Abstract
The magnitude and functional quality of antiviral CD8 T cell responses are critical for the efficacy of T cell based vaccines. Here, we investigate the influence of two popular viral vectors, adenovirus type 5 (Ad5) and modified vaccinia Ankara (MVA), on expansion, contraction and memory differentiation of HIV-1 Gag insert-specific CD8 T cell responses following immunization and show different patterns for the two recombinant viral vectors. The Ad5 vector primed 6-fold higher levels of insert-specific CD8 effector T cells than the MVA vector. The Ad5-primed effector cells also underwent less contraction (< 2-fold) than the MVA-primed cells (>5-fold). The Ad5-primed memory cells were predominantly CD62L negative (effector memory) whereas the MVA-primed memory cells were predominantly CD62L positive (central memory). Consistent with their memory phenotype, MVA-primed CD8 T cells underwent higher fold expansion than Ad5-primed CD8 T cells following a homologous or heterologous boost. Impressively, the Ad5 boost changed the quality of MVA-primed memory response such that they undergo less contraction with effector memory phenotype. However, the MVA boost did not influence the contraction and memory phenotype of Ad5-primed response. In conclusion, our results demonstrate that vaccine vector strongly influences the expansion, contraction and the functional quality of insert-specific CD8 T cell responses and have implications for vaccine development against infectious diseases.
Keywords: MVA, Ad5, CD8 T cells, Viral vectors
1. Introduction
The goal of vaccination is to elicit long lasting cellular and humoral immunity capable of rapidly expanding and restricting the replication of an infection. Naive CD8 T cells undergo clonal expansion following exposure to antigen and generate cytolytic effector cells. Following antigen clearance, these effector cells contract and progressively differentiate into long-lived memory cells [1]. Generation of functional effector and memory CD8 T cells is critical for the control of many viral infections. The functional quality as well as the frequency of memory CD8 T cells is important for their effector function and control of an infectious agent [2-5]. Two subsets of memory cells, central memory (TCM) and effector memory (TEM), have been defined based on their expression of the lymph node homing molecules CCR7 and CD62L [6]. TCM cells express CCR7/CD62L, preferentially home to lymph nodes, produce IL-2 and possess high proliferative capacity. On the other hand, TEM cells lack CCR7/CD62L expression, preferentially home to nonlymphoid tissues, predominantly produce IFN-γ and possess immediate killing function. A study in mice demonstrated that TCM have a greater capacity than TEM to persist in vivo and are more efficient in mediating protective immunity because of their high proliferative potential [7]. Similarly, an association between the frequency of SIV-specific TCM cells and control of SIV infection has been shown in macaques [8].
The lineage of memory T cell development is not fully understood. The above mouse study [7] proposed a linear differentiation model where, following antigen clearance, effector cells differentiate into TEM then convert to TCM. However, other studies have suggested the formation of TCM cells without passing through an effector-cell stage [6, 9-12]. More recent studies in mice have shown that the lineage differentiation of memory cells can be influenced by the initial T cell frequency, clonal competition and strength of stimulus [13-17].
Various factors influence the expansion, contraction and memory differentiation of antiviral CD8 T cells following antigen exposure [1]. These include instructive signals from antigen presenting cells (APC) and the cytokine environment [18]. Different viruses interact with innate immunity through different pattern recognition receptors that influence the instructive signals provided by the APC and the cytokine environment [19, 20]. Thus, the magnitude and functional quality of the memory pool elicited by different viral vectors are different. Here, we study the kinetics of expansion and contraction, and the functional quality of CD8 T cells elicited by recombinant adenovirus type 5 (Ad5) and modified vaccinia Ankara (MVA) viral vectors to understand the influence of these vaccine vectors on the magnitude and quality of insert-specific immunity in mice. Recombinants of both of these vectors are currently being tested in humans as candidate vaccines for multiple infectious diseases.
2. Materials and Methods
2.1 Immunizations
Six to 8 week old BALB/c mice were purchased from Charles River Laboratories (Wilmington, MA). Mice were immunized with either 1×106 or 1×107 pfu (titered on chicken embryo fibroblast cells) of MVA/HIV [21] or Ad5/HIV in 100μl volume of PBS intramuscularly by needle and syringe into each quadriceps (50μl/quadriceps). The MVA immunogen expressed HIV-1 Gag-Pol (chimera of HXB2 and BH10) under mH5 promoter and HIV Env (ADA, with a deletion of 115 amino acids of the cytoplasmic tail of gp41) under a synthetic promoter Psyn II. The Ad5 immunogen expressed HIV-1 clade B Gag and Env. Both genes are derived from the strain 89.6 and were expressed under the control of CMV promoter. The Ad5/HIV (E1−/E3−) was generated by homologous recombination between the pAdTrackCMVgagCMVenv and the Ad genomic plasmid pAdEasy-1 (Stratagene) in E. coli BJ5183. The plasmid pAdTrackCMVgagCMVenv was generated using gag cDNA obtained from Dr. Gary Nabel [22] and env cDNA from Dr. Richard Compans [23]. Both of these cDNAs have been codon-optimized for Rev-independent expression. The env cDNA has an ~150 amino acid cytoplasmic domain COOH-terminal truncation, which has been shown to increase cell surface expression [23] and the gag cDNA has a 68 amino acid COOH-terminal truncation. Following homologous recombination, candidate clones were screened by PacI restriction enzyme and sequenced. Positive clones were transfected into HEK 293 cells and the rescued virus was purified by double centrifugation on cesium chloride gradients, subjected to dialysis and titered on 293-AD cells using a standardized 50% tissue culture infectious dose (TCID50) assay.
2.2 Cell isolation
Blood was collected in 1 ml of 3.7% sodium citrate solution by retro orbital bleeding and diluted with 2 ml of RPMI 1640 containing 5% FBS. After lysis of red blood cells with ACK lysing buffer (Invitrogen corporation, Carlsbad, CA), leucocytes were washed and used for staining. Cells from multiple tissues were isolated as described previously [24]. Briefly, spleen and lymph nodes were mashed through a 100μm cell strainer (BD Falcon) using a plunger and collected in 15 ml conical centrifuge tube. Red blood cells were lysed and leucocytes were washed twice with RPMI 1640 containing 10% FBS before use. Lung and liver tissues were minced and homogenized using a sieve and plunger and passed through 100μm cell strainer with minimal force. The resulting suspension was collected in 50 ml centrifuge tube containing RPMI-1640/5% FBS and centrifuged at 300 x g for 10 min to remove the debris. The resulting pellet was digested with collagenase 100 U/ml (Worthington Biochemical Corporation, Lakewood, NJ) at 37°C for 40 min in RPMI-1640/5% FBS. Cells were pelleted by centrifugation and resuspended in 44% percoll (Sigma, St. Louis, MO) layered on 67% percoll and centrifuged at 600g. Cells at the interphase were collected and washed twice with RPMI 1640 containing 10% FBS before use.
2.3 Tetramer analysis
Gag specific CD8 T cells were enumerated by staining with H2-Kd tetrameric complexes that binds to TCR for the immunodominant Gag CD8 epitope AMQMLKETI[25]. Briefly, cells obtained from blood and tissues were stained with FITC conjugated anti-CD4 (clone RM4-5) and anti-CD19 (clone 1D3), PE conjugated anti-CD11a (clone 2D7), PerCP conjugated anti-CD8 (clone 53-6.7) (all from BD-Pharmingen, San Diego, CA) and APC conjugated Gag tetramer. Cells were washed twice in PBS containing 2% FBS and fixed in 0.2 ml of 1% Formaldehyde. Approximately 200,000 lymphocytes were acquired on a FACSCalibur (Becton Dickinson, San Jose, CA) and analyzed using FlowJo software (FlowJo, Ashland, OR). Tetramer+, CD8+, CD11a+, CD4−, CD19− cells were scored as tetramer positive cells. For the analysis of CD62L and CD127 positive cells, anti-CD11a antibody was replaced with antibody against CD62L (clone-MEL-14) or CD127 (clone-A7R34), respectively.
2.4 Intracellular cytokine staining analysis
Approximately 1×106 splenocytes were stimulated in 5 ml polypropylene tubes in 200 μl RPMI containing 10% FCS and 0.1μg/ml of Gag immunodominant peptide, AMQMLKETI. After 2 hrs, Golgi stop was added according to the manufacturers instructions in a volume of 10μl and the cells were cultured for an additional 4 hrs at 37°C. Cells were surface stained with antibody to mouse CD8 conjugated to PerCP (clone Ly-2) at 8°-10°C for 30 min., washed twice with cold PBS containing 2% FBS, and fixed and permeabilized with Cytofix/Cytoperm solution. Cells were then incubated with antibodies to mouse CD3 (clone 145-2C11), IFN-γ (clone XMG1.2) and IL-2 (clone JES6-5H4) conjugated to FITC, PE and APC, respectively in Perm wash solution for 30 min at 4°C. Cells were washed twice with Perm wash, once with plain PBS and resuspended in 1% Formaldehyde in PBS. All reagents were purchased from BD Pharmingen, San Diego, CA. Approximately 200,000 lymphocytes were acquired on the FACScalibur and analyzed using FlowJo software.
2.5 Detection of viral genomes
Total DNA was isolated from splenocytes using Qiagen DNA isolation kit. 2.5μl of DNA solution was used for PCR analysis. A nested PCR was used to amplify the Ad5 genome as described before [26]. This nested PCR assay amplifies the region 20721-21572 of the Ad2 sequence. The outer forward primer, 5′-ACC TTC TAT CTT AAT CAC A-3′ and the outer reverse primer, 5′-TTG GCG TAG AGA AGG TTT T-3′ in the first round of 45 PCR cycles produce an 852-bp product. The inner forward, 5′-GCC ATT ACC TTT GAC TCT TCT GT-3′ and inner reverse primers 5′-CCT GCT GAT ACT CCT TGT ATT TAG TAT C-3′ amplify a 286-bp final product in 30 cycles of PCR. Published [26] and unpublished (personal communication with Dr. Linda Gooding at Emory University) results have established that this nested PCR assay routinely detects five genome copies of all the species C adenoviruses including Ad5 and does not amplify representatives of other adenovirus serotypes.
MVA genome was screened using a nested PCR targeted at the thymidine kinase gene as described before [27]. Briefly, the first round of the PCR performed using the primers VTK1 (ATGAACGGCGGACATATTCAGTTG) and VTK-2 (TTATGAGTCGATGTAACACTTTCT), and was followed by a nested PCR on product from the first reaction using the primers NTK1 and NTK2 (ATAGCTCAATATAAATGCGTGAC and GCATTTCATACACACAGCAGTTA respectively). This should amplify a 335bp fragment from MVA genome. For MVA standard, known pfu of MVA/HIV was added to 2 million splenocytes, DNA was extracted and processed as described for test samples. Using this method we could detect 10 pfu of the recombinant MVA.
2.6 Statistical analysis
The student t-test was used for comparing the magnitude of CD8 T cells. Wilcoxon rank-sum test was used if data failed to meet the parametric assumptions. A two-sided p<0.05 was considered statistically significant. Statistical analyses were performed using S-PLUS 7.0.
3. Results
3.1 Ad5 vector primes higher magnitude of response that undergoes minimal contraction than MVA-primed response
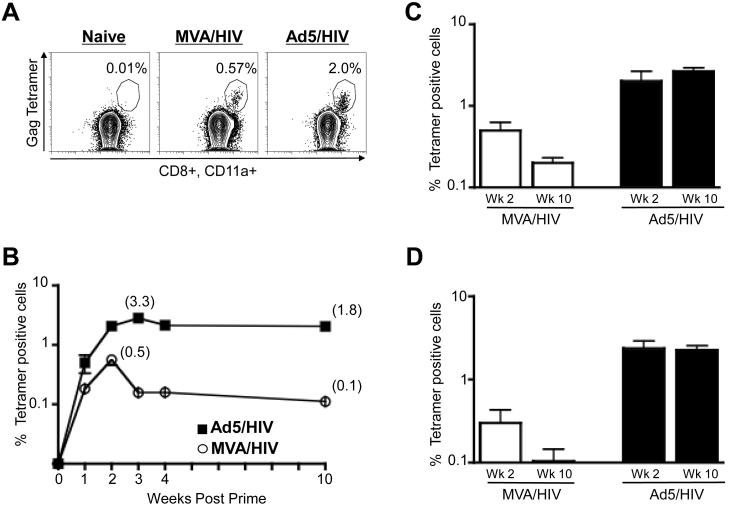
The Ad5 vector elicited a higher magnitude of Gag-specific CD8 T cell response than the MVA vector. We evaluated the frequency of Gag-specific CD8 T cell responses in peripheral blood of mice at various times following prime with 106 pfu of either recombinant Ad5 or MVA expressing HIV-1 clade B Gag (Fig. 1). The frequencies of Gag-specific CD8 T cells were enumerated using a MHC class I tetramer that identifies CD8 T cells specific for the immunodominant Gag epitope AMQMLKETI restricted by mouse MHC I molecule H2-Kd (Fig. 1). At one week following prime, low levels of tetramer positive cells could be detected in both Ad5 and MVA-primed mice. These responses underwent expansion and peaked at 2 weeks in MVA, and 2-3 weeks in Ad5-primed mice (Fig. 1A and 1B). At the peak response, the frequency of tetramer-specific cells in Ad5-primed mice was about 6-fold higher than in MVA-primed mice, mean of ~3.3% as opposed to 0.5% of total CD8 T cells (p<0.01). This was also true in the spleen of these mice (Fig. 1C).
Figure 1.
Magnitude of Gag tetramer-specific CD8 T cells in blood and spleen following priming with recombinant Ad5 and MVA vaccines. (A) Representative FACS plots demonstrating the frequency of Gag-tetramer-specific CD8 T cells raised by MVA/HIV (106 pfu, i.m.) or Ad5/HIV (106 pfu, i.m.) vaccines at 2 weeks following the prime in blood. PBMC were stained for CD8, CD4, CD20, CD11a and tetramer. CD8 T cells (CD4−, CD20−, CD8+) that were positive for tetramer and CD11ahi were scored as tetramer positive cells. The numbers on the FACS plots represent the frequency of tetramer positive cells as a percent of total CD8 T cells. (B) Temporal frequency of tetramer positive cells following the prime with 106 pfu of either vector in blood. Data represents mean values with standard error of mean (SEM) for a group of 5-10 mice. (C) Frequency of tetramer positive cells following prime with 106 pfu of either vector in spleen at the indicated time points. Data represent mean values with SEM for a group of 3-5 mice. (D) Frequency of tetramer positive cells following the prime with 107 pfu of either vector in blood. Data represents mean values with SEM for a group of 5 mice.
The peak CD8 response elicited by the MVA vector underwent a more marked contraction than the peak response elicited by the Ad5 vector (Fig. 1B and 1C). Contraction occurred within one week of the peak response. In the peripheral blood of Ad5 vaccinated mice, Gag-specific CD8 T cells contracted < 2-fold, whereas in MVA-primed mice they contracted ~5-fold (p<0.001). At ten weeks following prime, the magnitude of Gag tetramer-specific CD8 T cells in the Ad5-primed mice was about 18-fold higher than that in the MVA-primed mice (p=0.01). Likewise, spleen also showed comparable results (Fig. 1C). Additionally, similar patterns for magnitude and contraction were also observed in blood at 10-fold higher dose of these vectors (Fig. 1D). Collectively, these results demonstrate that Ad5 vectors prime higher frequencies of CD8 T cells that undergo less contraction than the CD8 T cell response primed by MVA.
3.2 MVA vector primes predominantly a central memory response whereas Ad5 vector primes an effector memory response
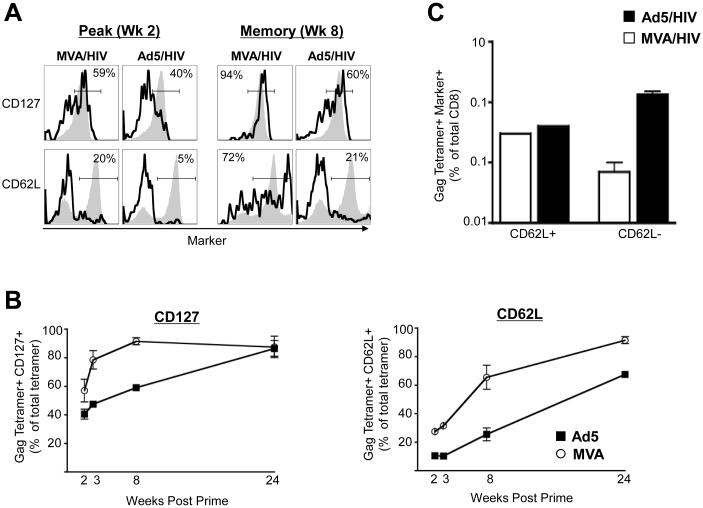
We next investigated the phenotype of the memory response elicited by MVA and Ad5 vectors following prime (Fig. 2). We measured temporal expression of the IL-7 receptor alpha (CD127) to study the differentiation of antigen-specific effector CD8 T cells (CD127−ve) into memory (CD127+ve) following vaccination. At two weeks following prime, ~ 40-60% of the total tetramer positive cells in MVA or Ad5-primed mice expressed CD127 (Fig. 2A and 2B). However, by 8 weeks following prime, the majority (greater than 90%) of the tetramer positive cells in the MVA-primed mice had differentiated into memory, whereas in Ad5-primed mice only ~ 60% of them had differentiated into memory (p=0.01). By 24 weeks post prime, the majority of tetramer positive cells in both groups had differentiated into memory (Fig. 2B). These results demonstrate that MVA-primed cells differentiate into memory more rapidly than Ad5-primed cells. However, because Ad5 vector primed higher frequency of tetramer positive cells, the frequency of total CD127 positive cells was marginally higher in Ad5-primed mice than MVA-primed mice (Fig. 2C).
Figure 2.
Phenotype and magnitude of the Gag tetramer-specific memory cells following priming with recombinant Ad5 and MVA vectors. (A) Representative FACS plots showing the frequency of Gag tetramer-specific CD8 T cells expressing either CD127 or CD62L raised by MVA/HIV (106 pfu, i.m.) or Ad5/HIV (106 pfu, i.m.) vaccines at 2 and 8 weeks following prime. Splenocytes were stained for CD8, CD3, tetramer and CD62L or CD127. CD8 T cells (CD3+, CD8+) were analyzed for expression of CD62L or CD127 and tetramer binding. Open black histograms represent tetramer positive cells and closed gray histograms represent total CD8 T cells. The numbers on the FACS plots represent the frequency of tetramer-positive cells that express CD62L or CD127 expressed as a percent of total tetramer-positive cells. (B) Temporal frequency of tetramer positive cells expressing either CD127 or CD62L following prime in spleen. The frequency of tetramer positive cells that express CD62L or CD127 expressed as a percent of total tetramer positive cells is shown. Data represent mean values for a group of 3-4 mice. (C) Magnitude of CD62L positive and negative cells in spleen at 8 weeks following prime. Frequency of tetramer+ CD62L+ or tetramer+ CD62L− cells were expressed as a percent of total CD8 T cells is shown. Data represents mean values with SEM for a group of 3-4 mice.
We further quantified the memory response as central (CD62L+) and effector (CD62L−) memory based on the expression of CD62L. As expected, at two weeks following prime (effector phase) the majority of tetramer positive cells in MVA or Ad5-primed mice did not express CD62L. However, by 8 weeks following prime about 60% of the MVA-primed cells expressed CD62L, whereas only 20% of the Ad5-primed cells expressed CD62L (p=0.05) (Fig. 2B). By 24 weeks following prime, the proportion of tetramer positive cells expressing CD62L had increased in both groups, but the difference between the two groups was maintained (Fig. 2B). These results demonstrate that the MVA vector predominantly primes a central memory response, whereas the Ad5 vector predominantly primes an effector memory response. However, at 8 weeks post prime, because of the higher number of memory cells in the Ad5- than MVA-primed mice, the frequency of central memory cells was similar in both groups and the frequency of effector memory cells was higher in the Ad5 group (Fig. 2C).
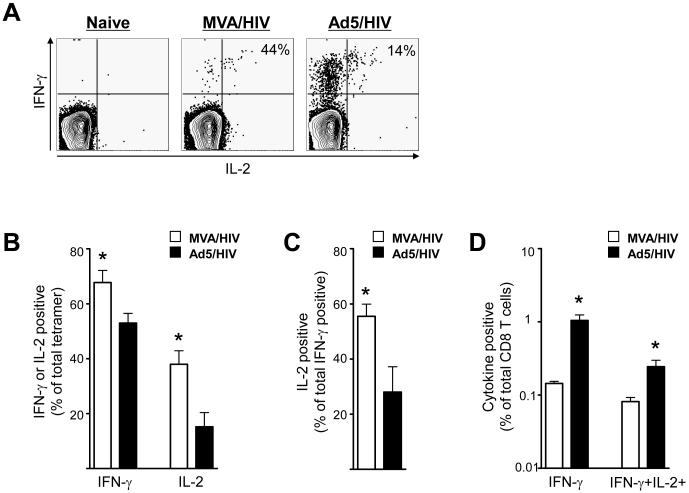
3.3 Higher proportion of MVA-primed than Ad5-primed cells produce IL-2
To understand the functional quality of vaccine elicited CD8 T cells, we next compared the ability of these cells to produce IFN-γ and IL-2 (Fig. 3). A higher proportion of Gag tetramer-specific CD8 T cells elicited by the MVA than Ad5 vector produced IFN-γ or IL-2. At 12 weeks following prime ~ 70% of MVA elicited tetramer positive cells produced IFN-γ, whereas only ~ 50% of Ad5-primed tetramer positive cells produced IFN-γ (Fig. 3B)(p<0.05). Similarly, ~ 40% of MVA elicited tetramer positive cells produced IL-2, whereas only < 20% of Ad5-primed tetramer positive cells produced IL-2 (Fig. 3B)(p<0.05). At this time, the proportion of IFN-γ positive cells that co-expressed IL-2 was higher for MVA-primed cells than Ad5-primed cells. About 55% of the total IFN-γ positive cells primed by MVA co-produced IL-2, whereas less than 30% of Ad5-primed IFN-γ positive cells co-produced IL-2 (Fig. 3A and 3C) (p<0.05). However, because of the higher number of Ad5-primed cells, by 12 weeks following prime, the frequency of Gag-specific IFN-γ producing cells was 7-fold and IFN-γ plus IL-2 producing cells was 3-fold higher in Ad5-primed than MVA-primed mice (Fig. 3D) (p<0.05).
Figure 3.
Cytokine production patterns of Gag-specific CD8 T cells following prime with Ad5 and MVA. (A) Representative FACS plots demonstrating the co-expression of IFN-γ and IL-2 by CD8 T cells raised by MVA and Ad5 vaccines at 12 weeks following prime. Splenocytes were stimulated with media alone or Gag immunodominant peptide and stained for CD8, CD3, IFN-γ and IL-2. CD8 T cells (CD3+, CD8+) were analyzed for expression of IFN-γ and IL-2. The numbers on the FACS plots represent the frequency of IL-2 positive cells that co-express IFN-γ, expressed as a percent of total IFN-γ positive cells. (B) Proportion of tetramer positive cells expressing either IFN-γ or IL-2 at 12 weeks following prime in spleen. The frequency of IFN-γ or IL-2 positive cells expressed as a percent of tetramer positive cells is shown. (C) Summary of the frequency of IL-2 positive cells that co-express IFN-γ expressed as a percent of total IFN-γ positive cells. (D) Magnitude of IFN-γ and IFN-γ plus IL-2 positive cells in spleen. Frequency of IFN-γ or IFN-γ plus IL-2 positive cells expressed as a percent of total CD8 T cells is shown. Data represents mean values with SEM for a group of 4 mice. * - Significantly different, p<0.05.
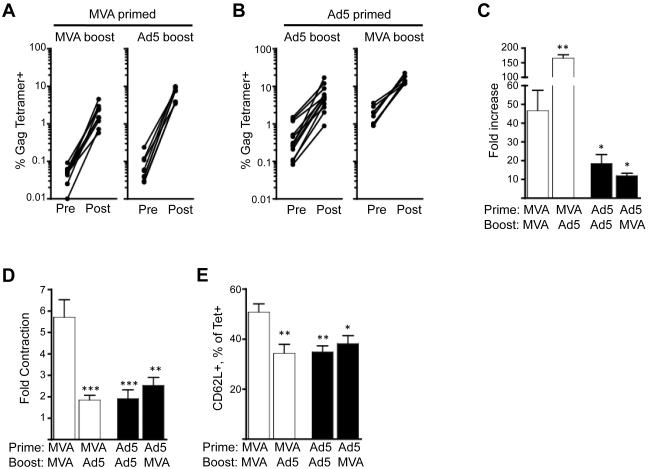
3.4 MVA-primed cells possess higher expansion potential and Ad5 changes properties of MVA-primed cells
We next determined the ability of Ad5 or MVA-primed memory cells to expand following encounter with antigen during a recall response. Both homologous and heterologous boosts were performed to control for the influence of vector-specific immunity on the boosting ability of the homologous vector. Both homologous and heterologous boosts resulted in rapid expansions of Gag-specific CD8 T cells (Fig. 4). The magnitude of Gag-specific CD8 cells was generally higher following a heterologous boost than a homologous boost (Fig. 4A, 4B). At one week following the boost, the magnitude of Gag tetramer-specific response ranged from 0.5-23% with MVA/MVA immunizations eliciting the lowest and Ad5/MVA immunizations eliciting the highest responses. The MVA-primed cells underwent higher fold expansion than Ad5 primed cells. This was true whether the MVA-primed mice were boosted with MVA or Ad5 (Fig. 4A). MVA-primed cells underwent a 47-fold expansion following MVA boost and a 163-fold expansion following an Ad5 boost (Fig. 4C). In contrast to MVA-primed cells, Ad5-primed cells underwent 12-18 fold expansion following either Ad5 or MVA boost (Fig. 4C).
Figure 4.
Expansion of Gag-specific CD8 T cells following boost in blood. MVA or Ad5 primed mice were boosted with either MVA or Ad5 at 8-10 weeks after the prime. (A) Expansion of MVA-primed Gag tetramer-specific CD8 T cells following MVA or Ad5 boost. (B) Expansion of Ad5-primed Gag tetramer-specific CD8 T cells following MVA or Ad5 boost. (C) Fold increase in the frequency of Gag tetramer-specific CD8 T cells following boost. Fold increase was calculated as a ratio of tetramer positive cells at week 1 post boost (post) and pre boost (pre). (D) Fold contraction of the frequency of Gag tetramer-specific CD8 T cells following boost. Fold contraction was calculated as a ratio of tetramer positive cells at week 1 post boost and week 10 post boost. (E) CD62L expression by Gag tetramer positive cells at 10 weeks post boost. ***, p<0.001; **, p<0.01; *, p<0.05; p values indicate significantly lower or higher responses compared to MVA/MVA group.
Following the boost, Ad5 but not MVA changed the contraction (Fig. 4D) and memory differentiation (Fig. 4E) patterns of responding primary memory cells. To understand the influence of Ad5 or MVA on the quality of primary memory response, we studied the contraction and memory differentiation of Gag-specific T cells following homologous and heterologous boosts. Consistent with the post prime observation, the Ad5-boosted cells underwent less contraction than MVA-boosted cells following the respective homologous boosts (Fig. 4D). In the Ad5/Ad5 group, the Gag-specific CD8 T cells contracted about 1.9 fold, whereas in the MVA/MVA mice they contracted about 6 fold. However, a different contraction pattern was observed following heterologous boosts. The Gag-specific CD8 T cells contracted about 2.5 and 1.9 fold in Ad5/MVA and MVA/Ad5 groups, respectively. These results demonstrate that the Gag-specific CD8 T cells undergo less contraction when Ad5 is used during either prime or boost. A similar pattern was also observed for expression of CD62L. A significantly higher proportion of MVA-primed and MVA-boosted cells expressed CD62L than Ad5-primed and Ad5-boosted or MVA-primed and Ad5-boosted cells (Fig. 4E).
3.5 Ad5 vector but not MVA vector persists in vivo
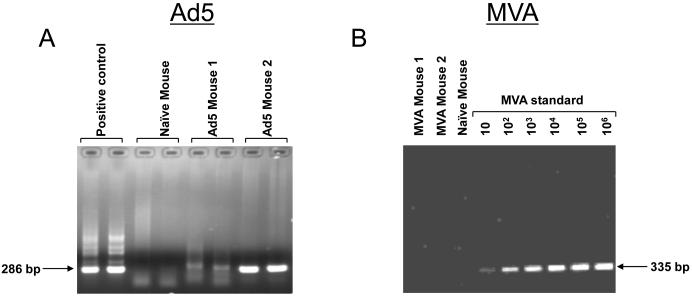
We next investigated the persistence of these two vectors in the spleen at 12 weeks following prime. Splenocytes were evaluated for the presence of viral genomes using a semi-quantitative PCR analysis. As can be seen in Figure 5, Ad5 virus genome could be detected at 12 weeks after the prime, whereas MVA genome could not be detected at this time. These results demonstrate that replication defective Ad5 vectors, but not MVA vectors, persist after immunization.
Figure 5.
Persistence of (A) Ad5 viral vector but not (B) MVA viral vector in spleen at 12 weeks following prime. DNA was isolated from splenocytes and analyzed for viral genomes using semi-quantitative PCR as described in methods. Analyses for Ad5 genome were performed in duplicates. MVA standard was generated as described in materials and methods.
4. Discussion
Our study investigating the influence of vaccine vector on the magnitude and quality of vaccine insert-specific memory responses demonstrates that the vaccine vector influences both the expansion and contraction of insert-specific effector CD8 T cells. The Ad5-primed CD8 T cells underwent higher expansion and lower contraction than MVA-primed CD8 T cells, which resulted in an18-fold higher frequency of memory cells in Ad5-primed mice than in MVA-primed mice. A similar expansion and contraction patterns for Ad5 were also evident in non-human primate studies that studied the immunogenicity of Ad5 recombinant expressing SIV Gag[28-31]. Interestingly, the minimal contraction observed in Ad5-primed mice differed from that observed for acute lymphocytic choriomeningitis virus (LCMV) infection-induced CD8 T cells, which undergo a 10-fold contraction following the initial expansion phase [32]. A 10-fold contraction has also been observed for CD8 T cells elicited by replication competent vaccinia virus in mice [33], monkeys [34] and humans [35-37]. These results suggest that the Ad5-primed CD8 T cells exhibit a different pattern of contraction compared to the CD8 T cells elicited by LCMV and poxvirus vectors. It is interesting to note that the Ad5 effect on contraction was dominant and could change the contraction pattern of MVA-primed cells in a MVA/Ad5 heterologous prime/boost modality.
Our study also demonstrates that recombinant Ad5 and MVA vectors prime different memory subsets of insert-specific CD8 T cells. The Ad5 vector primed predominantly an effector memory response, whereas the MVA vector primed predominantly a central memory response. Consistent with the memory phenotype, a higher proportion of MVA-primed cells produced IL-2 than Ad5-primed cells. Similarly, following a homologous as well as heterologous boost, MVA-specific cells underwent greater expansions than Ad5-primed cells. The lower expansion in Ad5-primed mice was not due to limiting levels of antigen presenting cells as a similar expansion was also observed for a 10-fold higher dose of the Ad5 boost (data not shown). Collectively these results demonstrate that the Ad5 vector predominantly elicits effector memory cells that undergo less expansion following antigen encounter, whereas the MVA vector predominantly primes central memory cells that undergo more expansion following antigen encounter.
Multiple reasons, including the level of antigen expression per cell, antigen persistence, infection of different dendritic cells and interaction with different toll-like receptors could have contributed to the differences between CD8 T cell responses elicited by Ad5 and MVA vectors. Higher level of antigen expression per cell by the recombinant vector can lead to priming of higher magnitude of T cell response. To understand the contribution of antigen expression, we quantitated the Gag expression on a per-cell basis in vitro by infecting 293T cells with either Ad5/HIV or MVA/HIV followed by flow cytometry based detection of Gag using fluorochrome conjugated anti-Gag Ab (clone KC57). We found that the mean fluorescence intensity of Gag positive cells was similar between Ad5 and MVA infected cells suggesting that higher expression of CD8 T cell response in Ad5 primed mice may not be due to higher levels of antigen expression (Supplementary Figure 1). Similarly, a prior study comparing the immunogenicity of Ad5 and vaccinia virus recombinant vectors in mice demonstrated that Ad5 elicits higher magnitude of antigen-specific IFNγ producing T cells despite producing less transgene product[38]. However, it is important to measure the Gag-expression in vivo in different cell types early following immunization with recombinant vectors to make more concrete conclusions about the contribution of level of antigen for enhancing immunogenicity.
To understand the role of antigen persistence on the memory differentiation we investigated the presence of viral genomes in spleen at 3 months following prime. Our results demonstrated that Ad5 but not MVA persist in vivo. These results are consistent with a recent report demonstrating the persistence of Ad5 vector and the expression of recombinant protein by the persisting vector both in mice and rhesus macaques [39]. MVA does not persist in vaccinated SCID mice and rhesus macaques [40]. Thus, the observed dominant effector memory response in Ad5-primed mice could be due to the persistence of Ad5. The length of antigen persistence during prime has been shown to influence the quality of memory CD8 T cell responses in mice [17]. Interestingly, however, the minimal contraction of the effector response in Ad5-primed mice may not be due only to the persistence of antigen, as effector CD8 T cells have been shown to undergo a 10-fold contraction despite the presence of high levels of persisting antigen in mice chronically infected with LCMV [41]. A similar contraction has also been observed for chronic SIV infections in macaques [42].
The mechanisms of persistence of Ad5 and how it influences the memory differentiation of elicited CD8 T cells is not completely understood. The cell types Ad5 targets can vary significantly between mice and humans due to differences in expression of CAR receptor and thus may influence the memory differentiation of elicited T cells in these two species. Similarly, it is also important to note that other adenovirus serotypes are being used as vaccine candidates[31, 43, 44], and the quality of memory T cells elicited by these vectors could be different from Ad5 due to differences in tropism, level of antigen expression and persistence. In conclusion, our results demonstrate that vaccine vector strongly influences the expansion, contraction and the functional quality of insert-specific CD8 T cell responses and have implications for vaccine development against infectious diseases.
Supplementary Material
5. Acknowledgements
We are grateful to Drs. B. Moss, P. Earl and L. Wyatt for providing MVA; Drs. L. Gooding and W. Huang for help with quantitation of adenoviral genomes in immunized mice. We thank Mrs. Helen Drake-Perrow for outstanding administrative support. We are thankful to the Yerkes Division of Research Resources for the consistent excellence of animal care support. Also, we thank the NIH AIDS Research and Reference reagent Program for the provision of peptides. This work was supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases grants R01 AI57029 to RA; P01 AI49364 to HR; and Yerkes National Primate Research Center base grant, P51 RR00165.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- [1].Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2(4):251–62. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- [2].Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006 Jun 15;107(12):4781–9. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007 Jul;13(7):843–50. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- [4].Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J Virol. 2007 Aug;81(16):8468–76. doi: 10.1128/JVI.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kannanganat S, Kapogiannis BG, Ibegbu C, Chennareddi L, Goepfert P, Robinson HL, et al. Human immunodeficiency virus type 1 controllers but not noncontrollers maintain CD4 T cells coexpressing three cytokines. J Virol. 2007 Nov;81(21):12071–6. doi: 10.1128/JVI.01261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- [7].Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003 Mar;4(3):225–34. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- [8].Vaccari M, Trindade CJ, Venzon D, Zanetti M, Franchini G. Vaccine-induced CD8+ central memory T cells in protection from simian AIDS. J Immunol. 2005 Sep 15;175(6):3502–7. doi: 10.4049/jimmunol.175.6.3502. [DOI] [PubMed] [Google Scholar]
- [9].Lauvau G, Vijh S, Kong P, Horng T, Kerksiek K, Serbina N, et al. Priming of memory but not effector CD8 T cells by a killed bacterial vaccine. Science. 2001 Nov 23;294(5547):1735–9. doi: 10.1126/science.1064571. [DOI] [PubMed] [Google Scholar]
- [10].Manjunath N, Shankar P, Wan J, Weninger W, Crowley MA, Hieshima K, et al. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J Clin Invest. 2001 Sep;108(6):871–8. doi: 10.1172/JCI13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000 Oct;1(4):311–6. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- [12].Iezzi G, Scheidegger D, Lanzavecchia A. Migration and function of antigen-primed nonpolarized T lymphocytes in vivo. J Exp Med. 2001 Apr 16;193(8):987–93. doi: 10.1084/jem.193.8.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bouneaud C, Garcia Z, Kourilsky P, Pannetier C. Lineage relationships, homeostasis, and recall capacities of central- and effector-memory CD8 T cells in vivo. J Exp Med. 2005 Feb 21;201(4):579–90. doi: 10.1084/jem.20040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005 Aug;6(8):793–9. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jabbari A, Harty JT. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J Exp Med. 2006 Apr 17;203(4):919–32. doi: 10.1084/jem.20052237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007 Jun;26(6):827–41. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sarkar S, Teichgraber V, Kalia V, Polley A, Masopust D, Harrington LE, et al. Strength of stimulus and clonal competition impact the rate of memory CD8 T cell differentiation. J Immunol. 2007 Nov 15;179(10):6704–14. doi: 10.4049/jimmunol.179.10.6704. [DOI] [PubMed] [Google Scholar]
- [18].Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003 Apr;3(4):269–79. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- [19].Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006 Feb 24;124(4):849–63. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- [20].Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R, et al. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med. 2006 Feb 20;203(2):413–24. doi: 10.1084/jem.20051720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wyatt LS, Earl PL, Liu JY, Smith JM, Montefiori DC, Robinson HL, et al. Multiprotein HIV type 1 clade B DNA and MVA vaccines: construction, expression, and immunogenicity in rodents of the MVA component. AIDS Res Hum Retroviruses. 2004 Jun;20(6):645–53. doi: 10.1089/0889222041217428. [DOI] [PubMed] [Google Scholar]
- [22].Huang Y, Kong WP, Nabel GJ. Human immunodeficiency virus type 1-specific immunity after genetic immunization is enhanced by modification of Gag and Pol expression. J Virol. 2001;75(10):4947–51. doi: 10.1128/JVI.75.10.4947-4951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yao Q, Bu Z, Vzorov A, Yang C, Compans RW. Virus-like particle and DNA-based candidate AIDS vaccines. Vaccine. 2003 Jan 30;21(7-8):638–43. doi: 10.1016/s0264-410x(02)00572-8. [DOI] [PubMed] [Google Scholar]
- [24].Lefrancois L, Lycke N. Current Protocols in Immunology. John Wiley & Sons, Inc.; New Jersy: 1996. [Google Scholar]
- [25].Ganguly S, Manicassamy S, Blackwell J, Pulendran B, Amara RR. Adenovirus type 5 induces vitamin A-metabolizing enzymes in dendritic cells and enhances priming of gut-homing CD8 T cells. Mucosal immunology. Feb 2; doi: 10.1038/mi.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Garnett CT, Erdman D, Xu W, Gooding LR. Prevalence and quantitation of species C adenovirus DNA in human mucosal lymphocytes. J Virol. 2002 Nov;76(21):10608–16. doi: 10.1128/JVI.76.21.10608-10616.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chantrey J, Meyer H, Baxby D, Begon M, Bown KJ, Hazel SM, et al. Cowpox: reservoir hosts and geographic range. Epidemiol Infect. 1999 Jun;122(3):455–60. doi: 10.1017/s0950268899002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shiver JW, Fu T, Chen L, Casimiro D, Davies ME, Evans RK, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency virus immunity. Nature. 2002;415:331–5. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- [29].Casimiro DR, Chen L, Fu T-M, Evans RK, Caulfield MJ, Davies M-E, et al. Comparative Immunogenicity in Rhesus Monkeys of DNA Plasmid, Recombinant Vaccinia Virus, and Replication-Defective Adenovirus Vectors Expressing a Human Immunodeficiency Virus Type 1 gag Gene. J Virol. 2003 June 1;77(11):6305–13. doi: 10.1128/JVI.77.11.6305-6313.2003. 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Santra S, Seaman MS, Xu L, Barouch DH, Lord CI, Lifton MA, et al. Replication-Defective Adenovirus Serotype 5 Vectors Elicit Durable Cellular and Humoral Immune Responses in Nonhuman Primates. J Virol. 2005 May 15;79(10):6516–22. doi: 10.1128/JVI.79.10.6516-6522.2005. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liu J, O/’Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457(7225):87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8(2):177–87. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- [33].Harrington LE, Most Rv R, Whitton JL, Ahmed R. Recombinant vaccinia virus-induced T-cell immunity: quantitation of the response to the virus vector and the foreign epitope. J Virol. 2002;76(7):3329–37. doi: 10.1128/JVI.76.7.3329-3337.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nigam P, Earl PL, Americo JL, Sharma S, Wyatt LS, Edghill-Spano Y, et al. DNA/MVA HIV-1/AIDS vaccine elicits long-lived vaccinia virus-specific immunity and confers protection against a lethal monkeypox challenge. Virology. 2007 Sep 15;366(1):73–83. doi: 10.1016/j.virol.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003 August 17; doi: 10.1038/nm917. 2003;10.1038/nm917:1-7. [DOI] [PubMed] [Google Scholar]
- [36].Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003 Nov 15;171(10):4969–73. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- [37].Amara RR, Nigam P, Sharma S, Liu J, Bostik V. Long-Lived Poxvirus Immunity, Robust CD4 Help, and Better Persistence of CD4 than CD8 T Cells. J Virol. 2004 April 15;78(8):3811–6. doi: 10.1128/JVI.78.8.3811-3816.2004. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Maeda K, West K, Hayasaka D, Ennis FA, Terajima M. Recombinant adenovirus vector vaccine induces stronger cytotoxic T-cell responses than recombinant vaccinia virus vector, plasmid DNA, or a combination of these. Viral Immunol. 2005;18(4):657–67. doi: 10.1089/vim.2005.18.657. [DOI] [PubMed] [Google Scholar]
- [39].Tatsis N, Fitzgerald JC, Reyes-Sandoval A, Harris-McCoy KC, Hensley SE, Zhou D, et al. Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines. Blood. 2007 Sep 15;110(6):1916–23. doi: 10.1182/blood-2007-02-062117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hanke T, McMichael AJ, Dennis MJ, Sharpe SA, Powell LA, McLoughlin L, et al. Biodistribution and persistence of an MVA-vectored candidate HIV vaccine in SIV-infected rhesus macaques and SCID mice. Vaccine. 2005 Feb 10;23(12):1507–14. doi: 10.1016/j.vaccine.2004.08.050. [DOI] [PubMed] [Google Scholar]
- [41].Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003 Apr;77(8):4911–27. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Amara RR, Villinger F, Altman JD, Lydy SL, O’Neil SP, Staprans S, et al. Control of a Mucosal Challenge and Prevention of AIDS by a Multiprotein DNA/MVA Vaccine. Science. 2001 April 6;292:69–74. doi: 10.1126/science.1058915. 2001. [DOI] [PubMed] [Google Scholar]
- [43].Barouch DH, Pau MG, Custers JH, Koudstaal W, Kostense S, Havenga MJ, et al. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol. 2004 May 15;172(10):6290–7. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- [44].Roberts DM, Nanda A, Havenga MJ, Abbink P, Lynch DM, Ewald BA, et al. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature. 2006 May 11;441(7090):239–43. doi: 10.1038/nature04721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.