Abstract
Nasal polyps are benign mucosal protrusions that expand into the nasal cavity. There are no any etiological factors that may explain the pathogenesis of nasal polyps but currently inflammation continues to be the major factor. As a result of inflammation, neutrophils become activated and migrate to the inflammatory area and form their bactericidal effects by producing free oxygen radicals. The objective of our study is to investigate the expression of myeloperoxydase enzyme, which is usually an indicator of leukocyte infiltration and is responsible in the formation of free oxygen radicals in polyp tissues and to determine its role in the pathogenesis of nasal polyps.
Keywords: Myeloperoxidase, Nasal polyps, Inflammation, Free oxygen radicals
Introduction
Nasal polyps are benign mucosal protrusions that expand into the nasal cavity. Nasal polyposis is a condition that affects the 1–4% of the community and leads to various complaints such as nasal obstruction, rhinorrhea and anosmia [1, 2]. There are no any etiological factors that may explain the pathogenesis of nasal polyps but currently inflammation continues to be the major factor. Nasal polyp tissues are histologically characterized by the infiltration of inflammatory cells such as neutrophils and eosinophils [3–5].
As a result of inflammation, neutrophils become activated and migrate to the inflammatory area and form their bactericidal effects by producing free oxygen radicals (FOR). FOR are necessary for normal immune defense and metabolic activity in humans but also they may cause tissue damage if they are excessively produced and they may play an important role in the pathogenesis of various diseases [6, 7]. Currently, there are several studies related with nasal polyps and their relationship with FOR [8, 9] but up to date and as much as we are informed, there are a limited number of direct studies related with the role of myeloperoxydase(MPO) which is responsible in the formation of FOR, in the pathogenesis of polyps [10].
The objective of our study is to investigate the expression of MPO enzyme, which is usually an indicator of leukocyte infiltration and is responsible in the formation of FOR in polyp tissues and to determine its role in the pathogenesis of nasal polyps.
Materials and methods
The study was carried out on a total of 54 cases including 30 patients who underwent an operation due to nasal polyps and 24 patients who underwent an operation due to septoplasty or concha hypertrophy at Dr. Lütfi Kırdar Kartal Educational and Research Hospital, second ENT Clinic.
Preoperative vital findings, examination findings of patients were recorded. Patients were diagnosed with nasal polyposis and chronic rhinosinusitis based on history, physical examination, nasal endoscopy and paranasal sinus CT. None of the patients had sinus surgery history. Patients were informed in general about the study and necessary permits were obtained.
During the operations, nasal polyp tissue and nasal mucosa samples were collected, fixed by formol and prepared for pathological examination.
Immunohistochemical Staining
Samples were fixed in formol, embedded in paraffin blocks, and 5-mm cross-sections were obtained. A cross-section was systemically stained with hematoxylen-eosin for standard histomorphological analysis. The same technique was applied both to nasal mucosa and polyps.
Cross-sections were deparaffinized for 1 h in an incubator at 60°C before immunohistochemical staining. They were allowed to wait in xylene for 2 × 5 min and then in absolute alcohol for 2 × 5 min. They were thoroughly washed with distilled water. 10% Citrate Buffer solution was prepared for antigen retrieval. Slides were placed inside a plastic container resistant to the microwave oven. 10% antigen retrieval solution was added. Microwave was operated at 750 and 350 W for 5 and 15 min, respectively. At the end of 15 min the container was taken out of the microwave and allowed to cool down to room temperature for 20 min. It was thoroughly washed with distilled water. It was subject to peroxidase blockage with 3% H2O2 for 20 min. Each section was reacted with the primary antibody for 90 min. The primary antibody used was human myeloperoxidase monoclonal andibody (MPO Ab-2) (Neomarkers, USA)
Immunohistochemical Assessment
Brown-color staining with MPO in the inflammatory cell cytoplasm was rated as mild (+), medium (++), and intense (+++) according to the intensity of the staining.
Statistical Analysis
SPSS (Statistical Package for Social Sciences) for Windows 10.0 software was used for statistical analysis. To evaluate the data derived from the study, the Chi-square test was used in the comparison of qualitative data, in addition to descriptive statistical methods (frequency). Results were evaluated at 95% confidence interval, and significance was found to be P < 0.05.
Results
29 of the patients were male and 25 were female. Their ages varied between 12 and 63 (average age 34). The study group included 17 male and 13 female patients, whose ages varied between 12 and 58 (average age 36). The control group included 12 male and 12 female patients, whose ages varied between 18 and 63 (average age 32).
Histopathological Results
Nasal polyp and nasal mucosa samples are stained with hematoxylene-eosin at a standard manner. While inflammatory findings were observed in nasal polyp samples, no sign of inflammation was observed in normal nasal mucosa samples. Nasal polyp and nasal mucosa samples were then stained with MPO antibody and evaluated.
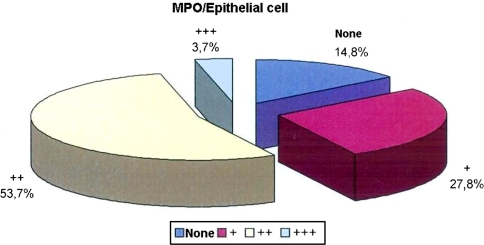
While no staining was observed in the epithelial cells in 14.8% of the cases with MPO, epithelial cell staining level with MPO was in +, ++, and +++ in 27.8, 53.7, and 3.7% of the cases, respectively (Table 1; Fig. 1).
Table 1.
Distribution of epithelial cells and inflammatory cells in all tissues stained with MPO
| n | % | ||
|---|---|---|---|
| MPO/Epithelial cell | None | 8 | 14.8 |
| + | 15 | 27.8 | |
| ++ | 29 | 53.7 | |
| +++ | 29 | 3.7 | |
| MPO/Inflammatory cell | None | 8 | 14.8 |
| + | 15 | 27.8 | |
| ++ | 27 | 50.0 | |
| +++ | 4 | 7.4 | |
Fig. 1.
Distribution of epithelial cell stained with MPO in all tissues
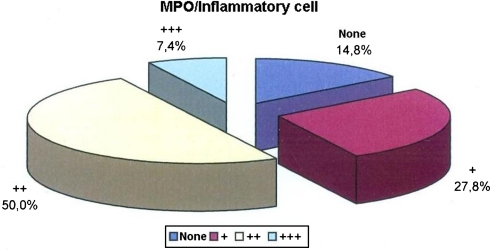
While no staining was observed in the inflammatory cells in 14.8% of the cases with MPO, inflammatory cell staining level with MPO was in +, ++, and +++ in 27.8, 50, and 7.4% of the cases, respectively (Table 1; Fig. 2).
Fig. 2.
Distrubution of inflammatory cells stained with MPO in all tissues
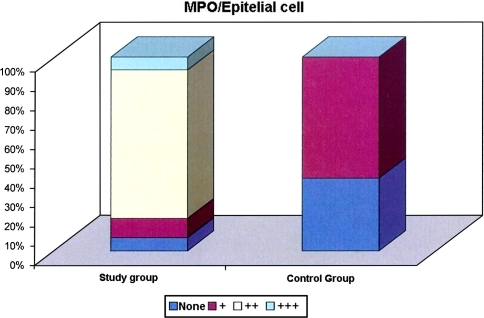
There is high statistically significant difference between the groups according to the distribution of epithelial cells stained with MPO (P < 0.01). While level of epithelial cells stained with MPO was ++ in 76.6% of the cases in the study group, such level is + and +++ positive in 10.0 and 6.6% of the cases, respectively. While no staining was observed in the epithelial cells in 37.5% of the cases with MPO in the control group, epithelial cell staining level with MPO was + in 62.5% of the cases (Table 2; Fig. 3).
Table 2.
Comparison of groups according to epithelial cell staining levels with MPO
| MPO/Epithelial cell | Patient group | Control group | Test stat. | ||
|---|---|---|---|---|---|
| n | % | n | % | p | |
| None | 2 | 6.6 | 9 | 37.5 | |
| + | 3 | 10.0 | 15 | 62.5 | χ2: 37.248 |
| ++ | 23 | 76.6 | – | – | P 0.001** |
| +++ | 2 | 6.6 | – | – | |
χ2 Q-Square Test; ** P < 0.01 significiant at advanced level Table
Fig. 3.
Distribution of epithelial cells stained with MPO between groups
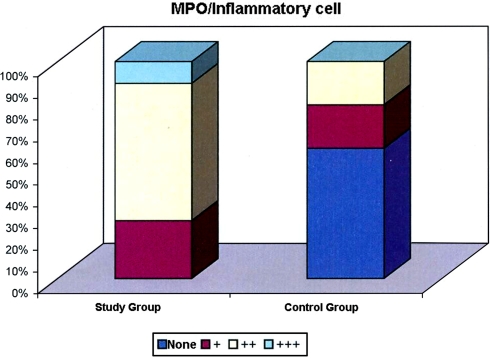
According to the distribution of inflammatory cells stained with MPO there is high statistically significant difference between the groups (P < 0.01). While the level of inflammatory cells stained with MPO is ++ in 63.3% of the cases in the study group, such level is + and +++ positive in 26.7 and 10.0% of the cases, respectively. There are no cases in the study group without inflammatory cell staining with MPO. While the level of inflammatory cells stained with MPO in 25% of the cases in the control group was +, the level of inflammatory cells stained with MPO in 25% of the cases is ++. The rate of cases without inflammatory cells stained with MPO in the control group is 50% (Table 3; Fig. 4).
Table 3.
Comparison of groups according to inflammatory cell levels stained with MPO
| MPO/Inflammatory cell | Patient group | Control group | Test stat. | ||
|---|---|---|---|---|---|
| n | % | n | % | P | |
| None | – | – | 12 | 50.0 | |
| + | 8 | 26.7 | 4 | 25.0 | χ2: 25.121 |
| ++ | 19 | 63.3 | 4 | 25.0 | |
| +++ | 3 | 10.0 | – | – | P 0.001** |
χ2 Q-Square Test ** P < 0.01 significant at advanced level
Fig 4.
Distrubution of inflammatory cells stained with MPO between groups
Discussion
Chronic infections of nasal and paranasal sinuses are a common condition in nasal polyp patients. There is a high ratio of neutrophil infiltration in the polyps of patients suffering from chronic rhinosinusitis. In a study carried out by Rudack et al., nasal mucosa of patients diagnosed with chronic rhinosinusitis with polyps were compared with the nasal mucosa of patients diagnosed with chronic rhinosinusitis without polyps, and the amount of neutrophils in the nasal polyp tissue was found intensive [11]. In another similar study conducted by Morinaka et al., the polyp and inferior turbinate mucosas were compared and the ratio of neutrophils was found significantly higher in polyps [12].
MPO is an enzyme, which occurs as a result of neutrophil activation, may lead to the formation of FOR such as H2O2, NO and especially, HOCL with other enzymes [13, 14]. Several studies were performed to investigate the relationship of MPO with chronic sinusitis, role of FOR in the development of nasal polyps and the effects of antioxidant agents on the development of nasal polyps [7, 15, 16]. In a study carried out by Demoly et al. regarding neutrophil infiltration in patients diagnosed with chronic sinusitis, investigators observed a high ratio of MPO and IL-8 in the sinus lavage of patients included in the patient and control group [15]. Dogru et al. investigated the role of FOR in the development of nasal polyps and reported that FOR may play a significant role in the pathogenesis of nasal polyps. 16 In another study performed by Okur et al. investigators demonstrated the production of FOR in the neutrophils of nasal polyps and determined a very high ratio of FOR production in nasal polyps [16].
As far as is known, there is only one study carried out directly on the relationship of MPO and the pathogenesis of nasal polyps, however, the number of patients enrolled in this study is very limited. In this study carried out by Van Zele et al. MPO levels in nasal polyps of patients with a cystic fibrosis was found elevated, however, in patients with nasal polyps without cystic fibrosis, no any significantly different results were observed when compared with the results obtained from chronic sinusitis patients regarding MPO levels [10].
In our study, MPO expression in epithelial and inflammatory cells of patients with nasal polyps were compared with the MPO expression in epithelial and inflammatory cells of patients enrolled in the control group. Consequently, we found a statistically significant difference between the two groups regarding the levels of MPO activity in both epithelial cells and inflammatory cells. According to these findings and especially the presence of FOR in nasal polyps indicate that MPO may play an important role in the pathogenesis of nasal polyposis. The condense condition of MPO expression in inflammatory cells of patients with nasal polyps make us think that the number of cells that participate in the inflammation process of nasal polyposis is huge and that the amount of FOR produced from these cells is very intensive. Similarly, the increased MPO expression in epithelial cells of patients who especially have nasal polyps, show that the epithelial damage among these patients is considerably high and that MPO enzyme may be responsible from the occurrence of this damage. We presume that, a different result obtained in the previous study can be explained due to the less number of patients.
Conclusion
FOR may lead to tissue damage when they are over-produced even though if they are necessary to provide normal immune defense and metabolic activity which is extremely important for humans to fight infectious agents. Results obtained from our study demonstrate that, accompanied by a persistent inflammation, increased amounts of MPO in the nasal mucosa and eventually, epithelial damage of FOR may play a significant role in the pathogenesis of nasal polyps.
References
- 1.Bachert C, Gevaert P, Holtappels G, Cuvelier C, Cauwenberge P. Nasal polyposis: from cytokines to growth. Am J Rhinol. 2000;14:279–290. doi: 10.2500/105065800781329573. [DOI] [PubMed] [Google Scholar]
- 2.Ozcan C, Polat A, Otag F, Gorur K. Does Helicobacter pylori play a role in etiology of nasal polyposis? Auris Nasus Larynx. 2009;14:427–430. doi: 10.1016/j.anl.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Pawankar R. Nasal polyposis: an update: editorial review. Curr Opin Allergy Clin Immunol. 2003;3:1–6. doi: 10.1097/00130832-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy DW, Bolger WE, Zinreich SJ (2001) Nasal polyposis In: Diseases of the sinuses, therapy and management. B.C.Decker, Philedelphia, pp 69–75
- 5.Drake-Lee A (1997) The pathogenesis of nasal polyps. In: Nasal polyps: epidemiology, pathogenesis and treatment. OceanSide Publications Inc., Rhode Islands, pp 57–64
- 6.Shukla GK, Mahajan A, Pandey S, et al. A study of free radicals and scaveging enzyme in tonsillitis. Boll Chim Farm. 1996;135:653–655. [PubMed] [Google Scholar]
- 7.Dogru H, Delibas N, Doner F, Tuz M, Uygur K. Free radical damage in nasal polyp tissue. Otolaryngol Head Neck Surg. 2001;124:570–572. doi: 10.1067/mhn.2001.115086. [DOI] [PubMed] [Google Scholar]
- 8.Kahveci OK, Derekoy FS, Yilmaz M, Serteser M, Altuntas A. The role of MMP-9 and TIMP-1 in nasal polyp formation. Swiss Med Wkly. 2008;138:684–688. doi: 10.4414/smw.2008.12459. [DOI] [PubMed] [Google Scholar]
- 9.Lu X, Zhang XH, Wang H, Long XB, You XJ, Gao QX, Cui YH, Liu Z. Expression of osteopontin in chronic rhinosinusitis with and without nasal polyps. Allergy. 2009;64:104–111. doi: 10.1111/j.1398-9995.2008.01829.x. [DOI] [PubMed] [Google Scholar]
- 10.Zele T, Claeys S, Gevaert P, Maele G, Holtappels G, Cauwenberge P, Bachert C. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:280–289. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 11.Rudack C, Sachse F, Alberty J. Chronic rhinosinusitis-need for further classification? Inflamm Res. 2004;53:111–117. doi: 10.1007/s00011-003-1232-2. [DOI] [PubMed] [Google Scholar]
- 12.Morinaka S, Nakamura H. Inflammatory cells in nasal mucosa and nasal polyps. Auris Nasus Larynx. 2000;27:59–64. doi: 10.1016/S0385-8146(99)00038-3. [DOI] [PubMed] [Google Scholar]
- 13.Thomas EL, Bozeman PM, Jefferson MM, King CC. Oxidation of bromide by the human leukocyte enzymes myeloperoxidase and eosinophil peroxidase: formation of bromamines. J Biol Chem. 1995;270:2906–2913. doi: 10.1074/jbc.270.7.2906. [DOI] [PubMed] [Google Scholar]
- 14.Barrette WC, Jr, Hannum DM, Wheeler WD, Hurst JK. General mechanism for the bacterial toxicity of hypochlorous acid: abolition of ATP production. Biochemistry. 1989;28:172–178. doi: 10.1021/bi00449a032. [DOI] [PubMed] [Google Scholar]
- 15.Demoly P, Crampette L, Mondain M, Enander I, Jones I, Bousquet J. Myeloperoxidase and interleukin-8 (IL-8) levels in chronic sinusitis. Clin Exp Allergy. 1997;27:672–675. doi: 10.1111/j.1365-2222.1997.tb01195.x. [DOI] [PubMed] [Google Scholar]
- 16.Okur E, Inanc F, Yildirim I, Kilinc M, Kilic MA. Malondialdehyde level and adenosine deaminase activity in nasal polyps. Otolaryngol Head Neck Surg. 2006;134:37–40. doi: 10.1016/j.otohns.2005.09.032. [DOI] [PubMed] [Google Scholar]