Abstract
This chapter provides an overview of neurosteroids, especially their impact on the brain, sex differences and therapeutic potentials. Neurosteroids are synthesized within the brain and rapidly modulate neuronal excitability. They are classified as pregnane neurosteroids such as allopregnanolone and allotetrahydrodeoxycorticosterone, and androstane neurosteroids, such as androstanediol and etiocholanone. Neurosteroids such as allopregnanolone are positive allosteric modulators of GABA-A receptors with powerful antiseizure activity in diverse animal models. Neurosteroids increases both synaptic and tonic inhibition. They are endogenous regulators of seizure susceptibility, anxiety and stress. Sulfated neurosteroids such as pregnenolone sulfate, which are negative GABA-Areceptor modulators, are memory-enhancing agents. Sex differences in susceptibility to brain disorders could be due to neurosteroids and sexual dimorphism in specific structures of the human brain. Synthetic neurosteroids that exhibit better bioavailability and efficacy and drugs that enhance neurosteroid synthesis have therapeutic potential in anxiety, epilepsy and other brain disorders. Clinical trials with the synthetic neurosteroid analog ganaxolone in the treatment of epilepsy have been encouraging. Neurosteroidogenic agents that lack benzodiazepine-like side effects show promise in the treatment of anxiety and depression.
Keywords: Allopregnanolone, androstanediol, deoxycorticosterone, epilepsy, ganaxolone, GABA-A receptor, sex differences, neurosteroid, progesterone, seizure susceptibility, testosterone
Introduction
Neurosteroids are steroids synthesized within the brain and modulate neuronal excitability by rapid non-genomic actions. The term ‘neurosteroids’, originally coined by the French physiologist Etienne Baulieu, is now widely used to refer to steroids that are synthesized in the brain. Circulating steroid hormones serve as precursors for the synthesis of neurosteroids, which are produced locally in the hippocampus and other brain structures (Baulieu and Robel, 1990). Based on structural features, neurosteroids can be classified as pregnane neurosteroids, such as allopregnanolone and allotetrahydrodeoxycorticosterone (THDOC), androstane neurosteroids, such as androstanediol and etiocholanone, and sulfated neurosteroids, such as pregnenolone sulfate (PS) and dehydroepiandrosterone sulfate (DHEAS). Steroid hormones have long been recognized to have sedative, anesthetic and antiseizure properties in animals and humans (Aird, 1944; Aird and Gordan, 1951; Gyermek et al., 1967; Green et al., 1978). Studies during the past two decades have uncovered that progesterone and deoxycorticosterone serve as precursors for the endogenous neurosteroids allopregnanolone (5α-pregnane-3α-ol-20-one) and THDOC (5α-pregnane-3α,21-diol-20-one), respectively (Reddy, 2003; 2009a). Testosterone-derived androgens such as androstanediol (5α-androstane-3α,17β-diol) and estradiol can be considered as neurosteroids (Reddy, 2008). Generally, the acute effects of neurosteroids are not related to interactions with classical steroid hormone receptors that regulate gene transcription. Moreover, neurosteroids are not themselves active at intracellular steroid receptors. They modulate brain excitability primarily by interaction with neuronal membrane receptors and ion channels, principally GABA-A receptors (Lambert et al., 2003; Reddy, 2003; Akk et al., 2009). Neurosteroids are endogenous regulators of neuronal excitability, and therefore provide tremendous opportunities for developing therapeutic approaches (Reddy and Kulkarni, 2000; Morrow, 2007). This chapter reviews the biosynthesis, mechanisms, pharmacology, sex differences and therapeutic potentials of neurosteroids and their synthetic analogs.
Biosynthesis of neurosteroids
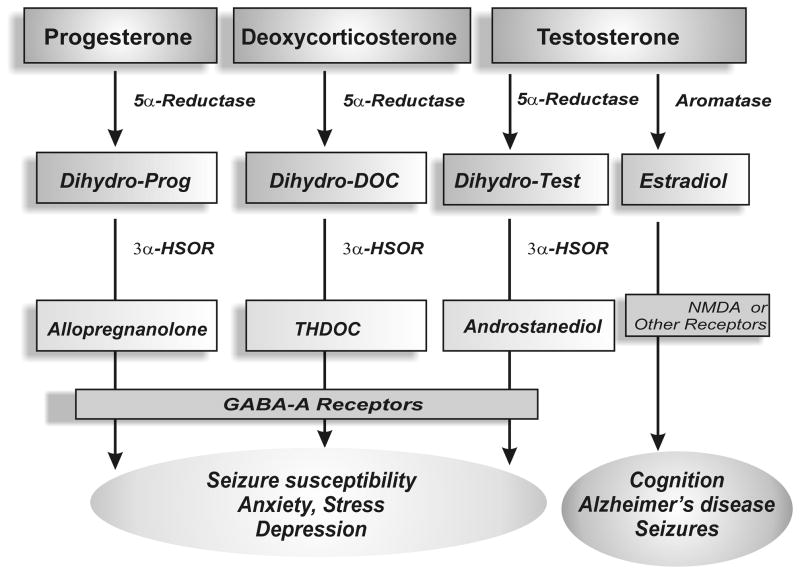
Neurosteroids are A-ring reduced metabolites of the steroid hormones progesterone, deoxycorticosterone and testosterone (Fig.1). The steroid precursors of neurosteroids are mainly synthesized in the gonads, adrenal gland, and feto-placental unit. Several neurosteroids including allopregnanolone, THDOC, and androstanediol are generated by sequential reduction of the parent steroid by 5α-reductase and 3α-hydroxysteroid oxidoreductase (3α-HSOR) (Fig. 2). These conversion steps occur in peripheral tissues such as reproductive endocrine tissues, liver, and skin that are rich in the two reducing activities (Do Rego et al., 2009). Since neurosteroids are highly lipophilic and can readily cross the blood-brain barrier, neurosteroids synthesized in peripheral tissues accumulate in the brain and can influence brain function (Schumacher et al., 1996).
Figure 1. Biosynthetic pathways of neurosteroids in the human brain and their impact on brain function.
5α-Reductase converts progesterone, testosterone and deoxycorticosterone into 5α-dihydro reduced steroids, which are then reduced further to 3α-hydroxylated neurosteroids by 3α-HSOR. Testosterone is converted into 17β-estradiol by the aromatase enzyme. These and related enzymes involved in neurosteroid biosynthesis and metabolism are present in the human brain.
Figure 2. Biosynthesis of progesterone- and testosterone-derived neurosteroids in the brain.
Androstanediol (5α-androstan-3α,17β-diol) is synthesized from testosterone by reduction at the 5-and 3-positions of the steroid A-ring. Allopregnanolone (5α-pregnan-3α-ol-20-one) is derived from progesterone by reduction at the 5- and 3-positions of the steroid A-ring. Androstanediol differs from allopregnanolone by a 17β-hydroxyl group instead of 17β-methyl-carbonyl group.
The emerging evidence support that neurosteroid biosynthetic enzymes are present in the human brain (Mensah-Nyagan et al., 1999; Stoffel-Wagner et al., 2000; Petratos et al., 2000; Do Rego et al., 2009). 5α-Reductase activity has been identified in both neurons and glial cells in the brain (Melcangi et al., 1998; Petratos et al., 2000). In humans, the 5α-reductase and 3α-HSOR enzymes have been found in neocortex and subcortical white matter as well as in hippocampal tissues (Stoffel-Wagner, 2001; Stoffel-Wagner et a., 2003). Thus, it is likely that neurosteroids can be formed from their parent hormonal steroids directly in the target brain region (Mellon et al., 2001; Reddy and Kulkarni, 2000). Steroid precursors readily enter the brain so that pools of peripherally synthesized precursors are readily available for local neurosteroid biosynthesis. Since the activity of the 3α-HSOR is far greater than that of the 5α-reductase, steroid 5α-reduction is the rate-limiting step in the biosynthesis of neurosteroids.
Neurosteroids are produced from their precursor steroids de novo by glial cells and principal neurons (Mensah-Nyagan et al., 1999; Agís-Balboa et al., 2006). Brain astrocytes and neurons express cytochrome P450 cholesterol side-chain cleavage enzyme (CYP450scc), which converts cholesterol to pregnenolone, an intermediate necessary for the synthesis of neurosteroids (Patte-Mensah et al., 2003). Moreover, 3β-hydroxysteroid dehydrogenase, an enzyme required for further conversion of pregnenolone to progesterone, has been demonstrated in the brain (Guennoun et al., 1995). Thus, the enzymes necessary for in situ synthesis of progesterone from cholesterol are present in the brain. Neurosteroidogenesis occurs in the brain regions such as cortex, hippocampus, and amygdala. Within these brain regions, neurosteroid synthetic enzymes are localized to glutamatergic principal neurons and not GABAergic inhibitory neurons (Agís-Balboa et al., 2006); thus, neurosteroids are synthesized within the same neurons that express their receptor targets. Allopregnanolone persists in the brain after adrenalectomy and gonadectomy or after pharmacological suppression of adrenal and gonadal secretions (Corpechot et al., 1993; Purdy et al., 1991), indicating that allopregnanolone can be synthesized de novo in the brain via 5α-reduction of progesterone. However, regulatory mechanisms underlying the neurosteroid biosynthesis in the brain remain unclear.
The biosynthesis of neurosteroids is controlled by the translocator protein (18 kD), formerly called peripheral or mitochondrial benzodiazepine receptor (Costa and Guidotti, 1991; Korneyev et al. 1993). The translocator protein is widely found in the peripheral tissues and in the brain. It is mainly located in the outer mitochondrial membrane and favors the transport of cholesterol to the inner mitochondrial membrane, ultimately promoting neurosteroid synthesis (Papadopoulos et al., 2006). Activation of this protein by certain ligands facilitates the intramitochondrial flux of cholesterol and thereby increases the availability of cholesterol to the CYP450scc, an enzyme located in the inner mitochondrial membrane that converts cholesterol into pregnenolone, which is a key intermediate for neurosteroid biosynthesis. The selective ligands of the translocator protein can stimulate neurosteroid biosynthesis in brain (Auta et al. 1993; Rupprecht et al., 2009), confirming the key role of the translocator protein in neurosteroidogenesis.
Mechanisms of neurosteroid actions
Generally, there is strong evidence that acute effects of neurosteroids are not related to interactions with classical steroid hormone receptors that regulate gene transcription. However, chronic effects of neurosteroids are due to both genomic (classical intracellular steroid receptors) and non-genomic rapid actions (ion channels and membrane receptors) in the brain. Furthermore, the genomic effects of neurosteroids are mainly due to their metabolic interconversion to traditional steroids (Rupprecht et al., 1993). Overall, neurosteroids are not themselves active at intracellular steroid receptors. They modulate brain excitability primarily by interaction with neuronal membrane receptors and ion channels (Reddy, 2003). This contention is supported by the following observations: First, the effects of neurosteroids occur rapidly (within minutes), whereas steroid hormone actions via intracellular steroid receptors are usually slow in onset and are of prolonged duration (Joels, 1997). Second, neurosteroids do not exhibit high-affinity interaction with nuclear steroid hormone receptors (Rupprecht et al., 1993; 1996). Metabolites of neurosteroids produced by intracellular oxidation of the 3α-hydroxyl group may nevertheless bind to steroid receptors. Third, our recent studies in progesterone receptor (PR) knockout mice conclusively demonstrate that the classical steroid receptor is not required for the sedative, anxiolytic, and anticonvulsant activity of progesterone and related neurosteroids (Reddy et al., 2004; 2005; Reddy and Apanites, 2005). Finally, neurosteroids have been demonstrated to directly modulate the activity of ligand-gated ion channels, most notably GABA-A receptors (Gee et al., 1995; Lambert et al., 2003).
Modulation of GABA-A receptors
The GABA-A receptor is a major target of neurosteroids (Fig.3). Neurosteroids can be positive or negative regulators of GABA-A receptor function, depending on the chemical structure of the steroid molecule (Majewska, 1992; Reddy, 2003). The GABA-A receptor, a subtype of receptor for the neurotransmitter GABA, mediates the bulk of synaptic inhibition in the central nervous system. Structurally, GABA-A receptors are heteropentamers with five protein subunits that form the chloride ion channels. There are seven different classes of subunits, some of which have multiple homologous variants (α1–6, β1–3, γ1–3, σ1–3, δ, ε, θ); most GABA-A receptors are composed of α, β and γ or δ subunits (Sieghart, 2006). GABA activates the opening of chloride ion channels, permitting chloride ion influx and, ultimately, hyperpolarization. GABA-A receptors prevent action potential generation by short-circuiting the depolarization produced by excitatory neurotransmission. There are two types of inhibitory neurotransmission mediated via GABA-A receptors: synaptic (phasic) and extrasynaptic (tonic) inhibition. Neurosteroids modulate both synaptic and extrasynaptic GABA-A receptors, and thereby potentiate both phasic and tonic currents. Phasic inhibition results from the activation of γ2-containing receptors at the synapse by intermittent release of high levels of GABA from presynaptic terminals. Tonic inhibition, in contrast, is mediated by the continuous activation of δ-containing receptors located outside the synaptic cleft by low levels of ambient GABA. The δ-subunit is found on the dendrites of hippocampal dentate gyrus granule cells and confers important functional characteristics to GABA-A receptors. In contrast to synaptic receptors, which are periodically activated by millimolar concentrations of GABA released from the axon terminals of GABAergic interneurons, extrasynaptic GABA-A receptors are activated by the stray GABA molecules that escape reuptake by GABA transporters. Tonic inhibition plays a unique role in controlling hippocampus excitability by “setting” the baseline excitability.
Figure 3. Neurosteroid potentiation of GABA-A receptor-mediated currents.
(A) Neurosteroids have specific allosteric binding site on the GABA-A receptors, which are pentameric structures made of 2α, 2β,1γ or δ subunit that form chloride ion channel. The binding site(s) for the neurosteroids is separate from that of the benzodiazepine and barbiturate sites. (B) The neurosteroid androstanediol causes an increase in GABA-gated chloride currents in acutely dissociated hippocampal CA1 neurons in the whole-cell patch-clamp electrophysiological recordings. (C) The concentration-dependent increase in GABA potentiation by androstanediol in CA1 neurons.
Neurosteroids such as allopregnanolone, THDOC, and androstanediol are potent positive allosteric modulators of GABA-A receptors (Fig. 3) (Reddy and Rogawski, 2002; Akk et al., 2009). In 1984, Harrison and Simmonds first demonstrated that alphaxolone enhances GABA-evoked responses that are mediated by GABA-A receptors (Harrison and Simmonds, 1984). The modulating effects of neurosteroids occur by binding to discrete sites on the GABA-A receptor that are located within the transmembrane domains of the α- and β-subunits (Hosie et al., 2006). The binding site for neurosteroids is proposed to be distinct from that of the GABA, benzodiazepine and barbiturate sites. Although the exact location of neurosteroid binding site is currently unknown, it has been shown that a highly conserved glutamine at position 241 in the M1 domain of the α-subunit plays a key role in neurosteroid modulation (Hosie et al., 2009). Exposure to neurosteroids enhances the open probability of the GABA-A receptor chloride channel, so that the mean open time is increased and the mean closed time is decreased. This increases the chloride current through the channel, ultimately resulting in a reduction of neuronal excitability.
The GABA-A receptors are believed to contain two sites for agonist GABA and show positive (or negative) cooperativity for some modulators. Recent studies have indicated the existence of at least three neurosteroid binding sites on the GABA-A receptor: one for allosteric enhancement of GABA-evoked currents by allopregnanolone, one for direct activation by allopregnanolone, and one for antagonist action of sulfated neurosteroids such as PS (Lambert et al., 2003; Hosie et al., 2007). Electrophysiological studies are extensively used to confirm that neurosteroids, at low (nM) concentrations, act as positive allosteric modulators of GABA-A receptor function (Harrison et al., 1987; Kokate et al., 1994; Wetzel et al., 1999). Hence, neurosteroids enhance the specific receptor binding of [3H]flunitrazepam, a benzodiazepine receptor agonist, and [3H]muscimol, a specific GABA-site agonist, and inhibit the binding of [35S]t-butylbicycloorthobenzoate (TBPS), a cage convulsant and noncompetitive GABA-A receptor antagonist. Neurosteroid enhancement of GABA-A receptor chloride currents occurs through increases in both the channel open frequency and channel open duration (Twyman and MacDonald, 1992; Lambert et al., 1995; Hosie et al., 2007). Thus, neurosteroids greatly enhance the probability of GABA-A receptor chloride channel opening that allows massive chloride ion influx, thereby promoting augmentation of inhibitory GABAergic transmission. These effects occur at physiological concentrations of neurosteroids. Thus, endogenous neurosteroid levels continuously modulate the function of GABA-A receptors.
There are strict structural requirements for neurosteroid modulation of GABA-A receptors. A hydrogen bond-donating 3α-hydroxy group on the steroid A-ring and a hydrogen bond accepting group (typically a keto moiety) on the D ring at either C20 of the pregnane steroid side chain or C17 of the androstane ring system are critical for positive activity at GABA-A receptors (Purdy et al., 1990; Lambert et al., 2003). The orientation of the C5 hydrogen group appears to be essential for increased potency, but less critical for activity (Morrow et al., 1990; Kokate et al., 1994; Xue et al., 1997).
Neurosteroids modulates most GABA-A receptor isoforms, including recombinant GABA-A receptors (Puia et al., 1990; Lambert et al., 2003). This distinguishes neurosteroids from benzodiazepines, which only act on GABAA receptors that (i) contain γ2-subunits and (ii) do not contain α4- or α6-subunits. In general, the specific α-subunit may influenceneurosteroid efficacy, whereas the γ-subunit type may affect both the efficacyand potency for neurosteroid modulation of GABA-A receptors (Lambert et al., 2003). However, GABA-A receptors that contain the δ subunit are more sensitive to neurosteroid-induced potentiation of GABA responses (Mihalek et al., 1999; Spigelman et al., 2002; Stell et al., 2003; Belelli et al., 2002; Wohlfarth et al., 2002). Mice lacking δ subunit shows drastically reduced sensitivity to neurosteroids (Mihalek et al., 1999). In electrophysiological and radioligand binding studies, δ-subunit-containing GABA-A receptors exhibit increased sensitivity to neurosteroids (Wohlfarth et al., 2002), suggesting a unique role of δ-subunit in neurosteroid modulation of GABA-A receptors. The δ-subunit does not contribute to the neurosteroid binding site, but appears to confer enhanced transduction of neurosteroid action after the neurosteroid has bound to the receptor. GABA-A receptors containing the δ-subunit have a low degree of desensitization and they are located perisynaptically/extrasynaptically. These properties cause them to be prime candidates for mediating “tonic” GABA-A receptor current that is activated by ambient concentrations of GABA in the extracellular space. Tonic GABA-A receptor current causes a steady inhibition of neurons and reduces their excitability. Indeed, GABA is a relatively low efficacy agonist of δ-containing GABA-A receptors even though it binds with high affinity (Glykys and Mody, 2007). Therefore, neurosteroids have an opportunity to markedly enhance the current generated byδ-containing GABA-A receptors even in the presence of saturating GABA concentrations. During neuronal activity, there is expected to be substantial release of GABA from active GABAergic interneurons that can interact with perisynaptic and extrasynaptic δ-subunit-containing GABA-A receptors. Overall, the robust effect of neurosteroids is likely to be due to their action on both synaptic and perisynaptic/extrasynaptic GABAA receptors.
At high concentrations (>10 μM), neurosteroids can directlyactivate GABA-A receptor channels in the absence of GABA (Lambert et al., 1995). In this respect, neurosteroids resemble barbiturates but not benzodiazepines (Rho et al., 1996). These direct actions, which are picrotoxin-sensitive (Kokate et al., 1994; Reddy and Rogawski, 2002), have pharmacological significance with exogenously administered neurosteroids, but are not likely to be related to the actions of endogenous neurosteroids which are present only at low nanomolar concentrations.
Neurosteroids that are sulfated at C3 have inhibitory actions on GABA-A receptors (Park-Chung et al., 1999). For example, PS and DHEAS block GABA-A receptors at low micromolar concentrations (Majewska, 1992). These “sulfated steroids” act as non-competitive antagonists of the GABA-A receptor by interacting with a site that is distinct from that of neurosteroids such as allopregnanolone and THDOC (Majewska and Schwartz, 1987; Majewska et al., 1990; Park-Chung et al., 1999). The steroid negative modulatory action on GABA-A receptors occurs through a reduction in channel opening frequency, although the precise mechanism of block is not well understood (Mienville and Vicini, 1989; Akk et al., 2001). Given their abundance in brain, it seems reasonable that PS and DHEAS could function as endogenous neuromodulators.
Modulation of glutamate and other neurotransmitter receptors
Some neurosteroids can modulate the N-methyl-D-apartate (NMDA) type glutamate receptors (Gibbs and Farb, 2004; Wang et al., 2007). The NMDA receptors exhibit at least two distinct sites for neurosteroid modulation, one of which mediates the effects of positive modulators, while the other mediates the effects of negative modulators. Sulfated neurosteroids PS and DHEAS have been shown to be potent allosteric agonists at NMDA receptor complex (Wu et al., 1991). Generally, high micromolar concentrations of PS and DHEAS are necessary to achieve actions on NMDA receptor-mediated currents. PS can potentiate NMDA-mediated responses, as assessed by electrophysiological recording (Bowlby, 1993) or measurements of NMDA-induced increases in intracellular Ca2+ in cultured neurons (Irwin et al., 1994). PS selectively augments NMDA receptor-mediated glutamate-induced depolarisation in chick spinal cord neurons, while inhibiting the GABA, glycine, and non-NMDA response. PS potentiation of NMDA response is distinct from the glycine modulatory site (Wu et al, 1991). PS and its derivatives potentiated, while 3α-hydroxy-5β-pregnan-20-one sulphate inhibited NMDA-induced elevations in intracellular Ca2+ (Irwin et al., 1994). PS has also been shown to potentiate the NMDA-mediated increase in intracellular Ca2+ in cultured chick cortical neurons (Fahey et al., 1995). PS modulation of the NMDA receptor is attributed to its ability to increase the fractional open time of NMDA-activated channels, by increasing the frequency of opening and the duration of channel opening. PS effects on NMDA receptors depend upon subunit composition, with the NR2A and NR2B subunits supporting a potentiating effect, while NR2C and NR2D subunits support inhibition (Malayev et al., 2002).
Binding and pharmacological studies revealed that neurosteroids such as pregnenolone, DHEAS, and PS interact with σ receptors, a distinct family of receptors present in high density in the brain (Su et al., 1988; Maurice et al., 1996). A crossed pharmacology between the effects of σ1 modulators and neurosteroids has been described, DHEAS and PS behaving as agonists and progesterone as an antagonist. DHEAS potentiate, where as PS inhibits the NMDA-induced [3H]norepinephrine, which are sensitive to σ receptor antagonists. DHEA potentiates the NMDA-evoked excitability of hippocampus neurons, an effect that could be blocked by the σ1 antagonist haloperidol and NE-100, as well as by progesterone (Maurice et al., 1996). Effects of PS and DHEAS on σ1 receptors may underlie presynaptic action inducing glutamate release.
Physiological and pharmacological effects and therapeutic potentials
The physiological and pharmacological profile of major neurosteroids is listed in Table 1. In general, neurosteroids that are 3α-hydroxy-pregnane derivatives such as allopregnanolone, pregnanolone, and THDOC elicit sedative, anxiolytic, and anticonvulsant actions (Reddy, 2003). PS and DHEAS are excitatory and produce memory enhancing and anxiogenic effects. Synthetic neurosteroids that show better pharmacokinetics and efficacy are evaluated for sedative and anxiolytic (minaxolone), anesthetic (alphaxolone) and antiepileptic (ganaxolone) effects (Table 2).
Table 1.
Pharmacological profile of major neurosteroids.
| Neurosteroid | Pharmacological Actions | Mechanism of Action |
|---|---|---|
| Allopregnanolone | Sedative-hypnotic Anxiolytic, anticonvulsant Antistress, neuroprotection |
Potentiation of GABA-A receptor function |
| THDOC | Sedative-hypnotic Anxiolytic, anticonvulsant Antistress, neuroprotection |
Potentiation of GABA-A receptor function |
| Androstanediol | Anxiolytic, anticonvulsant | Potentiation of GABA-A receptor function |
| Pregnenolone sulfate | Anxiogenic, proconvulsant Memory enhancing, neuroprotection |
Inhibition of GABA-A receptor function Enhanced NMDA receptor function |
| Dehydroepiandrosterone sulfate | Anxiogenic, proconvulsant Memory enhancing Neurogenesis, neuroprotection |
Inhibition of GABA-A receptor function Enhanced NMDA receptor function Anti-glucocorticoid action |
Table 2.
Therapeutic potentials of synthetic neurosteroids.
| Agent | Chemical Structure | Major Indication | Status |
|---|---|---|---|
| Alphaxolone |
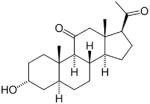
|
Anesthetic | Used in veterinary anesthesia |
| Minaxolone |

|
Sedative Anxiety | Phase I/II |
| Ganaxolone |

|
Epilepsy | Phase IIB trials |
Epilepsy
Neurosteroids are broad-spectrum anticonvulsant agents. They protect against seizures induced in animals by GABA-A receptor antagonists, pilocarpine-induced limbic seizures, and kindled seizures (Belleli et al., 1989; Kokate et al., 1994; Frye, 1995; Wieland et al., 1995; Reddy et al., 2004). Neurosteroids are highly effective in the pentylenetetrazol (PTZ) model of epilepsy. Some neurosteroids are highly effective in suppressing cocaine-, ethanol-, diazepam-, and neurosteroid-withdrawal seizures (Devaud et al., 1996; Tsuda et al., 1997; Reddy and Rogawski, 2001). At very high doses, neurosteroids also partially protect mice against maximal electroshock (MES)-induced seizures. Neurosteroids have differing potencies in various seizure models. In mice, the potency ranking is as follows (most sensitive to least sensitive): pilocarpine > bicuculline > PTZ > kindling > MES. Similarly, synthetic allopregnanolone analogs demonstrate comparable anticonvulsant efficacy (Hogenkamp et al., 1997; Carter et al., 1997). The protective index (TD50/ED50 values) of neurosteroids is comparable to those of clinically used antiepileptic drugs. The TD50 (the dose producing 50% motor toxicity) relates to the ability of neurosteroids to produce sedation/ataxia, not some other side effects. In addition to anticonvulsant activity, there is evidence that neurosteroids can retard the development of spontaneous recurrent seizures in animal models of epileptogenesis (Biagini et al., 2006, 2009).
The sulfated neurosteroids PS and DHEAS are proconvulsants partly because they block GABA-A receptors and facilitate NMDA receptor functions. In fact, acute intracerebroventricular or chronic systemic administration of these steroids reduces the PTZ seizure threshold and intracerebroventricular or intra-hippocampal administration can induce seizures and status epilepticus (Kokate et al., 1999; Williamson et al., 2004). The seizure facilitating effects of PS and DHEAS can be blocked by coadministration of allopregnanolone or other related neurosteroids that positively modulate GABA-A receptors, as well as by benzodiazepines and by NMDA receptor antagonists (Reddy and Kulkarni, 1998a). Moreover, exogenous application or endogenous stimulation of DHEAS modulates hippocampal GABA inhibition possibly by entraining hippocampal neurons to theta rhythm (Steffensen, 1995), suggesting a potential physiological relevance of the proconvulsant effects of DHEAS in animals.
Although natural neurosteroid can be used for therapeutic purpose in patients with epilepsy (Herzog, 1995; 1999), certain obstacles prevent the clinical use of endogenously occurring neurosteroids. First, natural neurosteroids such as allopregnanolone have low bioavailability because they are rapidly inactivated and eliminated by glucuronide or sulfate conjugation at the 3α-hydroxyl group. Secondly, the 3α-hydroxyl group of allopregnanolone may undergo oxidation to the ketone, restoring activity at steroid hormone receptors (Rupprecht et al., 1993). Synthetic neurosteroids, which are devoid of such hormonal actions, could provide a rational alternative approach to therapy (Reddy and Kulkarni, 2000). Ganaxolone (3α-hydroxy-3β-methyl-5α-pregnane-20-one), the 3β-methyl analog of allopregnanolone (Table 2), is a synthetic neurosteroid analog that overcomes these limitations (Carter et al., 1997). Like allopregnanolone, ganaxolone is a positive allosteric modulator of GABA-A receptors and is an effective anticonvulsant in the PTZ seizure test as well as in other anticonvulsant screening models (Carter et al., 1997; Gasior et al., 1997). However, ganaxolone is orally active and adequate blood levels can be maintained in human subjects with two or three daily doses (Monaghan et al., 1999). In addition, although ganaxolone is extensively metabolized, the potentially hormonally active 3-keto derivative is not formed. Preliminary evidence of the efficacy of ganaxolone in the treatment of human epilepsy supports a role for neurosteroids in epilepsy therapy (Reddy and Woodward, 2004).
Ganaxolone is the only neurosteroid-like agent that has been evaluated in human clinical trials for the treatment of epilepsy (Nohria et al., 2010). Ganaxolone modulates GABA-A receptors in a comparable fashion to allopregnanolone and has protective activity against clonic seizures induced by diverse chemoconvulsants including PTZ and limbic seizures in the 6-Hz model. In addition, a recent study in female amygdala kindled mice demonstrated suppression of behavioral and electrographic seizures with ED50 of 6.6 mg/kg (Reddy and Rogawski, 2010). In chronically treated rats, tolerance does not occur to the anticonvulsant activity of ganaxolone (Reddy and Rogawski, 2000).
Over the past decade, ganaxolone has been studied in various clinical trials to assess its efficacy and safety in the treatment of epilepsy (Laxer et al., 2000; Kerrigan et al., 2000; Peribone et al., 2007). A recent randomized, double-blind controlled study compared ganaxolone (500 mg t.i.d.) to placebo in 147 adults with partial onset seizures who were refractory to conventional antiepileptic drugs. The mean weekly seizure frequency, the primary endpoint, and the percent change from baseline in weekly seizure frequency were significantly improved in the ganaxolone group.
A randomized, placebo-controlled study of 56 infants and toddlers with refractory infantile spasms showed clear trends toward benefit in terms of reduction in seizure clusters, responder rate, resolution of hypsarrhythmia, and investigator’s global assessment, although the study did not reach significance with respect to its predefined outcome. More than 900 subjects have received ganaxolone in human clinical trials; common treatment-related adverse events are dizziness and fatigue, but discontinuation rates have generally been similar to that of placebo. Overall, ganaxolone appears to be an efficacious, well-tolerated and safe treatment for partial seizures that is probably also effective for infantile spasms.
Catamenial epilepsy
Catamenial epilepsy, the cyclical occurrence of seizure exacerbations near the time of menstruation or at other phases of the menstrual cycle, affects a high proportion of women of reproductive age with drug refractory epilepsy. Recent studies indicate that “catamenial” epilepsy affects from 39 to 60% of the women with epilepsy (Herzog et al., 2004; Bazan et la., 2005). Diagnosis of catamenial epilepsy is mainly based on the assessment of menstruation and seizure records for at least 2 cycles. The number of seizures in each phase is counted and a two-fold or greater increase in frequency is used as the criterion for diagnosis. There appears to be three patterns of catamenial epilepsy: perimenstrual, periovulatory and inadequate luteal-phase catamenial seizures, with the perimenstrual pattern most commonly observed (Herzog et al., 1997). Currently, there is no specific drug therapy for catamenial epilepsy; a detailed understanding of the patterns and pathophysiology is essential for the development of rational approaches for the prevention and treatment of catamenial epilepsy.
Catamenial epilepsy is observed in women with ovulatory or anovulatory cycles. A variety of mechanisms, such as fluctuations in antiepileptic drug levels, changes in water and electrolyte balance, and physiological variation in ovarian hormone secretion are thought to contribute to catamenial epilepsy, with hormonal changes (in the circulating levels of estrogens and progesterone) during the menstrual cycle certainly playing a large role in the increased seizure susceptibility (Bonucelli et al., 1989; Scharfman et al., 2006). There is emerging evidence that endogenous neurosteroids, including those derived from adrenal steroid hormones and circulating androgens, substantially influence seizure susceptibility (Tuveri et al., 2008; Reddy, 2009a). Because progesterone is a powerful anticonvulsant hormone, decreasing levels of progesterone (or neurosteroids) during the perimenstrual period could result in catamenial seizure exacerbation. Withdrawal from chronic progesterone or allopregnanolone has been shown to be associated with enhanced expression of GABA-A receptor α4 subunit (Smith et al., 1998), a molecular signal for increased neuronal excitability and seizure susceptibility. Studies in animal models of catamenial epilepsy indicate that following neurosteroid withdrawal there is enhanced susceptibility to chemoconvulsant seizures, marked reduction in the antiseizure potency of benzodiazepines and valproate, but an increase in the anticonvulsant potency of neurosteroids is evident in these models (Reddy et al., 2001; Reddy and Rogawski, 2001).
Conventional antiepileptic drugs are used in catamenial epilepsy therapy. However, catamenial seizures are often not successfully treated with these drugs. Hormonal agents, such as progesterone and neurosteroids, may provide rational therapy for catamenial epilepsy. Progesterone supplementation has been shown to reduce catamenial seizure frequency in women with epilepsy (Herzog, 1995; 1999). Using an animal model of catamenial epilepsy, we evaluated the neurosteroid “replacement” as an effective therapy for catamenial epilepsy (Reddy and Rogawski, 2000a, b). Our results show that neurosteroids that positively modulate GABA-A receptors have enhanced anticonvulsant potency in catamenial epilepsy, providing support for “neurosteroid replacement” therapy as a rational approach for effective treatment of catamenial epilepsy (Reddy and Rogawski, 2009). Although progesterone is an effective treatment, but it may be associated with undesired hormonal side effects. Neurosteroids and synthetic analogs such as ganaxolone might provide an effective approach for catamenial epilepsy therapy that is more reliable and does not expose patients to the risk of hormonal side effects (Reddy and Woodward, 2004). Pilot clinical studies indicated beneficial effect with ganaxolone, but it is not yet evaluated in controlled clinical trials.
Anxiety
There is considerable evidence for an involvement of neurosteroids in the etiology of anxiety disorders. Neurosteroids such as allopregnanolone and THDOC are potent anxiolytic agents (Crawley et al., 1986; Bitran et al., 1995; Wieland et al., 1995; Reddy and Kulkarni, 2000; Finn et al., 2003; Eser et al., 2008; Maguire et al., 2005). Progesterone also has anxiolytic activity in animal models (Reddy and Kulkarni, 1997). Administration of progesterone produces similar sedative-anxiolytic effects in men and women (Soderpalm et al., 2004). The allopregnanolone- and progesterone-induced anxiolytic effects can be blocked by picrotoxin (Reddy and Kulkarni, 1997), suggesting that GABA-A receptors mediate the anxiolytic properties of neurosteroids. Anxiolytic properties have also been demonstrated with the use of synthetic analogs of allopregnanolone (Vanover et al., 2000). Treatment with fluoxetine, a specific serotonin uptake inhibitor, dose-dependently increases brain allopregnanolone levels (Uzunov et al., 1996), suggesting that elevated neurosteroid synthesis could be involved in the anxiolytic and antidysphoric actions of fluoxetine. In patients with induced panic attacks, there is pronounced decrease in allopregnanolone levels, and elevated neurosteroids may counteract the occurrence of spontaneous panic attacks (Strohle et al., 2000; 2003). Therefore, replacement of neurosteroids by synthetic analogs or stimulation of endogenous neurosteroid synthesis might constitute a promising novel strategy for the treatment of anxiety disorders.
Neurosteroidogenic compounds might represent novel drugs for anxiety. The selective ligands of the translocator protein such as FGIN-1-27 (Auta et al. 1993), AC-5216 (Kita et al., 2009) and XBD173 (Rupprecht et al., 2009) potently stimulates neurosteroid biosynthesis in brain, and produces anxiolytic actions, most likely via activation of GABA-A receptors. These agents are proposed to produce anxiolytic effects without causing the side effects normally associated with conventional benzodiazepines such as sedation and tolerance. In human subjects, XBD173 exerted antipanic activity, but did not cause sedation or withdrawal symptoms (Rupprecht et al., 2009).
The sulphated neurosteroids PS and DHEAS have been shown to be anxiogenic effects (Reddy and Kulkarni, 1997). DHEAS is synthesized in the brain from DHEA, an adrenal steroid that decreases with aging. PS had a biphasic response on the plus-maze; at higher doses it caused an anxiogenic response while at lower doses it produced an anxiolytic response (Melchior and Ritzmann, 1994). PS is present in brain at a relatively high concentration compared with many other neurosteroids (Corpéchot et al., 1993; Vallee et al., 1997) and is presumably generated by local steroid sulfotransferases since charged steroid sulfates are unlikely to cross the blood–brain barrier.
Premenstrual syndrome
Premenstrual syndrome (PMS) is a chronic, cyclical disorder manifested by emotional and physical symptoms in the second half of the menstrual cycle. Premenstrual dysphoric disorder (PMDD) is more severe than PMS, with women reporting severe psychological symptoms of depression, anxiety, and mood swings, in addition to the more common complaints of bloating and breast pain. The etiology of PMS is unknown. For years, PMS was attributed to numerous abnormalities of ovarian hormone secretion during the luteal phase. Progesterone-derived neurosteroids may be important for the clinical manifestations of PMS (Schmidt et al., 1994; Rapkin et al., 1997; Bicikova et al., 1998). In normal women, allopregnanolone varies very similarly to progesterone throughout the menstrual cycle with greater levels in the luteal phase than in the follicular phase (Genazzani et al., 1998). Thus, allopregnanolone could play an important role in the pathophysiology of PMS. Serum concentrations of the progesterone metabolite allopregnanolone during the luteal phase are lower in women with PMS (Sundstrom et al., 1997; Monteleone et al., 2000; Girdler et al., 2001) and withdrawal from progesterone (allopregnanolone) increases anxiety in animal models (Smith et al., 1998). Both at baseline and after stress, an enhanced ratio of allopregnanolone/cortisol has been reported (Girdler et al., 2001). There is a marked insensitivity to benzodiazepine therapy in patients with PMS (Sundstrom et al., 1997), which might be due to the development of cross-tolerance between benzodiazepines and neurosteroid. Although neurosteroids represent promising approach for PMS, natural progesterone supplementation in women with PMS has no clear beneficial effect (Freeman et al., 1990; 1995). This could be due to several reasons, such as hormonal side effects, disruption of ovarian rhythms or conversion of progesterone to other neurosteroids with negative properties.
Stress
Neurosteroids are released during physiological stress (Reddy, 2003). Stress results in the hypothalamic release of corticotropin-releasing hormone (CRH), which liberates ACTH from the anterior pituitary. Along with cortisol, ACTH also enhances the synthesis of adrenal deoxycorticosterone (DOC) (Tan and Mulrow, 1975; Kater et al., 1989). DOC itself is inactive and must be activated by A-ring reduction. The neurosteroid THDOC is synthesized from circulating DOC by the same two sequential A-ring reductions that convert progesterone to allopregnanolone (Reddy and Rogawski, 2002). In contrast to allopregnanolone, which is present in the brain even after adrenalectomy and gonadectomy, THDOC appears to be derived nearly exclusively from adrenal sources (Purdy et al., 1991).
Plasma and brain levels of THDOC and allopregnanolone rise rapidly following acute stress (Purdy et al., 1991; Reddy and Rogawski, 2002). Plasma levels of THDOC normally fluctuate between 1–5nM, but increase to 15–30nM following acute stress and might reach 40–60nM during pregnancy (Concas et al., 1998; Vallee et al., 2000). Both circulating and brain levels of allopregnanolone and THDOC reach their peak during stress. Acute stressors such as swimming, foot shock or carbon dioxide exposure elicit an increase in allopregnanolone and THDOC concentrations in plasma and in brain (Barbaccia et al., 1996; 1997). Stress-induced increases in THDOC peak between 10 and 30 min after stressor in normal animals. Thus, THDOC can be considered a component of the “hypothalamic-pituitary-adrenal (HPA) axis” stress response system. Since 5α-dihydrodeoxycorticosterone and THDOC exhibits anticonvulsant activity in a variety of animal seizure models (Reddy and Rogawski, 2002), it is attractive to speculate that DOC-derived neurosteroids may play a physiological role in the homeostasis of stress.
Stress-induced neurosteroids affects seizure susceptibility (Reddy and Rogawski, 2002). Stress induced seizure protection could be due to circulating neurosteroids synthesized in peripheral tissues or to those produced locally in the brain. The stress-induced increase in seizure threshold and THDOC levels were abolished in adrenalectomized animals, indicating a strong relationship between seizure probability and adrenal-derived THDOC concentrations. Despite THDOC’s antiseizure effects in animals (Soubrie et al., 1980; Pericic et al., 2000; Reddy and Rogawski, 2002), however, stress has been reported to trigger seizure activity in persons with epilepsy (Temkin and Davis, 1984; Frucht et al., 2000). One possible explanation is that the extent of seizure susceptibility during stress depends on the balance between anticonvulsant (e.g. allopregnanolone and THDOC), and proconvulsant steroids (PS and DHEAS) or other factors (e.g. CRH) (Reddy, 2006). Stress induced seizures would thus occur when the balance of neurosteroids is shifted to favor proconvulsants rather than anticonvulsants.
Neurosteroids plays an important role in homoeostatic regulation of GABA-A receptors at puberty (Shen et al., 2010). The α4δ-containing GABA-A receptors are involved in puberty. This receptor, which is expressed extrasynaptically on the dendrites, normally has low expression in the brain, but displays a remarkable degree of plasticity. It is a sensitive target for endogenous neurosteroids such as allopregnanolone, which is released during stress. Expression of α4δ-containing GABA-A receptors in CA1 hippocampus is also tightly regulated by fluctuating levels of neurosteroids, as seen at the onset of puberty. Declining levels of inhibition resulting from the decrease in neurosteroids at puberty are compensated for by an increase in α4δ-containing receptors along the apical dendrites of CA1 hippocampal pyramidal cells, which reduces neuronal excitability by decreasing the input resistance.
Depression
Neurosteroids have a crucial role in depression. Animal studies showed that fluoxetine, a selective serotonin reuptake inhibitor and widely used antidepressant, increases brain levels of allopregnanolone (Uzunova et al., 1996), while direct administration of allopregnanolone alleviates depressive behavior in animal models of depression (Khisti et al., 2000; Khisti and Chopde, 2000). Consequently, there is emerging interest on the role of allopregnanolone in mediating antidepressant actions of fluoxetine and in the pathophysiology of depression. Protracted social isolation in mice produces certain behavioral symptoms that are found in clinical depression (Dong et al., 2001). Interestingly, the concentrations of allopregnanolone are decreased in the frontal cortex of these animals partly due to a diminished expression of 5α-reductase, a key enzyme in the synthesizing allopregnanolone and other 5α-reduced neurosteroids. Indeed, major depression in humans is associated with a dysequilibrium of endogenous neurosteroids. In depressed patients, plasma and cerebrospinal fluid allopregnanolone levels are reduced, while plasma concentrations of THDOC are higher (Uzunov et al., 1996; Strohle et al, 1999; 2000). Both decreased allopregnanolone and increased THDOC levels can be normalized by clinically effective treatment with fluoxetine (Strohle et al., 2000). Moreover, fluoxetine-like antidepressants markedly elevate levels of allopregnanolone most likely through direct activation of 3α-HSOR, a critical enzyme in the synthesis of 3α-reduced neurosteroids (Mellon et al., 2001).
Sulfated neurosteroids PS and DHEAS as well as DHEA have clear antidepressant effects in animals and humans (Reddy et al., 1998b; Urani et al., 2001; Wolkowitz et al., 1999). PS and DHEAS also enhance cognition in animals (Flood et al., 1992; Vallee et al., 1997; Reddy and Kulkarni, 1998b; Sabeti et al., 2007). DHEA, a precursor of DHEAS and a dietary supplement, has been widely investigated as a novel antidepressant (Wolkowitz et al., 1995; 1997). However, studies investigating DHEA and DHEAS concentrations in depression have yielded inconsistent results with both increase and decrease during major depression. Overall, there are indications that adjunct DHEA could produce beneficial effects in patients with depression.
Depression during pregnancy and in the postpartum period is common, devastating to mothers and their offspring, and poorly understood in terms of pathophysiology (Nemeroff, 2008). Pregnancy is associated with a marked rise in progesterone-derived neurosteroid levels, which decline rapidly after delivery. Because neuroactive steroids are anxiolytic and neurosteroid withdrawal causes enhanced anxiety behaviour (Smith et al., 2007), neurosteroids could play a key role in the pathophysiology of postpartum depression. Recently, Maguire and Mody (2008) provided strong evidence for a role for aberrant neurosteroid regulation of the GABA-A receptor subunit in the etiology of postpartum depression, presaging elucidation of the pathophysiology and development of treatments of this depression in women.
Learning and memory
Neurosteroids have been widely recognized to modulate learning and memory processes in young, aged and in pharmacological models of amnesia (Reddy, 2003). In early studies, PS infused into the basal magnocellular nucleus enhanced memory performance, whereas AP disrupted memory (Mayo et al., 1993). Pregnenolone, PS, DHEA, and DHEAS increased memory when injected systemically, centrally or into amygdala (Isaacson et al., 1995; Flood et al., 1992; 1995; Reddy and Kulkarni, 1998c). Also prolonged intracerebroventricular infusion of PS enhanced cognitive performance in mice (Ladurelle et al, 2000). PS has also been reported to enhance learning in the Morris water maze and improves acquisition and retention of a food search task (Isaacson et al., 1994). In contrast, post-training administration of allopregnanolone reduced retention in a conditioned odour task in rat pups (Zimmerberg and McDonald, 1996). PS and DHEAS produced attenuating effects on amnesic response induced by the muscarinic receptor agonist, scopolamine and the non-competitive NMDA receptor antagonist, dizocilpine, the competitive NMDA receptor antagonist, 3-(+)-2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid, and ethanol (Maurice et al., 1997; Cheney e al., 1995; Melchior and Ritzmann, 1997).
Normal aging and cognitive dysfunction is associated with decreased levels of DHEA and DHEAS (Orentretch et al., 1984; Roberts, 1995). Consequently, neurosteroids such as DHEA and DHEAS are implicated to play a role in the manifestations of Alzheimer’s disease. There is evidence that the concentrations of DHEA and DHEAS are decreased in patients suffering from Alzheimer’s disease (Sunderland et al., 1989; Nasman et al., 1991; Hillen et al., 2000; Murialdo et al., 2001). Interestingly, DHEA is available in the market as an antiaging drug. In preclinical studies, administration of DHEA and DHEAS improved retention performance in aged animals (Flood et al., 1995), indicating a pathological role for neurosteroids in dementia related cognitive disorders. PS, DHEA, and DHEAS dose-dependently attenuated the memory deficits induced by the beta-amyloid β25–35 related to Alzheimer’s disease, in a σ receptor antagonist haloperidol- or progesterone-sensitive manner (Maurice et al., 1998). Pregnenolone treatment as adjunctive for cognitive and negative symptoms in patients with schizophrenia has been investigated (Marx et al., 2009). Patients receiving pregnenolone demonstrated significantly greater improvements in cognitive scores, indicating the promising therapeutic potential of neurosteroids for these conditions.
Inhibitors of steroid sulfatase, an enzyme that converts sulphated steroids into free steroids, may alter the metabolism of neurosteroids and affects cognitive function. The steroid sulfatase inhibitor estrone-3-O-sulfamate and p-O-(sulfamoyl)-N-tetradecanoyl tyramine potentiated the antiamnesic effect of DHEAS (Li et al., 1995; 1997), suggesting that increasing the levels of endogenous sulfated neurosteroids via the inhibition of steroid sulfatase activity may enhance learning and memory function.
Alcohol withdrawal
Neurosteroids play an important role in the alcohol tolerance and withdrawal (Morrow et al., 2001; 2006). There is an essential correlation between the time course of ethanol-induced allopregnanolone production in the brain and specific behavioral and neural effects of ethanol. Furthermore, the anticonvulsant and inhibitory effects of ethanol can be completely prevented by a key inhibitor of neurosteroid biosynthesis (VanDoren et al., 2000). Allopregnanolone influences cognitive processing, spatial learning and memory and alters alcohol drinking behaviors in rodents. Furthermore, ethanol induction of allopregnanolone is diminished in tolerant and dependent animals. These effects are associated with increases in the sensitivity of GABA-A receptors to neurosteroids and suggest an important role in ethanol withdrawal (Morrow et al., 2006). Thus, it is suggested that neurosteroids contribute to ethanol action and this interaction may represent a new mechanism of ethanol action. The identification of neurosteroid signaling involved in ethanol action may lead to further developments in the field of alcoholism.
Role of Neurosteroids in Sex Differences in Susceptibility to Brain Disorders
Sex difference in susceptibility to certain brain disorders is one of the long-standing issues in neuroscience research, both at the basic and clinical levels. Anxiety and depression affects more women than men. There is now strong evidence of gender differences in the serotonin neurotransmitter system that may account for differences in rates of depression and anxiety in men and women and therapeutic responses to antidepressants. Sex differences have been confirmed in the dynamics of tobacco smoking and cessation in humans. These differences have been attributed to acute and chronic effects of ovarian steroid hormones on nicotinic receptors, although findings have not been conclusive. Robust sex differences in dopamine release to amphetamine administration have been reported in men and women. Young adult men release more dopamine and report greater “high” and “drug liking” following amphetamine administration compared with age-matched women.
Similar to other neurological diseases, epilepsy shows sex differences in incidence, progression and severity, as well as in responsiveness to therapy. There is strong evidence on gender- and age-related expression in many epileptic seizure syndromes. The incidence of epilepsy is generally higher in males than in females. More women than men are diagnosed with idiopathic generalized epilepsy, but localization-related symptomatic epilepsies are more frequent in men, and cryptogenic localization-related epilepsies are more frequent in women (Hauser, 1997; Christensen et al., 2005). Sex differences have been described in patients with temporal lobe epilepsy, with respect to distinct regional distribution of brain dysfunction during interictal periods as well as to the extent of neuronal damage. Women tend to have less structural atrophy than men, regardless of the seizure rate. During brain development, sex hormones have organizational effects leading to permanent differences between males and females in distinct brain regions. However, the precise mechanisms underlying the sex-dependent differentiation of the specific neuronal circuits, particularly brain regions involved in seizure control, are not clear. Many factors are involved in determining sex differences in seizure susceptibility, including the presence of sexual dimorphism in brain structures involved in seizure generation and control, in regional connectivity, sensitivity of neurotransmitter systems, receptor distribution, and dependence on hormonal milieu and on changes in sex hormone levels during the life span.
Hormones and Sex differences
Hormones influence neuronal development and neuronal circuit formation in the brain, and modulate its activity either through excitatory or inhibitory mechanisms (Veliskova, 2009). During embryonic and postnatal development, steroid hormones induce differentiation of discrete brain regions by modulation of specific neuronal and glial cell components directly involved in synaptogenesis and myelinogenesis. At the cellular level, sex differences in the nervous system include the size of nucleus and nucleolus in neurons, size of synaptic vesicles and terminals, and dendritic branching pattern – all leading to differences in gross structural volume, connectivity, and neurotransmitter distribution. These steroid hormone-dependent sex differences include brain areas such as preoptic area, amygdala, hippocampus, hypothalamus, cortex, substantia nigra, and striatum. Such sexual differences may develop during the critical period for sexual differentiation. During this developmental period, the CNS is more sensitive to the organizational effects of gonadal hormones (imprinting effect). Development of sexual dimorphism is mainly due to ‘chromosomal sex’ (XX or XY) and testosterone or its metabolites, which determine the male-type of brain organization.
Sexual dimorphism
Sex-specific differences in the development of seizure suppressing neuronal networks may partly account for sex related susceptibility to seizures (Cooke et al., 1999; Reddy, 2009b). The substantia nigra pars reticulate (SNR), a midbrain structure populated largely by GABAergic neurons, plays an important role in seizure control, in an age- and sex-dependent manner (Veliskova and Moshe, 2001; Ravizza et al., 2003). In particular, GABAergic activation of SNR at P15 has sex-specific features on seizure control. This sexual dimorphism may play a role in several other recognized sex differences in the development of SNR and in its regulatory role in seizures. Because the substantia nigra is an important brain nucleus involved in the expression of movement disorders, the two most common movement disorders affecting the substantia nigra – Parkinson’s disease and Tourette syndrome – also show gender differences and age-related onset. However, there is limited information about sexually dimorphism in brain regions that control seizure initiation or suppression (hippocampus and amygdala).
Neurosteroids and sex differences
Steroid hormones such as progesterone and testosterone play a key role in the gender-related differences in susceptibility to seizures. However, the precise mechanism underlying such sexual dimorphism is obscure. Many of the biological actions of steroid hormones are mediated through intracellular receptors. Studies have suggested that these sex differences in seizure sensitivity are due to gender-specific distribution of steroid hormones or other sexually dimorphic characteristics in specific brain areas relevant to epilepsy. For example, estradiol reduces seizure-induced hippocampal injury in ovariectomized female but not in male rats, suggesting that the effects of estradiol on seizure threshold and damage may be determined by sex-related differences in the hormonal environment. Neurosteroids may play a vital role in gender-related differences in seizure susceptibility (Reddy, 2009b). It is proposed that progesterone- and testosterone-derived neurosteroids could be involved in sexual dimorphism in neural excitability and seizure susceptibility. Both progesterone and allopregnanolone protects against experimental seizures in both male and female mice lacking progesterone receptors (Reddy et al., 2004). However, female mice exhibit significantly enhanced sensitivity to the protective activity of allopregnanolone as compared to males. In the pilocarpine seizure test, androstanediol has similar increased potency in female mice, which is not related to differences in pharmacokinetics of this neurosteroid. Significant sex differences in responses to neurosteroids are also evident during alcohol withdrawal. These results underscore the possible role of GABAergic neurosteroids in gender-related differences in seizure susceptibility and protection.
Stress affects seizure susceptibility in both genders. Acute stress activates hypothalamic-pituitary-adrenal stress axis, which ultimately increase the synthesis and secretion of neurosteroids. Deoxycorticosterone, a mineralocorticoid precursor for the neurosteroid THDOC, is also produced in the adrenal zona fasciculata. Consequently, stress induces THDOC to levels that can activate GABA-A receptors (Reddy, 2006). These findings have obvious implications for stress-sensitive conditions such as epilepsy and post-traumatic stress disorder. The extent of seizure susceptibility during stress, in both males and females, is likely to reflect the balance between endogenous anticonvulsants (such as neurosteroids) and proconvulsant factors (such as glucocorticoids). Gender differences in stress-induced seizures may arise due to differences in the secretion of anticonvulsant or proconvulsant steroid hormones.
Conclusions and future perspectives
Neurosteroids are endogenous modulators of neural excitability. The major pharmacological effects of neurosteroids occur largely as a result of their allosteric potentiation of GABA-A receptors. Experimental and clinical evidence suggest an endogenous role for neurosteroids in various neurological and psychiatric conditions such as epilepsy, anxiety and depression. Treatment of epilepsy, anxiety, depression and stress-sensitive conditions are among the clinical situations in which synthetic neurosteroid analogs may have clinical applications. Pathways of neurosteroid biosynthesis in the brain are better understood, but regulatory mechanisms are not well characterized. Much has been learned about the hormonal influence on brain function in females during puberty, menstrual cycle and menopause, but there is much more that is yet to be learned. Gender-related differences in epileptogenesis and epilepsy therapy are not well studied. The role of neurosteroids in gender-specific brain conditions certainly deserves further investigations. Although steroid hormones and sexually dimorphic brain structures play an important role in gender-related seizure susceptibility, the precise mechanisms underlying such sex differences in brain disorders remain unclear. Neurosteroid-based treatments are being developed for gender-specific conditions such as catamenial epilepsy. An NIH-funded study is currently determining progesterone efficacy in women with epilepsy. The main challenge in neurosteroid research is lack of specific antagonist(s) for neurosteroid sites on GABA-A receptors. Further studies are, therefore, clearly warranted to establish the molecular mechanisms of neurosteroid actions and their impact on the human brain.
Acknowledgments
The original research described in this article was supported in part by the National Institute of Health, National Institute of Neurological Disorders and Stroke Grants NS051398, NS052158, and NS071597.
References
- Agís-Balboa RC, Pinna G, Zhubi A, et al. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proceedings of the National Academy of Science USA. 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aird RB. The effect of desoxycorticosterone in epilepsy. The Journal of Nervous and Mental Disorders. 1944;99:501–510. [Google Scholar]
- Aird RB, Gordan GS. Anticonvulsive properties of desoxycorticosterone. Journal of the American Medical Association. 1951;145:715–719. doi: 10.1001/jama.1951.02920280027006. [DOI] [PubMed] [Google Scholar]
- Akk G, Bracamontes J, Steinbach JH. Pregnenolone sulfate block of GABA-A receptors, mechanism and involvement of a residue in the M2 region of the α subunit. The Journal of Physiology (Lond) 2001;532:673–684. doi: 10.1111/j.1469-7793.2001.0673e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Covey DF, Evers AS, Steinbach JH, Zorumski CF, Mennerick S. The influence of the membrane on neurosteroid actions at GABA-A receptors. Psychoneuroendocrinology. 2009;34(S1):S59–S66. doi: 10.1016/j.psyneuen.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auta J, Romeo A, Kozikowski A, Ma D, Costa E, Guidotti A. Participation of mitochondrial diazepam binding inhibitor receptors in the anticonflict, antineophobic and anticonvulsant action of 2-aryl-3-indoleacetamide, and imidazopyridine derivatives. Journal of Pharmacology and Experimental Therapeutics. 1993;265:649–656. [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Mostallino MC, Concas A, Purdy RH, Biggio G. Time-dependent changes in rat brain neuroactive steroid concentrations and GABAA receptor function after acute stress. Neuroendocrinology. 1996;63:166–172. doi: 10.1159/000126953. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Purdy RH, Mostallino MC, Concas A, Biggio G. The effects of inhibitors of GABAergic transmission and stress on brain and plasma allopregnanolone concentrations. British Journal of Pharmacology. 1997;120:1582–1588. doi: 10.1038/sj.bjp.0701046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu E-E, Robel P. Neuroactive steroids, a new brain function? The Journal of Steroid Biochemistry and Molecular Biology. 1990;37:395–403. doi: 10.1016/0960-0760(90)90490-c. [DOI] [PubMed] [Google Scholar]
- Bazan AC, Montenegro MA, Cendes F, Min LL, Guerreiro CA. Menstrual cycle worsening of epileptic seizures in women with symptomatic focal epilepsy. Arquivos de Neuro-Psiquiatria. 2005;63(3B):751–756. doi: 10.1590/s0004-282x2005000500006. [DOI] [PubMed] [Google Scholar]
- Belelli D, Bolger MB, Gee KW. Anticonvulsant profile of the progesterone metabolite 5α-pregnan-3α-ol-20-one. European Journal of Pharmacology. 1989;166:325–329. doi: 10.1016/0014-2999(89)90077-0. [DOI] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Biagini G, Baldelli E, Longo D, Pradelli L, Zini I, Rogawski MA, Avoli M. Endogenous neurosteroids modulate epileptogenesis in a model of temporal lobe epilepsy. Experimental Neurology. 2006;201:519–524. doi: 10.1016/j.expneurol.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Biagini G, Longo D, Baldelli E, Zoli M, Rogawski MA, Bertazzoni G, Avoli M. Neurosteroids and epileptogenesis in the pilocarpine model, Evidence for a relationship between P450scc induction and length of the latent period. Epilepsia. 2009;50(Suppl 1):53–58. doi: 10.1111/j.1528-1167.2008.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicikova M, Dibbelt L, Hill M, Hampl R, Starka L. Allopregnanolone in women with premenstrual syndrome. Hormone and Metabolic Research. 1998;30:227–230. doi: 10.1055/s-2007-978871. [DOI] [PubMed] [Google Scholar]
- Bitran D, Shiekh M, McLeod M. Anxiolytic effect of progesterone is mediated by the neuroactive steroid allopregnanolone at brain GABAA receptors. Journal of Neuroendocrinology. 1995;7:171–7. doi: 10.1111/j.1365-2826.1995.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Bonuccelli U, Melis GB, Paoletti AM, Fioretti P, Murri L, Muratorio A. Unbalanced progesterone and estradiol secretion in catamenial epilepsy. Epilepsy Research. 1989;3:100–106. doi: 10.1016/0920-1211(89)90037-5. [DOI] [PubMed] [Google Scholar]
- Bowlby MR. Pregnenolone sulfate potentiation of N-methyl-D-aspartate receptor channels in hippocampal neurons. Molecular Pharmacology. 1993;43:813–819. [PubMed] [Google Scholar]
- Carter RB, Wood PL, Wieland S, Hawkinson JE, Belelli D, Lambert JJ, White HS, Wolf HH, Mirsadeghi S, Tahir SH, Bolger MB, Lan NC, Gee KW. Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3α-hydroxy-3β-methyl-5α-pregnan-20-one), a selective, high-affinity, steroid modulator of the γ-aminobutyric acidA receptor. Journal of Pharmacology and Experimental Therapeutics. 1997;280:1284–1295. [PubMed] [Google Scholar]
- Cheney DL, Uzunov D, Guidotti A. Pregnenolone sulfate antagonizes dizocilpine amnesia, Role for allopregnanolone. NeuroReport. 1995;6:1697–1700. doi: 10.1097/00001756-199508000-00025. [DOI] [PubMed] [Google Scholar]
- Christensen J, Kjeldsen MJ, Anderson H, Friis ML, Sidenius P. Gender differences in epilepsy. Epilepsia. 2005;46:956–960. doi: 10.1111/j.1528-1167.2005.51204.x. [DOI] [PubMed] [Google Scholar]
- Concas A, Mostallino MC, Porcu P, Folesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of γ-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proceedings of the National Academy of Sciences USA. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke BM, Tabibnia G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. Proceedings of the National Academy of Sciences USA. 1999;96:7538–7540. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpechot C, Young J, Calvel M, Wehrey C, Veltz JN, Touyer G, Mouren M, Prasad VV, Banner C, Sjovall J, et al. Neuroactive steroids, 3(-hydroxy-5(-pregnan-20-one and its precursors in the brain, plasma and steroidogenic glands of male and female rats. Endocrinology. 1993;133:1003–1009. doi: 10.1210/endo.133.3.8365352. [DOI] [PubMed] [Google Scholar]
- Costa E, Guidotti A. Diazepam binding inhibitor (DBI), a peptide with multiple biological actions. Life Sciences. 1991;49:325–344. doi: 10.1016/0024-3205(91)90440-m. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Glowa JR, Majewska MD, Paul SM. Anxiolytic activity of an endogenous adrenal steroid. Brain Research. 1986;398:382–5. doi: 10.1016/0006-8993(86)91500-3. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Purdy RH, Finn DA, Morrow AL. Sensitization of (-aminobutyric acidA receptors to neuroactive steroids in rats during ethanol withdrawal. Journal of Pharmacology and Experimental Therapeutics. 1996;278:510–517. [PubMed] [Google Scholar]
- Do Rego JL, Seong JY, Burel D, Leprince J, Luu-The V, Tsutsui K, Tonon MC, Pelletier G, Vaudry H. Neurosteroid biosynthesis, enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Frontiers in Neuroendocrinology. 2009;30:259–301. doi: 10.1016/j.yfrne.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Dong E, Matsumoto K, Uzunova V, Sugaya I, Takahata H, Nomura H, Watanabe H, Costa E, Guidotti A. Brain 5(-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proceedings of the National Academy of Sciences USA. 2001;98:2849–54. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eser D, Baghai TC, Schüle C, Nothdurfter C, Rupprecht R. Neuroactive steroids as endogenous modulators of anxiety. Current Pharmaceutical Design. 2008;14:3525–33. doi: 10.2174/138161208786848838. [DOI] [PubMed] [Google Scholar]
- Fahey JM, Lindquist DG, Pritchard GA, Miller LG. Pregnenolone sulfate potentiation of NMDA-mediated increases in intracellular calcium in cultured chick cortical neurons. Brain Research. 1995;669:183–188. doi: 10.1016/0006-8993(94)01223-5. [DOI] [PubMed] [Google Scholar]
- Finn DA, Roberts AJ, Long S, Tanchuck M, Phillips TJ. Neuroactive steroid consumption has anxiolytic effects in mice. Pharmacology Biochemistry and Behavior. 2003;76:451–462. doi: 10.1016/j.pbb.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Flood JF, Morley JE, Roberts E. Pregnenolone sulfate enhances post-training memory processes when injected in very low doses into limbic system structures, The amygdala is by far the most sensitive. Proceedings of the National Academy of Sciences USA. 1995;92:10806–10810. doi: 10.1073/pnas.92.23.10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood JF, Morley JE, Roberts E. Memory-enhancing effects in male mice of pregnenolone and steroids metabolically derived from it. Proceedings of the National Academy of Sciences USA. 1992;89:1567–71. doi: 10.1073/pnas.89.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman E, Rickels K, Sondheimer SJ, Polansky M. Ineffectiveness of progesterone suppository treatment for premenstrual syndrome. The Journal of the American Medical Association. 1990;264:349–353. [PubMed] [Google Scholar]
- Freeman EW, Rickels K, Sondheimer SJ, Polansky M. A double-blind trial of oral progesterone, alprazolam, and placebo in treatment of severe premenstrual syndrome. The Journal of the American Medical Association. 1995;274:51–57. [PubMed] [Google Scholar]
- Frucht MM, Quigg M, Schwaner C, Fountain NB. Distribution of seizure precipitants among epilepsy syndromes. Epilepsia. 2000;41:1534–1539. doi: 10.1111/j.1499-1654.2000.001534.x. [DOI] [PubMed] [Google Scholar]
- Frye CA. The neuroactive steroid 3(,5(-THP has anti-seizure and possible neuroprotective effects in an animal model of epilepsy. Brain Research. 1995;696:113–120. doi: 10.1016/0006-8993(95)00793-p. [DOI] [PubMed] [Google Scholar]
- Gasior M, Carter RB, Goldberg SR, Witkin JM. Anticonvulsant and behavioral effects of neuroactive steroids alone and in conjunction with diazepam. Journal of Pharmacology and Experimental Therapeutics. 1997;282:543–553. [PubMed] [Google Scholar]
- Genazzani AR, Petraglia F, Bernardi F, Casarosa E, Salvestroni C, Tonetti A. Circulating levels of allopregnanolone in humans, gender, age and endocrine influences. Journal of Clinical Endocrinology and Metabolism. 1998;83:2099–2103. doi: 10.1210/jcem.83.6.4905. [DOI] [PubMed] [Google Scholar]
- Gibbs TT, Farb DH. Direct modulation of amino acid receptors by neuroactive steroids, physiological and pharmacological implications. In: Smith SS, editor. Neurosteroids Effects in the Central Nervous System, Role of the GABAA receptor. CRC press; Washington: 2004. pp. 339–358. [Google Scholar]
- Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biological Psychiatry. 2001;49:788–97. doi: 10.1016/s0006-3223(00)01044-1. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. Activation of GABA-A receptors, views from outside the synaptic cleft. Neuron. 2007;56:763–70. doi: 10.1016/j.neuron.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Green CJ, Halsey MJ, Precious S, Wardley-Smith B. Alphaxolone-alphadolone anesthesia in laboratory animals. Laboratory Animals. 1978;12:85–89. doi: 10.1258/002367778780953206. [DOI] [PubMed] [Google Scholar]
- Guennoun R, Fiddes RJ, Gouezou M, Lombes M, Baulieu EE. A key enzyme in the biosynthesis of neuroactive steroids, 3(-hydroxysteroid dehydrogenase/(5-Δ4-isomerase (3β-HSD), is expressed in rat brain. Molecular Brain Research. 1995;30:287–300. doi: 10.1016/0169-328x(95)00016-l. [DOI] [PubMed] [Google Scholar]
- Gyermek L, Genther G, Fleming N. Some effects of progesterone and related steroids on the central nervous system. International Journal of Neuropharmacology. 1967;6:191–198. doi: 10.1016/0028-3908(67)90005-6. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Simmonds MA. Modulation of the GABA receptor complex by a steroid anesthetic. Brain Research. 1984;323:287–292. doi: 10.1016/0006-8993(84)90299-3. [DOI] [PubMed] [Google Scholar]
- Harrison NL, Majewska MD, Harrington JW, Barker JL. Structure-activity relationships for steroid interactions with the γ-aminobutyric acidA receptor complex. Journal of Pharmacology and Experimental Therapeutics. 1987;241:346–353. [PubMed] [Google Scholar]
- Hauser WA. Incidence and prevalence. In: Engel J Jr, Pedley TA, editors. Epilepsy, A Comprehensive Textbook. Lippincott-Raven Publishers; Philadelphia: 1997. pp. 47–57. [Google Scholar]
- Herzog AG, Harden CL, Liporace J, Pennell P, Schomer DL, Sperling M, Fowler K, Nikolov B, Shuman S, Newman M. Frequency of catamenial seizure exacerbation in women with localization-related epilepsy. Annals of Neurology. 2004;56:431–434. doi: 10.1002/ana.20214. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia. 1997;38:1082–1088. doi: 10.1111/j.1528-1157.1997.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Progesterone therapy in women with complex partial and secondary generalized seizures. Neurology. 1995;45:1600–1662. doi: 10.1212/wnl.45.9.1660. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Progesterone therapy in women with epilepsy, a 3-year follow-up. Neurology. 1999;52:1917–1918. doi: 10.1212/wnl.52.9.1917-a. [DOI] [PubMed] [Google Scholar]
- Hillen T, Lun A, Reischies FM, Borchelt M, Steinhagen-Thiessen E, Schaub RT. DHEA-S plasma levels and incidence of Alzheimer’s disease. Biological Psychiatry. 2000;47:161–163. doi: 10.1016/s0006-3223(99)00217-6. [DOI] [PubMed] [Google Scholar]
- Hogenkamp DJ, Hasan Tihar SH, Hawkinson JE, Upasani RB, Alauddin M, Kimbrough CL, Acosta-Burruel M, Whittemore ER, Woodward RM, Lan NC, Gee KW, Bolger MB. Synthesis and in vitro activity of 3β-substituted-3α-hydroxypregnan-20-ones, allosteric modulators of the GABAA receptor. Journal of Medicinal Chemistry. 1997;40:61–72. doi: 10.1021/jm960021x. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, Smart TG. Neurosteroid binding sites on GABA-A receptors. Pharmacology and Therapeutics. 2007;116:7–19. doi: 10.1016/j.pharmthera.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Clarke L, da Silva H, Smart TG. Conserved site for neurosteroid modulation of GABA-A receptors. Neuropharmacology. 2009;56:149–154. doi: 10.1016/j.neuropharm.2008.07.050. [DOI] [PubMed] [Google Scholar]
- Irwin RP, Lin SZ, Rogawski MA, Purdy RH, Paul SM. Steroid potentiation and inhibition of N-methyl-D-aspartate receptor-mediated intracellular Ca2+ responses, structure-activity studies. Journal of Pharmacology and Experimental Therapeutics. 1994;271:677–682. [PubMed] [Google Scholar]
- Isaacson RL, Varner JA, Baars JM, De Wied D. The effects of pregnenolone sulfate and ethylestrenol on retention of a passive avoidance task. Brain Research. 1995;689:79–84. doi: 10.1016/0006-8993(95)00493-a. [DOI] [PubMed] [Google Scholar]
- Isaacson RL, Yoder PE, Varner J. The effects of pregnenolone on acqusition and retention of a food search task. Behavioral and Neural Biology. 1994;61:170–176. doi: 10.1016/s0163-1047(05)80071-8. [DOI] [PubMed] [Google Scholar]
- Joels M. Steroid hormones and excitability in the mammalian brain. Frontiers in Neuroendocrinology. 1997;18:2–48. doi: 10.1006/frne.1996.0144. [DOI] [PubMed] [Google Scholar]
- Kater CE, Biglieri EG, Brust N, Chang B, Hirai J, Irony I. Stimulation and suppression of the mineralocorticoid hormones in normal subjects and adrenocortical disorder. Endocrine Reviews. 1989;10:149–164. doi: 10.1210/edrv-10-2-149. [DOI] [PubMed] [Google Scholar]
- Kerrigan JF, Shields WD, Nelson TY, Bluestone DL, Dodson WE, Bourgeois BF, Pellock JM, Morton LD, Monaghan EP. Ganaxolone for treating intractable infantile spasms, a multicenter, open-label, add-on trial. Epilepsy Research. 2000;42:133–139. doi: 10.1016/s0920-1211(00)00170-4. [DOI] [PubMed] [Google Scholar]
- Khisti RT, Chopde CT. Serotonergic agents modulate antidepressant-like effect of the neuroactive steroid 3α-hydroxy-5α-pregnan-20-one in mice. Brain Research. 2000;865:291–300. doi: 10.1016/s0006-8993(00)02373-8. [DOI] [PubMed] [Google Scholar]
- Khisti RT, Chopde CT, Jain SP. Antidepressant-like effect of the neuroactive steroid 3α-hydroxy-5α-pregnan-20-one in mice forced swim test. Pharmacology Biochemistry and Behavior. 2000;67:137–43. doi: 10.1016/s0091-3057(00)00300-2. [DOI] [PubMed] [Google Scholar]
- Kita A, Kinoshita T, Kohayakawa H, Furukawa K, Akaike A. Lack of tolerance to anxiolysis and withdrawal symptoms in mice repeatedly treated with AC-5216, a selective TSPO ligand. Progress in Neuropsychopharmacology & Biological Psychiatry. 2009;33:1040–1045. doi: 10.1016/j.pnpbp.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Juhng KN, Kirkby RD, Llamas J, Yamaguchi S, Rogawski MA. Convulsant actions of the neuroactive steroid pregnenolone sulfate in mice. Brain Research. 1999;831:119–124. doi: 10.1016/s0006-8993(99)01287-1. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neuroactive steroids, correlation with γ-aminobutyric acid-evoked chloride current potentiation. Journal of Pharmacology and Experimental Therapeutics. 1994;270:1223–1229. [PubMed] [Google Scholar]
- Korneyev A, Pan BS, Polo A, Romeo E, Guidotti A, Costa E. Stimulation of brain pregnenolone synthesis by mitochondrial diazepam binding inhibitor receptor ligands in vivo. Journal of Neurochemistry. 1993;61:1515–1524. doi: 10.1111/j.1471-4159.1993.tb13647.x. [DOI] [PubMed] [Google Scholar]
- Ladurelle N, Eychenne B, Denton D, Blair-West J, Schumacher M, Robel P, Baulieu E. Prolonged intracerebroventricular infusion of neurosteroids affects cognitive performances in the mouse. Brain Research. 2000;858:371–379. doi: 10.1016/s0006-8993(00)01953-3. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Hill-Venning C, Peters JA. Neuroactive steroids and GABA-A receptor function. Trends in Pharmacological Sciences. 1995;16:295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. Neurosteroid modulation of GABAA receptors. Progress in Neurobiology. 2003;71:67–80. doi: 10.1016/j.pneurobio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Laxer K, Blum D, Abou-Khalil BW, Morrell MJ, Lee DA, Data JL, Monaghan EP, et al. Assessment of ganaxolone’s anticonvulsant activity using a randomized, double-blind, presurgical trial design. Epilepsia. 2000;41:1187–1194. doi: 10.1111/j.1528-1157.2000.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Li P-K, Rhodes ME, Burke AM, Johnson DA. Memory enhancement mediated by the steroid sulfatase inhibitor (p-O-sulfamoyl)-N-tetradecanoyl tyramine. Life Sciences. 1997;60:PL45–51. doi: 10.1016/s0024-3205(96)00621-2. [DOI] [PubMed] [Google Scholar]
- Li PK, Rhodes ME, Jagannathan S, Johnson DA. Reversal of scopolamine induced amnesia in rats by the steroid sulfatase inhibitor estrone-3-O-sulfamate. Cognitive Brain Research. 1995;2:251–254. doi: 10.1016/0926-6410(95)90016-0. [DOI] [PubMed] [Google Scholar]
- Maguire J, Mody I. GABA-AR plasticity during pregnancy, relevance to postpartum depression. Neuron. 2008;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA-A receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nature Neuroscience. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Majewska MD. Neuroactive steroids, endogenous bimodal modulators of the GABA-A receptor. Mechanism of action and physiological significance. Progress in Neurobiology. 1992;38:379–395. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Schwartz RD. Pregnenolone sulfate, an endogenous antagonist of the γ-aminobutyric acid receptor complex in brain? Brain Research. 1987;404:355–360. doi: 10.1016/0006-8993(87)91394-1. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Demirgoren S, Spivak CE, London ED. The neuroactive steroid dehydroepiandrosterone sulfate is an allosteric antagonist of the GABA-A receptor. Brain Research. 1990;526:143–146. doi: 10.1016/0006-8993(90)90261-9. [DOI] [PubMed] [Google Scholar]
- Malayev A, Gibbs TT, Farb DH. Inhibition of the NMDA response by pregnenolone sulphate reveals subtype selective modulation of NMDA receptors by sulphated steroids. British Journal of Pharmacology. 2002;135:901–909. doi: 10.1038/sj.bjp.0704543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx CE, Keefe RS, Buchanan RW, Hamer RM, Kilts JD, Bradford DW, Strauss JL, Naylor JC, Payne VM, Lieberman JA, Savitz AJ, Leimone LA, Dunn L, Porcu P, Morrow AL, Shampine LJ. Proof-of-concept trial with the neurosteroid pregnenolone targeting cognitive and negative symptoms in schizophrenia. Neuropsychopharmacology. 34:1885–903. doi: 10.1038/npp.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice T, Junien JL, Privat A. Dehydroepiandrosterone sulfate attenuates dizocilpine-induced learning impairment in mice via σ1 receptors. Behavioral Brain Research. 1997;83:159–164. doi: 10.1016/s0166-4328(97)86061-5. [DOI] [PubMed] [Google Scholar]
- Maurice T, Roman FJ, Privat A. Modulation by neurosteroids of the in vivo (+)-[3H]SKF-10,047 binding to σ1 receptors in the mouse forebrain. Journal of Neuroscience Research. 1996;46:734–743. doi: 10.1002/(SICI)1097-4547(19961215)46:6<734::AID-JNR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Maurice T, Su TP, Privat A. Sigma1 (σ1) receptor agonists and neurosteroids attenuate β25–35-amyloid peptide-induced amnesia in mice through a common mechanism. Neuroscience. 1998;83:413–428. doi: 10.1016/s0306-4522(97)00405-3. [DOI] [PubMed] [Google Scholar]
- Mayo W, Dellu F, Robel P, Cherkaoui J, Le Moal M, Baulieu EE, Simon H. Infusion of neurosteroids into the nucleus basalis magnocellularis affects cognitive processes in the rat. Brain Research. 1993;607:324–328. doi: 10.1016/0006-8993(93)91524-v. [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Poletti A, Cavarretta I, Celotti F, Colciago A, Magnaghi V, Motta M, Negri-Cesi P, Martini L. The 5α-reductase in the central nervous system, expression and modes of control. Journal of Steroid Biochemistry and Molecular Biology. 1998;65:295–299. doi: 10.1016/s0960-0760(98)00030-2. [DOI] [PubMed] [Google Scholar]
- Melchior CL, Ritzmann RF. Pregnenolone and pregnenolone sulfate, alone and with ethanol, in mice on the plus-maze. Pharmacology Biochemistry and Behavior. 1994;48:893–897. doi: 10.1016/0091-3057(94)90197-x. [DOI] [PubMed] [Google Scholar]
- Melchior CL, Ritzmann RF. Neurosteroids block the memory-impairment effects of ethanol in mice. Pharmacology Biochemistry and Behavior. 1997;53:51–56. doi: 10.1016/0091-3057(95)00197-2. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD, Compagnone NA. Biosynthesis and action of neuroactive steroids. Brain Research Reviews. 2001;37:3–12. doi: 10.1016/s0165-0173(01)00109-6. [DOI] [PubMed] [Google Scholar]
- Mensah-Nyagan AG, Do-Rego JL, Beaujean D, Luu-The V, Pelletier G, Vaudry H. Neuroactive steroids, expression of steroidogenic enzymes and regulation of steroid biosynthesis in the central nervous system. Pharmacological Reviews. 1999;51:63–81. [PubMed] [Google Scholar]
- Mienville JM, Vicini S. Pregnenolone sulfate antagonizes GABAA receptor-mediated currents via a reduction of channel opening frequency. Brain Research. 1989;489:190–194. doi: 10.1016/0006-8993(89)90024-3. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type A receptor delta subunit knockout mice. Proceedings of the National Academy of Sciences USA. 1999;96:12905–129010. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan EP, McAculey JW, Data JL. Ganaxolone, a novel positive allosteric modulator of the GABAA receptor complex for the treatment of epilepsy. Expert Opinion on Investigational Drugs. 1999;8:1663–1671. doi: 10.1517/13543784.8.10.1663. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Luisi S, Tonetti A, Bernardi F, Genazzani AD, Luisi M, Petraglia F, Genazzani AR. Allopregnanolone concentrations and premenstrual syndrome. European Journal of Endocrinology. 2000;142:269–273. doi: 10.1530/eje.0.1420269. [DOI] [PubMed] [Google Scholar]
- Morrow AL. Recent developments in the significance and therapeutic relevance of neuroactive steroids--Introduction to the special issue. Pharmacology & Therapeutics. 2007;116:1–6. doi: 10.1016/j.pharmthera.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, Porcu P, Boyd KN, Grant KA. Hypothalamic-pituitary-adrenal axis modulation of GABAergic neuroactive steroids influences ethanol sensitivity and drinking behavior. Dialogues in Clinical NeuroSciences. 2006;8:463–477. doi: 10.31887/DCNS.2006.8.4/amorrow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, Pace JR, Purdy RH, Paul SM. Characterization of steroid interactions withγ-aminobutyric acid receptor-gated chloride ion channels, evidence for multiple steroid recognition sites. Molecular Pharmacology. 1990;37:263–270. [PubMed] [Google Scholar]
- Morrow AL, VanDoren MJ, Penland SN, Matthews DB. The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Res Reviews. 2001;37:98–109. doi: 10.1016/s0165-0173(01)00127-8. [DOI] [PubMed] [Google Scholar]
- Murialdo G, Barreca A, Nobili F, Rollero A, Timossi G, Gianelli MV, Copello F, Rodriguez G, Polleri A. Relationships between cortisol, dehydroepiandrosterone sulphate and insulin-like growth factor-I system in dementia. Journal of Endocrinology Investigation. 2001;24:139–146. doi: 10.1007/BF03343833. [DOI] [PubMed] [Google Scholar]
- Nasman B, Olsson T, Backstrom T, Eriksson S, Grankvist K, Viitanen M, Bucht G. Serum dehydroepiandrosterone sulfate in Alzheimer’s disease and in multi-infarct dementia. Biological Psychiatry. 1991;30:684–690. doi: 10.1016/0006-3223(91)90013-c. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Understanding the pathophysiology of postpartum depression, implications for the development of novel treatments. Neuron. 2008;59:185–186. doi: 10.1016/j.neuron.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Nohria V, Tsai J, Shaw K, Rogawski MA, Pieribone VA, Farfel G. Ganaxolone, in Bialer M, et al., Progress report on new antiepileptic drugs, A summary of the Tenth Eilat Conference (EILAT X) Epilepsy Research. 2010 doi: 10.1016/j.eplepsyres.2010.09.001. in press. [DOI] [PubMed] [Google Scholar]
- Orentretch N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations through adulthood. Journal of Clinical Endocrinology and Metabolism. 1984;59:551–555. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapère JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18kDa), new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends in Pharmacological Sciences. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Park-Chung M, Malayev A, Purdy RH, Gibbs TT, Farb DH. Sulfated and unsulfated steroids modulate γ-aminobutyric acidA receptor function through distinct sites. Brain Research. 1999;830:72–87. doi: 10.1016/s0006-8993(99)01381-5. [DOI] [PubMed] [Google Scholar]
- Patte-Mensah C, Kappes V, Freund-Mercier MJ, Tsutsui K, Mensah-Nyagan AG. Cellular distribution and bioactivity of the key steroidogenic enzyme, cytochrome P450side chain cleavage, in sensory neural pathways. Journal of Neurochemistry. 2003;86:1233–1246. doi: 10.1046/j.1471-4159.2003.01935.x. [DOI] [PubMed] [Google Scholar]
- Pericić D, Svob D, Jazvinsćak M, Mirković K. Anticonvulsive effect of swim stress in mice. Pharmacology Biochemistry and Behavior. 2000;66:879–886. doi: 10.1016/s0091-3057(00)00267-7. [DOI] [PubMed] [Google Scholar]
- Petratos S, Hirst JJ, Mendis S, Anikijenko P, Walker DW. Localization of p450scc and 5α-reductase type-2 in the cerebellum of fetal and newborn sheep. Developmental Brain Research. 2000;123:81–86. doi: 10.1016/s0165-3806(00)00076-6. [DOI] [PubMed] [Google Scholar]
- Pieribone VA, Tsai J, Soufflet C, Rey E, Shaw K, Giller E, Dulac O. Clinical evaluation of ganaxolone in pediatric and adolescent patients with refractory epilepsy. Epilepsia. 2007;48:1870–1874. doi: 10.1111/j.1528-1167.2007.01182.x. [DOI] [PubMed] [Google Scholar]
- Puia G, Santi M, Vicini S, Pritchett DB, Purdy RH, Paul SM, Seeburg PH, Costa E. Neuroactive steroids act on recombinant human GABAA receptors. Neuron. 1990;4:759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Blinn JR, Paul SM. Synthesis, metabolism, and pharmacological activity of 3α-hydroxy steroids which potentiate GABA-receptor-mediated chloride ion uptake in rat cerebral cortical synaptoneurosomes. Journal of Medicinal Chemistry. 1990;33:1572–1581. doi: 10.1021/jm00168a008. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proceedings of National Academy of Sciences USA. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapkin AJ, Morgan M, Goldman L, Brann DW, Simone D, Mahesh VB. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstetrics & Gynecology. 1997;190:709–714. doi: 10.1016/S0029-7844(97)00417-1. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Friedman LK, Moshe SL, Veliskova J. Sex differences in GABAergic system in rat substantia nigra pars reticulate. International Journal of Developmental Neuroscience. 2003;21:245–254. doi: 10.1016/s0736-5748(03)00069-8. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Pharmacology of endogenous neuroactive steroids. Critical Reviews in Neurobiology. 2003;15:197–234. doi: 10.1615/critrevneurobiol.v15.i34.20. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of ganaxolone after neurosteroid withdrawal in a rat model of catamenial epilepsy. Journal of Pharmacology and Experimental Therapeutics. 2000a;294:909–915. [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. Journal of Pharmacology and Experimental Therapeutics. 2000b;295:1241–1248. [PubMed] [Google Scholar]
- Reddy DS, Apanites LA. Anesthetic effects of progesterone are undiminished in progesterone receptor knockout mice. Brain Research. 2005;1033:96–101. doi: 10.1016/j.brainres.2004.11.026. [DOI] [PubMed] [Google Scholar]
- Reddy DS, O’Malley BW, Rogawski MA. Anxiolytic activity of progesterone in progesterone receptor knockout mice. Neuropharmacology. 2005;48:14–24. doi: 10.1016/j.neuropharm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Woodward R. Ganaxolone, A prospective overview. Drugs of the Future. 2004;29:227–242. [Google Scholar]
- Reddy DS, Kim Y-H, Rogawski MA. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia. 2001;42:328–336. doi: 10.1046/j.1528-1157.2001.10100.x. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy. Epilepsia. 2001;42:303–310. doi: 10.1046/j.1528-1157.2001.10200.x. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Physiological role of adrenal deoxycorticosterone-derived neuroactive steroids in stress-sensitive conditions. Neuroscience. 2006;138:911–20. doi: 10.1016/j.neuroscience.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Mass spectrometric quantification and physiological-pharmacological activity of androgenic neurosteroids. Neurochemistry International. 2008;52(4–5):541–553. doi: 10.1016/j.neuint.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. The role of neurosteroids in the pathophysiology and treatment of catamenial epilepsy. Epilepsy Research. 2009a;85:1–30. doi: 10.1016/j.eplepsyres.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Steroid hormones and sex differences in seizure susceptibility. In: Schwartzkroin Philip., editor. Encyclopedia of Basic Epilepsy Research. Vol. 1. Oxford: Academic Press; 2009b. pp. 526–533. [Google Scholar]
- Reddy DS, Kulkarni SK. Differential anxiolytic effects of neuroactive steroids in the mirrored chamber behavior test in mice. Brain Research. 1997;752:61–71. doi: 10.1016/s0006-8993(96)01447-3. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. Proconvulsant effects of neurosteroid pregnenolone sulfate and dehydroepiandrosterone sulfate in mice. European Journal of Pharmacology. 1998a;345:55–59. doi: 10.1016/s0014-2999(98)00034-x. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Neurosteroid replacement therapy for catamenial epilepsy. Neurotherapeutics. 2009;6:392–401. doi: 10.1016/j.nurt.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Castenada DA, O’Malley BW, Rogawski MA. Antiseizure activity of progesterone and neurosteroids in progesterone receptor knockout mice. Journal of Pharmacology and Experimental Therapeutics. 2004;310:230–239. doi: 10.1124/jpet.104.065268. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kaur G, Kulkarni SK. Sigma (σ1) receptor mediated anti-depressant-like effects of neuroactive steroids in the Porsolt forced swim test. NeuroReport. 1998b;9:3069–73. doi: 10.1097/00001756-199809140-00028. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. The effects of neuroactive steroids on acquisition and retention of a modified passive-avoidance learning task in mice. Brain Research. 1998c;791:108–16. doi: 10.1016/s0006-8993(98)00085-7. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. Development of neuroactive steroid-based novel psychotropic drugs. Progress in Medicinal Chemistry. 2000;37:135–175. doi: 10.1016/s0079-6468(08)70059-6. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Stress-induced deoxycorticosterone-derived neuroactive steroids modulates GABA-A receptor function and seizure susceptibility. The Journal of Neuroscience. 2002;42:3795–3805. doi: 10.1523/JNEUROSCI.22-09-03795.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Ganaxolone suppression of behavioral and electrographic seizures in the mouse amygdala kindling model. Epilepsy Research. 2010;89:254–260. doi: 10.1016/j.eplepsyres.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho JM, Donevan SD, Rogawski MA. Direct activation of GABA-A receptors by barbiturates in cultured rat hippocampal neurons. The Journal of Physiology. 1996;497(Pt 2):509–522. doi: 10.1113/jphysiol.1996.sp021784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts E. Pregnenolone, from Selye to Alzheimer and a model of the pregnenolone sulfate binding site on the GABA-A receptor. Biochemical Pharmacology. 1995;49:1–16. doi: 10.1016/0006-2952(94)00258-n. [DOI] [PubMed] [Google Scholar]
- Romeo E, Brancati A, De Lorenzo A, Fucci P, Furnari C, Pompili E, Sasso GF, Spalletta G, Troisi A, Pasini A. Marked decrease of plasma neuroactive steroids during alcohol withdrawal. Clinical Neuropharmcology. 1996;19:366–369. doi: 10.1097/00002826-199619040-00011. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Berning B, Hauser CAE, Holsboer F, Reul JMHM. Steroid receptor-mediated effects of neuroactive steroids, characterization of structure-activity relationship. European Journal of Pharmacology. 1996;303:227–234. doi: 10.1016/0014-2999(96)00036-2. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Rammes G, Eser D, Baghai TC, Schüle C, Nothdurfter C, Troxler T, Gentsch C, Kalkman HO, Chaperon F, Uzunov V, McAllister KH, Bertaina-Anglade V, La Rochelle CD, Tuerck D, Floesser A, Kiese B, Schumacher M, Landgraf R, Holsboer F, Kucher K. Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science. 2009;325(5939):490–493. doi: 10.1126/science.1175055. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Reul JM, Trapp T, van Steensel B, Wetzel C, Damm K, Zieglgansberger W, Holsboer F. Progesterone receptor-mediated effects of neuroactive steroids. Neuron. 1993;11:523–530. doi: 10.1016/0896-6273(93)90156-l. [DOI] [PubMed] [Google Scholar]
- Sabeti J, Nelson TE, Purdy RH, Gruol DL. Steroid pregnenolone sulfate enhances NMDA-receptor-independent long-term potentiation at hippocampal CA1 synapses, role for L-type calcium channels and sigma-receptors. Hippocampus. 2007;17:349–369. doi: 10.1002/hipo.20273. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ. The influence of gonadal hormones on neuronal excitability, seizures and epilepsy in the female. Epilepsia. 2006;47:1423–1440. doi: 10.1111/j.1528-1167.2006.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Purdy RH, Moore PH, Jr, Paul SM, Rubinow DR. Circulating levels of anxiolytic steroids in the luteal phase in women with premenstrual syndrome and in control subjects. Journal of Clinical Endocrinology & Metabolism. 1994;79:1256–60. doi: 10.1210/jcem.79.5.7962316. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Robel P, Baulieu EE. Development and regeneration of the nervous system, a role for neuroactive steroids. Developmental Neuroscience. 1996;18:16–21. doi: 10.1159/000111391. [DOI] [PubMed] [Google Scholar]
- Shen H, Sabaliauskas N, Sherpa A, Fenton AA, Stelzer A, Aoki C, Smith SS. A critical role for alpha4betadelta GABA-A receptors in shaping learning deficits at puberty in mice. Science. 2010;327(5972):1515–1518. doi: 10.1126/science.1184245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W. Structure, pharmacology, and function of GABA-A receptor subtypes. Advances in Pharmacology. 2006;54:231–263. doi: 10.1016/s1054-3589(06)54010-4. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JMH, Li X. GABA-A receptor α4-subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Smith SS, Shen H, Gong QH, Zhou X. Neurosteroid regulation of GABA-A receptors, focus on the α4 and δ subunits. Pharmacology & Therapeutics. 2007;116:58–76. doi: 10.1016/j.pharmthera.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderpalm AH, Lindsey S, Purdy RH, Hauger R, de Wit H. Administration of progesterone produces mild sedative-like effects in men and women. Psychoneuroendocrinology. 2004;29:339–354. doi: 10.1016/s0306-4530(03)00033-7. [DOI] [PubMed] [Google Scholar]
- Soubrie P, Thiebot MH, Jobert A, Montastruc JL, Hery F, Hamon M. Decreased convulsant potency of picrotoxin and pentetrazol and enhanced [3H]flunitrazepam cortical binding following stressful manipulations in rats. Brain Research. 1980;189:505–517. doi: 10.1016/0006-8993(80)90109-2. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Li Z, Banerjee PK, Mihalek RM, Homanics GE, Olsen RW. Behavior and physiology of mice lacking the GABAA-receptor δ subunit. Epilepsia. 2002;43(Suppl 5):3–8. doi: 10.1046/j.1528-1157.43.s.5.8.x. [DOI] [PubMed] [Google Scholar]
- Steffensen SC. Dehydroepiandrosterone sulfate suppresses hippocampal recurrent inhibition and synchronizes neuronal activity to theta rhythm. Hippocampus. 1995;5:320–328. doi: 10.1002/hipo.450050405. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proceedings of National Academy of Sciences USA. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel-Wagner B. Neuroactive steroid metabolism in the human brain. European Journal of Endocrinology. 2001;145:669–679. doi: 10.1530/eje.0.1450669. [DOI] [PubMed] [Google Scholar]
- Stoffel-Wagner B, Beyenburg S, Watzka MS, Blumcke I, Bauer J, Schramm J, Bidlingmaier F, Elger CE. Expression of 5α-reductase and 3α-hydroxisteroid oxidoreductase in the hippocampus of patients with chronic temporal lobe epilepsy. Epilepsia. 2000;41:140–147. doi: 10.1111/j.1528-1157.2000.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Stoffel-Wagner B, Watzka M, Steckelbroeck S, Ludwig M, Clusmann H, Bidlingmaier F, Casarosa E, Luisi S, Elger CE, Beyenburg S. Allopregnanolone serum levels and expression of 5α-reductase and 3α-hydroxysteroid dehydrogenase isoforms in hippocampal and temporal cortex of patients with epilepsy. Epilepsy Research. 2003;54:11–19. doi: 10.1016/s0920-1211(03)00036-6. [DOI] [PubMed] [Google Scholar]
- Strohle A, Pasini A, Romeo E, Hermann B, Spalletta G, di Michele F, Holsboer F, Rupprecht R. Fluoxetine decreases concentrations of 3α, 5α-tetrahydrodeoxycorticosterone (THDOC) in major depression. Journal of Psychiatric Research. 2000;34:183–186. doi: 10.1016/s0022-3956(00)00006-6. [DOI] [PubMed] [Google Scholar]
- Strohle A, Romeo E, di Michele F, Pasini A, Hermann B, Gajewsky G, Holsboer F, Rupprecht R. Induced panic attacks shift γ-aminobutyric acid type A receptor modulatory neuroactive steroid composition in patients with panic disorder, preliminary results. Archives of General Psychiatry. 2003;60:161–8. doi: 10.1001/archpsyc.60.2.161. [DOI] [PubMed] [Google Scholar]
- Strohle A, Romeo E, Hermann B, Pasini A, Spalletta G, di Michele F, Holsboer F, Rupprecht R. Concentrations of 3 alpha-reduced neuroactive steroids and their precursors in plasma of patients with major depression and after clinical recovery. Biological Psychiatry. 1999;45:274–277. doi: 10.1016/s0006-3223(98)00328-x. [DOI] [PubMed] [Google Scholar]
- Su TP, London ED, Jaffee JH. Steroid binding at sigma receptors suggesting a link between endocrine, nervous and immune systems. Science. 1988;240:219–221. doi: 10.1126/science.2832949. [DOI] [PubMed] [Google Scholar]
- Sunderland T, Merril CR, Harrington MG, Lawlor BA, Molchan SE, Martinez R, Murphy DL. Reduced plasma dehydroepiandrosterone concentrations in Alzheimer’s disease. Lancet. 1989;2(8662):570. doi: 10.1016/s0140-6736(89)90700-9. [DOI] [PubMed] [Google Scholar]
- Sundstrom I, Nyberg S, Bäckström T. Patients with premenstrual syndrome have reduced sensitivity to midazolam compared to control subjects. Neuropsychopharmacology. 1997;17:370–381. doi: 10.1016/S0893-133X(97)00086-9. [DOI] [PubMed] [Google Scholar]
- Tan SY, Mulrow PJ. The contribution of the zona fasciculata and glomerulosa to plasma 11-deoxycorticosterone levels in man. Journal of Clinical Endocrinology and Metabolism. 1975;41:126–130. doi: 10.1210/jcem-41-1-126. [DOI] [PubMed] [Google Scholar]
- Temkin NR, Davis GR. Stress as a risk factor for seizures among adults with epilepsy. Epilepsia. 1984;25:450–456. doi: 10.1111/j.1528-1157.1984.tb03442.x. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Suzuki T, Misawa M. Modulation of the decrease in the seizure threshold of pentylenetetrazole in diazepam-withdrawn mice by the neuroactive steroid 5α-pregnan-3α,21-diol-20-one (alloTHDOC) Addiction Biology. 1997;2:455–460. doi: 10.1080/13556219772516. [DOI] [PubMed] [Google Scholar]
- Tuveri A, Paoletti AM, Orrù M, Melis GB, Marotto MF, Zedda P, Marrosu F, Sogliano C, Marra C, Biggio G, Concas A. Reduced serum level of THDOC, an anticonvulsant steroid, in women with perimenstrual catamenial epilepsy. Epilepsia. 2008;49:1221–1229. doi: 10.1111/j.1528-1167.2008.01555.x. [DOI] [PubMed] [Google Scholar]
- Twyman RE, Macdonald RL. Neuroactive steroid regulation of GABAA receptor single-channel kinetic properties of mouse spinal cord neurons in culture. The Journal of Physiology. 1992;456:215–245. doi: 10.1113/jphysiol.1992.sp019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urani A, Roman FJ, Phan VL, Su TP, Maurice T. The antidepressant-like effect induced by sigma (σ1)-receptor agonists and neuroactive steroids in mice submitted to the forced swimming test. Journal of Pharmacology and Experimental Therapeutics. 2001;298:1269–79. [PubMed] [Google Scholar]
- Uzunov DP, Cooper TB, Costa E, Guidotti A. Fluoxetine-elicited changes in brain neuroactive steroid content measured by negative ion mass fragmentograpy. Proceedings of National Academy of Sciences USA. 1996;93:12599–12604. doi: 10.1073/pnas.93.22.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallée M, Mayo W, Darnaudéry M, Corpéchot C, Young J, Koehl M, Le Moal M, Baulieu EE, Robel P, Simon H. Neuroactive steroids, deficient cognitive performance in aged rats depends on low pregnenolone sulfate levels in the hippocampus. Proceedings of National Academy of Sciences USA. 1997;94:14865–14870. doi: 10.1073/pnas.94.26.14865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. The Journal of Neuroscience. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanover KE, Rosenzweig-Lipson S, Hawkinson JE, Lan NC, Belluzzi JD, Stein L, Barrett JE, Wood PL, Carter RB. Characterization of the anxiolytic properties of a novel neuroactive steroid, Co 2-6749 (GMA-839; WAY-141839; 3α, 21-dihydroxy-3β-trifluoromethyl-19-nor-5β-pregnan-20-one), a selective modulator of γ-aminobutyric acidA receptors. Journal Pharmacology and Experimental Therapeutics. 2000;295:337–345. [PubMed] [Google Scholar]
- Veliskova J, Moshe SL. Sexual dimorphism and developmental regulation of substantia nigra function. Annals of Neurology. 2001;50:596–601. doi: 10.1002/ana.1248. [DOI] [PubMed] [Google Scholar]
- Veliskova J. Sex differences in seizure susceptibility. In: Schwartzkroin Philip., editor. Encyclopedia of Basic Epilepsy Research. Oxford: Academic Press; 2009. pp. 1–4. [Google Scholar]
- Wang C, Marx CE, Morrow AL, Wilson WA, Moore SD. Neurosteroid modulation of GABAergic neurotransmission in the central amygdala, a role for NMDA receptors. Neuroscience Letters. 2007;415:118–123. doi: 10.1016/j.neulet.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel CH, Vedder H, Holsboer F, Zieglgansberger W, Deisz RA. Bidirectional effects of the neuroactive steroid tetrahydrodeoxycorticosterone on GABA-activated Cl− currents in cultured rat hypothalamic neurons. British Journal of Pharmacology. 1999;127:863–868. doi: 10.1038/sj.bjp.0702597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland S, Belluzzi JD, Stein L, Lan NC. Comparative behavioral characterization of the neuroactive steroids 3α-OH,5α-pregnan-20-one and 3α-OH,5β-pregnan-20-one in rodents. Psychopharmacology. 1995;118:65–71. doi: 10.1007/BF02245251. [DOI] [PubMed] [Google Scholar]
- Williamson J, Mtchedlishvili Z, Kapur J. Characterization of the convulsant action of pregnenolone sulfate. Neuropharmacology. 2004;46:56–864. doi: 10.1016/j.neuropharm.2003.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABAA receptors containing the δ subunit. The Journal of Neuroscience. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowitz OM, Reus VI, Keebler A, Nelson N, Friedland M, Brizendine L, Roberts E. Double-blind treatment of major depression with dehydroepiandrosterone. The American Journal of Psychiatry. 1999;156:646–649. doi: 10.1176/ajp.156.4.646. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Reus VI, Roberts E, Manfredi F, Chan T, Ormiston S, Johnson R, Canick J, Brizendine L, Weingartner H. Antidepressant and cognition-enhancing effects of DHEA in major depression. Annals of the New York Academy of Sciences. 1995;774:337–9. doi: 10.1111/j.1749-6632.1995.tb17403.x-i1. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Reus VI, Roberts E, Manfredi F, Chan T, Raum WJ, Ormiston S, Johnson R, Canick J, Brizendine L, Weingartner H. Dehydroepiandrosterone (DHEA) treatment of depression. Biological Psychiatry. 1997;41:311–8. doi: 10.1016/s0006-3223(96)00043-1. [DOI] [PubMed] [Google Scholar]
- Wu FS, Gibbs TT, Farb DH. Pregnenonolone sulfate, a positive allosteric modulator at the N-methyl-D-aspartate receptor. Molecular Pharmacology. 1991;40:333–336. [PubMed] [Google Scholar]
- Xue BG, Whittemore ER, Park CH, Woodward RM, Lan NC, Gee KW. Partial agonism by 3α,21-dihydroxy-5β-pregnan-20-one at the γ-aminobutyric acidA receptor neuroactive steroid site. Journal of Pharmacology and Experimental Therapeutics. 1997;281:1095–1101. [PubMed] [Google Scholar]
- Zimmerberg B, McDonald BC. Prenatal alcohol exposure influences the effects of neuroactive steroids on separation-induced ultrasonic vocalizations in rat pups. Pharmacology Biochemistry and Behavior. 1996;55:531–547. doi: 10.1016/s0091-3057(96)00281-x. [DOI] [PubMed] [Google Scholar]