Abstract
Bacterial and yeast encoded cytosine deaminases (bCD and yCD, respectively) are widely investigated suicide enzymes used in combination with the prodrug 5-fluorocytosine (5FC) to achieve localized cytotoxicity. Yet characteristics such as poor turnover rates of 5FC (bCD) and enzyme thermolability (yCD) preclude their full therapeutic potential. We previously applied regio-specific random mutagenesis and computational design to create novel bCD and yCD variants with altered substrate preference (bCD1525) or increased thermostability (yCDdouble, yCDtriple) to aid in overcoming these limitations. Others have utilized pathway engineering in which the microbial enzyme uracil phosphoribosyltransferase (UPRT) is fused with its respective CD, creating bCD/bUPRT or yCD/yUPRT. In this study, we evaluated whether the overlay of CD mutants onto their respective CD/UPRT fusion construct would further enhance 5FC activation, cancer cell prodrug sensitivity and bystander activity in vitro and in vivo. We show that all mutant fusion enzymes allowed for significant reductions in IC50 values relative to their mutant CD counterparts. However, in vivo the CD mutants displayed enhanced tumor growth inhibition capacity relative to the mutant fusions, with bCD1525 displaying the greatest tumor growth inhibition and bystander activity. In summary, mutant bCD1525 appears to be the most effective of all bacterial or yeast CD or CD/UPRT enzymes examined and as such is likely to be the best choice to significantly improve the clinical outcome of CD/5FC suicide gene therapy applications.
Keywords: cytosine deaminase, uracil phosphoribosyltransferase, suicide gene therapy, bystander effect, 5-fluorocytosine
Introduction
The cytosine deaminase (CD; EC 3.5.4.1)/5-fluorocytosine (5FC) system is one of the most well-characterized enzyme/prodrug systems in suicide gene therapy (SGT). Found primarily in microbes such as Escherichia coli and Saccharomyces cerevisiae, CD is a pyrimidine salvage pathway enzyme responsible for catalyzing the hydrolytic deamination of cytosine to form uracil and ammonia[1]. Similarly, CD (codA in E. coli, FCY1 in S. cerevisiae) deaminates the prodrug 5FC into the potent antimetabolite and radiosensitizing agent, 5-fluorouracil (5FU). 5FU is further converted to the irreversible thymidylate synthase inhibitor, 5FdUMP, leading to depleted dTTP pools, DNA synthesis inhibition, and subsequent apoptosis[2-4]. Its cytotoxic activity is also extended by passive diffusion from CD-expressing to neighboring untransfected cells resulting in a phenomenon known as the bystander effect. This phenomenon is an essential part of SGT and serves to compensate, at least in part, for inefficient gene transfer seen with current delivery systems[1].
The CD/5FC system was originally developed using bacterial CD (bCD)[5]. Although promising, the therapeutic application of this system has been limited by the poor turnover rate (kcat) of 5FC by bCD. Alternatively, yeast CD (yCD) was later evaluated and shown to display superior kinetic properties toward 5FC (22-fold lower Km for 5FC) and increase the efficacy of the CD/5FC system approach[6, 7]. However, the thermolabile nature of yCD at 37°C precludes its full therapeutic potential. To overcome this limitation, computational design studies aimed at repacking the hydrophobic core of yCD identified two enzyme mutants (yCDdouble and yCDtriple) with increased half-lives and melting temperatures that significantly increased the sensitivity of cancer cells to 5FC treatment as compared to wild-type yCD[8, 9]. Similarly, to alleviate the enzymatic limitations seen with bCD, regio-specific random mutagenesis studies targeting two regions flanking the bCD active site identified a superior bCD mutant, bCD1525, with improved kinetic properties to wild-type bCD. With a 19-fold shift in substrate preference for 5FC, bCD1525 significantly reduced IC50 levels, improved the bystander effect, and prevented tumor growth at 5FC levels where tumors expressing wild-type bCD were unresponsive[10].
In microbes, 5FU is directly converted to the downstream metabolite 5FUMP by uracil phosphoribosyltransferase (UPRT; EC 2.4.2.9). UPRT (upp in E. coli, FUR1 in S. cerevisiae) is a member of the pyrimidine salvage pathway and is responsible for catalyzing the synthesis of UMP or 5FUMP from uracil or 5FU, respectively[11, 12]. Overexpression of UPRT in multiple cancer cell lines resulted in a marked increase in 5FU sensitization. However, it has been shown that systemic treatment of 5FU can lead to gastrointestinal and hematological toxicity[13, 14]. In addition, some human tumor cell lines display resistance to 5FU as a result of possible defects in downstream cellular metabolism or overexpression of 5FU catabolic enzymes[15]. To circumvent 5FU systemic toxicity and bypass 5FU resistance, co-expression of CD with UPRT and expression of bCD or yCD fused to UPRT (bCD/bUPRT and yCD/yUPRT) have both been tested and shown to enhance 5FC-mediated cell killing[16-20].
In the present study, we evaluated whether the overlay of previously created yeast and bacterial CD mutants onto their corresponding CD/UPRT fusion construct would result in a cooperative increase in 5FC metabolism and cancer cell prodrug sensitivity in vitro and in vivo. Our data suggest that although previous studies have shown wild-type CD/UPRT fusions enhance prodrug sensitivity in vitro and in vivo compared to their CD counterparts, UPRT activity in fusion with our previously identified CD mutants negatively influences bystander activity in vivo and diminishes the therapeutic value of the CD/5FC system. Taken together, this comparative study reveals that the optimized bacterial mutant bCD1525 alone displays ideal cell and tumor ablative characteristics for suicide gene therapy applications.
Materials and Methods
Materials
Oligonucleotides used to mutate and/or sequence bCD, yCD, bUPRT and yUPRT were obtained from Integrated DNA Technologies (Coralville, IA). Restriction endonucleases and T4 DNA ligase were purchased from New England Biolabs (Beverly, MA). The Wizard PCR prep kit (Promega, Madison, WI), HiSpeed Plasmid Midi Kit (Qiagen, Valencia, CA) and StrataPrep EF Plasmid Midikit (Stratagene, La Jolla, CA) were all used for DNA purification. Alamar Blue was purchased from Serotec Limited (Oxford, UK). All cell culture reagents were purchased from Gibco (Carlsbad, CA), and all other reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted.
Bacterial strains
Escherichia coli strain XL1-Blue [F′∷Tn10 proA+B+ lacIq D(lacZ) M15/recA1 endA1 gyrA96 (NaIr) thi hsdR17 (rk-mk+) supE44 relA1 lac] and NM522 [F+ lacIq Δ(lacZ)-M15 proA+B+/supE thiΔ(lac-proAB) Δ(hsdMS-mcrB)5(rk-mk-McrBC-)] were used for cloning and mutagenesis procedures. E. coli strains BM604 [HfrH thi galE Δ (attλ-bio) deoA103 deoC argA lysA cytR upp udp pyrF30] and GIA39 [thr- dadB3 fhuA21 codA1 lacY1 tsk-95 glnV44(AS) λ- pyrF101 his-108 argG6 ilvA634 thi-1 deoC1 glt-15] were first lysogenized with λDE3 according to manufacturer protocol (Novagen, Madison, WI) and then used for UPRT and CD genetic complementation, respectively. The BM604 strain, a strain deficient in UPRT activity and orotidine 5-phosphate decarboxylase, was a kind gift from F. Bringel[21, 22], and the GIA39 strain, a strain deficient in CD and orotidine 5-phosphate decarboxylase, was obtained from the E. coli Genetic Stock Center (CGSC # 5594).
Construction of bCD/bUPRT and yCD/yUPRT fusion mutant constructs and genetic complementation
The bCD/bUPRT fusion gene was first removed from pORF-codA∷upp (Invivogen, San Diego, CA), which carries functional bCD and bUPRT, using NcoI/HinDIII and subcloned into NcoI/HinDIII digested pETHT[23]. The yCD/yUPRT fusion gene was isolated as an XhoI(blunt-ended)/NotI fragment from pCI-neoFCU1 (Transgene, France) and subcloned into EcoRI(blunt-ended)/NotI digested pETHT. Both bCD/bUPRT and yCD/yUPRT were then subjected to mutagenesis using the QuikChange site-directed mutagenesis kit from Stratagene (La Jolla, CA) to create the respective mutant fusion counterparts. Two pairs of oligonucleotides (MB455 5′-GGATTGATCTGCAAATCGCCGCTTTCCCTCAGG-3′/MB456 5′-CCTGAGGGAAAGCGGCGATTTGCAGATCAATCC-3′ and MB457 5′-CTTTGGTCACGATGATGTCTGCGGTCCGTGGTAT-3′/MB458 5′-ATACCACGGACCGCAGACATCATCGTGACCAAAG-3′) were used to introduce the bCD1525 (V152A/F316C/D317G) sequence. Oligonucleotide pairs (MB462 5′-GCCTATGAGGAGGCGCTCTTAGGTTACAAAGAGGG-3′/MB463 5′-CCCTCTTTGTAACCTAAGAGCGCCTCCTCATAGGC-3′ and MB466 5′-GACGATGAGAGGTGTAAAAAGCTCATGAAACAATTTATCG-3′/MB467 5′-CGATAAATTGTTTCATGAGCTTTTTACACCTCTCATCGTC-3′) were used to introduce the yCDdouble (A23L/I140L) sequence, while yCDtriple (A23L/V108I/I140L) used (MB464 5′-GGTATTCCACGCTGTGTTATCGGT-3′/MB465 5′-CGTTCTCACCGATAACACAGCGTGGAATACC-3′) in addition to those used for the yCDdouble sequence. DNA sequencing analysis confirmed the presence of correct mutations in the mutant fusion constructs. The resulting plasmids were designated pETHT:bCD1525/bUPRT, pETHT:yCDdouble/yUPRT and pETHT:yCDtriple/yUPRT, respectively. The construction of pETHT:bCD, pETHT:bCD1525, pETHT:yCD, pETHT:yCDdouble and pETHT:yCDtriple were as previously described[9,10]. For complementation studies, E. coli strains GIA39(DE3) or BM604(DE3) harboring either pETHT, wild-type or mutant CD, or wild-type or mutant CD/UPRT constructs were grown at 37°C on either CD[24,25] or UPRT[21,22] complementation plates.
Construction of mammalian expression vectors
The bCD/bUPRT fusion gene was subcloned from pETHT:bCD/bUPRT into EcoRV/XhoI digested pcDNA6/myc-HisB (Invitrogen, Carlsbad, CA) as an NcoI(blunt-ended)/XhoI fragment, while the yCD/yUPRT fusion gene was subcloned from pETHT:yCD/yUPRT into EcoRV/NotI digested pcDNA6/myc-HisB as an NcoI(blunt ended)/NotI fragment creating pcDNA:bCD/bUPRT and pcDNA:yCD/yUPRT, respectively. Following restriction enzyme verification, DNA sequencing analysis confirmed the presence of both fusion genes. Both pcDNA:bCD/bUPRT and pcDNA:yCD/yUPRT were then subjected to mutagenesis using the Stratagene QuikChange site-directed mutagenesis kit to create their mutant fusion counterparts as described above. Resulting plasmids were designated pcDNA:bCD1525/bUPRT, pcDNA:yCDdouble/yUPRT and pcDNA:yCDtriple/yUPRT. DNA sequencing analysis confirmed the presence of correct mutations in the mutant fusion constructs. The construction of pcDNA:bCD, pcDNA:yCD, and their mutant constructs was described previously by Fuchita et al. (2009) and Stolworthy et al. (2008), respectively.
Cell line
Rat C6 glioma cells were purchased from ATCC (Manasass, VA) and grown in Dulbecco's Modified Essential Medium containing 5% fetal bovine serum, 1mM sodium pyruvate, 10mM HEPES, 100μM nonessential amino acids, 100U/mL penicillin G, 10μg/mL streptomycin sulfate, 292μg/mL L-glutamine, 100μM sodium citrate and 0.0014% NaCl. Cells were maintained in a humidified incubator at 37°C in 5% CO2.
In vitro cytotoxicity assay
Stable transfectants were created by the addition of 1μg purified endotoxin-free DNA (Stratagene, La Jolla, CA) (pcDNA (empty vector), pcDNA:bCD, pcDNA:bCD1525, pcDNA:bCD/bUPRT, pcDNA:bCD1525/bUPRT, pcDNA:yCD, pcDNA:yCDdouble, pcDNA:yCDtriple, pcDNA:yCD/yUPRT, pcDNA:yCDdouble/yUPRT, or pcDNA:yCDtriple/yUPRT) to 1×105 rat C6 glioma cells by lipofection using FuGENE 6 transfection reagent (Roche Diagnostic, Penzberg, Germany) at a 3:1 ratio as described by the manufacturer. Stable transfectants were selected by culture in media supplemented with 4μg/mL blasticidin.
Immunoblot analysis using rabbit polyclonal antiserum followed by goat anti-rabbit-AP conjugated secondary antibody (Bio-Rad, Hercules, CA) was performed to determine protein expression levels, as previously described[9]. Antisera to purified bCD, bUPRT or yCD was generated by Harlan Laboratories (Indianapolis, IN), while antiserum for yUPRT was generated by Invitrogen (Carlsbad, CA). An AP Conjugate Substrate Kit (Bio-Rad) was used for colorimetric detection. Stable transfectants confirmed by immunoblot were used for in vitro cytotoxicity assays. Pools of transfectants were plated at an initial density of 500 cells/well in 96-well microtiter plates. Following cell adherence overnight, 5FC (0-10mM) was added in sets of eight wells for each concentration tested. After a 7 day incubation period, cell viability was determined by the addition of the redox indicator dye Alamar Blue and fluorometric readings at 530/590nm using a Biotek Synergy HT microplate reader several hours later as described by the manufacturer. Data were plotted with the standard error of the mean and at least three replicates were performed.
In vitro bystander experiment
Rat C6 glioma cells stably transfected with pcDNA were mixed at different ratios with stable transfectants harboring pcDNA:bCD, pcDNA:bCD1525, pcDNA:bCD/bUPRT, pcDNA:bCD1525/bUPRT, pcDNA:yCD, pcDNA:yCDdouble, pcDNA:yCDtriple, pcDNA:yCD/yUPRT, pcDNA:yCDdouble/yUPRT, or pcDNA:yCDtriple/yUPRT. Mixed cells were then plated at an initial density of 500 cells/well in 96-well microtiter plates. Following cell adherence overnight, 4 or 10mM 5FC was added to the plated cells. Cell survival was determined as described above.
Xenograft tumor model
Pools of rat C6 glioma cells stably transfected with pcDNA, pcDNA:bCD1525, pcDNA:bCD/bUPRT, pcDNA:bCD1525/bUPRT, pcDNA:yCDdouble, pcDNA:yCDtriple, pcDNA:yCD/yUPRT, or pcDNA:yCDdouble/yUPRT (0.5×106 cells in 200μl PBS at pH 7.2, n=5 for each group) were injected subcutaneously into the left and right flanks of five to six week old female, athymic NCr-nu/nu mice (National Cancer Institute, Frederick, MD). For the bystander xenograft tumor model experiments, pools of C6 glioma cells stably transfected with pcDNA:bCD1525, pcDNA:bCD1525/bUPRT, pcDNA:yCDdouble, pcDNA:yCDdouble/yUPRT, pcDNA:yCDtriple, or pcDNA:yCDtriple/UPRT were mixed with pools of C6 glioma cells stably transfected with pcDNA at a ratio of 80:20 (vector : suicide gene) then subcutaneously injected into the left and right flanks of nude mice (n=5). When tumors reached 3-4mm in diameter (day 0), PBS or 5FC at 375mg kg-1 was administered via intraperitoneal injection twice a day for up to 21 days. Tumor volume was monitored by caliper measurement (length, width, and height) every other day, starting at day 0, until mice were euthanized. Tumor volume was calculated using the formula: 4/3π ((width × length × height)/2) and analyzed for statistical significance using the Student's T-test. All mice with heavy tumor burden were sacrificed in compliance with IACUC recommended practices and guidelines.
Results
Genetic complementation
To determine whether bCD or yCD mutations overlaid onto their respective CD/UPRT constructs maintained dual functionality, previously established E. coli genetic complementation assays evaluating CD or UPRT activity were performed using GIA39 (DE3) or BM604 (DE3) E. coli, respectively (see Materials and Methods). In these assays, pETHT, pETHT:bCD, pETHT:bCD1525, pETHT:bCD/bUPRT, pETHT:bCD1525/bUPRT, pETHT:yCD, pETHT:yCDdouble, pETHT:yCDtriple, pETHT:yCD/yUPRT, pETHT:yCDdouble/yUPRT and pETHT:yCDtriple/yUPRT were used to transform E. coli and bacteria were then plated onto appropriate selective media supplemented with carbenicillin. For CD genetic complementation, all CD and CD/UPRT constructs were able to complement the CD deficiency of GIA39 (DE3) and the CD/UPRT fusion constructs were able to complement the UPRT deficiency of BM604 (DE3). Cells harboring pETHT were unable to complement the deficiency of either E. coli strain and all cultures grew on non-selective media (data not shown). These results indicate all wild-type and mutant fusion constructs maintain dual functionality, which is in accordance to previous studies [16, 17].
In vitro prodrug sensitivity assays
To examine the ability of mutant fusion enzymes to sensitize cancer cells to 5FC, wild-type or mutant CD or CD/UPRT constructs were first cloned into the expression vector pcDNA and the resulting plasmids were used to transfect rat C6 glioma cells. Pools of stable transfectants were then exposed to a wide concentration range of 5FC (0-10mM) and cell survival determined after 7 days (Figure 1). Consistent with a previous report by Fuchita et al. (2009)[10], bCD1525-transfected cells conferred increased sensitivity to 5FC compared to wild-type bCD-transfected cells. This sensitivity is a direct reflection of the increased 5FU levels produced in the bCD1525-transfected cells[10]. While wild-type bCD/bUPRT-transfected cells failed to lower IC50 levels in comparison to bCD1525, bCD1525/bUPRT-transfected cells displayed a 4.5-fold decrease (<0.1mM) in IC50 value (Figure 1A). As previously reported by Stolworthy et al. (2008)[9], both yCDdouble and yCDtriple had lower IC50 values than wild-type yCD-transfected C6 cells. Wild-type yCD/yUPRT displayed a similar IC50 value to yCDtriple (1.8mM) and a 2.6-fold lower IC50 than yCDdouble (4.75mM). The yCD/yUPRT fusion mutants further lowered IC50 values 3.3-fold (yCDdouble/yUPRT) and 6-fold (yCDtriple/yUPRT) compared to wild-type yCD/yUPRT. Interestingly, of the two yCD/yUPRT fusion mutants, yCDdouble/yUPRT showed the greatest reduction in IC50 (8.5-fold) in comparison to its yCD mutant counterpart, yCDdouble. Both yCDdouble/yUPRT and yCDtriple/yUPRT exhibited similar IC50 values of 0.55 and 0.3mM, respectively (Figure 1B). When wild-type or mutant CD/UPRT-transfected cells were analyzed together, bCD1525/bUPRT-transfected cells displayed the greatest degree of 5FC sensitivity (Figure 1C).
Figure 1.

Sensitivity of wild-type or mutant CD or CD/UPRT-transfected rat C6 glioma cells to 5FC. Pools of stable transfectants containing pcDNA, (A) wild-type or mutant bCD or bCD/bUPRT, (B) wild-type or mutant yCD or yCD/yUPRT, or (C) wild-type or mutant bCD/bUPRT or yCD/yUPRT were evaluated for 5FC sensitivity. After 7 days of 5FC treatment, cell survival was measured by Alamar Blue treatment according to the manufacturer's protocol with untreated (control) samples (0mM 5FC) set to 100% cell survival. Each data point (mean ± SEM; n = 3; performed with 24 replicates) is expressed as a percentage of the value for control samples.
In vitro bystander activity analyses
One key feature of the CD/5FC system that poses a potential advantage over other SGT systems, such as the HSVTK/GCV system, is the potent bystander effect that results from the passive diffusion of 5FU from transfected to untransfected cancer cells. This potent bystander effect can help overcome poor transduction efficiencies seen with current delivery systems and may increase the likelihood of complete tumor eradication[26, 27]. In the case of the CD/UPRT fusion constructs, UPRT activity may induce bystander characteristics that more heavily rely on alternative mechanisms of action, such as the transfer of active metabolites via gap junctions, such as that observed with the HSVTK/GCV system. To investigate the ability of mutant CD/UPRT fusion constructs to elicit a potent bystander effect, cocultures of vector and wild-type or mutant CD or CD/UPRT-transfected cells were mixed at different ratios and subjected to a set concentration of 5FC for 7 days (see Materials and Methods). Whereas a cell population containing 15% bCD1525-transfected cells is sufficient to induce 50% cell killing, bCD1525/bUPRT-transfected cells failed to display significant bystander cell killing activity at the 5FC concentration used (Figure 2A). Alternatively, cell populations containing yCDdouble/yUPRT or yCDtriple/yUPRT-transfected cells elicited a robust bystander effect as indicated by 50% tumor cell killing when only 10-15% of the cell population expressed these mutant fusion enzymes. Indeed, when all wild-type or mutant CD/UPRT constructs were compared together, the yeast fusion mutants demonstrated the most significant bystander activity (Figure 2B). Both wild-type bCD/bUPRT and yCD/yUPRT showed slight to modest cell killing effects (Figure 2B), while yCDdouble and yCDtriple failed to display significant bystander activity at the 5FC concentration used (data not shown). Clonogenic assays[28] examining bystander cell populations containing 100% suicide gene-transfected cells revealed these populations are unable to form colonies (data not shown). Thus detection of some survival for the yeast fusion mutants (Figure 2B) most likely reflects an artifact of the Alamar Blue dye assays rather than cell viability.
Figure 2.

In vitro bystander analyses of rat C6 glioma transfectants treated with 5FC. Pools of C6 cells containing pcDNA were mixed with cells harboring (A) wild-type or mutant bCD or bCD/bUPRT or (B) wild-type or mutant bCD/bUPRT or yCD/yUPRT at different ratios and subjected to 4mM (A) or 10mM (B) 5FC for 7 days. Cell survival was measured by Alamar Blue treatment and each data point (mean ± SEM; n = 3; performed with 24 replicates) is expressed as a percentage of the value for control wells containing 100% pcDNA-transfected cells.
Xenograft tumor model
Constructs that conferred the best combined cell killing and bystander activity were selected for further characterization in an in vivo xenograft tumor model. The yCDdouble/yUPRT construct was chosen for further in vivo characterization over yCDtriple/yUPRT as both displayed similar in vitro prodrug sensitivity and bystander activity, yet yCDdouble/yUPRT showed the greatest improvement over its respective yCD mutant counterpart. Figure 3 illustrates tumor growth in athymic NCr-nu/nu mice treated with 5FC (375mg kg-1, twice a day) or phosphate buffered saline (PBS) over time. In Figure 3A, mice bearing bCD1525 or bCD1525/bUPRT-transfected tumors showed indistinguishable growth rates to those bearing vector-transfected tumors when treated with PBS. However, when mice bearing bCD1525 or bCD1525/bUPRT-transfected tumors were treated with 5FC a significant growth restriction emerged starting day 4 (P≤0.05) in comparison to those bearing vector-transfected tumors. Mice bearing bCD1525-transfected tumors and treated with 5FC displayed the greatest restriction in tumor growth (day 16 mean tumor volume: 27.16mm3) (Figure 3B; Table 1). Figure 3C shows all PBS treated groups to have statistically insignificant differences in growth rates. In contrast, tumor cell growth rates in mice bearing yCDdouble or yCDdouble/yUPRT-transfected tumors were significantly reduced starting day 8 relative to mice bearing vector-transfected tumors when treated with 5FC. Following the same trend as the bCD1525 versus bCD1525/bUPRT in vivo results, the single gene construct yCDdouble displayed the greatest ability to restrict tumor growth (day 16 mean tumor volume: 269.31mm3) (Figure 3D; Table 2). Neither wild-type bCD/bUPRT nor yCD/yUPRT-transfected tumors displayed significant sensitivity to 5FC (data not shown). Based on tumor growth inhibition rates, bCD1525-transfected tumors appear to be the most sensitive to 5FC treatment of all tested constructs. All PBS treated mice and 5FC treated mice bearing vector-transfected tumors were sacrificed at day 16 or 18 due to heavy tumor burden.
Figure 3.
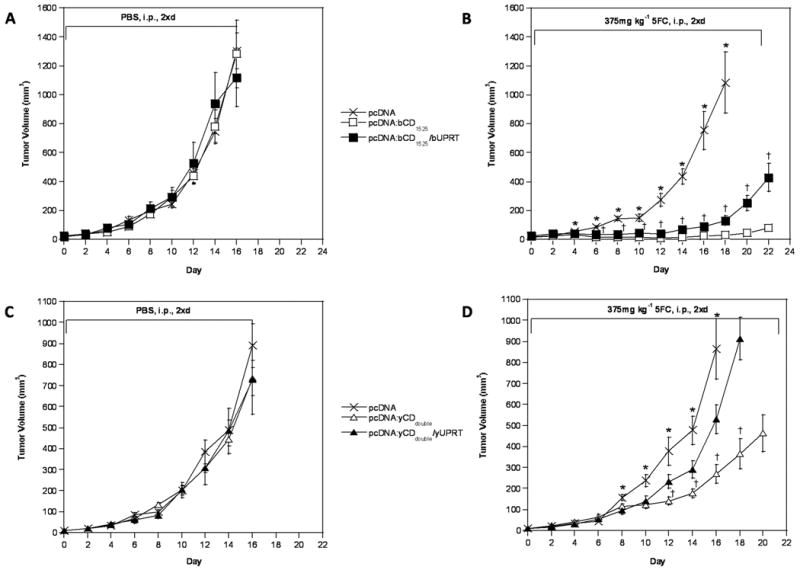
Xenograft tumor growth course of bCD or yCD mutant or mutant fusion enzymes. Pools of stably transfected rat C6 glioma cells expressing pcDNA (×), pcDNA:bCD1525 (□), pcDNA:bCD1525/bUPRT (■), pcDNA:yCDdouble (△), or pcDNA:yCDdouble/yUPRT (▲) were used to seed tumors in nude mice (n = 5 for each group). When tumor size reached 3-4mm (Day 0), PBS (A,C) or 375mg kg-1 5FC (B, D) was administered intraperitoneally twice a day for up to 21 days. Asterisks (*) denote statistical significance (P≤0.05) in tumor sizes between mice bearing mutant CD or CD/UPRT-transfected cells to those bearing pcDNA-transfected cells, while (†) denotes statistical significance (P≤0.05) in tumor sizes between mice bearing mutant CD-transfected cells to those bearing mutant CD/UPRT-transfected cells (B,D).
Table 1.
In vivo growth of rat C6 tumor cells transfected with pcDNA, pcDNA:bCD1525 or pcDNA:bCD1525/bUPRT in response to PBS or 5FC treatment.
| Mean tumor volume (mm3) | |||
|---|---|---|---|
| Day 0 | Day 16 | ||
| 5FC (375mg kg-1) | PBS | 5FC (375mg kg-1) | |
| pcDNA | 14.98 (1.26)a | 1303.79 (123.55) | 754.86 (131.38)** |
| bCD1525 | 18.37 (4.09) | 1282.14 (234.76) | 27.16 (4.63) |
| bCD1525/bUPRT | 24.17 (4.53) | 1119.38 (200.54) | 87.42 (20.44)** |
Standard error mean.
P≤0.01 (bCD1525 vs. bCD1525/bUPRT tumor volume) or 0.001 (bCD1525 vs. pcDNA tumor volume) (n = 5).
5FC, 5-fluorocytosine; bCD, bacterial cytosine deaminase; bUPRT, bacterial uracil phosphoribosyltransferase; PBS, phosphate buffered saline.
Table 2.
In vivo growth of rat C6 tumor cells transfected with pcDNA, pcDNA:yCDdouble or pcDNA:yCDdouble/yUPRT in response to PBS or 5FC treatment.
| Mean tumor volume (mm3) | |||
|---|---|---|---|
| Day 0 | Day 16 | ||
| 5FC (375mg kg-1) | PBS | 5FC (375mg kg-1) | |
| pcDNA | 8.82 (0.91)a | 890.51 (103.22) | 864.23 (142.17)** |
| yCDdouble | 11.75 (1.4) | 735.92 (83.99) | 269.31 (43.47) |
| yCDdouble/yUPRT | 10.55 (1.07) | 728.28 (166.02) | 529.37 (67.67)** |
Standard error mean.
P≤0.05 (yCDdouble vs. yCDdouble/yUPRT tumor volume) or 0.005 (yCDdouble vs. pcDNA tumor volume) (n = 5).
5FC, 5-fluorocytosine; yCD, yeast cytosine deaminase; yUPRT, yeast uracil phosphoribosyltransferase; PBS, phosphate buffered saline.
Bystander xenograft tumor model
As the xenograft tumor model results did not directly substantiate our in vitro sensitivity assay results, we next sought to determine whether the tumor microenvironment may alter or influence bystander activity in vivo. To this end, an 80:20 mixture of vector transfected cells to cells harboring previously tested mutant CD or mutant CD/UPRT fusion gene were injected into athymic NCr-nu/nu mice. Figure 4 illustrates tumor growth in mice treated with 5FC (375mg kg-1, twice a day) or PBS over time. Results show bCD1525 to display the greatest bystander activity and response to 5FC treatment as indicated by substantial tumor growth inhibition (day 16 mean tumor volume: 179.57mm3), while bCD1525/bUPRT also exhibited a significant bystander killing effect (day 16 mean tumor volume: 432.51 mm3). Conversely, yCDdouble/yUPRT did not evoke sufficient bystander activity able to suppress tumor growth and yCDdouble displayed minimal bystander activity (significance starting day 14 (P≤0.05) in comparison to PBS treated vector: yCDdouble-expressing tumors) (Figure 4B, Table 3). Similar results were seen with yCDtriple and yCDtriple/yUPRT (data not shown). All tumors from PBS treated mice groups grew at approximately the same rate (P≥0.05) (Figure 4A). The variability in tumor volumes at day 16 may be attributed to necrotic centers or the amorphous nature of the large tumors that prevented consistent measurements.
Figure 4.

Bystander xenograft tumor model of bCD or yCD mutant and mutant fusion enzymes. Pools of rat C6 glioma cells transfected with pcDNA were mixed with cells stably transfected with pcDNA:bCD1525 (□), pcDNA:bCD1525/bUPRT (■), pcDNA:yCDdouble (△), or pcDNA:yCDdouble/yUPRT (▲) and then used to seed tumors in nude mice (n = 5 for each group). When tumor size reached 3-4mm (Day 0), PBS (A) or 375mg kg-1 5FC (B) was administered intraperitoneally twice a day for up to 21 days. Asterisks (*) denote statistical significance (P≤0.05) in tumor sizes between mice bearing 20% bCD1525-transfected cells to those bearing 20% yCDdouble or yCDdouble/yUPRT-transfected cells, while (†) denotes statistical significance (P≤0.05) in tumor sizes between mice bearing 20% bCD1525-transfected cells to those bearing 20% bCD1525/bUPRT-transfected cells (B).
Table 3.
Bystander in vivo growth of rat C6 tumors containing an 80:20 ratio of vector transfected (pcDNA) to transfected (yCDdouble, yCDdouble/yUPRT, bCD1525, or bCD1525/bUPRT) cells in response to PBS or 5FC treatment.
| Mean tumor volume (mm3) | |||
|---|---|---|---|
| Day 0 | Day 16 | ||
| 5FC (375mg kg-1) | PBS | 5FC (375mg kg-1) | |
| yCDdouble | 10.42 (0.96)a | 1010.8 (128.84) | 718.88 (82.2)** |
| yCDdouble/yUPRT | 6.78 (0.76) | 705.98 (170.6) | 810.62 (142.01)** |
| bCD1525 | 8.88 (1.19) | 1066.5 (196.17) | 179.57 (28.9) |
| bCD1525/bUPRT | 8.23 (0.65) | 638.0 (70.33) | 432.51 (53.68)** |
Standard error mean.
P≤0.005 (bCD1525 vs. bCD152S/bUPRT tumor volume) or 0.0005 (bCD1525 vs. yCDdouble and yCDdouble/yUPRT tumor volumes) (n = 5).
5FC, 5-fluorocytosine; bCD or yCD, bacterial or yeast cytosine deaminase; bUPRT or yUPRT, bacterial or yeast uracil phosphoribosyltransferase; PBS, phosphate buffered saline.
Discussion
Although multiple preclinical studies have demonstrated the feasibility of suicide gene therapy for cancer treatment, it is evident that further improvements are necessary to achieve clinical success. To this end, we and other research teams have utilized enzyme and pathway engineering strategies to enhance the catalytic properties or increase the stability of multiple suicide enzymes. These engineered enzymes have been shown to lower necessary prodrug doses used during therapy, enhance cancer cell prodrug sensitivity and improve bystander activity (reviewed in Ardiani et al., manuscript in preparation). Our primary goal for this study was to evaluate whether the combination of enzyme and pathway engineering strategies using the CD/5FC approach would further augment prodrug activation and cooperatively increase cancer cell prodrug sensitivity. This comparative analysis study was also used as a means to identify the most suitable bacterial or yeast wild-type or mutant CD or CD/UPRT enzyme for clinical application.
Earlier studies directly comparing wild-type bacterial and yeast CD revealed that, despite its thermolability, the use of yCD enhanced HT29 human colon cancer cell radiosensitization, the bystander effect, and the overall therapeutic value of the CD/5FC treatment strategy over bCD[6, 7]. Later studies comparing wild-type bCD, yCD, and the yCD/yUPRT fusion in a rat hepatoma model confirmed these findings and also provided evidence that the yCD/yUPRT fusion further enhanced cancer cell 5FC sensitivity in vitro and in vivo over both bCD and yCD alone[29]. Prior to these studies, the yCD/yUPRT fusion was characterized in multiple cancer cell lines and shown to significantly improve bystander activity in vitro relative to wild-type yCD[17]. Similarly, glioblastoma and colon cancer cell lines expressing the bCD/bUPRT fusion displayed enhanced 5FC sensitivity and bystander activity in vitro[30,31] and significant tumor growth inhibition in vivo[30] over wild-type bCD. Our results recapitulate the superiority of the CD/UPRT fusions over their wild-type CD counterparts and also reveal that wild-type yCD/yUPRT-transfected rat C6 glioma cells are slightly more sensitive to 5FC than wild-type bCD/bUPRT-transfected cells and maintain greater bystander activity. The combination of elevated yCD activity[17], more efficient kinetic parameters toward 5FC[6], and fusion to yUPRT[17,29] may account for these enhanced in vitro results. In the context of wild-type CD or CD/UPRT enzymes, all combined results indicate the yCD/yUPRT fusion to be the most effective prodrug activation system.
An advantage of SGT over conventional treatments such as systemic chemotherapy is its ability to selectively convert prodrugs at targeted tumor sites resulting in local, high antimetabolite concentrations. However, use of the CD/5FC system to date has been limited to only a handful of conducted clinical trials[32-34]. These trials have been hindered in part by inefficient gene delivery and poor prodrug activation at the 5FC doses used. Previously, we reported that yCDdouble and yCDtriple-transfected rat C6 glioma cells and several bCD1525-transfected cell lines were rendered more sensitive to 5FC than wild-type CD-transfected cells at low 5FC concentrations[9, 10]. Our current data are consistent with these reports and also show that bCD1525 displayed the lowest relative IC50 value of all CD mutants tested. Furthermore, bCD1525 was the only CD mutant able to display significant bystander activity in vitro at the 5FC dose tested. Wild-type yCD, although previously revealed to significantly increase the therapeutic effect of the CD/5FC system relative to wild-type bCD, showed minimal relative therapeutic value in vitro as compared to the bacterial mutant bCD1525. When tested in vivo, our xenograft and bystander tumor model studies reiterated the superior antitumor and bystander activity of bCD1525 relative to the yCD mutant, yCDdouble, at a low 5FC concentration. Similar results for yCDdouble were seen with yCDtriple in the bystander xenograft tumor model study (data not shown). As a single gene, the bacterial mutant bCD1525 is therefore the most effective CD mutant tested and its use in cancer gene therapy may allow for reduced, less debilitating 5FC treatment doses for tumor ablation. Based on previous work with these variants[9, 10] and the reports by many other investigators regarding the ability to sensitize many different cancer cell lines to 5FC using CD or CD/UPRT fusions[3-7, 13, 15-20, 26,27, 29-37,38] the results shown here will likely be relevant to a number of cancer types.
As current delivery systems pose a major limitation for successful cancer gene therapy, a robust bystander effect is crucial for its successful clinical application[1]. By introducing UPRT activity to the CD/5FC system, it is feasible that more efficient conversion of 5FU to its active metabolites may diminish available, diffusible 5FU pools thereby reducing the bystander effect and in turn its therapeutic value. However, as described above, for wild-type CD/UPRT constructs the presence of UPRT appears to enhance bystander activity in vitro. This phenomenon is not likewise observed when the CD mutants are present in the CD/UPRT fusion constructs.
Although it displayed the greatest reduction in IC50 value of all constructs tested, bCD1525/bUPRT lacked significant bystander activity in vitro. Prior HPLC studies measuring 5FU levels released into culture medium revealed SW480 colon cancer cells expressing wild-type bCD or bCD/bUPRT produce similar 5FU levels; yet, the bCD/bUPRT fusion displayed superior bystander activity to bCD alone[30]. While the concerted action of bCD and bUPRT may account for the ability of bCD1525/bUPRT to confer increased cancer cell 5FC sensitivity in vitro, it may also account for the decreased bystander activity seen in vitro and in vivo for bCD1525/bUPRT relative to bCD1525 alone. In this case, the transport of 5FUMP and other downstream metabolites to neighboring cells may be restricted by the inability of charged molecules to diffuse across membranes, thus diminishing the bystander activity. Our in vitro and in vivo bystander results are consistent with this hypothesis.
Like bCD1525/bUPRT, both yCDdouble/yUPRT and yCDtriple/yUPRT displayed significant reductions in IC50 values compared to their CD mutant counterparts. However, unlike bCD1525/bUPRT, the yeast fusion mutants exhibited increased bystander activity in vitro (data not shown). This increased bystander activity may be directly related to elevated CD activity and extracellular 5FU levels. For example, previous studies have shown that cells co-expressing yCD and yUPRT enzymes fail to exhibit enhanced bystander cell killing, or increased 5FU levels, relative to those expressing yCD alone [17]. Enhanced yCD half-life and melting temperature may also be contributing factors. Indeed, the fusion of yCD with other proteins has been shown to prolong yCD half-life and increase its thermostability[35, 36]. For instance, fusion of wild-type yCD to the hyaluronan binding domain of tumor necrosis factor alpha-stimulated gene-6 (TSG-6) prolonged the enzymatic activity of yCD at physiological temperatures and increased its apparent melting temperature[35]. This may explain the similar IC50 values seen between wild-type yCD/yUPRT and the thermostabilized yCDtriple. Our in vitro results also point to the overlay of yCD mutants onto yCD/yUPRT as a potential means to further elevate yCD melting temperature or half-life activity as the mutant fusions displayed lower IC50 values relative to the yCD mutants alone. This was more prevalent for yCDdouble/yUPRT presumably due to the already increased half-life of yCDtriple (117 hrs at 50°C) compared to yCDdouble (21 hrs at 50°C)[8]. Although superior in cell killing and bystander activity in vitro, decreased amounts of diffusible 5FU due to UPRT activity may account for the decreased therapeutic value of yCDdouble/yUPRT compared to yCDdouble alone in vivo. We are currently investigating 5FC metabolite levels produced from mutant CD and CD/UPRT fusion transfected cells using HPLC analysis to ascertain the metabolic flux in these various transfected cells lines as a means to correlate antimetabolite levels with cell killing potency.
In conclusion, this comparative analysis study demonstrates that while enzyme and pathway engineering approaches separately enhance 5FC-mediated cancer cell death for the CD/5FC system, their concomitant use may negatively influence the system's therapeutic value. With its enhanced catalytic properties and striking antitumor activity at low prodrug doses, the bacterial mutant bCD1525 has promising clinical application and is a prime candidate for use in combinatorial strategies. Most notably, bCD1525 may selectively enhance adjuvant radiation therapy as its product, 5FU, is a potent radiosensitizer[2, 37]. Likewise antineoplastic agents[19], immunomodulatory molecules[38,39], and oncolytic virus delivery vehicles[40] have all enhanced the therapeutic outcome of the CD/5FC treatment strategy. Thus, a multimodal approach using bCD1525 may move suicide gene therapy from a promising, alternative anticancer strategy to a potent treatment modality for complete tumor ablation.
Acknowledgments
This work was supported by the National Institutes of Health through grants R01CA85939 (M.E. Black) and T32GM008336 (A.J. Johnson).
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Olga G, Gabi UD. Gene directed enzyme/prodrug therapy of cancer: historical appraisal and future prospectives. J Cell Physiol. 2001;187(1):22–36. doi: 10.1002/1097-4652(2001)9999:9999<::AID-JCP1060>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 2.Longley DB, Harkin DP, Johnston PG. 5-Fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3(5):330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 3.Huber BE, Austin EA, Richards CA, Davis ST, Good SS. Metabolism of 5-fluorocytosine to 5-fluorouracil in human colorectal tumor cells transduced with the cytosine deaminase gene: significant antitumor effects when only a small percentage of tumor cells express cytosine deaminase. Proc Natl Acad Sci U S A. 1994;91(17):8302–8306. doi: 10.1073/pnas.91.17.8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mullen CA, Coale MM, Lowe R, Blaese RM. Tumors expressing the cytosine deaminase suicide gene can be eliminated in vivo with 5-fluorocytosine and induce protective immunity to wild type tumor. Cancer Res. 1994;54(6):1503–1506. [PubMed] [Google Scholar]
- 5.Austin EA, Huber BE. A first step in the development of gene therapy for colorectal carcinoma: cloning, sequencing, and expression of Escherichia coli cytosine deaminase. Mol Pharmacol. 1993;43(3):380–387. [PubMed] [Google Scholar]
- 6.Kievit E, Bershad E, Ng E, et al. Superiority of yeast over bacterial cytosine deaminase for enzyme/prodrug gene therapy in colon cancer xenografts. Cancer Res. 1999;59(7):1417–1421. [PubMed] [Google Scholar]
- 7.Kievit E, Nyati MK, Ng E, et al. Yeast cytosine deaminase improves radiosensitization and bystander effect by 5-fluorocytosine of human colorectal cancer xenografts. Cancer Res. 2000;60(23):6649–6655. [PubMed] [Google Scholar]
- 8.Korkegian A, Black ME, Baker D, Stoddard BL. Computational thermostabilization of an enzyme. Science. 2005;308(5723):857–860. doi: 10.1126/science.1107387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stolworthy TS, Korkegian AM, Willmon CL, et al. Yeast cytosine deaminase mutants with increased thermostability impart sensitivity to 5-fluorocytosine. J Mol Biol. 2008;377(3):854–869. doi: 10.1016/j.jmb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuchita M, Ardiani A, Zhao L, Serve K, Stoddard BL, Black ME. Bacterial cytosine deaminase mutants created by molecular engineering show improved 5-fluorocytosine-mediated cell killing in vitro and in vivo. Cancer Res. 2009;69(11):4791–4799. doi: 10.1158/0008-5472.CAN-09-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lundegaard C, Jensen KF. Kinetic mechanism of uracil phosphoribosyltransferase from Escherichia coli and catalytic importance of the conserved proline in the PRPP binding site. Biochemistry. 1999;38(11):3327–3334. doi: 10.1021/bi982279q. [DOI] [PubMed] [Google Scholar]
- 12.Kern L, de Montigny J, Jund R, Lacroute F. The FUR1 gene of Saccharomyces cerevisiae: cloning, structure and expression of wild-type and mutant alleles. Gene. 1990;88(2):149–157. doi: 10.1016/0378-1119(90)90026-n. [DOI] [PubMed] [Google Scholar]
- 13.Kanai F, Kawakami T, Hamada H, et al. Adenovirus-mediated transduction of Escherichia coli uracil phosphoribosyltransferase gene sensitizes cancer cells to low concentrations of 5-fluorouracil. Cancer Res. 1998;58(9):1946–1951. [PubMed] [Google Scholar]
- 14.Vauthey JN, de Marsh R, Cendan JC, Chu NM, Copeland EM. Arterial therapy of hepatic colorectal metastases. Br J Surgery. 1996;83:447–455. doi: 10.1002/bjs.1800830405. [DOI] [PubMed] [Google Scholar]
- 15.Harris JD, Gutierrez AA, Hurst HC, Sikora K, Lemoine NR. Gene therapy for cancer using tumour-specific prodrug activation. Gene Ther. 1994;1(3):170–175. [PubMed] [Google Scholar]
- 16.Tiraby M, Cazaux C, Baron M, Drocourt D, Reynes JP, Tiraby G. Concomitant expression of E. coli cytosine deaminase and uracil phosphoribosyltransferase improves the cytotoxicity of 5-fluorocytosine. FEMS Microbiol Lett. 1998;167(1):41–49. doi: 10.1111/j.1574-6968.1998.tb13205.x. [DOI] [PubMed] [Google Scholar]
- 17.Erbs P, Regulier E, Kintz J, et al. In vivo cancer gene therapy by adenovirus-mediated transfer of a bifunctional yeast cytosine deaminase/uracil phosphoribosyltransferase fusion gene. Cancer Res. 2000;60(14):3813–3822. [PubMed] [Google Scholar]
- 18.Xing L, Sun X, Deng X, et al. Expression of the bifunctional suicide gene CDUPRT increases radiosensitization and bystander effect of 5-FC in prostate cancer cells. Radiother Oncol. 2009;92(3):345–352. doi: 10.1016/j.radonc.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gopinath P, Ghosh S. Apoptotic induction with bifunctional E. coli cytosine deaminase-uracil phosphoribosyltransferase mediated suicide gene therapy is synergized by curcumin treatment in vitro. Mol Biotechnol. 2008;39(1):39–48. doi: 10.1007/s12033-007-9026-3. [DOI] [PubMed] [Google Scholar]
- 20.Koyama F, Sawada H, Hirao T, Fujii H, Hamada H, Nakano H. Combined suicide gene therapy for human colon cancer cells using adenovirus-mediated transfer of Escherichia coli cytosine deaminase gene and Escherichia coli uracil phosphoribosyltransferase gene with 5-fluorocytosine. Cancer Gene Ther. 2000;7(7):1015. doi: 10.1038/sj.cgt.7700189. [DOI] [PubMed] [Google Scholar]
- 21.Andersen PS, Smith JM, Mygind B. Characterization of the upp gene encoding uracil phosphoribosyltransferase of Escherichia coli K12. Eur J Biochem. 1992;204(1):51–56. doi: 10.1111/j.1432-1033.1992.tb16604.x. [DOI] [PubMed] [Google Scholar]
- 22.Arsene-Ploetze F, Nicoloff H, Kammerer B, Martinussen J, Bringel F. Uracil salvage pathway in Lactobacillus plantarum: transcription and genetic studies. J Bacteriol. 2006;188(13):4777–4786. doi: 10.1128/JB.00195-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brady WA, Kokoris MS, Fitzgibbon M, Black ME. Cloning, characterization, and modeling of mouse and human guanylate kinases. J Biol Chem. 1996;271(28):16734–16740. doi: 10.1074/jbc.271.28.16734. [DOI] [PubMed] [Google Scholar]
- 24.Mahan SD, Ireton GC, Stoddard BL, Black ME. Alanine-scanning mutagenesis reveals a cytosine deaminase mutant with altered substrate preference. Biochemistry. 2004;43(28):8957–8964. doi: 10.1021/bi049720z. [DOI] [PubMed] [Google Scholar]
- 25.Mahan SD, Ireton GC, Knoeber C, Stoddard BL, Black ME. Random mutagenesis and selection of Escherichia coli cytosine deaminase for cancer gene therapy. Protein Eng Des Sel. 2004;17(8):625–633. doi: 10.1093/protein/gzh074. [DOI] [PubMed] [Google Scholar]
- 26.Kuriyama S, Masui K, Sakamoto T, et al. Bystander effect caused by cytosine deaminase gene and 5-fluorocytosine in vitro is substantially mediated by generated 5-fluorouracil. Anticancer Res. 1998;18(5A):3399–3406. [PubMed] [Google Scholar]
- 27.Lawrence TS, Rehemtulla A, Ng EY, Wilson M, Trosko JE, Stetson PL. Preferential cytotoxicity of cells transduced with cytosine deaminase compared to bystander cells after treatment with 5-flucytosine. Cancer Res. 1998;58(12):2588–2593. [PubMed] [Google Scholar]
- 28.Franken NAP, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1(5):2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 29.Graepler F, Lemken ML, Wybranietz WA, et al. Bifunctional chimeric SuperCD suicide gene - YCD:YUPRT fusion is highly effective in a rat hepatoma model. World J Gastoenterol. 2005;11(44):6910–6919. doi: 10.3748/wjg.v11.i44.6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung-Faye GA, Chen MJ, Green NK, et al. In vivo gene therapy for colon cancer using adenovirus-mediated transfer of the fusion gene cytosine deaminase and uracil phosphoribosyltransferase. Gene Ther. 2001;8(20):1547–1554. doi: 10.1038/sj.gt.3301557. [DOI] [PubMed] [Google Scholar]
- 31.Bourbeau D, Lavoie G, Nalbantoglu J, Massie B. Suicide gene therapy with an adenovirus expressing the fusion gene CD∷UPRT in human glioblastomas: different sensitivities correlate with p53 status. J Gene Med. 2004;6(12):1320–1332. doi: 10.1002/jgm.611. [DOI] [PubMed] [Google Scholar]
- 32.Pandha HS, Martin LA, Rigg A, et al. Genetic prodrug activation therapy for breast cancer: a phase I clinical trial of erbB-2-directed suicide gene expression. J Clin Oncol. 1999;17(7):2180–2189. doi: 10.1200/JCO.1999.17.7.2180. [DOI] [PubMed] [Google Scholar]
- 33.Crystal RG, Hirschowitz E, Lieberman M, et al. Phase I study of direct administration of a replication deficient adenovirus vector containing the E. coli cytosine deaminase gene to metastatic colon carcinoma of the liver in association with the oral administration of the pro-drug 5-fluorocytosine. Hum Gene Ther. 1997;8(8):985–1001. doi: 10.1089/hum.1997.8.8-985. [DOI] [PubMed] [Google Scholar]
- 34.Freytag SO, Stricker H, Pegg J, et al. Phase I study of replication-competent adenovirus-mediated double-suicide gene therapy in combination with conventional-dose three-dimensional conformal radiation therapy for the treatment of newly diagnosed, intermediate to high-risk prostate cancer. Cancer Res. 2003;63(21):7497–7506. [PubMed] [Google Scholar]
- 35.Park JI, Cao L, Platt VM, et al. Antitumor therapy mediated by 5-fluorocytosine and a recombinant fusion protein containing TSG-6 hyaluronan binding domain and yeast cytosine deaminase. Mol Pharm. 2009;6(3):801–812. doi: 10.1021/mp800013c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Senter PD, Su PC, Katsuragi T, et al. Generation of 5-fluorouracil from 5-fluorocytosine by monoclonal antibody-cytosine deaminase conjugates. Bioconjug Chem. 1991;2(6):447–451. doi: 10.1021/bc00012a012. [DOI] [PubMed] [Google Scholar]
- 37.Khil MS, Kim JH, Mullen CA, Kim SH, Freytag SO. Radiosensitization by 5-fluorocytosine of human colorectal carcinoma cells in culture transduced with cytosine deaminase gene. Clin Cancer Res. 1996;2(1):53–57. [PubMed] [Google Scholar]
- 38.Khatri A, Husaini Y, Ow K, Chapman J, Russell PJ. Cytosine deaminase-uracil phosphoribosyltransferase and interleukin (IL)-12 and IL-18: a multimodal anticancer interface marked by specific modulation in serum cytokines. Clin Cancer Res. 2009;15(7):2323–2334. doi: 10.1158/1078-0432.CCR-08-2039. [DOI] [PubMed] [Google Scholar]
- 39.Ito S, Natsume A, Shimato S, et al. Human neural stem cells transduced with IFN-[beta] and cytosine deaminase genes intensify bystander effect in experimental glioma. Cancer Gene Ther. 2009 doi: 10.1038/cgt.2009.80. [DOI] [PubMed] [Google Scholar]
- 40.Foloppe J, Kintz J, Futin N, et al. Targeted delivery of a suicide gene to human colorectal tumors by a conditionally replicating vaccinia virus. Gene Ther. 2008;15(20):1361–1371. doi: 10.1038/gt.2008.82. [DOI] [PubMed] [Google Scholar]


