The cerebral vasculature rapidly adapts to changes in perfusion pressure (cerebral autoregulation), regional metabolic requirements of the brain (neurovascular coupling), autonomic neural activity, and humoral factors (cerebrovascular reactivity). Regulation of cerebral blood flow (CBF) is therefore highly controlled and involves a wide spectrum of regulatory mechanisms that together work to maintain optimum oxygen and nutrient supply. It is well-established that the cerebral vasculature is highly sensitive to changes in arterial blood gases, in particular the partial pressure of arterial carbon dioxide (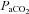 ). The teleological relevance of this unique feature of the brain is likely to lie in the need to tightly control brain pH and its related impact on ventilatory control at the level of the central chemoreceptors. Changes in arterial blood gases, in particular those that cause hypoxaemia and hypercapnia, also lead to widespread effects on the systemic vasculature often leading to sympathoexcitation and related blood pressure (BP) elevations via vasoconstriction (Ainslie et al. 2005).
). The teleological relevance of this unique feature of the brain is likely to lie in the need to tightly control brain pH and its related impact on ventilatory control at the level of the central chemoreceptors. Changes in arterial blood gases, in particular those that cause hypoxaemia and hypercapnia, also lead to widespread effects on the systemic vasculature often leading to sympathoexcitation and related blood pressure (BP) elevations via vasoconstriction (Ainslie et al. 2005).
In this issue of The Journal of Physiology an elegant study by Battisti-Charbonney and co-workers provide a relevant example of integrative human physiology (Battisti-Charbonney et al. 2011). Using continuous bilateral measurements of blood flow velocity in the middle cerebral arteries (as a surrogate index of CBF) and BP, the authors gauged the CBF responses to CO2 changes under the background condition of either hyperoxia or hypoxia. The key findings indicate that the relationship between CBF velocity over a wide range of end-tidal 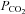 (
(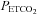 ) values during hypocapnia (
) values during hypocapnia (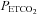 : ∼25 mmHg) and hyperoxic or hypoxic rebreathing (
: ∼25 mmHg) and hyperoxic or hypoxic rebreathing (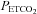 : 55–60 mmHg and 45–50 mmHg, respectively) are optimally fitted using a sigmoid (logistic) curve rather than a linear curve. Above the upper limits of CO2 reactivity (i.e. near the threshold (∼55 to 60 mmHg) where CBF velocity has plateaued despite further elevations in
: 55–60 mmHg and 45–50 mmHg, respectively) are optimally fitted using a sigmoid (logistic) curve rather than a linear curve. Above the upper limits of CO2 reactivity (i.e. near the threshold (∼55 to 60 mmHg) where CBF velocity has plateaued despite further elevations in 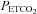 ) linear elevations in BP then progressed, presumably via chemoreflex-induced elevations in sympathetic nerve activity (SNA). Notably, the authors are the first to integrate this logistic and linear fitting approach to document the influence of
) linear elevations in BP then progressed, presumably via chemoreflex-induced elevations in sympathetic nerve activity (SNA). Notably, the authors are the first to integrate this logistic and linear fitting approach to document the influence of 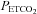 and related changes in mean arterial pressure (MAP) on CBF. Collectively, these experiments demonstrate that rebreathing tests – when analysed as described – may provide an estimate of the cerebrovascular response to CO2 (and O2) at a constant BP, as well as an estimate of the cerebrovascular passive response to both BP and CO2.
and related changes in mean arterial pressure (MAP) on CBF. Collectively, these experiments demonstrate that rebreathing tests – when analysed as described – may provide an estimate of the cerebrovascular response to CO2 (and O2) at a constant BP, as well as an estimate of the cerebrovascular passive response to both BP and CO2.
In the broader context of integrative physiology, these findings are noteworthy on many levels. For example, impairment in cerebrovascular reactivity to CO2 and failure to effectively counter-regulate (or autoregulate) against systemic BP fluctuations could lead to a predisposition to adverse cerebrovascular events such as stroke, infarct extension and haemorrhagic transformation of existing strokes (Aries et al. 2010). However, the critical physiological and methodological consideration is that traditional tests to assess cerebrovascular reactivity to CO2 or cerebrovascular autoregulation treat these factors as separate identities. Clearly they are not: elevations in 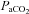 will lead to sympathoexcitation and increases in BP via vasoconstriction (Ainslie et al. 2005). The latter, as exampled by Battisti-Charbonney and co-workers, will have independent effects on CBF from those of
will lead to sympathoexcitation and increases in BP via vasoconstriction (Ainslie et al. 2005). The latter, as exampled by Battisti-Charbonney and co-workers, will have independent effects on CBF from those of 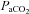 (Lucas et al. 2010). Conversely, emerging evidence indicates that acute changes in BP may then impact on alveolar ventilation and thus
(Lucas et al. 2010). Conversely, emerging evidence indicates that acute changes in BP may then impact on alveolar ventilation and thus 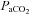 , in part via the aptly named ‘ventilatory baroreflex’ (Stewart et al. 2011). Moreover, because the brain is relatively pressure-passive (Lucas et al. 2010) and since elevations in
, in part via the aptly named ‘ventilatory baroreflex’ (Stewart et al. 2011). Moreover, because the brain is relatively pressure-passive (Lucas et al. 2010) and since elevations in 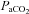 also ‘impair’ the brain's capability to defend against BP changes (Panerai et al. 1999), considerations of BP as a critical determinant of CBF is warranted in these conditions. An example of these integrated changes in
also ‘impair’ the brain's capability to defend against BP changes (Panerai et al. 1999), considerations of BP as a critical determinant of CBF is warranted in these conditions. An example of these integrated changes in 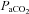 and BP occur in a myriad everyday activities: postural change, coughing, laughing, defecation, exercise, sexual activity, to name but a few. The merit of the newly proposed method as a useful clinical tool to explore the separate and combined quantification of the cerebrovascular reactivity to CO2 and BP needs to be established. However, consideration of the combined influence of both
and BP occur in a myriad everyday activities: postural change, coughing, laughing, defecation, exercise, sexual activity, to name but a few. The merit of the newly proposed method as a useful clinical tool to explore the separate and combined quantification of the cerebrovascular reactivity to CO2 and BP needs to be established. However, consideration of the combined influence of both 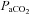 and BP on the brain would seem meritorious from a systems physiology viewpoint.
and BP on the brain would seem meritorious from a systems physiology viewpoint.
In summary, in view of the article by Battisti-Charbonney et al., we have attempted to highlight some of the common factors that independently, synergistically and often antagonistically participate in the regulation of CBF. Research exploring these complex interactions is currently lacking. Future studies with particular focus on these integrative physiological mechanisms are clearly warranted in both health and disease states.
References
- Ainslie PN, Ashmead JC, Ide K, Morgan BJ, Poulin MJ. J Physiol. 2005;566:613–624. doi: 10.1113/jphysiol.2005.087320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aries MJ, Elting JW, De Keyser J, Kremer BP, Vroomen PC. Stroke. 2010;41:2697–2704. doi: 10.1161/STROKEAHA.110.594168. [DOI] [PubMed] [Google Scholar]
- Battisti-Charbonney A, Fisher J, Duffin J. J Physiol. 2011;589:3039–3048. doi: 10.1113/jphysiol.2011.206052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas SJ, Tzeng YC, Galvin SD, Thomas KN, Ogoh S, Ainslie PN. Hypertension. 2010;55:698–705. doi: 10.1161/HYPERTENSIONAHA.109.146290. [DOI] [PubMed] [Google Scholar]
- Panerai RB, Deverson ST, Mahony P, Hayes P, Evans DH. Physiol Meas. 1999;20:265–275. doi: 10.1088/0967-3334/20/3/304. [DOI] [PubMed] [Google Scholar]
- Stewart JM, Rivera E, Clarke DA, Baugham IL, Ocon AJ, Taneja I, Terilli C, Medow MS. Am J Physiol Heart Circ Physiol. 2011;300:H1492–H1500. doi: 10.1152/ajpheart.01217.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


