Abstract
Dietary intervention strategies have proven to be an effective means of decreasing several risk factors associated with the development of atherosclerosis. Endothelial cell dysfunction influences vascular inflammation and is involved in promoting the earliest stages of lesion formation. Caveolae are lipid raft microdomains abundant within the plasma membrane of endothelial cells and are responsible for mediating receptor-mediated signal transduction. Caveolae have been implicated in the regulation of enzymes associated with several key signaling pathways capable of determining intracellular redox status. Diet and plasma-derived nutrients may modulate an inflammatory outcome by interacting with and altering caveolae-associated cellular signaling. For example, omega-3 fatty acids and several polyphenolics have been shown to improve endothelial cell function by decreasing the formation of ROS and increasing NO bioavailability, events associated with altered caveolae composition. Thus, nutritional modulation of caveolae-mediated signaling events may provide an opportunity to ameliorate inflammatory signaling pathways capable of promoting the formation of vascular diseases, including atherosclerosis.
Link of functional caveolae to the pathology atherosclerosis
Caveolae play an important regulatory role in vascular inflammation and appear to be required for the pathology of atherosclerosis. In fact, caveolin-1 deficient mice are protected against atherogenic lesion formation [1]. It is widely accepted that atherosclerosis is the result of sustained chronic low-grade inflammation. Endothelial cell dysfunction contributes significantly to vascular inflammation and is thus an important mediator involved in the development of atherosclerotic plaques. Endothelial dysfunction may result in response to inflammatory stimuli including circulating cytokines, and oxidized low-density lipoproteins (oxLDL). Upon activation, the endothelium increases expression of adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1). Concomitant increases in the secretion of chemokines including monocyte chemoattractant protein-1 (MCP-1) culminate in the recruitment, attachment, and transendothelial migration of circulating monocytes into the subendothelial space, and their subsequent ingestion of lipids to form foam cells.
Caveolae are cholesterol and sphingolipid enriched microdomains responsible of mediating signal transduction across the plasma membrane, and are particularly abundant in vascular endothelial cells [2]. Caveolin-1, a 22 kDa integral membrane protein localized to caveolae, is capable of influencing cellular signaling events by interacting with the caveolin binding motif (CBM) of cytosolic proteins through its scaffolding domain (amino acids 82–101) [3]. Caveolae have been implicated as mediators of vascular inflammation by facilitating the formation of reactive oxygen species (ROS) and decreasing nitric oxide (NO) bioavailability in response to endothelial cell injury or inflammatory stimuli. Thus, caveolae may provide an important regulatory platform for nutritional intervention strategies and attenuation of inflammatory parameters.
Caveolin-1 and endothelial activation
A critical role for endothelial caveolin-1 in the development of atherosclerosis was elegantly demonstrated by reconstituting caveolin-1 specifically in endothelial cells of apoE−/−/cav-1−/− mice [4]. To this effect, cav-1−/−/apoE−/− mice have reduced lipid accumulation in the subendothelial space compared to apoE−/− mice despite increased non-HDL plasma cholesterol levels [1], elucidating a role for caveolae in the uptake of proatherogenic lipoproteins. Caveolae facilitate the development of atherosclerosis through its integral involvement in inflammatory signaling networks within the vascular endothelium. Caveolin-1 deficiency was associated with downregulation of proatherogenic VCAM-1 and CD36 scavenger receptor [1]. When primary human retinal vascular endothelial cells were exposed to either IL-1β or TNF-α, lipid raft disruption with the cholesterol depleting agent methyl-β-cyclodextrin markedly attenuated NFκB dependent signaling events [5]. Caveolin-1 was necessary for mediating TNF-α induced NFκB dependent induction of cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2) in vitro [6]. Alternatively, in the human derived EA.hy926 endothelial cell line, lipid raft disruption with methyl-β-cyclodextrin did not prevent TNF-α induced degradation of IκBα, a measure of NFκB activation [7]. A CBM within the carboxy-terminal intracellular domain of toll-like receptor 4 (TLR4) was identified and found to be critical for regulating its activity [8]. Correspondingly, cav-1−/− mice exhibit attenuated NFκB activity in response to lipopolysacharide (LPS) leading to decreased polymorphonuclear sequestration and lung injury [9]. However, using a cav-1−/−/eNOS−/− (endothelial nitric oxide synthase) mouse model, the role of caveolin-1 in mediating acute lung injury in response to LPS was found to be dependent upon eNOS inhibition [10].
Caveolae and eNOS signaling: regulation of cellular oxidative stress
Endothelial cell dysfunction is characterized by a decrease in NO bioavailability, and increased production of ROS. Accordingly, a decrease in NO bioavailability is both necessary and sufficient to cause endothelial dysfunction and activation of inflammatory signaling pathways [11, 12]. eNOS is a Ca2+/calmodulin dependent enzyme [13] responsible for constitutive NO production in the vasculature, and its activity is susceptible to modulation by a diverse array of extracellular stimuli. Caveolin-1 has long been known to negatively regulate eNOS function [14]. To this effect, caveolin-1 is capable of limiting NO bioavailability in response to interleukin-6 (IL-6) [15] and LPS [10]. Tetrahydrobiopterin (BH4) is an indispensible cofactor necessary for NO production by eNOS, and plays a critical role in preventing eNOS uncoupling leading to formation of potentially damaging ROS in the form of superoxide [16]. Caveolin-1 has recently been shown to negatively regulate guanosine triphosphate cyclohydrolase I, the rate limiting enzyme responsible for de novo synthesis of BH4 [17]. Interestingly, caveolin-1 protein expression is elevated in the aortas of diet induced obese rats fed a high fat diet, and this was correlated with a decrease in eNOS activity [18]. TNF-α induced NFκB activation and subsequent expression of inflammatory mediators VCAM-1 and MCP-1 requires NADPH oxidase activation and ensuing production of ROS [19, 20]. Caveolin-1 was found to be necessary for NADPH oxidase derived ROS production, [21, 22] and through the formation of endocytic vesicles termed ‘redoxosomes,’ was capable of facilitating the interleukin-1β (IL-1β) dependent activation of NFκB [21]. Caveolin-1 levels are increased in response to inflammatory stimuli including tumor necrosis factor-alpha (TNF-α) [6] and LPS [23], potentially leading to decreased NO bioavailability and increased ROS production. Thus, caveolae appear to be an important intermediary for determining the response to inflammatory stimuli within the endothelium.
Caveolae and Nrf2 signaling: regulation of antioxidant defense pathways
Caveolae may also play an integral role in regulating cellular antioxidant defense pathways necessary for eliminating potentially damaging ROS from cells. The transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) is a master regulator of antioxidant and cytoprotective defenses responsible for cellular detoxification of ROS. Sulforaphane dependent Nrf2 activation attenuated LPS induced endothelial activation in aortas of C57BL/6 mice [24]. Treating rat glioma C6 cells with the NO donor SNAP lead to the formation of a nitrated guanine nucleotide, 8-nitroguanosine 3′,5′-cyclic monophosphate (8-nitro-cGMP), causing S-guanylation of Keap1, the cellular inhibitor of Nrf2, and subsequent Nrf2 activation [25]. Hence, the prospect of an increased antioxidative capacity in caveolin-1 deficient mice is intriguing knowing their predilection for exaggerated NO production in the vasculature [26]. Heme oxygenase 1 (HO-1) catalyzes the breakdown of heme to form carbon monoxide (CO), free iron and biliverdin, eventually forming bilirubin (BR) through the action biliverdin reductase. CO and BR are known to have antiatherosclerotic and antioxidative properties respectively [27]. RAW264.7 macrophages expressing caveolin-1 antisense mRNA exhibited increased HO-1 activity in response to LPS when compared to the control group [8], suggesting caveolin-1 may be a negative regulator of HO-1. Likewise, thioredoxin reductase 1 (TrxR1) is a thiol metabolizing protein responsible for reducing cellular compounds including thioredoxin. TrxR1 has been implicated in the regulation of redox sensitive pathways capable of influencing eNOS activity [28] and thus may be an important player in preventing endothelial cell injury. TrxR1 binds the caveolin-1 scaffolding domain through its CBM and caveolin-1 acts as an endogenous inhibitor of TrxR1 activity both in vitro and in vivo [29]. Thus, increased levels of caveolin-1 may decrease the antioxidative capacity of the endothelium, further exacerbating the inflammatory response.
Caveolae and regulation of mitochondrial function
The development of atherosclerosis is correlated with increased mitochondrial DNA (mtDNA) damage, and mtDNA damage may precede the earliest stages of lesion development [30]. Impaired mitochondrial respiration in response to extensively oxLDL resulted in elevated intracellular ROS in porcine aortic endothelial cells [31]. Oxidized LDL has been shown to deplete caveolae of cholesterol causing translocation of both eNOS and caveolin-1 from caveolae, consequently preventing acetylcholine induced eNOS activation [32]. Furthermore, mitochondrial biogenesis is dependent upon eNOS activation in response to calorie restriction [33], suggesting a role for caveolae in the regulation of mitochondrial function. To this extent, the caveolar signaling complexes were able to influence mitochondrial ATP-sensitive K+ (mitoKATP) channels in response to bradykinin [34], resulting in cardioprotection. AMP-activated protein kinase (AMPK) is a cellular energy sensor that, through the NAD+-dependent type III deacetylase SIRT1, is capable of augmenting mitochondrial function by activating the master regulator of mitochondrial activity peroxisome proliferator activated receptor-gamma coactivator 1 alpha (PGC-1α) [35]. PGC-1α responds to elevated intracellular oxidative stress by inducing the production of mitochondrial detoxification enzymes including glutathione peroxidase, manganese superoxide dismutase (SOD2), and catalase [36]. Accordingly, wild type but not α1-AMPK deficient mice, when administered the AMPK agonist 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside (AICAR), were protected against LPS induced endothelial dysfunction [37]. Likewise, AICAR also prevented TNF-α induced NF-κB activation in HUVECs [38]. Caveolin-1 was found to play a critical role in facilitating AMPK dependent eNOS activation in bovine aortic endothelial cells in response to agonist stimulation with either VEGF or S1P [39]. Furthermore, caveolin-1 silencing lead to an increase in the p-AMPK/AMPK ratio; elucidating a role for caveolin-1 as a negative regulator of AMPK activity. Hence, caveolae may play a fundamental role in mediating AMPK regulation of mitochondrial function, and perhaps most importantly, mitochondrial redox status.
Nutritional modulation of caveolae function
Omega-3 polyunsaturated fatty acids
It has become evident that targeted disruption of caveolae through nutritional modulation by bioactive compounds found in food may confer cardioprotection by decreasing inflammation within the vasculature. A diet rich in omega-3 polyunsaturated fatty acids (PUFAs) is thought to aid in the prevention of developing cardiovascular diseases including atherosclerosis. The anti-inflammatory properties of fish oil and in particular omega-3 fatty acids have been extensively considered [40]. Accordingly, dietary supplementation with docosahexaenoic acid (DHA, 22:6 n - 3), and to a lesser extent eicosapentaenoic acid (EPA, 20:5 n – 3), attenuates atherosclerotic lesion formation and apoE−/− mice [41]. Omega-3 PUFA’s may alter the lipid environment of raft microdomains and subsequently alter downstream signaling events [42, 43]. It has been proposed that PUFAs, and DHA in particular, may be imparted with an inherent aversion for cholesterol resulting in lipid raft disruption [44]. Indeed, treatment of human endothelial cells with DHA resulted in substantially augmented membrane lipids in both total membrane phospholipid and caveolar fractions [5]. DHA enrichment of caveolae resulted in decreased lipid raft cholesterol and reduced ICAM-1 expression in response to TNF-α. Alternatively, pretreatment of human umbilical vein endothelial cells with DHA significantly reduced TNF-α induced monocyte rolling, adhesion and transendothelial cell migration without affecting adhesion molecule expression [45]. Both DHA and EPA are capable of displacing caveolin-1 and eNOS from caveolae in endothelial cells, an event associated with increased NO production [46, 47]. DHA is also able to modulate TLR4 activation in response to LPS or lauric acid in RAW264.7 cells by preventing receptor dimerization and recruitment into lipid rafts [48]. It was further demonstrated that DHA inhibited NADPH oxidase derived superoxide production necessary for TLR4 activation. Conversely, linoleic acid, an inflammatory omega-6 fatty acid, is capable of increasing caveolin-1 expression levels and exacerbating TNF-α dependant endothelial cell activation [6]. Similarly, exposing human microvascular endothelial cells to saturated fatty acids such as palmitate can lead to superoxide production in a TLR4 dependent manner [49]. Therefore, omega-3 PUFAs appear to exert their anti-inflammatory effects, at least in part, through attenuation of raft dependent inflammatory signaling events while omega-6 PUFAs and saturated fatty acids may aggravate disease progression.
Plant-derived polyphenols
Dietary polyphenols are abundant in fruits and vegetables and can also be found in red wine, tea, and dark chocolate. Recently, the efficacy of decreasing several markers of cardiovascular disease using various flavonoids including quercetin and (−)-epicatechin was demonstrated in vivo [50]. Similarly, several dietary phenolics have demonstrated the ability to lessen the degree of endothelial activation in response to inflammatory stimuli in vitro [51, 52]. In view of that, functional caveolae may be necessary for the efficient uptake of various phenolics including resveratrol [53]. Interestingly, metabolites of dietary quercetin have been shown to selectively accumulate in macrophage laden atherosclerotic lesions [54]. It seems feasible to speculate that caveolae may provide a platform to facilitate selective uptake of certain phenolics at the site of lesion development given the propensity for caveolin-1 upregulation at sites of inflammation [55]. However, more studies are needed to either confirm or negate this possibility. Resveratrol has been shown to stimulate mitochondrial biogenesis in primary human coronary arterial endothelial cells, and this was dependant on both NO and SIRT1 [56]. Furthermore, resveratrol dependent stimulation of NO production in endothelial cells was found to be lipid raft dependent and associated with increased phosphorylation of caveolin-1, c-Src, and eNOS [57]. The isoflavone daidzein was able to enhance ACh-induced vasorelaxation in aortic rings from male rats [58, 59]. Daidzein pretreatment was associated with decreased caveolin-1 and increased calmodulin levels, presumably accounting for the increased eNOS activity. Similarly, green tea polyphenols were also able to reduce caveolin-1 levels in vitro; a property attributed to epigallocatechin-3-gallate’s (EGCG) ability to both suppress p38MAPK and activate ERK1/2 signaling pathways [60]. EGCG pretreatment of porcine endothelial cells blocked linoleic acid induced increases in caveolin-1 and cyclooxygenase 2 (COX)-2 expression [61]. Moreover, EGCG stimulated NO production in endothelial cells in vitro while inducing vasorelaxation in mesenteric vascular beds isolated from Wistar Kyoto rats ex vivo [62]. AICAR induced AMPK activation stimulated NO production by phosphorylating eNOS at Ser-1177 [63]. EGCG treatment of primary mouse hepatocytes was able to trigger the ROS dependent activation of AMPK leading to phosphorylation of downstream targets including acetyl-CoA carboxylase [64], conceivably accounting for the increase in NO bioavailability in EGCG treated endothelial cells. Perhaps most importantly, both EGCG and quercetin have been shown to preferentially accumulate in the mitochondria, providing protection against mitochondrial oxidative damage [65, 66]. Bearing in mind the inhibitory role caveolin-1 plays in modulating the activity of both AMPK and eNOS, an axis in which caveolae act as signaling platforms able to facilitate the actions of EGCG would seem plausible. Intriguingly, quercetin was able to increase both eNOS expression and activity in spontaneously hypertensive (SH) rats but this was in the absence of any changes in caveolin-1 protein isolated from aortic rings [67]. Chronic administration of genistein to SH rats also improved endothelium-dependent vasodilation in response to acetylcholine without affecting caveolin-1 levels [68]. However, considering the disparate roles caveolin-1 may play in facilitating atherogenesis in endothelial cells and surrounding vascular smooth muscle cells [69] one should proceed with caution when attempting to derive conclusions from data obtained in vivo.
Conclusion
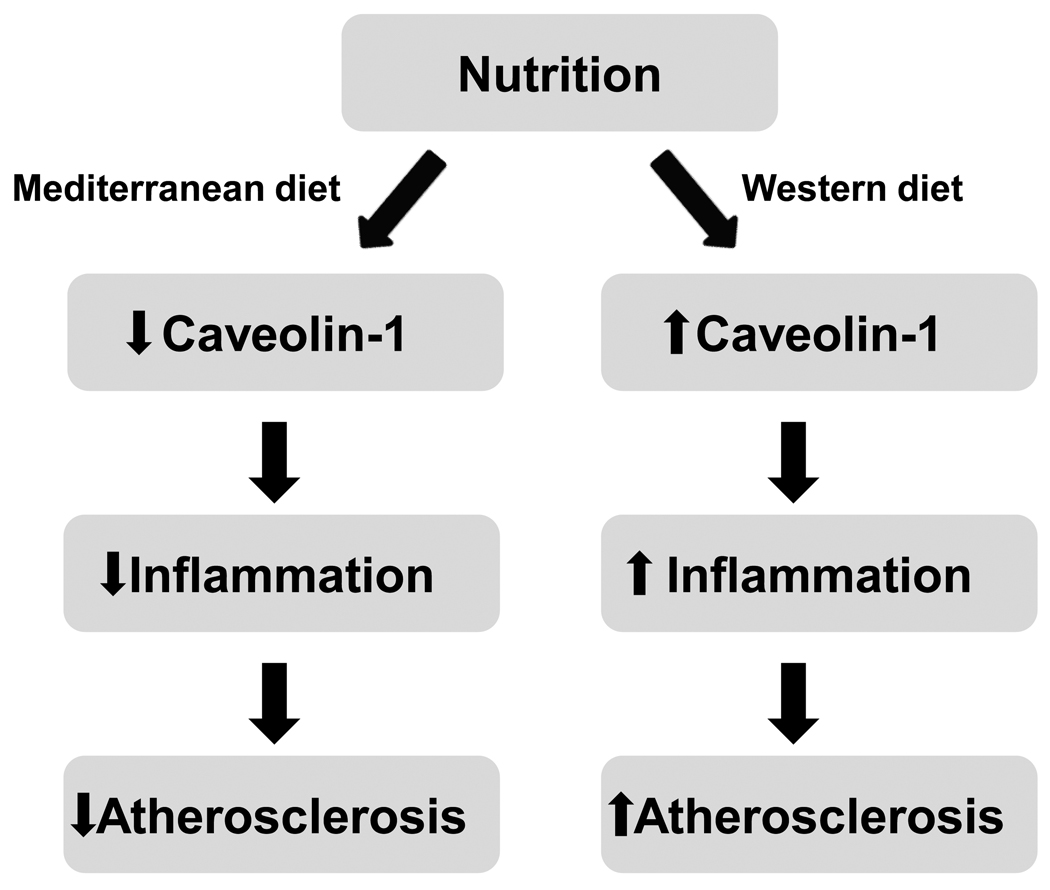
Caveolae are an important regulatory platform capable of mediating signal transduction across the lipid bilayer. Caveolin-1 appears to promote endothelial activation, leading to increased lipid accumulation in the subendothelial space and enhanced monocyte/macrophage recruitment. Inflammatory stimuli can lead to increased expression of caveolin-1, the major protein component of caveolae. Caveolin-1 upregulation may potentiate endothelial cell activation by providing a platform to facilitate the increased production of ROS and subsequent decrease in NO bioavailability. Caveolin-1 expression may further exacerbate the inflammatory response through inhibition of upstream regulators of antioxidant defense enzymes. Moreover, as the critical role of mitochondrial function in vascular diseases continues to emerge, so will the task of its modulation by lipid rafts and bioactive nutrients. Nutritional modulation of caveolae may provide an opportunity to disrupt inflammatory signaling events and attenuate endothelial cell dysfunction during the early pathology of atherosclerosis (Figure 1). Omega-3 PUFAs, such as DHA, have potent anti-inflammatory effects, and are capable of modifying the lipid raft microenvironment. Similarly, several polyphenolic compounds are able to interrupt caveolae mediated signaling events, increasing NO production and dampening vascular inflammation. More studies are warranted to substantiate the critical role of caveolae as a regulatory platform for nutritional modulation of inflammatory diseases.
Figure 1.
Nutritional modulation of caveolae may alter intracellular signaling events associated with vascular inflammation. An anti-inflammatory diet rich in omega-3 PUFAs, fruits, and vegetables (e.g., similar to a Mediterranean diet) may alter caveolae, which may lead to cardioprotection. Alternatively, a Western style diet rich in saturated fats, cholesterol, and omega-6 PUFAs, may increase caveolae and promote inflammation and the development of atherosclerosis.
Acknowledgements
This work was supported in part by NIH/NIEHS grant P42ES007380, and the University of Kentucky Agricultural Experiment Station.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frank PG, Lee H, Park DS, Tandon NN, Scherer PE, Lisanti MP. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:98–105. doi: 10.1161/01.ATV.0000101182.89118.E5. [DOI] [PubMed] [Google Scholar]
- 2.Mineo C, Shaul PW. Circulating cardiovascular disease risk factors and signaling in endothelial cell caveolae. Cardiovasc Res. 2006;70:31–41. doi: 10.1016/j.cardiores.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 3.Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem. 1997;272:6525–6533. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Hernando C, Yu J, Suarez Y, Rahner C, Davalos A, Lasuncion MA, et al. Genetic evidence supporting a critical role of endothelial caveolin-1 during the progression of atherosclerosis. Cell Metab. 2009;10:48–54. doi: 10.1016/j.cmet.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W, Jump DB, Esselman WJ, Busik JV. Inhibition of cytokine signaling in human retinal endothelial cells through modification of caveolae/lipid rafts by docosahexaenoic acid. Invest Ophthalmol Vis Sci. 2007;48:18–26. doi: 10.1167/iovs.06-0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, Lim EJ, Toborek M, Hennig B. The role of fatty acids and caveolin-1 in tumor necrosis factor alpha-induced endothelial cell activation. Metabolism. 2008;57:1328–1339. doi: 10.1016/j.metabol.2008.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Alessio A, Al-Lamki RS, Bradley JR, Pober JS. Caveolae participate in tumor necrosis factor receptor 1 signaling and internalization in a human endothelial cell line. Am J Pathol. 2005;166:1273–1282. doi: 10.1016/S0002-9440(10)62346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang XM, Kim HP, Nakahira K, Ryter SW, Choi AM. The heme oxygenase-1/carbon monoxide pathway suppresses TLR4 signaling by regulating the interaction of TLR4 with caveolin-1. J Immunol. 2009;182:3809–3818. doi: 10.4049/jimmunol.0712437. [DOI] [PubMed] [Google Scholar]
- 9.Garrean S, Gao XP, Brovkovych V, Shimizu J, Zhao YY, Vogel SM, et al. Caveolin-1 regulates NF-kappaB activation and lung inflammatory response to sepsis induced by lipopolysaccharide. J Immunol. 2006;177:4853–4860. doi: 10.4049/jimmunol.177.7.4853. [DOI] [PubMed] [Google Scholar]
- 10.Mirza MK, Yuan J, Gao XP, Garrean S, Brovkovych V, Malik AB, et al. Caveolin-1 deficiency dampens Toll-like receptor 4 signaling through eNOS activation. Am J Pathol. 176:2344–2351. doi: 10.2353/ajpath.2010.091088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clapp BR, Hingorani AD, Kharbanda RK, Mohamed-Ali V, Stephens JW, Vallance P, et al. Inflammation-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc Res. 2004;64:172–178. doi: 10.1016/j.cardiores.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Spiecker M, Peng HB, Liao JK. Inhibition of endothelial vascular cell adhesion molecule-1 expression by nitric oxide involves the induction and nuclear translocation of IkappaBalpha. J Biol Chem. 1997;272:30969–30974. doi: 10.1074/jbc.272.49.30969. [DOI] [PubMed] [Google Scholar]
- 13.Venema RC, Sayegh HS, Arnal JF, Harrison DG. Role of the enzyme calmodulin-binding domain in membrane association and phospholipid inhibition of endothelial nitric oxide synthase. J Biol Chem. 1995;270:14705–14711. doi: 10.1074/jbc.270.24.14705. [DOI] [PubMed] [Google Scholar]
- 14.Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem. 1997;272:18522–18525. doi: 10.1074/jbc.272.30.18522. [DOI] [PubMed] [Google Scholar]
- 15.Hung MJ, Cherng WJ, Hung MY, Wu HT, Pang JH. Interleukin-6 inhibits endothelial nitric oxide synthase activation and increases endothelial nitric oxide synthase binding to stabilized caveolin-1 in human vascular endothelial cells. J Hypertens. 28:940–951. doi: 10.1097/HJH.0b013e32833992ef. [DOI] [PubMed] [Google Scholar]
- 16.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, et al. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du YH, Chen AF. A new role for caveolin-1: regulation of guanosine triphosphate cyclohydrolase I and tetrahydrobiopterin in endothelial cells. Hypertension. 2009;53:115–117. doi: 10.1161/HYPERTENSIONAHA.108.123356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang N, Ying C, Xu M, Zuo X, Ye X, Liu L, et al. High-fat diet up-regulates caveolin-1 expression in aorta of diet-induced obese but not in diet-resistant rats. Cardiovasc Res. 2007;76:167–174. doi: 10.1016/j.cardiores.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 19.Chen XL, Zhang Q, Zhao R, Medford RM. Superoxide, H2O2, and iron are required for TNF-alphainduced MCP-1 gene expression in endothelial cells: role of Rac1 and NADPH oxidase. Am J Physiol Heart Circ Physiol. 2004;286:H1001–H1007. doi: 10.1152/ajpheart.00716.2003. [DOI] [PubMed] [Google Scholar]
- 20.Weber C, Erl W, Pietsch A, Strobel M, Ziegler-Heitbrock HW, Weber PC. Antioxidants inhibit monocyte adhesion by suppressing nuclear factor-kappa B mobilization and induction of vascular cell adhesion molecule-1 in endothelial cells stimulated to generate radicals. Arterioscler Thromb. 1994;14:1665–1673. doi: 10.1161/01.atv.14.10.1665. [DOI] [PubMed] [Google Scholar]
- 21.Oakley FD, Smith RL, Engelhardt JF. Lipid rafts and caveolin-1 coordinate interleukin-1beta (IL-1beta)-dependent activation of NFkappaB by controlling endocytosis of Nox2 and IL-1beta receptor 1 from the plasma membrane. J Biol Chem. 2009;284:33255–33264. doi: 10.1074/jbc.M109.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singleton PA, Pendyala S, Gorshkova IA, Mambetsariev N, Moitra J, Garcia JG, et al. Dynamin 2 and c-Abl are novel regulators of hyperoxia-mediated NADPH oxidase activation and reactive oxygen species production in caveolin-enriched microdomains of the endothelium. J Biol Chem. 2009;284:34964–34975. doi: 10.1074/jbc.M109.013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tiruppathi C, Shimizu J, Miyawaki-Shimizu K, Vogel SM, Bair AM, Minshall RD, et al. Role of NFkappaB-dependent caveolin-1 expression in the mechanism of increased endothelial permeability induced by lipopolysaccharide. J Biol Chem. 2008;283:4210–4218. doi: 10.1074/jbc.M703153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zakkar M, Van der Heiden K, Luong le A, Chaudhury H, Cuhlmann S, Hamdulay SS, et al. Activation of Nrf2 in endothelial cells protects arteries from exhibiting a proinflammatory state. Arterioscler Thromb Vasc Biol. 2009;29:1851–1857. doi: 10.1161/ATVBAHA.109.193375. [DOI] [PubMed] [Google Scholar]
- 25.Fujii S, Sawa T, Ihara H, Tong KI, Ida T, Okamoto T, et al. The critical role of nitric oxide signaling, via protein S-guanylation and nitrated cyclic GMP, in the antioxidant adaptive response. J Biol Chem. doi: 10.1074/jbc.M110.145441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, Marks CB, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 27.Loboda A, Jazwa A, Grochot-Przeczek A, Rutkowski AJ, Cisowski J, Agarwal A, et al. Heme oxygenase-1 and the vascular bed: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2008;10:1767–1812. doi: 10.1089/ars.2008.2043. [DOI] [PubMed] [Google Scholar]
- 28.Sugiyama T, Michel T. Thiol-metabolizing proteins and endothelial redox state: differential modulation of eNOS and biopterin pathways. Am J Physiol Heart Circ Physiol. 298:H194–H201. doi: 10.1152/ajpheart.00767.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volonte D, Galbiati F. Inhibition of thioredoxin reductase 1 by caveolin 1 promotes stress-induced premature senescence. EMBO Rep. 2009;10:1334–1340. doi: 10.1038/embor.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ballinger SW, Patterson C, Knight-Lozano CA, Burow DL, Conklin CA, Hu Z, et al. Mitochondrial integrity and function in atherogenesis. Circulation. 2002;106:544–549. doi: 10.1161/01.cir.0000023921.93743.89. [DOI] [PubMed] [Google Scholar]
- 31.Roy Chowdhury SK, Sangle GV, Xie X, Stelmack GL, Halayko AJ, Shen GX. Effects of extensively oxidized low-density lipoprotein on mitochondrial function and reactive oxygen species in porcine aortic endothelial cells. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00433.2009. [DOI] [PubMed] [Google Scholar]
- 32.Blair A, Shaul PW, Yuhanna IS, Conrad PA, Smart EJ. Oxidized low density lipoprotein displaces endothelial nitric-oxide synthase (eNOS) from plasmalemmal caveolae and impairs eNOS activation. J Biol Chem. 1999;274:32512–32519. doi: 10.1074/jbc.274.45.32512. [DOI] [PubMed] [Google Scholar]
- 33.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 34.Quinlan CL, Costa AD, Costa CL, Pierre SV, Dos Santos P, Garlid KD. Conditioning the heart induces formation of signalosomes that interact with mitochondria to open mitoKATP channels. Am J Physiol Heart Circ Physiol. 2008;295:H953–H961. doi: 10.1152/ajpheart.00520.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 37.Schulz E, Dopheide J, Schuhmacher S, Thomas SR, Chen K, Daiber A, et al. Suppression of the JNK pathway by induction of a metabolic stress response prevents vascular injury and dysfunction. Circulation. 2008;118:1347–1357. doi: 10.1161/CIRCULATIONAHA.108.784298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hattori Y, Suzuki K, Hattori S, Kasai K. Metformin inhibits cytokine-induced nuclear factor kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension. 2006;47:1183–1188. doi: 10.1161/01.HYP.0000221429.94591.72. [DOI] [PubMed] [Google Scholar]
- 39.Levine YC, Li GK, Michel T. Agonist-modulated regulation of AMP-activated protein kinase (AMPK) in endothelial cells. Evidence for an AMPK -> Rac1 -> Akt -> endothelial nitric-oxide synthase pathway. J Biol Chem. 2007;282:20351–20364. doi: 10.1074/jbc.M702182200. [DOI] [PubMed] [Google Scholar]
- 40.Chapkin RS, Kim W, Lupton JR, McMurray DN. Dietary docosahexaenoic and eicosapentaenoic acid: emerging mediators of inflammation. Prostaglandins Leukot Essent Fatty Acids. 2009;81:187–191. doi: 10.1016/j.plefa.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang TM, Chen CJ, Lee TS, Chao HY, Wu WH, Hsieh SC, et al. Docosahexaenoic acid attenuates VCAM-1 expression and NF-kappaB activation in TNF-alpha-treated human aortic endothelial cells. J Nutr Biochem. doi: 10.1016/j.jnutbio.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Chapkin RS, McMurray DN, Davidson LA, Patil BS, Fan YY, Lupton JR. Bioactive dietary long-chain fatty acids: emerging mechanisms of action. Br J Nutr. 2008;100:1152–1157. doi: 10.1017/S0007114508992576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma DW, Seo J, Davidson LA, Callaway ES, Fan YY, Lupton JR, et al. n-3 PUFA alter caveolae lipid composition and resident protein localization in mouse colon. FASEB J. 2004;18:1040–1042. doi: 10.1096/fj.03-1430fje. [DOI] [PubMed] [Google Scholar]
- 44.Wassall SR, Stillwell W. Docosahexaenoic acid domains: the ultimate non-raft membrane domain. Chem Phys Lipids. 2008;153:57–63. doi: 10.1016/j.chemphyslip.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 45.Schaefer MB, Wenzel A, Fischer T, Braun-Dullaeus RC, Renner F, Dietrich H, et al. Fatty acids differentially influence phosphatidylinositol 3-kinase signal transduction in endothelial cells: impact on adhesion and apoptosis. Atherosclerosis. 2008;197:630–637. doi: 10.1016/j.atherosclerosis.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Li Q, Zhang Q, Wang M, Zhao S, Ma J, Luo N, et al. Eicosapentaenoic acid modifies lipid composition in caveolae and induces translocation of endothelial nitric oxide synthase. Biochimie. 2007;89:169–177. doi: 10.1016/j.biochi.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 47.Li Q, Zhang Q, Wang M, Liu F, Zhao S, Ma J, et al. Docosahexaenoic acid affects endothelial nitric oxide synthase in caveolae. Arch Biochem Biophys. 2007;466:250–259. doi: 10.1016/j.abb.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 48.Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang DH. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem. 2009;284:27384–27392. doi: 10.1074/jbc.M109.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maloney E, Sweet IR, Hockenbery DM, Pham M, Rizzo NO, Tateya S, et al. Activation of NF-kappaB by palmitate in endothelial cells: a key role for NADPH oxidase-derived superoxide in response to TLR4 activation. Arterioscler Thromb Vasc Biol. 2009;29:1370–1375. doi: 10.1161/ATVBAHA.109.188813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loke WM, Proudfoot JM, Hodgson JM, McKinley AJ, Hime N, Magat M, et al. Specific dietary polyphenols attenuate atherosclerosis in apolipoprotein E-knockout mice by alleviating inflammation and endothelial dysfunction. Arterioscler Thromb Vasc Biol. 30:749–757. doi: 10.1161/ATVBAHA.109.199687. [DOI] [PubMed] [Google Scholar]
- 51.Yamagata K, Miyashita A, Matsufuji H, Chino M. Dietary flavonoid apigenin inhibits high glucose and tumor necrosis factor alpha-induced adhesion molecule expression in human endothelial cells. J Nutr Biochem. 21:116–124. doi: 10.1016/j.jnutbio.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 52.Lee YW, Lee WH. Protective effects of genistein on proinflammatory pathways in human brain microvascular endothelial cells. J Nutr Biochem. 2008;19:819–825. doi: 10.1016/j.jnutbio.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 53.Yang HL, Chen WQ, Cao X, Worschech A, Du LF, Fang WY, et al. Caveolin-1 enhances resveratrol-mediated cytotoxicity and transport in a hepatocellular carcinoma model. J Transl Med. 2009;7:22. doi: 10.1186/1479-5876-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawai Y, Nishikawa T, Shiba Y, Saito S, Murota K, Shibata N, et al. Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries: implication in the anti-atherosclerotic mechanism of dietary flavonoids. J Biol Chem. 2008;283:9424–9434. doi: 10.1074/jbc.M706571200. [DOI] [PubMed] [Google Scholar]
- 55.Wu CC, Wang SH, Kuan II, Tseng WK, Chen MF, Wu JC, et al. OxLDL upregulates caveolin-1 expression in macrophages: Role for caveolin-1 in the adhesion of oxLDL-treated macrophages to endothelium. J Cell Biochem. 2009;107:460–472. doi: 10.1002/jcb.22144. [DOI] [PubMed] [Google Scholar]
- 56.Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H13–H20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klinge CM, Wickramasinghe NS, Ivanova MM, Dougherty SM. Resveratrol stimulates nitric oxide production by increasing estrogen receptor alpha-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J. 2008;22:2185–2197. doi: 10.1096/fj.07-103366. [DOI] [PubMed] [Google Scholar]
- 58.Woodman OL, Missen MA, Boujaoude M. Daidzein and 17 beta-estradiol enhance nitric oxide synthase activity associated with an increase in calmodulin and a decrease in caveolin-1. J Cardiovasc Pharmacol. 2004;44:155–163. doi: 10.1097/00005344-200408000-00003. [DOI] [PubMed] [Google Scholar]
- 59.Sobey CG, Weiler JM, Boujaoude M, Woodman OL. Effect of short-term phytoestrogen treatment in male rats on nitric oxide-mediated responses of carotid and cerebral arteries: comparison with 17beta-estradiol. J Pharmacol Exp Ther. 2004;310:135–140. doi: 10.1124/jpet.103.063255. [DOI] [PubMed] [Google Scholar]
- 60.Li Y, Ying C, Zuo X, Yi H, Yi W, Meng Y, et al. Green tea polyphenols down-regulate caveolin-1 expression via ERK1/2 and p38MAPK in endothelial cells. J Nutr Biochem. 2009;20:1021–1027. doi: 10.1016/j.jnutbio.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 61.Zheng Y, Lim EJ, Wang L, Smart EJ, Toborek M, Hennig B. Role of caveolin-1 in EGCG-mediated protection against linoleic-acid-induced endothelial cell activation. J Nutr Biochem. 2009;20:202–209. doi: 10.1016/j.jnutbio.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim JA, Formoso G, Li Y, Potenza MA, Marasciulo FL, Montagnani M, et al. Epigallocatechin gallate, a green tea polyphenol, mediates NO-dependent vasodilation using signaling pathways in vascular endothelium requiring reactive oxygen species and Fyn. J Biol Chem. 2007;282:13736–13745. doi: 10.1074/jbc.M609725200. [DOI] [PubMed] [Google Scholar]
- 63.Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol Chem. 2003;278:31629–31639. doi: 10.1074/jbc.M212831200. [DOI] [PubMed] [Google Scholar]
- 64.Collins QF, Liu HY, Pi J, Liu Z, Quon MJ, Cao W. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5'-AMP-activated protein kinase. J Biol Chem. 2007;282:30143–30149. doi: 10.1074/jbc.M702390200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schroeder EK, Kelsey NA, Doyle J, Breed E, Bouchard RJ, Loucks FA, et al. Green tea epigallocatechin 3-gallate accumulates in mitochondria and displays a selective antiapoptotic effect against inducers of mitochondrial oxidative stress in neurons. Antioxid Redox Signal. 2009;11:469–480. doi: 10.1089/ars.2008.2215. [DOI] [PubMed] [Google Scholar]
- 66.Fiorani M, Guidarelli A, Blasa M, Azzolini C, Candiracci M, Piatti E, et al. Mitochondria accumulate large amounts of quercetin: prevention of mitochondrial damage and release upon oxidation of the extramitochondrial fraction of the flavonoid. J Nutr Biochem. 21:397–404. doi: 10.1016/j.jnutbio.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 67.Sanchez M, Galisteo M, Vera R, Villar IC, Zarzuelo A, Tamargo J, et al. Quercetin downregulates NADPH oxidase, increases eNOS activity and prevents endothelial dysfunction in spontaneously hypertensive rats. J Hypertens. 2006;24:75–84. doi: 10.1097/01.hjh.0000198029.22472.d9. [DOI] [PubMed] [Google Scholar]
- 68.Vera R, Sanchez M, Galisteo M, Villar IC, Jimenez R, Zarzuelo A, et al. Chronic administration of genistein improves endothelial dysfunction in spontaneously hypertensive rats: involvement of eNOS, caveolin and calmodulin expression and NADPH oxidase activity. Clin Sci (Lond) 2007;112:183–191. doi: 10.1042/CS20060185. [DOI] [PubMed] [Google Scholar]
- 69.Hardin CD, Vallejo J. Caveolins in vascular smooth muscle: form organizing function. Cardiovasc Res. 2006;69:808–815. doi: 10.1016/j.cardiores.2005.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]