Abstract
Objectives
HIV disease is associated with increased arterial stiffness, which may be related to inflammation provoked by HIV-related immune perturbation. We assessed the association of T cell markers of immune activation and immunosenescence with carotid artery stiffness among HIV-infected women.
Methods
Among 114 HIV-infected and 43 HIV-uninfected women, we measured CD4+ and CD8+ T cell populations expressing activation (CD38+HLA-DR+) and senescence (CD28-CD57+) markers. We then related these measures of immune status with parameters of carotid artery stiffness, including decreased distensibility, and increased Young’s elastic modulus, as assessed by B-mode ultrasound.
Results
HIV infection was associated with increased CD4+ T cell activation, CD8+ T cell activation and CD8+ T cell senescence. Among HIV-infected women, adjusted for age, HIV medications, and vascular risk factors, higher CD4+CD38+HLA-DR+ T cell frequency was associated with decreased carotid artery distensibility (β= −2.00, 95% confidence interval [CI]= −3.86,−0.14, P=0.04) and increased Young’s modulus (β=1.00, 95% CI=0.03,1.97, P=0.04). These associations were affected little by further adjustment for CD4+ T cell count and viral load. Among HIV-infected women, higher frequencies of immunosenescent T cells, including CD4+CD28-CD57+ and CD8+CD28-CD57+ T cells, were also associated with decreased arterial distensibility. Among HIV-uninfected women, frequencies of activated or senescent T cells were not significantly associated with measures of carotid stiffness.
Discussion
T cell activation and senescence are associated with arterial stiffness, suggesting that pro-inflammatory populations of T cells may produce functional or structural vascular changes in HIV-infected women.
Arterial stiffness is often increased in the presence of established cardiovascular risk factors including aging, diabetes, hypertension and chronic kidney disease [1]. HIV infection, particularly when associated with severe immunodeficiency, has recently been added to the list of conditions known to be associated with vascular stiffening in adults [2–5]. This effect of HIV infection is evident even in HIV-infected children, who have decreased carotid artery distensibility without concomitant increases in carotid artery wall thickness [6].
Many of the diseases that produce premature arterial stiffness, including HIV infection and Kawasaki disease [7], are inflammatory conditions. Markers of inflammation have been correlated with the degree of vascular stiffness in various populations including stroke survivors [8], patients with renal disease [9] and obstructive sleep apnea [10], and apparently healthy adults [11, 12]. We hypothesized that increased arterial stiffness in patients infected with HIV may be associated with pro-inflammatory T cell subsets, including T cells expressing markers of activation and senescence. In the setting of HIV infection, activated T cells are abundant and are thought to be an important source of inflammation. Activated CD4+ T cells are known to localize to arterial tissues in regions affected by vascular disease[13] and may in turn produce inflammatory mediators which activate matrix-degrading enzymes such as matrix metalloproteinase-1 (MMP-1), MMP-3, and MMP-9 [14–16]. In HIV-infected persons, senescence markers are found on a large frequency of CD8+ T cells, and to a lesser extent on CD4+ T cells. Senescent T cells are apoptosis-resistant and, like activated T cells, may secrete pro-inflammatory mediators. We recently showed that among HIV-infected patients, higher frequencies of T cells expressing markers of activation (CD38, HLA-DR) and senescence (presence of CD57, absence of CD28) were associated with greater arterial wall thickness [17]. In the present study, we examined whether increased carotid artery stiffness, which we assessed from measurements of arterial diameters obtained by B-mode ultrasound, was also related to T cell activation and senescence.
Methods
Study population
The Women’s Interagency HIV Study (WIHS) is a prospective multicenter study of 3766 HIV-infected and HIV-uninfected women at six urban US field centers. HIV-infected and HIV-uninfected WIHS participants were initially recruited in 1994–1995, and additional women were added to the cohort in 2001–2002. All WIHS participants are invited to complete study visits every six months for collection of biological specimens, questionnaire data and clinical measurements. In April 2004, all WIHS participants were invited to participate in a carotid artery ultrasound substudy, which was completed by ~75% of both HIV-infected and HIV-uninfected women. Institutional Review Board approval and informed consent were obtained.
Carotid artery ultrasound
High resolution B-mode carotid artery ultrasound was used to image the far wall of the right common carotid artery. Standardized carotid artery ultrasound images were centrally measured by automated computerized edge detection software (Prowin, Patents, 2005, 2006)[17–20]. We obtained measurements of right common carotid artery diameters at systole (DS) and diastole (DD). Right distal common carotid artery intima-media thickness (cIMT) was assessed [17, 20]. Blood pressure was measured in the brachial artery at the same time as carotid ultrasound (average of five measurements) in order to measure systolic and diastolic pressures and pulse pressure (PP). We calculated two indices of arterial stiffness, including distensibility and Young’s elastic modulus. We calculated carotid artery distensibility as:
in units of (10−6 * Newtons−1 * meters2)[2, 19, 21]. We estimated Young’s elastic modulus [19], in units of (105 * Newtons * meters−2), as: PP/DD × 0.5 × DD/cIMTD, where PP = pulse pressure, DD = percent arterial dilation over the cardiac cycle, DD = arterial diameter at diastole, and cIMTD = common carotid artery intima-media thickness at diastole. From a repeated-measures study we estimated the coefficient of variation as 1.8% for cIMT (intraclass correlation coefficient [ICC] = 0.98), 2.2% for carotid diameters (ICC = 0.96), and 8.8% for blood pressures (ICC = 0.65 – 0.73).
Laboratory assays
Plasma HIV RNA levels were quantified using nucleic acid sequence based amplification commercial assays, and total peripheral CD4+ T cell counts were measured with standard flow cytometric methods. C-reactive protein levels were measured using nephelometry (Dade Behring, Deerfield, IL). Laboratory values included glucose, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), triglyceride, and low-density lipoprotein cholesterol (LDL-c) levels, which were measured centrally. T cell activation (CD38+, HLA-DR+) and senescence (CD28-, CD57+) markers were measured by immunophenotyping performed at the UCSF Core Immunology Laboratory, as previously described[17], for a random sample of 114 HIV-infected women and 43 HIV-uninfected women. HIV-infected and HIV-uninfected groups were matched by age and race/ethnicity, and represented a sample of participants 40 years and older who were enrolled in the WIHS cohort, were free of clinical vascular disease, had sufficient peripheral blood mononuclear cells (PBMCs) for flow cytometry measurements, and had available carotid ultrasound measurements within several months of collection of PBMCs.
Clinical variables
Key cardiovascular and HIV-related covariates were obtained by self-report and direct measurements. Seated blood pressure was measured using a standardized protocol. Hypertension was defined as either systolic blood pressure >=140 mm Hg, diastolic blood pressure >=90 mm Hg, or self-reported physician’s diagnosis of hypertension. Diabetes was defined as either fasting glucose levels >=126 mg/dL, or self-reported physician’s diagnosis of diabetes. Body mass index was calculated as weight in kilograms divided by the square of height in meters. We defined use of major drug categories, including protease inhibitors (PIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs) and nucleoside reverse transcriptase inhibitors (NRTIs), using medication questionnaire data.
Data analyses
CD4+ T cell activation was defined as % of CD4+ T cells expressing CD38 and HLA-DR, and CD8+ T cell activation as % of CD8+ T cells expressing CD38 and HLA-DR. CD4+ T cell senescence was defined as % of CD4+ T cells expressing CD57 and with an absence of CD28, and CD8+ T cell senescence was defined as % of CD8+ T cells expressing CD57 and with an absence of CD28. We examined Pearson correlations between the two different measures of carotid artery stiffness, and among frequencies of activated T cells and senescent T cells, C-reactive protein levels, total CD4+ T cell counts and HIV RNA measurements. Logarithmic transformations were applied to T cell variables, HIV RNA levels and C-reactive protein levels, whereas carotid artery stiffness parameters were untransformed. To examine the association of T cell activation and senescence with carotid artery distensibility and Young’s modulus, multivariable linear regression models were used, with vascular stiffness parameters (distensibility and Young’s elastic modulus) as the dependent variables and frequencies of activated or senescent T cells as the main independent variables of interest. Lower levels of distensibility and higher values of Young’s modulus are indicative of increased vascular stiffness. Multivariable models included adjustment for age, smoking, body mass index, use of major classes of antiretroviral medications, and standard cardiovascular risk factors including HDL-c, LDL-c, and plasma glucose. In a subsequent model, we adjusted for CD4+ T cell count and HIV RNA. C-reactive protein level was also examined both as an adjustment variable, and also as an independent predictor variable in relation to carotid artery distensibility and Young’s elastic modulus. Finally, we examined whether carotid artery stiffness was increased in the group that had both T cell activation and T cell senescence above the median values.
Among HIV-infected women, comparing individuals above versus below the median frequency of activated or senescent T cells, we had 80% power to detect a 0.52 standard deviation difference in carotid stiffness outcomes, while among HIV-uninfected women the detectable difference was 0.86 standard deviations. Analyses were performed using Stata version 11 (StataCorp LP, College Station, TX), SAS (version 9.2, SAS Institute, Cary, NC) and GraphPad Prism version 5.00 (GraphPad Software, San Diego, CA). All statistical tests were two-sided.
Results
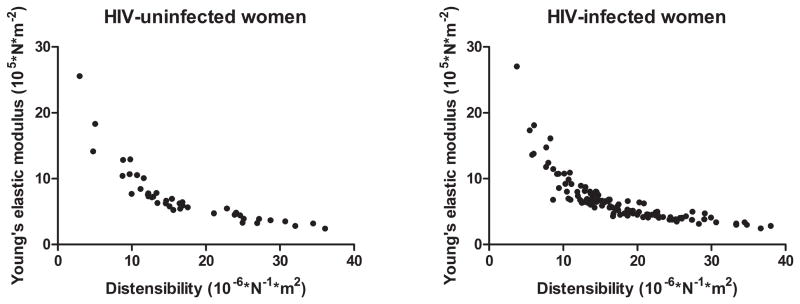
Among HIV-infected women, 36% were not currently receiving antiretroviral treatment, 39% were treated and had detectable viremia, and 25% were treated and did not have detectable HIV. As previously described[17], HIV-infected women had higher frequencies of activated CD4+ and CD8+ T cells, and higher frequency of senescent CD8+ T cells, as compared with HIV-uninfected women (Table 1). Other HIV-related and cardiovascular variables appear in Table 1. Pearson correlations between carotid artery distensibility and Young’s elastic modulus were in the range of r = −0.75 to −0.80 (Figure 1).
Table 1.
Characteristics of HIV-infected women and HIV-uninfected women
| HIV-uninfected women | HIV-infected women | |||||
|---|---|---|---|---|---|---|
| N = 43 | N = 114 | |||||
| Median | IQR | Median | IQR | P | ||
| Age, years | 47 | 42 – 51 | 46 | 43 – 50 | 0.92 | |
| CD4+CD38+DR+ T cells, %* | 2.5 | 1.7 – 3.1 | 7.7 | 4.6 – 14.0 | <0.01 | |
| CD8+CD38+DR+ T cells, %* | 7.3 | 4.2 – 13.1 | 28.9 | 17.6 – 41.2 | <0.01 | |
| CD4+CD28-CD57+ T cells, %* | 1.8 | 0.3 – 4.7 | 2.5 | 0.7 – 6.6 | 0.07 | |
| CD8+CD28-CD57+ T cells, %* | 22.7 | 12.8 – 40.0 | 35.8 | 25.1 – 46.8 | <0.01 | |
| C-reactive protein, μg/mL | 3.1 | 1.5 – 6.4 | 2.7 | 1.0 – 6.3 | 0.50 | |
| Current viral load/mL in thousands | n/a | 1.3 | 0.08 – 16 | n/a | ||
| Peak viral load/mL in thousands | n/a | 59 | 15 – 290 | n/a | ||
| Current CD4+ T cell count, cells/mm3 | 965 | 812 – 1,298 | 384 | 227 – 582 | <0.01 | |
| Nadir CD4+ T cell count, cells/mm3 | 782 | 676 – 912 | 210 | 109 – 339 | <0.01 | |
| LDL-c, mg/dL | 104 | 80 – 117 | 96 | 79 – 113 | 0.23 | |
| HDL-c, mg/dL | 56 | 44 – 64 | 45 | 33 – 56 | <0.01 | |
| Total cholesterol, mg/dL | 177 | 156 – 201 | 168 | 146 – 196 | 0.16 | |
| Plasma glucose, mg/dL | 91 | 80 – 102 | 86 | 80 – 96 | 0.31 | |
| BMI categories | <25 | 26% | 32% | 0.05 | ||
| 25–30 | 26% | 39% | ||||
| >30 | 48% | 29% | ||||
| No. of PIs used (current) | 0 | n/a | 55% | n/a | ||
| 1 | 27% | |||||
| 2 | 18% | |||||
| Any NRTIs use (current) | n/a | 63% | n/a | |||
| Any NNRTI use (current) | n/a | 24% | n/a | |||
| History of diabetes | 33% | 22% | 0.15 | |||
| Lipid-lowering drug use | 2% | 7% | 0.26 | |||
| Hypertension | 44% | 40% | 0.66 | |||
| Hepatitis C antibody positive | 40% | 49% | 0.28 | |||
| Current smoking | 70% | 48% | 0.02 | |||
| Race | White/Other | 9% | 9% | 0.88 | ||
| Hispanic | 23% | 27% | ||||
| African American | 68% | 64% | ||||
IQR, interquartile range; BMI, body mass index; HAART, highly active antiretroviral therapy; PI, protease inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor
Data on CD38+HLA-DR+ and CD28-CD57+ subsets were expressed as percent of T cells expressing these markers.
Figure 1. Scatterplot of carotid artery distensibility and Young’s elastic modulus.
Left panel, HIV-uninfected women. Right panel, HIV-infected women. Pearson correlations between carotid artery distensibility and Young’s elastic modulus were r = −0.79 (95% confidence interval = −0.88 to −0.65, p < 0.0001) among HIV-uninfected women and r = −0.76 (95% confidence interval = −0.83 to −0.67, p < 0.0001) among HIV-infected women.
Correlation among immune status, C-reactive protein level, and HIV RNA
Low CD4+ T cell count was associated with increased activation of CD4+ T cells (r = −0.72, P < 0.0001, and to a lesser extent increased activation of CD8+ T cells (r = −0.32, P < 0.01) (Table 2). HIV RNA was positively correlated with CD4+ and CD8+ T cell activation (r = 0.44 – 0.46, P < 0.0001). C-reactive protein levels had no significant correlation with T cell parameters or HIV RNA.
Table 2.
Correlation of T cell activation, T cell senescence, total peripheral CD4+ T cell count, HIV RNA and C-reactive protein among HIV-infected women
| CD8+ T cell activation (CD38+HLA- DR+) | CD4+ T cell senescence (CD28-CD57+) | CD8+ T cell senescence (CD28-CD57+) | CD4+ count | HIV RNA | C-reactive protein | ||
|---|---|---|---|---|---|---|---|
| CD4+ T cell activation (CD38+HLA-DR+) | r | 0.66 | 0.29 | 0.23 | −0.72 | 0.44 | −0.01 |
| P | <0.0001 | 0.01 | 0.01 | <0.0001 | <0.0001 | 0.92 | |
| CD8+ T cell activation (CD38+HLA-DR+) | r | 0.09 | 0.45 | −0.32 | 0.46 | 0.03 | |
| P | 0.32 | <0.0001 | 0.01 | <0.0001 | 0.75 | ||
| CD4+ T cell senescence (CD28-CD57+) | r | 0.41 | −0.14 | −0.08 | 0.01 | ||
| P | <0.0001 | 0.13 | 0.41 | 0.94 | |||
| CD8+ T cell senescence (CD28-CD57+) | r | −0.02 | 0.02 | 0.07 | |||
| P | 0.84 | 0.80 | 0.45 | ||||
| CD4+ count | r | −0.49 | 0.15 | ||||
| P | <0.0001 | 0.13 | |||||
| HIV RNA | r | −0.09 | |||||
| P | 0.36 |
Pearson correlations were obtained, using log-transformed data for T cell activation, T cell senescence, CD4+ T cell count, HIV RNA and C-reactive protein.
T cell activation and senescence as predictors of carotid artery stiffness
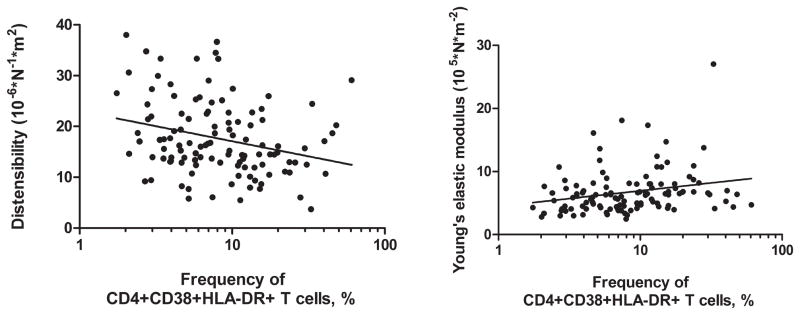
Among HIV-infected women, higher frequencies of CD4+CD38+HLA-DR+ T cells were associated with decreased carotid artery distensibility and with increased Young’s elastic modulus (Figure 2), and these associations were statistically significant (P = 0.04) after adjustment for age and other cardiovascular risk factors (Table 3). In subsequent models that also included HIV RNA and CD4+ T cell count as adjustment variables, the point estimates of association were almost completely unchanged, although confidence intervals and p-values were slightly larger (Table 4). In the HIV-infected group, women with higher frequencies of CD4+CD28-CD57+ and CD8+CD28-CD57+ T cells had reduced carotid artery distensibility after adjustment for cardiovascular and HIV-related variables (Table 4). However, significant associations between T cell senescence markers and arterial stiffness were not seen when Young’s modulus rather than distensibility was defined as the outcome variable (Table 3 and Table 4).
Figure 2. Among HIV-infected women, correlation of % CD4+CD38+HLA-DR+ with carotid artery distensibility (left panel) and Young’s elastic modulus (right panel).
Linear regression was used to estimate intercepts and slopes as follows: distensibility = 23.0 – 5.9 log(% CD4+CD38+HLA-DR+), with 95% confidence intervals for the slope = −9.8 to −2.1. Young’s elastic modulus = 4.5 + 2.4 × log(% CD4+CD38+HLA-DR+), with 95% confidence intervals for the slope = 0.6 to 4.3.
Table 3.
Association of T cell activation (CD38+HLA-DR+) and senescence (CD28-CD57+) with carotid artery distensibility and Young’s elastic modulus among HIV-infected women
| Change in distensibility per unit change of predictor | 95% CI | P | Change in Young’s modulus per unit change of predictor | 95% CI | P | |
|---|---|---|---|---|---|---|
| CD4+CD38+HLA-DR+ T cells | −2.00 | −3.86, −0.14 | 0.04 | 1.00 | 0.03, 1.97 | 0.04 |
| CD8+CD38+HLA-DR+ T cells | −1.91 | −4.42, 0.60 | 0.13 | 0.85 | −0.46, 2.16 | 0.20 |
| CD4+CD28-CD57+ T cells | −0.85 | −1.76, 0.05 | 0.06 | 0.29 | −0.18, 0.77 | 0.23 |
| CD8+CD28-CD57+ T cells | −2.22 | −5.13, 0.69 | 0.13 | 0.56 | −0.97, 2.09 | 0.47 |
CI, confidence interval.
Change in stiffness parameter (distensibility or Young’s elastic modulus) associated with log-transformed values of T cell activation and senescence was estimated in linear regression models, after adjustment for age, body mass index, smoking, use of antiretroviral medications, and circulating levels of high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and glucose.
Table 4.
Association of T cell activation and senescence with measures of carotid artery stiffness among HIV-infected women, after adjustment for total CD4+ T cell count and HIV RNA
| Change in distensibility per unit change in predictor | 95% confidence interval | P | Change in Young’s modulus per unit change in predictor | 95% confidence interval | P | |
|---|---|---|---|---|---|---|
| CD4+CD38+HLA-DR+ T cells | −2.12 | −4.14, −0.11 | 0.04 | 1.00 | −0.06, 2.05 | 0.06 |
| CD8+CD38+HLA-DR+ T cells | −1.73 | −3.96, 0.49 | 0.13 | 0.80 | −0.36, 1.96 | 0.18 |
| CD4+CD28-CD57+ T cells | −0.78 | −1.57, 0 | 0.05 | 0.32 | −0.09, 0.73 | 0.13 |
| CD8+CD28-CD57+ T cells | −2.61 | −5.15, −0.07 | 0.04 | 0.88 | −0.46, 2.21 | 0.19 |
| CD4+ T cell count (per 100 cells/mL) | 0.01 | −0.04, 0.06 | 0.78 | 0.01 | −0.02, 0.03 | 0.73 |
| HIV RNA (per log10 copies/mL) | −0.65 | −1.94, 0.65 | 0.32 | 0.40 | −0.28, 1.07 | 0.24 |
Adjusted for age, body mass index, smoking, use of antiretroviral medications, CD4+ count, HIV RNA, and T cell activation or senescence
We then examined the association of carotid artery stiffness with frequencies of activated CD4+ T cells and senescent CD4+ T cells when examined jointly in the same model. Mean carotid artery distensibility was 16.6 ×10−6 m2/N among HIV-infected women who were above the median value of both CD4+CD38+HLA-DR+ T cell frequency and CD4+CD28-CD57+ T cell frequency. In comparison, mean distensibility was in the range of 19.1 ×10−6 to 19.6 ×10−6 m2/N among the groups that only had CD4+CD38+HLA-DR+ T cell frequency above the median, that only had CD4+CD28-CD57+ T cell frequency above the median, or that had neither above the median. Adjusted for age and cardiovascular risk factors, comparing HIV-infected women with both CD4+ T cell activation and CD4+ T cell senescence above the median values versus those with both CD4+ T cell activation and senescence below the median values, the adjusted difference in distensibility was −2.5 ×10−6 m2/N (95% confidence interval −6.4 ×10−6 to 1.3 ×10−6, P = 0.21).
Adjustment for C-reactive protein had minimal effect on the associations of T cell activation and senescence markers with carotid artery stiffness, nor was C-reactive protein level a significant predictor of carotid artery distensibility or Young’s modulus (data not shown).
Among HIV-uninfected women, no significant associations of T cell activation or senescence with carotid distensibility or Young’s modulus were observed (data not shown).
Discussion
Among HIV-infected women, cellular markers of immune activation (defined by presence of CD38 and HLA-DR on circulating CD4+ T cells) were associated with increased carotid artery stiffness. Arterial stiffness was defined as low levels of distensibility and high values of Young’s elastic modulus as measured by B-mode carotid artery ultrasound. The associations between activated CD4+ T cell frequency and vascular stiffness persisted after adjustment for HIV RNA and total peripheral CD4+ T cell count, suggesting that for a given CD4+ T cell count and viral load, higher CD4+ T cell activation was an independent predictor of vascular stiffness. The association between T cell activation and carotid artery stiffness was also shown to be independent of standard vascular risk factors, including plasma lipids, which are often abnormal in HIV-infected individuals. These results agree with prior evidence linking HIV disease with increased vascular stiffness, particularly among individuals with relatively severe immune perturbation. Further, they support the hypothesis that pro-inflammatory populations of T cells may produce functional and/or structural arterial changes in HIV-infected patients with relatively severe damage to the immune system.
HIV infected adults have elevated levels of circulating pro-inflammatory cytokines and a high frequency of circulating CD4+ and CD8+ T cells that express markers of chronic activation, including CD38 and HLA-DR. These T cell abnormalities may be only partially reversed with effective HIV treatment [22–24]. While many studies of HIV-infected individuals have shown an association between immunological complications of HIV infection and increased arterial wall thickness [17, 25, 26], our study supports prior evidence that other types of pathological vascular changes beyond intimal-medial thickening may develop as a result of chronic HIV infection. Vasculitides of various types, which are typically localized to specific vessels in persons at advanced stages of immunocompromise, and which are possibly causally related to T lymphocyte infiltration[27], are a relatively uncommon complication of HIV infection. More recently, studies have suggested that chronic HIV infection may produce changes in vascular tissues leading to loss of normal arterial elasticity and vasoreactivity. Carotid artery specimens from HIV-infected patients treated surgically for asymptomatic carotid artery stenosis may have degradation of elastic fibers and inflammatory infiltration of the vascular wall [28]. In addition, uncontrolled HIV replication is associated with reduced brachial artery reactivity, which suggests that impaired endothelial function may also contribute to increased vascular stiffness in HIV-infected persons [29]. In our data, CD4+CD38+HLA-DR+ T cell frequency was associated with carotid artery distensibility, which is calculated from changes in arterial diameters across the cardiac cycle, as well as with Young’s elastic modulus, which is a different parameter calculated from arterial diameters that also includes standardization to the arterial intima-media thickness. This study is further evidence of a link between HIV disease and vascular pathological changes that may be distinct from atherosclerosis and that occur independently of dyslipidemia and other cardiometabolic risk factors, which were controlled for as potential confounders.
This study has several limitations. First, the cross-sectional nature of the study makes it difficult to establish a cause and effect relationship. Although the most likely explanation for our observations is that inflammation in HIV infected women causes vascular changes, it is conceivable that vascular dysfunction could affect the immune system in some way. It is also possible that unmeasured factors associated with HIV infection may affect both vascular and T cell functions. Second, although the clinical importance of reduced carotid distensibility as well as other non-invasive measures of vascular stiffness has been shown in patient populations with premature vascular disease [1], the long-term importance of these vascular parameters in predicting future cardiovascular events among HIV-infected adults is unknown. Third, the study was limited to a population which, despite being female and relatively young, had a high burden of cardiovascular risk factors including obesity and smoking. Therefore the results may not be generalizable to men or to populations with different constellations of cardiovascular risk factors. Fourth, we did not have information on regulatory T cells, so were unable to assess the possibility of a confounding effect on the observed associations. Finally, these observations need to be corroborated in larger cohorts. We had relatively limited sample size, so our study only had adequate power to detect a moderate association between activation or senescence and carotid artery stiffness, particularly among HIV-uninfected women.
In summary, our findings suggest that measures of T cell activation, which are known to predict immunologic HIV disease progression [30, 31], are also associated with increased vascular stiffness among HIV-infected women. Neither cardiometabolic risk factors nor exposure to antiretroviral medications explained the finding between HIV-associated immune perturbation and vascular stiffness measures. This association was also independent of standard clinical measures of HIV disease status including total peripheral CD4+ T cell count and circulating HIV RNA levels. Therefore, our data support the hypothesis that pro-inflammatory populations of T cells may lead to functional or structural changes in the peripheral vasculature. Because effective antiretroviral treatment can reduce these markers of immune perturbation, although not fully reverse them, the findings support the rationale for treating HIV infection to limit immune system damage and also potentially to benefit cardiovascular health.
Acknowledgments
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington DC, Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange). The Helen Riaboff Whiteley Center at Friday Harbor Laboratories is gratefully acknowledged for facilitating the completion of this work.
Funding: This work was supported by the National Institute of Allergy and Infectious Diseases [grant numbers UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, UO1-AI-42590] and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development [grant number UO1-HD-32632]. This study was co- funded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Additional co-funding was provided by the National Heart, Lung and Blood Institute (grant numbers 1R01HL095140, 1R01HL083760 to R.C.K.). Funding support was also provided by the National Center for Research Resources (UCSF-CTSI grant number UL1 RR024131), NIH/NIAID funding to the UCSF-GIVI Center for AIDS Research (P30AI027763) and NCR funding to the UCSF Clinical and Translational Science Institute (UL1RR024131). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Statement of conclusions: Prior evidence suggests that patients infected with HIV may have increased risk of clinical cardiovascular events as well as subclinical structural and functional vascular changes. Among a cohort of HIV-infected women, we studied the association of carotid artery stiffness with expression of markers of T cell activation (CD38+HLA-DR+). Our findings suggest that activation of CD4+ T cells is associated with increased vascular stiffness among HIV-infected women. These findings are important because these immunologic perturbations almost universally affect patients with advanced HIV disease, and may also be elevated in those on effective antiretroviral therapy.
Disclosures: None
Prior presentation: The work described herein was presented, in part, at the 2010 Conference on Retroviruses and Opportunistic Infections (San Francisco).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blacher J, Pannier B, Guerin AP, et al. Carotid arterial stiffness as a predictor of cardiovascular and all-cause mortality in end-stage renal disease. Hypertension. 1998;32:570–574. doi: 10.1161/01.hyp.32.3.570. [DOI] [PubMed] [Google Scholar]
- 2.Seaberg EC, Benning L, Sharrett AR, et al. Association between human immunodeficiency virus infection and stiffness of the common carotid artery. Stroke. 2010;41:2163–2170. doi: 10.1161/STROKEAHA.110.583856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho JE, Deeks SG, Hecht FM, et al. Initiation of antiretroviral therapy at higher nadir CD4+ T-cell counts is associated with reduced arterial stiffness in HIV-infected individuals. AIDS. 2010;24:1897–1905. doi: 10.1097/QAD.0b013e32833bee44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lekakis J, Ikonomidis I, Palios J, et al. Association of highly active antiretroviral therapy with increased arterial stiffness in patients infected with human immunodeficiency virus. Am J Hypertension. 2009;22:828–834. doi: 10.1038/ajh.2009.90. [DOI] [PubMed] [Google Scholar]
- 5.Schillaci G, DeSocio GV, Pucci G, et al. Aortic stiffness in untreated adult patients with human immunodeficiency virus infection. Hypertension. 2008;52:308–313. doi: 10.1161/HYPERTENSIONAHA.108.114660. [DOI] [PubMed] [Google Scholar]
- 6.Bonnet D, Aggoun Y, Szezepanski I, Bellal N, Blanche S. Arterial stiffness and endothelial dysfunction in HIV-infected children. Aids. 2004;18:1037–1041. doi: 10.1097/00002030-200404300-00012. [DOI] [PubMed] [Google Scholar]
- 7.Ooyanagi R, Fuse S, Tomita H, et al. Pulse wave velocity and ankle brachial index in patients with Kawasaki disease. Pediatr Int. 2004;46:398–402. doi: 10.1111/j.1442-200x.2004.01929.x. [DOI] [PubMed] [Google Scholar]
- 8.Tuttolomondo A, DiRaimondo D, Pecoraro R, et al. Immune-inflammatory markers and arterial stiffness indexes in subjects with acute ischemic stroke. Atherosclerosis. 2010;213:311–318. doi: 10.1016/j.atherosclerosis.2010.08.065. [DOI] [PubMed] [Google Scholar]
- 9.Tseke P, Grapsa E, Stamatelopoulos K, et al. Atherosclerotic risk factors and carotid stiffness in elderly asymptomatic HD patients. Int Urol Nephrol. 2006;38:801–809. doi: 10.1007/s11255-006-9000-1. [DOI] [PubMed] [Google Scholar]
- 10.Kasai T, Inoue K, Kumagai T, et al. Plasma Pentraxin3 and Arterial Stiffness in Men With Obstructive Sleep Apnea. Am J Hyperten. 2010 doi: 10.1038/ajh.2010.248. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Yasmin, McEniery CM, Wallace S, et al. C-reactive protein is associated with arterial stiffness in apparently healthy individuals. Arterioscler Thromb Vasc Biol. 2004;24:969–974. doi: 10.1161/01.ATV.zhq0504.0173. [DOI] [PubMed] [Google Scholar]
- 12.Nagano M, Nakamura M, Sato K, et al. Association between serum C-reactive protein levels and pulse wave velocity: a population-based cross-sectional study in a general population. Atherosclerosis. 2005;180:189–195. doi: 10.1016/j.atherosclerosis.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Hansson GK, Holm J, Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989;135:169–175. [PMC free article] [PubMed] [Google Scholar]
- 14.Andersson J, Libby P, Hansson GK. Adaptive immunity and atherosclerosis. Clin Immunol. 2010;134:33–46. doi: 10.1016/j.clim.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Lehoux S, Lemarie CA, Esposito B, Lijnen HR, Tedgui A. Pressure-induced matrix metalloproteinase-9 contributes to early hypertensive remodeling. Circulation. 2004;109:1041–1047. doi: 10.1161/01.CIR.0000115521.95662.7A. [DOI] [PubMed] [Google Scholar]
- 16.Medley TL, Kingwell BA, Gatzka CD, Pillay P, Cole TJ. Matrix metalloproteinase-3 genotype contributes to age-related aortic stiffening through modulation of gene and protein expression. Circ Res. 2003;92:1254–1261. doi: 10.1161/01.RES.0000076891.24317.CA. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan RC, ES, Landay AL, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis. 2011;203:452–463. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selzer RH, Hodis HN, Kwong-Fu H, et al. Evaluation of computerized edge tracking for quantifying intima-media thickness of the common carotid artery from B-mode ultrasound images. Atherosclerosis. 1994;111:1–11. doi: 10.1016/0021-9150(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 19.Selzer RH, Mack WJ, Lee PL, Kwong-Fu H, Hodis HN. Improved common carotid elasticity and intima-media thickness measurements from computer analysis of sequential ultrasound frames. Atherosclerosis. 2001;154:185–193. doi: 10.1016/s0021-9150(00)00461-5. [DOI] [PubMed] [Google Scholar]
- 20.Hodis HN, Mack WJ, Lobo RA, et al. Estrogen in the prevention of atherosclerosis. A randomized, double- blind, placebo-controlled trial. Ann Intern Med. 2001;135:939–953. doi: 10.7326/0003-4819-135-11-200112040-00005. [DOI] [PubMed] [Google Scholar]
- 21.Lage SG, Kopel L, Monachini MC, et al. Carotid arterial compliance in patients with congestive heart failure secondary to idiopathic dilated cardiomyopathy. Am J Cardiol. 1994;74:691–695. doi: 10.1016/0002-9149(94)90311-5. [DOI] [PubMed] [Google Scholar]
- 22.Palella FJ, Jr, Gange SJ, Benning L, et al. Inflammatory biomarkers and abacavir use in the Women’s Interagency HIV Study and the Multicenter AIDS Cohort Study. AIDS. 2010;24:1657–1665. doi: 10.1097/QAD.0b013e3283389dfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neuhaus J, Jacobs DR, Jr, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197:126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan RC, Kingsley LA, Gange SJ, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS. 2008;22:1615–1624. doi: 10.1097/QAD.0b013e328300581d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsue PY, Lo JC, Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109:1603–1608. doi: 10.1161/01.CIR.0000124480.32233.8A. [DOI] [PubMed] [Google Scholar]
- 27.Chetty R. Vasculitides associated with HIV infection. J Clin Pathol. 2001;54:275–278. doi: 10.1136/jcp.54.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Regina G, Impedovo G, Angiletta D, et al. Surgical experience with carotid stenosis in young HIV-1 positive patients under antiretroviral therapy: an emerging problem? Eur J Vasc Endovasc Surg. 2005;29:167–170. doi: 10.1016/j.ejvs.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 29.Torriani FJ, Komarow L, Parker RA, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol. 2008;52:569–576. doi: 10.1016/j.jacc.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 31.Deeks SG, Kitchen CM, Liu L, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]