Abstract
Pemetrexed (ALIMTA) is a folate anti-metabolite that has been approved for the treatment of non-small cell lung cancer, and has been shown to stimulate autophagy. In the present study, we sought to further understand the role of autophagy in the response to pemetrexed and to test if combination therapy could enhance the level of toxicity through altered autophagy in tumor cells. The multi-kinase inhibitor sorafenib (NEXAVAR), used in the treatment of renal and hepatocellular carcinoma, suppresses tumor angiogenesis and promotes autophagy in tumor cells. We found that sorafenib interacted in a greater than additive fashion with pemetrexed to increase autophagy and to kill a diverse array of tumor cell types. Tumor cell types that displayed high levels of cell killing after combination treatment showed elevated levels of AKT, p70 S6K and/or phosphorylated mTOR, in addition to Class III RTKs such as PDGFRβ and VEGFR1, known in vivo targets of sorafenib. In xenograft and in syngeneic animal models of mammary carcinoma and glioblastoma, the combination of sorafenib and pemetrexed suppressed tumor growth without deleterious effects on normal tissues or animal body mass. Taken together, the data suggest that premexetred and sorafenib act synergistically to enhance tumor killing via the promotion of a toxic form of autophagy that leads to activation of the intrinsic apoptosis pathway, and predict that combination treatment represents a future therapeutic option in the treatment of solid tumors.
Introduction
The anti-folate drug pemetrexed (abbreviated in this manuscript as “PTX”) (ALIMTA®) was developed as an inhibitor of thymidylate synthase (TS) (1–4). Pemetrexed also has at least one other target that becomes apparent from a continued anti-proliferative effect of drug treatment in cell cultures exposed to exogenous thymidine, which prevents the cytotoxic effects of TS inhibition (1, 2). The identity of any secondary target(s) for pemetrexed could be of considerable interest as the drug exhibits clinical responses in non-small cell lung cancers, which is an unusual activity for folate anti-metabolites (5, 6). Subsequently, the secondary target was shown to be the folate-dependent enzyme, aminoimidazole-carboxamide ribonucleotide formyl-transferase (AICART) (1, 2). Pemetrexed inhibition of AICART elevated the levels of ZMP, a substrate of the AICART reaction. Accumulation of ZMP caused activation of AMP-activated protein kinase with subsequent inhibition of mammalian target of rapamycin (mTOR) and the induction of autophagy (1, 2).
There are two main types of programmed cell death: Type I, also called apoptosis refers specifically to an ATP energy-dependent, genetically controlled process involving transcription of specific proteins and leading eventually to a cell’s demise. Propagation of a type I apoptotic signal may occur via either the extrinsic or the intrinsic pathway (7–9). In the extrinsic pathway trimerization of a death receptor e.g. CD95 recruits and activates caspase-8 via the death inducing signaling complex (DISC). DISC formation and activation of procaspase 8 is suppressed by c-FLIP-l and c-FLIP-s (9). In some caspase-8 may cleave the pro-apoptotic protein BID into its active form, tBID. After activation, tBID translocates to the mitochondria, where it contributes to mitochondrial membrane permeabilization, and cytochrome c and apoptosis inducing factor (AIF) release (10, 11). Cytochrome c binds to Apaf-1 that associates with pro-caspase 9 and permits pro-caspase 9 to auto-catalyze its activation. Caspase 9 cleaves pro-caspase 3; after cleavage, caspase-3 translocates to the nucleus, followed by DNA fragmentation, carried out by DFF40 /45. The intrinsic apoptosis pathway comprises the mitochondrial portion of the extrinsic pathway. After an intracellular insult, BCL-2 pro-apoptotic family members such as BAK and BAX translocate to the mitochondria, inactivating the anti-apoptotic BCL-2 family proteins such as BCL-XL and MCL-1 (12, 13). This allows BAX and BAK to form multimers (pores) which lead to mitochondrial membrane permeabilization. Inhibition of the intrinsic apoptosis pathway has previously been shown to suppress the cyto-toxicity of several TS inhibitors, including pemetrexed (14, 15).
Type II programmed cell death, also called autophagy is a ubiquitous process that occurs in all eukaryotes (16, 17). Autophagy is a non-selective process in which cytoplasm and organelles are (apparently) randomly assorted into the autophagosome, where they are degraded. The process is activated by extracellular and intracellular stimuli (18, 19). Simplistically, there are three types of autophagy: micro-autophagy, macroautophagy, and chaperone-mediated autophagy (CMA) (20–25). Macroautophagy is mediated by two ubiquitin-like conjugation systems, ATG12-ATG5 and ATG8 (microtubule-associated protein 1 light-chain 3, LC3)- phosphatidyl-ethanolamine (PE) (26–30). A GFP-conjugated form of LC3 (ATG8) thus provides a useful tool for the study of autophagy. After autophagosome formation, this structure fuses with an acidic endosome. The proteins in the lumen of this compartment are then degraded.
Based on the system and the drugs/stimulus being studied, autophagy can either act to protect tumor cells from a toxic stress or can facilitate the toxicity of the stress; we have published evidence for both “protective” and “toxic” forms of autophagy based on the stimulus and cell type being examined (31–38). Apoptosis pathways have been linked with autophagy, e.g. knock down of caspase 8 can induce autophagic death (30). Beclin1 contains a BH3 domain that binds to BCL-2 / BCL-XL / MCL-1 and release of Beclin1 from these proteins permits induction of autophagy (26–30). The ser/thr kinase mTOR acts as one gate-keeper in the autophagy process, exerting an inhibitory effect; mTOR acts both in a signal transduction cascade that activates anti-autophagic transcription and translation and by inhibiting the ATG proteins directly via their phosphorylation (39, 40). The PI3K Class I / AKT pathway is involved in down-regulation of autophagy, by activation of mTOR whereas Beclin1 and the Class III type PI3K complex are positive regulators of autophagy (41).
Sorafenib (Bay 43-9006, Nexavar®; a RAF family kinase inhibitor) is a multi-kinase inhibitor that was originally developed as an inhibitor of RAF-1, a component of the ERK1/2 pathway, but which was subsequently shown to inhibit multiple other kinases, including class III tyrosine kinase receptors such as platelet-derived growth factor, vascular endothelial growth factor receptors 1 and 2, c-Kit and FLT3 (31–35, and references therein). Anti-tumor effects of sorafenib in renal cell carcinoma and in hepatoma have been ascribed to anti-angiogenic actions of this agent through inhibition of the growth factor receptors (31–35, and references therein). However, several groups, including ours, have shown in vitro that sorafenib kills human leukemia cells at concentrations below the maximum achievable dose (Cmax) of 15–20 µM, through a mechanism involving down-regulation of the anti-apoptotic BCL-2 family member MCL-1. In these studies sorafenibmediated MCL-1 down-regulation occurred through a translational rather than a transcriptional or post-translational process that was mediated by endoplasmic reticulum (ER) stress signaling and the regulation of autophagy. More recently, we have shown that sorafenib-mediated inhibition of PDGFRβ plays a key role in the ability of this agent to promote autophagy in tumor cells (35).
The present studies attempted to define whether pemetrexed (PTX) toxicity was modified by the drug-induced induction of autophagy, and whether sorafenib, a drug that also is known to modulate autophagy, could interact with pemetrexed to kill tumor cells.
Materials and Methods
Materials
Sorafenib tosylate was purchased from Eton Bioscience Inc., (San Diego, CA). Pemetrexed was purchased from LC Laboratories (Woburn, MA). Trypsin-EDTA, DMEM, RPMI, penicillin-streptomycin were purchased from GIBCOBRL (GIBCOBRL Life Technologies, Grand Island, NY). All cells, except for primary human GBM cells and MCF7 and MCF7F, were purchased from the ATCC and were not further validated beyond that claimed by ATCC. Cells were re-purchased every ~6 months. GBM cells came from the repository at the Mayo Clinic. MCF7 cells were obtained by Dr. Nephew from their primary source (U. Michigan, Ann Arbor). MCF7F cells were generated as noted in [Fan et al. (2006) Cancer Res. 66:11954-66]. Plasmids to express active p70 S6K and active mTOR were purchased from Addgene (Boston, MA). Commercially available validated short hairpin RNA molecules to knock down RNA / protein levels were from Qiagen (Valencia, CA). Reagents and performance of experimental procedures were described in (31–38).
Methods
Culture and in vitro exposure of cells to drugs
All cell lines were cultured at 37 °C (5% (v/v CO2) in vitro using RPMI supplemented with dialyzed 5% (v/v) fetal calf serum and 10% (v/v) Non-essential amino acids. Cells growing in “complete” fetal calf serum that contains thymidine were gradually weaned into dialyzed serum lacking thymidine over 2 weeks and were then used for experimental analyses for the following 3 weeks before discarding (1, 2). Cells were re-isolated in thymidine-less media as required. For short term cell killing assays, immunoblotting studies, cells were plated at a density of 3 × 103 per cm2 (~2 × 105 cells per well of a 12 well plate) and 48h after plating treated with various drugs, as indicated. In vitro pemetrexed and sorafenib treatments were from 100 mM stock solutions of each drug and the maximal concentration of Vehicle (DMSO) in media was 0.02% (v/v). Cells were not cultured in reduced serum media during any study in this manuscript.
In vitro cell treatments, microscopy, SDS-PAGE and Western blot analysis
For in vitro analyses of short-term cell death effects, cells were treated with Vehicle or pemetrexed/sorafenib for the indicated times in the Figure legends. For apoptosis assays where indicated, cells were isolated at the indicated times, and either subjected to trypan blue cell viability assay by counting in a light microscope or fixed to slides, and stained using a commercially available Diff Quick (Geimsa) assay kit. Alternatively, the Annexin V/propidium iodide assay was carried to determine cell viability out as per the manufacturer's instructions (BD PharMingen) using a Becton Dickinson FACScan flow cytometer (Mansfield, MA). Pemetrexed / sorafenib lethality, as judged by annexin-PI, was first evident ~12h after drug exposure (data not shown).
For SDS PAGE and immunoblotting, cells were plated at 5 × 105 cells / cm2 and treated with drugs at the indicated concentrations and after the indicated time of treatment, lysed in whole-cell lysis buffer (0.5 M Tris-HCl, pH 6.8, 2%SDS, 10% glycerol, 1% β-mercaptoethanol, 0.02% bromophenol blue), and the samples were boiled for 30 min. The boiled samples were loaded onto 10–14% SDS-PAGE and electrophoresis was run overnight (10–100 µg/lane based on the gel size. Proteins were electrophoretically transferred onto 0.22 µm nitrocellulose, and immunoblotted with various primary antibodies against different proteins. All immunoblots were visualized using an Odyssey Infrared Imager. For presentation, immunoblots were digitally assessed using the provided Odyssey Imager software (the data sets presented are the −Fold increase +/− SEM (n = 3) in expression of the indicated protein compared to GAPDH loading control; for phospho-proteins the −Fold increase +/− SEM (at least n = 3) is normalized to the total protein level of the indicated kinase or substrate). Errors are not numerically shown due to space restrictions in the Figure panels; any indicated significant differences between the expression / phosphorylation levels of proteins are indicated by an asterisk or other annotation and have a p < 0.05. Images have their color removed and Figures generated in MicroSoft PowerPoint.
Transfection of cells with siRNA or with plasmids
For Plasmids
Cells were plated as described above and 24h after plating, transfected. For mouse embryonic fibroblasts (2–5µg) or other cell types (0.5µg) plasmids expressing a specific mRNA (or siRNA) or appropriate vector control plasmid DNA was diluted in 50µl serum-free and antibiotic-free medium (1 portion for each sample). Concurrently, 2µl Lipofectamine 2000 (Invitrogen), was diluted into 50µl of serum-free and antibiotic-free medium (1 portion for each sample). Diluted DNA was added to the diluted Lipofectamine 2000 for each sample and incubated at room temperature for 30 min. This mixture was added to each well / dish of cells containing 200µl serum-free and antibiotic-free medium for a total volume of 300 µl, and the cells were incubated for 4 h at 37 °C. An equal volume of 2× medium was then added to each well. Cells were incubated for 48h, then treated with pemetrexed / sorafenib.
Transfection with siRNA
Cells were plated in 60 mm dishes from a fresh culture growing in log phase as described above, and 24h after plating transfected. Prior to transfection, the medium was aspirated and 1 ml serum-free medium was added to each plate. For transfection, 10 nM of the annealed siRNA, the positive sense control doubled stranded siRNA targeting GAPDH or the negative control (a “scrambled” sequence with no significant homology to any known gene sequences from mouse, rat or human cell lines) were used. Ten nM siRNA (scrambled or experimental) was diluted in serum-free media. Four µl Hiperfect (Qiagen) was added to this mixture and the solution was mixed by pipetting up and down several times. This solution was incubated at room temp for 10 min, then added dropwise to each dish. The medium in each dish was swirled gently to mix, then incubated at 37 °C for 2h. One ml of 10% (v/v) serum-containing medium was added to each plate, and cells were incubated at 37 °C for 48h before re-plating (50 × 103 cells each) onto 12-well plates. Cells were allowed to attach overnight, then treated with pemetrexed / sorafenib (0–48h). Trypan blue exclusion / TUNEL / flow cytometry assays and SDSPAGE/immunoblotting analyses were performed at the indicated time points.
Recombinant adenoviral vectors; infection in vitro
We generated and purchased previously described recombinant adenoviruses to modulate protein expression and to express constitutively activated and dominant negative proteins, dominant negative caspase 9, and BCL-XL (Vector Biolabs, Philadelphia, PA). Cells were infected with these adenoviruses at an approximate m.o.i. of 50. Cells were further incubated for 24 hours to ensure adequate expression of transduced gene products prior to drug exposures.
Microscopy for LC3-GFP expression
Cells were transfected with a plasmid to express an LC3-GFP fusion protein, and were then cultured for 24 h. Cells were then treated with drugs, as indicated/ LC3-GFP transfected cells were visualized at the indicated time points on the Zeiss Axiovert 200 microscope using the FITC filter.
Animal studies
Animal studies in flank and brain are performed under approved protocols by the VCU IACUC. For studies with human mammary carcinoma cells, athymic Nu/Nu mice (8 week old, female) were injected, into the 4th mammary fat pad, with 1.0 × 107 BT474 cells. Tumors of ~75 mm3 grew over the following month. Animals were segregated into tumor volumes of approximate equivalent mean tumor size and standard error. The animals were administered vehicle diluent (cremophore), sorafenib (25 mg/kg), pemetrexed (50 mg/kg) or the drug combination by oral gavage once every day for 5 days. We performed the experiment twice (n = 2) with a total of 10 animals per treatment group. Tumor volumes are measured every two-three days as indicated. For studies with mouse mammary tumor cells Balb/c mice (8 week old, female) were obtained from the NCI and animals injected into the 4th mammary fat pad with 1.0 × 107 4T1 cells. Five days after implantation the animals were administered vehicle diluent (cremophore), sorafenib, pemetrexed or the drug combination (as above) by oral gavage for 5 days. The volumes of the tumors in each group were calculated 14 days after the final drug treatment. We performed the experiment twice (n = 2) with a total of 10 animals per treatment group.
For studies using GBM6-luciferase; athymic female were anesthetized via intraperitoneal administration of (ketamine, 40 mg/kg; xylazine, 3 mg/kg) and immobilized in a stereotactic frame (David Kopf Instruments, Tujunga, CA). A 24-gauge needle attached to a Hamilton syringe was inserted into the right basal ganglia to a depth of 3.5-mm and then withdrawn 0.5-mm to make space for tumor cell accumulation. The entry point at the skull was 2-mm lateral and 1-mm dorsal to the bregma. Intracerebral injection of 0.5 × 106 GBM6-luc glioma cells (~40 mice per cell line per separate experiment) in 2 µl of Dulbecco's modified Eagle's medium was performed over 10 minutes. The skull opening was enclosed with sterile bone wax and the skin incision was closed using sterile surgical staples. The animals were administered vehicle diluent (cremophore), sorafenib (25 mg/kg), pemetrexed (50 mg/kg) or the drug combination by oral gavage once every day for 5 days. We performed the experiment twice (n = 2) with a total of 12 animals per treatment group.
For normal tissue toxicity studies, animals were administered vehicle diluent (cremophore) or with sorafenib (25 mg/kg) and pemetrexed (50 mg/kg) in combination by oral gavage once every day for 5 days. Animals were rested for two days and then treated for an additional 5 days with vehicle or drugs. Two days after cessation of drug treatment animals were humanely sacrificed and tissues (brain, lung, liver, heart, kidney, spleen) isolated, 10 µM sections taken, and H&E stained. For immunohistochemistry and staining of fixed tumor sections; post sacrifice, tumors were fixed in OCT compound (Tissue Tek); cryostat sectioned (Leica) as 10 µm sections. Data shown are representative slides from several sections from the same tumor with multiple tumors (from multiple animals; and multiple experiments) having been examined (n = at least 3–6 animals-tumors per group).
Data analysis
Comparison of the effects of various treatments was performed using ANOVA and the Student’s t test. Differences with a p-value of < 0.05 were considered statistically significant. Experiments shown are the means of multiple individual points (± SEM). Statistical examination of in vivo animal survival data utilized log rank statistical analyses between the different treatment groups.
Results
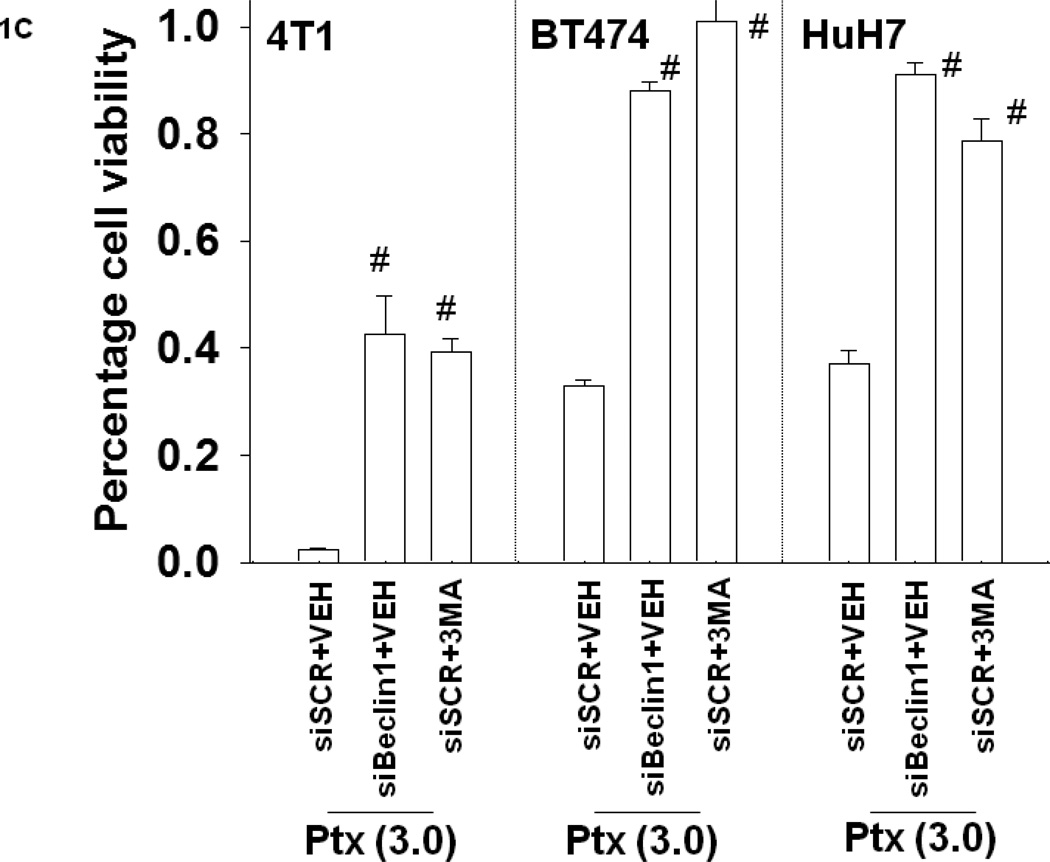
Mammary, hepatoma and lung carcinoma cells were grown in thymidine-less serum, conditions that replicate growth conditions of an in situ tumor in a patient. Cells were treated with low clinically relevant doses of the anti-folate pemetrexed (PTX) and in response cells rapidly increased their levels of autophagy as judged by vesicularization of a green fluorescent protein tagged form of LC3 (ATG8) (LC3-GFP) and by increased processing of LC3 to LC3II (Figure 1A and Figure S1) (1, 2). Drug-induced autophagy was first observed at a higher pemetrexed dose within 6h of treatment and autophagy was noted at all doses within 12h. The induction of autophagy was blocked by a small molecule inhibitor of the class III PI3K Vps34, 3 methyl-adenine (3MA), or by knock down of Beclin1 or ATG5 (data not shown, Figure S2). Within 24h, treatment of breast cancer cells with pemetrexed caused a dose-dependent reduction in cell viability as judged by Annexin-PI flow-cytometry, an effect that was blocked by 3MA or by knock down of Beclin1 (Figures 1B and 1C).
Figure 1. Pemetrexed induces autophagy and tumor cell killing that is suppressed by knock down of Beclin1 or treatment with 3-methyl adenine.
Panel A. H460 and 4T1 cells were transfected with a plasmid to express LC3-GFP. Twenty four h after transfection cells were treated with vehicle (PBS) or pemetrexed (0.03–3.0 µM). Cells were examined under a fluorescent microscope (X40) at the indicated times after drug exposure and the mean number of vesicles in 40 selected random cells in triplicate wells calculated (n = 2 studies, +/− SEM; * p < 0.05 greater than vehicle control value). Upper inset: H460 cells, 12h after pemetrexed treatment (0.3 µM) treatment were isolated and immunoblotting performed to determine the expression and lipidation / conversion of LC3 to LC3II. Panels B and C. H460, 4T1, BT474 and HuH7 cells as indicated are transfected with siRNAs (si-scramble, siSCR; siBeclin1; 20 nM). Twenty four h after transfection cells were treated with vehicle (PBS) or pemetrexed (0.03–3.0 µM). As indicated, just prior to pemetrexed treatment, cells were also treated with vehicle (PBS) or with 3-methyl adenine (1 mM). Cells were isolated 24h after treatment and viability determined by annexin-PI staining / flow cytometry (n = 3, +/− SEM; # p < 0.05 greater than corresponding vehicle treated or siSCR transfected cell value). Data in Panel C is presented without the control “1.00” survival bars for clarity.
Sorafenib (Sor) is a multi-kinase inhibitor whose biologic actions have often been tied to inhibition of class III receptor tyrosine kinases, e.g. vascular endothelial growth factor receptors (VEGFRs) and platelet derived growth factor receptor beta (PDGFRβ) (35). We and others have also noted that sorafenib can stimulate autophagy through inhibition of class III RTK’s and that in a dose-dependent effect this response can either be a protective form of autophagy or a toxic form of autophagy (e.g. 35,36). Hence, we next determined whether sorafenib enhanced, or suppressed, pemetrexed toxicity. Sorafenib and pemetrexed interacted in a greater than additive fashion to increase the number of autophagic vesicles in tumor cells that correlated with increased processing of LC3 to LC3II and that was inhibited by knock down of Beclin1 (Figure 2A). Sorafenib enhanced the toxicity of pemetrexed in a dose-dependent fashion in short-term 24h cell viability assays using multiple tumor cells from a diverse range of tissue types (Figures 2B and 2C; Figures S3–S7). Knock down of Beclin1 blocked the pemetrexed + sorafenib drug combination -stimulated induction of autophagy and suppressed the cytotoxic interaction between sorafenib + pemetrexed (Figures 2A and 2D; Figure S8). Incubation of cells with 3MA also suppressed pemetrexed + sorafenib –induced toxicity (data not shown).
Figure 2. Pemetrexed interacts with sorafenib in a dose-dependent fashion to increase autophagy and tumor cell killing that is suppressed by knock down of Beclin1.
Panel A. BT474, 4T1 and HuH7 cells as indicated are transfected with siRNAs (si-scramble, siSCR; siBeclin1; 20 nM) and with a plasmid to express LC3-GFP. Twenty four h after transfection cells were treated with vehicle (PBS) or pemetrexed (0.03–3.0 µM) and/or vehicle (DMSO) or sorafenib. Twelve h after drug exposure cells were examined under a fluorescent microscope (X40) at the indicated times after drug exposure and the mean number of vesicles in 40 random cells in triplicate calculated per experiment (n = 2 studies, +/− SEM; * p < 0.05 less than corresponding siSCR value). Panel B. HuH7 and H460 cells were treated as indicated with vehicle (PBS) or pemetrexed and/or vehicle (DMSO) or sorafenib. Viability was determined in triplicate 24h later by trypan blue exclusion (HuH7, H460) or annexin-PI staining, as noted in the panel (n = 2, +/− SEM; ¶ p < 0.05 greater than vehicle control value). Panel C. Parental MCF7 and fluvestrant resistant MCF7 cells (MCF7F) were treated as indicated with vehicle (PBS) or pemetrexed and/or vehicle (DMSO) or sorafenib. Viability was determined in triplicate 24h later by trypan blue exclusion (n = 2, +/− SEM; ¶ p < 0.05 greater than vehicle control value; ¶¶ p < 0.05 greater than corresponding value in parental MCF7 cells). Panel D. BT474 and HuH7 cells as indicated are transfected with siRNAs (si-scramble, siSCR; siBeclin1; 20 nM). Twenty four h after transfection in triplicate cells were treated with vehicle (PBS) or pemetrexed (0.03–3.0 µM) and/or vehicle (DMSO) or sorafenib. Viability was determined in triplicate 24h later by trypan blue exclusion (n = 2, +/− SEM; * p < 0.05 less than corresponding siSCR value).
It has been noted by the Moran laboratory that pemetrexed treatment increases the intracellular concentration of the chemical ZMP (AICAr monophosphate; 5-Aminoimidazole-4-carboxamide-1-β-D-Ribofuranosyl monophosphate) resulting in ZMP-induced activation of the AMP-activated protein kinase (AMPK); AMPK activation in turn acts to suppress mTOR activity (1, 2). Reduced mTOR activity has been associated with elevated levels of autophagy. Because of these findings, we investigated whether suppression of mTOR function by use of rapamycin altered pemetrexed lethality (1, 2, 39, 40). Rapamycin (Rap) enhanced pemetrexed toxicity in multiple tumor cell types (Figure S9). Thus suppression of the PI3K-mTOR pathway at the level of a growth factor receptor or at the level of mTOR could enhance pemetrexed lethality.
The development estrogen independence in recurrent mammary tumors; i.e. those tumors initially diagnosed as being ER+ and thus chronically treated with Tamoxifen, has frequently been observed. For ER+ postmenopausal breast cancer patients the development of other “purer” anti-estrogen therapeutics such as Faslodex (Fulvestrant, ICI 182,780) has also been beneficial though even these purer anti-estrogen modalities have potential to fail with the delayed outgrowth of estrogen independent breast cancer cells (42). We next determined the impact of Fulvestrant resistance on the lethality of the pemetrexed + sorafenib drug combination in a well characterized ER+ breast cancer cell line; a cell line isolated from a pleural effusion, MCF7.
In agreement with a role for autophagy in the regulation of mammary tumor cell survival following sorafenib + pemetrexed, in parental MCF7 and fulvestrant resistant MCF7 cells (MCF7F) sorafenib or the drug combination increased LC3II processing and decreased p62 levels (Figure 3A). Parental MCF7 cells are known to be haplotype for expression of the autophagy regulatory gene Beclin1 and it has been postulated in clinical samples from breast cancer and lung cancer patients that loss of Beclin 1 facilitates mammary / lung tumorigenesis (e.g. 43). Basal Beclin1 levels were significantly elevated in MCF7F compared to parental MCF7 cells; similar findings were also noted for ATG5-ATG12 levels. In parental MCF7 cells drug combination treatment increased Beclin1 and ATG5-ATG12 expression to approximately the same extent as the basal protein levels in MCF7F cells (Figures 3A). Beclin1 and ATG5-ATG12 levels were not further enhanced by the drug treatment in MCF7F cells.
Figure 3. Fulvestrant resistant MCF7 cells express higher levels of autophagy markers and mitochondrial protective proteins; protection of the mitochondria blocks pemetrexed + sorafenib toxicity.
Panel A. MCF7 and MCF7F cells, 24h after plating, were treated with vehicle (PBS) or pemetrexed (1.0 µM) and/or vehicle (DMSO) or sorafenib (3.0 µM). Cells were isolated 24h after drug exposure and subjected to SDS PAGE and immunoblotting against the indicated proteins as described in the Methods section. The intensity of immunostaining was normalized to either GAPDH for proteins or for phospho-proteins to the dephosphorylated protein; these values were then arbitrarily normalized with the intensity value for each protein or phosphoprotein equivalent in vehicle treated MCF7 cells to 1.00 (n = 3 +/− SEM; * p < 0.05 less than corresponding vehicle control value; @ p < 0.05 greater than corresponding vehicle control value in parental MCF7; # p < 0.05 greater than corresponding vehicle control value). Panel B. MCF7 and MCF7F cells were infected with either empty vector virus (Ad.cmv); with a virus to express dominant negative caspase 9 (Ad.dnCasp9); or with viruses to express a BCL-XL or c-FLIP-s (Ad.BCL-XL; Ad.c-FLIP). Twenty four h after infection cells were treated with vehicle (PBS) or pemetrexed (0.03–3.0 µM) and/or vehicle (DMSO) or sorafenib. Viability was determined in triplicate 48h later by trypan blue exclusion (n = 2, +/− SEM; * p < 0.05 less than corresponding vehicle control value).
In addition to altering expression of autophagy regulatory proteins, drug combination exposure of MCF7 and MCF7F cells also reduced expression of the mitochondrial protective proteins MCL-1 and BCL-XL (Figure 3B); these are proteins we have previously implicated in breast cancer cells to sequester Beclin1 and reduce the ability of tumor cells to induce autophagy, as well as to suppress apoptosis. Over-expression of BCL-XL or expression of dominant negative caspase 9, but not expression of c-FLIP-s; significantly suppressed drug combination cytotoxicity, implying that autophagy was feeding in to the intrinsic apoptosis pathway at the level of the mitochondrion and that the extrinsic pathway was not involved in killing (Figure 3B).
Based on our in vitro cell survival data and expression data for autophagy regulatory proteins, we next examined the relative phosphorylation and expression levels of signal transduction proteins after pemetrexed + sorafenib treatment; proteins whose expression and activity has been associated by others and ourselves in a variety of systems to correlate to altered levels of autophagy and apoptosis. Tumor cell types that displayed high levels of cell killing after pemetrexed + sorafenib exposure, such as MCF7F, H460 and HuH7 tended to exhibit significantly elevated basal levels of AKT, p70 S6K and/or mTOR phosphorylation (Figure 4A; Figure S10).
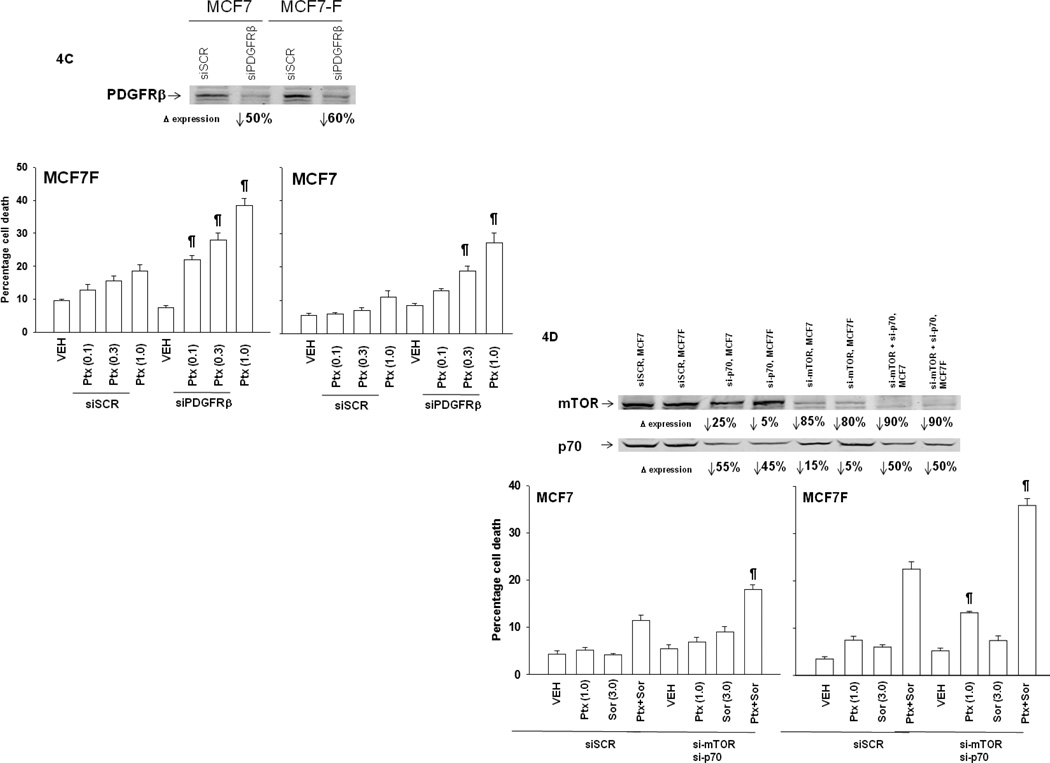
Figure 4. Knock down of PDGFRβ, mTOR or p70 S6K enhances pemetrexed toxicity and inhibition of ERK1/2 suppresses drug combination toxicity.
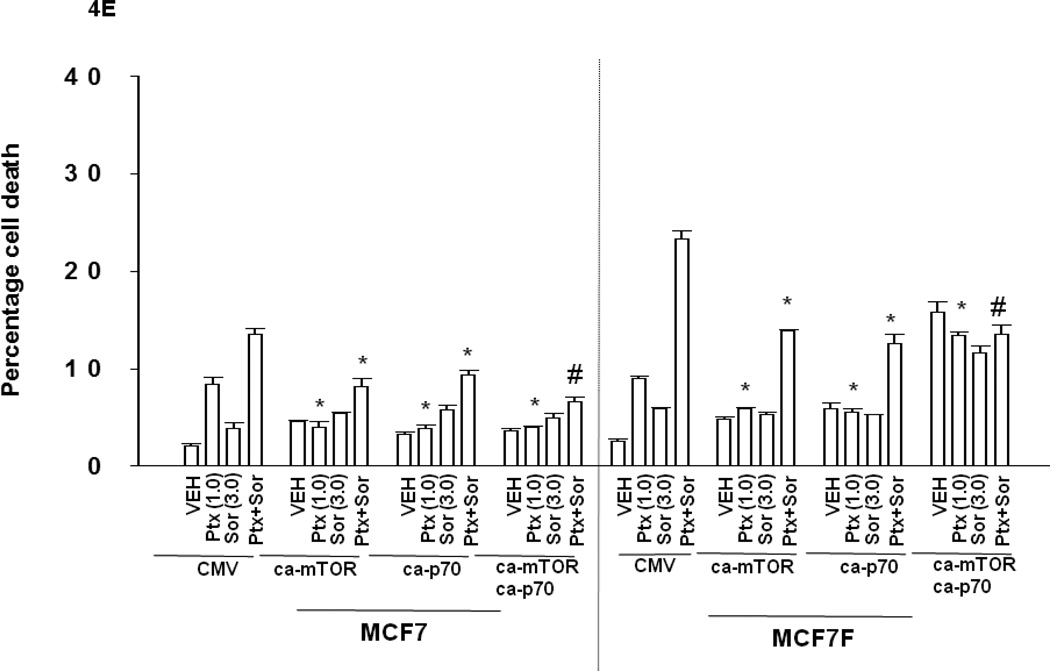
Panel A. Tumor cells, as indicated, growing in log phase were isolated 24h after plating and subjected to SDS PAGE and immunoblotting against the indicated proteins as described in the Methods section. The intensity of immunostaining was normalized to either GAPDH for proteins or for phospho-proteins to the equivalent dephosphorylated protein; these values were then normalized with the intensity value of each protein / phosphor-protein in BT474 cells defined as 1.00 (+/− SEM, n = 3) (# p < 0.05 greater than value in BT474; @ p < 0.05 greater than value in parental MCF7 and BT474; + p < 0.05 greater than value in parental MCF7. Panel B. MCF7 and MCF7F cells, 24h after plating, were treated with vehicle (PBS) or pemetrexed (1.0 µM) and/or vehicle (DMSO) or sorafenib (3.0 µM). Cells were isolated 24h after drug exposure and subjected to SDS PAGE and immunoblotting against the indicated proteins as described in the Methods section. The intensity of immunostaining was normalized to either GAPDH for proteins or for phospho-proteins to the dephosphorylated protein; these values were then arbitrarily normalized with the intensity value for each protein or phospho-protein equivalent in vehicle treated MCF7 cells to 1.00 (n = 3 +/− SEM; * p < 0.05 less than corresponding vehicle control value; @ p < 0.05 greater than corresponding vehicle control value in parental MCF7; # p < 0.05 greater than corresponding vehicle control value). Panel C. MCF7 and MCF7F cells are transfected in triplicate with siRNAs (si-scramble, siSCR; PDGFRβ; 20 nM). Twenty four h after transfection cells were treated with vehicle (PBS) or pemetrexed (0.1–1.0 µM). Viability was determined in triplicate 48h later by trypan blue exclusion (n = 2, +/− SEM; ¶ p < 0.05 greater than corresponding siSCR value). Upper immunoblots: siRNA transfected cells are treated with drugs and isolated 12h after drug treatment and immunoblotting performed for levels of SRC Y416 and PDGFRβ. Panel D. MCF7 and MCF7F cells are transfected with siRNAs (si-scramble, siSCR; si-mTOR; si-p70 S6K; 20 nM). Twenty four h after transfection cells were treated with vehicle (PBS) or pemetrexed (0.1–1.0 µM). Viability was determined in triplicate 48h later by trypan blue exclusion (n = 2, +/− SEM; ¶ p < 0.05 greater than corresponding siSCR value). Panel E. MCF7 and MCF7F cells were transfected with either empty vector plasmid (CMV) or with plasmids to express a constitutively activated form of p70 S6K and/or a constitutively activated form of mTOR. Twenty four h after transfection cells were treated with vehicle (PBS) or pemetrexed (0.03–3.0 µM) and/or vehicle (DMSO) or sorafenib. Viability was determined in triplicate 48h later by trypan blue exclusion (n = 3, +/− SEM; * p < 0.05 less than corresponding CMV value; # p < 0.05 less than difference in corresponding ca-mTOR or ca-p70 S6K).
Cells that were more sensitive to the pemetrexed + sorafenib drug combination, such as MCF7F and HuH7 also tended to display elevated expression levels of Class III RTKs such as PDGFRβ and VEGFR1; known in vivo targets of sorafenib. Thus, based on these findings, and those in Figure 2C with estrogen-dependent and fulvestrant-resistant MCF7 cells, we further explored the signaling responses and viability changes of tumor cells after pemetrexed + sorafenib treatment.
We found that fulvestrant resistant MCF7 breast cancer cells (MCF7F) over-expressed the class III RTK PDGFRβ, had elevated levels of ERK1/2, p70 S6K and mTOR activity, and were more sensitive to drug combination toxicity compared to their estrogen dependent counterparts (Figures 2C, 4A, 4B). Treatment of parental MCF7 cells with pemetrexed and sorafenib, but not the individual drugs, modestly suppressed T421/S424 and mTOR S2448 phosphorylation and strongly increased ERK1/2 phosphorylation (Figure 4B). Treatment of MCF7F cells with pemetrexed more effectively reduced p70 S6K T421/S424 phosphorylation than sorafenib, and sorafenib more effectively reduced p70 S6K T389 and mTOR S2448 phosphorylation than pemetrexed. Knock down of PDGFRβ, or of p70 S6K or mTOR enhanced pemetrexed and pemetrexed + sorafenib lethality (Figures 4C and 4D). Thus for PDGFRβ, the observed elevated receptor expression and elevated drug toxicity in MCF7F cells, define this protein as one molecular marker for a tumor cell response to the sorafenib and pemetrexed drug combination. Because sorafenib and pemetrexed treatment reduced p70 S6K and mTOR activity, we hypothesized that expression of constitutively activated forms of p70 S6K and mTOR would reduce drug cytotoxicity. Expression of a constitutively active form of p70 S6K and of mTOR significantly reduced the toxic effects of pemetrexed and sorafenib treatment (Figure 4E).
All the prior studies in the manuscript have been using 2D in vitro cultures of tumor cells. As our ultimate goal is to translate the pemetrexed + sorafenib drug combination into the clinic, we determined whether sorafenib and pemetrexed interacted in vivo to suppress tumor cell growth in various tumor model systems. In orthotopic established HER2 positive BT474 human mammary carcinoma tumors growing in the 4th mammary fat pad, sorafenib significantly reduced tumor growth whereas pemetrexed had little impact on tumor mass (Figure 5A). Combined exposure to sorafenib and pemetrexed significantly reduced tumor growth below that of sorafenib treatment alone and almost abolished tumor growth. The alterations in tumor growth data correlated in sections of the respective tumors with increased cleavage of pro-caspase 3 and TUNEL staining, a reduction in proliferation (Ki67) and a manifest disruption of tumor cyto-architecture (Figure 5B).
Figure 5. Pemetrexed and sorafenib interact to suppress breast cancer tumor growth in orthotopic human and rodent syngeneic model systems.
Panel A. BT474 tumors were established in the 4th mammary fat pad of athymic mice (~75 mm3). Animals were treated with vehicle, sorafenib (sor), pemetrexed (ptx) or both drugs simultaneously as described in Methods. Animals were treated for 5 days with drugs and tumor volumes measured every two-three days as indicated, and mean tumor volumes plotted (n = 2 studies; 8 animals per group total +/− SEM; * p < 0.05 less than corresponding vehicle control value; % p < 0.05 less than corresponding sorafenib value). Panel B. BT474 tumors fourteen days after drug exposure were fixed, sectioned and stained as described in Methods. Ki67 measures tumor cell proliferative rate; TUNEL and cleaved caspase 3 the levels of tumor cell apoptosis within the tumors. Panel C. 4T1 tumors were injected into the 4th mammary fat pad of BALB/c mice. Five days after implantation the animals were administered vehicle, sorafenib (sor), pemetrexed (ptx) or both drugs simultaneously as described in Methods for 5 days. The volumes of the tumors in each group were calculated 14 days after the final drug treatment (n = 2 studies; 8 animals per group total +/− SEM; * p < 0.05 less than ptx or sor treated tumor values).
In the spontaneous mouse mammary tumor cell line 4T1, that is HER1 dependent for growth, we noted in tumors growing in the 4th mammary fat pad that sorafenib and pemetrexed also interacted to suppress tumor growth (Figure 5C). Our in vivo data using 4T1 cells was in agreement with in vitro apoptosis data in Figure S5. In an orthotopic model of human GBM, in a primary GBM tumor cell isolate that displays invasive capabilities compared to commercially available established cell lines i.e. GBM6-luc that expresses EGFR vIII, we noted that treatment with pemetrexed + sorafenib significantly suppressed tumor cell growth during and shortly following drug exposure (Figure S11, ; p < 0.05). Many days after drug exposure, tumors still exhibited a high level of apoptosis (Figure S12). Finally, as we wish to move our drug combination into the clinic, consideration of normal tissue toxicity effects needed to be made. Thus we determined whether combined sorafenib and pemetrexed treatment had any deleterious effects on normal mouse tissues. Pemetrexed + sorafenib treatment did not reduce the body mass of animals carrying GBM6-luc, BT474 tumors or in animals lacking tumors (Figures S13–S15) Two weeks of pemetrexed + sorafenib treatment did not result in any obvious normal tissue toxicity as judged using H&E staining of sectioned organs and examination of nuclear morphology (apoptosis) or of tissue integrity (Figures S16–S18).
Discussion
The anti-folate pemetrexed was recently shown to elevate ZMP levels thereby activating AMPK, which in turn caused inactivation of mTOR and to increased levels of autophagy within a tumor cell (1, 2). The precise role of autophagy in survival or death within these studies was not investigated. The mTOR pathway is, in part, responsible for regulating energy metabolism, and a number of tumor suppressor proteins play a central role in fine-tuning the activity of this pathway including PI3K, PTEN, LKB1, AMPK and mTORC1. The present studies sought to understand the role of autophagy in the response of tumor cells to pemetrexed and to understand how the levels of autophagy and ER stress caused by pemetrexed could be manipulated to cause additional tumor cell killing. i.e. by combined treatment with the multi-RTK inhibitor, sorafenib.
In a genetically diverse group of tumor cells from a wide range of malignancies we found that pemetrexed caused a toxic form of autophagy, as judged using 3MA and Beclin1 knock down. The multi-kinase inhibitor sorafenib inhibited PDGFRβ and knock down of PDGFRβ or sorafenib treatment of cells further promoted pemetrexed –induced autophagy that was causal in the additional levels of tumor cell killing caused by the drug combination. The drug combination exhibited elevated anti-tumor effects in vivo compared to the individual agents in several animal orthotopic model systems of breast cancer, and also in an orthotopic model of invasive primary human glioblastoma.
As previously published by this laboratory and other groups, autophagy can be shown either act to protect cells from a toxic stress or can facilitate the toxicity of the stress all of which appears to be based on the stimulus and the tumor cell type being examined (31–38). In the majority of cells, the single agent toxicity of pemetrexed was dependent on autophagy. More significantly, however, for the future translational development of this drug combination, in at least one tumor cell type, the HCT116 and DLD1 colorectal carcinoma lines, we noted that pemetrexed-induced autophagy was a weakly protective response to single agent lethality; nonetheless, sorafenib also potentiated pemetrexed –induced autophagy and cell killing in these tumor cells in a manner consistent with the other cell lines / types examined in the majority of our studies (Park, Bareford, Moran and Dent, Unpublished findings).
Pemetrexed and sorafenib treatment caused inactivation of the p70 S6K and mTOR protein kinases, as judged by their de-phosphorylation at multiple regulatory sites. Of note, though, was that the protein kinases believed to phosphorylate these particular sites in p70 S6K and mTOR either exhibited little change in their phosphorylation / activity i.e. AKT, or paradoxically displayed elevated activity i.e. ERK1/2. In this regard, it has been shown that p70 S6K and mTOR inactivation and ERK1/2 activation have all been linked to enhanced autophagy levels (44–49). Expression of activated forms of p70 S6K and mTOR significantly reduced the toxicity of sorafenib and pemetrexed exposure. Collectively our data leads us to conclude that sustained inhibition of the p70 S6K / mTOR signaling module following sorafenib + pemetrexed exposure plays a central role in drug combination lethality. Whether the altered p70 S6K activity in our system also results in differential translation of terminal oligo-pyrimidine tracts in 5’UTR (5’-TOP) mRNAs, molecules which have recently been linked to increased autophagy, will require additional detailed experimentation (50).
Apoptosis and autophagy pathways are intimately inter-twined processes. Based on a literature search the primary pathway of pemetrexed –induced cell killing has been postulated to occur via the intrinsic pathway although there is relatively little information in the literature, and tested with molecular tools, to completely prove this assertion (1, 2, 51). There are several mechanisms by which endoplasmic reticulum stress, autophagy and apoptosis can interact to alter cell viability, most notably through PKR-like endoplasmic reticulum (PERK) –dependent inhibition of translation thereby reducing levels of protective proteins with short half-lives such as MCL-1; through altering the sequestration of Beclin1 via its BH3 domain with several protective BCL-2 family proteins (BCL-2, BCL-XL, MCL-1); and by cleavage and activation of BID by lysosomal proteases such as cathepsins and calpains (52, 53). Sorafenib + pemetrexed treatment reduced both MCL-1 and BCL-XL protein levels which would lead to both reduced levels of protective proteins permitting activation of toxic BH3 domain proteins such as BAX and BAK, as well as causing greater levels of un-sequestered Beclin1. In other studies we have recently demonstrated that drug-induced release of Beclin1 from MCL-1 using the MCL-1 inhibitor obatoclax facilitates the binding of Beclin1 to the class III PI3K vps34, thereby promoting autophagy, an effect that has been shown to either preserve or diminish cell viability based on the stimulus and cell type being examined (31–38). Increased formation of acidic lysosomes, as the final end product of autophagic flux, have potential to lead to release of active cathepsin and calpain proteases into the cytosol where pro-apoptotic proteins such as BID can then be cleaved / activated (37). Other groups using a diverse variety of stimuli have observed similar lysosomal protease-dependent events (52–54). Beclin1 and ATG5 have potential to both be cleaved by cysteine proteases resulting in metabolized proteins that no-longer promote autophagy but instead facilitate activation of other toxic BH3 domain proteins leading to pore formation in the outer mitochondrial membrane, causing release of cytochrome c with resultant activation of pro-caspase 9 and induction of apoptosis (55). At present we do not precisely know, following pemetrexed + sorafenib treatment, how high levels of autophagy at the molecular level facilitate mitochondria-dependent apoptosis. These studies await detailed examination in a subsequent study.
It is well known that MCF7 cells, and many primary breast cancers, have a haplotype insufficiency in Beclin1 and thus are less effective at inducing autophagy than non-transformed mammary epithelial cells (56). These findings would suggest that loss of autophagy in breast cancer facilitates tumor formation. We noted that MCF7F cells expressed higher basal levels of Beclin1, as well as of ATG5-ATG12, compared to parental MCF7 cells arguing that a portion of the process by which MCF7 cells maintain their viability in the face of estrogen deprivation / loss of ER signaling is due to an increased ability to induce a protective form of autophagy. In agreement with this finding, agents which act to suppress the later lysosomal fusion stage of autophagy, e.g. chloroquine, can facilitate the anti-tumor effects of the anti-estrogen tamoxifen (57). We noted that MCF7F cells were more susceptible to pemetrexed + sorafenib lethality; a lethality that was dependent on a toxic form of autophagy. At least one key molecular marker for the enhanced response in MCF7F cells to sorafenib was due to their having over-expression of PDGFRβ compared to parental MCF7 cells. Collectively, our data argue that the initial autophagic survival response of breast cancer cells following anti-estrogen therapy, which selects for tumor cells that over-express Beclin1, may subsequently facilitate tumor cell killing by drug combinations that utilize toxic forms of autophagy to achieve their therapeutic effects (58).
In several orthotopic models of breast cancer we demonstrated that pemetrexed and sorafenib interacted to suppress tumor growth that correlated with increased levels of apoptosis within the tumor. Using invasive primary human GBM cells that express an oncogene associated with shorter patient survival, EGFR vIII, we also observed a significant reduction in tumor growth and prolongation of survival. In the clinic, sorafenib is approved for treatment of renal and hepatocellular carcinomas and is believed to act by suppressing tumor angiogenesis i.e. through inhibition of VEGFR family receptors. Pemetrexed is approved for treatment and maintenance therapy of non-small cell lung cancer and mesothelioma. Based on the data presented in the present manuscript we are preparing to develop and implement a protocol for an open label Phase I trial combining sorafenib and pemetrexed in patients with recurrent solid tumors that should open at VCU Massey Cancer Center by Fall of 2011.
Supplementary Material
Acknowledgements
Support for the present study was provided; to P.D. from PHS grants (R01-DK52825; P01-CA104177; R01-CA108325; R01-CA141703; R01-CA150214), The Jim Valvano “V” foundation, and Department of Defense Award (DAMD17-03-1-0262 and W81XWH-10-1-0009); to S.G. from PHS grants (R01-CA63753; R01-CA77141) and a Leukemia Society of America grant 6405-97; to R.G.M. from PHS grants (R01-CA39687; R01-CA140416); to KPN (NCI U54CA113001). P.D. is The Universal Inc. Professor in Signal Transduction Research.
Abbreviations
- ERK
extracellular regulated kinase
- MEK
mitogen activated extracellular regulated kinase
- PI3K
phosphatidyl inositol 3 kinase
- ca
constitutively active
- dn
dominant negative
- Sor
sorafenib
- PTX
premetrexed
- ER
endoplasmic reticulum
- MAPK
mitogen activated protein kinase
- PDFR
platelet derived factor receptor
- PTEN
phosphatase and tensin homologue on chromosome ten
- ROS
reactive oxygen species
- CMV
empty vector plasmid or virus
- si
small interfering
- SCR
scrambled
- PP
protein phosphatase
- IP
immunoprecipitation
- Ad
adenovirus
- VEH
vehicle
References
- 1.Racanelli AC, Rothbart SB, Heyer CL, Moran RG. Therapeutics by cytotoxic metabolite accumulation: pemetrexed causes ZMP accumulation, AMPK activation, and mammalian target of rapamycin inhibition. Cancer Res. 2009;69:5467–5474. doi: 10.1158/0008-5472.CAN-08-4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothbart SB, Racanelli AC, Moran RG. Pemetrexed indirectly activates the metabolic kinase AMPK in human carcinomas. Cancer Res. 2010;70:10299–10309. doi: 10.1158/0008-5472.CAN-10-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jarmuła A. Antifolate Inhibitors of Thymidylate Synthase as Anticancer Drugs. Mini Rev Med Chem. 2010 Sep 21; doi: 10.2174/13895575110091211. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Fleeman N, Bagust A, McLeod C, Greenhalgh J, Boland A, Dundar Y, Dickson R, Tudur Smith C, Davis H, Green J, Pearson M. Pemetrexed for the first-line treatment of locally advanced or metastatic non-small cell lung cancer. Health Technol Assess. 2010;14 S1:47–53. doi: 10.3310/hta14Suppl1/07. [DOI] [PubMed] [Google Scholar]
- 5.Chattopadhyay S, Zhao R, Krupenko SA, Krupenko N, Goldman ID. The inverse relationship between reduced folate carrier function and pemetrexed activity in a human colon cancer cell line. Mol Cancer Ther. 2006;5:438–449. doi: 10.1158/1535-7163.MCT-05-0243. [DOI] [PubMed] [Google Scholar]
- 6.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 7.Peter ME, Krammer PH. The CD95 (APO-1/Fas) DISC and beyond. Cell Death Differ. 2003;10:26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- 8.Reed JC. Apoptosis-targeted therapies for cancer. Cancer Cell. 2003;3:17–22. doi: 10.1016/s1535-6108(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 9.Hyer ML, Samuel T, Reed JC. The FLIP-side of Fas signaling. Clin Cancer Res. 2006;12:5929–5931. doi: 10.1158/1078-0432.CCR-06-2098. [DOI] [PubMed] [Google Scholar]
- 10.Grinberg M, Sarig R, Zaltsman Y, Frumkin D, Grammatikakis N, Reuvany E, Gross A. tBid homooligomerizes in the mitochondrial membrane to induce apoptosis. J Biol Chem. 2002;277:12237–12245. doi: 10.1074/jbc.M104893200. [DOI] [PubMed] [Google Scholar]
- 11.Esposti MD. The roles of Bid. Apoptosis. 2002;7:433–440. doi: 10.1023/a:1020035124855. [DOI] [PubMed] [Google Scholar]
- 12.Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7:1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- 13.Gross A, Jockel J, Wei MC, Korsmeyer SJ. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borralho PM, Kren BT, Castro RE, da Silva IB, Steer CJ, Rodrigues CM. MicroRNA-143 reduces viability and increases sensitivity to 5-fluorouracil in HCT116 human colorectal cancer cells. FEBS J. 2009;276:6689–6700. doi: 10.1111/j.1742-4658.2009.07383.x. [DOI] [PubMed] [Google Scholar]
- 15.Vandermeers F, Hubert P, Delvenne P, Mascaux C, Grigoriu B, Burny A, Scherpereel A, Willems L. Valproate, in combination with pemetrexed and cisplatin, provides additional efficacy to the treatment of malignant mesothelioma. Clin Cancer Res. 2009;15:2818–2828. doi: 10.1158/1078-0432.CCR-08-1579. [DOI] [PubMed] [Google Scholar]
- 16.Dice JF. Chaperone-mediated autophagy. Autophagy. 2007;3:295–299. doi: 10.4161/auto.4144. [DOI] [PubMed] [Google Scholar]
- 17.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yorimitsu T, Nair U, Yang Z, Klionsky DJ. Endoplasmic Reticulum Stress Triggers Autophagy. J Biol Chem. 2006;281:30299–30304. doi: 10.1074/jbc.M607007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogata M, Hino SI, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaushik S, Cuervo AM. Methods to monitor chaperone-mediated autophagy. Methods Enzymol. 2009;452:297–324. doi: 10.1016/S0076-6879(08)03619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaushik S, Cuervo AM. Autophagy as a cell-repair mechanism: activation of chaperone-mediated autophagy during oxidative stress. Mol Aspects Med. 2006;27:444–454. doi: 10.1016/j.mam.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizushima N, Yamamoto A, Hatano M, Kobayashi Y, Kabeya Y, Suzuki K, Tokuhisa T, Ohsumi Y, Yoshimori T. Dissection of Autophagosome Formation using Apg5-deficient Mouse Embryonic Stem Cells. J Cell Biol. 2001;152:657–668. doi: 10.1083/jcb.152.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuma A, Mizushima N, Ishihara N, Ohsumi Y. Formation of the 350-kDa Apg12-Apg5-Apg16 multimeric complex, mediated by Apg16 oligomerization, is essential for autophagy in yeast. J Biol Chem. 2002;277:18619–18625. doi: 10.1074/jbc.M111889200. [DOI] [PubMed] [Google Scholar]
- 24.George MD, Baba M, Scott SV, Mizushima N, Garrison BS, Ohsumi Y, Klionsky DJ. Apg5p functions in the sequestration step in the cytoplasm-to-vacuole targeting and macroautophagy pathways. Mol Biol Cell. 2000;11:969–982. doi: 10.1091/mbc.11.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqi FH, Underwood BR, Winslow AR, Rubinsztein DC. Regulation of Mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90:1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- 27.Mizushima N, Levine B. Autophagy in mammalian development and differentiation. Nat Cell Biol. 2010;12:823–830. doi: 10.1038/ncb0910-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010;12:814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tooze SA, Jefferies HB, Kalie E, Longatti A, McAlpine FE, McKnight NC, Orsi A, Polson HE, Razi M, Robinson DJ, Webber JL. Trafficking and signaling in mammalian autophagy. IUBMB Life. 2010;62:503–508. doi: 10.1002/iub.334. [DOI] [PubMed] [Google Scholar]
- 30.Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, Baehrecke EH, Lenardo MJ. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 31.Yacoub A, Hamed HA, Allegood J, Mitchell C, Spiegel S, Lesniak MS, Ogretmen B, Dash R, Sarkar D, Broaddus WC, Grant S, Curiel DT, Fisher PB, Dent P. PERK-dependent regulation of ceramide synthase 6 and thioredoxin play a key role in mda-7/IL-24-induced killing of primary human glioblastoma multiforme cells. Cancer Res. 2010;70:1120–1129. doi: 10.1158/0008-5472.CAN-09-4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park MA, Yacoub A, Rahmani M, Zhang G, Hart L, Hagan MP, Calderwood SK, Sherman MY, Koumenis C, Spiegel S, Chen CS, Graf M, Curiel DT, Fisher PB, Grant S, Dent P. OSU-03012 stimulates PKR-like endoplasmic reticulum-dependent increases in 70-kDa heat shock protein expression, attenuating its lethal actions in transformed cells. Mol Pharmacol. 2008;73:1168–1184. doi: 10.1124/mol.107.042697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker T, Mitchell C, Park MA, Yacoub A, Graf M, Rahmani M, Houghton PJ, Voelkel-Johnson C, Grant S, Dent P. Sorafenib and vorinostat kill colon cancer cells by CD95-dependent and -independent mechanisms. Mol Pharmacol. 2009;76:342–355. doi: 10.1124/mol.109.056523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin AP, Park MA, Mitchell C, Walker T, Rahmani M, Thorburn A, Häussinger D, Reinehr R, Grant S, Dent P. BCL-2 family inhibitors enhance histone deacetylase inhibitor and sorafenib lethality via autophagy and overcome blockade of the extrinsic pathway to facilitate killing. Mol Pharmacol. 2009;76:327–341. doi: 10.1124/mol.109.056309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park MA, Reinehr R, Häussinger D, Voelkel-Johnson C, Ogretmen B, Yacoub A, Grant S, Dent P. Sorafenib activates CD95 and promotes autophagy and cell death via Src family kinases in gastrointestinal tumor cells. Mol Cancer Ther. 2010;9:2220–2231. doi: 10.1158/1535-7163.MCT-10-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin AP, Mitchell C, Rahmani M, Nephew KP, Grant S, Dent P. Inhibition of MCL-1 enhances lapatinib toxicity and overcomes lapatinib resistance via BAK-dependent autophagy. Cancer Biol Ther. 2009;8:2084–2096. doi: 10.4161/cbt.8.21.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yacoub A, Park MA, Gupta P, Rahmani M, Zhang G, Hamed H, Hanna D, Sarkar D, Lebedeva IV, Emdad L, Sauane M, Vozhilla N, Spiegel S, Koumenis C, Graf M, Curiel DT, Grant S, Fisher PB, Dent P. Caspase-, cathepsin-, and PERK-dependent regulation of MDA-7/IL-24-induced cell killing in primary human glioma cells. Mol Cancer Ther. 2008;7:297–313. doi: 10.1158/1535-7163.MCT-07-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yacoub A, Park MA, Hanna D, Hong Y, Mitchell C, Pandya AP, Harada H, Powis G, Chen CS, Koumenis C, Grant S, Dent P. OSU-03012 promotes caspase-independent but PERK-, cathepsin B-, BID-, and AIFdependent killing of transformed cells. Mol Pharmacol. 2006;70:589–603. doi: 10.1124/mol.106.025007. [DOI] [PubMed] [Google Scholar]
- 39.Paglin S, Lee NY, Nakar C, Fitzgerald M, Plotkin J, Deuel B, Hackett N, McMahill M, Sphicas E, Lampen N, Yahalom J. Rapamycin-sensitive pathway regulates mitochondrial membrane potential, autophagy, and survival in irradiated MCF-7 cells. Cancer Res. 2005;65:11061–11070. doi: 10.1158/0008-5472.CAN-05-1083. [DOI] [PubMed] [Google Scholar]
- 40.Sini P, James D, Chresta C, Guichard S. Simultaneous inhibition of mTORC1 and mTORC2 by mTOR kinase inhibitor AZD8055 induces autophagy and cell death in cancer cells. Autophagy. 2010;6 doi: 10.4161/auto.6.4.11671. In press. [DOI] [PubMed] [Google Scholar]
- 41.Funderburk SF, Wang QJ, Yue Z. The Beclin 1-VPS34 complex--at the crossroads of autophagy and beyond. Trends Cell Biol. 2010;20:355–362. doi: 10.1016/j.tcb.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kansra S, Yamagata L, Sneade L, Foster L, Ben-Jonathan N. Differential effects of estrogen receptor antagonists on pituitary lactotroph proliferation and prolactin release. Mol Cell Endocrinol. 2005;239:27–36. doi: 10.1016/j.mce.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 43.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 44.Schmid K, Bago-Horvath Z, Berger W, Haitel A, Cejka D, Werzowa J, Filipits M, Herberger B, Hayden H, Sieghart W. Dual inhibition of EGFR and mTOR pathways in small cell lung cancer. Br J Cancer. 2010;103:622–628. doi: 10.1038/sj.bjc.6605761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, Fan Z. The epidermal growth factor receptor antibody cetuximab induces autophagy in cancer cells by downregulating HIF-1alpha and Bcl-2 and activating the beclin 1/hVps34 complex. Cancer Res. 2010;70:5942–5952. doi: 10.1158/0008-5472.CAN-10-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu WK, Cho CH, Lee CW, Wu YC, Yu L, Li ZJ, Wong CC, Li HT, Zhang L, Ren SX, Che CT, Wu K, Fan D, Yu J, Sung JJ. Macroautophagy and ERK phosphorylation counteract the anti-proliferative effect of proteasome inhibitor in gastric cancer cells. Autophagy. 2010;6:228–238. doi: 10.4161/auto.6.2.11042. [DOI] [PubMed] [Google Scholar]
- 47.Schmid K, Bago-Horvath Z, Berger W, Haitel A, Cejka D, Werzowa J, Filipits M, Herberger B, Hayden H, Sieghart W. Dual inhibition of EGFR and mTOR pathways in small cell lung cancer. Br J Cancer. 2010;103:622–628. doi: 10.1038/sj.bjc.6605761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beffy P, Del Carratore R, Masini M, Furling D, Puymirat J, Masiello P, Simili M. Altered signal transduction pathways and induction of autophagy in human myotonic dystrophy type 1 myoblasts. Int J Biochem Cell Biol. 2010 doi: 10.1016/j.biocel.2010.08.010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 49.Aoki H, Takada Y, Kondo S, Sawaya R, Aggarwal BB, Kondo Y. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: role of Akt and extracellular signal-regulated kinase signaling pathways. Mol Pharmacol. 2007;72:29–39. doi: 10.1124/mol.106.033167. [DOI] [PubMed] [Google Scholar]
- 50.Chu C, Shatkin AJ. Apoptosis and autophagy induction in mammalian cells by small interfering RNA knockdown of mRNA capping enzymes. Mol Cell Biol. 2008;28:5829–5836. doi: 10.1128/MCB.00021-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stravopodis DJ, Karkoulis PK, Konstantakou EG, Melachroinou S, Thanasopoulou A, Aravantinos G, Margaritis LH, Anastasiadou E, Voutsinas GE. Thymidylate synthase inhibition induces p53-dependent and p53-independent apoptotic responses in human urinary bladder cancer cells. J Cancer Res Clin Oncol. 2010 doi: 10.1007/s00432-010-0891-y. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maiuri MC, Criollo A, Tasdemir E, Vicencio JM, Tajeddine N, Hickman JA, Geneste O, Kroemer G. BH3-only proteins and BH3 mimetics induce autophagy by competitively disrupting the interaction between Beclin 1 and Bcl-2/Bcl-X(L) Autophagy. 2007;3:374–676. doi: 10.4161/auto.4237. [DOI] [PubMed] [Google Scholar]
- 53.Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, Tasdemir E, Pierron G, Troulinaki K, Tavernarakis N, Hickman JA, Geneste O, Kroemer G. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007;26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Motyl T, Gajkowska B, Zarzyńska J, Gajewska M, Lamparska-Przybysz M. Apoptosis and autophagy in mammary gland remodeling and breast cancer chemotherapy. J Physiol Pharmacol. 2006;57 S7:17–32. [PubMed] [Google Scholar]
- 55.Luo S, Rubinsztein DC. Apoptosis blocks Beclin 1-dependent autophagosome synthesis: an effect rescued by Bcl-xL. Cell Death Differ. 2010;17:268–277. doi: 10.1038/cdd.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Z, Chen B, Wu Y, Jin F, Xia Y, Liu X. Genetic and epigenetic silencing of the beclin 1 gene in sporadic breast tumors. BMC Cancer. 2010;10:98–110. doi: 10.1186/1471-2407-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amaravadi RK, Yu D, Lum JJ, Bui T, Christophorou MA, Evan GI, Thomas-Tikhonenko A, Thompson CB. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest. 2007;117:326–336. doi: 10.1172/JCI28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roy S, Debnath J. Autophagy and Tumorigenesis. Semin Immunopathol. 2010 doi: 10.1007/s00281-010-0213-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.