Abstract
Magnesium (Mg2+) transport across membranes plays an essential role in cellular growth and survival. TRPM7 is the unique fusion of a Mg2+ permeable pore with an active cytosolic kinase domain, and is considered a master regulator of cellular Mg2+ homeostasis. We previously found that the genetic deletion of TRPM7 in DT40 B-cells results in Mg2+ deficiency and severe growth impairment, which can be rescued by supplementation with excess extracellular Mg2+. Here, we show that gene expression of the Mg2+ selective transporter MagT1 is upregulated in TRPM7−/− cells. Furthermore, overexpression of MagT1 in TRPM7−/− cells augments their capacity to uptake Mg2+, and improves their growth behavior in the absence of excess Mg2+.
Keywords: MagT1, Mg2+ homeostasis, channel-kinases, TRPM7 complementation, DT40 B cells
1. Introduction
As the most abundant intracellular divalent cation, Mg2+ is essential for a multitude of physiological processes, including protein synthesis and cell proliferation [1,2]. Transport of Mg2+ across membranes plays an important role in maintaining cellular equilibrium [3], yet the molecular mechanisms underlying vertebrate Mg2+ homeostasis have just begun to be characterized [4,5]. In this study we focus on the ability of one Mg2+ transporter, MagT1 (Magnesium transporter subtype 1), to complement the deletion of TRPM7 (Transient Receptor Potential Melastatin 7), an ion channel shown to play a major role in regulating cellular Mg2+ homeostasis [5,6].
TRPM7 and its closest relative, TRPM6, are the only examples of ion channels covalently linked to a kinase domain. Biophysical studies have shown that TRPM7 is a channel selective for divalent cations and can be inhibited by intracellular Mg2+ or MgATP [7]. The genetic disruption of TRPM7 in DT40 B-cells results in reduced intracellular Mg2+ levels and cell growth arrest under physiological concentrations of Mg2+ (0.5–1 mM). Importantly, this phenotype can be reversed by supplementation with high (10 mM) extracellular Mg2+, but not by adding Ca2+, Mn2+, Zn2+, or Ni2+ to the culture media [8]. Similarly, patients deficient in TRPM6 suffer from lethal seizures, but can live normally if nutritionally supplemented with Mg2+ [9,10]. Others have demonstrated that in C. elegans and zebrafish, TRPM7 homologues are also involved in Mg2+ regulation [11,12]. TRPM7− deficiency in mice is embryonic lethal, though rescue by supplemental Mg2+ was not tested in this study [13]. Recently, a different group published that embryonic stem cells lacking TRPM7 kinase show minimal TRPM7 channel activity and a proliferation arrest phenotype that, similar to the TRPM7-deficient DT40 cells, can be rescued by Mg2+ supplementation [14].
Currently, the only known selective plasma membrane Mg2+ transporter is MagT1. It has been demonstrated to be essential for vertebrate Mg2+ influx [3], and is broadly expressed in human tissues [15]. It was originally discovered as an upregulated transcript in mouse distal convoluted tubule (MDCT) cells grown under hypomagnesic conditions [16]. MagT1 was also recently captured in a screen designed to identify human molecules that can complement for the deficiency in the Alr1p Mg2+-transporter in yeast [15]. Knockdown of MagT1 leads to early developmental arrest in zebrafish embryos, and excess supplemental Mg2+ was shown to rescue this developmental block [15]. These studies and others also revealed that varying concentrations of extracellular Mg2+ can regulate expression of MagT1 in cell lines and murine tissues [17,18].
Given these reports, we asked whether MagT1 levels are increased in TRPM7−/− DT40 cells, possibly to help compensate for the negative effect of TRPM7-deficiency on intracellular [Mg2+], and show here that this is indeed the case. Furthermore, we find that overexpression of recombinant MagT1 in TRPM7−/− cells partially rescues the ability of these cells to uptake Mg2+, as well as their growth-defect in the absence of Mg2+ supplementation. Our results lend further credentials to the notion that MagT1 is a potent Mg2+ transporter pathway in vertebrates.
2. Materials and Methods
2.1. Molecular Biology
The coding sequence of chicken MagT1 was extracted from DT40 B cell cDNA using oligos designed from the gallus gallus MagT1 published sequence (GenBank ID: NM_001006435) 5’- ACGTGGTACCACTCATTAGGAAACTGTATGGATATCC -3’ and 5’- ACGTAAGCTTATGGCGGCGCTGCCGGTACTTGTG -3’. Chicken MagT1 was then cloned into the pcDNA4/TO-C-term-FLAG expression vector (KpnI/HindIII), allowing the expression of the C-terminally FLAG-tagged transporter.
2.2. DT40 Cell Line Construction, Cell Culture, and Growth Curves
The generation of DT40 TRPM7−/− cells, the cloning of human TRPM7 WT, and the establishment of stable inducible expression of hTRPM7 in TRPM7−/− DT40 cells have been previously described [8]. TRPM7−/− DT40 cells expressing the tet –repressor were transfected with pcDNA4/TO-constructs encoding C-terminally FLAG-tagged chicken MagT1. Cells were maintained in chemically defined HyQ CCM1 media supplemented with 10 mM MgCl2, P/S, Glu, 1% chicken serum, and 1mg/ml Zeocin (Invitrogen), 50 µg/ml Blasticidin (Invivogen). Growth curves were generated using customized Mg2+ free CCM1 media supplemented with 0.5 mM MgCl2 and 1% chicken serum.
2.3. Real-time Quantitative PCR analyses
DT40 WT and TRPM7−/− cells were cultured in Mg2+ deficient media for 0, 4, or 24 hours. Total RNA was isolated from cells using RNeasy (Qiagen) and converted to cDNA using Superscript III RT (Invitrogen). Quantitative RT-PCR was carried out using Sybr-Green Master Mix and DNA Engine Opticon 2 (BioRad formerly MJ Research). Primers used for detection of MagT1 (For 5’- GTGAACTATATCCATGGAAGC -3’ and Rev 5’- TCCTAAAGTAACACCACCATTG -3’) and GAPDH control (For 5'- TTGTTTCCTGGTATGACAATGAGTTT-3', Rev 5'-CTCACTCCTTGGATGCCATGT -3’).
2.4. Immunoblotting
Stable cell lines expressing MagT1 were lysed in 0.5% Triton lysis buffer, and proteins immunoprecipitated using anti-Flag M2 agarose beads at 4°C overnight under constant rotation. Beads were washed 3× with lysis buffer and proteins were eluted by boiling in 2× sample buffer. Proteins were separated by SDS-PAGE using 12.5% polyacrylamide gels, transferred to a polyvinylidene difluoride membrane, and analyzed by anti-FLAG (Sigma) immunoblotting.
2.5. Mg2+ Measurements
Intracellular concentrations of Mg2+ were measured using a QM-6/2003 spectrofluorometer (Photon Technology International). As previously described [19], cells were grown in chemically defined media supplemented with 10 mM MgCl2 and when required for induction of gene expression, treated with doxicycline 48 hours before being placed in 0.5 mM MgCl2. At 14–18 hours, 4×106 cells per sample were loaded with 1µg/ml Mag-Fura-2 (Invitrogen) for 30 min at room temperature in 0.5 mM MgCl2 buffer (135 mM NaCl, 5 mM KCl, 1 mM CaCl2, 5.6 mM Glucose, 10 mM Hepes, adjust pH to 7.5, then add 0.1% bovine serum albumin). The cells were washed, placed in a cuvette with a stirbar, and measured every 2 s at 340/380 nm excitation accompanied by monitoring 510 nm emission light for 10 min, with 9.5 mM or 19.5 mM of MgCl2 added directly into the cuvette at t=40s. The area under the curve was calculated by averaging the fluorescence-ratio (F340/F380) of the first 39s (before MgCl2 addition) to obtain baseline average. The baseline was subtracted from each timepoint in the indicated response (40–600s) and these values were summed and normalized to TRPM7−/−. The SEM was calculated for each group and a two-tailed t-test performed on four separate experiments.
3. Results
3.1. TRPM7−/− DT40 cells show higher MagT1 gene expression levels than wildtype cells
TRPM7−/− DT40 cells become Mg2+ deficient and die unless their culture medium is supplemented with 10–20 fold higher [Mg2+]e than the normal 0.5–1 mM physiologic levels provided to DT40 wildtype (WT) cells. Since it has been previously demonstrated that MagT1 is upregulated under hypomagnesic conditions, we hypothesized that TRPM7-deficiency might also elicit an increase in MagT1 gene expression. To test this idea, using quantitative RT-PCR, we analyzed chicken MagT1 specific transcript levels in WT vs. TRPM7−/− DT40 cells under varying Mg2+ levels.
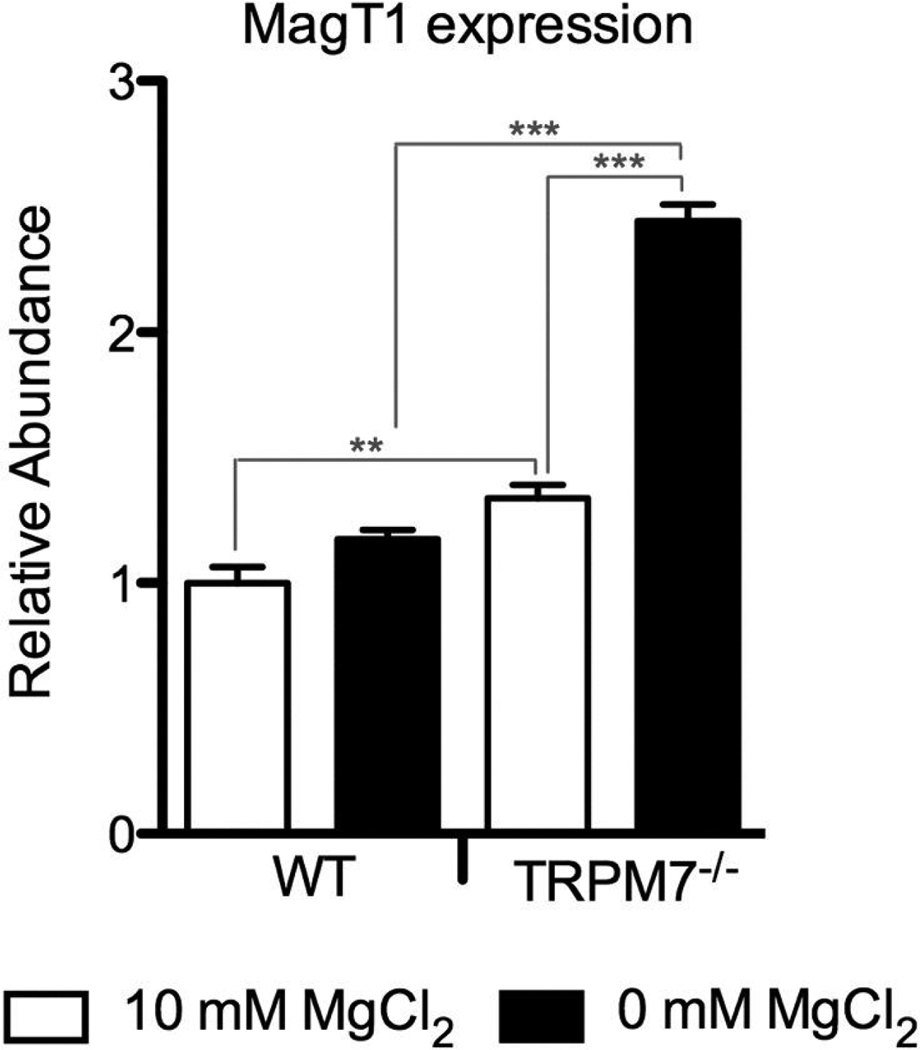
Since TRPM7−/− cells need to be maintained in 10 mM MgCl2 to ensure their survival, we cultured the WT cells under the same conditions for better comparison. As shown in Fig. 1, we detect MagT1 gene expression in WT DT40 cells at 10 mM MgCl2, and its level is elevated when the cells are cultured in Mg2+ free medium for 24hrs (p< 0.05). In the same experiment, we also found that MagT1 gene expression is further increased in TRPM7−/− cells, even in 10 mM MgCl2, but most substantially when TRPM7−/− cells were deprived of Mg2+ (p<0.01 and p<0.001 respectively, as compared to WT DT40s under the same Mg2+ conditions). We therefore conclude from these results that in an effort to compensate for the Mg2+ deficiency caused by the absence of TRPM7, TRPM7−/− DT40s are upregulating expression of the Mg2+ transporter MagT1. Although the added amount of MagT1 does not appear to be sufficient to rescue growth of the TRPM7−/− cells under normal levels of Mg2+, it might contribute to the rescue of their growth under 10 mM MgCl2.
Figure 1. TRPM7−/− DT40 cells show higher MagT1 gene expression levels than wildtype cells.
Quantitative RT-PCR was performed using total RNA from wildtype and TRPM7−/− DT40 cells maintained in media containing either 10 mM Mg2+, or cultured without Mg2+ for 24 hours. GAPDH levels were used as internal reference. Samples were measured in triplicate with error bars indicating SEM. Graph is from a representative experiment of four. *p < 0.05, **p < 0.01, ***p < 0.001.
3.2. Complementation with MagT1 partially restores growth in TRPM7−/− cells under physiologic extracellular [Mg2+]
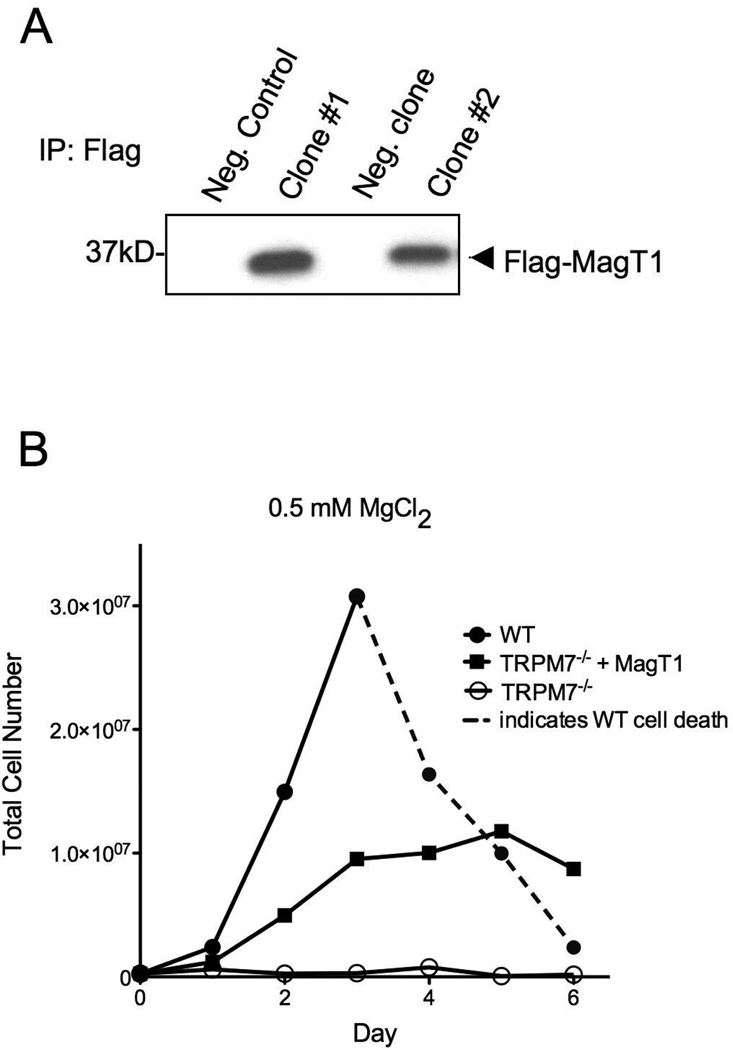
Based on the observations that TRPM7−/− cells exhibit higher levels of MagT1-transcript, we asked the question whether overexpression of recombinant MagT1 would allow TRPM7−/− cells to grow under physiologic concentrations of Mg2+ (0.5–1 mM). To this aim, chicken MagT1 cDNA was isolated from WT DT40 cells and cloned into an expression vector that adds a C-terminal Flag-tag for easy detection of the overexpressed protein by immuno-Western blot. We subsequently transfected TRPM7−/− DT40 cells cultured in 10 mM MgCl2 with the MagT1 construct, selected for zeocin-resistant clones, and screened for those with stable expression of flag-tagged MagT1. Two MagT1-expressing clones are shown in Fig. 2A, and clone #2 was used for subsequent experiments.
Figure 2. MagT1 overexpression partially reverses growth defect of TRPM7−/− DT40 cells.
A) Western Blot showing stable expression of Flag-tagged MagT1 in TRPM7−/− cell clones. Flag-MagT1 was immunoprecipitated using anti-Flag M2-agarose beads, and blot was probed with anti-Flag antibody. TRPM7−/− parental cell line was used as negative control. Positive clone #2 was selected for all subsequent experiments. B) Growth curves recorded in parallel of following DT40 cell lines: Wildtype, TRPM7−/−, or TRPM7−/− overexpressing MagT1, all grown in the presence of 0.5 mM Mg2+ for 6 days. Dotted line indicates cell death.
In order to monitor the growth of the MagT1-overxpressing TRPM7−/− DT40 cells in comparison to TRPM7−/− and WT DT40s, all cell lines were grown in parallel for 6 days in chemically defined media containing either 10 mM or 0.5 mM MgCl2. As anticipated, these cell lines show similar growth behavior under 10 mM Mg2+ (not shown to improve graph clarity). However, at 0.5 mM MgCl2, as previously reported, TRPM7−/− cells do not grow [8], whereas growth is partially restored in TRPM7−/− cells overexpressing MagT1, albeit not to WT levels. Thus, overexpression of the MagT1 Mg2+ transporter is beneficial to DT40 cells lacking TRPM7, although given the different biological properties of these two molecules, it is perhaps not surprising that the complementation is only partial.
3.3. Overexpression of MagT1 increases intracellular Mg2+ recovery in TRPM7−/− cells
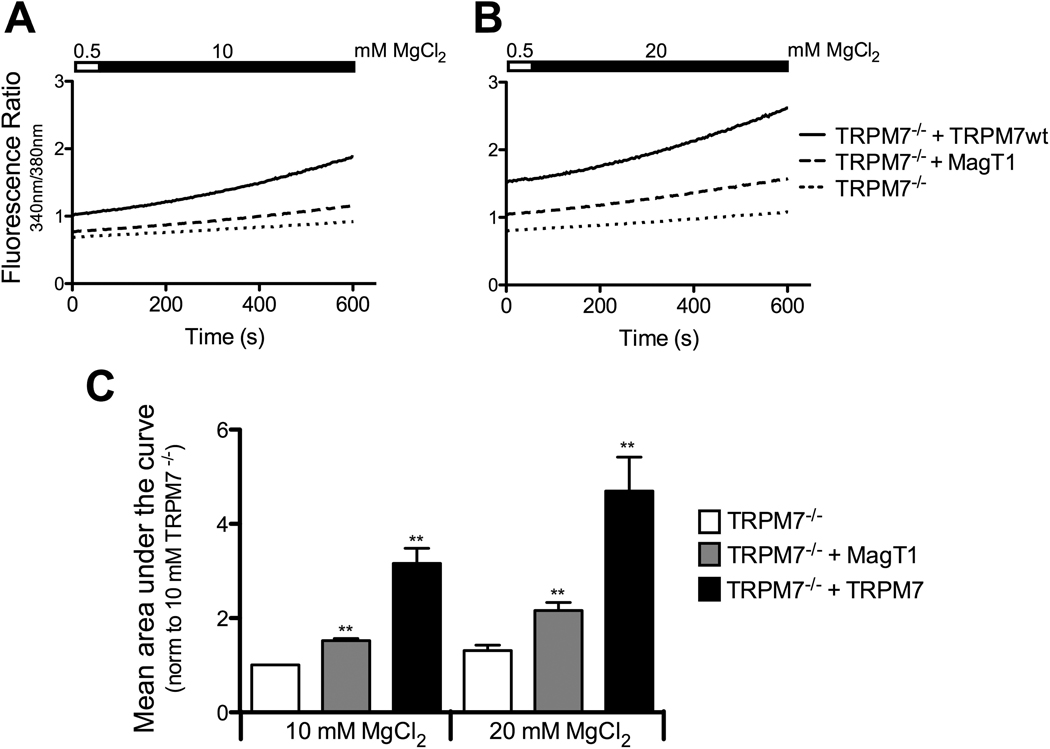
To further characterize the functional impact of MagT1-overexpression in TRPM7−/− cells, we wanted to investigate whether MagT1 alters the capacity of TRPM7−/− cells to uptake Mg2+. We therefore performed Mg2+ add back experiments in our DT40 cell lines cultured under hypomagnesic conditions, and monitored changes in cytosolic free Mg2+ levels by loading the cells with the Mg2+ sensitive fluorescent dye Mag-fura, as previously described [19]. All cells were preincubated in 0.5 mM MgCl2 for 14–18 hours, and cytosolic Mg2+ was measured after the addition of either 9.5 mM (Fig. 3A), or 19.5 mM MgCl2 (Fig. 3B). Notably, baseline fluorescence ratio in TRPM7−/− cells already appear to reflect lower levels of intracellular free Mg2+ than in cells complemented with MagT1 or TRPM7. Following 14–18hrs at 0.5 mM MgCl2, TRPM7−/− cells show the least cytosolic Mg2+ recovery when MgCl2 is added, as previously published [8]. When complemented with MagT1, Mg2+ uptake is increased in TRPM7−/− cells, and as would be expected, Mg2+ uptake is strongest when TRPM7 is reintroduced into these cells. The addition of 19.5 mM instead of 9.5 mM MgCl2 results in similar effects, but more pronounced, indicating that the amplitude of the Mg2+ uptake correlates with the amount of added extracellular MgCl2 (Fig. 3B,C). In sum, these results show that the improved growth behavior of MagT1-complemented TRPM7−/− DT40 cells is accompanied by an amelioration of the Mg2+ uptake capacity of these cells, suggesting that this is the mechanism by which MagT1 compensates for the absence of TRPM7.
Figure 3. Overexpression of MagT1 increases intracellular Mg2+ recovery in TRPM7−/− cells.
A) Changes in free cytosolic Mg2+ in DT40 wildtype cells in comparison to TRPM7−/− DT40 cells complemented with TRPM7 or MagT1. All cell lines were cultured under hypomagnesic conditions (0.5 mM Mg2+) for 14–18 hours, and then loaded with the Mg2+ sensitive fluorescent dye Mag-fura for 30 minutes in a 0.5 mM Mg2+ buffer, and samples analyzed using a fluorometer. 9.5 mM MgCl2 were added to the samples as indicated by the black filling in the bar above the graph. Shown trace is representative of four separate experiments. B) Experimental design as in A, except that 19.5 mM MgCl2 were added. Data is representative of six separate experiments. C) Quantification of the results presented under A and B where bars represent mean area under the curve normalized to TRPM7−/− cells at 10 mM final concentration. For statistical analysis, results were compared to values obtained from TRPM7−/− cells at the corresponding MgCl2 concentrations. Data shown are from 4 individual experiments, the mean ± SEM; **p < 0.01.
4. Discussion
In this study, we demonstrate that cells lacking the regulator of Mg2+ homeostasis, TRPM7, increase gene expression of the Mg2+ transporter MagT1, indicating that MagT1 function might be beneficial in situations of TRPM7-deficiency. This notion is further supported by our results showing that overexpression of MagT1 enables TRPM7−/− cells to regain viability and some growth under normal levels of Mg2+. This is in contrast to TRPM7−/− cells that require extracellular Mg2+ levels an order of magnitude higher than physiologic concentrations.
Our findings about MagT1 gene expression regulation are reminiscent of work done in HEK and MDCT cells where MagT1 expression was also upregulated in response to hypomagnesia [15,16]. Together with our data, these studies suggest that when cells are sensing suboptimal extracellular Mg2+, they respond by transcriptional upregulation of MagT1, probably in an effort to avoid intracellular Mg2+ deficiency by increasing Mg2+ uptake. Importantly, TRPM7 itself is not required to mediate this hypomagnesia-induced MagT1 upregulation given that this takes place in DT40 cells deprived of TRPM7. Since TRPM7 is described as a master regulator of Mg2+ regulation, it could be required to sense environmental Mg2+ availability and elicit upregulation of cellular Mg2+ transport capacity, but this appears not to be the case for MagT1. From our review of the literature, MagT1 gene expression does not appear to be uniformly regulated in all tissues. Other studies have shown that in low concentrations of Mg2+, mammary epithelial MagT1 expression remains unchanged [20], while rumen epithelial cells actually downregulate MagT1 expression [17,18]. These results imply that there may be differential regulation of Mg2+ and its transport machinery in various cell types.
We observed that overexpression of MagT1 in TRPM7−/− cells is capable of augmenting growth and increasing cytosolic Mg2+ recovery in the absence of supplemental Mg2+. This suggests that MagT1 enhances Mg2+ accumulation in TRPM7−/− cells, and supports the role of MagT1 as a Mg2+ influx system. These results further establish TRPM7−/− DT40 cells as a valid model system to test functionality of Mg2+ transport pathways. This experimental approach has been used previously to demonstrate that SLC41A2, another putative Mg2+ transporter, can indeed function as such. Similarly to MagT1, SLC41A2 restores partial growth of the TRPM7−/− cells under normagnesic conditions [21]. In the past, we have also used this system to demonstrate that TRPM7’s closest homologue and only other known channel-kinase fusion, TRPM6, cannot functionally compensate for TRPM7 in DT40s [19]. Together, the only partial TRPM7-complementations reported with either SLC41A2 or MagT1 suggest that TRPM7 remains essential for DT40s to proliferate and accrue Mg2+ when grown in physiological concentrations of Mg2+. Future studies will need to determine whether overexpression of multiple Mg2+ transporters in the absence of TRPM7 can lead to a complete rescue of the growth phenotype without supplemental Mg2+. In summary, our data suggests that MagT1 is an integral component of Mg2+ uptake in TRPM7−/− DT40s.
Highlights.
-
-
We analyzed the role of the Mg2+ transporter MagT1 in DT40 B cells lacking the Mg2+ regulator TRPM7.
-
-
Gene expression of MagT1 is upregulated in TRPM7−/− cells.
-
-
Overexpressing MagT1 in TRPM7−/− cells partially rescues their growth in the absence of excess Mg2+.
-
-
Overexpressing MagT1 in TRPM7−/− cells partially rescues their ability to uptake Mg2+.
Acknowledgements
This work was supported by National Institutes of Health Predoctoral Traineeship (NIH T32 AI07405), and by grants R01GM068801 (ALP), K08AI060926 and R01GM90123 (CS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rubin H. Magnesium: The missing element in molecular views of cell proliferation control. Bioessays. 2005;27:311–320. doi: 10.1002/bies.20183. [DOI] [PubMed] [Google Scholar]
- 2.Wolf FI, Trapani V. Cell (patho)physiology of magnesium. Clin Sci (Lond) 2008;114:27–35. doi: 10.1042/CS20070129. [DOI] [PubMed] [Google Scholar]
- 3.Quamme GA. Molecular identification of ancient and modern mammalian magnesium transporters. Am J Physiol Cell Physiol. 2010;298:C407–C429. doi: 10.1152/ajpcell.00124.2009. [DOI] [PubMed] [Google Scholar]
- 4.Romani AM. Magnesium homeostasis in mammalian cells. Front Biosci. 2007;12:308–331. doi: 10.2741/2066. [DOI] [PubMed] [Google Scholar]
- 5.Schmitz C, Deason F, Perraud AL. Molecular components of vertebrate Mg2+-homeostasis regulation. Magnes Res. 2007;20:6–18. [PubMed] [Google Scholar]
- 6.Schlingmann KP, Waldegger S, Konrad M, Chubanov V, Gudermann T. TRPM6 and TRPM7--Gatekeepers of human magnesium metabolism. Biochim Biophys Acta. 2007;1772:813–821. doi: 10.1016/j.bbadis.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Nadler MJ, et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz C, et al. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- 9.Schlingmann KP, et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet. 2002;31:166–170. doi: 10.1038/ng889. [DOI] [PubMed] [Google Scholar]
- 10.Walder RY, et al. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet. 2002;31:171–174. doi: 10.1038/ng901. [DOI] [PubMed] [Google Scholar]
- 11.Teramoto T, Sternick LA, Kage-Nakadai E, Sajjadi S, Siembida J, Mitani S, Iwasaki K, Lambie EJ. Magnesium excretion in C. elegans requires the activity of the GTL-2 TRPM channel. PLoS One. 2010;5:e9589. doi: 10.1371/journal.pone.0009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elizondo MR, Budi EH, Parichy DM. trpm7 regulation of in vivo cation homeostasis and kidney function involves stanniocalcin 1 and fgf23. Endocrinology. 2010;151:5700–5709. doi: 10.1210/en.2010-0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin J, Desai BN, Navarro B, Donovan A, Andrews NC, Clapham DE. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science. 2008;322:756–760. doi: 10.1126/science.1163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryazanova LV, et al. TRPM7 is essential for Mg(2+) homeostasis in mammals. Nat Commun. 2010;1:109. doi: 10.1038/ncomms1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou H, Clapham DE. Mammalian MagT1 and TUSC3 are required for cellular magnesium uptake and vertebrate embryonic development. Proc Natl Acad Sci U S A. 2009;106:15750–15755. doi: 10.1073/pnas.0908332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goytain A, Quamme GA. Identification and characterization of a novel mammalian Mg2+ transporter with channel-like properties. BMC Genomics. 2005;6:48. doi: 10.1186/1471-2164-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schweigel M, Kolisek M, Nikolic Z, Kuzinski J. Expression and functional activity of the Na/Mg exchanger, TRPM7 and MagT1 are changed to regulate Mg homeostasis and transport in rumen epithelial cells. Magnes Res. 2008;21:118–123. [PubMed] [Google Scholar]
- 18.Schweigel M, Kuzinski J, Deiner C, Kolisek M. Rumen epithelial cells adapt magnesium transport to high and low extracellular magnesium conditions. Magnes Res. 2009;22:133–150. [PubMed] [Google Scholar]
- 19.Schmitz C, Dorovkov MV, Zhao X, Davenport BJ, Ryazanov AG, Perraud AL. The channel kinases TRPM6 and TRPM7 are functionally nonredundant. J Biol Chem. 2005;280:37763–37771. doi: 10.1074/jbc.M509175200. [DOI] [PubMed] [Google Scholar]
- 20.Wolf FI, Trapani V, Simonacci M, Mastrototaro L, Cittadini A, Schweigel M. Modulation of TRPM6 and Na(+)/Mg(2+) exchange in mammary epithelial cells in response to variations of magnesium availability. J Cell Physiol. 2010;222:374–381. doi: 10.1002/jcp.21961. [DOI] [PubMed] [Google Scholar]
- 21.Sahni J, Nelson B, Scharenberg AM. SLC41A2 encodes a plasma-membrane Mg2+ transporter. Biochem J. 2007;401:505–513. doi: 10.1042/BJ20060673. [DOI] [PMC free article] [PubMed] [Google Scholar]