Abstract
Objective
To test the hypothesis that in vivo transgene expression mediated by single intra-articular injection of adeno-associated virus serotype 2 (AAV2) persists within intra-articular tissues 1-year post-injection and can be externally controlled using an AAV2-based tetracycline-inducible gene regulation system containing the tetracycline response element (TRE) promoter.
Methods
Sprague-Dawley rats received intra-articular injections of AAV2-CMV-GFP and AAV2-CMV-Luc into their right and left knees, respectively. Luciferase expression was evaluated over 1-year using bioluminescence imaging. After sacrifice, tissues were analyzed for GFP+ cells by fluorescent microscopy. To study external control of intra-articular AAV-transgene expression, another set of rats were co-injected with AAV2-TRE-Luc and AAV2-CMV-reverse-tetracycline-controlled transactivator (rtTA) into the right knees, and AAV2-CMV-Luc and AAV2-CMV-rtTA into the left knees. Rats received oral doxycycline (Dox), an analogue of tetracycline, for 7 days. Luciferase expression was assessed by bioluminescence imaging.
Results
Luciferase expression was localized to the injected joint and persisted throughout the 1-year study period. Abundant GFP+ cells were observed within intra-articular soft tissues. Transgene expression in AAV2-TRE-Luc injected joints was upregulated by oral administration of Dox, and downregulated following its removal, at 14-days and 13-months post-AAV injection.
Conclusions
This longitudinal in vivo study shows that sustained and stable AAV-mediated intra-articular transgene expression can be achieved through a single intra-articular injection and can be controlled using a tetracycline-controlled inducible AAV system in a normal rat knee model. Highly regulatable long-term intra-articular transgene expression is of potential clinical utility for development of treatment strategies for chronic intra-articular disease processes such as inflammatory and degenerative arthritis.
Keywords: adeno-associated virus, gene therapy, cartilage, injection, doxycycline
Introduction
Prevention of articular cartilage degradation or treatment of its damage in arthritis remains challenging due to the limited self-repair potential of articular cartilage. Currently, no therapeutic methods exist for complete re-establishment of cartilage function. Delivery of therapeutic agents that could promote articular cartilage repair or prevent its further degradation once damaged is an attractive therapeutic option. Protein biologics can be delivered systemically, or locally by direct injection or through polymer based delivery systems 1. However, due to the short half-life of proteins, administration of supra-physiological doses and/or repeated delivery are often necessary, significantly increasing the cost of these approaches. An attractive alternative is to deliver the genetic information to cells within the joint and engineer them to produce the therapeutic protein in situ.
Naked DNA, retrovirus, adenovirus, and herpes virus-based vectors have been explored for gene transduction in vivo; however, most were rendered suboptimal due to safety, efficacy, and duration issues. Recombinant adeno-associated virus (AAV) derived from an endemic and non-pathogenic parvovirus is emerging as a promising delivery vehicle for musculoskeletal tissues, with the advantages of sustained transgene expression, reduced potential for host immune response, and the capacity to transduce both dividing and non-dividing cells in vitro and in vivo 2. Different serotypes of AAV exist, each having different preferential targets 2, 3. AAV5 has better transduction efficiency in rodent arthritic joints; however, AAV2 is currently used in human clinical trials for arthritis 4–7.
Many studies have genetically engineered cells ex vivo for implantation in vivo. Although this has led to cartilage repair in animal models 8–12, culturing cells ex vivo prolongs the time before treatment and is also very expensive in a clinical setting. An alternative method would be to deliver the genetic material intra-articularly by direct injection and rely on the cells within the joint to produce the therapeutic factor. AAV vectors have a strong tropism for synoviocytes in vitro 13–16. However, few studies have identified the exact in vivo localization of the transgene, after intra-articular injection of AAV 17–22. This is important to identify which cells or tissues are infected by AAV after intra-articular injection and to use this information to develop optimal treatment strategies for intra-articular disease processes such as inflammatory and degenerative arthritis.
Knowledge of the duration of transgene expression is also important to develop efficient therapies. It has been reported that parkinsonian nonhuman primates having received AAV2 encoding human L-amino acid decarboxylase (L-DOPA) into their brains were still expressing the transgene after 8 years 23. AAV-mediated erythropoietin delivery to rhesus monkey skeletal muscle has led to transgene expression for more than 6 years 24. Long-term transgene expression may be critical for disease treatment, but may not be ideal for cartilage repair strategies, where a growth factor may only be needed for a limited time period. In cases where a growth factor is needed for a specific period of time or concentration, it may be advantageous to use an inducible gene expression system, where the transgene expression can be controlled pharmacologically by oral administration of the inducing agent. The tetracycline-inducible gene regulation system has been efficient both in vitro and in vivo 24–26. Doxycycline (Dox), an analog of tetracycline, is widely accepted as an inducer due to its safe use in humans 27. Only few studies have investigated the use of an AAV-based tetracycline-inducible gene system in the knee joint after direct injection.
Given the potential translational value of AAV for human clinical use, the aim of the present study was to investigate the presence, persistence, and ability to externally control transgene expression when using AAV2 delivered by a single intra-articular injection into the rat diarthrodial joint. The immunocompetent rat was chosen since it is a good animal model for future studies that will test the repair of articular cartilage, the prevention of its degradation, or the treatment of inflammatory arthritis by AAV-mediated gene therapy.
Materials and Methods
AAV vector production
Double-stranded serotype 2 AAV (AAV2) vectors were produced using the three-plasmid cotransfection method 28. Depending on the AAV2 vector being produced, the first plasmid used was either the AAV-CMV-Luc plasmid with the luciferase (Luc) gene driven by the CMV promoter, the AAV-CMV-enhanced green fluorescent protein (GFP) plasmid, the AAV-CMV- reverse tetracycline-controlled transactivator (rtTA) plasmid, or the AAV-TRE-Luc plasmid with the Luc gene driven by the tetracycline response element (TRE) promoter. The second plasmid used in the cotransfection was the pXX6 plasmid, which contains the helper genes from adenovirus, and the third plasmid was the pXX2, which supplies AAV2 rep protein and capsid protein 28. Vector purification was performed as previously described and AAV genomic titers were determined by DNA dot-blot assay 28.
In vitro infection of rat articular chondrocytes
Primary rat articular chondrocytes were extracted from the tibial and femoral cartilage of 3-month-old male Sprague Dawley rats. Cartilage samples were washed in phosphate buffered saline (PBS; Invitrogen) with 2% penicillin-streptomycin (pen/strep; Invitrogen). To digest the cartilage, thin shavings were incubated in 0.2% pronase (Calbiochem) solution in F-12 medium (Invitrogen) supplemented with 5% fetal bovine serum (FBS) and 1% pen/strep for 90 minutes in a dry 37°C incubator. This was followed by overnight digestion in 0.025% collagenase P (Roche Applied Science) in F-12 medium supplemented with 5% FBS and 1% pen/strep. Recovered chondrocytes were seeded as monolayers in a T-75 flask in 50% Dulbecco’s modified Eagle medium/50% F-12 medium (DMEM/F-12; Invitrogen) supplemented with 10% FBS, and 1% pen/strep under normal growth conditions (37°C, 5% CO2). Cells were grown to passage four and plated onto 96-well plates at a density of 2.0 × 104 cells/cm2 in growth medium (DMEM/F-12, 10% FBS, 1% pen/strep). On the following day, chondrocytes in plain DMEM/F-12 were infected with 3.2 × 105 vector genomes per cell (vg/cell) of AAV-CMV-GFP, AAV-CMV-Luc, or AAV-CMV-rtTA + AAV-TRE-Luc for one hour, after which the medium was changed to growth medium. This dose was selected because it gave the greatest gene expression in an in vitro dose response study ranging from 4 × 104 – 3.2 × 105 vg/cell (data not shown). After 2 days, cells infected with AAV-TRE-Luc were stimulated with Dox at concentrations of 0, 1, or 2 μg/ml for 24 hours. Luciferase transgene expression was measured 3 days post-transduction by adding 50 μl of luciferin (30 mg/ml; D-Luciferin Firefly, Caliper Life Sciences) per well and imaging after 5 minutes with the IVIS® 200 optical imaging system (Xenogen Corp., Hopkinton, MA, USA). GFP signal was visualized on the same day using the epi-fluorescent Eclipse TE-2000U inverted microscope (Nikon).
Animal experiments
All animal experiments were performed following a University of Pittsburgh Institutional Animal Care and Use Committee approved protocol. The study was divided into two parts.
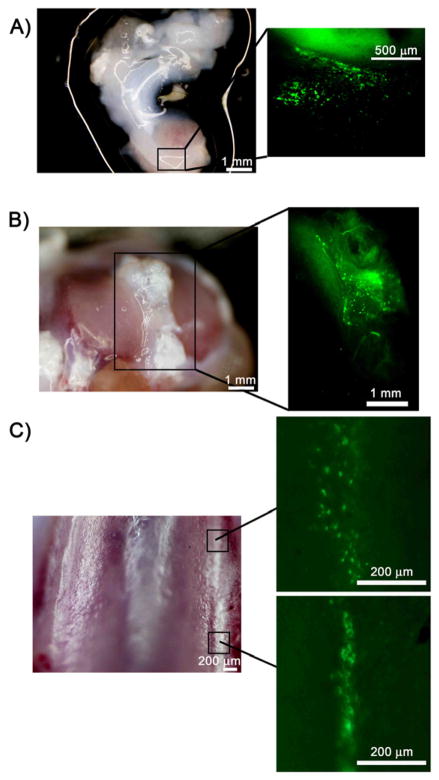
In the first study, 9 male Sprague Dawley rats (3 months old) received a 50 μl intra-articular injection containing 2.5 × 1010 vg of AAV-CMV-GFP or AAV-CMV-Luc into the right and left knee joint, respectively. For longitudinal evaluation of luciferase expression, rats were anesthetized with isoflurane inhalation, followed by a 50 μl intra-articular injection of luciferin (30 mg/ml). After 5 minutes, to allow proper distribution of luciferin, rats were placed in an IVIS® 200 optical imaging system. Photon emissions in the region of interest were quantified using Living Image software V.3.0 (Xenogen Corp.), and bioluminescent flux was reported as photons/sec. Rats were imaged every other week for four months, and monthly thereafter, until one-year post-AAV injection. Rats were sacrificed at either 1 week (N=1), 2 weeks (N=2), 3 months (N=1), 4 months (N=1) or 1 year (N=4) after AAV injection. On the day of sacrifice, rats received an intraperitoneal injection of 100 μl of luciferin to determine whether any signal was present outside of the intra-articular space. They also received an intra-articular injection of luciferin and imaged as previously described. The joint was then opened to image the intra-articular tissues. Tissues within the joint were also analyzed for GFP+ cells using a fluorescent stereomicroscope (MVX-10 MacroView Systems, Olympus, Japan) equipped with a DP71 camera (Olympus). All GFP images in Figure 4 had the contrast increased by the same value in Adobe Photoshop to improve the GFP signal for print.
Figure 4.
GFP+ cells in tissues harvested from the right knee joint of rats injected with AAV-CMV-GFP and imaged with a stereomicroscope. (A) Brightfield image (left panel) of meniscal tissue with the area located within the square enlarged to show the GFP+ cells (right panel). (B) Brightfield image (left panel) of tibial plateau. Area within the square is magnified in the right panel and shows GFP+ cells within the soft tissue. (C) Brightfield image of the trochlea (left panel). GFP+ cells were sparse in articular cartilage. Two small areas with GFP+ cells were found on the trochlear ridge in this animal (right panels).
In the second animal experiment, the right and left knees of 4 male Sprague Dawley rats (3 months old) were divided into experimental and control groups. In the right knee, each rat received a 50 μl intra-articular injection containing 2.5×1010 vg of AAV-TRE-Luc and 2.5×1010 vg of AAV-CMV-rtTA. In the left knee, each rat received a 50 μl intra-articular injection containing 2.5×1010 vg of AAV-CMV-Luc and 2.5×1010 vg of AAV-CMV-rtTA, to have the same number of vg in both knees. To induce expression of luciferase in the right knee joint (injected with AAV-TRE-Luc), rats were administered drinking water containing Dox at a concentration of 2 mg/ml for 7 days, followed by its removal. To ensure fresh supply and prolong the half-life of Dox, the water was replaced every day and protected from light with amber drinking bottles. Rats were imaged twice a week as described above. Addition of Dox to the drinking water was performed 14 days, 8 months and 13 months post-AAV injection.
Statistical analysis
In vitro studies were repeated three times with 3–9 replicates. A representative experiment is shown in Figure 1 (n=9). In vivo studies were conducted and data observed using independent animals, with N=4–8 for Figure 2 due to animals being sacrificed at different time points during the study and N=4 for Figure 5. All data are represented as median and interquartile range boxplots with percentiles calculated using Tukey Hinge. The significant level used was p < .05, unless otherwise noted for post-hoc comparisons. Assumptions of parametric data were tested for all data: normality of data distribution using Shapiro-Wilk test and homogeneity of variance using Levene’s test. For parametric data (Figure 5), one-way ANOVA with post-hoc Tukey was used. For non-parametric independent data (Figure 1), a Kruskal-Wallis test and post-hoc pairwise Mann-Whitney U with a Bonferroni correction was used. p-values less than .008 (.05/6 total comparisons) were considered significant. For non-parametric data in the longitudinal study (Figure 2), Friedman’s ANOVA test was used with the Wilcoxon test and a Bonferroni correction applied for post-hoc analysis of each consecutive time point. p-values less than .005 (.05/10 comparisons) were considered significant. Statistical evaluations were performed with SPSS software.
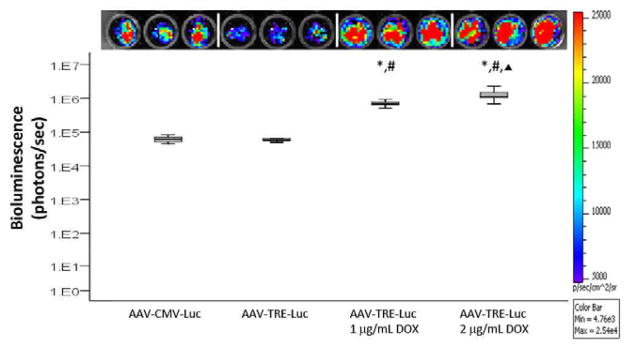
Figure 1.
In vitro response of rat articular chondrocytes to AAV2. Rat articular chondrocytes were infected with AAV-CMV-Luc or AAV-TRE-Luc, stimulated with Dox (0, 1 or 2 μg/ml) for 24 hours, and luciferase signal was detected. Upper panel: Image of bioluminescence signal from the cells infected with (left to right) AAV-CMV-Luc, AAV-TRE-Luc (no Dox), AAV-TRE-Luc stimulated with Dox (1 μg/ml) and AAV-TRE-Luc stimulated with Dox (2 μg/ml). Lower panel: Median and interquartile range of bioluminescence for the different cell groups. n=9. *p<.001 for AAV-TRE-Luc: 1 μg/ml Dox vs. AAV-TRE-Luc and for AAV-TRE-Luc: 2 μg/ml Dox vs. AAV-TRE-Luc. #p<.001 for AAV-TRE-Luc: 1 μg/ml Dox vs. AAV-CMV-Luc and for AAV-TRE-Luc: 2 μg/ml Dox vs. AAV-CMV-Luc. ▲ p<.001 for AAV-TRE-Luc: 2 μg/ml Dox vs. AAV-TRE-Luc: 1 μg/ml Dox.
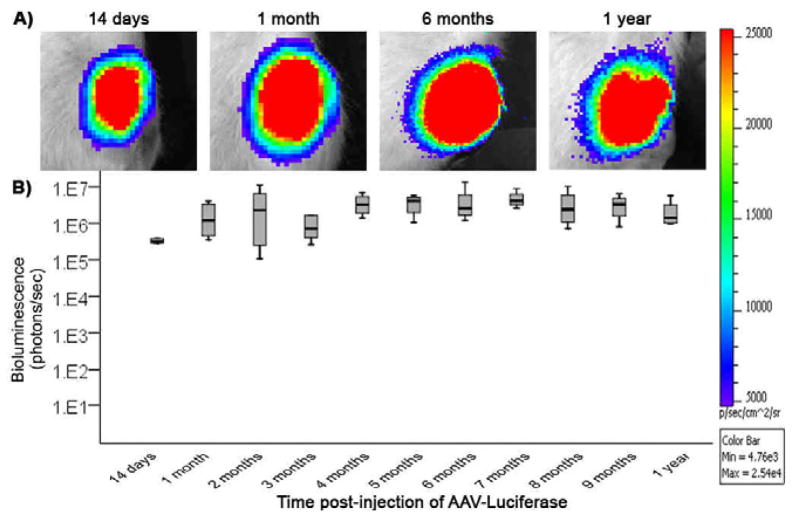
Figure 2.
Duration of in vivo transgene expression after a single intra-articular injection of AAV2-CMV-Luc was observed and quantified using bioluminescence imaging to detect luciferase activity. (A) Bioluminescent images of the left knee joint of a representative rat at 14 days, 1 month, 6 months and 1 year after a single intra-articular injection of AAV-CMV-Luc. (B) Median and interquartile range of bioluminescence observed in the left knee joint of all rats in the study over 1-year following intra-articular injection of AAV-CMV-Luc. N=4–8 rats (N=8 at 14 days, N=6 at 1 and 2 months, N=5 at 3 months, and N=4 from 4 months to 1 year). No significant difference was observed between the different time points.
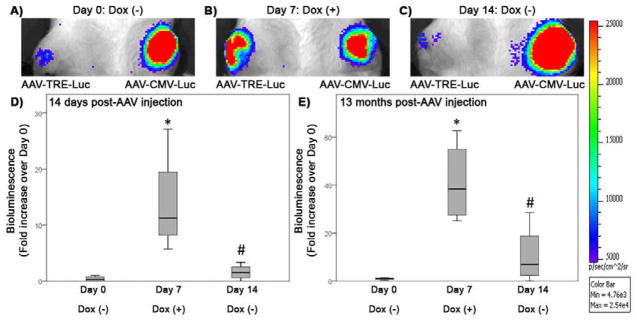
Figure 5.
Controlled in vivo transgene expression following a single intra-articular injection of AAV2. (A,B,C) Bioluminescent images of a representative rat that received AAV-TRE-Luc in the right knee and AAV-CMV-Luc in the left knee 14 days prior. Images shown were taken (A) before addition of Dox to the drinking water (Day 0), (B) 7 days after Dox was added to the drinking water (Day 7) and (C) 7 days after Dox was removed from the drinking water (Day 14). Luciferase expression was stable in the knee joint injected with AAV-CMV-Luc, but varied with Dox administration in the knee joints injected with AAV-TRE-Luc. (D) Bioluminescence measured in the knee joint 14 days post-AAV-TRE-Luc injection. Bioluminescence is represented as a median fold increase over Day 0, when no Dox was present in the drinking water, and interquartile range. Luciferase expression increased with addition of Dox to the drinking water after 7 days (Day 7: Dox (+)). *p=.015 compared to Day 0: Dox (−). After removal of Dox from the drinking water for 7 days (Day 14: Dox (−)), the luciferase signal decreased. #p=.023 compared to Day 7: Dox (+). (E) Median and interquartile range of bioluminescence measured 13 months post-injection with AAV-TRE-Luc. Luciferase expression increased 7 days after Dox was present in the drinking water (Day 7: Dox (+)). *p=.003 compared to Day 0: Dox (−)). Luciferase expression decreased 7 days after removal of Dox (Day 14: Dox (−)). #p=.016 compared to Day 7: Dox (+). N=4 rats.
Results
In vitro response of rat articular chondrocytes to AAV2 vectors
GFP expression was visualized in articular chondrocytes infected with AAV-CMV-GFP as early as 24 hours post-infection (data not shown). Articular chondrocytes infected with AAV-CMV-Luc had luciferase expression (Figure 1). Infection of articular chondrocytes with AAV-TRE-Luc led to weak bioluminescence when no Dox was added (Figure 1, AAV-TRE-Luc). This signal was significantly increased when the cells were cultured with Dox at concentrations of 1 μg/ml and 2 μg/ml for 24 hours (Figure 1, *p<.001 for AAV-TRE-Luc: 1 μg/ml Dox and AAV-TRE-Luc: 2 μg/ml Dox compared to AAV-TRE-Luc). The bioluminescence observed in vitro with the AAV-TRE-Luc vector and addition of Dox was also greater than that obtained with the AAV-CMV-Luc vector (Figure 1, #p<.001 for AAV-TRE-Luc: 1 μg/ml Dox and AAV-TRE-Luc: 2 μg/ml Dox compared to AAV-CMV-Luc). A significant difference was also observed between the two concentrations of Dox tested (Figure 1, ▲ p<.001 for AAV-TRE-Luc: 2 μg/ml Dox compared to AAV-TRE-Luc: 1 μg/ml Dox).
Duration of in vivo transgene expression after a single intra-articular injection of AAV2
The IVIS® 200 optical imaging system enabled in vivo tracking of luciferase expression in the same rat knee joints over a 1-year period. Bioluminescence was visualized as early as 14 days after intra-articular injection of AAV-CMV-Luc and persisted in all animals for 1 year, the study end point. Figure 2A shows bioluminescent images from a representative animal at 14 days, 1 month, 6 months, and 1 year after intra-articular injection of AAV-CMV-Luc. In all study animals, the luciferase signal remained constant throughout the duration of the study (Figure 2B). The contralateral AAV-CMV-GFP injected knees did not show luciferase signal during the study. Intraperitoneal injection of luciferin and imaging before sacrifice also indicated that no luciferase signal was present outside the injected knee area.
Localization of in vivo transgene expression after a single intra-articular injection of AAV2
To localize luciferase signal, knees were imaged intact (Figure 3A) and after exposing the intra-articular space (Figure 3B). Bioluminescence was concentrated in two areas (Figure 3B). The majority of the signal was found in the exposed infrapatellar fat pad area, with some signal in the intra-articular space containing the ligaments and meniscus. No bioluminescence was observed in articular cartilage.
Figure 3.
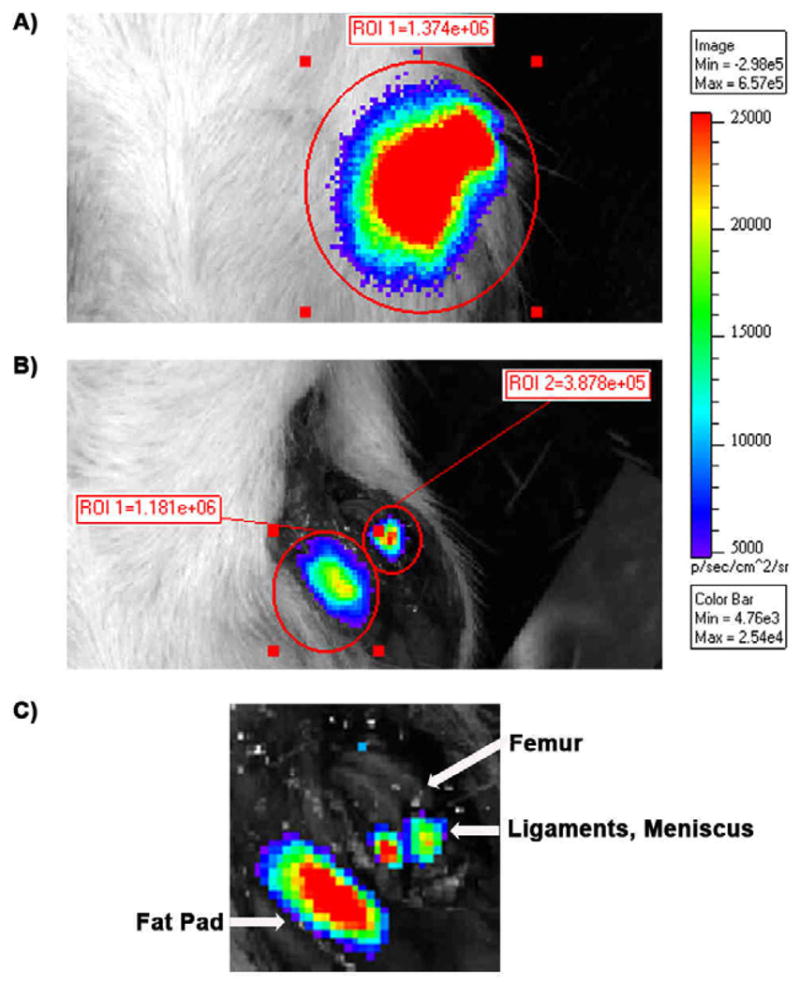
Localization of in vivo luciferase expression after a single intra-articular injection of AAV-CMV-Luc by using the IVIS optical imaging system. (A) Luciferase was detected in the intact left knee joint. (B) Exposure of the intra-articular space of the same knee joint in (A) revealed that the luciferase signal was located in 2 major areas. (C) A higher magnification of (B) indicated that the signal was mainly found in the infrapatellar fat pad and in the soft tissues between the femur and tibia, such as the ligaments and meniscus.
Tissues retrieved from the AAV-CMV-GFP-injected side provided more information on the localization of cells expressing the transgene. Abundant GFP+ cells were found in soft tissues such as synovium, infrapatellar fat pad and tissues within the intra-articular joint space. Figure 4A shows a large area of GFP+ cells within meniscus, while Figure 4B shows GFP+ cells in soft tissues surrounding the tibial plateau. GFP+ cells could also be seen in articular cartilage, although fewer cells could be found when compared to soft tissues. As seen in Figure 4C, two small areas containing GFP+ cells were found on the trochlear ridge of the femur. Large areas of GFP+ cells in articular cartilage were not found in any of the study animals.
Controlled in vivo transgene expression following a single intra-articular injection of AAV2
Persistent luciferase expression was observed for the duration of the experiment, in all knee joints injected with AAV-CMV-Luc (Figure 5A, B, and C). Intra-articular injection of AAV-TRE-Luc led to a weak luciferase signal before addition of Dox to the drinking water of the rats (Figure 5A). Fourteen days post-AAV injection, Dox was added to the drinking water and led to a significant increase in the luciferase signal after 7 days (Figure 5B and D, median fold increase of 11.28 and interquartile range of 16.34 (25th-percentile = 8.20, 75th-percentile = 19.49) and *p=.015 compared to Day 0: Dox(−)). Removal of Dox significantly decreased the signal 7 days later (Figure 5C and D, # p=.023 compared to Day 7: Dox (+)). When measured 8 months post-AAV injection, addition of Dox to the drinking water of the rats for 7 days increased luciferase expression by a median fold increase of 4.31 and interquartile range of 5.45 (25th-percentile = 2.16, 75th-percentile = 6.04) (p=.099 compared to Day 0: Dox(−)). The luciferase signal returned to pre-Dox values after the inducer had been removed for 7 days. The induction cycle performed 13 months after the initial injection of AAV also showed increased luciferase signal on the AAV-TRE-Luc injected side 7 days after addition of Dox to the drinking water (Figure 5E, median fold increase of 38.43 and interquartile range of 32.46 (25th-percentile = 27.48, 75th-percentile = 54.90) and *p=.003 compared to Day 0: Dox (−)). Removal of Dox decreased the signal to baseline levels (Figure 5E, #p=.016 compared to Day 7: Dox (+)).
Discussion
Prevention of articular cartilage degradation or promotion of its repair in inflammatory and degenerative arthritis remains challenging. Therapeutic strategies that rely on the delivery of growth factors to enhance repair or inhibitory molecules that would prevent degradation are of interest. Ideally, the therapeutic protein should be produced in situ, to obtain a persistent and stable concentration, thus limiting the need for purification processes, repeated injections, and supraphysiological doses, which are not cost effective. Gene therapy is a promising alternative to the delivery of therapeutic factors, as it provides the genetic material necessary for protein synthesis in situ. In this study, we analyzed important aspects for efficient intra-articular gene delivery. These included the persistence and localization of transgene expression after a single intra-articular injection of AAV2, as well as the ability to control the transgene expression over time when using an inducible AAV2 vector.
Efficacy of AAV2 for in vitro chondrocyte transduction was evaluated using GFP and luciferase as reporter genes. In accordance with other studies on mice, rabbit, equine and human chondrocytes 21, 29–31, rat articular chondrocytes were also capable of uptaking the AAV2 vector and producing the transgene protein, suggesting that AAV2 could be used to infect rat chondrocytes in vivo. The ability to induce transgene expression with addition of Dox to the cell culture medium demonstrated the potential to control the timing of transgene expression.
Studies in nonhuman primates indicate that administration of AAV to the brain leads to transgene expression for as long as 8 years 23, yet few studies have assessed the persistence of transgene expression after AAV administration to the rat knee joint past a few months 16, 18, 22, 32. Intra-articular injections into the rat stifle joint, while challenging, have previously been reported by our group and others 18, 19, 32, 33. Given this experience, we believe that AAV was delivered intra-articularly in this study, although it is possible that some leakage or partial extra-articular injection may have occurred. By using bioluminescence, the same animal could be imaged repeatedly, providing a clear profile of transgene persistence and biodistribution. All animals had stable luciferase transgene expression for up to 1 year following intra-articular injection of AAV-CMV-Luc. A recent study employing recombinant lentivirus or adenovirus vectors did not observe such stable transgene expression in knees of immunocompetent rats 34. They observed strong transgene expression that diminished over a 3-week period. Injection of the same vectors into athymic rats led to an initially high transgene expression, which decreased to 20% and remained at that level for 6 months 34. Prolonged transgene expression in the current study suggests lower immunogenicity of AAV2 than lentivirus or adenovirus. Stability of transgene expression following AAV2 injection into the rat knee joint supports a potential role for AAV2 gene therapy in treatment strategies for inflammatory or degenerative arthritis by facilitating the synthesis of therapeutic factors within the joint space.
Consistent with previous reports of AAV injection into the knee joint of various animals, both luciferase and GFP transgene expressions were mostly localized to soft tissues 16, 17, 19, 20, 22. GFP imaging with the stereomicroscope provided a more precise localization than the bioluminescence imaging system and indicated that few sparse areas of GFP+ cells could be found in articular cartilage. A recent study employing self-complementary AAV2 injected into the intra-articular joint of guinea pigs also demonstrated transgene expression by articular chondrocytes 20. Previous studies using AAV have shown higher levels of transgene expression in diseased cartilage compared to normal cartilage 17, 19. Seeing that intact and undamaged articular cartilage is unlikely to require treatment, future studies will test AAV in rat models of articular cartilage damage, as it will more closely mimic the osteoarthritic environment. Abundant transgene expression in soft tissues suggests that cells within these tissues, such as those within the infrapatellar fat pad, can uptake the AAV2 and lead to transgene expression. Given the low turn over rate of these cells, intra-articular delivery of AAV2 targeted to soft tissues is of value for anti-inflammatory treatment strategies for arthritic conditions. For articular cartilage repair, it may be necessary to target more specifically the chondrocytes. This may be especially important if delivering genes encoding for bone morphogenetic proteins, as their expression in soft tissues could result in ectopic mineralization. We and others are exploring strategies for targeted delivery of transgenes to articular chondrocytes and chondral defects 12, 35, 36.
Lack of luciferase signal in the contralateral control knee that did not receive AAV-CMV-Luc, and lack of signal anywhere else in the animal suggests that intra-articular injection limits AAV2 delivery to tissues within the joint and does not travel systemically. This is advantageous, as bioactive factors present from viral transgene expression would not affect tissues outside of the joint, and possibly lead to negative side effects.
The dose of AAV used in this study of 2.5 × 1010 vg/knee joint was chosen based on what has previously been shown to be effective in rats 32. This prior study employed 1010 vg of AAV/rat knee joint and reported transgene expression for at least 100 days. The dose chosen for our rat study will need to be increased to be effective in a human joint which is larger in size and in volume of synovial fluid, resulting in a larger volume of distribution.
Others have shown it is feasible to administer AAV to humans through intra-articular injection. Human clinical trials for rheumatoid arthritis using a recombinant, single-stranded AAV2 vector have performed intra-articular injections at doses ranging from 1011 to 1013 vg/ml and 5 ml was injected per knee joint 7, 37. The authors reported these dosages to be safe and feasible, and although some improvement in patient-reported outcomes was observed, the transgene expression was not found to be sufficient. It should be noted that humans have serum IgG and neutralizing factors against different types of AAV, representing a limiting factor to AAV gene therapy 38. In moving towards direct intra-articular injection of AAV to larger joints, it will be important to determine the optimal dose of AAV to deliver and the serotype that will face the least amount of neutralizing antibodies.
Studies involving inducible gene therapy for musculoskeletal application have mainly focused on genetic engineering of cells ex vivo, followed by their implantation in vivo 25, 39–41. Direct viral gene delivery is easier to administer, and does not require the additional time and cost associated with ex vivo cell-mediated gene delivery. As shown in this study, the transgene expression can last for up to a year, which is not the case with implanted cells 12. By persisting for a prolonged period of time, there is the possibility to perform multiple cycles of transgene expression with a single injection of AAV, as shown in this study.
We observed increased transgene expression with addition of Dox to the drinking water and reduction of transgene expression following its removal at both 14 days and 13 months post-AAV injection. A similar trend was observed after 8 months. These data demonstrate the persistence of the transgene and controllability of its expression. The variability in observed transgene expression between the 14 days, 8 months and 13 months time points could reflect type II error due to the small sample size or potentially be related to a difference in Dox intake. Adding Dox to the drinking water while simple and shown in this study to be capable of controlling in vivo intra-articular transgene expression may however lead to some degree of variability since free water intake is less well controlled than oral administration as a pill or measured suspension. Future studies with inducible gene expression systems may need to use direct injection of Dox in small animals and oral suspension or pill administration in larger animals.
Induction of gene expression 13 months post-AAV injection is of clinical relevance when a therapeutic protein needs to be re-administered to further promote cartilage repair, or in cases where cartilage resumes degrading or inflammation increases and another round of treatment is necessary. An inducible gene expression system is also of interest for the delivery of multiple factors. Different biological molecules could be synthesized at different time points, depending on the regulatory transcriptional activator. This would provide a controlled environment for cartilage repair, where multiple growth factors are involved. A limitation of the inducible AAV2 gene expression system used in this study is the observed leakiness without the addition of Dox. This can potentially be addressed in future studies by newer rtTA vectors that have been developed to reduce basal activity and that require less Dox to be activated 42.
In summary, AAV2 delivered by a single intra-articular injection to a normal rat knee joint led to persistent and stable transgene expression that was mainly localized to soft tissues of the joint, with some expression in chondrocytes. Intra-articular injection of an inducible AAV2 vector demonstrated the ability to regulate in vivo transgene expression by oral administration of Dox, a compound that is safely used in humans. This regulation was possible immediately after delivering the AAV to the knee joint, and 13 months later. Ability to achieve stable yet highly regulatable long-term intra-articular transgene expression is of potential clinical utility for development of new treatment strategies for intra-articular disease processes such as inflammatory and degenerative arthritis.
Acknowledgments
The authors would like to thank Dr. Stephen H. Thorne for his assistance in optimizing the IVIS® imaging system for this study. The authors would also like to thank Drs. Veronica Yao and Michael J. O’Malley for technical assistance and Dr. James Irrgang for advice with statistical analysis.
Funding source
This work was funded by National Institutes of Health (NIH) RO1 AR051963 (CRC), NIH RC2 AR058929 (CRC), an Arthritis Foundation postdoctoral fellowship (KAP) and NIH T32-EB001026 (HHL/RPB).
Footnotes
Contributions
The hypotheses were conceived by CRC and XX. The study was designed by KAP and CRC. KAP, HHL, AMH, CM, ZY, and CQ performed data acquisition. KAP, HHL, AMH, CM, XX, and CRC performed data analysis and interpretation. The article was written by KAP, HHL, and CRC, and critically reviewed by all authors. All authors approved the final version of the article. CRC (chucr@upmc.edu) takes responsibility for the integrity of the work.
Conflict of interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ye X, Yang D. Recent advances in biological strategies for targeted drug delivery. Cardiovasc Hematol Disord Drug Targets. 2009;9:206–21. doi: 10.2174/187152909789007025. [DOI] [PubMed] [Google Scholar]
- 2.Coura Rdos S, Nardi NB. The state of the art of adeno-associated virus-based vectors in gene therapy. Virol J. 2007;4:99. doi: 10.1186/1743-422X-4-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16:1073–80. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- 4.Adriaansen J, Fallaux FJ, de Cortie CJ, Vervoordeldonk MJ, Tak PP. Local delivery of beta interferon using an adeno-associated virus type 5 effectively inhibits adjuvant arthritis in rats. J Gen Virol. 2007;88:1717–21. doi: 10.1099/vir.0.82603-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adriaansen J, Khoury M, de Cortie CJ, Fallaux FJ, Bigey P, Scherman D, et al. Reduction of arthritis following intra-articular administration of an adeno-associated virus serotype 5 expressing a disease-inducible TNF-blocking agent. Ann Rheum Dis. 2007;66:1143–50. doi: 10.1136/ard.2006.064519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khoury M, Adriaansen J, Vervoordeldonk MJ, Gould D, Chernajovsky Y, Bigey P, et al. Inflammation-inducible anti-TNF gene expression mediated by intra-articular injection of serotype 5 adeno-associated virus reduces arthritis. J Gene Med. 2007;9:596–604. doi: 10.1002/jgm.1053. [DOI] [PubMed] [Google Scholar]
- 7.Mease PJ, Wei N, Fudman EJ, Kivitz AJ, Schechtman J, Trapp RG, et al. Safety, tolerability, and clinical outcomes after intraarticular injection of a recombinant adeno-associated vector containing a tumor necrosis factor antagonist gene: results of a phase 1/2 Study. J Rheumatol. 2010;37:692–703. doi: 10.3899/jrheum.090817. [DOI] [PubMed] [Google Scholar]
- 8.Gelse K, Muhle C, Franke O, Park J, Jehle M, Durst K, et al. Cell-based resurfacing of large cartilage defects: long-term evaluation of grafts from autologous transgene-activated periosteal cells in a porcine model of osteoarthritis. Arthritis Rheum. 2008;58:475–88. doi: 10.1002/art.23124. [DOI] [PubMed] [Google Scholar]
- 9.Hidaka C, Goodrich LR, Chen CT, Warren RF, Crystal RG, Nixon AJ. Acceleration of cartilage repair by genetically modified chondrocytes over expressing bone morphogenetic protein-7. J Orthop Res. 2003;21:573–83. doi: 10.1016/S0736-0266(02)00264-4. [DOI] [PubMed] [Google Scholar]
- 10.Kuroda R, Usas A, Kubo S, Corsi K, Peng H, Rose T, et al. Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthritis Rheum. 2006;54:433–42. doi: 10.1002/art.21632. [DOI] [PubMed] [Google Scholar]
- 11.Mason JM, Breitbart AS, Barcia M, Porti D, Pergolizzi RG, Grande DA. Cartilage and bone regeneration using gene-enhanced tissue engineering. Clin Orthop Relat Res. 2000:S171–8. doi: 10.1097/00003086-200010001-00023. [DOI] [PubMed] [Google Scholar]
- 12.Pagnotto MR, Wang Z, Karpie JC, Ferretti M, Xiao X, Chu CR. Adeno-associated viral gene transfer of transforming growth factor-beta1 to human mesenchymal stem cells improves cartilage repair. Gene Ther. 2007;14:804–13. doi: 10.1038/sj.gt.3302938. [DOI] [PubMed] [Google Scholar]
- 13.Bandara G, Mueller GM, Galea-Lauri J, Tindal MH, Georgescu HI, Suchanek MK, et al. Intraarticular expression of biologically active interleukin 1-receptor-antagonist protein by ex vivo gene transfer. Proc Natl Acad Sci U S A. 1993;90:10764–8. doi: 10.1073/pnas.90.22.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bandara G, Robbins PD, Georgescu HI, Mueller GM, Glorioso JC, Evans CH. Gene transfer to synoviocytes: prospects for gene treatment of arthritis. DNA Cell Biol. 1992;11:227–31. doi: 10.1089/dna.1992.11.227. [DOI] [PubMed] [Google Scholar]
- 15.Hung GL, Galea-Lauri J, Mueller GM, Georgescu HI, Larkin LA, Suchanek MK, et al. Suppression of intra-articular responses to interleukin-1 by transfer of the interleukin-1 receptor antagonist gene to synovium. Gene Ther. 1994;1:64–9. [PubMed] [Google Scholar]
- 16.Hiraide A, Yokoo N, Xin KQ, Okuda K, Mizukami H, Ozawa K, et al. Repair of articular cartilage defect by intraarticular administration of basic fibroblast growth factor gene, using adeno-associated virus vector. Hum Gene Ther. 2005;16:1413–21. doi: 10.1089/hum.2005.16.1413. [DOI] [PubMed] [Google Scholar]
- 17.Goater J, Muller R, Kollias G, Firestein GS, Sanz I, O'Keefe RJ, et al. Empirical advantages of adeno associated viral vectors in vivo gene therapy for arthritis. J Rheumatol. 2000;27:983–9. [PubMed] [Google Scholar]
- 18.Izal I, Acosta CA, Ripalda P, Zaratiegui M, Ruiz J, Forriol F. IGF-1 gene therapy to protect articular cartilage in a rat model of joint damage. Arch Orthop Trauma Surg. 2008;128:239–47. doi: 10.1007/s00402-007-0407-7. [DOI] [PubMed] [Google Scholar]
- 19.Pan RY, Xiao X, Chen SL, Li J, Lin LC, Wang HJ, et al. Disease-inducible transgene expression from a recombinant adeno-associated virus vector in a rat arthritis model. J Virol. 1999;73:3410–7. doi: 10.1128/jvi.73.4.3410-3417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santangelo KS, Baker SA, Nuovo G, Dyce J, Bartlett JS, Bertone AL. Detectable reporter gene expression following transduction of adenovirus and adeno-associated virus serotype 2 vectors within full-thickness osteoarthritic and unaffected canine cartilage in vitro and unaffected guinea pig cartilage in vivo. J Orthop Res. 2010;28:149–55. doi: 10.1002/jor.20975. [DOI] [PubMed] [Google Scholar]
- 21.Ulrich-Vinther M, Stengaard C, Schwarz EM, Goldring MB, Soballe K. Adeno-associated vector mediated gene transfer of transforming growth factor-beta1 to normal and osteoarthritic human chondrocytes stimulates cartilage anabolism. Eur Cell Mater. 2005;10:40–50. doi: 10.22203/ecm.v010a05. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe S, Imagawa T, Boivin GP, Gao G, Wilson JM, Hirsch R. Adeno-associated virus mediates long-term gene transfer and delivery of chondroprotective IL-4 to murine synovium. Mol Ther. 2000;2:147–52. doi: 10.1006/mthe.2000.0111. [DOI] [PubMed] [Google Scholar]
- 23.Hadaczek P, Eberling JL, Pivirotto P, Bringas J, Forsayeth J, Bankiewicz KS. Eight years of clinical improvement in MPTP-lesioned primates after gene therapy with AAV2-hAADC. Mol Ther. 2010;18:1458–61. doi: 10.1038/mt.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rivera VM, Gao GP, Grant RL, Schnell MA, Zoltick PW, Rozamus LW, et al. Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood. 2005;105:1424–30. doi: 10.1182/blood-2004-06-2501. [DOI] [PubMed] [Google Scholar]
- 25.Gafni Y, Pelled G, Zilberman Y, Turgeman G, Apparailly F, Yotvat H, et al. Gene therapy platform for bone regeneration using an exogenously regulated, AAV-2-based gene expression system. Mol Ther. 2004;9:587–95. doi: 10.1016/j.ymthe.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992;89:5547–51. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roushan MR, Amiri MJ, Janmohammadi N, Hadad MS, Javanian M, Baiani M, et al. Comparison of the efficacy of gentamicin for 5 days plus doxycycline for 8 weeks versus streptomycin for 2 weeks plus doxycycline for 45 days in the treatment of human brucellosis: a randomized clinical trial. J Antimicrob Chemother. 2010;65:1028–35. doi: 10.1093/jac/dkq064. [DOI] [PubMed] [Google Scholar]
- 28.Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72:2224–32. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodrich LR, Choi VW, Carbone BA, McIlwraith CW, Samulski RJ. Ex vivo serotype-specific transduction of equine joint tissue by self-complementary adeno-associated viral vectors. Hum Gene Ther. 2009;20:1697–702. doi: 10.1089/hum.2009.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi H, Kato K, Miyake K, Hirai Y, Yoshino S, Shimada T. Adeno-associated virus vector-mediated anti-angiogenic gene therapy for collagen-induced arthritis in mice. Clin Exp Rheumatol. 2005;23:455–61. [PubMed] [Google Scholar]
- 31.Yokoo N, Saito T, Uesugi M, Kobayashi N, Xin KQ, Okuda K, et al. Repair of articular cartilage defect by autologous transplantation of basic fibroblast growth factor gene-transduced chondrocytes with adeno-associated virus vector. Arthritis Rheum. 2005;52:164–70. doi: 10.1002/art.20739. [DOI] [PubMed] [Google Scholar]
- 32.Pan RY, Chen SL, Xiao X, Liu DW, Peng HJ, Tsao YP. Therapy and prevention of arthritis by recombinant adeno-associated virus vector with delivery of interleukin-1 receptor antagonist. Arthritis Rheum. 2000;43:289–97. doi: 10.1002/1529-0131(200002)43:2<289::AID-ANR8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 33.Chu CR, Coyle CH, Chu CT, Szczodry M, Seshadri V, Karpie JC, et al. In vivo effects of single intra-articular injection of 0. 5% bupivacaine on articular cartilage. J Bone Joint Surg Am. 2010;92:599–608. doi: 10.2106/JBJS.I.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gouze E, Gouze JN, Palmer GD, Pilapil C, Evans CH, Ghivizzani SC. Transgene persistence and cell turnover in the diarthrodial joint: implications for gene therapy of chronic joint diseases. Mol Ther. 2007;15:1114–20. doi: 10.1038/sj.mt.6300151. [DOI] [PubMed] [Google Scholar]
- 35.Carlisle RC, Benjamin R, Briggs SS, Sumner-Jones S, McIntosh J, Gill D, et al. Coating of adeno-associated virus with reactive polymers can ablate virus tropism, enable retargeting and provide resistance to neutralising antisera. J Gene Med. 2008;10:400–11. doi: 10.1002/jgm.1161. [DOI] [PubMed] [Google Scholar]
- 36.Lee HH, Haleem AM, Yao V, Li J, Xiao X, Chu CR. Release of Bioactive Adeno-Associated Virus from Fibrin Scaffolds: Effects of Fibrin Glue Concentration. Tissue Eng Part A. 2011 doi: 10.1089/ten.tea.2010.0586. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mease PJ, Hobbs K, Chalmers A, El-Gabalawy H, Bookman A, Keystone E, et al. Local delivery of a recombinant adenoassociated vector containing a tumour necrosis factor alpha antagonist gene in inflammatory arthritis: a phase 1 dose-escalation safety and tolerability study. Ann Rheum Dis. 2009;68:1247–54. doi: 10.1136/ard.2008.089375. [DOI] [PubMed] [Google Scholar]
- 38.Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF, et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther. 2010;21:704–12. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 39.Moutsatsos IK, Turgeman G, Zhou S, Kurkalli BG, Pelled G, Tzur L, et al. Exogenously regulated stem cell-mediated gene therapy for bone regeneration. Mol Ther. 2001;3:449–61. doi: 10.1006/mthe.2001.0291. [DOI] [PubMed] [Google Scholar]
- 40.Peng H, Usas A, Gearhart B, Young B, Olshanski A, Huard J. Development of a self-inactivating tet-on retroviral vector expressing bone morphogenetic protein 4 to achieve regulated bone formation. Mol Ther. 2004;9:885–94. doi: 10.1016/j.ymthe.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 41.Wubbenhorst D, Dumler K, Wagner B, Wexel G, Imhoff A, Gansbacher B, et al. Tetracycline-regulated bone morphogenetic protein 2 gene expression in lentivirally transduced primary rabbit chondrocytes for treatment of cartilage defects. Arthritis Rheum. 2010;62:2037–46. doi: 10.1002/art.27461. [DOI] [PubMed] [Google Scholar]
- 42.Zhou X, Vink M, Klaver B, Berkhout B, Das AT. Optimization of the Tet-On system for regulated gene expression through viral evolution. Gene Ther. 2006;13:1382–90. doi: 10.1038/sj.gt.3302780. [DOI] [PubMed] [Google Scholar]