Abstract
Selective inhibitors of human peptide deformylase (HsPDF) are predicted to constitute a new class of antitumor agents. We report the identification of benzofuran-4,5-diones as the first known selective HsPDF inhibitors and we describe their selectivity profile in a panel of metalloproteases. We characterize their struture activity relationships for antitumor activity in a panel of cancer cell lines, and we assess their in vivo efficacy in a mouse xenograft model. Our results demonstrate that selective HsPDF inhibitors based on the benzofuran-4,5-dione scaffold constitute a novel class of antitumor agents that are potent in vitro and in vivo.
Keywords: Human peptide deformylase; Benzofuran-4,5-diones; Structure activity relationships; Fluorescence polarization; Antiproliferative agents
During protein synthesis in prokaryotes, the N-formyl group of nascent peptides is removed from most peptides in order to yield mature proteins. Consequently, PDF activity is essential to bacterial growth [1,2]. Since until recently PDF was thought to be absent from eukaryotes, PDF has constituted an attractive target for the development of antibotics [3]. However, the demonstration of the existence of a functional human analogue of PDF [4-6] raises concerns over the use of non selective PDF inhibitors as antibacterial agents in humans.
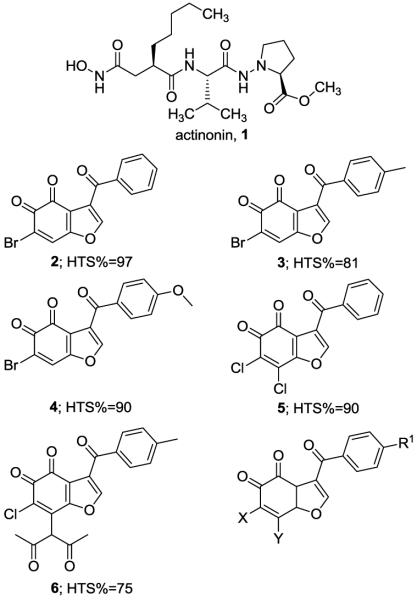
Following the observation that HsPDF inhibition by actinonin (1) and actinonin analogs or by specific siRNA knockdown of expression is associated with antiproliferative effect in cancer cells [7], we speculated that HsPDF inhibitors could constitute a new class of antitumor agents. However, most currently known PDF inhibitors such as actinonin consist of a peptidomimetic backbone attached to a hydroxamic acid moiety, and this class of compounds is typically associated with poor selectivity across metalloproteases [8-11]. In addition, their poor bioavailability precludes their use in vivo as antitumor agents. The crystal structure of an N-terminal truncated, catalytically active HsPDF revealed structural differences between HsPDF and EcPDF such as a characteristic entrance to the active site that provide a rationale for the identification of selective HsPDF inhibitors [12]. For this reason, we developed and validated a strategy that would allow us to identify novel non peptidomimetic and non hydroxamic acid based inhibitors of HsPDF [13], and we subsequently embarked in the screening of a library of 200,000 small molecules using our proven strategy. Among the confirmed positives identified in this campaign were 5 compounds (2-6) belonging to the chemical scaffold of benzofuran-4,5-diones (Fig. 1). All 5 compounds induced ≥ 75% inhibition at 10 μM in our fluorescence polarization-based assay for HsPDF (Fig. 1) in absence of any optical interference, which was measured as previously described [13]. In addition, the 5 benzofuran-4,5-diones identified during primary screening were confirmed as functional inhibitors of HsPDF using a methodology previously described [13]. While the benzofuran moiety is included in inhibitors of various enzymes, to our knowledge, no inhibitory activity toward any PDF and no antitumor activity has previously been described for the chemical scaffold of benzofuran-4,5-diones. In order to expand the limited structure activity relationships of benzofuran-4,5-diones gathered during primary screening, we initiated exploratory chemistry efforts aimed at defining the importance of the halogen substitutions at α- and β-positions on the 4,5-orthodione moiety.
Figure 1.
Chemical structure of actinonin, 1; chemical structure and percentage inhibition (HTS%) of confirmed positives in primary screen belonging to the benzofuran-4,5-dione scaffold, 2-6; general chemical structure of the primary hits belonging to the benzofuran-4,5-dione scaffold.
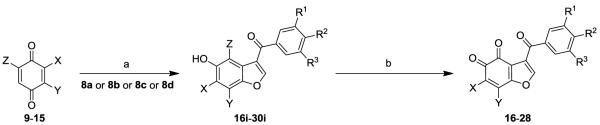
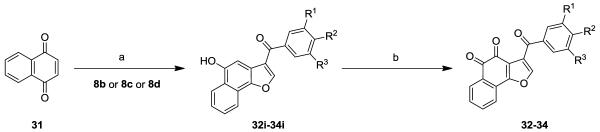
For the synthesis of 13 novel benzofuran-4,5-dione derivatives and 3 napthofurandione derivatives, we engaged in a strategy relying on acid catalyzed reaction of substituted enaminones with appropriately halogenated 1,4-quinones [14-17] to provide a general construct of substituted 5-hydroxy benzofuran and naphthofuran derivatives, followed by oxidation with a suitable oxidant. Toward this end, substituted acetophenones 7a-7d were reacted with dimethyl formamide dimethyl acetal at 150°C in DMF to give the enaminones 8a-8d[18] in 63-88% yield (Scheme 1). The enaminones 8a-8d were reacted with appropriately halogenated 1,4-quinones and hydroquinone in acetic acid as a solvent to give the corresponding 5-hydroxybenzofuran derivatives 16i-30i [19,20] (Scheme 2), as well as the corresponding 5-hydroxynaphthofuran derivatives 32i-34i [20] (Scheme 3) in variable yields. The oxidation of 5-hydroxybenzofuran derivatives 16i-28i and 5-hydroxynaphthofuran derivatives 32i-34i was best accomplished via either nitric acid [22,23] or with Dess-Martin’s periodinane, to give the corresponding substituted 4,5-benzofurandiones 16-28 with 40 to 57% yield and the 4,5-naphthofurandiones 32-34 with 38 to 43% yield.
Scheme 1.
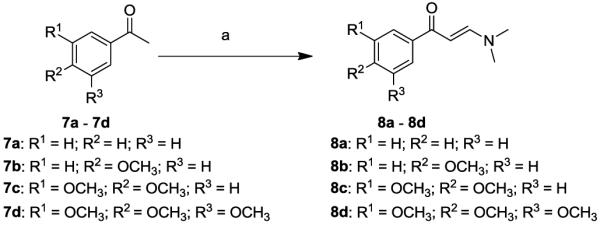
Synthesis of the enaminones 8a-8d. Reagents and conditions: (a) DMF-DMA, 150°C, 20-30h
Scheme 2.
Synthesis of benzofuran-4,5-diones. Reagents and conditions: (a) AcOH, rt; (b) For 16, 17, 19, 21, 28: Dess-Martin periodinane, DMSO, 0°C→rt, 20 min; For 5, 20, 22-27: HNO3, AcOH, rt→65°C, 3h. Z = Br or Cl, matching X and Y.
Scheme 3.
Synthesis of naphtofurandiones. Reagents and conditions: (a) AcOH, rt; (b) HNO3, AcOH, rt→50°C, 30 min.
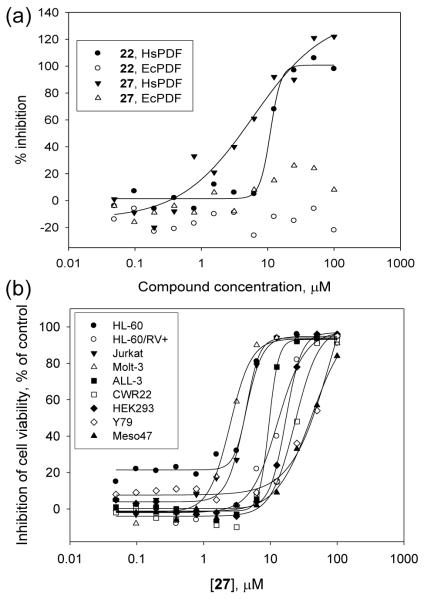
We evaluated the potency of the 13 novel benzofuran-4,5-dione derivatives toward HsPDF and EcPDF as well as their selectivity profile using a previously validated methodology [10]. We found that all the benzofuran-4,5-dione derivatives we have synthesized inhibit HsPDF with an IC50 ranging from 5.2 to 65 μM (Table 1). In contrast, when we characterized the potency of the 5-hydroxybenzofuran intermediates of synthesis 16i-30i, we found that they were all inactive toward HsPDF up to 100 μM (Suppl. Table 1). This result clearly indicates that the orthodione moiety in the benzofuran-4,5-dione scaffold is important for activity. Similarly, the three napthofurandiones 32, 33 and 34 we have synthesized have no activity toward any of the metalloproteases tested (Suppl. Table 2), suggesting that substituting the benzofurandione moiety by a naphtofurandione moiety abrogates activity. Interestingly, since the benzofurandiones 16 and 17 lacking a halogen substituent at α- and β-positions on the 4,5-orthodione moiety are potent toward HsPDF (Table 1), we can conclude that halogens in those positions are not required for activity, and that the naphtofurandiones 32, 33 and 34 are not inactive because they lack halogens, but rather because the benzofuran-4,5-dione moiety is necessary for activity. While halogen substitutions at α- and β-positions on the 4,5-orthodione moiety are not required for HsPDF activity, they do seem to increase the potency of benzofuran-4,5-diones toward HsPDF, since the top six most potent derivatives we have synthesized harbor at least one chloro or one bromo substituent in those positions. Similarly, we observe that the top three most potent derivatives have two or three methoxy substituents on the benzoyl moiety (Table 1), indicating that those substituents could be responsible for the formation of hydrogen bonds with the target. With an IC50 of 5.2 μM toward HsPDF, 27 is the most potent HsPDF inhibitor we have characterized. When we assessed the selectivity of benzofuran-4,5-diones, we found that only one of the derivatives we have synthesized was weakly active toward EcPDF (23, IC50 = 67 μM), while 12 out of 13 had no measurable activity toward EcPDF (Table 1). This result contrasts with the broad activity of benzofuran-4,5-diones toward the human enzyme and demonstrates that this class of compounds is selective for the human PDF compared to the bacterial enzyme. To our knowledge, benzofuran-4,5-diones constitute the first class of compounds with such a specificity, since all currently known HsPDF inhibitors are peptidomimetic- and hydroxamic acid-based that inhibit both HsPDF and EcPDF [6,8,24], and tend to exhibit low specificity among metalloproteases [8-11]. When we further characterized the selectivity profile of benzofuran-4,5-diones at 100 μM in a panel of metalloproteases, we found that the newly synthesized compounds are not only selective for HsPDF compared to EcPDF, but also demonstrate selectivity for HsPDF among other metalloproteases such as APN and MMP-1; as an example, 22 had no significant activity toward EcPDF and MMP-1, while being potent toward HsPDF (IC50 = 15 μM) (Table 1, Fig. 2a). Similarly, 16, 17 and 28 completely inhibited HsPDF while having low activity toward EcPDF, APN and MMP-1 (Table 1). Altogether, our results demonstrate that benzofuran-4,5-diones constitute the first known selective inhibitors of HsPDF. With an IC50 of 5.2 μM toward HsPDF and no significant activity toward the bacterial enzyme (Table 1, Figure 2a), 27 is the most potent and selective HsPDF inhibitor we have characterized.
Table 1.
Potency and selectivity profile of benzofuran-4,5-dione derivatives.
 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compd | X | Y | R1 | R2 | R3 | HsPDF (IC50, μM) |
EcPDF (IC50, μM) |
HsPDF (%I) |
EcPDF (%I) |
APN (%I) |
MMP-1 (%I) |
| 1 | - | - | - | - | - | 2.7 | 0.14* | 98 | 99 | 95 | 97 |
| 16 | H | H | H | H | H | 59 | >100 | 100 | 32 | 5 | 0 |
| 17 | H | H | H | OMe | H | 34 | >100 | 100 | 24 | 4 | 6 |
| 20 | Cl | H | H | OMe | H | 40 | >100 | 100 | 11 | 54 | 11 |
| 28 | Br | H | H | OMe | H | 32 | >100 | 100 | 5 | 22 | 26 |
| 19 | H | Cl | H | OMe | H | 16 | >100 | 100 | 13 | 68 | 10 |
| 21 | H | Br | H | OMe | H | 15 | >100 | 100 | 26 | 64 | 8 |
| 5 | Cl | Cl | H | H | H | 45 | >100 | 100 | 65 | 101 | 41 |
| 22 | Cl | Cl | H | OMe | H | 15 | >100 | 88 | 1 | 50 | 0 |
| 25 | Br | Br | H | OMe | H | 65 | >100 | 100 | 22 | 41 | 23 |
| 23 | Cl | Cl | OMe | OMe | H | 6.1 | 67 | 100 | 63 | 99 | 46 |
| 26 | Br | Br | OMe | OMe | H | 10 | >100 | 100 | 53 | 96 | 46 |
| 24 | Cl | Cl | OMe | OMe | OMe | 25 | >100 | 100 | 27 | 50 | 32 |
| 27 | Br | Br | OMe | OMe | OMe | 5.2 | >100 | 100 | 32 | 50 | 38 |
%I: % Inhibition at 100 μM, average of duplicates; 100: ≥100 %I; 0: ≤0 %I
as assessed in the FLUO functional assay [13]
Figure 2.
(a) Dose response curves for 22 and 27 toward HsPDF and EcPDF. (b) Cytotoxicity profiling of 27.
To confirm that the novel HsPDF inhibitors we have identified have no antibacterial activity as predicted by their lack of activity toward EcPDF, we determined the MIC of compounds 5 and 22 in a panel of 15 bacterial strains. We found that none of the compounds had measurable antibacterial activity toward 14 out of the 15 strains tested (MIC > 64 μg/mL), while actinonin was active in 12 out of the 15 strains tested as expected (Suppl. Table 3). This important result confirms that benzofuran-4,5-diones constitute the first known HsPDF inhibitors that specifically target the human enzyme and lack antibacterial activity, presumably because they are structurally different than actinonin and its derivatives and do not contain a hydroxamic acid moiety.
To verify the hypothesis that HsPDF inhibitors may constitute novel anticancer agents, we assessed the cytotoxicity profile of the 13 novel benzofuran-4,5-dione derivatives in a panel of nine well characterized cancer cell lines using a method previously described [25]. We found that all the benzofuran-4,5-dione derivatives we have synthesized have cytotoxic activity toward at least five out of nine of the cancer cell lines tested, with an IC50 ranging from 2.8 to 74 μM (Table 2). Interestingly, the most potent HsPDF inhibitor we have identified was also the most potent compound in the viability assay: 27 was active across all the cancer cell lines tested, with IC50 ranging from 2.8 to 37 μM, including the multidrug resistant cell line HL-60/RV+ (IC50 = 12 μM) (Table 2, Fig. 2).
Table 2.
Cytotoxicity profile of benzofuran-4,5-dione derivatives.
| Compd | HL-60 | HL-60/RV+ | Jurkat | Molt-3 | ALL-3 | CWR22 | HEK293 | Y79 | Meso47 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 16 | 100 | 40 | 45 | N.D. | 25 | 15 | N.D. | 100 |
| 16 | 21 | 22 | 8.1 | 7.3 | 22 | 30 | 23 | 30 | 63 |
| 17 | 17 | 27 | 8.9 | 9.1 | 29 | 35 | 21 | 31 | 100 |
| 20 | 32 | 67 | 57 | 47 | 34 | 69 | 100 | 67 | 71 |
| 28 | 48 | 67 | 20 | 4 | 48 | 71 | 54 | 100 | 71 |
| 19 | 17 | 48 | 13 | 11 | 47 | 59 | 40 | 74 | 100 |
| 21 | 10 | 38 | 12 | 11 | 35 | 46 | 36 | 63 | 100 |
| 5 | 72 | 100 | 46 | 31 | 69 | 72 | 100 | 100 | 100 |
| 22 | 10 | 43 | 6.1 | 4.1 | 27 | 50 | 30 | 66 | 100 |
| 25 | 53 | 100 | 55 | 40 | 70 | 70 | 100 | 68 | 100 |
| 23 | 69 | 100 | 67 | 71 | 69 | 68 | 100 | 66 | 72 |
| 26 | 42 | 64 | 28 | 25 | 36 | 100 | 100 | 100 | 100 |
| 24 | 36 | 55 | 5.9 | 7.0 | 49 | 67 | 67 | 64 | 100 |
| 27 | 5.4 | 12 | 2.8 | 2.9 | 10 | 21 | 16 | 33 | 37 |
IC50, μM ; 100: ≥100 μM ; N.D.: not determined
In summary, our findings provide guidelines for the development of selective HsPDF inhibitors active at the cellular level and altogether our results validate our strategy, in that we have designed novel and selective HsPDF inhibitors that have cell-based anticancer activity.
In a pilot study, we assessed the in vivo efficacy of 27 in a mouse xenograft model using human promyelocytic leukemia HL-60 cells. Our lead compound delayed the growth of HL-60 tumors by up to 40% (Fig. 3). To put this result in perspective, actinonin – which has a reported IC50 of 43 nM toward HsPDF[7] – must be administered twice a day for two weeks at the large dose of 250 mg/kg to delay tumor growth[7]. This difference in potency could be attributed to a better bioavailability compared to the peptidomimetic actinonin but further studies are needed to address this question. In light of this observation, our results are encouraging considering that 27, a benzofuran-4,5-dione derivative of first generation, was potent in vivo at a dose of 15 mg/kg.
Figure 3.
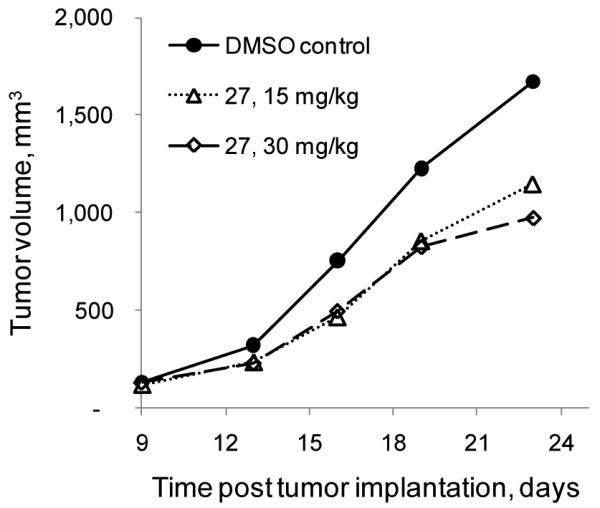
In vivo efficacy of 27 in a mouse xenograft model using HL-60 cells.
In conclusion, our findings strongly suggest that derivatives of the benzofuran-4,5-dione scaffold could constitute a new class of potent antitumor agents selective for HsPDF.
Supplementary Material
Acknowledgments
The authors thank Constantin Radu and the other members of the High Throughput Screening Core Facility for their help during the course of this study. The authors are greatful to Kumaraswamy Sorra, Benarjee Velaga, Srinivas Oruganti and Narinder Mohal for the chemical synthesis of the new derivatives described in this study. The HTS Core Facility is partially supported by Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research, the Experimental Therapeutics Center of MSKCC, the William Randolph Hearst Fund in Experimental Therapeutics, the Lilian S Wells Foundation and by an NIH/NCI Cancer Center Support Grant 5 P30 CA008748-44. This study was funded by NIH grant 1R21NS57008 (HD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Supplementary tables, experimental procedures, spectroscopic data and NMR data for all compounds are available as supplementary material.
References
- 1.Mazel D, Pochet S, Marliere P. EMBO J. 1994;13:914. doi: 10.1002/j.1460-2075.1994.tb06335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meinnel T, Blanquet S. J. Bacteriol. 1994;176:7387. doi: 10.1128/jb.176.23.7387-7390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meinnel T, Patiny L, Ragusa S, Blanquet S. Biochemistry. 1999;38:4287. doi: 10.1021/bi982622r. [DOI] [PubMed] [Google Scholar]
- 4.Serero A, Giglione C, Sardini A, Martinez-Sanz J, Meinnel T. J. Biol. Chem. 2003;278:52953. doi: 10.1074/jbc.M309770200. [DOI] [PubMed] [Google Scholar]
- 5.Lee MD, Antczak C, Li Y, Sirotnak FM, Bornmann WG, et al. Biochem. Biophys. Res. Commun. 2003;312:309. doi: 10.1016/j.bbrc.2003.10.123. [DOI] [PubMed] [Google Scholar]
- 6.Leeds JA, Dean CR. Curr. Opin. Pharmacol. 2006;6:445. doi: 10.1016/j.coph.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Lee MD, She Y, Soskis MJ, Borella CP, Gardner JR, Hayes PA, Dy BM, Heaney ML, Philips MR, Bornmann WG, Sirotnak FM, Scheinberg DA. J. Clin. Invest. 2004;114:1107. doi: 10.1172/JCI22269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kontogiorgis C, Papaioannou P, Hadjipavlou-Litina D. Curr. Med. Chem. 2005;12:339. doi: 10.2174/0929867053363243. [DOI] [PubMed] [Google Scholar]
- 9.Saghatelian A, Jessani N, Joseph A, Humphrey M, Cravatt B. Proc. Natl. Acad. Sci. U.S.A. 2004;101:10000. doi: 10.1073/pnas.0402784101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antczak C, Radu C, Djaballah H. J. Biomol. Screen. 2008;13:285. doi: 10.1177/1087057108315877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Overall C, Kleifeld O. Nat. Rev. Cancer. 2006;6:227. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- 12.Escobar-Alvarez S, Goldgur Y, Yang G, Ouerfelli O, Li Y, Scheinberg DA. J. Mol. Biol. 2009;5:1211. doi: 10.1016/j.jmb.2009.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antczak C, Shum D, Escobar S, Bassit B, Kim E, Seshan VE, Wu N, Yang G, Ouerfelli O, Li YM, Scheinberg DA, Djaballah H. J. Biomol. Screen. 2007;12:521. doi: 10.1177/1087057107300463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atkinson R, Mare P, Larsen D. J. Chem. Soc., Perkin Trans. 2. 1983;1983:271. [Google Scholar]
- 15.Singh V, Sapehiyia V, Kad G. ChemInform. 2003;34 [Google Scholar]
- 16.Lopez-Alvarado P, Avendano C, Menendez J. ChemInform. 2003;34 [Google Scholar]
- 17.Barrero A, Alvarez-Manzaneda E, Chahboun R, Diaz C. Synlett. 2000:1561. [Google Scholar]
- 18.Lin Y, Lang S., Jr J. Org. Chem. 1980;45:4857. [Google Scholar]
- 19.Cheng J. International patent WO/2005/011670
- 20.Cheng X, Liu X. J. Comb. Chem. 2007;9:906. doi: 10.1021/cc070015u. [DOI] [PubMed] [Google Scholar]
- 21.Al-Mousawi S, Mohammed Abdel-Khalik M, EI-Sherbiny S, John E, Elnagdi M. J. Heterocycl. Chem. 2001;38:949. [Google Scholar]
- 22.Grinev A, Khun‘Shchi-tszyun’ A, Terent’ev A. J. Gen. Chem. USSR. 1962;32:1931. [Google Scholar]
- 23.Grinev A, Arkhangel’skaya N, Uretskaya G, Vlasova T. Chem. Heterocycl. Compd. 1975;11:639. [Google Scholar]
- 24.Nguyen K, Hu X, Colton C. Biochemistry. 2003;42:9952. doi: 10.1021/bi0346446. [DOI] [PubMed] [Google Scholar]
- 25.Shum D, Radu C, Kim E, Cajuste M, Shao Y, Seshan VE, Djaballah H. J. Enzyme Inhib. Med. Chem. 2008;23:931. doi: 10.1080/14756360701810082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.