Abstract
Although surgical aortic valve replacement is the standard therapy for severe aortic stenosis (AS), about one third of patients are considered inoperable due to unacceptable surgical risk. Under medical treatment alone these patients have a very poor prognosis with a mortality rate of 50% at 2 years. Transcatheter aortic valve implantation (TAVI) has been used in these patients, and has shown robust results in the only randomized clinical trial of severe AS treatment performed so far. In this review, we will focus on the two commercially available systems: Edwards SAPIEN valve and CoreValve Revalving system. Both systems have demonstrated success rates of over 90% with 30-d mortality rates below 10% in the most recent transfemoral TAVI studies. Moreover, long-term studies have shown that the valves have good haemodynamic performance. Some studies are currently exploring the non-inferiority of TAVI procedures vs conventional surgery in high-risk patients, and long-term clinical results of the percutaneous valves. In this article we review the current status of TAVI including selection of patients, a comparison of available prostheses, results and complications of the procedure, clinical outcomes, and future perspectives.
Keywords: Aortic valve stenosis, Heart valve prosthesis, Transcatheter aortic valve implantation, Non-coronary intervention, Aortic valve replacement
INTRODUCTION
Degenerative aortic stenosis (AS) is the most frequent acquired heart valve disease, with a prevalence of 4.6% in adults aged 75 years or more, and is the most common indication for valve surgery[1-3]. Surgical aortic valve replacement (SVR) is the current treatment of choice in symptomatic AS. There is a huge worldwide experience with SVR, which has resulted in improved survival in historical comparisons with a low rate of mortality in low-risk patients[4-6]. However, about one third of patients with AS referred for surgery are rejected, mainly because of their high surgical risk[7,8].
Transcatheter aortic valve implantation (TAVI) was developed as an alternative for those patients, and consists of a conventional aortic valvuloplasty followed by the implantation of a biological prosthetic valve stitched to a metallic stent and crimped on a catheter. The implantation is performed inside the native valve, rejecting the native leaflets between the stent and the walls, instead of the surgical technique of replacing the diseased valve with a prosthetic valve, with the advantage of not requiring open-heart surgery.
Since the first-in-man TAVI in 2002, this technology has grown to currently become a true alternative to surgery in patients with severe AS rejected for surgery[9,10]. Moreover, transfemoral TAVI has become the first therapy for AS to demonstrate improved survival and non-inferiority compared to surgery in a randomized trial[11,12]. This trial randomized patients with unacceptable surgical risk to medical treatment including valvuloplasty vs transfemoral TAVI, and showed an absolute reduction in mortality of 20% at 1 year. The other arm of the trial showed non-inferiority when compared to SVR[12]. It is noteworthy that TAVI technology was developed on an extremely high-risk population, and this should be taken into account when analyzing the initial outcomes of TAVI procedures.
In this review, we will focus on TAVI procedures using the two commercially available systems: Edwards SAPIEN (ES) and Medtronic CoreValve ReValving System (CS). We will review the current status of TAVI procedures: selection of candidates, a comparison of available prostheses, results and complications of the procedure, clinical outcomes, and future perspectives.
CURRENT TRANSCATHETER VALVES
There are two commercially available valves for transcatheter implantation (Figures 1 and 2). The main characteristics and differences between these valves are shown in Table 1. The Edwards-SAPIEN (Edwards Lifesciences, Irvine, USA) system uses a bovine pericardial valve sutured to a metallic stent frame which is balloon-expandable. From the early Cribier-Edwards model this device evolved to the THV valve and finally, to the current XT model, which is delivered in the new, low profile, NovaFlex catheter system. Conversely, the CoreValve ReValving system (Medtronic Inc., Minneapolis, USA) uses a porcine pericardial valve in a larger and self-expandable nitinol frame which covers both the left ventricular outflow tract (LVOT) and the aortic root. Currently, the third generation CS system is commercially available. Prostheses sizes are different: ES uses 23 mm valves for aortic annulus (measured from hinge to hinge of the leaflets) of 18-21.5 mm and 26 mm from 21.5-25 mm, whereas CS uses 26 mm valves for 20-23 mm annulus and 29 mm valves for annulus of 24-27 mm. A larger (29 mm) ES valve is expected for the transapical approach in 2011, and the release of new CS 23 mm and ES 20 mm sizes is anticipated.
Figure 1.
Corevalve Revalving System.
Figure 2.
Edwards SAPIEN XT valve, 23 mm. A and B: Two views of the valve before implantation, in the final, deployed position; C: View of the valve crimped on the delivery catheter (18F).
Table 1.
Comparative characteristics of the Edwards SAPIEN and Corevalve ReValving System valves
| Features | Edwards SAPIEN XT | Medtronic core valve |
| Manufacturer | Edwards Lifesciences | Medtronic |
| Stent | Cobalt Chromium | Nitinol |
| Valve leaflets | Bovine pericardium | Porcine pericardium |
| Implantation | Balloon-expandable | Self-expandable |
| Repositionable | No | Partially (prior to release) |
| Retrievable | No | No |
| Fixation | Aortic annulus | Aortic annulus and ascending aorta |
| Available diameters (mm) | 23, 26 | 26, 29 |
| Recommended annulus diameter (mm) | 18-25 | 20-27 |
| Delivery system diameter | 18F (23) and 19F (26) | 18 F |
| Minimum required arterial diameter (mm) | 6 | 6 |
| Alternative to transfemoral | Transapical | Trans-subclavian |
| Permanent pacemaker implantation | < 10% | 25%-35% |
Both systems utilize the arterial retrograde access to the aortic root and require a conventional aortic valvuloplasty prior to final implantation of the valve[13]. Initially, ES catheters were bigger (22-24 French), but currently both systems have comparable 18-19 F transfemoral delivery systems. For patients with inadequate diameters in the femoral arteries, CS has developed the surgical subclavian approach, and ES the transapical access. There are also isolated case reports of implants through a surgical approach using the ascending aorta or the retroperitoneal iliac artery as entry points[14]. Recommended medical treatment after implantation is aspirin indefinitely and clopidogrel for 1 to 3 mo after the procedure.
PATIENT SELECTION
The selection of candidates for TAVI is crucial for the success of the TAVI programme. A team of clinical cardiologists, interventional cardiologists, heart surgeons and anaesthesiologists is needed. The multidisciplinary approach to these patients is essential to the success of the programme[15]. Patients should have severe tri-leaflet native-valve AS with an area < 1 cm2 or < 0.6 cm2/m2. Unsuitability for surgery is established by a predicted mortality in EuroSCORE > 20% or STS Score > 10%, or other conditions that preclude conventional SVR such as porcelain aorta, frailty, advanced liver or renal disease, or previous patent left internal mammary artery grafts[16,17]. The decision on each patient’s surgical risk should be individualized, but basically, any contraindication for sternotomy, cardiopulmonary bypass, cardioplegic cardiac arrest or aortic clamping may be indications for TAVI.
The patient assessment protocol used at our institution includes three main tests. (1) Catheterization: Coronary angiography is performed to exclude significant coronary disease and aortography and femoral angiography are also performed[18]. If significant coronary lesions are present, they should be revascularized percutaneously and the TAVI procedure is usually deferred for ≥ 1 mo; (2) Echocardiography: The aortic valve annulus diameter measured by echocardiography should fall into the available prosthesis size range (Table 1). Transesophageal echocardiography is more accurate in sizing the aortic annulus than transthoracic echocardiography; and (3) Computed tomography: vascular computerized tomography with three-dimensional reconstructions of the infrarrenal aorta to the femoral arteries is performed, and those patients with diameters < 6 mm, or excessive calcification and/or tortuosity are excluded from the trans-femoral approach. Other authors also propose computerized tomography of the aortic root and the whole aorta, but the usefulness of this test is not well-established[19]. Exclusion criteria for TAVI, other than inadequate femoral access or apical thrombus for the transapical approach, are recent myocardial infarction, congenital bicuspid valve (although there are some reports of successful cases[20]) and very severe impairment in left ventricular ejection fraction (LVEF ≤ 20%).
TRANSFEMORAL PROCEDURE
TAVI is performed in a hybrid or interventional cardiology room in a sterile environment, and under general anaesthesia (although some groups perform TAVI under sedation without general anaesthesia and intubation). Fluoroscopic, angiographic and transesophageal echocardiographic monitoring is needed. The retrograde, transarterial route is currently preferred over the initial transvenous and transeptal antegrade approach[13]. Arterial access can be accomplished by surgical cutdown of the femoral artery, or now typically by true percutaneous puncture. Further arterial access is needed for blood pressure monitoring and aortic root angiography. A transvenous pacemaker is placed in the right ventricle to perform rapid (around 200 bpm) pacing, needed to avoid prosthesis displacement during implantation. A conventional balloon valvuloplasty is performed, and immediately afterwards the prosthesis is released (inflating the balloon in the ES system or withdrawing the sheath in the CS). Angiography, echocardiography and/or direct gradient measuring verify the success of the implant. All catheters are removed and the access site is closed surgically or with percutaneous suture closure devices. The pacemaker is left in position because delayed auriculoventricular (AV) blocks have been described. In our centre, with the ES valve, the pacemaker is removed after monitoring for 24 h when no new bundle branch or AV block has occurred. CS usually needs a longer monitoring time.
NON-FEMORAL APPROACHES
The most common is the transapical approach, designed initially for the ES valve[21], although the first-in-man transapical implantation of a CS valve has also been reported[22]. The left ventricular apex is directly punctured through a left lateral mini-thoracotomy, a high-support guidewire is placed across the aortic valve, and a 26 F catheter is inserted in the left ventricle, after which the procedure is similar to the transfemoral access but with a different delivery catheter[22]. The subclavian access for CS consists of a direct surgical dissection of the subclavian artery and insertion of the catheter, after which the procedure follows the transfemoral approach[23]. The subclavian approach is still considered off-label, similar to the direct transaortic surgical approach. Following reduction in the profile of the catheters, non-femoral access is needed in around 30% of patients. One advantage of these approaches is more direct handling of the catheter due to the shorter distance to the target, however, as it is more invasive, the results are still slightly poorer at medium-term follow-up. However, it must be taken into account that patients referred for transapical access are systematically described as a higher risk compared to the transfemoral population across studies.
COMPLICATIONS OF THE PROCEDURE
Valve malapposition and/or embolization
Valve malapposition or embolization rates were ≤ 2% in the most recent studies. The ES valve is not repositionable once expanded, whereas the CS is partially repositionable as some adjustment of the final position is possible when only the distal half of the prosthesis is released. These figures will probably remain stable until a fully retrievable valve is developed. Prevention of this complication is crucial and fine measurements of the aortic annulus, as well as the calcifications, which are frequently asymmetrical, of the aortic root are necessary[24,25]. On the other hand, the operator has to be extremely cautious during positioning and implantation of the valve.
Aortic regurgitation
Aortic regurgitation is frequently found after the procedure. The mechanism of aortic regurgitation is usually due to the presence of small paravalvular leaks because of incomplete apposition of the valve, due to severe nodular calcifications. In most cases, the grade is trace or mild, with minimal clinical consequences. Only 5% of procedures result in severe aortic regurgitation, which may be treated by a second, valve-in-valve procedure or with conventional surgery. With the ES system, aortic regurgitation is frequently improved with a second, higher volume, balloon inflation within the valve. Deaths due to severe aortic regurgitation (probably associated with significant valve malapposition) were more frequent in CS than in ES (10% vs 0%, P = 0.03) in a recent pooled analysis[26]. In follow-up studies, no increase in the degree of aortic regurgitation was found, remaining stable or improving after the procedure.
Conversion to open heart surgery
The rate of conversion to open heart surgery or the need for haemodynamic support is also ≤ 2% across published data. Currently it is not recommended that these procedures be performed in centres without cardiac surgery backup. Both complications are predictors of higher mortality across published series[24,27].
Access site complications
Access site complications are the most frequent complications in transfemoral procedures. These complications reach 40% in some series, with a great variety of severity, from small haematomas to severe bleedings, tears or even avulsions of the femoral vessels. While most of the data comes from series with larger delivery systems (RetroFlex 22-24 F for ES), the impact of the reduction in the gauge of delivery catheters is assumed but still needs to be determined[28]. The ES valve has higher reported rates of these complications than the CS, and are linked to higher mortality[26], however, most of the data on ES come from the early, larger systems. Currently both systems use comparable sizes of catheters (Table 1), and data from the last generation of devices concerning this issue are awaited. Careful selection of patients, with comprehensive analysis of the femoral and iliac anatomy, and identification of size, calcification and tortuosity decrease these complications. Patients with inappropriate femoral anatomy should be directed towards transapical or subclavian approaches. It is advisable to have experience in peripheral interventions and/or to have the backup of vascular surgeons to help solve incidental problems with the access site. In the Placement of AoRTic TraNscathetER Valve Trial (PARTNER) trial (ES valve randomized vs SVR), there were more major bleedings (19.5% vs 9.3%, P < 0.01) but fewer major vascular site complications (3.2% vs 11%, P < 0.01 in the SVR group at 1 mo[12].
Stroke
Cerebrovascular event rates are reported to be below 5% in most series; these figures are fairly low bearing in mind the advanced age and high prevalence of atherosclerosis in the TAVI population (Stroke rates in SVR are usually reported to be over 5% in the elderly). Studies with magnetic resonance before and after a TAVI procedure showed that subclinical cerebrovascular ischemia occurs frequently (73%-84%) during TAVI[29]. These results have also been reported with SVR, at a rate of 40%-50% and are mostly clinically silent, with unknown long-term consequences[30]. Some studies have suggested that the transapical approach, avoiding manipulation of catheters along the aorta, is related to a lower rate of stroke compared to transfemoral access, but results are inconclusive[31]. An embolic deflection device deployed through radial access has been tested in humans as a protection device[32]. In the recently presented PARTNER trial, TAVI was associated with a higher rate of the composite outcome “all stroke or transient ischemic attack” (5.5% vs 2.4%, P = 0.04) compared with SVR at 1 mo; with no differences in the individual components of the outcome[12].
Myocardial infarction and coronary obstruction
The incidence of myocardial infarction during TAVI is highly variable, ranging from 0.2%-18%, however, this information is biased by the absence of a common definition for myocardial infarction after TAVI. The question of which rise in cardiac markers after a TAVI procedure should be “acceptable” is still unanswered. To ensure a more reliable outcome definition, the reported rates of coronary ostia obstruction are always below 1%. The usual mechanism of the obstruction is not due to jailing of the ostia, but rather displacement of the native aortic valve leaflets, severely calcified and distorted, over the coronary ostia. Manufacturers and independent investigators recommend measuring the distance between the aortic annulus and the coronary ostia, but there are no specific recommendations to prevent this complication[33].
Acute kidney injury
The reported incidence of acute kidney injury ranges from 12%-28%[34]. This complication has been identified as a predictor of mortality in several studies[27,35]. The need for haemodialysis after TAVI ranges from 2.5%-7.4%. Acute kidney injury in patients undergoing a TAVI procedure can be due to a combination of several factors: the injection of contrast media needed for angiography, severe hypotension during certain procedures, manipulation of large catheters in atherosclerotic aortas resulting in microembolization of cholesterol crystals, and an important prevalence of chronic kidney disease in this population.
Need for permanent pacemaker implantation
TAVI is highly associated with new intraventricular conduction abnormalities and the need for permanent pacemaker insertion. The underlying mechanism is trauma over the AV node and the bundle of His generated by the radial forces of the stent[36]. The need for a permanent pacemaker is clearly different between the two systems: < 10% with ES vs near 30% with CS. The proposed explanation for this is that the CS is longer and is usually situated lower in the LVOT. There is no identified strategy to prevent this complication, but some predictors of the need for permanent pacemaker implantation have been identified, such as small aortic annulus, use of CS over ES and the development of transient AV block during implantation[37]. Interestingly, in the recently reported results from the PARTNER trial with ES valves, no differences in new pacemaker implantations were found (3.8% TAVI vs 3.6% SVR at 1 mo, P = 0.89)[12].
Cardiac tamponade
This complication is usually related to a perforation in the left ventricle wall due to the guidewire or in the right ventricle due to the temporary pacemaker lead. In a recent study, cardiac tamponade was reported more frequently as a cause of death with the CS valves, probably linked to the higher rate of AV block and longer time with a temporary pacemaker lead after the procedure[26]. Rupture of the aortic annulus has been reported but it is a rare complication.
PATIENT OUTCOMES
Reported procedural success and available mortality rates at 30 d and 1 year are shown in Table 2. We have chosen studies published only in the past 2 years to show the results of the latest generation of valves and after the learning curve. They are mostly registries, and most have a relatively selected population. Globally, the success rate is above 90%, whereas mortality rate at 30 d is below 10% for transfemoral and around 15% for transapical. Mortality rates at 1 year are still highly variable (Table 2). A recent German registry including 697 patients in a real-world population, mixing CS and ES valves (84% CS) and 96% by femoral access resulted in a mortality rate of 12.4% at 30 d[38].
Table 2.
Clinical outcomes across the most recent published studies
| Year published | Patients | Valve | Access | Procedural success (%) | 30-d mortality (%) | 1-yr mortality (%) | |
| PARTNER EU[55] | 2010 | 61 | ES | TF | 91 | 8.1 | 21.3 |
| SOURCE Registry[56] | 2010 | 463 | ES | TF | 95.2 | 6.3 | - |
| PARTNER cohort B[11] | 2010 | 179 | ES | TF | - | 5 | 30.7 |
| Rodés-Cabau et al[24] | 2010 | 168 | ES | TF | 90.5 | 9.5 | 25 |
| PARTNER cohort A[12] | 2011 | 244 | ES | TF | - | 3.3 | 22.2 |
| PARTNER EU[55] | 2010 | 69 | ES | TA | 91 | 18.8 | 51.7 |
| SOURCE Registry[56] | 2010 | 575 | ES | TA | 92.7 | 10.3 | - |
| Rodés-Cabau et al[24]1 | 2010 | 177 | ES | TA | 96.1 | 11.3 | 23 |
| Wong et al[45]1 | 2010 | 60 | ES | TA | 98.3 | 18.3 | - |
| PARTNER cohort A[12] | 2011 | 104 | ES | TA | - | 3.8 | 29 |
| Grube et al[43] | 2008 | 1022 | CS | TF | 91.2 | 10.8 | - |
| Piazza et al[57] | 2008 | 646 | CS | TF | 97.2 | 8 | - |
| Avanzas et al[58] | 2010 | 108 | CS | 103 TF/5 TS | 98.1 | 7.4 | 17.7 |
| Tamburino et al[27] | 2011 | 663 | CS | 599 TF/64 TS | 98 | 5.4 | 15 |
Dr. Wong and Dr. Rodés-Cabau are from the same centre, probably patients overlapped in these two studies;
Results referred to the third generation Corevalve ReValving System (CS) device only. ES: Edwards SAPIEN; TF: Transfemoral; TA: Transapical; TS: Trans-subclavian.
The PARTNER trial is the first randomized trial of TAVI. The remarkable results of cohort B (transfemoral TAVI with ES valve vs medical treatment including valvuloplasty in patients rejected from surgery) showed an absolute reduction in mortality at 1 year of 20% (50.7% in medical treatment vs 30.7% in TAVI group, P < 0.05)[11]. In cohort A, 699 patients with high surgical risk were assessed for transfemoral access and then randomized 1:1 to transfemoral TAVI vs SVR (492 patients) or transapical TAVI vs SVR (207 patients). The primary endpoint, non-inferiority of TAVI with ES valve in all-cause mortality at 1 year, was met (24.2% vs 26.8%, P = 0.001 for non-inferiority). The transfemoral TAVI subgroup was also non-inferior to SVR. TAVI was associated with more strokes and major vascular complications, whereas SVR had more major bleedings and new onset atrial fibrillation[12].
Left ventricular ejection fraction improves after TAVI in patients with impaired function prior to the procedure[39]. Moreover, the increase in LVEF is higher in TAVI patients when compared to SVR patients[40]. In addition, left ventricular mass decreased and diastolic dysfunction improved after TAVI[41]. Mitral regurgitation, which is usually present in some degree, remains unchanged in most patients (61% in one study), although some may experience a change[42]. Severe mitral regurgitation has been identified as a poor prognostic factor and we think that it should be considered as an exclusion criterion for the procedure.
In less than 10 years of the use of this technique we have seen a remarkable drop in procedure failure and mortality rates. This rapid mastering of the procedure is mainly explained by two factors. One is the development of a new generation of devices with reduction in catheter sizes and better deliverability. Studies comparing the first and last generation of the devices have demonstrated a significant reduction in procedure failure and mortality rates[43,44]. The other factor is the training of interventional cardiologists or surgeons who want to start a TAVI programme, which usually involves a course at an experienced centre, followed by surveillance of the first cases by a proctor. This approach has largely contributed to shortening the learning curve and rapidly improving the results of TAVI procedures in naïve centres. Also, the learning curve has contributed to improving the selection of candidates for the procedure.
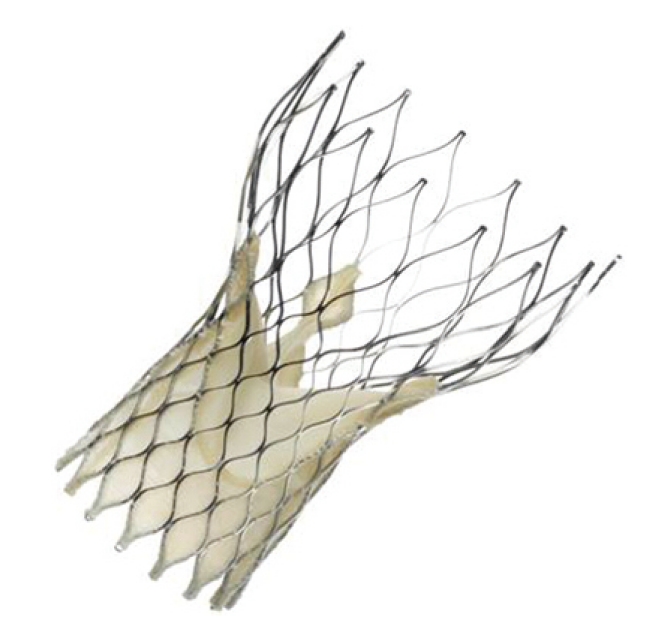
The importance of the learning curve has been highlighted by some groups, making a comparison between early and late experience, and obtaining a relative reduction in death and complications of 50%-70%[43,45,46]. Figure 3 shows the improvement in outcomes from studies published during the last 5 years[26].
Figure 3.
Decrease in 1-mo and procedural mortality observed through the years (from studies published in 2004 to 2010) in patients undergoing transcatheter aortic valve implantation. From Moreno et al[26].
Some authors have tried to identify predictors of procedure success. In a two-centre, German experience with 168 patients, good pre-procedure functional status (Karnofsky index) was identified as the only independent predictor of in-hospital survival[47]. In a large (663 patients) multicentre Italian series, conversion to open heart surgery, cardiac tamponade, major access site complications, LVEF < 40%, prior balloon valvuloplasty, and diabetes mellitus were independent predictors of mortality at 30 d. In addition, prior stroke, postprocedural paravalvular leak ≥ 2, prior acute pulmonary edema, and chronic kidney disease were independent predictors of mortality between 30 d and 1 year[27]. The Canadian experience identified pulmonary hypertension, severe mitral regurgitation and the need for haemodynamic support as 30-d mortality predictors with the ES valve[24]. Periprocedural acute kidney injury is also proposed as a 30-d and 1-year predictor of mortality[35].
The long-term durability of these valves has been addressed only in small studies, due to the newness of the technique. Theoretically, and accordingly to the manufacturer’s wear test, both CS and ES valves are designed to last ≥ 10 years. All published studies agree with their good durability and preserved haemodynamic function with effective orifice areas over 1.5 cm2 and no significant change in gradients or new aortic regurgitation at 3 years[48,49].
OFF-LABEL INDICATIONS
As with other new devices, some experienced centres have tried to explore the outer limits of the current indications for these valves. The “valve-in-valve” procedures were developed to avoid redo cardiac surgery in elderly, high-risk patients with degenerated bioprostheses, usually in the aortic position, with acceptable results[50]. It is also a common last-resource technique for unsuccessful TAVI procedures with severe paravalvular leaks[51]. Isolated case reports of valve-in-valve procedures of mitral bioprostheses have also been published[52]. Another proposed procedure is the valve-in-ring, in which a transcatheter prosthesis is inserted inside a failed annuloplasty. In addition, transcatheter valves have been successfully implanted in tricuspid or pulmonary positions[53,54]. Further investigations in this field are warranted.
FUTURE VALVES AND PERSPECTIVES
Several new valves are in different phases of experimental, clinical, or feasibility investigation. Most of the new models have the self-expanding technology. These will improve delivery of the valve, minimize paravalvular leaks, and allow for reposition or recovery of the implanted valve. Unfortunately, there is still a paucity of information and clinical data for these valves. Table 3 shows the potential advantages and published experience of the valves that are currently under development.
Table 3.
Future valves under development
| Name | Manufactured by | Country | Advantages and published experience |
| AorTx™ | Hansen Medical | USA | Fully retrievable |
| Direct Flow™ | Direct Flow Medical Inc | USA | Fully retrievable, inflatable fabric cuff around the valve that seals the aortic annulus. 6 patients implanted[59] |
| Engager™ | Medtronic | USA | Specifically designed for transapical access. Easy positioning, better fixation (hooks). 30 implants in tricuspid position[53] |
| HLT™ | Heart Leaflet Technologies | USA | ”Flow-through” configuration that does not create obstruction. No need for rapid pacing |
| JenaValve™ | JenaValve | Germany | Repositionable, clipping of the native leaflets. No need for rapid pacing. First-in-man |
| Lotus™ | Sadra Medical / Boston Scientific | USA | Fully repositionable, self-centreing, early leaflet function before final release. First-in-man[60] |
| Paniagua™ | Endoluminal technology Research | USA | Low profile catheter. First retrograde implantation in the world[61] |
| St Jude™ | St Jude Medical | USA | Additional binding in the ascending aorta. Early stages. No human implants yet |
| ValveXchange™ | ValveXchange Inc | USA | Permanent support frame and exchangeable leaflet set. Early stages. No human implants yet |
In the next few years we will probably see a drop in the “high risk” threshold of patients selected for TAVI, possibly in direct competition with SVR. With the accumulated experience, risk scores for mortality and morbidity in TAVI procedures will be developed. New valves will probably come onto the market, reducing the costs of the procedure and providing advantages such as simplification of the procedure, widening of the valve size range, reduction in catheters (albeit a balance between catheter gauge and quality of the stent/leaflets will closely follow) and a further fall in complication rates. Off-label indications, such as valve-in-valve procedures and implantation in other valve rings will generate more literature. Cost-effectiveness studies will also clarify the final position of the TAVI procedure in modern cardiology. Results from many ongoing studies like the pivotal trials of CS vs SVR (one ongoing in the US and the SURTAVI trial in preparation in Europe), a small study of valve-in-valve in failing aortic bioprostheses with CS (REDO study), and some post market registries from both systems are eagerly awaited.
ACKNOWLEDGMENTS
Dr. Salinas receives an educational grant from the Spanish Society of Cardiology.
Footnotes
Peer reviewer: Vignendra Ariyarajah, MD, Division of Interventional Cardiology, 5320G, Cath Lab, Gibbon Building, 111 South 11 th, Thomas Jefferson University Hospital, Philadelphia, PA 19107, United States; Alfredo E Rodriguez, MD, PhD, FACC, FSCAI, Heard Institutional Cardiology, Otamendi Hospital, Post Graduate School of Medicine, Cardiac Unit, Azcuenaga 870, Buenos Aires, C1115AAB, Argentina; Ulrich Nellessen, Professor, Medical Director, Chief Department of Cardiology, Johanniter-Krankenhaus Genthin-Stendal GmbH, Wendstraße 31, 39576 Stendal, Germany
S- Editor Cheng JX L- Editor Webster JR E- Editor Zheng XM
References
- 1.Aronow WS, Kronzon I. Prevalence and severity of valvular aortic stenosis determined by Doppler echocardiography and its association with echocardiographic and electrocardiographic left ventricular hypertrophy and physical signs of aortic stenosis in elderly patients. Am J Cardiol. 1991;67:776–777. doi: 10.1016/0002-9149(91)90542-s. [DOI] [PubMed] [Google Scholar]
- 2.Lester SJ, Heilbron B, Gin K, Dodek A, Jue J. The natural history and rate of progression of aortic stenosis. Chest. 1998;113:1109–1114. doi: 10.1378/chest.113.4.1109. [DOI] [PubMed] [Google Scholar]
- 3.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 4.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O’Gara PT, et al. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118:e523–e661. doi: 10.1161/CIRCULATIONAHA.108.190748. [DOI] [PubMed] [Google Scholar]
- 5.Vahanian A, Baumgartner H, Bax J, Butchart E, Dion R, Filippatos G, Flachskampf F, Hall R, Iung B, Kasprzak J, et al. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J. 2007;28:230–268. doi: 10.1093/eurheartj/ehl428. [DOI] [PubMed] [Google Scholar]
- 6.Ross J, Braunwald E. Aortic stenosis. Circulation. 1968;38:61–67. doi: 10.1161/01.cir.38.1s5.v-61. [DOI] [PubMed] [Google Scholar]
- 7.Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Bärwolf C, Levang OW, Tornos P, Vanoverschelde JL, Vermeer F, Boersma E, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24:1231–1243. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 8.Bach DS, Siao D, Girard SE, Duvernoy C, McCallister BD, Gualano SK. Evaluation of patients with severe symptomatic aortic stenosis who do not undergo aortic valve replacement: the potential role of subjectively overestimated operative risk. Circ Cardiovasc Qual Outcomes. 2009;2:533–539. doi: 10.1161/CIRCOUTCOMES.109.848259. [DOI] [PubMed] [Google Scholar]
- 9.Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, Derumeaux G, Anselme F, Laborde F, Leon MB. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106:3006–3008. doi: 10.1161/01.cir.0000047200.36165.b8. [DOI] [PubMed] [Google Scholar]
- 10.Webb J, Cribier A. Percutaneous transarterial aortic valve implantation: what do we know? Eur Heart J. 2011;32:140–147. doi: 10.1093/eurheartj/ehq453. [DOI] [PubMed] [Google Scholar]
- 11.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 12.Smith CR. Transcatheter vs surgical aortic valve replacement in high risk patients with severe aortic stenosis: Results from the PARTNER trial; ACC congress; 2011 Apr 3; New Orleans. Available from: http://my.americanheart.org/idc/groups/ahamah-public/@wcm/@sop/@scon/documents/downloadable/ucm_425332.pdf. [Google Scholar]
- 13.Webb JG, Chandavimol M, Thompson CR, Ricci DR, Carere RG, Munt BI, Buller CE, Pasupati S, Lichtenstein S. Percutaneous aortic valve implantation retrograde from the femoral artery. Circulation. 2006;113:842–850. doi: 10.1161/CIRCULATIONAHA.105.582882. [DOI] [PubMed] [Google Scholar]
- 14.Latsios G, Gerckens U, Grube E. Transaortic transcatheter aortic valve implantation: a novel approach for the truly “no-access option” patients. Catheter Cardiovasc Interv. 2010;75:1129–1136. doi: 10.1002/ccd.22378. [DOI] [PubMed] [Google Scholar]
- 15.Dobarro D, Moreno R, Filgueiras D, Calvo L, López-Fernández T, Sánchez-Recalde A, Jiménez-Valero S, Galeote G, Gómez-Rubín Mdel C, Moreno-Gómez I, et al. [Implantation of aortic valvular prosthesis via transfemoral catheter. Evaluation of candidates undergoing the procedure] Med Clin (Barc) 2009;133:414–421. doi: 10.1016/j.medcli.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 16.Edwards FH, Grover FL, Shroyer AL, Schwartz M, Bero J. The Society of Thoracic Surgeons National Cardiac Surgery Database: current risk assessment. Ann Thorac Surg. 1997;63:903–908. doi: 10.1016/s0003-4975(97)00017-9. [DOI] [PubMed] [Google Scholar]
- 17.Roques F, Michel P, Goldstone AR, Nashef SA. The logistic EuroSCORE. Eur Heart J. 2003;24:881–882. doi: 10.1016/s0195-668x(02)00799-6. [DOI] [PubMed] [Google Scholar]
- 18.Moreno R, Calvo L, Filgueiras D, López T, Sánchez-Recalde A, Jiménez-Valero S, Galeote G, López-Sendón JL. [Percutaneous implantation of aortic valve prosthesis in patients with symptomatic severe aortic stenosis rejected for surgical valve replacement] Rev Esp Cardiol. 2008;61:1215–1219. [PubMed] [Google Scholar]
- 19.Leipsic J, Wood D, Manders D, Nietlispach F, Masson JB, Mayo J, Al-Bugami S, Webb JG. The evolving role of MDCT in transcatheter aortic valve replacement: a radiologists’ perspective. AJR Am J Roentgenol. 2009;193:W214–W219. doi: 10.2214/AJR.08.2230. [DOI] [PubMed] [Google Scholar]
- 20.Ferrari E, Locca D, Sulzer C, Marcucci C, Rizzo E, Tozzi P, von Segesser LK. Successful transapical aortic valve implantation in a congenital bicuspid aortic valve. Ann Thorac Surg. 2010;90:630–632. doi: 10.1016/j.athoracsur.2009.12.080. [DOI] [PubMed] [Google Scholar]
- 21.Lichtenstein SV, Cheung A, Ye J, Thompson CR, Carere RG, Pasupati S, Webb JG. Transapical transcatheter aortic valve implantation in humans: initial clinical experience. Circulation. 2006;114:591–596. doi: 10.1161/CIRCULATIONAHA.106.632927. [DOI] [PubMed] [Google Scholar]
- 22.Lange R, Schreiber C, Götz W, Hettich I, Will A, Libera P, Laborde JC, Bauernschmitt R. First successful transapical aortic valve implantation with the Corevalve Revalving system: a case report. Heart Surg Forum. 2007;10:E478–E479. doi: 10.1532/HSF98.20071140. [DOI] [PubMed] [Google Scholar]
- 23.Ruge H, Lange R, Bleiziffer S, Hutter A, Mazzitelli D, Will A, Schreiber C, Laborde JC, Bauernschmitt R. First successful aortic valve implantation with the CoreValve ReValving System via right subclavian artery access: a case report. Heart Surg Forum. 2008;11:E323–E324. doi: 10.1532/HSF98.20081021. [DOI] [PubMed] [Google Scholar]
- 24.Rodés-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Feindel CM, Osten M, Natarajan MK, Velianou JL, Martucci G, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. 2010;55:1080–1090. doi: 10.1016/j.jacc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 25.Al Ali AM, Altwegg L, Horlick EM, Feindel C, Thompson CR, Cheung A, Carere RG, Humphries K, Ye J, Masson JB, et al. Prevention and management of transcatheter balloon-expandable aortic valve malposition. Catheter Cardiovasc Interv. 2008;72:573–578. doi: 10.1002/ccd.21667. [DOI] [PubMed] [Google Scholar]
- 26.Moreno R, Calvo L, Salinas P, Dobarro D, Santiago JV, Sanchez-Recalde A, Galeote G, Riera L, Moreno-Gomez I, Mesa J, et al. causes-peri-operative-mortality-after-transcatheter-aortic-valve-implantation-pooled-analys. J Invasive Cardiol. 2011;23:180–184. [PubMed] [Google Scholar]
- 27.Tamburino C, Capodanno D, Ramondo A, Petronio AS, Ettori F, Santoro G, Klugmann S, Bedogni F, Maisano F, Marzocchi A, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. 2011;123:299–308. doi: 10.1161/CIRCULATIONAHA.110.946533. [DOI] [PubMed] [Google Scholar]
- 28.Kahlert P, Al-Rashid F, Weber M, Wendt D, Heine T, Kottenberg E, Thielmann M, Kühl H, Peters J, Jakob HG, et al. Vascular access site complications after percutaneous transfemoral aortic valve implantation. Herz. 2009;34:398–408. doi: 10.1007/s00059-009-3252-3. [DOI] [PubMed] [Google Scholar]
- 29.Ghanem A, Müller A, Nähle CP, Kocurek J, Werner N, Hammerstingl C, Schild HH, Schwab JO, Mellert F, Fimmers R, et al. Risk and fate of cerebral embolism after transfemoral aortic valve implantation: a prospective pilot study with diffusion-weighted magnetic resonance imaging. J Am Coll Cardiol. 2010;55:1427–1432. doi: 10.1016/j.jacc.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 30.Knipp SC, Matatko N, Schlamann M, Wilhelm H, Thielmann M, Forsting M, Diener HC, Jakob H. Small ischemic brain lesions after cardiac valve replacement detected by diffusion-weighted magnetic resonance imaging: relation to neurocognitive function. Eur J Cardiothorac Surg. 2005;28:88–96. doi: 10.1016/j.ejcts.2005.02.043. [DOI] [PubMed] [Google Scholar]
- 31.Astarci P, Glineur D, Kefer J, D’Hoore W, Renkin J, Vanoverschelde JL, El Khoury G, Grandin C. Magnetic resonance imaging evaluation of cerebral embolization during percutaneous aortic valve implantation: comparison of transfemoral and trans-apical approaches using Edwards Sapiens valve. Eur J Cardiothorac Surg. 2011:Epub ahead of print. doi: 10.1016/j.ejcts.2010.11.070. [DOI] [PubMed] [Google Scholar]
- 32.Nietlispach F, Wijesinghe N, Gurvitch R, Tay E, Carpenter JP, Burns C, Wood DA, Webb JG. An embolic deflection device for aortic valve interventions. JACC Cardiovasc Interv. 2010;3:1133–1138. doi: 10.1016/j.jcin.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 33.Piazza N, de Jaegere P, Schultz C, Becker AE, Serruys PW, Anderson RH. Anatomy of the aortic valvar complex and its implications for transcatheter implantation of the aortic valve. Circ Cardiovasc Interv. 2008;1:74–81. doi: 10.1161/CIRCINTERVENTIONS.108.780858. [DOI] [PubMed] [Google Scholar]
- 34.Bagur R, Webb JG, Nietlispach F, Dumont E, De Larochellière R, Doyle D, Masson JB, Gutiérrez MJ, Clavel MA, Bertrand OF, Pibarot P, Rodés-Cabau J. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J. 2010;31:865–874. doi: 10.1093/eurheartj/ehp552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sinning JM, Ghanem A, Steinhäuser H, Adenauer V, Hammerstingl C, Nickenig G, Werner N. Renal function as predictor of mortality in patients after percutaneous transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2010;3:1141–1149. doi: 10.1016/j.jcin.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 36.Moreno R, Dobarro D, López de Sá E, Prieto M, Morales C, Calvo Orbe L, Moreno-Gomez I, Filgueiras D, Sanchez-Recalde A, Galeote G, et al. Cause of complete atrioventricular block after percutaneous aortic valve implantation: insights from a necropsy study. Circulation. 2009;120:e29–e30. doi: 10.1161/CIRCULATIONAHA.109.849281. [DOI] [PubMed] [Google Scholar]
- 37.Bleiziffer S, Ruge H, Hörer J, Hutter A, Geisbüsch S, Brockmann G, Mazzitelli D, Bauernschmitt R, Lange R. Predictors for new-onset complete heart block after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2010;3:524–530. doi: 10.1016/j.jcin.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 38.Zahn R, Gerckens U, Grube E, Linke A, Sievert H, Eggebrecht H, Hambrecht R, Sack S, Hauptmann KE, Richardt G, et al. Transcatheter aortic valve implantation: first results from a multi-centre real-world registry. Eur Heart J. 2011;32:198–204. doi: 10.1093/eurheartj/ehq339. [DOI] [PubMed] [Google Scholar]
- 39.Ewe SH, Ajmone Marsan N, Pepi M, Delgado V, Tamborini G, Muratori M, Ng AC, van der Kley F, de Weger A, Schalij MJ, et al. Impact of left ventricular systolic function on clinical and echocardiographic outcomes following transcatheter aortic valve implantation for severe aortic stenosis. Am Heart J. 2010;160:1113–1120. doi: 10.1016/j.ahj.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Clavel MA, Webb JG, Rodés-Cabau J, Masson JB, Dumont E, De Larochellière R, Doyle D, Bergeron S, Baumgartner H, Burwash IG, et al. Comparison between transcatheter and surgical prosthetic valve implantation in patients with severe aortic stenosis and reduced left ventricular ejection fraction. Circulation. 2010;122:1928–1936. doi: 10.1161/CIRCULATIONAHA.109.929893. [DOI] [PubMed] [Google Scholar]
- 41.Tzikas A, Geleijnse ML, Van Mieghem NM, Schultz CJ, Nuis RJ, van Dalen BM, Sarno G, van Domburg RT, Serruys PW, de Jaegere PP. Left ventricular mass regression one year after transcatheter aortic valve implantation. Ann Thorac Surg. 2011;91:685–691. doi: 10.1016/j.athoracsur.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 42.Tzikas A, Piazza N, van Dalen BM, Schultz C, Geleijnse ML, van Geuns RJ, Galema TW, Nuis RJ, Otten A, Gutierrez-Chico JL, et al. Changes in mitral regurgitation after transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2010;75:43–49. doi: 10.1002/ccd.22197. [DOI] [PubMed] [Google Scholar]
- 43.Grube E, Buellesfeld L, Mueller R, Sauren B, Zickmann B, Nair D, Beucher H, Felderhoff T, Iversen S, Gerckens U. Progress and current status of percutaneous aortic valve replacement: results of three device generations of the CoreValve Revalving system. Circ Cardiovasc Interv. 2008;1:167–175. doi: 10.1161/CIRCINTERVENTIONS.108.819839. [DOI] [PubMed] [Google Scholar]
- 44.Détaint D, Lepage L, Himbert D, Brochet E, Messika-Zeitoun D, Iung B, Vahanian A. Determinants of significant paravalvular regurgitation after transcatheter aortic valve: implantation impact of device and annulus discongruence. JACC Cardiovasc Interv. 2009;2:821–827. doi: 10.1016/j.jcin.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Wong DR, Ye J, Cheung A, Webb JG, Carere RG, Lichtenstein SV. Technical considerations to avoid pitfalls during transapical aortic valve implantation. J Thorac Cardiovasc Surg. 2010;140:196–202. doi: 10.1016/j.jtcvs.2009.07.081. [DOI] [PubMed] [Google Scholar]
- 46.Webb JG, Altwegg L, Masson JB, Al Bugami S, Al Ali A, Boone RA. A new transcatheter aortic valve and percutaneous valve delivery system. J Am Coll Cardiol. 2009;53:1855–1858. doi: 10.1016/j.jacc.2008.07.075. [DOI] [PubMed] [Google Scholar]
- 47.Buellesfeld L, Wenaweser P, Gerckens U, Mueller R, Sauren B, Latsios G, Zickmann B, Hellige G, Windecker S, Grube E. Transcatheter aortic valve implantation: predictors of procedural success--the Siegburg-Bern experience. Eur Heart J. 2010;31:984–991. doi: 10.1093/eurheartj/ehp570. [DOI] [PubMed] [Google Scholar]
- 48.Gurvitch R, Wood DA, Tay EL, Leipsic J, Ye J, Lichtenstein SV, Thompson CR, Carere RG, Wijesinghe N, Nietlispach F, et al. Transcatheter aortic valve implantation: durability of clinical and hemodynamic outcomes beyond 3 years in a large patient cohort. Circulation. 2010;122:1319–1327. doi: 10.1161/CIRCULATIONAHA.110.948877. [DOI] [PubMed] [Google Scholar]
- 49.Ye J, Cheung A, Lichtenstein SV, Nietlispach F, Albugami S, Masson JB, Thompson CR, Munt B, Moss R, Carere RG, et al. Transapical transcatheter aortic valve implantation: follow-up to 3 years. J Thorac Cardiovasc Surg. 2010;139:1107–1113, 1113.e1. doi: 10.1016/j.jtcvs.2009.10.056. [DOI] [PubMed] [Google Scholar]
- 50.Pasic M, Unbehaun A, Dreysse S, Buz S, Drews T, Kukucka M, Hetzer R. Transapical aortic valve implantation after previous aortic valve replacement: Clinical proof of the “valve-in-valve” concept. J Thorac Cardiovasc Surg. 2010:Epub ahead of print. doi: 10.1016/j.jtcvs.2010.09.049. [DOI] [PubMed] [Google Scholar]
- 51.Ussia GP, Barbanti M, Ramondo A, Petronio AS, Ettori F, Santoro G, Klugmann S, Bedogni F, Maisano F, Marzocchi A, et al. The valve-in-valve technique for treatment of aortic bioprosthesis malposition an analysis of incidence and 1-year clinical outcomes from the italian CoreValve registry. J Am Coll Cardiol. 2011;57:1062–1068. doi: 10.1016/j.jacc.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 52.Seiffert M, Franzen O, Conradi L, Baldus S, Schirmer J, Meinertz T, Reichenspurner H, Treede H. Series of transcatheter valve-in-valve implantations in high-risk patients with degenerated bioprostheses in aortic and mitral position. Catheter Cardiovasc Interv. 2010;76:608–615. doi: 10.1002/ccd.22618. [DOI] [PubMed] [Google Scholar]
- 53.Falk V, Walther T, Schwammenthal E, Strauch J, Aicher D, Wahlers T, Schäfers J, Linke A, Mohr FW. Transapical aortic valve implantation with a self-expanding anatomically oriented valve. Eur Heart J. 2011;32:878–887. doi: 10.1093/eurheartj/ehq445. [DOI] [PubMed] [Google Scholar]
- 54.Vezmar M, Chaturvedi R, Lee KJ, Almeida C, Manlhiot C, McCrindle BW, Horlick EM, Benson LN. Percutaneous pulmonary valve implantation in the young 2-year follow-up. JACC Cardiovasc Interv. 2010;3:439–448. doi: 10.1016/j.jcin.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Lefèvre T, Kappetein AP, Wolner E, Nataf P, Thomas M, Schächinger V, De Bruyne B, Eltchaninoff H, Thielmann M, Himbert D, et al. One year follow-up of the multi-centre European PARTNER transcatheter heart valve study. Eur Heart J. 2011;32:148–157. doi: 10.1093/eurheartj/ehq427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomas M, Schymik G, Walther T, Himbert D, Lefèvre T, Treede H, Eggebrecht H, Rubino P, Michev I, Lange R, et al. Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: A European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation. 2010;122:62–69. doi: 10.1161/CIRCULATIONAHA.109.907402. [DOI] [PubMed] [Google Scholar]
- 57.Piazza N, Grube E, Gerckens U, den Heijer P, Linke A, Luha O, Ramondo A, Ussia G, Wenaweser P, Windecker S, et al. Procedural and 30-day outcomes following transcatheter aortic valve implantation using the third generation (18 Fr) corevalve revalving system: results from the multicentre, expanded evaluation registry 1-year following CE mark approval. EuroIntervention. 2008;4:242–249. doi: 10.4244/eijv4i2a43. [DOI] [PubMed] [Google Scholar]
- 58.Avanzas P, Muñoz-García AJ, Segura J, Pan M, Alonso-Briales JH, Lozano I, Morís C, Suárez de Lezo J, Hernández-García JM. Percutaneous implantation of the CoreValve self-expanding aortic valve prosthesis in patients with severe aortic stenosis: early experience in Spain. Rev Esp Cardiol. 2010;63:141–148. doi: 10.1016/s1885-5857(10)70031-1. [DOI] [PubMed] [Google Scholar]
- 59.Low RI, Bolling SF, Yeo KK, Ebner A. Direct flow medical percutaneous aortic valve: proof of concept. EuroIntervention. 2008;4:256–261. [PubMed] [Google Scholar]
- 60.Buellesfeld L, Gerckens U, Grube E. Percutaneous implantation of the first repositionable aortic valve prosthesis in a patient with severe aortic stenosis. Catheter Cardiovasc Interv. 2008;71:579–584. doi: 10.1002/ccd.21470. [DOI] [PubMed] [Google Scholar]
- 61.Paniagua D, Condado JA, Besso J, Vélez M, Burger B, Bibbo S, Cedeno D, Acquatella H, Mejia C, Induni E, et al. First human case of retrograde transcatheter implantation of an aortic valve prosthesis. Tex Heart Inst J. 2005;32:393–398. [PMC free article] [PubMed] [Google Scholar]