Academic Press; Norfolk, UK: 2010. [Google Scholar]
- 19.Steere A.C., Coburn J., Glickstein L. The emergence of Lyme disease. J Clin Invest. 2004;113(April (8)):1093–1101. doi: 10.1172/JCI21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang G., Aguero-Rosenfeld M.E., Wormser G.P., Schwartz I. Detection of Borrelia burgdorferi. In: Samuels D.S., Radolf J.D., editors. Borrelia, molecular biology, host interaction and pathogenesis. Caister Academic Press; Norfolk, UK: 2010. pp. 443–466. [Google Scholar]
- 21.Fikrig E., Barthold S.W., Kantor F.S., Flavell R.A. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990;250:553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- 22.Sigal L.H., Zahradnik J.M., Lavin P., Patella S.J., Bryant G., Haselby R. A vaccine consisting of recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease. N Engl J Med. 1998;339(4):216–222. doi: 10.1056/NEJM199807233390402. [DOI] [PubMed] [Google Scholar]
- 23.Steere A.C., Sikand V.K., Meurice F., Parenti D.L., Fikrig E., Schoen R.T. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. N Engl J Med. 1998;339(4):209–215. doi: 10.1056/NEJM199807233390401. [DOI] [PubMed] [Google Scholar]
- 24.Luke C.J., Carner K., Liang X., Barbour A.G. An OspA-based DNA vaccine protects mice against infection with Borrelia burgdorferi. J Infect Dis. 1997;175(January (1)):91–97. doi: 10.1093/infdis/175.1.91. [DOI] [PubMed] [Google Scholar]
- 25.Gipson C.L., Davis N.L., Johnston R.E., de Silva A.M. Evaluation of venezuelan equine encephalitis (VEE) replicon-based outer surface protein A (OspA) vaccines in a tick challenge mouse model of Lyme disease. Vaccine. 2003;21(September (25–26)):3875–3884. doi: 10.1016/s0264-410x(03)00307-4. [DOI] [PubMed] [Google Scholar]
- 26.Scheckelhoff M.R., Telford S.R., Hu L.T. Protective efficacy of an oral vaccine to reduce carriage of Borrelia burgdorferi (strain N40) in mouse and tick reservoirs. Vaccine. 2006;24(March (11)):1949–1957. doi: 10.1016/j.vaccine.2005.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marconi R.T., Earnhart C.G. Lyme disease vaccines. In: Samuels D.S., Radolf J.D., editors. Borrelia, molecular biology, host interaction and pathogenesis. Caister Academic Press; Norfolk, UK: 2010. pp. 461–480. [Google Scholar]
- 28.Barthold S.W., Diego C., Philipp M.T. Animal models of borreliosis. In: Samuels D.S., Radolf J.D., editors. Borrelia, molecular biology, host interaction and pathogenesis. Caister Academic Press; Norfolk, UK: 2010. pp. 353–405. [Google Scholar]
- 29.Barthold S.W., Beck D.S., Hansen G.M., Terwilliger G.A., Moody K.D. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990;162:133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- 30.Johnson R.C., Marek N., Kodner C. Infection of Syrian hamsters with Lyme disease spirochetes. J Clin Microbiol. 1984;20(December (6)):1099–1101. doi: 10.1128/jcm.20.6.1099-1101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson R.C., Kodner C., Russell M., Duray P.H. Experimental infection of the hamster with Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:258–263. doi: 10.1111/j.1749-6632.1988.tb31859.x. [DOI] [PubMed] [Google Scholar]
- 32.Weis J.J., Bockenstedt L.K. Host response. In: Samuels D.S., Radolf J.D., editors. Borrelia, molecular biology, host interaction and pathogenesis. Caister Academic Press; Norfolk, UK: 2010. pp. 413–441. [Google Scholar]
- 33.Connolly S.E., Benach J.L. The versatile roles of antibodies in Borrelia infections. Nat Rev Microbiol. 2005;3(May (5)):411–420. doi: 10.1038/nrmicro1149. [DOI] [PubMed] [Google Scholar]
- 34.Bryksin A.V., Godfrey H.P., Carbonaro C.A., Wormser G.P., Aguero-Rosenfeld M.E., Cabello F.C. Borrelia burgdorferi BmpA, BmpB, and BmpD proteins are expressed in human infection and contribute to P39 immunoblot reactivity in patients with Lyme disease. Clin Diagn Lab Immunol. 2005;12(August (8)):935–940. doi: 10.1128/CDLI.12.8.935-940.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang F.T., Jacobs M.B., Bowers L.C., Philipp M.T. An immune evasion mechanism for spirochetal persistence in Lyme borreliosis. J Exp Med. 2002;195(4):415–422. doi: 10.1084/jem.20011870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pal U., Wang P., Bao F., Yang X., Samanta S., Schoen R. Borrelia burgdorferi basic membrane proteins A and B participate in the genesis of Lyme arthritis. J Exp Med. 2008;205(January (1)):133–141. doi: 10.1084/jem.20070962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwan T.G., Piesman J., Golde W.T., Dolan M.C., Rosa P.A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci U S A. 1995;92(7):2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathiesen M.J., Christiansen M., Hansen K., Holm A., Asbrink E., Theisen M. Peptide-based OspC enzyme-linked immunosorbent assay for serodiagnosis of Lyme borreliosis. J Clin Microbiol. 1998;36(December (12)):3474–3479. doi: 10.1128/jcm.36.12.3474-3479.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roessler D., Hauser U., Wilske B. Heterogeneity of BmpA (P39) among European isolates of Borrelia burgdorferi sensu lato and influence of interspecies variability on serodiagnosis. J Clin Microbiol. 1997;35(November (11)):2752–2758. doi: 10.1128/jcm.35.11.2752-2758.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bockenstedt L.K., Hodzic E., Feng S., Bourrel K.W., de Silva A., Montgomery R.R. Borrelia burgdorferi strain-specific Osp C-mediated immunity in mice. Infect Immun. 1997;65(November (11)):4661–4667. doi: 10.1128/iai.65.11.4661-4667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gilmore R.D., Jr., Kappel K.J., Dolan M.C., Burkot T.R., Johnson B.J. Outer surface protein C (OspC), but not P39, is a protective immunogen against a tick-transmitted Borrelia burgdorferi challenge: evidence for a conformational protective epitope in OspC. Infect Immun. 1996;64(June (6)):2234–2239. doi: 10.1128/iai.64.6.2234-2239.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Probert W.S., Lefebvre R.B. Protection of C3H/HeN mice from challenge with Borrelia burgdorferi through active immunization with OspA, OspB or OspC but not OspD or the 83-kilodalton antigen. Infect Immun. 1994;62:1920–1926. doi: 10.1128/iai.62.5.1920-1926.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schloer G.M., Hanson R.P. Relationship of plaque size and virulence for chickens of 14 representative Newcastle disease virus strains. J Virol. 1968;2(January (1)):40–47. doi: 10.1128/jvi.2.1.40-47.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elias A.F., Stewart P.E., Grimm D., Caimano M.J., Eggers C.H., Tilly K. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect Immun. 2002;70(April (4)):2139–2150. doi: 10.1128/IAI.70.4.2139-2150.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DiNapoli J.M., Ward J.M., Cheng L., Yang L., Elankumaran S., Murphy B.R. Delivery to the lower respiratory tract is required for effective immunization with Newcastle disease virus-vectored vaccines intended for humans. Vaccine. 2009;27(March (10)):1530–1539. doi: 10.1016/j.vaccine.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kolakofsky D., Pelet T., Garcin D., Hausmann S., Curran J., Roux L. synthesis and the requirement for hexamer genome length: the rule of six revisited. J Virol. 1998;72(February (2)):891–899. doi: 10.1128/jvi.72.2.891-899.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kozak M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15(October (20)):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang Z., Krishnamurthy S., Panda A., Samal S.K. High-level expression of a foreign gene from the most 3′-proximal locus of a recombinant Newcastle disease virus. J Gen Virol. 2001;82(July (Pt 7)):1729–1736. doi: 10.1099/0022-1317-82-7-1729. [DOI] [PubMed] [Google Scholar]
- 49.Iorio R.M., Syddall R.J., Sheehan J.P., Bratt M.A., Glickman R.L., Riel A.M. Neutralization map of the hemagglutinin-neuraminidase glycoprotein of Newcastle disease virus: domains recognized by monoclonal antibodies that prevent receptor recognition. J Virol. 1991;65(September (9)):4999–5006. doi: 10.1128/jvi.65.9.4999-5006.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pal U., Yang X., Chen M., Bockenstedt L.K., Anderson J.F., Flavell R.A. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J Clin Invest. 2004;113(January (2)):220–230. doi: 10.1172/JCI19894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang X., Coleman A.S., Anguita J., Pal U. A chromosomally encoded virulence factor protects the Lyme disease pathogen against host-adaptive immunity. PLoS Pathog. 2009;5(March (3)):e1000326. doi: 10.1371/journal.ppat.1000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson R.C., Kodner C., Russell M., Duray P.H. Active immunization of hamsters against experimental infection with Borrelia burgdorferi. Infect Immun. 1986;54:897. doi: 10.1128/iai.54.3.897-898.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allan W.H., Gough R.E. A standard haemagglutination inhibition test for Newcastle disease (1). A comparison of macro and micro methods. Vet Rec. 1974;95(August (6)):120–123. doi: 10.1136/vr.95.6.120. [DOI] [PubMed] [Google Scholar]
- 54.Nelson C.B., Pomeroy B.S., Schrall K., Park W.E., Lindeman R.J. An outbreak of conjunctivitis due to Newcastle disease virus (NDV) occurring in poultry workers. Am J Public Health Nations Health. 1952;42(June (6)):672–678. doi: 10.2105/ajph.42.6.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lovrich S.D., La Fleur R.L., Jobe D.A., Johnson J.C., Asp K.E., Schell R.F. Borreliacidal OspC antibody response of canines with Lyme disease differs significantly from that of humans with Lyme disease. Clin Vaccine Immunol. 2007;14(May (5)):635–637. doi: 10.1128/CVI.00431-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lovrich S.D., Jobe D.A., Schell R.F., Callister S.M. Borreliacidal OspC antibodies specific for a highly conserved epitope are immunodominant in human lyme disease and do not occur in mice or hamsters. Clin Diagn Lab Immunol. 2005;12(June (6)):746–751. doi: 10.1128/CDLI.12.6.746-751.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liang F.T., Brown E.L., Wang T., Iozzo R.V., Fikrig E. Protective niche for Borrelia burgdorferi to evade humoral immunity. Am J Pathol. 2004;165(September (3)):977–985. doi: 10.1016/S0002-9440(10)63359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rudd P.M., Elliott T., Cresswell P., Wilson I.A., Dwek R.A. Glycosylation and the immune system. Science. 2001;291(March (5512)):2370–2376. doi: 10.1126/science.291.5512.2370. [DOI] [PubMed] [Google Scholar]
- 59.Granoff D.M. Review of meningococcal group B vaccines. Clin Infect Dis. 2010;50(March (Suppl. 2)):S54–S65. doi: 10.1086/648966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roehrig J.T., Piesman J., Hunt A.R., Keen M.G., Happ C.M., Johnson B.J. The hamster immune response to tick-transmitted Borrelia burgdorferi differs from the response to needle-inoculated, cultured organisms. J Immunol. 1992;149(December (11)):3648–3653. [PubMed] [Google Scholar]
- 61.Dubska L., Literak I., Kocianova E., Taragelova V., Sverakova V., Sychra O. The importance of synanthropic wild birds in distribution of Borrelia spirochetes: analysis of spring collections of Ixodes ricinus ticks feeding on passerine birds in the Czech Republic. Appl Environ Microbiol. 2010;(December) [Google Scholar]
- 62.Dubska L., Literak I., Kocianova E., Taragelova V., Sychra O. Differential role of passerine birds in distribution of Borrelia spirochetes, based on data from ticks collected from birds during the postbreeding migration period in Central Europe. Appl Environ Microbiol. 2009;75(February (3)):596–602. doi: 10.1128/AEM.01674-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Comstedt P., Bergstrom S., Olsen B., Garpmo U., Marjavaara L., Mejlon H. Migratory passerine birds as reservoirs of Lyme borreliosis in Europe. Emerg Infect Dis. 2006;12(July (7)):1087–1095. doi: 10.3201/eid1207.060127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taragel’ova V., Koci J., Hanincova K., Kurtenbach K., Derdakova M., Ogden N.H. Blackbirds and song thrushes constitute a key reservoir of Borrelia garinii, the causative agent of borreliosis in Central Europe. Appl Environ Microbiol. 2008;74(February (4)):1289–1293. doi: 10.1128/AEM.01060-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hanincova K., Taragelova V., Koci J., Schafer S.M., Hails R., Ullmann A.J. Association of Borrelia garinii and B. valaisiana with songbirds in Slovakia. Appl Environ Microbiol. 2003;69(May (5)):2825–2830. doi: 10.1128/AEM.69.5.2825-2830.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


