Abstract
Chemokines and their receptors play a critical role in orchestrating the immune response during experimental autoimmune encephalomyelitis (EAE). Expression of CCR4 and its ligand CCL22 has been observed in ongoing disease. Here we describe a role for CCR4 in EAE, illustrating delayed and decreased disease incidence in CCR4−/− mice corresponding with diminished CNS infiltrate. Peripheral T cell responses were unaltered in CCR4−/− mice; rather, disease reduction was related to reduced CD11b+Ly6Chi inflammatory macrophage (iMϕ) numbers and function. These results provide evidence that CCR4 regulates EAE development and further supports the involvement of CCR4 in iMϕ effector function.
Keywords: Experimental Autoimmune Encephalomyelitis, Multiple Sclerosis, Central Nervous System, Inflammatory Macrophage, Chemokine Receptor, CCR4
1. Introduction
Experimental autoimmune encephalomyelitis (EAE) is a T helper cell mediated autoimmune disease of the central nervous system (CNS) that shares many disease characteristics with multiple sclerosis (MS) (Hickey, 1999). The disease manifests itself clinically as ascending hind limb paralysis, induced by immunization with myelin antigen emulsified in complete Freund’s adjuvant (CFA) or encephalitogenic CD4+ T cell transfer (Baron et al., 1993; Bettelli et al., 2003; Whitham et al., 1991). This results in infiltration of mononuclear cells, including but not limited to; CD4+ T cells, CD8+ T cells, macrophages (Mϕ) and dendritic cells (DC) into the spinal cord resulting in inflammation and demyelination (Abdul-Majid et al., 2003; Bailey et al., 2007; Brosnan et al., 1981; Hickey et al., 1983; Jensen et al., 1992).
Chemokines are small molecular weight chemoattractants that are produced under steady state and pathological conditions to maintain or influence cell recruitment to specific tissues (Forster et al., 2008; Huang et al., 2001; Karpus et al., 1995). Chemokines can function in differentiation and maturation of leukocytes, in addition to their well described role in cell trafficking and accumulation (Karpus et al., 1997; Marsland et al., 2005). Various chemokines and their cognate receptors are expressed during EAE, and have been shown to be important for disease progression (Carlson et al., 2008; Dogan et al., 2011; Elhofy et al., 2009; Fife et al., 2000; Fife et al., 2001; Huang et al., 2001; Karpus and Kennedy, 1997; Rottman et al., 2000). CCR4 and CCL22 transcripts are known to be expressed in the CNS of mice during EAE (Columba-Cabezas et al., 2002). Recently, we demonstrated inhibition of CCL22 ameliorated EAE in SJL mice by reducing the CNS accumulation of the inflammatory macrophage (iMϕ) population and/or reducing the inflammatory function of that cellular population (Dogan et al., 2011).
In the present study, we sought to distinguish between these two possibilities by utilizing CCR4−/− mice to determine whether CCR4 regulates iMϕ tissue accumulation or inflammatory function. Macrophages are critical for the development of EAE, as evidenced by depletion studies which resulted in greatly diminished disease (Huitinga et al., 1993). Additionally, our lab and others have demonstrated ameliorated disease in CCR2−/− and CCL2−/− mice due to defective macrophage migration (Fife et al., 2000; Huang et al., 2001). The Ly6Chi subset of macrophages are considered inflammatory and are pathogenic during EAE (King et al., 2009), thus inhibition of this specific population of macrophages may provide a more strategic targeting for disease inhibition. Here we report that CCR4-deficient mice display a delay in clinical onset, while maintaining normal peripheral T cell responses. These data support a role for CCR4 in regulating iMϕ effector function during EAE.
2. Materials and Methods
Mice
Female C57BL/6 (H-2b) mice were purchased from Harlan Sprague Dawley (Indianapolis, IN). CCR4−/− (H-2b) homozygous knockout mice were previously described (Chvatchko et al., 2000) and were a gift from Dr. Amanda Proudfoot. All mice were maintained in the Center for Comparative Medicine at Northwestern University. Mice were 6-8 weeks old at the initiation of experiments and were maintained on standard laboratory chow and water. Animal care was in accordance with Northwestern University Animal Care and Use committee and Public Health Service policies.
Antigen and antibodies
MOG 35-55 (MEVGWYRSPFSRVVHLYRNGK) (Slavin et al., 1998) was purchased from Peptides International (Louisville, KY). Amino acid composition was verified by mass spectrometry, and purity was determined to be >98%. Fluorochrome-conjugated monoclonal antibodies to murine CD45 (Ly-5), CD4 (RM4-5), CD11b (M1/70), Ly6C (AL-21), IL-17A (TC11-18H10), TNF (MP6-XT22), and IL-4 (BVD4-1D11) were purchased from BD PharMingen (San Diego, CA). Fluorochrome-conjugated monoclonal antibody to murine CCR4 (2G12) was purchased from BioLegend (San Diego, CA), and the isotype control antibody fluorochrome-conjugated antibody to hamster IgG was purchased from Caltag Laboratories (Burlingame, CA). Fluorochrome-conjugated monoclonal antibodies to murine IFNγ (XMG1.2) and Foxp3 (FJK-16s) were purchased from eBioscience (San Diego, CA).
Histology
Mice were anesthetized, perfused intracardially with ice-cold PBS and spinal cords harvested. Spinal cords were embedded in optimal cutting temperature compound (OCT) and 8-10 μm sections were cut on a cryostat. Frozen sections were stained using standard H&E methodology as previously described (Karpus et al., 1995).
Disease induction and clinical evaluation
For active EAE, mice were immunized with 100 μg of MOG 35-55 emulsified in CFA containing 4 mg/ml Mycobacterium tuberculosis (Difco, Kansas City, MO) subcutaneously. Mice were given 200 ng of pertussis toxin (List Biological Laboratories) intraperitoneally on days 0 and 2 post immunization. Animals were graded daily according to clinical severity using the following scale: grade 0, no abnormality; grade 1, limp tail; grade 2, limp tail and hind limb weakness; grade 3, partial hind limb paralysis; grade 4, complete hind limb paralysis; grade 5, death (Miller et al., 2010).
ELISA
CCL22 expression was quantified from spleen, draining lymph nodes (DLN), and spinal cord samples homogenized in PBS containing 0.05% Tween-20 (Sigma-Aldrich) and clarified by centrifugation at 400 × g for 10 minutes. Levels of CCL22 from homogenates were quantified by ELISA. Briefly, flat bottom 96 well plates (Nunc, Rochester, NY) were coated with 200 ng/well monoclonal rat anti-mouse CCL22 (R&D Systems, Minneapolis, MN) in PBS overnight at 4 °C. Nonspecific binding sites were blocked with 2% BSA in PBS for 1 h at 37 °C. Dilutions of tissue homogenate were added in triplicate for 4 h at 37°C, plates were washed and 5 ng/well biotinylated goat-anti mouse CCL22 (R&D Systems) was added for 1 h at 37 °C. Plates were developed using streptavidin-peroxidase at a 1:5000 dilution (Zymed, San Francisco, CA) for 30 minutes at 37 °C, followed by TMB substrate-chromogen (Dako, Denmark) and the reaction was stopped using sulfuric acid. The absorbance was read on a plate reader at 450 nm and CCL22 levels were quantified based on a standard curve expressed as pg/ml.
Flow Cytometry
Spinal cords were isolated from the CNS of mice anesthetized and perfused intracardially with ice cold PBS. Mononuclear cells were isolated as previously described (Pope et al., 1996). Briefly, tissues were dissociated in Hank’s Balanced Salt Solution (HBSS) by passage through a metal screen (100 mesh) and centrifuged at 1200 rpm for 10 minutes at 4 °C. The pellet was resuspended in a 30% isotonic Percoll gradient (Pharmacia Biotech, NJ) and centrifuged at 1200 rpm for 10 minutes at room temperature. Spleens and DLN were also subjected to mechanical disruption through metal screens, centrifuged and resuspended in Dulbecco’s Modified Eagle Medium (DMEM). Red blood cells (RBC) from spleen samples were lysed with Tris-NH4Cl (pH 7.3) and resuspended in DMEM. Cells were collected and washed followed by incubation with specific antibodies to extracellular antigens. For intracellular flow cytometry cells were treated in culture medium with 50 ng/ml phorbol myristate acetate (PMA) and 500 ng/ml ionomycin (Sigma-Aldrich) for 5 h, and 1 μl of 1000× monensin (Ebioscience) was added 2 h into stimulation. Cells were stained for extracellular antigens and then fixed with 2% paraformaldehyde for 10 minutes at room temperature. Cells were permeabilized in 0.5% saponin solution and then stained intracellularly for cytokines and transcription factors. Data was collected on a FACSCanto flow cytometer (BD Biosciences) in the Interdepartmental Immunobiology Center Flow Cytometry facility at Northwestern University and was analyzed offline using FCS Express software (DeNovo Software, Thornhill, Ontario, Canada).
Statistical analysis
Clinical disease differences, sample mean, standard deviation and statistical significance were calculated using Prism 5.0 (Graphpad). Statistical significance between sample means was analyzed using the Student’s t test and differences in disease incidence were analyzed using χ2. P values <0.05 were considered significant.
3. Results
CCR4 and its ligand, CCL22, are elevated in the periphery and CNS during EAE
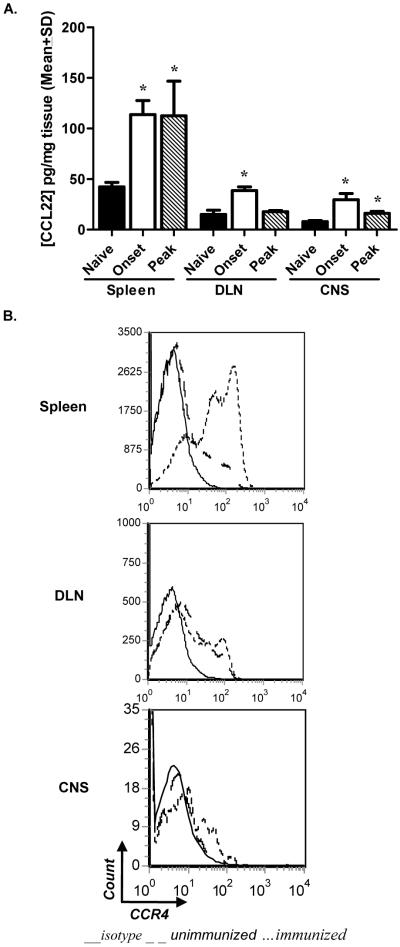
Previous reports have described an increase in CCR4 and CCL22 transcripts during EAE in the CNS of C57BL/6 mice (Columba-Cabezas et al., 2002). Although there are two known CCR4 ligands, CCL17 (TARC) (Imai et al., 1996) and CCL22 (MDC) (Andrew et al., 1998), only CCL22 has been shown to be highly expressed and play a functional role during disease (Columba-Cabezas et al., 2002; Dogan et al., 2011). We set out to evaluate expression of this receptor/ligand pair before and after disease induction in both the periphery and CNS of wild type (WT) mice. Tissue homogenates were harvested at key points in disease and analyzed for CCL22 by ELISA. Protein levels were found to be significantly increased over unimmunized controls at onset of clinical disease in each tissue tested, and at peak of disease in the spleen and CNS (Fig. 1A). We also evaluated CCR4 expression on leukocytes from DLN, spleens, and spinal cords after disease induction. Flow cytometric analysis comparing unimmunized and immunized animals illustrate a marked increase in CCR4 expression on leukocytes in both the periphery and CNS post immunization (Fig. 1B). These data confirm CCR4 and its corresponding ligand CCL22 are expressed in the target organ during EAE in C57BL/6 mice, suggesting a role in disease development.
Figure 1. CCR4 and CCL22 expression correlates with EAE development.
C57BL/6 mice were immunized with MOG35-55 emulsified in CFA and killed at the indicated stage(s) of disease. Unimmunized mice served as a baseline control. (A) CCL22 from spleen, DLN, and spinal cord homogenates were measured by ELISA. Protein expression was significantly increased over baseline in all tissues analyzed. The data are expressed as mean pg/mg of tissue (±SD) from individual mice. (B) CCR4 expression by leukocytes pre- and post-immunization was evaluated at peak of disease in spleens, DLN, and spinal cords by flow cytometric analysis, displayed as histograms from a combined forward versus side-scatter and CD45+ gate. The data is representative of two experiments, n=3 mice/group. *, p<0.05.
Delayed disease onset in the absence of CCR4
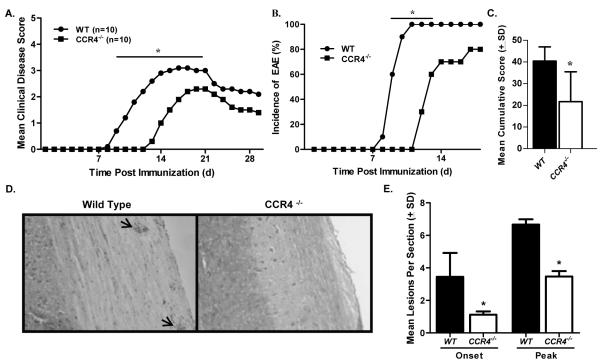
We confirmed leukocyte expression of CCR4 in key tissues following disease induction (Fig. 1B), next we investigated whether this receptor plays a functional role in the development of disease. To test this we examined EAE in the absence of CCR4, immunizing WT C57BL/6 and CCR4−/− mice with MOG35-55 in CFA and monitoring clinical disease development. The results illustrated in Fig. 2A indicate CCR4−/− mice develop significantly delayed disease as compared to WT controls. This delay in onset was associated with a decrease in disease incidence at early time points (Fig. 2B), as well as a decrease in mean cumulative score (Fig. 2C). Table 1 summarizes clinical disease courses from three individual experiments. The clinical disease pattern is similar in each experiment, illustrating a significant delay in the mean day of onset and mean day of peak disease observed in the CCR4−/− group. In addition, there was a significant reduction in disease incidence in CCR4−/− mice, when calculated from the number of mice that developed EAE in all three experiments.
Figure 2. Disease is delayed in CCR4−/− mice.
WT and CCR4−/− mice were immunized with MOG35-55 emulsified in CFA and monitored for clinical disease development. (A) CCR4−/− mice showed a significant decrease in clinical disease as compared to WT mice at early time points (days 9-17). The data is expressed as daily mean clinical disease score post immunization. N=10 mice per group. (B) Disease incidence was significantly diminished in CCR4−/− mice early in disease. (C) Mean cumulative disease score (±SD) was calculated for each group and determined to be significantly reduced in CCR4−/− mice. (D) Knockout mice showed reduced histological disease as determined by microscopic analysis of H&E stained spinal cord sections at onset and peak of clinical disease. Representative photomicrographs (original magnification, 100×) of spinal cord sections from WT and CCR4−/− mice at onset of clinical disease. Arrows indicate mononuclear cell infiltrate. (E) Quantitative analysis of histological disease was performed by calculating the mean number (±SD) of inflammatory foci per section at onset and peak of disease. These results are representative of three separate experiments. *, p<0.05.
Table 1.
Clinical Disease Analysis
| Mice | Incidencea | Mean Onsetb | Mean Peakc | Mean Peak Scored | |
|---|---|---|---|---|---|
| Experiment 1 | WT | 11/12 | 8.5±.8 | 12.8±1.0 | 3.1±.6 |
| CCR4−/− | 7/11 | 12.3±1.3e | 14.9±1.1e | 3.0±.8 | |
| Experiment 2 | WT | 10/10 | 9.4±.8 | 13.5±1.1 | 3.2±.6 |
| CCR4−/− | 8/10 | 14.3±2.0e | 16.9±1.8e | 3.0±.8 | |
| Experiment 3 | WT | 6/6 | 8.7±1.6 | 12.8±.8 | 3.0±.6 |
| CCR4−/− | 4/6 | 13.0±1.4e | 16.3±2.0e | 3.0±.8 |
Incidence describes the number of mice in each group that developed clinical disease.
Mean onset refers to the average day of initial clinical disease ±SD.
Mean peak is the average day mice reached their maximum disease score ±SD.
Mean peak score is the average maximum disease score ±SD.
P<0.05, two-tailed Student’s t test.
In order to investigate the effect CCR4-defiency had on CNS inflammation, spinal cords from a similar experiment were harvested from WT and CCR4−/− mice at onset and peak clinical disease, as determined by WT disease development. Tissues were sectioned and examined for inflammatory infiltrate by H&E stain. The results, shown in Fig. 2D, represent spinal cord sections from WT and CCR4−/− mice and illustrate less mononuclear cell infiltration (arrows) in knockout mice. Quantification of the mean number of inflammatory lesions per section reveals significantly fewer loci at onset and peak of disease in CCR4-deficient spinal cords (Fig. 2E). Thus the delay in clinical disease displayed by CCR4−/− mice is associated with a reduction in inflammatory lesion number.
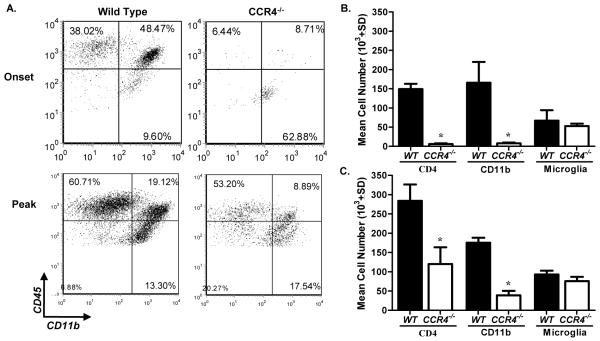
We assessed the leukocyte composition of the CNS infiltrate using flow cytometric analysis. Representative flow dot plots illustrating CNS mononuclear cells indicate a decrease in the percentage of lymphocytes (CD45hiCD11b−) at onset, and a decrease in the percentage of infiltrating monocytes (CD45hiCD11b+) at both onset and peak clinical disease in CCR4−/− mice (Fig. 3A). Quantification of CNS-infiltrating lymphocytes and monocytes revealed significantly decreased numbers of cells at both onset (Fig. 3B) and peak (Fig. 3C) clinical disease, while the mean number of microglia remained equivalent. These data support a role for CCR4 in the regulation of EAE.
Figure 3. Mononuclear cell accumulation is decreased in CCR4−/− mice.
WT and CCR4−/− were killed at onset and peak of WT clinical disease. Spinal cords were harvested and subjected to flow cytometric analysis. (A) Representative flow dot plots of CNS-infiltrating mononuclear cells from WT and CCR4−/− mice. Relative proportions of lymphocytes (CD45hiCD11b−), infiltrating monocytes (CD45hiCD11b+) and microglia (CD45loCD11b+) at onset and peak clinical disease are displayed. Dot plots are derived from a combined forward versus side-scatter and CD45+ gate. (B) Quantitative analysis of mice from each group indicate a significant decrease in macrophage and lymphocyte numbers in the CNS of CCR4−/− as compared to WT control mice at onset (C) and peak clinical disease. The data is based on two independent flow cytometric analyses, n=3. *, p<0.05.
Peripheral T cell response to antigen is unaltered in CCR4−/− mice
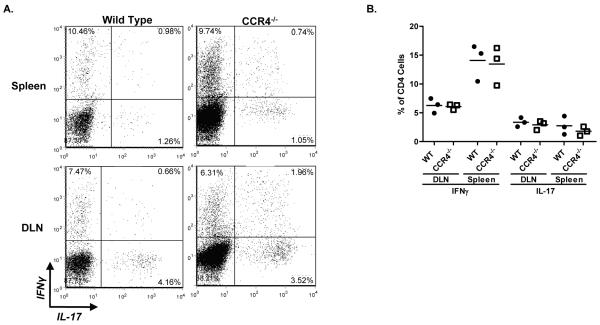
In order to determine the mechanism by which CCR4-deficiency delays disease, we examined mice at the time point at which the discrepancy between clinical scores was maximal. To address whether CCR4 deficiency affected the peripheral T cell response, DLN and spleens were evaluated for Th1 and Th17 populations at the onset of WT disease. Intracellular flow cytometric analysis illustrates similar percentages of CD4+ IFNγ- and IL-17-producing cells in the WT and CCR4−/− periphery (Fig. 4A). Likewise, calculation of the mean percentage of peripheral Th1 and Th17 cells reveal no statistical differences between groups (Fig. 4B). These results indicate CCR4 is not involved in regulating a Th1 or Th17 response to myelin antigen.
Figure 4. CCR4 deficiency does not affect the peripheral T helper cell response.
Immunized WT and CCR4−/− mice were killed at onset based on WT clinical disease. Peripheral Th1 and Th17 responses were evaluated by intracellular flow cytometric analysis. (A) Representative flow dot plots displaying IFNγ- and IL-17-producing cells in peripheral lymphoid organs of WT and CCR4−/− mice. The dot plots are derived from a combined forward versus side-scatter and CD45+CD4+ gate. (B) The mean percentage of CD4+ IFNγ- and IL-17-producing cells (±SD) was calculated, and indicates no statistical difference in Th1 or Th17 percentages between groups. Data representative of two separate experiments, n=3.
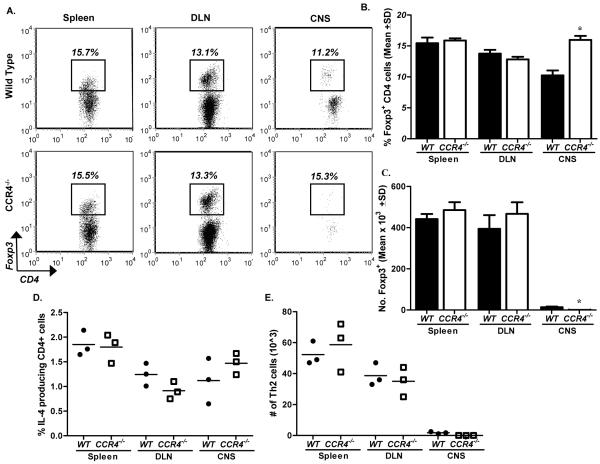
We also explored the possibility that the absence of CCR4 resulted in defective Treg and/or Th2 cell trafficking during EAE. Treg and Th2 populations preferentially express CCR4 (Imai et al., 1999; Lee et al., 2005), hence CCR4-deficiency had the potential to affect trafficking of these cells to tissue-specific sites where immune responses could be down-regulated. Representative flow dot plots gated on CD4+ cells illustrate similar Foxp3 percentages in the periphery of WT and CCR4−/− mice during early disease development (Fig. 5A). Similarly, quantification of the mean percentage (Fig. 5B) and mean cell number (Fig. 5C) of Foxp3+ cells reveal no significant differences in the spleen or DLN. There was a significant increase in the percentage of Foxp3+ cells in the CNS of CCR4−/− animals; however, the absolute cell number was decreased (Fig. 5A/B). Diminished numbers of Foxp3+ cells in the target organ would not explain the lag in disease observed in CCR4−/− mice, as Tregs are known to ameliorate EAE (Yu et al., 2005).
Figure 5. Treg and Th2 numbers remain unaltered in CCR4−/− mice.
Immunized WT and CCR4−/− mice were killed at the onset of WT clinical disease. Treg and Th2 numbers were assessed by intracellular flow cytometric analysis. (A) Representative flow dot plots of Foxp3 expression in the periphery and spinal cord of WT and CCR4−/− animals. The data is derived from a combined forward versus side-scatter and CD45+CD4+ gate. (B) Mean percentage (±SD) of CD4+ cells expressing Foxp3 and (C) quantification of the mean number (±SD) of CD4+Foxp3+ cells in the periphery and spinal cord of each group. (D) Mean percentage (±SD) of CD4+ cells expressing IL-4 and (E) quantification of the mean number (±SD) of CD4+IL-4+ cells in the periphery and spinal cord of each group. The data is representative of two experiments, n=3 mice/group. *, p<0.05.
Additionally, we examined Th2 populations following immunization of CCR4−/− and WT mice. Th2 cells were identified as CD4+ IL-4-producing cells, as assessed by intracellular flow cytometric analysis. The mean percentage (Fig. 5D) and mean number (Fig. 5E) of Th2 cells from each group was calculated, and reveal no statistical difference in any of the tissues examined. These results provide no evidence that the absence of CCR4 affects Treg or Th2 cell accumulation during acute EAE.
Diminished iMϕ population in CCR4−/− mice
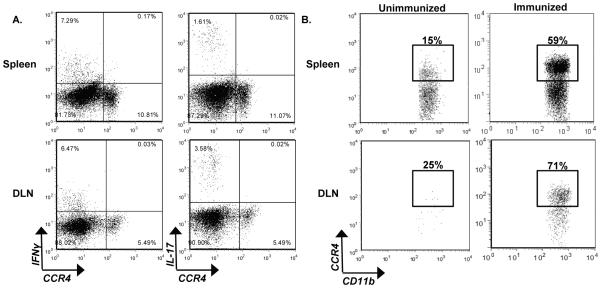
We did not detect a difference in Th1 or Th17 populations in the periphery of CCR4−/− mice post priming (Fig. 4); however, the possibility remained that these cells require CCR4 to traffic to the CNS. Therefore, we evaluated the expression of CCR4 on Th1 and Th17 effector cells in the periphery during active disease via intracellular flow cytometric analysis. Representative flow dot plots reveal neither effector T cell population that expresses IFN-γ or IL-17 express CCR4 post immunization (Fig. 6A). These results indicate that CCR4 is unlikely to be involved in Th1 or Th17 trafficking to the CNS during EAE.
Figure 6. CCR4 is expressed by iMϕ post immunization.
WT mice were immunized and killed at peak clinical disease. CCR4 expression on pathogenic cell populations was evaluated by flow cytometric analysis. (A) Representative flow dot plots of WT spleens and DLN displaying IFNγ and IL-17 versus CCR4. The data is derived from a combined forward versus side-scatter and CD45+CD4+ gate. (B) Representative flow dot plots from unimmunized and immunized WT mice, illustrate CCR4 expression by CD11b+Ly6Chi cells in the spleen and DLN. The data is derived from a CD45+CD11b+Ly6Chi gate. The data is representative of two experiments, n=3 mice/group.
While CCR4 is often associated with Th2 and Treg populations (Imai et al., 1999; Lee et al., 2005), a variety of cell types express this receptor (Abi-Younes et al., 2001; Meyer et al., 2007; Stolberg et al., 2011). Importantly, there is a growing body of literature describing a role for CCR4 in iMϕ function (Dogan et al., 2011; Ness et al., 2006b; Trujillo et al., 2008; Yogo et al., 2009). We explored the possibility that the iMϕ population generated post-immunization in EAE express CCR4. This population of iMϕ has previously been shown to be pathogenic in EAE and is characterized by their expression of CD11b+Ly6Chi (King et al., 2009). The data presented in Figure 6B indicates that the majority of CD11b+Ly6Chi cells found in the periphery at the onset of disease express CCR4. These results support a possible role of CCR4 in regulating iMϕ function during EAE. Since CCR4−/− mice display a lag in disease development, we hypothesized that CCR4 may be important iMϕ trafficking and/or effector function during EAE.
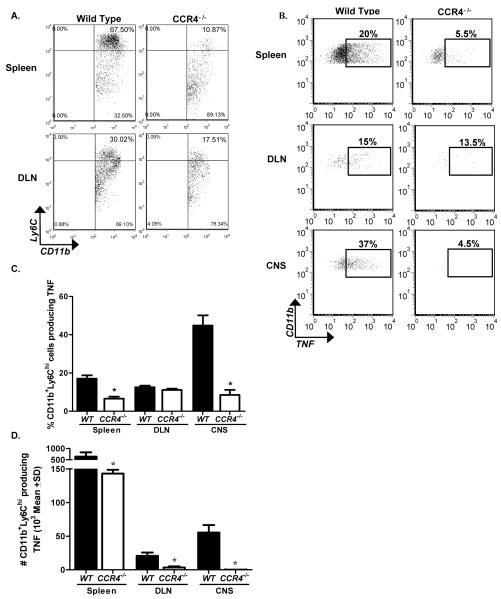
In order to determine a possible role for CCR4 in iMϕ migration, we evaluated the percentage of CD11b+Ly6Chi cells in the periphery post antigen priming in WT and CCR4−/− mice. Flow cytometric analysis of peripheral lymphoid organs illustrates the proportion of CD11b+ cells expressing Ly6Chi were greatly diminished in the periphery of CCR4−/− animals. This data illustrates CCR4−/− mice have a decreased iMϕ population in the periphery early in disease, when clinical disease severity most differs from WT controls.
M1 macrophages are characterized by the genes they express; including, iNOS, IL-12, and TNF (Gordon and Taylor, 2005) and thus are considered iMϕ. We assessed TNF expression by CD11b+Ly6Chi iMϕ using intracellular flow cytometric analysis to establish whether these were indeed inflammatory in nature and whether the absence of CCR4 affected that inflammatory nature. The results presented in Figure 7B demonstrate that in addition to a diminished CD11b+Ly6Chi cell population in CCR4−/− mice, there was a decrease in the percentage of those cells that produce TNF. Diminished TNF expression was evident in both the spleen and CNS at this early time point. Further analysis of CD11b+Ly6ChiTNF+ cells at disease onset showed a significant decrease in the mean percentage of cells in the spleen and spinal cord of CCR4−/− mice (Fig. 7C). Even more profound was the decreased mean CD11b+Ly6ChiTNF+ cell number in spleens, DLN and spinal cords of CCR4-deficient mice (Fig. 7D). Taken together these data strongly suggest a role for CCR4 in iMϕ function during EAE pathogenesis.
Figure 7. Diminished iMϕ population in CCR4−/− mice.
WT and CCR4−/− mice were immunized with MOG35-55 emulsified in CFA and monitored for clinical disease. Mice were killed at onset corresponding to WT disease. (A) Representative flow dot plots illustrate the percentage of Ly6Chi cells in the spleen, DLN, and CNS of WT and CCR4−/− mice. Data are derived from a forward versus side scatter and CD45+CD11b+ gate. (B) Representative flow dot plots display the percentage of CD11b+Ly6ChiTNF+ cells in the periphery and spinal cord of WT and CCR4−/− mice. Data derived from a forward versus side scatter and CD45+CD11b+Ly6Chi gate. (C) Quantification of the mean percentage (±SD) (D) and mean number (±SD) of CD11b+Ly6ChiTNF+ cells in the periphery and spinal cord of WT and CCR4−/− mice. The data are representative of two similar experiments, n=3 mice/group. *, p<0.05
4. Discussion
Chemokine and chemokine receptors are key players in orchestrating an immune response and are important for the development of many autoimmune diseases (Engelhardt, 2008; McDonald, 2009). The present investigation focuses on the contribution of CCR4 to the development of EAE and expands upon the body of literature describing a role for CCR4 in macrophage regulation (Chvatchko et al., 2000; Dogan et al., 2011; Garcia et al., 2003; Ishii et al., 2008; Trujillo et al., 2008; Yogo et al., 2009). It has been proposed that CCL22 and CCR4 may play a role in EAE as affected mice express both receptor and ligand in the CNS correlating with disease development (Columba-Cabezas et al., 2002) and neutralization of CCL22 inhibited EAE development (Dogan et al., 2011). We confirmed CCR4 and its ligand CCL22 are present in the periphery and CNS of C57BL/6 mice post disease induction (Fig. 1). To determine the mechanism of CCR4 regulation of EAE we compared the clinical disease course of WT and CCR4−/− mice. CCR4-deficiency resulted in significantly delayed clinical disease (Fig. 2) with a significant decrease in CNS-infiltrating mononuclear cells (Fig. 3).
One possible explanation for the delay in disease observed in CCR4−/− mice is that CCR4 is involved in the generation or trafficking of pathogenic T lymphocytes to the CNS. It has previously been reported that CCL3 (Karpus and Kennedy, 1997; Karpus et al., 1997) and CCL2 (Gu et al., 2000) play a role in Th1 and Th2 development, respectively. We investigated the possibility that CCR4 was involved in T cell polarization by determining the peripheral T cell response that resulted post disease induction. Specifically, we looked at Th1 (Kuchroo et al., 1993) and Th17 (Bettelli et al., 2008; Dardalhon et al., 2008) populations, as they are thought to mediate EAE progression (Abromson-Leeman et al., 2007). We did not detect a difference in the percentage of Th1 or Th17 cells in DLN or spleens of CCR4−/− mice as compared to WT mice (Fig. 4), thus arguing against the idea that CCR4 regulated Th polarization during disease development. Additionally, we explored the possibility that CCR4 is necessary for Th1 or Th17 trafficking to the CNS, by assessing CCR4 expression on Th1 and Th17 cells prior to migration. Flow cytometric evaluation illustrated a lack of CCR4 expression on IFNγ- and IL-17-producing CD4 cells (Fig. 6A). These results show that it is highly unlikely CCR4 is involved in trafficking of pathogenic lymphocytes to the CNS during EAE. Therefore, we conclude that CCR4 is not required for development of antigen-specific Th1 or Th17 responses and it is not involved in the chemoattraction of these cells to the CNS.
CCR4 has been associated with Th2 and Treg populations (Lee et al., 2005; Vestergaard et al., 2000; Yang et al., 2006), therefore, the second possibility to explain reduced EAE in CCR4−/− mice is that CCR4−/− mice have a defect in the ability of Th2 or Treg to traffic or accumulate thus altering the intrinsic regulation of the disease process. This is an unlikely mechanism for the delayed clinical disease displayed by CCR4−/− mice as lack of CNS Treg accumulation would likely lead to increased clinical severity (Stephens et al., 2005). Nevertheless, it is possible a defect in trafficking of Treg to the CNS in response to CCL22 expressed during the disease process (Columba-Cabezas et al., 2002; Dogan et al., 2011) could result in accumulation in the periphery and suppression of the peripheral immune response thereby affecting the development of EAE. When we investigated whether Treg or Th2 cell numbers were altered in CCR4−/− immunized to develop EAE we found no significant differences in the number of either T cell subset in the periphery of WT and knockout mice (Fig. 5). Together, these data demonstrate that CCR4 does not regulate EAE by affecting the accumulation of Th2 or Treg cells.
Macrophages are a major constituent of the inflammatory infiltrate in the CNS and have proven critical to the development of EAE (Brosnan et al., 1981; Huitinga et al., 1995). Ly6Chi is a marker for inflammatory macrophages (Hatakeyama et al., 1994) which are considered pathogenic during disease (King et al., 2009). Therefore, the third major possibility for CCR4 regulation of EAE was whether the receptor affected CNS macrophage accumulation and/or effector function. Indeed, we found a large portion of the CD11b+Ly6Chi population post immunization express CCR4 (Fig. 6B). If CCR4 affected EAE by the regulation of CNS iMϕ accumulation, then we would have expected similar numbers of iMϕ in the periphery (DLN and spleen) of WT and CCR4−/− mice but decreased numbers in the CNS of CCR4−/− mice compared to controls. We found fewer iMϕ (CD11b+Ly6Chi) numbers in DLN and spleens of CCR4−/− mice after EAE induction compared to WT controls (Fig. 7A). Therefore, there is no strong evidence that CCR4 is regulating EAE through CNS iMϕ accumulation despite the original reports indicating that CCR4−/− mice have a defect in Mϕ migration (Chvatchko et al., 2000) and anti-CCL22 inhibited Mϕ migration in a cecal ligation and puncture model of bacterial immunity (Matsukawa et al., 2000).
In addition to its role in chemoattraction, CCR4 appears to be important for iMϕ effector function (Dogan et al., 2011; Ness et al., 2006b). Specifically, it has been hypothesized that CCR4 signaling plays a role in promoting the M1 inflammatory phenotype (Trujillo et al., 2008) including TNF production in association with TLR stimulation (Ishii et al., 2008). Moreover, Jakubzick et al. (Jakubzick et al., 2004) demonstrated that neutralization of CCR4 ligands in a murine model of schistosomiasis resulted in a Mϕ cytokine phenotype resembling M2 (Mantovani et al., 2004). In support of the idea that CCR4 regulates EAE through modulation of effector function in our present report, we noted there were significantly fewer TNF-producing Ly6Chi iMϕ in both the periphery and CNS of knockout animals (Fig. 7D). In the present report we used TNF as a marker for an inflammatory macrophage phenotype. These results confirm the findings that in vivo anti-CCL22 neutralization, and therefore lack of CCR4 signaling, resulted in decreased expression of TNF by iMϕ (Dogan et al., 2011). In addition, we have previously demonstrated that when CCR4 signaling is affected by neutralizing CCL22 in vivo, the Ly6Chi macrophage population made more IL-10 (Dogan et al., 2011). It is known that administration of IL-10, deletion of IL10 or administration of cells producing IL-10 affects the development of EAE (Bettelli et al., 1998; Rott et al., 1994; Segal et al., 1998; Zhang et al., 2004). Therefore it is plausible that in the present experiments, a shift from inflammatory to anti-inflammatory cytokines resulted in delayed disease development seen in the CCR4−/− mice (Fig. 1). We favor the mechanism that ligand-dependent stimulation of CCR4 up-regulates iMϕ effector function and promotes an M1 phenotype which is critical for the induction of EAE as opposed an M2 phenotype which has the potential to dampen development of clinical disease. Support for the idea that a shift in the balance between M1 and M2 macrophage phenotypes can regulate EAE derives from a previous study where macrophages activated by stimuli resulting in an M2 phenotype could confer protection from development of severe EAE (Tierney et al., 2009). Furthermore, adoptive transfer of M2 monocytes has been shown to reverse ongoing EAE (Weber et al., 2007). Finally, tt has been recently demonstrated that the balance of circulating M1 and M2 Mϕ predicted the severity of EAE development and administration of M2-macrophages to animals with EAE resulted in amelioration of clinical disease (Mikita et al., 2011).
King and colleagues (King et al., 2009) have demonstrated that CD11b+ Ly6Chi monocytes are precursors of both CNS macrophages and dendritic cells (DC) and have the propensity to home to sites of active inflammation. Furthermore, this phenotype has been shown to give rise to the DC population that traffic to sites of Listeria infection and is involved in bacterial clearance via their inflammatory effector function (Serbina et al., 2003). These examples raise the question of whether CCR4 signaling in LyC6hi iMϕ promotes differentiation to DC or regulation of DC to affect either their inflammatory or anti-inflammatory functions. Possibilities of CCR4 regulation of Mϕ and DC function include modulation of inflammatory/anti-inflammatory cytokine gene expression patterns (Ishii et al., 2008; Ness et al., 2006a; Trujillo et al., 2008) and cell surface molecules such as PD1/PDL1 which is known to play a role in the pathogenesis of EAE (Carter et al., 2007; Liang et al., 2003; Schreiner et al., 2008; Zhu et al., 2006).
This report is the first to demonstrate a role for CCR4 in EAE, and expands on a growing body of literature suggesting CCR4 modulates macrophage function (Dogan et al., 2011; Ishii et al., 2008; Jakubzick et al., 2004; Ness et al., 2006b; Trujillo et al., 2008). This could have implications for MS, as CCL22 cerebrospinal fluid levels are elevated in female MS patients, suggesting a possible role for the ligand in disease progression (Galimberti et al., 2008a). Additionally, a single nucleotide polymorphism (SNP) in the promoter and coding sequence of CCL22 appears to confer a decreased risk of developing MS (Galimberti et al., 2008b). Human inflammatory monocytes are known to express CCR4 (Geissmann et al., 2003) and are a key player in MS pathogenesis (Bruck et al., 1995), thus inhibiting this population has the potential to ameliorate ongoing disease. CCR4 antagonists have already been shown to inhibit macrophage migration in a peritonitis disease model (Burdi et al., 2007), and we are currently exploring these compounds as therapeutic modalities for ongoing autoimmune disease.
Acknowledgements
The study was supported by NIH grant R01NS34510 (WJK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdul-Majid KB, Wefer J, Stadelmann C, Stefferl A, Lassmann H, Olsson T, Harris RA. Comparing the pathogenesis of experimental autoimmune encephalomyelitis in CD4−/− and CD8−/− DBA/1 mice defines qualitative roles of different T cell subsets. J Neuroimmunol. 2003;141:10–19. doi: 10.1016/s0165-5728(03)00210-8. [DOI] [PubMed] [Google Scholar]
- Abi-Younes S, Si-Tahar M, Luster AD. The CC chemokines MDC and TARC induce platelet activation via CCR4. Thromb Res. 2001;101:279–289. doi: 10.1016/s0049-3848(00)00402-3. [DOI] [PubMed] [Google Scholar]
- Abromson-Leeman S, Ladell DS, Bronson RT, Dorf ME. Heterogeneity of EAE mediated by multiple distinct T-effector subsets. J Neuroimmunol. 2007;192:3–12. doi: 10.1016/j.jneuroim.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew DP, Chang MS, McNinch J, Wathen ST, Rihanek M, Tseng J, Spellberg JP, Elias CG. STCP-1 (MDC) CC chemokine acts specifically on chronically activated Th2 lymphocytes and is produced by monocytes on stimulation with Th2 cytokines IL-4 and IL-13. Journal of Immunology. 1998;161:5027–5038. [PubMed] [Google Scholar]
- Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ T(H)-17 cells in relapsing EAE. Nat Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- Baron JL, Madri JA, Ruddle NH, Hashim G, Janeway CA., Jr. Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Das MP, Howard ED, Weiner HL, Sobel RA, Kuchroo VK. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. The Journal of Immunology. 1998;161:3299–3306. [PubMed] [Google Scholar]
- Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of TH17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Pagany M, Weiner HL, Linington C, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan CF, Bornstein MB, Bloom BR. The effects of macrophage depletion on the clinical and pathologic expression of experimental allergic encephalomyelitis. Journal of Immunology. 1981;126:614–620. [PubMed] [Google Scholar]
- Bruck W, Porada P, Poser S, Rieckmann P, Hanefeld F, Kretzschmar HA, Lassmann H. Monocyte/macrophage differentiation in early multiple sclerosis lesions. Ann Neurol. 1995;38:788–796. doi: 10.1002/ana.410380514. [DOI] [PubMed] [Google Scholar]
- Burdi DF, Chi S, Mattia K, Harrington C, Shi Z, Chen S, Jacutin-Porte S, Bennett R, Carson K, Yin W, Kansra V, Gonzalo JA, Coyle A, Jaffee B, Ocain T, Hodge M, LaRosa G, Harriman G. Small molecule antagonists of the CC chemokine receptor 4 (CCR4) Bioorg Med Chem Lett. 2007;17:3141–3145. doi: 10.1016/j.bmcl.2007.03.030. [DOI] [PubMed] [Google Scholar]
- Carlson T, Kroenke M, Rao P, Lane TE, Segal B. The Th17-ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease. J Exp Med. 2008;205:811–823. doi: 10.1084/jem.20072404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LL, Leach MW, Azoitei ML, Cui J, Pelker JW, Jussif J, Benoit S, Ireland G, Luxenberg D, Askew GR, Milarski KL, Groves C, Brown T, Carito BA, Percival K, Carreno BM, Collins M, Marusic S. PD-1/PD-L1, but not PD-1/PD-L2, interactions regulate the severity of experimental autoimmune encephalomyelitis. Journal of Neuroimmunology. 2007;182:124–134. doi: 10.1016/j.jneuroim.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Chvatchko Y, Hoogewerf AJ, Meyer A, Alouani S, Juillard P, Buser R, Conquet F, Proudfoot AE, Wells TN, Power CA. A key role for CC chemokine receptor 4 in lipopolysaccharide-induced endotoxic shock. J Exp Med. 2000;191:1755–1764. doi: 10.1084/jem.191.10.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Columba-Cabezas S, Serafini B, Ambrosini E, Sanchez M, Penna G, Adorini L, Aloisi F. Induction of macrophage-derived chemokine/CCL22 expression in experimental autoimmune encephalomyelitis and cultured microglia: implications for disease regulation. J Neuroimmunol. 2002;130:10–21. doi: 10.1016/s0165-5728(02)00170-4. [DOI] [PubMed] [Google Scholar]
- Dardalhon V, Korn T, Kuchroo VK, Anderson AC. Role of Th1 and Th17 cells in organ-specific autoimmunity. J Autoimmun. 2008 doi: 10.1016/j.jaut.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan RN, Long N, Forde E, Dennis K, Kohm AP, Miller SD, Karpus WJ. CCL22 regulates experimental autoimmune encephalomyelitis by controlling inflammatory macrophage accumulation and effector function. J Leukoc Biol. 2011;89:93–104. doi: 10.1189/jlb.0810442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhofy A, Depaolo RW, Lira SA, Lukacs NW, Karpus WJ. Mice deficient for CCR6 fail to control chronic experimental autoimmune encephalomyelitis. J Neuroimmunol. 2009;213:91–99. doi: 10.1016/j.jneuroim.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt B. Immune cell entry into the central nervous system: Involvement of adhesion molecules and chemokines. J Neurol Sci. 2008 doi: 10.1016/j.jns.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Fife BT, Huffnagle GB, Kuziel WA, Karpus WJ. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:899–905. doi: 10.1084/jem.192.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife BT, Kennedy KJ, Paniagua MC, Lukacs NW, Kunkel SL, Luster AD, Karpus WJ. CXCL10 (IFN-gamma-inducible protein-10) control of encephalitogenic CD4+ T cell accumulation in the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2001;166:7617–7624. doi: 10.4049/jimmunol.166.12.7617. [DOI] [PubMed] [Google Scholar]
- Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- Galimberti D, Fenoglio C, Comi C, Scalabrini D, De Riz M, Leone M, Venturelli E, Cortini F, Piola M, Monaco F, Bresolin N, Scarpini E. MDC/CCL22 intrathecal levels in patients with multiple sclerosis. Mult Scler. 2008a;14:547–549. doi: 10.1177/1352458507084268. [DOI] [PubMed] [Google Scholar]
- Galimberti D, Scalabrini D, Fenoglio C, De Riz M, Comi C, Venturelli E, Cortini F, Piola M, Leone M, Dianzani U, D’Alfonso S, Monaco F, Bresolin N, Scarpini E. Gender-specific influence of the chromosome 16 chemokine gene cluster on the susceptibility to Multiple Sclerosis. J Neurol Sci. 2008b;267:86–90. doi: 10.1016/j.jns.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Garcia GE, Xia Y, Harrison J, Wilson CB, Johnson RJ, Bacon KB, Feng L. Mononuclear cell-infiltrate inhibition by blocking macrophage-derived chemokine results in attenuation of developing crescentic glomerulonephritis. Am J Pathol. 2003;162:1061–1073. doi: 10.1016/S0002-9440(10)63903-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407–411. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- Hatakeyama S, Iwabuchi K, Ogasawara K, Good RA, Onoe K. The murine c-fgr gene product associated with Ly6C and p70 integral membrane protein is expressed in cells of a monocyte/macrophage lineage. Proc Natl Acad Sci U S A. 1994;91:3458–3462. doi: 10.1073/pnas.91.8.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey WF. The pathology of multiple sclerosis: a historical perspective. J Neuroimmunol. 1999;98:37–44. doi: 10.1016/s0165-5728(99)00079-x. [DOI] [PubMed] [Google Scholar]
- Hickey WF, Gonatas NK, Kimura H, Wilson DB. Identification and quantitation of T lymphocyte subsets found in the spinal cord of the Lewis rat during acute experimental allergic encephalomyelitis. Journal of Immunology. 1983;131:2805–2809. [PubMed] [Google Scholar]
- Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med. 2001;193:713–726. doi: 10.1084/jem.193.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huitinga I, Damoiseaux JG, Dopp EA, Dijkstra CD. Treatment with anti-CR3 antibodies ED7 and ED8 suppresses experimental allergic encephalomyelitis in Lewis rats. Eur J Immunol. 1993;23:709–715. doi: 10.1002/eji.1830230321. [DOI] [PubMed] [Google Scholar]
- Huitinga I, Ruuls SR, Jung S, Van Rooijen N, Hartung HP, Dijkstra CD. Macrophages in T cell line-mediated, demyelinating, and chronic relapsing experimental autoimmune encephalomyelitis in Lewis rats. Clin Exp Immunol. 1995;100:344–351. doi: 10.1111/j.1365-2249.1995.tb03675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T, Nagira M, Takagi S, Kakizaki M, Nishimura M, Wang J, Gray PW, Matsushima K, Yoshie O. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Immunol. 1999;11:81–88. doi: 10.1093/intimm/11.1.81. [DOI] [PubMed] [Google Scholar]
- Imai T, Yoshida T, Baba M, Nishimura M, Kakizaki M, Yoshie O. Molecular cloning of a novel T cell-directed CC chemokine expressed in thymus by signal sequence trap using Epstein-Barr virus vector. J.Biol.Chem. 1996;271:21514–21521. doi: 10.1074/jbc.271.35.21514. [DOI] [PubMed] [Google Scholar]
- Ishii M, Hogaboam CM, Joshi A, Ito T, Fong DJ, Kunkel SL. CC chemokine receptor 4 modulates Toll-like receptor 9-mediated innate immunity and signaling. Eur J Immunol. 2008;38:2290–2302. doi: 10.1002/eji.200838360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubzick C, Wen H, Matsukawa A, Keller M, Kunkel SL, Hogaboam CM. Role of CCR4 Ligands, CCL17 and CCL22, During Schistosoma mansoni Egg-Induced Pulmonary Granuloma Formation in Mice. American Journal of Pathology. 2004;165:1211–1221. doi: 10.1016/S0002-9440(10)63381-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MA, Arnason BG, Toscas A, Noronha A. Preferential increase of IL-2R+ CD4+ T cells and CD45RB- CD4+ T cells in the central nervous system in experimental allergic encephalomyelitis. J Neuroimmunol. 1992;38:255–261. doi: 10.1016/0165-5728(92)90018-g. [DOI] [PubMed] [Google Scholar]
- Karpus WJ, Kennedy KJ. MIP-1alpha and MCP-1 differentially regulate acute and relapsing autoimmune encephalomyelitis as well as Th1/Th2 lymphocyte differentiation. J Leukoc Biol. 1997;62:681–687. [PubMed] [Google Scholar]
- Karpus WJ, Lukacs NW, Kennedy KJ, Smith WS, Hurst SD, Barrett TA. Differential CC chemokine-induced enhancement of T helper cell cytokine production. Journal of Immunology. 1997;158:4129–4136. [PubMed] [Google Scholar]
- Karpus WJ, Lukacs NW, McRae BL, Strieter RM, Kunkel SL, Miller SD. An important role for the chemokine macrophage inflammatory protein-1 alpha in the pathogenesis of the T cell-mediated autoimmune disease, experimental autoimmune encephalomyelitis. J Immunol. 1995;155:5003–5010. [PubMed] [Google Scholar]
- King IL, Dickendesher TL, Segal BM. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 2009;113:3190–3197. doi: 10.1182/blood-2008-07-168575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchroo VK, Martin CA, Greer JM, Ju ST, Sobel RA, Dorf ME. Cytokines and adhesion molecules contribute to the ability of myelin proteolipid protein-specific T cell clones to mediate experimental allergic encephalomyelitis. Journal of Immunology. 1993;151:4371–4382. [PubMed] [Google Scholar]
- Lee I, Wang L, Wells AD, Dorf ME, Ozkaynak E, Hancock WW. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med. 2005;201:1037–1044. doi: 10.1084/jem.20041709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang SC, Latchman YE, Buhlmann JE, Tomczak MF, Horwitz BH, Freeman GJ, Sharpe AH. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol. 2003;33:2706–2716. doi: 10.1002/eji.200324228. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in Immunology. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Marsland BJ, Battig P, Bauer M, Ruedl C, Lassing U, Beerli RR, Dietmeier K, Ivanova L, Pfister T, Vogt L, Nakano H, Nembrini C, Saudan P, Kopf M, Bachmann MF. CCL19 and CCL21 induce a potent proinflammatory differentiation program in licensed dendritic cells. Immunity. 2005;22:493–505. doi: 10.1016/j.immuni.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Matsukawa A, Hogaboam CM, Lukacs NW, Lincoln PM, Evanoff HL, Kunkel SL. Pivotal role of the CC chemokine, macrophage-derived chemokine, in the innate immune response. J Immunol. 2000;164:5362–5368. doi: 10.4049/jimmunol.164.10.5362. [DOI] [PubMed] [Google Scholar]
- McDonald JR. Selective chemokine receptor-targeted depletion of pathological cells as a therapeutic strategy for inflammatory, allergic and autoimmune diseases. Recent Pat Inflamm Allergy Drug Discov. 2009;3:177–187. doi: 10.2174/187221309789257423. [DOI] [PubMed] [Google Scholar]
- Meyer EH, Wurbel MA, Staton TL, Pichavant M, Kan MJ, Savage PB, DeKruyff RH, Butcher EC, Campbell JJ, Umetsu DT. iNKT cells require CCR4 to localize to the airways and to induce airway hyperreactivity. J Immunol. 2007;179:4661–4671. doi: 10.4049/jimmunol.179.7.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikita J, Dubourdieu-Cassagno N, Deloire MS, Vekris A, Biran M, Raffard G, Brochet B, Canron MH, Franconi JM, Boiziau C, Petry KG. Altered M1/M2 activation patterns of monocytes in severe relapsing experimental rat model of multiple sclerosis. Amelioration of clinical status by M2 activated monocyte administration. Mult Scler. 2011;17:2–15. doi: 10.1177/1352458510379243. [DOI] [PubMed] [Google Scholar]
- Miller SD, Karpus WJ, Davidson TS. Experimental autoimmune encephalomyelitis in the mouse. Curr Protoc Immunol. 2010 doi: 10.1002/0471142735.im1501s88. Chapter 15, Unit 15 11. [DOI] [PubMed] [Google Scholar]
- Ness TL, Ewing JL, Hogaboam CM, Kunkel SL. CCR4 Is a Key Modulator of Innate Immune Responses. The Journal of Immunology. 2006a;177:7531–7539. doi: 10.4049/jimmunol.177.11.7531. [DOI] [PubMed] [Google Scholar]
- Ness TL, Ewing JL, Hogaboam CM, Kunkel SL. CCR4 is a key modulator of innate immune responses. J Immunol. 2006b;177:7531–7539. doi: 10.4049/jimmunol.177.11.7531. [DOI] [PubMed] [Google Scholar]
- Pope JG, Karpus WJ, VanderLugt C, Miller SD. Flow cytometric and functional analyses of central nervous system-infiltrating cells in SJL/J mice with Theiler’s virus-induced demyelinating disease. Evidence for a CD4+ T cell-mediated pathology. J Immunol. 1996;156:4050–4058. [PubMed] [Google Scholar]
- Rott O, Fleischer B, Cash E. Interleukin-10 prevents experimental allergic encephalomyelitis in rats. European Journal of Immunology. 1994;24:1434–1440. doi: 10.1002/eji.1830240629. [DOI] [PubMed] [Google Scholar]
- Rottman JB, Slavin AJ, Silva R, Weiner HL, Gerard CG, Hancock WW. Leukocyte recruitment during onset of experimental allergic encephalomyelitis is CCR1 dependent. Eur J Immunol. 2000;30:2372–2377. doi: 10.1002/1521-4141(2000)30:8<2372::AID-IMMU2372>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Schreiner B, Bailey SL, Shin T, Chen L, Miller SD. PD-1 ligands expressed on myeloid-derived APC in the CNS regulate T-cell responses in EAE. Eur J Immunol. 2008;38:2706–2717. doi: 10.1002/eji.200838137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal BM, Dwyer BK, Shevach EM. An Interleukin (IL)-10/IL-12 Immunoregulatory Circuit Controls Susceptibility to Autoimmune Disease. Journal of Experimental Medicine. 1998;187:537–546. doi: 10.1084/jem.187.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-Producing Dendritic Cells Mediate Innate Immune Defense against Bacterial Infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- Slavin A, Ewing C, Liu J, Ichikawa M, Slavin J, Bernard CC. Induction of a multiple sclerosis-like disease in mice with an immunodominant epitope of myelin oligodendrocyte glycoprotein. Autoimmunity. 1998;28:109–120. doi: 10.3109/08916939809003872. [DOI] [PubMed] [Google Scholar]
- Stephens LA, Gray D, Anderton SM. CD4+CD25+ regulatory T cells limit the risk of autoimmune disease arising from T cell receptor crossreactivity. Proc Natl Acad Sci U S A. 2005;102:17418–17423. doi: 10.1073/pnas.0507454102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolberg VR, Chiu BC, Schmidt BM, Kunkel SL, Sandor M, Chensue SW. CC Chemokine Receptor 4 Contributes to Innate NK and Chronic Stage T Helper Cell Recall Responses during Mycobacterium bovis Infection. Am J Pathol. 2011;178:233–244. doi: 10.1016/j.ajpath.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney JB, Kharkrang M, La Flamme AC. Type II-activated macrophages suppress the development of experimental autoimmune encephalomyelitis. Immunol Cell Biol. 2009;87:235–240. doi: 10.1038/icb.2008.99. [DOI] [PubMed] [Google Scholar]
- Trujillo G, O’Connor EC, Kunkel SL, Hogaboam CM. A novel mechanism for CCR4 in the regulation of macrophage activation in bleomycin-induced pulmonary fibrosis. Am J Pathol. 2008;172:1209–1221. doi: 10.2353/ajpath.2008.070832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard C, Bang K, Gesser B, Yoneyama H, Matsushima K, Larsen CG. A Th2 chemokine, TARC, produced by keratinocytes may recruit CLA+CCR4+ lymphocytes into lesional atopic dermatitis skin. J Invest Dermatol. 2000;115:640–646. doi: 10.1046/j.1523-1747.2000.00115.x. [DOI] [PubMed] [Google Scholar]
- Weber MS, Prod’homme T, Youssef S, Dunn SE, Rundle CD, Lee L, Patarroyo JC, Stuve O, Sobel RA, Steinman L, Zamvil SS. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med. 2007;13:935–943. doi: 10.1038/nm1620. [DOI] [PubMed] [Google Scholar]
- Whitham RH, Bourdette DN, Hashim GA, Herndon RM, Ilg RC, Vandenbark AA, Offner H. Lymphocytes from SJL/J mice immunized with spinal cord respond selectively to a peptide of proteolipid protein and transfer relapsing demyelinating experimental autoimmune encephalomyelitis. J Immunol. 1991;146:101–107. [PubMed] [Google Scholar]
- Yang ZZ, Novak AJ, Stenson MJ, Witzig TE, Ansell SM. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood. 2006;107:3639–3646. doi: 10.1182/blood-2005-08-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogo Y, Fujishima S, Inoue T, Saito F, Shiomi T, Yamaguchi K, Ishizaka A. Macrophage derived chemokine (CCL22), thymus and activation-regulated chemokine (CCL17), and CCR4 in idiopathic pulmonary fibrosis. Respir Res. 2009;10:80. doi: 10.1186/1465-9921-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Gregg RK, Bell JJ, Ellis JS, Divekar R, Lee HH, Jain R, Waldner H, Hardaway JC, Collins M, Kuchroo VK, Zaghouani H. Specific T regulatory cells display broad suppressive functions against experimental allergic encephalomyelitis upon activation with cognate antigen. J Immunol. 2005;174:6772–6780. doi: 10.4049/jimmunol.174.11.6772. [DOI] [PubMed] [Google Scholar]
- Zhang X, Koldzic DN, Izikson L, Reddy J, Nazareno RF, Sakaguchi S, Kuchroo VK, Weiner HL. IL-10 is involved in the suppression of experimental autoimmune encephalomyelitis by CD25+CD4+ regulatory T cells. Int.Immunol. 2004;16:249–256. doi: 10.1093/intimm/dxh029. [DOI] [PubMed] [Google Scholar]
- Zhu B, Guleria I, Khosroshahi A, Chitnis T, Imitola J, Azuma M, Yagita H, Sayegh MH, Khoury SJ. Differential Role of Programmed Death-1 Ligand and Programmed Death-2 Ligand in Regulating the Susceptibility and Chronic Progression of Experimental Autoimmune Encephalomyelitis. The Journal of Immunology. 2006;176:3480–3489. doi: 10.4049/jimmunol.176.6.3480. [DOI] [PubMed] [Google Scholar]