Abstract
Purpose
Globular adiponectin (gAd) is a type of adipocytokine, which is mainly produced by adipose tissue. It has been reported that gAd acts as a pro- as well as an anti-inflammatory factor. Interleukin (IL)-6 and IL-8 are pro-inflammatory cytokines. To investigate the role of gAd on periodontal tissues, the expression of adiponectin receptor 1 (AdipoR1) and the effect of gAd on the expression of IL-6 and IL-8 were investigated in periodontal ligament (PDL) and gingival fibroblasts.
Methods
PDL and gingival fibroblasts were cultured from human periodontal tissues. gAd derived from Escherichia coli and murine myeloma cells were used. The expression of AdipoR1 was estimated by reverse transcription-polymerase chain reaction and western blot. The expression of cytokines was measured by enzyme-linked immunosorbent assay.
Results
PDL and gingival fibroblasts expressed both mRNA and protein of AdipoR1. gAd derived from E. coli increased the production of IL-6 and IL-8, but polymyxin B, an inhibitor of lipopolysaccharide (LPS), inhibited IL-6 and IL-8 production induced by gAd in both types of cells. gAd derived from murine myeloma cells did not induce IL-6 and IL-8 production in those cells. gAd derived from E. coli contained higher levels of LPS than gAd derived from murine myeloma cells. LPS increased production of IL-6 and IL-8 in PDL and gingival fibroblasts, but pretreatment of cells with gAd derived from murine myeloma cells did not inhibit LPS-induced IL-6 and IL-8 expression.
Conclusions
Our results suggest that PDL and gingival fibroblasts express AdipoR1 and that gAd does not act as a modulator of IL-6 and IL-8 expression in PDL and gingival fibroblasts.
Keywords: Adiponectin, Periodontal ligament, Fibroblasts, Receptors, Cytokines
INTRODUCTION
Periodontitis is an inflammatory disease caused by bacteria and the inflammatory/immune response to bacteria. Epidemiological studies have suggested that periodontitis is intimately related with obesity [1-2] and diabetes [3]. Several studies have reported that adipocytokines, which are produced by adipocytes, can modulate inflammation [4] and insulin sensitivity [5]. Therefore, the role of adipocytokines in the pathogenesis of periodontitis has become an area of interest in research.
Adiponectin is a type of adipocytokine, produced mainly by adipose tissue [6,7]. Studies have shown that adiponectin is also expressed in skeletal muscle cells and cardiac and synovial fibroblasts [4,8,9]. There are two types of adiponectin: a full-length adiponectin (fAd) and a globular adiponectin (gAd) [6]. fAd consists of an N-terminal signal peptide, a variable domain, a collagen-like domain, and a C-terminal C1q-like globular domain [4]. gAd consists of a globular domain that is cleaved from fAd by proteolysis [7]. Two receptors for adiponectin have been reported: adiponectin receptor 1 (AdipoR1) is a high affinity receptor for gAd, and AdipoR2 is an intermediate affinity receptor for gAd and fAd [10].
Until now, the role of adiponectin in inflammation has not been clear. Some studies have demonstrated that adiponectin has a pro-inflammatory effect, but others showed an anti-inflammatory effect or did not show any effects. The pro-inflammatory effects of adiponectin have been suggested by demonstrating its cytokine-induction activity. Research has shown that fAd stimulates the production of interleukin (IL)-8 and monocyte chemoattractant protein-1 in monocytes and endothelial cells [11]. In addition, fAd has been shown to stimulate production of IL-6 and IL-8 by rheumatoid synovial fibroblasts, suggesting that adiponectin is involved in the pathogenesis of rheumatoid arthritis [12,13]. Studies have also shown that gAd induces the production of IL-1β, IL-6, IL-8, and TNFα in macrophages [14,15] and IL-6 in cardiac fibroblasts [16]. However, other studies have demonstrated that adiponectin has an anti-inflammatory effect by showing its induction of tolerance to lipopolysaccharide (LPS) and inhibition of phagocytosis in macrophages. Pretreatment with gAd reduced LPS-induced production of IL-6 and TNFα in macrophages, suggesting that adiponectin causes macrophages to become resistant to LPS [14]. Treatment of macrophages with adiponectin inhibited macrophage phagocytosis, suggesting that adiponectin suppresses macrophage function and that inhibitionby adiponectin of macrophages may contribute to termination of the inflammatory reaction [17]. However, other studies have not been able to demonstrate the induction of IL-6 and TNFα and the suppression of LPS-induced IL-6 and TNFα production by adiponectin in macrophages, suggesting that some adiponectin effects, such as the inhibition and stimulation of cytokine (IL-6 and TNFα) production in macrophages, may be confused by LPS contamination [18,19].
Interestingly, adiponectin plasma levels are high in healthy humans and decreased in individuals with obesity and type 2 diabetes [20,21]. One study found a trend toward decreased serum levels of adiponectin in periodontitis patients [22]. Moreover, infection with periodontopathogen such as Porphyromonas gingivalis was shown to cause decreased serum levels of adiponectin in type 2 diabetic mice [23]. In another study, antimicrobial periodontal treatment (APT) not only ameliorated periodontitis but also increased serum levels of adiponectin in type 2 diabetes mellitus patients. It has been proposed that amelioration of periodontitis by APT might cause an increase in serum levels of adiponectin in type 2 diabetic subjects [24]. Pretreatment of mice with gAd was shown to suppress Aggregatibacter actinomycetemcomitans LPS-induced TNFα expression in a murine macrophage cell line [25]. gAd was also found to suppress osteoclast formation induced by LPS of A. actinomycetemcomitans [26]. Those reports suggest that, in periodontitis, adiponectin may function as an inhibitor of both LPS-mediated cytokine expression in macrophages and LPS-mediated osteoclast formation. Periodontal ligament (PDL) and gingival fibroblasts play an important role in the pathogenesis of periodontitis by producing pro-inflammatory cytokines such as IL-6 and IL-8 [27]. To this date, however, the role of adiponectin in PDL and gingival fibroblasts has not been clearly determined. In this study, the expression of adiponectin AdipoR1 and the effect of gAd on the expression of IL-6 and IL-8 were investigated in PDL and gingival fibroblasts.
MATERIALS AND METHODS
Culture of cells
Human PDL fibroblasts were prepared from extracted teeth for orthodontic treatment as described previously [28]. The protocol was approved by the Institutional Review Board of Yonsei Dental Hospital (IRB No. 2-2010-0005). Using blades, PDL tissue fragments were removed from the middle one third of the extracted tooth roots and washed three times with α-modified Eagle's medium (α-MEM, Gibco BRL, San Diego, CA, USA) containing antibiotics of 300 mg/mL streptomycin, 300 unit/mL penicillin, and 0.75 mg/mL amphotericin-B (Gibco BRL). PDL tissue fragments were placed in 100-mm dishes containing α-MEM supplemented with the antibiotics of 100 mg/mL streptomycin, 100 unit/mL penicillin, 0.25 mg/mL amphotericin-B, and 20% fetal bovine serum (FBS, Gibco BRL). The culture media was replaced every 2 days until cells grew from the fragments, after which the cultures were maintained with α-MEM containing antibiotics and 10% FBS. Upon reaching 70% confluence, the cells were removed with 0.25% trypsin and passaged to 100-mm dishes. Gingival fibroblasts were prepared from gingival tissues excised for crown lengthening. Gingival tissues were cut into small fragments, and then the tissue fragments were incubated in 2.4 U dispase II (Roche, Mannheim, Germany) and 0.25% collagenase (Roche) for 1 hour at 37℃. The tissue fragments were then separated into epithelium and connective tissue. Connective tissue fragments were placed in 100-mm dishes containing Dulbecco's modified Eagle's medium (DMEM, Gibco BRL) supplemented with antibiotics and 10% FBS and were cultured according to the same procedure as PDL fibroblasts. The cells used in this study had been passaged 5 times. THP-1 monocytes and MDA-MB231 breast cancer cells were maintained in Roswell Park Memorial Institute 1640 medium (Gibco BRL) and DMEM each containing both antibiotics and 10% FBS.
Western blotting
PDL fibroblasts, gingival fibroblasts or MDA-MB231 (5×105 cells/well) were cultured in 6-well culture plates. THP-1 monocytes (5×105 cells/well) were plated in 6-well plates and treated with phorbol 12-myristate 13-acetate (PMA, 100 ng/mL, Sigma-Aldrich Co., St. Louis, MO, USA) for 24 hours to induce the differentiation of monocytes into macrophages. After reaching confluence, the whole cells were lysed with lysis buffer (20 mM Tris-HCl, pH7.5, 150 mM NaCl, 1 mM Na2EDTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 µg/mL leupeptin, and 1 mM phenylmethylsulfonyl fluoride; Cell Signaling Technology Inc., Danvers, MA, USA). After centrifugation at 12,000×g at 4℃, the supernatant was used for the protein assay. Protein concentrations were determined with bovine serum albumin protein assay kits (Bio-Rad Laboratories Inc., Hercules, CA, USA). Forty micrograms of protein per sample of cell extract were run on a 10% SDS-polyacrylamide gel electrophoresis and then electroblotted onto a polyvinylidene fluoride membrane (Bio-Rad Laboratories Inc.). The membranes were blocked with 5% skim milk in tris-buffered saline (10 mM Tris-HCl, 166 mM NaCl, pH 7.4) for 1 hr, rinsed, and then incubated overnight with primary antibodies against adiponectin (R&D Systems Inc., Minneapolis, MN, USA), AdipoR1 (Abcam plc, Cambridge, UK), or actin (SantaCruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1:1,000. Secondary antibody, peroxidase-conjugated anti-rabbit antibody (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA), was used at 1:2,500. Protein bands were visualized by an ECL kit (Amersham, Buckinghamshire, UK).
Reverse transcription-polymerase chain reaction (RT-PCR)
PDL fibroblasts or gingival fibroblasts (5×105 cells/well) were cultured in 6-well culture plates. THP-1 monocytes (5×105 cells/well) were plated in 6-well plates and treated with PMA (100 ng/mL) for 24 hours to induce the differentiation of monocytes into macrophages. After reaching confluence, total RNA in PDL fibroblasts, gingival fibroblasts, or THP-1 macrophages was isolated with Trizol reagent (Invitrogen, Carlsbad, CA, USA). Two micrograms of total RNA was converted to cDNA using an RT premix kit (Bioneer, Seoul, Korea) in accordance with the manufacturer's instructions. The cDNA was amplified with i-StarTaq (Intron Biotech, Seongnam, Korea). The primers used for RT-PCR were as follows: AdipoR1sense primer, 5'-CCTTTCCCCAAGCTGAAGCTGC-3' and antisense primer, 5'-CCTTGACAAAGCCCTCAGCGAT-3'; GAPDH sense primer, 5'-CGGAGTCAACGGATTTGGTCGTAT-3' and antisense primer, 5'-AGCCTTCTCCATGGTGGTGAAGAC-3'. For AdipoR1, the reaction mixtures were subjected to 35 cycles of denaturation and annealing at 60℃. For GAPDH, the reaction mixtures were subjected to 28 cycles of denaturation and annealing at 56℃. The PCR products were then resolved by electrophoresis in a 1% agarose gel containing ethidium bromide.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay
Cell viability was measured using an MTT assay. PDL fibroblasts or gingival fibroblasts (1.7×105 cells/well) were seeded in 24-well plates and maintained in media containing antibiotics and 10% FBS. After being maintained for 7 hours, the cells were starved in serum-free media for 20 hours and then treated with the indicated concentration of polymyxin B for 24 hours. After treatment, 20 µL of MTT solution (5 mg/mL in PBS, Sigma-Aldrich Co.) was added to each well. After incubation for 4 hours, the medium was aspirated, and 200 µL of dimethylsulfoxide was added to each well to dissolve MTT-formazan crystals. The plates were shaken for 5 minutes, and the absorbance was measured on an MRX II microplate reader (Dynatech Laboratories Inc., Chantilly, VA, USA) at 570 nm.
Enzyme-linked immunosorbent assay (ELISA)
PDL fibroblasts or gingival fibroblasts (1.7×105 cells/well) were plated in 24-well culture plates and maintained in media containing antibiotics and 10% FBS for 7 hours. THP-1 monocytes were plated in 24-well plates and treated with PMA for 24 hours to induce the differentiation of monocytes into macrophages. After being maintained for the indicated time, the cells were starved in serum-free media for 20 hours and then treated with myeloma cell-derived recombinant gAd (M-gAd, R&D systems), Escherichia coli-derived gAd (E-gAd, Phoenix Pharmaceuticals, Burlingame, USA), or LPS (Sigma-Aldrich Co.) for 24 hours. For polymyxin B treatment, cells were pretreated with polymyxin B for 1 hour and then treated with M-gAd, E-gAd, or LPS for 24 hours. For estimating the induction of tolerance to LPS by gAd, the cells were pretreated with gAd for 24 hours and then treated with LPS for an additional 24 hours. After the treatment, the levels of IL-6 or IL-8 were measured in the culture supernatants of PDL fibroblasts, gingival fibroblasts, or THP-1 macrophages using an ELISA kit (BioLegend, San Diego, CA, USA) in accordance with the manufacturer's instructions.
Statistical analysis
The results were analyzed using one-way analysis of variance and Tukey's test. A P-value of <0.05 was considered to indicate statistical significance.
RESULTS
Expression of adiponectin and adiponectin receptor
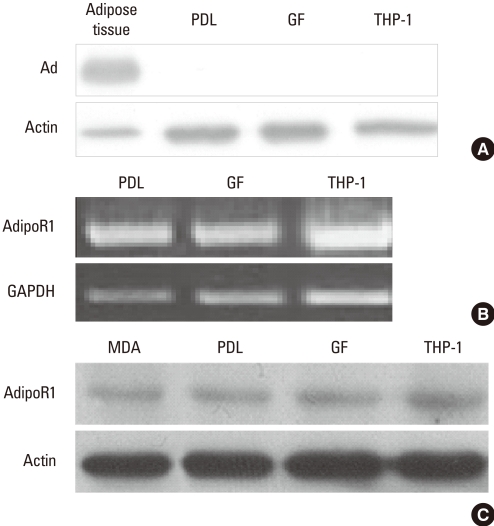
Expression of adiponectin was estimated by western blot. Subcutaneous adipose tissue from the inguinal region expressed adiponectin, but PDL fibroblasts, gingival fibroblasts, and THP-1 macrophages did not show adiponectin expression (Fig. 1A). Expression of AdipoR1 was estimated by RT-PCR and western blot. Like THP-1 macrophages, which have been known to express AdipoR1, PDL and gingival fibroblasts expressed mRNA of AdipoR1 (Fig. 1B) [29]. Similar to THP-1 macrophages and the breast cancer cell line MDA-MB231,which are positive controls for the expression of AdipoR1, PDL and gingival fibroblasts also expressed the protein of AdipoR1 (Fig. 1C) [30].
Figure 1.
Expression of adiponectin and adiponectin receptor 1 in periodontal ligament and gingival fibroblasts. Expression of adiponectin (Ad) in adipose tissue, periodontal ligament (PDL) fibroblasts, gingival fibroblasts (GF), and THP-1 macrophages was estimated by western blot (A). Expression of adiponectin receptor 1 (AdipoR1) in PDL fibroblasts, GF, MDA-MB231 breast cancer cell line (MDA), and THP-1 macrophages was estimated by reverse transcription-polymerase chain reaction (B) and western blot (C).
Effect of gAd on expression of IL-6 and IL-8 in PDL cells and gingival fibroblasts
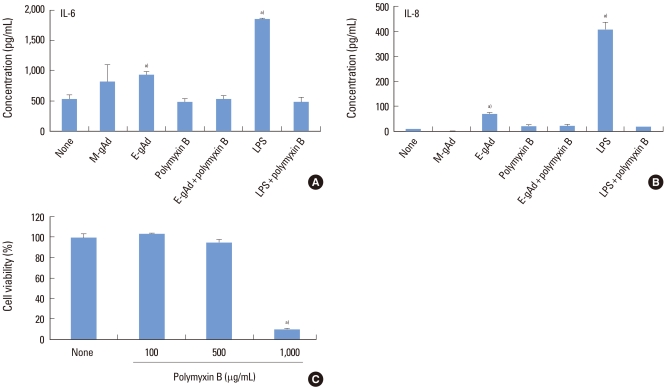
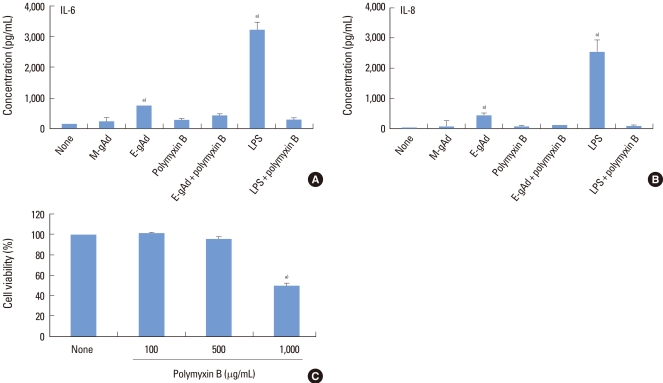
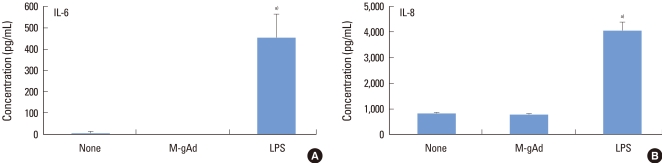
The effect of gAd on the expression of IL-6 and IL-8 was estimated in PDL and gingival fibroblasts by ELISA. Treatment with E. coli-derived gAd increased the expression of IL-6 and IL-8 in PDL fibroblasts, but treatment with myeloma cell line-derived gAd did not increase the expression of IL-6 and IL-8 (Fig. 2A, B). Polymyxin B is an inhibitor of LPS. Polymyxin B blocked IL-6 and IL-8 expression induced by E. coli-derived gAd at a concentration of 100 µg/mL which polymyxin B inhibited LPS activity (Fig. 2A, B). Treatment of gingival fibroblasts with E. coli- or myeloma cell-derived gAd showed results similar to those of PDL fibroblasts (Fig. 3A, B). Polymyxin B did not have a cytotoxic effect on PDL and gingival fibroblasts at concentrations less than or equal to 500 µg/mL (Figs. 2C, 3C). LPS increased IL-6 and IL-8 expression in THP-1 macrophages, but myeloma cell-derived gAd did not stimulate expression of those cytokines (Fig. 4).
Figure 2.
Effect of globular adiponectin on the expression of interleukin (IL)-6 and IL-8 in periodontal ligament fibroblasts. Periodontal ligament fibroblasts were treated with murine myeloma cell-derived globular adiponectin (M-gAd, 15 µg/mL), Escherichia coli-derived gAd (E-gAd, 15 µg/mL), or lipopolysaccharide (LPS) (100 ng/mL) in the presence or absence of polymyxin B (100 µg/mL) for 24 hours. Levels of IL-6 (A) and IL-8 (B) in culture supernatants were assayed by enzyme-linked immunosorbent assay. Cell viability treated with polymyxin B was estimated by an 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium assay (C). a)Significantly different from untreated cells (P<0.05).
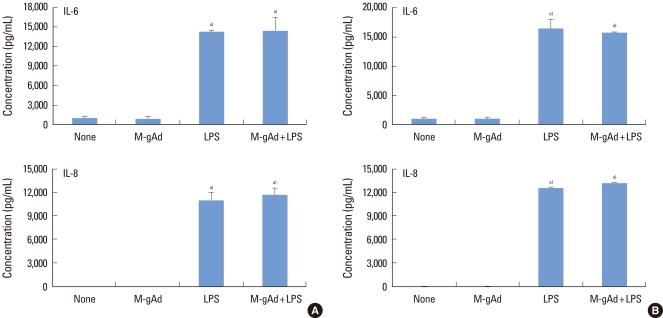
Figure 3.
Effect of globular adiponectin on the expression of interleukin (IL)-6 and IL-8 in gingival fibroblasts. Gingival fibroblasts were treated with murine myeloma cell-derived globular adiponectin (M-gAd, 15 µg/mL), Escherichia coli-derived gAd (E-gAd, 15 µg/mL), or lipopolysaccharide (LPS) (100 ng/mL) in the presence or absence of polymyxin B (100 µg/mL) for 24 hours. Levels of IL-6 (A) and IL-8 (B) in culture supernatants were assayed by enzyme-linked immunosorbent assay. The effect of gAd on the expression of cytokines was compared with LPS. Cell viability treated with polymyxin B was estimated by an 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium assay (C). a)Significantly different from untreated cells (P<0.05).
Figure 4.
Effect of globular adiponectin on the production of interleukin (IL)-6 and IL-8 in THP-1 macrophages. THP-1 macrophages were cultured for 24 hours in the presence of murine myeloma cell-derived globular adiponectin (M-gAd) or lipopolysaccharide (LPS). The levels of IL-6 (A) and IL-8 (B) in culture supernatants were assayed by enzyme-linked immunosorbent. a)Significantly different from untreated cells (P<0.05).
Effect of gAd on LPS-induced IL-6 and IL-8 expression
To estimate the induction of tolerance to LPS by gAd, PDL and gingival fibroblasts were pretreated with myeloma cell-derived gAd before exposure to LPS. LPS increased the expression of IL-6 and IL-8 in PDL fibroblasts, but pretreatment of cells with gAd did not decrease or increase the expression of IL-6 and IL-8 induced by LPS (Fig. 5A). Gingival fibroblasts showed results similar to those of PDL fibroblasts (Fig. 5B).
Figure 5.
Effect of globular adiponectin on the production of interleukin (IL)-6 and IL-8 induced by lipopolysaccharide (LPS) in periodontal ligament and gingival fibroblasts. Periodontal ligament fibroblasts (A) and gingival fibroblasts (B) were cultured for 24 hours in the presence of murine myeloma cell-derived globular adiponectin (M-gAd, 15 µg/mL) and then stimulated for 24 hours with LPS (100 ng/mL). The levels of IL-6 and IL-8 in the culture supernatant were assayed by enzyme-linked immunosorbent. a)Significantly different from untreated cells (P<0.05).
DISCUSSION
The alteration in serum levels of adiponectin according to periodontal status and the effect of adiponectin on periodontopathogen-induced cytokine expression by macrophages and osteoclast formation have been estimated [23-25], but few studies have addressed the effect of adiponectin on the cells of periodontal tissue such as PDL and gingival fibroblasts. In this study, it was shown that PDL and gingival fibroblasts express AdipoR1 and that gAd does not affect the production of IL-6 and IL-8 in those cells.
Although the main source of adiponectin is adipocytes, synovial and cardiac fibroblasts also express adiponectin [4,8,31]. In this study, we also found expression of adiponectin in adipocytes, but PDL and gingival fibroblasts did not express adiponectin like synovial and cardiac fibroblasts. AdipoR1 is most highly expressed in skeletal muscle [4], but its expression has also been detected in synovial and cardiac fibroblasts [8,12,13]. As a result, we looked for the expression of AdipoR1 in PDL and gingival fibroblasts. Similar to synovial and cardiac fibroblasts, PDL and gingival fibroblasts expressed not only the mRNA but also the protein of AdipoR1.
IL-6 induces the differentiation of B cells to antibody-secreting plasma cells and stimulates bone resorption [32,33]. IL-8 stimulates the attraction and activation of neutrophils and bone resorption [32]. The IL-6 and IL-8 levels of periodontitis patients are higher than those of healthy patients [32]. That evidence suggests that IL-6 and IL-8 play an important role in the pathogenesis of periodontitis. In this study, PDL and gingival fibroblasts expressed AdipoR1, which is a receptor for gAd [10]. The serum level of adiponectin was found to be 12.4±5.1 µg/mL in humans with healthy gingival [34]. As a result, we investigated the effect of gAd on IL-6 and IL-8 expression of those cells at a concentration of 15 µg/mL. Sources of commercially available recombinant gAd are E. coli and murine myeloma cells. Therefore, we investigated the effect of E. coli- and murine myeloma cell-derived gAd on the expression of those cytokines. E. coli-derived gAd increased IL-6 and IL-8 production in PDL and gingival fibroblasts. However, polymyxin B, an inhibitor of LPS, blocked not only the production of IL-6 and IL-8 induced by LPS, but also the production of those cytokines induced by E. coli-derived gAd. Analysis of E. coli-derived gAd by a limulus amebocyte lysate assay revealed contamination with LPS (data not shown). That suggests that the effect of E. coli-derived gAd is due to contaminated LPS. Murine myeloma cell-derived gAd contained lower levels of LPS than E. coli-derived gAd (data not shown). To confirm the effect of gAd, the effect of murine myeloma cell-derived gAd was estimated. Murine myeloma cell-derived gAd did not show an increase of IL-6 and IL-8 production in PDL or gingival fibroblasts. Those results indicate that gAd does not affect IL-6 and IL-8 production in PDL and gingival fibroblasts.
gAd induced IL-6 and IL-8 secretion by THP-1 macrophages [14,15]. However, in this study, gAd derived from murine myeloma cells did not stimulate those cytokines in the same cell lines. Similar to our results, the outcome of one study showed that fAd-induced IL-6 production was blocked by polymyxin B in macrophages, suggesting that fAd does not induce IL-6 induction and that evaluation of certain effects of adiponectin may be entangled by LPS contamination [19]. Our results also support the proposal that LPS contamination be considered when estimating the function of adiponectin in inflammation.
It has been reported that pretreatment of macrophages with adiponectin induces tolerance to LPS, suggesting that adiponectin has anti-inflammatory properties [14]. However, in another study, the tolerance effect of adiponectin on LPS-induced cytokine expression was not found [18]. Our results showed that pretreatment of PDL and gingival fibroblasts with murine myeloma cell-derived gAd did not decrease LPS-induced IL-6 and IL-8 expression, suggesting that gAd does not induce tolerance to LPS in PDL and gingival fibroblasts.
Previous reports have shown that adiponectin stimulates the production of IL-6 and IL-8 by rheumatoid synovial fibroblasts, indicating that the pro-inflammatory effects of adiponectin might play a role in the pathogenesis of rheumatoid arthritis [9,12,13]. In this study, however, we could not find the pro-inflammatory effect of adiponectin in PDL and gingival fibroblasts in periodontal tissue. Recently, Yamaguchi et al. [35] reported expression of AdipoR1 in gingival fibroblasts, but they did not estimate either the expression of AdipoR1 in PDL cells or the effect of adiponectin on the expression of cytokines in those cells. This study is meaningful in its demonstration of the expression of AdipoR1 in both PDL and gingival fibroblasts, its estimation of the effect of gAd on IL-6 and IL-8 expression, and its suggestion of the significance of LPS contamination in estimating the function of gAd.
In conclusion, our results showed that PDL and gingival fibroblasts expressed AdipoR1, and gAd did not affect the expression of IL-6 and IL-8 in those cells. Although gAd did not have a modulating effect on IL-6 and IL-8 expression in those cells, expression of AdipoR1 indicates that gAd may have other effects on PDL and gingival fibroblasts, which will be studied in the future.
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (KRF-2008-313-E00584).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Ekuni D, Yamamoto T, Koyama R, Tsuneishi M, Naito K, Tobe K. Relationship between body mass index and periodontitis in young Japanese adults. J Periodontal Res. 2008;43:417–421. doi: 10.1111/j.1600-0765.2007.01063.x. [DOI] [PubMed] [Google Scholar]
- 2.Han DH, Lim SY, Sun BC, Paek DM, Kim HD. Visceral fat area-defined obesity and periodontitis among Koreans. J Clin Periodontol. 2010;37:172–179. doi: 10.1111/j.1600-051X.2009.01515.x. [DOI] [PubMed] [Google Scholar]
- 3.Mealey BL, Rose LF. Diabetes mellitus and inflammatory periodontal diseases. Curr Opin Endocrinol Diabetes Obes. 2008;15:135–141. doi: 10.1097/MED.0b013e3282f824b7. [DOI] [PubMed] [Google Scholar]
- 4.Sun Y, Xun K, Wang C, Zhao H, Bi H, Chen X, et al. Adiponectin, an unlocking adipocytokine. Cardiovasc Ther. 2009;27:59–75. doi: 10.1111/j.1755-5922.2008.00069.x. [DOI] [PubMed] [Google Scholar]
- 5.Pittas AG, Joseph NA, Greenberg AS. Adipocytokines and insulin resistance. J Clin Endocrinol Metab. 2004;89:447–452. doi: 10.1210/jc.2003-031005. [DOI] [PubMed] [Google Scholar]
- 6.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 7.Lago F, Dieguez C, Gómez-Reino J, Gualillo O. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol. 2007;3:716–724. doi: 10.1038/ncprheum0674. [DOI] [PubMed] [Google Scholar]
- 8.Huang D, Yang C, Wang Y, Liao Y, Huang K. PARP-1 suppresses adiponectin expression through poly(ADP-ribosyl) ation of PPAR gamma in cardiac fibroblasts. Cardiovasc Res. 2009;81:98–107. doi: 10.1093/cvr/cvn264. [DOI] [PubMed] [Google Scholar]
- 9.Tan W, Wang F, Zhang M, Guo D, Zhang Q, He S. High adiponectin and adiponectin receptor 1 expression in synovial fluids and synovial tissues of patients with rheumatoid arthritis. Semin Arthritis Rheum. 2009;38:420–427. doi: 10.1016/j.semarthrit.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 11.Rovin BH, Song H. Chemokine induction by the adipocyte-derived cytokine adiponectin. Clin Immunol. 2006;120:99–105. doi: 10.1016/j.clim.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Tang CH, Chiu YC, Tan TW, Yang RS, Fu WM. Adiponectin enhances IL-6 production in human synovial fibroblast via an AdipoR1 receptor, AMPK, p38, and NF-kappa B pathway. J Immunol. 2007;179:5483–5492. doi: 10.4049/jimmunol.179.8.5483. [DOI] [PubMed] [Google Scholar]
- 13.Kitahara K, Kusunoki N, Kakiuchi T, Suguro T, Kawai S. Adiponectin stimulates IL-8 production by rheumatoid synovial fibroblasts. Biochem Biophys Res Commun. 2009;378:218–223. doi: 10.1016/j.bbrc.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Tsatsanis C, Zacharioudaki V, Androulidaki A, Dermitzaki E, Charalampopoulos I, Minas V, et al. Adiponectin induces TNF-alpha and IL-6 in macrophages and promotes tolerance to itself and other pro-inflammatory stimuli. Biochem Biophys Res Commun. 2005;335:1254–1263. doi: 10.1016/j.bbrc.2005.07.197. [DOI] [PubMed] [Google Scholar]
- 15.Tsatsanis C, Zacharioudaki V, Androulidaki A, Dermitzaki E, Charalampopoulos I, Minas V, et al. Peripheral factors in the metabolic syndrome: the pivotal role of adiponectin. Ann N Y Acad Sci. 2006;1083:185–195. doi: 10.1196/annals.1367.013. [DOI] [PubMed] [Google Scholar]
- 16.Liao W, Yu C, Wen J, Jia W, Li G, Ke Y, et al. Adiponectin induces interleukin-6 production and activates STAT3 in adult mouse cardiac fibroblasts. Biol Cell. 2009;101:263–272. doi: 10.1042/BC20080117. [DOI] [PubMed] [Google Scholar]
- 17.Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723–1732. [PubMed] [Google Scholar]
- 18.Neumeier M, Weigert J, Schäffler A, Wehrwein G, Müller-Ladner U, Schölmerich J, et al. Different effects of adiponectin isoforms in human monocytic cells. J Leukoc Biol. 2006;79:803–808. doi: 10.1189/jlb.0905521. [DOI] [PubMed] [Google Scholar]
- 19.Turner JJ, Smolinska MJ, Sacre SM, Foxwell BM. Induction of TLR tolerance in human macrophages by adiponectin: does LPS play a role? Scand J Immunol. 2009;69:329–336. doi: 10.1111/j.1365-3083.2008.02224.x. [DOI] [PubMed] [Google Scholar]
- 20.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 21.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 22.Furugen R, Hayashida H, Yamaguchi N, Yoshihara A, Ogawa H, Miyazaki H, et al. The relationship between periodontal condition and serum levels of resistin and adiponectin in elderly Japanese. J Periodontal Res. 2008;43:556–562. doi: 10.1111/j.1600-0765.2008.01085.x. [DOI] [PubMed] [Google Scholar]
- 23.Nishihara R, Sugano N, Takano M, Shimada T, Tanaka H, Oka S, et al. The effect of Porphyromonas gingivalis infection on cytokine levels in type 2 diabetic mice. J Periodontal Res. 2009;44:305–310. doi: 10.1111/j.1600-0765.2008.01130.x. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto S, Ogawa H, Soda S, Hirayama S, Amarasena N, Aizawa Y, et al. Effect of antimicrobial periodontal treatment and maintenance on serum adiponectin in type 2 diabetes mellitus. J Clin Periodontol. 2009;36:142–148. doi: 10.1111/j.1600-051X.2008.01359.x. [DOI] [PubMed] [Google Scholar]
- 25.Kamio N, Akifusa S, Yamaguchi N, Nonaka K, Yamashita Y. Anti-inflammatory activity of a globular adiponectin function on RAW 264 cells stimulated by lipopolysaccharide from Aggregatibacter actinomycetemcomitans. FEMS Immunol Med Microbiol. 2009;56:241–247. doi: 10.1111/j.1574-695X.2009.00573.x. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi N, Kukita T, Li YJ, Martinez Argueta JG, Saito T, Hanazawa S, et al. Adiponectin inhibits osteoclast formation stimulated by lipopolysaccharide from Actinobacillus actinomycetemcomitans. FEMS Immunol Med Microbiol. 2007;49:28–34. doi: 10.1111/j.1574-695X.2006.00164.x. [DOI] [PubMed] [Google Scholar]
- 27.Scheres N, Laine ML, de Vries TJ, Everts V, van Winkelhoff AJ. Gingival and periodontal ligament fibroblasts differ in their inflammatory response to viable Porphyromonas gingivalis. J Periodontal Res. 2010;45:262–270. doi: 10.1111/j.1600-0765.2009.01229.x. [DOI] [PubMed] [Google Scholar]
- 28.Lee YS, Bak EJ, Kim M, Park W, Seo JT, Yoo YJ. Induction of IL-8 in periodontal ligament cells by H(2)O (2) J Microbiol. 2008;46:579–584. doi: 10.1007/s12275-008-0182-3. [DOI] [PubMed] [Google Scholar]
- 29.Chinetti G, Zawadski C, Fruchart JC, Staels B. Expression of adiponectin receptors in human macrophages and regulation by agonists of the nuclear receptors PPARalpha, PPARgamma, and LXR. Biochem Biophys Res Commun. 2004;314:151–158. doi: 10.1016/j.bbrc.2003.12.058. [DOI] [PubMed] [Google Scholar]
- 30.Dos Santos E, Benaitreau D, Dieudonne MN, Leneveu MC, Serazin V, Giudicelli Y, et al. Adiponectin mediates an antiproliferative response in human MDA-MB 231 breast cancer cells. Oncol Rep. 2008;20:971–977. [PubMed] [Google Scholar]
- 31.Ehling A, Schäffler A, Herfarth H, Tarner IH, Anders S, Distler O, et al. The potential of adiponectin in driving arthritis. J Immunol. 2006;176:4468–4478. doi: 10.4049/jimmunol.176.7.4468. [DOI] [PubMed] [Google Scholar]
- 32.Okada H, Murakami S. Cytokine expression in periodontal health and disease. Crit Rev Oral Biol Med. 1998;9:248–266. doi: 10.1177/10454411980090030101. [DOI] [PubMed] [Google Scholar]
- 33.Preshaw PM, Taylor JJ. How has research into cytokine interactions and their role in driving immune responses impacted our understanding of periodontitis? J Clin Periodontol. 2011;38(Suppl 11):60–84. doi: 10.1111/j.1600-051X.2010.01671.x. [DOI] [PubMed] [Google Scholar]
- 34.Saito T, Yamaguchi N, Shimazaki Y, Hayashida H, Yonemoto K, Doi Y, et al. Serum levels of resistin and adiponectin in women with periodontitis: the Hisayama study. J Dent Res. 2008;87:319–322. doi: 10.1177/154405910808700416. [DOI] [PubMed] [Google Scholar]
- 35.Yamaguchi N, Hamachi T, Kamio N, Akifusa S, Masuda K, Nakamura Y, et al. Expression levels of adiponectin receptors and periodontitis. J Periodontal Res. 2010;45:296–300. doi: 10.1111/j.1600-0765.2009.01222.x. [DOI] [PubMed] [Google Scholar]