Abstract
Purpose
Hemorrhagic metastatic brain tumors are not rare, but little is known about the surgical outcome following treatment. We conducted this study to determine the result of the surgical outcome of hemorrhagic metastatic brain tumors.
Materials and Methods
From July 2001 to December 2008, 21 patients underwent surgery for hemorrhagic metastatic brain tumors at our institution. 15 patients had lung cancer, 3 had hepatocellular carcinoma, and the rest had rectal cancer, renal cell carcinoma, and sarcoma. 20 patients had macroscopic hemorrhage in the tumors, and one patient had intracerebral hemorrhage surrounding the tumor. A retrospective clinical review was conducted focusing on the patterns of presenting symptoms and signs, as well as local recurrence following surgery.
Results
Among 21 hemorrhagic brain metastases, local recurrence developed in two patients. The 12 month progression free survival rate was 86.1%. Mean time to progression was 20.8 months and median survival time after surgery was 11.7 months.
Conclusion
The results of our study showed that hemorrhagic metastatic brain tumors rarely recurred after surgery. Surgery should be considered as a good treatment option for hemorrhagic brain metastasis, especially in cases with increased intracranial pressure or severe neurologic deficits.
Keywords: Neoplasm metastasis, Brain, Hemorrhage, Local neoplasm recurrences, Surgery
Introduction
In pathological and radiological studies the frequency of spontaneous intracerebral hemorrhage (ICH) in intracranial neoplasms ranges from 1.4 to 10% with an average of 2-3% [1-4]. In general, any type of intracranial neoplasm can cause ICH, but the frequency varies widely among different tumor types [1-4]. Fast growing and highly vascularized neoplasms with an irregular and fragile vascular architecture are most frequently associated with ICH [5,6]. Metastatic tumors or malignant gliomas are the most common type of these tumors. Hemorrhage was a complication in 14% of patients with brain metastases compared to 0.8% of patients with gliomas [7]. Metastatic malignant melanoma bled in approximately 50% of the cases, whereas metastatic adenocarcinoma, squamous carcinoma, and anaplastic carcinoma had significantly lower bleeding rates [7].
There are several reports that have described the negative effects of intratumoral hemorrhage on tumor control after stereotactic radiosurgery [8-10]. Furthermore, most of these patients presented with acute stroke-like symptoms and signs. Therefore, surgery could be a more suitable treatment option in case of hemorrhagic metastasis because surgery can relieve neurologic deficits immediately. Although hemorrhage from metastatic brain tumors has often been observed, there is no study to date that has addressed the effect of intratumoral hemorrhage on surgical outcomes. To determine the result of surgical outcomes of hemorrhagic metastatic brain tumors, we reviewed the clinical records of patients treated with surgery for hemorrhagic brain metastases at our institution retrospectively.
Materials and Methods
From July 2001 to December 2008, 228 metastatic brain tumor surgeries were performed at our institution. Among them, 21 patients showed hemorrhagic metastatic brain tumors. Clinical data and imaging studies of 21 hemorrhagic metastatic brain tumors were reviewed retrospectively. All tumors were preoperatively examined with computed tomography or magnetic resonance imaging (MRI). Neuro-navigation-guided microsurgical excision was performed. The resection status was determined according to the margin biopsy and MRI performed within 48 hours after the craniotomy. Follow up in our outpatient department included neurological examination and contrast-enhanced cranial MRI every three months. The mean follow up period was 12.6 months (range, 1 to 62 months) after surgery. Immediate postoperative systemic chemotherapy was done to control primary disease. Postoperative radiotherapy (RT) was delayed until the recurrence of brain lesions, which was in accordance with our policy. When primary disease was well controlled, adjuvant RT was done immediately. Local and distant recurrence and survival times after brain surgery were analyzed. Local recurrence was defined as recurrence at the site of an original metastasis treated by surgery, and distant recurrence was defined as recurrence at other sites in the brain unrelated to the surgery. For survival analysis, we used the Kaplan-Meier method. Survival time was calculated after the day of brain surgery.
Results
1. Clinical characteristics
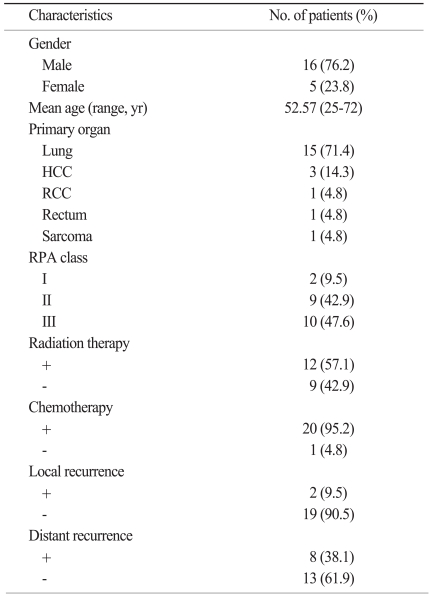
During the study period, 228 patients received metastatic brain tumor surgery. The primary diseases were lung cancer in 158 patients (69.2%), breast cancer in 18 patients (7.9%), colorectal cancer in 17 patients (7.5%), liver and renal cancer in 10 patients (4.4%), sarcoma and gynecologic cancer in 3 patients (1.3%), and stomach, esophagus, and thyroid cancer in 2 patients (0.9%). Other three patients were melanoma, testicular cancer, and unknown primary cancer. Among them, 21 patients had hemorrhagic metastatic brain tumors (9.2%). Patient characteristics are summarized in Table 1. Sixteen patients (76.2%) were male, and five were female. The mean age was 52.6 years (range, 25 to 72 years). Fifteen patients (71.4%) had lung cancer, three patients (14.3%) had liver cancer, and the others had colorectal cancer (4.8%), renal cancer (4.8%), and sarcoma (4.8%). Twenty patients showed macroscopic hemorrhage in the tumor and one patient showed macroscopic hemorrhage immediately surrounding the tumor. All patients except one received chemotherapy. RT was done in 12 patients. Among them, preoperative RT was done in two patients, immediate adjuvant postoperative RT was done in six patients, and delayed RT was done in four patients after local or distant recurrence developed. Fifteen patients had single brain metastasis, and six patients had multiple metastases. Surgical resection was done for only single symptomatic hemorrhagic lesions among multiple brain metastases. All patients showed improved neurological symptoms immediately after surgery.
Table 1.
Characteristics of patients with hemorrhagic brain metastasis
HCC, hepatocellular carcinoma; RCC, renal cell carcinoma; RPA, Recursive Partitioning Analysis.
2. Local recurrence
Among 21 hemorrhagic brain metastases, local recurrence developed only in two (9.5%) patients. The 12 month recurrence free survival rates were 86.1%. One patient suffered from hepatocellular carcinoma. Tumor bleeding occurred 5 months after brain RT. Local recurrence occurred 10 months of surgery. A second surgery was performed for the local recurrence, but 3 months later the metastatic mass recurred. The patient expired 19 months after initial brain surgery. The other patient who showed local recurrence suffered from lung cancer. He showed repeated intra-tumoral hemorrhage at one week intervals. He showed local and distant metastases only 1 month after surgery. He also showed rapid progression of the primary disease and expired 2 months after surgery.
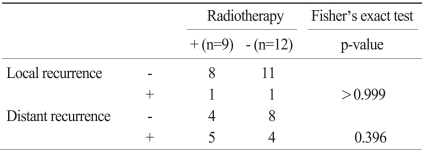
Among 9 RT-naïve patients, one patient showed local recurrence. Among 12 patients who were treated with RT, one patient showed local recurrence. There was no significant difference in local recurrence rate between RT-naïve and RT patients (Fisher's exact test, p>0.999) (Table 2).
Table 2.
Local and distant brain recurrence according to radiotherapy
3. Distant recurrence
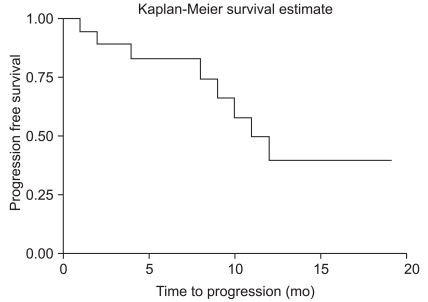
Eight (38.1%) patients showed distant brain recurrence. Seven patients suffered from lung cancer. One patient had renal cell carcinoma. The median time to progression (TTP) was 11 months after surgery. Twelve-month progression free survival rate was 39.7% (Fig. 1).
Fig. 1.
Distant recurrence free survival curve of hemorrhagic brain metastasis.
Among nine RT-naïve patients, five patients showed distant brain recurrence (55.6%). However, among 12 patients who received RT, four patients showed distant brain recurrence (33.3%), but there was no statistical significance (Fisher's exact test, p=0.396) (Table 2).
4. Overall survival
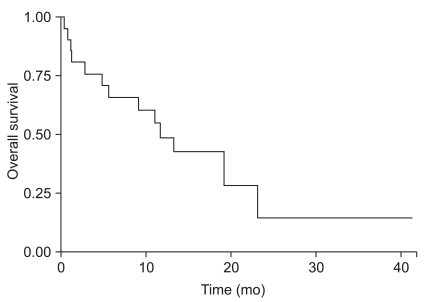
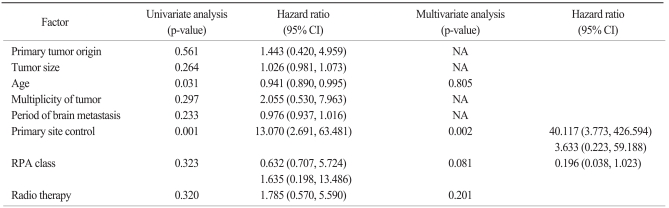
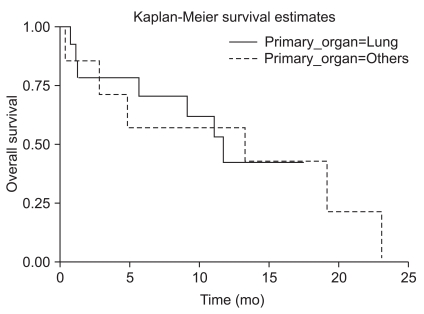
The median survival time was 11.7 months, and 12-month survival rate was 48.2% (Fig. 2). Factors affecting overall survival were analyzed in univariate and multivariate analysis using Cox regression model (Table 3). Prognostic factors that were analyzed included primary tumor origin, tumor size, patient age, number of lesions, time to develop brain metastases, extent and status of primary and systemic disease, Recursive Partitioning Analysis class, and history of RT. There were no significant differences in overall survival among different primary cancers (Fig. 3). Statistically significant prognostic factors were patient age (p=0.031) and primary disease control (p=0.001) in univariate analysis. However, in multivariate analysis, the only significant prognostic factor was primary disease control (p=0.002).
Fig. 2.
Overall survival curve of hemorrhagic brain metastasis.
Table 3.
Factors affecting overall survival in univariate and multivariate survival analysis (Cox regression model)
CI, confidence interval; NA, not assessed; RPA, Recursive Partitioning Analysis.
Fig. 3.
Overall survival curve of hemorrhagic brain metastasis based on primary cancers.
Discussion
It is not unusual for intracranial neoplasms to undergo spontaneous hemorrhage, regardless of whether they are intrinsic brain tumors or metastatic lesions. Prospective series indicate that brain tumors present as hemorrhagic lesions 2-3% of the time [2]. Similarly, in prospective series, a presenting spontaneous intra-cerebral hemorrhage can be associated with an intracranial tumor approximately 7% of the time [2]. In Kondziolka's series, intra-tumoral hemorrhage developed in 24% (36/147) of metastatic brain tumors [1]. Twenty-five percent (9/36) of the patients showed macroscopic hemorrhage [1]. However, only 2.7% of metastatic tumors exhibited acute symptoms [1]. According to Maiuri et al. [7], metastases are the most frequent cause of tumor-related ICH followed by glioblastoma. Among the metastases, malignant melanomas are most commonly observed. Metastases of choriocarcinoma represent a special feature because they directly erode cerebral vessels causing hemorrhage [11,12]. Because recurrent ICH occurs in all types of tumors, several authors have stressed the importance of complete tumor resection [4,13]. Furthermore, there are several reports that describe the negative effect of intra-tumoral hemorrhage on tumor control after stereotactic radiosurgery [8-10]. Therefore, surgery may be a more suitable treatment option in case of hemorrhagic metastasis.
Various theories for the etiology of intra-tumoral hemorrhage have been proposed previously. These include endothelial proliferation with vascular obliteration, vessel compression and/or distortion due to rapid tumor growth, vessel necrosis, invasion of vessel walls by the tumor, and increased venous pressure associated with increased intracranial pressure [14-17]. Common histological features of tumors that bled include tumor necrosis as well as the vascular changes of vessel-wall hyalinization, degeneration or necrosis of vessel walls, thrombosis, the presence of many thin-walled vessels, and ruptured vessels [1]. Recently, Jung et al. [18], reported that vascular endothelial growth factor and matrix metalloproteinases have a pathophysiological role in metastatic brain tumor-associated intracerebral hemorrhage. The goal of surgery for brain metastases is local disease control that results in stabilization or improvement of clinical symptoms. Local recurrences occur despite the best standard therapies. In studies of surgery and external beam radiation therapy, the recurrence rate at the site of surgery was reported to be 10% to 34% [19-22]. The local recurrence rate of a brain metastasis is likely to increase with prolonged survival time in cancer patients due to the advent of highly effective biological and other related targeted therapies. However, there is no study that addressed the effect of intra-tumoral hemorrhage on local recurrence following surgery.
Local recurrence following resection is believed to develop from microscopic, infiltrative tumor cells left behind at the time of surgery, beyond the circumference of the tumor removed. In a recent study reported from M. D. Anderson, there was a higher risk of local recurrence of brain metastasis with piecemeal resection than with en bloc resection, and tumor volume was also a negative prognostic factor [23]. In their report, tumors that were removed by piecemeal resection were significantly larger [23]. These two negative prognostic factors associated with local recurrence may be inter-related [23]. Previously, we reported that microscopic total resection of brain metastases that included removal of tumor cells infiltrating adjacent brain parenchyma to a depth of 5 mm at the tumor circumference, with resection margins pathologically confirmed to be free of tumor cells, significantly reduced local recurrence [24]. In this study, there were only two local recurrence episodes among 21 hemorrhagic brain metastases patients. Hemorrhagic tumors usually have a good dissection plane, so it is relatively easy to resect them completely. Another possible reason for the lower local recurrence rate may be disruption of the blood supply to the tumor and subsequent tumor cell necrosis when intra-tumor hemorrhage occurs.
Conclusion
The results of this study showed that hemorrhagic metastasis rarely recurred after surgery regardless of treatment with adjuvant therapy. Further prospective studies for a larger number of patients are required to determine adjuvant therapy after surgery for hemorrhagic brain metastases.
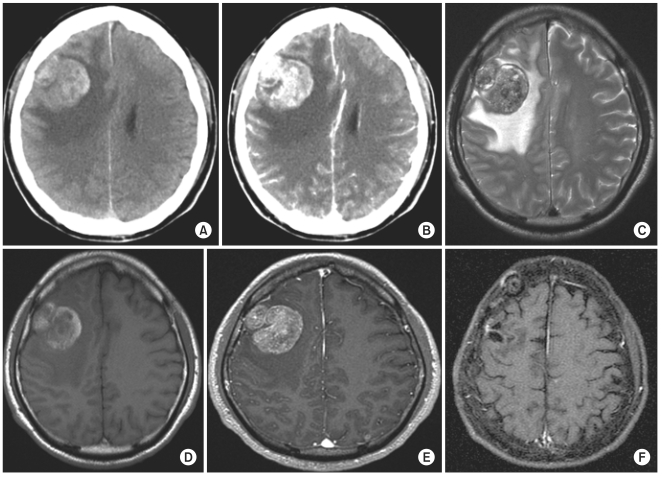
Fig. 4.
Illustrative case. A 55-yr-old male with non small cell lung cancer with hemorrhagic brain metastasis showed no local recurrence 49 mo after surgery without postoperative radiation therapy. However, multiple small distant metastases had developed. (A) Pre-operative non-contrast enhanced computed tomography (CT). (B) Pre-operative contrast-enhanced CT. (C) Pre-operative T2-weighted magnetic resonance imaging (MRI). (D) Pre-operative T1-weighted MRI. (E) Pre-operative gadolinium enhanced T1-weighted MRI. (F) Gadolinium enhanced T1-weighted MRI after 49 mo after surgery.
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Kondziolka D, Bernstein M, Resch L, Tator CH, Fleming JF, Vanderlinden RG, et al. Significance of hemorrhage into brain tumors: clinicopathological study. J Neurosurg. 1987;67:852–857. doi: 10.3171/jns.1987.67.6.0852. [DOI] [PubMed] [Google Scholar]
- 2.Schrader B, Barth H, Lang EW, Buhl R, Hugo HH, Biederer J, et al. Spontaneous intracranial haematomas caused by neoplasms. Acta Neurochir (Wien) 2000;142:979–985. doi: 10.1007/s007010070052. [DOI] [PubMed] [Google Scholar]
- 3.Wakai S, Yamakawa K, Manaka S, Takakura K. Spontaneous intracranial hemorrhage caused by brain tumor: its incidence and clinical significance. Neurosurgery. 1982;10:437–444. doi: 10.1227/00006123-198204000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Cheng MH, Lin JW. Intracranial meningioma with intratumoral hemorrhage. J Formos Med Assoc. 1997;96:116–120. [PubMed] [Google Scholar]
- 5.Cheng SY, Nagane M, Huang HS, Cavenee WK. Intracerebral tumor-associated hemorrhage caused by overexpression of the vascular endothelial growth factor isoforms VEGF121 and VEGF165 but not VEGF189. Proc Natl Acad Sci U S A. 1997;94:12081–12087. doi: 10.1073/pnas.94.22.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liwnicz BH, Wu SZ, Tew JM., Jr The relationship between the capillary structure and hemorrhage in gliomas. J Neurosurg. 1987;66:536–541. doi: 10.3171/jns.1987.66.4.0536. [DOI] [PubMed] [Google Scholar]
- 7.Maiuri F, D'Andrea F, Gallicchio B, Carandente M. Intracranial hemorrhages in metastatic brain tumors. J Neurosurg Sci. 1985;29:37–41. [PubMed] [Google Scholar]
- 8.Lavine SD, Petrovich Z, Cohen-Gadol AA, Masri LS, Morton DL, O'Day SJ, et al. Gamma knife radiosurgery for metastatic melanoma: an analysis of survival, outcome, and complications. Neurosurgery. 1999;44:59–64. doi: 10.1097/00006123-199901000-00031. [DOI] [PubMed] [Google Scholar]
- 9.Redmond AJ, Diluna ML, Hebert R, Moliterno JA, Desai R, Knisely JP, et al. Gamma Knife surgery for the treatment of melanoma metastases: the effect of intratumoral hemorrhage on survival. J Neurosurg. 2008;109(Suppl):99–105. doi: 10.3171/JNS/2008/109/12/S16. [DOI] [PubMed] [Google Scholar]
- 10.Seung SK, Sneed PK, McDermott MW, Shu HK, Leong SP, Chang S, et al. Gamma knife radiosurgery for malignant melanoma brain metastases. Cancer J Sci Am. 1998;4:103–109. [PubMed] [Google Scholar]
- 11.van den Doel EM, van Merriënboer FJ, Tulleken CA. Cerebral hemorrhage from unsuspected choriocarcinoma. Clin Neurol Neurosurg. 1985;87:287–290. doi: 10.1016/0303-8467(85)90137-4. [DOI] [PubMed] [Google Scholar]
- 12.Kidd D, Plant GT, Scaravilli F, McCartney AC, Stanford M, Graham EM. Metastatic choriocarcinoma presenting as multiple intracerebral haemorrhages: the role of imaging in the elucidation of the pathology. J Neurol Neurosurg Psychiatry. 1998;65:939–941. doi: 10.1136/jnnp.65.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwama T, Ohkuma A, Miwa Y, Sugimoto S, Itoh T, Takada M, et al. Brain tumors manifesting as intracranial hemorrhage. Neurol Med Chir (Tokyo) 1992;32:130–135. doi: 10.2176/nmc.32.130. [DOI] [PubMed] [Google Scholar]
- 14.Hirano A, Matsui T. Vascular structures in brain tumors. Hum Pathol. 1975;6:611–621. doi: 10.1016/s0046-8177(75)80045-1. [DOI] [PubMed] [Google Scholar]
- 15.McCormick WF, Ugajin K. Fatal hemorrhage into a medulloblastoma: case report. J Neurosurg. 1967;26:78–81. doi: 10.3171/jns.1967.26.1part1.0078. [DOI] [PubMed] [Google Scholar]
- 16.Scatliff JH, Radcliffe WB, Pittman HH, Park CH. Vascular structure of glioblastomas. Am J Roentgenol Radium Ther Nucl Med. 1969;105:795–805. doi: 10.2214/ajr.105.4.795. [DOI] [PubMed] [Google Scholar]
- 17.Weller RO, Foy M, Cox S. The development and ultrastructure of the microvasculature in malignant gliomas. Neuropathol Appl Neurobiol. 1977;3:307–322. [Google Scholar]
- 18.Jung S, Moon KS, Jung TY, Kim IY, Lee YH, Rhu HH, et al. Possible pathophysiological role of vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) in metastatic brain tumor-associated intracerebral hemorrhage. J Neurooncol. 2006;76:257–263. doi: 10.1007/s11060-005-6876-z. [DOI] [PubMed] [Google Scholar]
- 19.Armstrong JG, Wronski M, Galicich J, Arbit E, Leibel SA, Burt M. Postoperative radiation for lung cancer metastatic to the brain. J Clin Oncol. 1994;12:2340–2344. doi: 10.1200/JCO.1994.12.11.2340. [DOI] [PubMed] [Google Scholar]
- 20.Burt M, Wronski M, Arbit E, Galicich JH. Resection of brain metastases from non-small-cell lung carcinoma: results of therapy. Memorial Sloan-Kettering Cancer Center Thoracic Surgical Staff. J Thorac Cardiovasc Surg. 1992;103:399–410. [PubMed] [Google Scholar]
- 21.Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 22.Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 23.Patel AJ, Suki D, Hatiboglu MA, Abouassi H, Shi W, Wildrick DM, et al. Factors influencing the risk of local recurrence after resection of a single brain metastasis. J Neurosurg. 2010;113:181–189. doi: 10.3171/2009.11.JNS09659. [DOI] [PubMed] [Google Scholar]
- 24.Yoo H, Kim YZ, Nam BH, Shin SH, Yang HS, Lee JS, et al. Reduced local recurrence of a single brain metastasis through microscopic total resection. J Neurosurg. 2009;110:730–736. doi: 10.3171/2008.8.JNS08448. [DOI] [PubMed] [Google Scholar]