Abstract
To evaluate the short-term outcomes and safety of a newly introduced drug-eluting ethmoid stent. Prospective study of 23 patients with a total of 40 implanted ethmoid sinuses. Two tertiary care medical facility. Twenty-three patients with medically refractory chronic rhinosinusitis were treated with patient-appropriate endoscopic sinus surgery, with the modification of treating the ethmoid sinuses with an ethmoid stent infused with triamcinolone, instead of conventional endoscopic ethmoidectomy. Patients were then followed up over 6 months. Outcomes were assessed by interval changes in 20-item Sino-Nasal Outcome Test (SNOT-20) and Lund–MacKay CT scores. Safety was determined by adverse events. Overall, the pre-op SNOT-20 mean score was 2.18, versus post-op score of 1.02, an improvement of 1.16 that was both statistically (P < 0.001) and clinically significant. Ethmoid-specific and side-specific Lund–MacKay mean scores both also showed statistically significant improvements. Pre-op ethmoid-specific Lund–MacKay mean score was 1.93, versus post-op score of 1.10, an improvement of 0.83 (P < 0.001). Pre-op side-specific Lund–MacKay mean score was 5.75, compared with post-op score of 2.95, an improvement of 2.80 (P < 0.001). There were no significant intra-op or post-op complications encountered. This triamcinolone-infused ethmoid stent appears safe and effective in treating chronic ethmoid sinus disease within the defined follow-up period. The ability to deliver medication directly to diseased mucosa holds wide-ranging potential.
Keywords: Drug, Elution, Sinus, Stent, Ethmoid
Introduction
Surgery for chronic rhinosinusitis (CRS) is often recommended if patients do not respond adequately to medical therapy. With the introduction of the endoscope and an improved understanding of sinus physiology, endoscopic sinus surgery (ESS) has supplanted external approaches as the preferred operative technique. Over the past 8 years, there has been a trend back toward surgical conservatism, led initially with the introduction of the MIST procedure [1]. In September 2005, Balloon SinuplastyTM (Acclarent, Inc.) was introduced as a minimally invasive option to widen sinus ostia and their transition spaces while preserving the corresponding mucosa. The outcome, efficacy, and safety of the balloon dilation technology as applied to the frontal, maxillary and sphenoid sinuses has been well vetted and reported [2–9]. However, due to the complex drainage patterns and interconnected nature of the ethmoid complex, the balloon technology was not initially suited to treat chronic ethmoid disease [10].
The standard surgical treatment for chronic inflammatory disease of the ethmoid sinus is endoscopic ethmoidectomy performed with a microdebrider, through-cutting forceps, or a combination of the two [11]. However, the widespread use of these tools has been known to cause middle meatal scarring, cerebrospinal fluid leak, orbital emphysema and potential visual change or loss [12]. Furthermore, refractory and residual edematous/polypoidal tissue in the ethmoid region must then be managed with nasal rinses and systemic and/or topical steroids. Systemic steroids, while useful for anti-inflammatory effect, can result in gastritis, hyperglycemia, osteoporosis, immunosuppression, mood changes, headache and lethargy. Topical steroids have been shown to be ineffective in reaching or remaining in the ethmoid roof long enough to work satisfactorily.
A newly developed surgical tool, the Relieva StratusTMMicroFlow Spacer (Acclarent, Inc.), has recently been introduced into clinical practice as an option for minimally invasive treatment of chronic ethmoid mucosal disease. This device is easily and temporarily implanted into the ethmoid complex to enable local targeted delivery of a therapeutic agent for a sustained period of time. The device is then simply removed in the office setting.
Though there is potential to instill the device with any liquid agent, it is currently FDA-approved for use only with saline. The use of steroids (i.e. Kenalog-40 or triamcinolone acetonide 40 mg/ml) is presently considered “off-label” and at the discretion of the operating surgeon (CE Mark has been obtained for use with triamcinolone acetonide 40 mg/ml). In this article, we present an initial report on the Relieva StratusTMMicroFlow Spacer including device components, current indications and operative technique for its use in the treatment of chronic ethmoid inflammatory disease.
Device
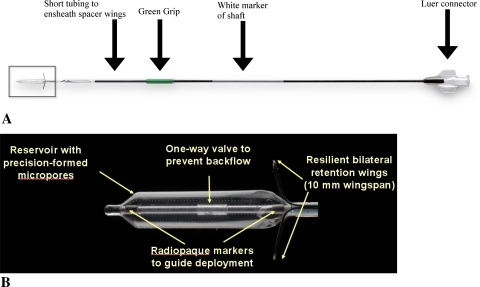
The system consists of two main components: (i) the MicroFlow Spacer and (ii) the Deployment Guide. The MicroFlow Spacer is a membrane reservoir that surrounds a catheter shaft. The reservoir has several hundred precision-formed micropores that permit slow seepage of an instilled therapeutic agent into the targeted region. The catheter shaft has a one-way valve to prevent backflow and a set of flexible retention wings to secure the device within the ethmoid bulla (Fig. 1a, b). The Deployment Guide consists of an access probe and a delivery sheath that work in concert to prepare the ethmoid complex for Spacer implantation (Fig. 2).
Fig. 1.
MicroFlow Spacer. a Reservoir plus shaft and b reservoir enlarged
Fig. 2.
Deployment Guide with access probe (top) and delivery sheath (bottom)
Indications
Indications include chronic mucosal inflammation involving the anterior ethmoid and/or posterior ethmoid sinuses in a previously unoperated patient. Contraindications include patients with absent or very small ethmoid bulla, dehiscent fovea ethmoidalis, prolapsing orbital tissues due to defects in the lamina, pathology in the ethmoid complex that should be surgically removed (i.e. extensive polyposis), allergy to the instilled drug agent or components of the device, or hyperostotic bony ethmoid partitions.
Preoperative Assessment
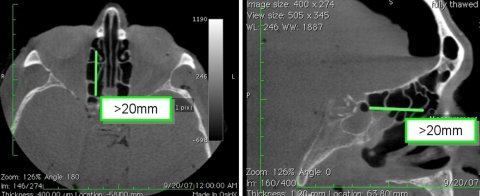
Patients must have a distance of greater than 20 mm between the anterior face of the ethmoid bulla and the anterior face of the sphenoid sinus, as the device is currently available in only one length. Measurement of this distance is best made on the sagittal or axial view of the sinus CT (Fig. 3).
Fig. 3.
Required distance between anterior wall of ethmoid bulla and anterior face of sphenoid
Technique
Patient preparation, anesthesia, and room setup are performed according to standard endoscopic sinus surgery protocol. C-arm fluoroscopy is recommended to help guide Spacer placement initially, although its use may be phased out depending on the surgeon’s experience.
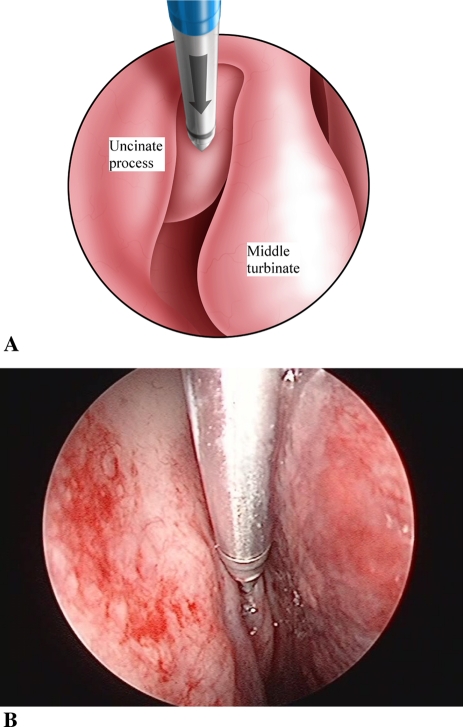
The MicroFlow Spacer is first checked and prepared by injecting 1 cc of air (before using saline or therapeutic agent) through the Spacer to prepare the one-way valve. The delivery sheath is loaded onto the access probe to prepare for insertion. It is recommended that the Spacer be placed after all other components of ESS are completed. The anterior face of the ethmoid bulla is exposed by either uncinectomy, gentle medialization of the middle turbinate with a Freer’s elevator, or resection of the lateral lamella of an obstructing concha bullosa.
Under direct endoscopic view, the access probe is positioned on the inferior and medial aspect of the anterior face of the ethmoid bulla (Fig. 4). This location is chosen to avoid the possibility of piercing the skull base and lamina papyracea during access probe insertion. To help establish the correct angle and trajectory for insertion of the access probe, a “shark-fin handle” has been developed. Proper insertion requires keeping the top of the “shark fin” handle parallel to the floor of the nasal cavity (Fig. 5) and, if using fluoroscopy (lateral ± AP view), following the two radio-opaque markers on the delivery system tip in relation to the visualized skull base, lamina papyracea, and anterior face of sphenoid. Proper insertion depth is achieved when the blue marker on the delivery sheath is endoscopically visualized to abut the anterior wall of the ethmoid bulla (Fig. 6). The access probe is then withdrawn while stabilizing the delivery sheath with the other hand. This does not require direct vision as the endoscope is removed for this two-handed maneuver.
Fig. 4.
Access probe in place on ethmoid bulla (a schematic, b endoscopic view)
Fig. 5.
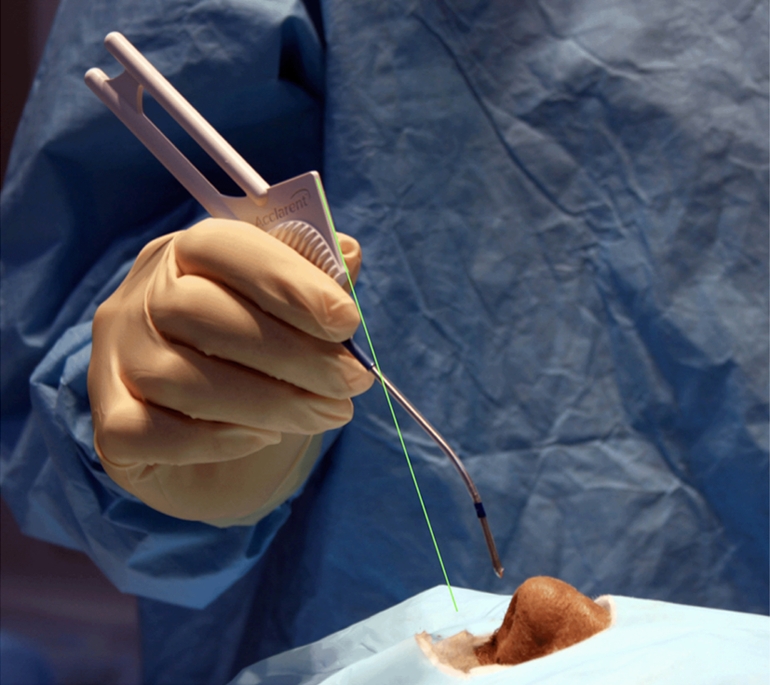
Initial access probe position—line of trajectory, as guided by shark fin, follows floor of nose
Fig. 6.
Final access probe position. a Endoscopic view and b fluoroscopic view
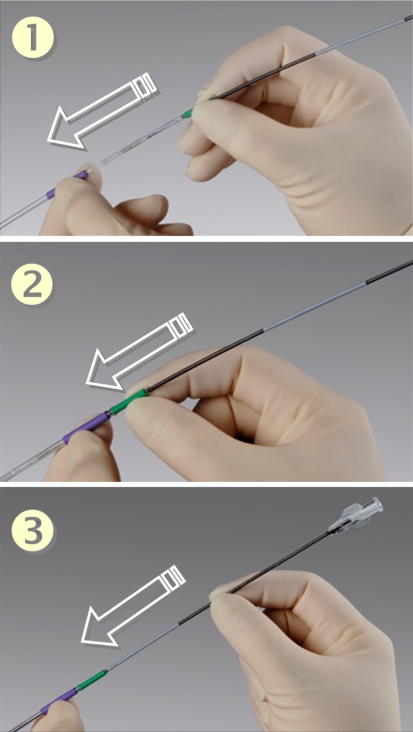
The second component of this system, the MicroFlow Spacer, is now inserted through the delivery sheath. The MicroFlow Spacer is advanced until the green grip of the Spacer meets the purple grip of the delivery sheath, then the catheter shaft is advanced until the white marker of the shaft meets the green grip (Fig. 7). This will ensure that the wings of the Spacer are within the front wall of the ethmoid bulla.
Fig. 7.
MicroFlow Spacer insertion through delivery sheath
Only then is the delivery sheath withdrawn completely by sliding it backwards over the Spacer catheter. This action will simultaneously deploy the retention wings behind the anterior wall of the ethmoid bulla thereby securing the Spacer. The endoscope should then be used to re-examine the position of the Spacer to ensure the retention wings are not visible outside the ethmoid bulla. Any concerns about the final position of the Spacer can be eliminated with a quick fluoroscopic check (Fig. 8). When the delivery sheath is withdrawn, a suture loop will also be unveiled from the anterior aspect of the Spacer. This loop can be used to suture the Spacer to the middle turbinate (serving as additional security against device migration, but is rarely needed), or used to retrieve the device after use.
Fig. 8.
Final MicroFlow Spacer position (lateral fluoroscopic view)
The Spacer is now ready to receive the therapeutic agent. If Kenalog®-40 (triamcinolone acetonide injectable solution, USP, 40 mg/ml, Bristol-Myers Squibb Company) is used, it must first be shaken to fully suspend the particles. 0.3 ml is then drawn into a 1.0 ml syringe and injected into the Spacer reservoir through the standard connector at the proximal end of the Spacer catheter.
Using straight or angled endoscopic scissors, the Spacer catheter shaft is transected (Fig. 9) just anterior to the head of the middle turbinate to ensure that the cut end will not irritate the turbinate. In addition, this protruding end helps to prevent postoperative lateralization of the middle turbinate and to facilitate device retrieval in the office setting. We also suggest the surgeon either eliminate or only use resorbable middle meatal packing to prevent accidental displacement of the Spacer.
Fig. 9.
MicroFlow Spacer catheter after cutting the shaft (endoscopic view)
The Spacer may be left in place for 14-28 days (FDA clearance is for 14 day implantation, while CE mark is for 28 day implantation). Our initial studies suggest that patients tolerate the device well with minimal awareness of its presence. At the desired time, the device is removed in the office. A topical decongestant and anesthetic are sprayed into the nose and the cut end of the catheter and/or suture loop is grabbed with a bayonet or Blakeskey forceps and gently pulled anteriorly. The retention wings collapse inward, allowing the entire device to be extracted from the ethmoid complex. The resultant hole in the bulla is generally quite small (<3 mm) and appears to re-mucosalize within a few weeks.
Discussion
Treatment of chronic inflammatory disease of the ethmoid sinus is challenging with its multiple bony partitions, proximity to critical structures like the skull base and lamina papyracea, and relatively high incidence of residual and/or refractory mucosal disease.
The MicroFlow Spacer System is a promising minimally invasive option for the targeted local delivery of pharmaceutical agents for prolonged periods of time, thus avoiding systemic medication side effects. In the event symptoms recur or disease persists, the option of a second Spacer or conventional endoscopic ethmoidectomy is not precluded.
It must also be noted that this system does not attempt to remove mucosa or bone and is therefore not appropriate for certain conditions (i.e. extensive panpolyposis). Investigational studies are currently underway to assess efficacy and long-term outcomes with the MicroFlow Spacer device when combined with triamcinolone acetonide (TA) 40 mg/ml. Preliminary clinical data of patients treated with unilateral or bilateral Spacers filled with TA has identified trace levels of TA in their plasma within 1 h after implantation and for as long as the Spacers are retained in situ.
Summary
Chronic ethmoid sinusitis is currently surgically treated with endoscopic ethmoidectomy. There is a subset of CRS patients without nasal polyps who may be amenable to less invasive surgery. This article introduces the Relieva StratusTMMicroFlow Spacer to serve as an alternate means of treating chronic ethmoid sinusitis via targeted and sustained delivery of a therapeutic agent to the ethmoid sinus mucosa. Appropriate patient selection, familiarity with device components, and proper insertion technique will ensure excellent outcomes through minimizing surgical and systemic drug morbidity.
References
- 1.Setliff RC. Minimally invasive sinus surgery: rationale and technique. Otolaryngol Clin North Am. 1996;29:115–129. [PubMed] [Google Scholar]
- 2.Brown CL, Bolger WE. Safety and feasibility of balloon catheter dilation of paranasal sinus ostia: a preliminary investigation. Ann Otol Rhinol Laryngol. 2006;115:293–299. doi: 10.1177/000348940611500407. [DOI] [PubMed] [Google Scholar]
- 3.Bolger WE, Brown CL, Church CA, Goldberg AN, Karanfilov B, Kuhn FA, et al. Safety and outcomes of balloon catheter sinusotomy: a multicenter 24-week analysis in 115 patients. Otolaryngol Head Neck Surg. 2007;137(1):10–20. doi: 10.1016/j.otohns.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Friedman M, Schalch P, Lin HC, Mazloom N, Neidich M, Joseph NJ. Functional endoscopic dilatation of the sinuses: patient satisfaction, postoperative pain, and cost. Am J Rhinol. 2008;22(2):204–209. doi: 10.2500/ajr.2008.22.3155. [DOI] [PubMed] [Google Scholar]
- 5.Kuhn FA, Church CA, Goldberg AN, Levine HL, Sillers MJ, Vaughan WC, et al. Balloon catheter sinusotomy: one-year follow-up—outcomes and role in functional endoscopic sinus surgery. Otolaryngol Head Neck Surg. 2008;139:S27–S37. doi: 10.1016/j.otohns.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Levine HL, Sertich AP, Hoisington DR, Weiss RL, Pritikin J. PatiENT registry study group: multicenter registry of balloon catheter sinusotomy outcomes for 1,036 patients. Ann Otol Rhinol Laryngol. 2008;117(4):263–270. doi: 10.1177/000348940811700405. [DOI] [PubMed] [Google Scholar]
- 7.Melroy CT. The balloon dilating catheter as an instrument in sinus surgery. Otolaryngol Head Neck Surg. 2008;139:S23–S26. doi: 10.1016/j.otohns.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Vaughan CW. Review of balloon sinuplasty. Curr Opin Otolaryngol Head Neck Surg. 2008;16:2–9. doi: 10.1097/MOO.0b013e3282f5e955. [DOI] [PubMed] [Google Scholar]
- 9.Weiss RL, Church CA, Kuhn FA, Levine HL, Sillers MJ, Vaughan WC. Long-term outcome analysis of balloon catheter sinusotomy: two-year follow-up. Otolaryngol Head Neck Surg. 2008;139:S38–S46. doi: 10.1016/j.otohns.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Friedman M, Schalch P. Functional endoscopic dilatation of the sinuses (FEDS): patient selection and surgical technique. Operat Tech Otolaryngol Head Neck Surg. 2006;17(2):126–134. doi: 10.1016/j.otot.2006.03.005. [DOI] [Google Scholar]
- 11.Siow JK, Kadash BA, Jochen AW. Balloon sinuplasty: a current hot topic in rhinology. Eur Arch Otorhinolaryngol. 2008;256:509–511. doi: 10.1007/s00405-008-0605-0. [DOI] [PubMed] [Google Scholar]
- 12.McMains KC. Safety in endoscopic sinus surgery. Curr Opin Otolaryngol Head Neck Surg. 2008;16(3):247–251. doi: 10.1097/MOO.0b013e3282fdccad. [DOI] [PubMed] [Google Scholar]