Abstract
To study the outcomes of split electrode array cochlear implantation in ossified cochlea using the CAP scoring system. Retrospective case study. Tertiary referral center. Six cochleae in three adult and three pediatric patients with ossification. Intervention(s): All Patients underwent cochlear implantation with a split electrode array system. Major outcome parameter(s): Number of electrodes inserted during surgery, number of functioning electrodes on follow-up and auditory performance evaluation using the CAP score—Category of Auditory Perception [TSC Revised Version, based on Nottingham CI Program, 1995]. Six patients (three children and three adults) had insertion of split electrode array system. The mean number of electrodes inserted were 18.3 (range 15–21) and functioning electrodes at follow-up were mean of 14.3 (range 7–21). Auditory performance was measured using CAP score at 1 year post implant follow up, mean score in children was six and that in adult was eight. One pt had facial nerve twitching which was corrected by switching off the concerned electrode. No complications in sort of facial palsy or vestibular disorder were observed. Patients of ossified cochlea having profound deafness do well with split electrode array cochlear implantation as evaluated with CAP scoring system. The split electrode array results in more number of electrodes within the cochlear lumen. Retro graded apical array insertion has less chances of facial nerve stimulation as it is placed away from the nerve.
Keywords: Split electrode array, Category of auditory perception (CAP), Cochlear ossification
Introduction
Meningitis is a common cause of acquired profound sensorineural hearing loss in up to 10% of children [1] and is associated with high incidence (15–20%) of cochlear ossification [2–4]. The occurrence of ossification virtually guarantees that hearing will not be restored, making cochlear implantation an important treatment option. Other etiologies of cochlear ossification include otosclerosis, chronic otitis media, autoimmune, and idiopathic processes.
Ossification in labyrinthitis ossificans occurs initially in the basal turn of the cochlea [5]. This location is the site of entry of the electrode array. Ossification may prevent a full length insertion of standard electrode array which may adversely affect the post implant performance [6, 7].
The split array implant system allows insertion of near to normal number of electrodes without major anatomical changes in middle or inner ear. Split arrays are manufactured by Med-El (Innsbruck, Austria), Cochlear (Sydney, Australia) and Nurelec. This study summarizes our experience with the split electrode array implantation in ossified cochlea.
Study Design
This is a retrospective study of patients who underwent cochlear implantation in ossified cochlea with either the Med-El or Cochlear split electrode array system at PD Hinduja hospital.
Patients
In our experience of 630 implants between Nov 1996 to Nov 2008, we have identified six patients who met the above criteria. Four patients were post lingual deaf while two were pre lingual deaf. The average duration of deafness in post lingual group was 6.7 years, ranging from 1 to 10 years. The duration of deafness in pre lingual group consisting of two patients was 2 and 4 years, respectively (Table 1). Three adults and three children with a mean age of 19.8 years (range 3–41). Meningitis was the etiological factor for deafness in five cases while one was due to otosclerosis. Five cases underwent implantation with the Nucleus 24 Double Array (Cochlear, Ltd.) Device while one patient had a Split Array C40 + GB (Med-El Corporation).
Table 1.
Patients and outcomes
| Patient number | Age in years | Pre/Post lingual deafness | Etiology of deafness | Duration of deafness (years) | Apical array insertion technique | CAP score | |
|---|---|---|---|---|---|---|---|
| Pre operative | 1 year post operative | ||||||
| 1 | 3 | Pre lingual | Meningitis | 2 | Ante graded | 1 | 7 |
| 2 | 5 | Pre lingual | Meningitis | 4 | Ante graded | 1 | 8 |
| 3 | 30 | Post lingual | Otosclerosis | 8 | Retro graded | 2 | 11 |
| 4 | 30 | Post lingual | Meningitis | 10 | Ante graded | 2 | 8 |
| 5 | 10 | Post lingual | Meningitis | 1 | Ante graded | 1 | 2 |
| 6 | 41 | Post lingual | Meningitis | 8 | Retro graded | 1 | 8 |
All patients had pre operative high resolution MRI done which is a useful guide for patency of Cochlear lumen and anatomy. All six cases in our study group had severe obliteration of Cochlear lumen.
Surgical Technique
Cochlear implantation with a split array is performed through a posterior tympanotomy approach. After identifying the facial nerve, facial recess is widely opened.
The first cochleostomy is made just anterior and little inferior to the round window for insertion in the basal turn of the cochlea. In cochlear ossification cases the basal turn of cochlea is drilled along the inferior wall of cochlea (approx 8–9 mm), taking care of avoiding Modiolus and the carotid. The basal turn dips deeply as it approaches the carotid and runs under the tensor semi canal then turns inferiorly in front of the oval window. In cases where lumen was obliterated by fibrous tissue, a pick was used to clear it.
While performing the second cochleostomy, Incus bar, Incus and stapes suprastructure was removed for adequate exposure. After dislocating the IS joint, the stapes supra structure is removed taking care to stabilize the footplate by gentle pressure using suction tip over a cotton ball. Using 1 mm diamond burr opening was made in second turn of cochlea just anterior to oval window, below the process cochleariformis. After drilling the two cochleostomies, first the basal array is inserted in the inferior tunnel created in basal turn of cochlea. The apical array is then inserted in ante grade (Directed away from basal turn of cochlea) (Fig. 1) or retro grade (Directed towards the basal turn) (Fig. 2) fashion from the second cochleostomy.
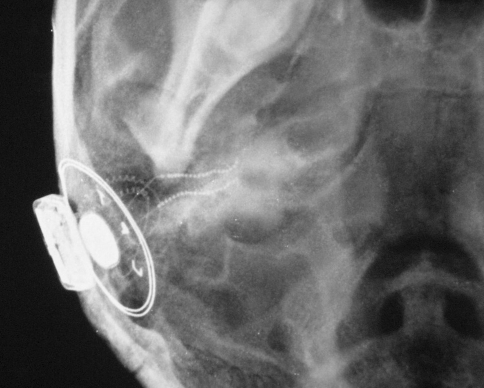
Fig. 1.
Post operative X ray showing Ante graded positioning of the apical array
Fig. 2.
Post operative X ray showing retro graded positioning of the apical array
Both the cochleostomies are then packed with tissue- fascia to secure the electrode and to prevent perilymph leaks. Intraoperative neural telemetry is performed to confirm device integrity, and a plain cranium radiograph (modified Stenvers view) is obtained to confirm and document the position of the electrode array(s).
Four of cases we had to drill the basal turn of cochlea to reach patent lumen, in three cases we encountered fibrous tissue which was cleared using a curved pick.
Results
Total of Six patients (three children and three adults) had split array electrode insertion. Age varied from 3 to 41 years. Deafness was prelingual in two cases and four cases had post lingual deafness (Table 1). Duration of deafness in post lingual cases varied from 1 to 10 years with a mean of 6.7 years. The duration of deafness in pre lingual group consisting of two patients was 2 and 4 years, respectively (Table 1). A mean of 9.8 (range, 9–11) electrodes were inserted basally and 8.5 (range, 5–11) electrodes were inserted apically, for a total mean of 18.3 (range, 15–21) (Table 2). At follow-up, the mean number of electrodes in use was 14.3 (range, 7–21). All patients had profound deafness before implantation. Auditory performance outcome was evaluated using CAP score (Table 3) at 1 yr post implant follow up, mean score in children was 5.6 (range 2–8) and that in adult was 9 (range 9–11). Four of the cases had ante grade insertion (Fig. 1) of apical array while two had retrograde insertion (Fig. 2). Etiology of deafness was meningitis in five cases while one implantee had otosclerosis. One case (patient no. 4) had post operative facial nerve stimulation in form of twitching which was resolved by switching off three concerned electrodes during mapping. We did not encounter any operative complications such as facial nerve palsy, vestibular dysfunction, wound infection, cerebrospinal fluid leak.
Table 2.
Electrode status
| Patient number | Electrodes inserted during surgery | Active electrodes on follow up | ||
|---|---|---|---|---|
| Basal array | Apical array | Total electrodes | ||
| 1 | 11 | 9 | 20 | 15 |
| 2 | 11 | 10 | 21 | 21 |
| 3 | 10 | 5 | 15 | 14 |
| 4 | 9 | 11 | 20 | 19 |
| 5 | 9 | 8 | 17 | 10 |
| 6 | 9 | 8 | 17 | 7 |
Table 3.
Category of auditory perception (CAP)
Mapping
Neural response telemetry was done at the operative suite, which demonstrated integrity of all electrodes we were able to insert during surgery; first mapping was done 4 week post surgery. We had an average of 14.3 electrodes functioning (range 7–21), in one case we had facial nerve stimulation in form of twitching which was corrected by switching of three concerned electrodes during first mapping. Mapping was performed at the audiology dept of P D Hinduja hospital, at intervals of 4 week. We have follow up ranging from 1–2 years. We did not observe any deterioration in number of active electrodes in follow up.
Evaluation Measures
The auditory percept evaluation following implant was done by CAP scoring system. Category of Auditory Perception, CAP [TSC Revised Version, based on Nottingham CI Program, 1995] is a twelve point hierarchical scale of auditory performance ranging from No awareness of environmental sound to Conversation on telephone with unfamiliar speaker (Table 3). We have implantees who come from various linguistic background, considering this the CAP scoring scale is easily adaptable, comprehensive for professionals and significant others. This scoring system is highly reproducible and delivers valid outcome measures. This scoring was done on 6 monthly interval after switch on, we have analyzed results at 1 year follow up of our study group, mean score in children was 5.6 (range 2–8) and that in adult was 9 (range 9–11). The Preoperative CAP score measured just before surgery varied from 1 to 2.
Discussion
Cochlear lumen obliteration by pathologic new bone formation leads to profound deafness. Cochlear implantation forms a major treatment option for such cases. Common causes of cochlear obliteration include post meningitis, auto immune inner ear diseases and otosclerosis. Cochlear lumen obliteration may preclude adequate insertion of standard electrode array. Increase in degree of obstruction leads to significant reduction in number of active electrode within the lumen which adversely affects the auditory outcomes [6, 7]. Axon PR, Temple RH, Saeed SR and Ramsden RT in their study of cochlear ossification after meningitis have classified patients based on extent of ossification [15]. According to their study ossification fell into three groups: Gross ossification of the scala tympani and variable amounts of the scala vestibuli; partial ossification localized to the basal turn of the scala tympani; and no ossification. All of our study group patients are placed in the first group i.e. Gross ossification of the scala tympani which precluded adequate insertion of standard electrode array. Many modified insertion techniques have been developed for insertion of usable electrodes in cochlear lumen. In cases where obliteration is limited to basal turn, standard array can be inserted by extended cochleostomy and clearing the lumen. Scala vestibuli, if patent provides with alternate channel for complete insertion. The option of total drill out around the modiolus has been described by Gantz et al. [8], Steernson and Gary [9] and Balkany et al. [10]. In cases of complete obliteration of cochlear lumen the double array allows for more number of electrode placement in the cochlear lumen and also results in increased number of active electrodes on follow up.
In our experience of 630 implants, we evaluate every case with a high resolution CT scan and an MRI. Cases in which we suspect cochlear lumen obliteration based on imaging and clinical history, we also obtained an MRI just prior to surgery to assess the progress of ossification. We had the option of split electrode implant along with standard array at every suite where we suspected cochlear ossification. During surgery, after the first cochleostomy near round window was performed we used a depth guage to measure the approximate length of insertion possible. The standard array insertion was performed where adequate insertion was possible as guided by depth guage, in rest we went ahead with performing the second cochleostomy near the oval window and inserted the split array system. In our short experience of retro graded apical array insertion (directed towards the basal turn) (Fig. 2), we believe this has less chances of facial nerve stimulation as its placed away from the nerve as compared to ante grade insertion (Fig. 1). Med-El Corporation (Innsbruck, Austria) has the C40 + BG that consist of a five and a seven split electrode array system. The Cochlear Corporation (Sydney, Australia) has the nucleus 24 double array that contains 11 electrodes on each array. Both arrays are designed for implantation into two separate cochleostomies with the goal of maximizing the number of available electrodes available. The split electrode system allowed more number of functional electrodes within cochlea as seen in our results. Basal array insertion had a mean of 9.8 (range, 9–11) electrodes, where as the apical array insertion had a mean 8.5 (range, 5–11) electrodes, for a total mean of 18.3 (range, 15–21) (Table 2). At follow-up, the mean number of electrodes in use was 14.3 (range, 7–21). Evaluating the Auditory outcome with the CAP scoring system (Table 3), five of our cases had significant gain in auditory perception as measured by CAP score which varied from 8 to 11 for these cases. One case had marginal benefit from double array implantation which reflected through his CAP score of two at 1 year follow up. The Preoperative CAP score measured just before surgery varied from 1 to 2 (mean 1.3). Comparing the outcomes using CAP score parameter we find an average gain of 8 points (CAP score scale, Table 3) in adult and a five point gain in children. Nikolopoulos et al. have published the speech perception outcome following cochlear implantation in prelingually deaf children using CAP score as the evaluating parameter [11].
Lenarz et al. [12] in 2001 published their series of patients implanted with the Nucleus Double Array cochlear implant. This was a prospective trail in which speech performance was tested with and without use of the additional array implanted into the second turn. Testing conditions were (1) basal array active only, (2) apical array active only, and (3) both arrays active. All patients performed better with both arrays active together as compared with either of the array functioning. This showed a marked improvement in outcome from the additional electrodes.
Bauer and Roland [13] published the results of ten children undergoing split array insertion. They have data of eight patients implanted with Med-El split arrays: six prelingually deafened and two postlingually deafened. Five children had full insertions. The auditory performance evaluation was done using the IT-MAIS parameter.
Roland and Coelho [14] published their experience with standard and double array implantation. This is a study of eight patients (four children and four adults) having a double-array electrode implantion. The mean number of electrodes inserted and active at follow-up was 18.1 and 16.3, respectively. Auditory performances were evaluated on basis of open set speech performance.
Cochlear ossification is thought to be progressive. In general it progresses from base to apex and results in complete labyrinthine ossificans [16–18]. Its effects on cochlear signal transmission and functioning of implanted electrode will be clear on a long term follow up of these cases. Nabili et al. in their study related to chronology of labyrinthitis ossificans conclude that ossification and osteoneogenesis within the cochlear lumen begins as early as 3 weeks and is active for a period of at least 12 months [19].
Conclusion
Patients of ossified cochlea having profound deafness do well with split electrode array cochlear implantation as evaluated with CAP scoring system. The split electrode array results in more number of electrodes within the cochlear lumen. Retro graded apical array insertion has less chances of facial nerve stimulation as it is placed away from the nerve.
Summary
Patients with ossified cochlea having profound deafness do well with split electrode array implant. This study evaluates the outcome of split electrode array implant in cases of ossified cochlea using the CAP scoring system. The studies in past have evaluated the outcomes with parameters such as Speech perception threshold, IT-MAIS, loudness balance test.
References
- 1.Shelton MM, Marks WA. Bacterial meningitis: an update. Neurol Clin. 1990;8:605–617. [PubMed] [Google Scholar]
- 2.Nadol JB, Jr, Hsu W. Histopathologic correlation of spiral ganglion cell count and new bone formation in the cochlea following meningogenic labyrinthitis and deafness. Ann Otol Rhinol Laryngol. 1991;100:111–113. doi: 10.1177/000348949110000904. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim RA, Linthicum FH., Jr Labyrinthitis ossificans and cochlear implants. Arch Otolaryngol. 1980;106:111–113. doi: 10.1001/archotol.1980.00790260043012. [DOI] [PubMed] [Google Scholar]
- 4.Green JD, Marion MS, Hinjosa R. Labyrinthitis ossificans: histopathologic consideration for cochlear implantation. Otolaryngol Head Neck Surg. 1991;104:320–326. doi: 10.1177/019459989110400306. [DOI] [PubMed] [Google Scholar]
- 5.Keithely EM, Harris JP. Late sequelae of cochlear infection. Laryngoscope. 1996;106:341–345. doi: 10.1097/00005537-199603000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Hartrampf R, et al. Insertion depth of the nucleus electrode array and relative performance. Ann Otol Rhinol Laryngol Suppl. 1995;166:277–280. [PubMed] [Google Scholar]
- 7.Geier LL, Norton SJ. The effects of limiting the number of nucleus 22 cochlear implant electrodes programmed on speech perception. Ear Hear. 1992;13:340–348. doi: 10.1097/00003446-199210000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Gantz BJ, McCabe BF, Tyler RS. Use of multichannel cochlear implants in obstructed and obliterated cochleas. Otolaryngol Head Neck Surg. 1988;98:72–81. doi: 10.1177/019459988809800113. [DOI] [PubMed] [Google Scholar]
- 9.Steenerson RL, Gary LB. Multichannel cochlear implantation in children with cochlear ossification. Am J Otol. 1999;20:442–444. [PubMed] [Google Scholar]
- 10.Balkany T, Luntz M, Telischi FF, Hodges AV. Intact canal wall drill-out procedure for implantation of the totally ossified cochlea. Am J Otol. 1997;18(6 Suppl):S58–S59. [PubMed] [Google Scholar]
- 11.Nikolopoulous TP, Archbold SM. The development of auditory perception in children following cochlear implantation. Int J Paediatr Otorhinolaryngol 1999. 1999;49(Sppl.1):189–191. doi: 10.1016/S0165-5876(99)00158-5. [DOI] [PubMed] [Google Scholar]
- 12.Lenarz T, Lesinski-Schiedat A, Weber BP, Issing PR, et al. The nucleus double array cochlear implant: a new concept for the obliterated cochlea. Otol Neurotol. 2001;22:24–32. doi: 10.1097/00129492-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Bauer PW, Roland PS. Clinical results with the Med-El compressed and split arrays in the United States. Laryngoscope. 2004;114:428–433. doi: 10.1097/00005537-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Roland, Thomas J, Jr, Coelho Partial and double-array implantation of the ossified cochlea. Otol Neurotol. 2008;29(8):1068–1075. doi: 10.1097/MAO.0b013e318188e8ea. [DOI] [PubMed] [Google Scholar]
- 15.Axon PR, Temple RH, Saeed SR, Ramsden RT, et al. Cochlear Ossification After Meningitis. American Journal of Otology. 1998;19(6):724–729. [PubMed] [Google Scholar]
- 16.Paparella MM, Sugiura S. The pathology of suppurative labyrinthitis. Ann Otol Rhinol Laryngol. 1967;76:554–586. doi: 10.1177/000348946707600303. [DOI] [PubMed] [Google Scholar]
- 17.Jackler RK, Bates GJ (1989) Medical and surgical considerations of cochlear implantation in children. Cochlear implant in young children. Little, Brown and Co, Boston, pp 153–181
- 18.Jackler RK, et al. Cochlear patency problems in cochlear implantation. Laryngoscope. 1987;97:801–805. doi: 10.1288/00005537-198707000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Nabili, Vishad BS, et al. Chronology of labyrinthitis ossificans induced by streptococcus pneumoniae meningitis. Laryngoscope. 1999;109(6):931–935. doi: 10.1097/00005537-199906000-00017. [DOI] [PubMed] [Google Scholar]