Non-technical summary
Motor cortical output is suppressed by two cortical inhibitory systems, short interval intracortical inhibition (SICI) and long interval intracortical inhibition (LICI). SICI is decreased in the presence of LICI. However, there is a long-standing argument whether this is caused by a true interaction between them or is due to simple saturation of the inhibitory effects that occur at common cortical elements which both inhibitory systems target. We addressed this question by recording the descending corticospinal waves in the subjects with implanted epidural electrodes. The results suggest that there are inhibitory interactions between LICI and SICI.
Abstract
Abstract
A subthreshold conditioning stimulation (CS) suppresses the motor-evoked potential (MEP) generated by a test stimulation (TS) at interstimulus intervals (ISIs) of 1–5 ms in a paired-pulse transcranial magnetic stimulation (TMS) protocol, a phenomenon termed short interval intracortical inhibition (SICI). Intracortical facilitation (ICF) occurs at ISIs of 7–30 ms. Long interval intracortical inhibition (LICI) is elicited with suprathreshold CS preceding the TS at ISIs of 50–200 ms. Previous studies showed that SICI is decreased in the presence of LICI but whether this is due to changes in descending indirect waves (I-waves) induced by LICI or true inhibitory interactions between LICI and SICI has not been resolved. To address this issue, we recorded I-waves in two patients with implanted cervical epidural electrodes and investigated how SICI and ICF changed I-waves in the presence of LICI. SICI alone reduced late I-waves but in the presence of LICI, neither the I-waves nor the MEP were further inhibited by SICI. ICF alone increased MEP amplitude but the I-waves were not facilitated. There was no change of ICF in the presence of LICI compared with ICF alone. We conclude that decreased SICI in the presence of LICI is not due to changes in I-wave content induced by LICI and is caused by their interactions at the cortical level.
Introduction
The excitability of corticospinal neurons is modulated by intracortical inhibitory and excitatory circuits in the human primary motor cortex (M1). The balance and interactions between these circuits determine the final output from M1 (Hallett, 2007; Chen et al. 2008). Studies with transcranial magnetic stimulation (TMS) showed that a subthreshold conditioning stimulation (CS) suppresses subsequent suprathreshold test stimulation (TS) at interstimulus intervals (ISIs) of 1–5 ms, a phenomenon termed short interval intracortical inhibition (SICI). Facilitation occurs with a similar protocol but at ISIs of 7–30 ms and is termed intracortical facilitation (ICF) (Kujirai et al. 1993). Pharmacological studies suggested that SICI is mediated by GABAA receptors (Ziemann et al. 1996a). Glutamate is involved in ICF (Ziemann et al. 1998; Schwenkreis et al. 1999) but subcortical or spinal activities may also be involved (Di Lazzaro et al. 2006). On the other hand, CS at suprathreshold intensity inhibits the TS at ISIs of 50–200 ms and this is termed long interval intracortical inhibition (LICI). Studies with recordings of corticospinal waves have confirmed that LICI at ISIs longer than 50 ms is due to the cortical inhibitory connections (Nakamura et al. 1997; Chen et al. 1999; Di Lazzaro et al. 2002). LICI is mediated by GABAB receptors (Werhahn et al. 1999; McDonnell et al. 2006).
Previous studies using a triple-pulse TMS protocol demonstrated that SICI was suppressed in the presence of LICI, both with the target muscle at rest and during voluntary contraction (Sanger et al. 2001; Ni et al. 2007; Cash et al. 2010). TMS of the M1 with a single pulse elicits a series of periodic, high-frequency descending corticospinal waves. These waves are caused by synaptic activities of interneurons in M1, which project to the corticospinal neurons and are termed indirect (I) waves (Di Lazzaro et al. 2004). The multiple I-waves are classified as early (e.g. I1) or late (e.g. I3). Both LICI and SICI inhibit the late I-waves but not early I-waves (Nakamura et al. 1997; Di Lazzaro et al. 1998b, 2002). This leads to the argument that the inhibitory interaction between LICI and SICI may simply be due to saturation of the inhibitory effects on late I-waves caused by the first inhibitory system (LICI) such that the second inhibitory system (SICI) is not able to inhibit the late I-waves further (Ni et al. 2011b). This issue cannot be resolved with conventional motor-evoked potential (MEP) recording. To address this important issue, we recorded I-waves in a triple-pulse TMS protocol in two patients with implanted epidural electrodes. We hypothesize that: (1) it is possible to compensate for reduction in I-waves caused by LICI by increasing the stimulus intensity to generate late I-waves with amplitudes comparable to single-pulse TMS; (2) In the presence of LICI and late I-waves, SICI would fail to inhibit the late I-waves. If these hypotheses are correct, the results will support the notion that the reduction of SICI in the presence of LICI is due to the interaction between these two circuits at the cortical level.
Methods
Subjects
Two subjects with implantation of cervical epidural electrodes were studied. Subject 1 was a 41-year-old man who suffered a left hand crush injury 5 years earlier with subsequent surgical repairs and resultant left upper extremity pain syndrome that failed best medical management. He underwent a C7 laminotomy and was implanted with a spinal cord electrode in the epidural space. Subject 2 was a 30-year-old man who had a right elbow fracture 4 years earlier. He subsequently developed deafferentation pain in the right ulnar nerve distribution. He failed medical therapy and had C7–T1 laminotomy and placement of an epidural spinal cord electrode. Both subjects had no central nervous system diseases and had normal neurological examination other than in the affected arm. Both subjects provided written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the University Health Network (Toronto) Research Ethics Board.
Recording
Experiments were performed 2 days after implantation of epidural electrodes during the trial screening period, when the electrode connections were externalized. Recordings were made simultaneously from the epidural electrode and from the first dorsal interosseous (FDI) muscle. Since the epidural electrodes were implanted slightly away from the midline toward the affected side to achieve the desired clinical effect, recordings were made from the affected arm in both subjects (left for Subject 1 and right for Subject 2) to obtain more reliable epidural potentials with larger amplitudes. Epidural potentials were recorded between the most proximal (contact 3) and most distal (contact 0) of the four electrode contacts. These contacts were 4 mm in diameter and were 10 mm apart (Resume Lead Model 3587A; Medtronic, Minneapolis, MN, USA). The distal contact was connected to the reference input of the amplifier. Surface electromyograms (EMGs) were obtained via two 9-mm-diameter Ag–AgCl electrodes, with the active electrode over the muscle belly and the reference electrode placed on the metacarpophalangeal joint of the index finger. The signal was amplified (1 k for EMG and 10 k for epidural potential), band-pass filtered (2 Hz–2.5 kHz, Intronix Technologies Corporation Model 2024F, Bolton, Ontario, Canada), digitized at 5 kHz by an analog-to-digital interface (Micro1401, Cambridge Electronics Design, Cambridge, UK) and stored on a computer for off-line analysis. In addition, subjects were provided with audio-visual feedback of background EMG activity to maintain constant relaxation of the FDI muscle during the experiment.
Transcranial magnetic stimulation
TMS was delivered to the contralateral M1 (right M1 for Subject 1, left M1 for Subject 2) using a triple-pulse protocol. Four Magstim 200 stimulators (Magstim, Whitland, Dyfed, UK) were connected via three Bistim modules in a ‘pyramid’ set-up (Sanger et al. 2001). The output of each of the two pairs of stimulators was connected to one Bistim module. The outputs from the two Bistim modules were directed to a third Bistim module which in turn was connected to a 7 cm figure-of-eight coil. This set-up allowed us to deliver up to four pulses with different stimulus intensities through the same coil at very short ISIs. The coil was placed at the optimal position for eliciting MEP from the FDI muscle where slightly suprathreshold stimulation produces the largest MEP in the target muscle. The handle of the coil pointed backwards and rotated about 45 deg to the mid-sagittal line. The induced current in the brain was perpendicular to the central sulcus in a posterior–anterior direction and was optimal to activate the corticospinal neurons trans-synaptically (Werhahn et al. 1994; Kaneko et al. 1996).
Experimental set-up
Each trial consisted of up to three TMS pulses. We named the pulses CS100, CS2 or CS10 and TS (Fig. 1). A TS was delivered in each trial. The TS used the intensity of ‘1 mV’ which was defined as the minimum stimulator output to produce MEPs of more than 1 mV in amplitude in at least 5 out of 10 trials when the FDI muscle was completely relaxed. CS100 was delivered 100 ms preceding TS and was used to elicit LICI. The intensity of CS100 was also set at ‘1 mV’. CS2 and CS10 were used to elicit SICI and ICF. They were delivered 2 ms and 10 ms before TS. The intensity of CS2 and CS10 was set at 95% of active motor threshold. Active motor threshold was determined with the FDI muscle contracting at 20% maximum and was defined as the minimum stimulator output that produced MEPs of >200 μV in at least 5 out of 10 trials. In some experimental conditions, we adjusted the TS intensity so that the TS was able to generate MEPs of ‘1 mV’ in the presence of preceding CS100. The adjusted TS was named TS’. The experimental configuration included 10 conditions: TS (TS alone), CS2–TS (SICI alone), CS10–TS (ICF alone), CS100–TS (LICI alone), TS’ (TS alone with adjusted intensity), CS2–TS’ (SICI alone with adjusted TS intensity), CS10–TS’ (ICF alone with adjusted TS intensity), CS100–TS’ (LICI alone with adjusted TS intensity), CS100–CS2–TS’ (SICI in the presence of LICI) and CS100–CS10–TS’ (ICF in the presence of LICI). Each experimental condition was repeated 20 times in a random order (200 trials in total).
Figure 1. Example of descending waves and MEPs recorded in different experimental conditions in Subject 1.
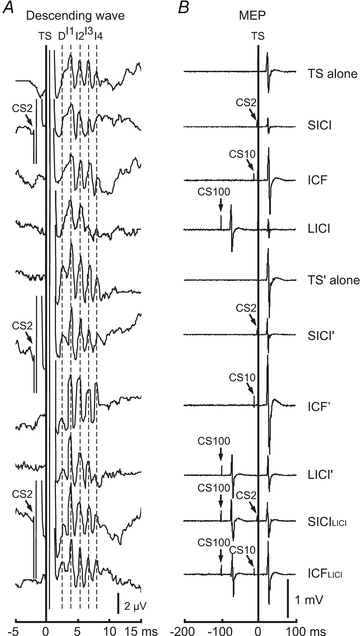
Up to three stimuli were employed in different experimental conditions. TS (test stimulus, marked with vertical line) was delivered in each trial. The time for delivery of the TS was defined as time 0. CS (conditioning stimuli) are named CS2, CS10 and CS100 according to the interstimulus interval between CS and TS. They are marked with arrows. The left panel (A) shows descending wave recordings from −5 ms to 15 ms and the right panel (B) shows MEP recordings from −200 ms to 100 ms. To simplify the figure CS10 and CS100 are not marked in descending wave recordings. Dashed lines indicate the peaks of direct (D-wave) and indirect waves (I1–I4 waves). TS alone generated MEP of ∼1 mV and produces I1–I4 waves. SICI (short interval intracortical inhibition) represents trial in which TS was conditioned by a subthreshold CS2. ICF (intracortical facilitation) represents trial in which TS was conditioned by a subthreshold CS10. LICI (long interval intracortical inhibition) represents trial in which TS was conditioned by a suprathreshold CS100. Note that late I-waves (I3, I4) were suppressed but the I1-wave was not affected by SICI and LICI. ICF facilitated MEP but had little effect on I-waves. TS’ alone with higher intensity produced larger MEP and descending waves. A D-wave was also generated by TS’. SICI’, ICF’ and LICI’ represent trials of SICI, ICF and LICI with TS’. In addition to the I3- and I4-waves, the I2-wave was also inhibited by SICI’ and LICI’. MEP amplitudes for LICI’ and TS alone were matched to ∼1 mV. SICILICI and ICFLICI represent trials of SICI and ICF conditioned by LICI. Note that none of the descending wave was further inhibited in SICILICI compared to LICI’.
Data and statistical analysis
Both the latencies and amplitudes of descending waves and MEPs were measured. The I-waves were identified by comparing their latencies with the direct wave (D-wave) latency. The D-wave was elicited by stimulating M1 with the handle of the TMS coil pointing laterally. The induced current in the M1 was lateral–medially directed (Di Lazzaro et al. 2001). The stimulus intensity to generate 1 mV MEP in the lateral–medial direction was higher than that in the posterior–anterior direction. I-wave latencies were measured from the TMS delivery to the peak. The amplitude was measured from the peak to the next trough. MEP latency was measured from TMS delivery to the MEP onset and amplitude was measured peak to peak. The ratio of CS2–TS/TS represents the baseline SICI (SICI alone). CS2–TS’/TS’ represents the SICI with adjusted TS intensity (SICI alone’). Similarly, LICI alone (CS100–TS/TS) and LICI alone’ (CS100–TS’/TS’) were used to evaluate the effect of the preceding CS100 with different TS intensities. CS100–CS2–TS’/CS100–TS’ represents the SICI in the presence of LICI (SICILICI). ICF alone, ICF alone’ and ICFLICI were calculated in a similar way. Ratios for each I-wave and MEP were calculated. Ratios less than 1 indicate inhibition, and ratios greater than 1 indicate facilitation. Values were expressed as mean ± standard deviation.
Statistical analysis was performed for each subject separately. To determine whether the conditioned responses (I-waves and MEP) were inhibited or facilitated, they were compared to test responses by an unpaired t test. Bonferroni's correction was applied as the same measurement (e.g. I1-wave amplitude) was compared under three different experimental conditions (e.g. SICI alone, SICI alone’ and SICILICI for SICI measurement). We also compared the inhibition ratios for I-waves and MEP between SICI and SICI’ and between LICI and LICI’ using an unpaired t test. A P value less than 0.05 after correction (multiplied by 3) was considered significant.
Results
I-waves and MEP latencies
D-wave latency was 2.4 ms in Subject 1. ‘1 mV’ intensity was 62% of stimulator output. The TS produced four descending waves with peak latencies of 3.8 (I1), 5.3 (I2), 6.7 (I3) and 8.0 ms (I4). The MEP latency was 19.6 ms. In the presence of LICI, TS’ was adjusted to 71% of stimulator output. It produced an additional peak at D-wave latency (Fig. 1). D-wave latency was 2.5 ms in Subject 2. ‘1 mV’ intensity was 70% of stimulator output. It produced three descending waves with peak latencies of 4.1 (I1), 5.6 (I2) and 7.1 ms (I3). The MEP latency was 22.7 ms. TS’ intensity was 82% of stimulator output. No D-wave was generated by TS or TS’ with adjusted intensity in Subject 2.
SICI in the presence of LICI
Figure 1 shows the recordings in Subject 1. The I-waves and MEP amplitudes are shown in Table 1 and results of the statistical analyses are listed in Table 2. SICI produced by CS2 inhibited MEP generated by TS (P < 0.001). However, only I3- and I4-waves were significantly smaller in CS2–TS-induced trials compared to TS alone (P < 0.001 for both waves). I1- and I2-waves were not affected. LICI generated by CS100 also significantly inhibited I3- and I4-waves (P < 0.001 for both comparisons), resulting in a reduction in MEP (P < 0.001). MEP with adjusted TS intensity (TS’) was also inhibited by SICI generated by CS2 (P < 0.001). This is caused by suppression of I2-, I3- and I4-waves (P < 0.001 for all comparisons). LICI produced by CS100 also suppressed I2-, I3- and I4-waves (P < 0.001 for all comparisons) and reduced MEP amplitude (P < 0.001) generated by TS’. In addition, the difference in inhibition ratios between SICI and SICI’ and between LICI and LICI’ were significant for the I2-wave (P < 0.05 for both comparisons), but were not significant for other I-waves and for MEP. Importantly, with adjustment in TS intensities, the experimental conditions of TS alone and CS100–TS’ had similar I3- and I4-waves and MEP amplitudes, but neither MEP nor any I-wave was further inhibited by SICI in the presence of LICI (CS100–CS2–TS’ compared to CS100–TS’). Conversely, the I3-wave was facilitated in Subject 1 (P < 0.05).
Table 1.
Amplitudes of descending waves and MEPs in different experimental conditions
| Subject 1 | Subject 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Descending wave (μV) | Descending wave (μV) | ||||||||
| Experimental condition | I1 | I2 | I3 | I4 | MEP (mV) | I1 | I2 | I3 | MEP (mV) |
| TS | 3.38 ± 0.62 | 2.59 ± 0.52 | 2.82 ± 0.37 | 1.44 ± 0.23 | 1.05 ± 0.33 | 5.67 ± 0.85 | 3.26 ± 0.42 | 2.01 ± 0.22 | 1.20 ± 0.25 |
| CS2–TS | 3.38 ± 0.77 | 2.36 ± 0.58 | 1.31 ± 0.26 | 0.72 ± 0.14 | 0.41 ± 0.25 | 5.38 ± 1.09 | 2.45 ± 0.47 | 1.23 ± 0.27 | 0.63 ± 0.27 |
| CS10–TS | 3.57 ± 0.70 | 2.74 ± 0.68 | 3.31 ± 0.43 | 1.56 ± 0.42 | 1.70 ± 0.55 | 6.01 ± 1.93 | 3.48 ± 1.05 | 1.90 ± 0.77 | 2.62 ± 1.56 |
| CS100–TS | 2.95 ± 0.72 | 2.82 ± 0.74 | 1.51 ± 0.30 | 0.79 ± 0.21 | 0.50 ± 0.24 | 5.55 ± 1.25 | 2.44 ± 0.52 | 1.49 ± 0.31 | 0.70 ± 0.23 |
| TS′ | 5.77 ± 1.15 | 4.87 ± 0.74 | 3.72 ± 0.32 | 2.44 ± 0.23 | 1.85 ± 0.45 | 7.22 ± 1.03 | 4.05 ± 0.47 | 2.64 ± 0.41 | 2.04 ± 0.40 |
| CS2–TS′ | 5.13 ± 1.46 | 3.28 ± 0.92 | 2.36 ± 0.77 | 1.18 ± 0.52 | 0.92 ± 0.41 | 7.11 ± 1.57 | 2.96 ± 0.47 | 1.64 ± 0.72 | 1.11 ± 0.53 |
| CS10–TS′ | 5.69 ± 1.57 | 4.88 ± 1.32 | 3.60 ± 0.32 | 2.45 ± 0.47 | 2.63 ± 0.88 | 7.24 ± 1.68 | 4.22 ± 0.63 | 2.78 ± 0.49 | 3.30 ± 1.52 |
| CS100–TS′ | 4.87 ± 1.05 | 3.56 ± 0.77 | 2.08 ± 0.45 | 1.26 ± 0.29 | 0.94 ± 0.35 | 7.02 ± 1.26 | 3.01 ± 0.57 | 1.84 ± 0.30 | 1.33 ± 0.54 |
| CS100–CS2–TS′ | 5.08 ± 1.30 | 3.21 ± 0.99 | 2.77 ± 0.56 | 1.31 ± 0.33 | 0.92 ± 0.44 | 6.85 ± 1.44 | 2.79 ± 0.72 | 1.68 ± 0.49 | 1.27 ± 0.75 |
| CS100–CS10–TS′ | 4.89 ± 1.56 | 3.87 ± 1.36 | 2.32 ± 0.39 | 1.31 ± 0.47 | 1.65 ± 0.83 | 7.23 ± 1.58 | 3.37 ± 0.78 | 1.95 ± 0.44 | 2.59 ± 1.22 |
Values represent mean ± standard deviation.
Table 2.
Statistical analysis for the different intracortical circuits measured with descending waves and MEPs in different experimental conditions
| Subject 1 | Subject 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Descending wave | Descending wave | ||||||||
| Intracortical circuit | I1 | I2 | I3 | I4 | MEP | I1 | I2 | I3 | MEP |
| SICI alone | 100.0% | 91.1% | 46.4% | 50.1% | 39.2% | 94.9% | 75.2% | 61.2% | 52.5% |
| (0) | (−1.3) | (−14.9***) | (−12.0***) | (−6.9***) | (−0.9) | (−5.7***) | (−10.0***) | (−6.9***) | |
| ICF alone | 105.6% | 105.8% | 117.4% | 108.5% | 161.9% | 106.0% | 106.7% | 94.5% | 218.3% |
| (0.9) | (0.8) | (3.9**) | (1.1) | (4.5**) | (0.7) | (0.9) | (−0.6) | (4.0**) | |
| LICI alone | 87.3% | 108.9% | 53.5% | 54.9% | 47.6% | 97.9% | 74.8% | 74.1% | 58.3% |
| (−2.0) | (1.1) | (−12.3***) | (−9.3***) | (−6.0***) | (−0. 4) | (−5.5***) | (−6.1***) | (−6.6***) | |
| SICI alone’ | 88.9% | 67.4% | 63.4% | 48.7% | 49.7% | 98.5% | 73.1% | 62.1% | 54.4% |
| (−1.5) | (−6.0***) | (−7.3***) | (−9.9***) | (−6.8***) | (−0.3) | (−7.3***) | (−5.4***) | (−6.3***) | |
| ICF alone’ | 98.6% | 100.2% | 96.8% | 100.4% | 142.2% | 100.3% | 104.2% | 105.3% | 161.8% |
| (−0.2) | (0) | (−1.2) | (0.1) | (3.5*) | (0.1) | (1.0) | (1.0) | (3.6*) | |
| LICI alone’ | 84.4% | 73.1% | 55.9% | 51.6% | 50.8% | 97.2% | 74.3% | 69.7% | 65.2% |
| (−2.5) | (−5.5***) | (−13.3***) | (−14.2***) | (−7.1***) | (−0.5) | (−6.3***) | (−7.0***) | (−4.7***) | |
| SICILICI | 104.2% | 89.9% | 133.3% | 104.1% | 97.9% | 97.6% | 92.7% | 91.3% | 95.5% |
| (0.6) | (−1.2) | (4.3**) | (0.5) | (−0.2) | (−0.4) | (−1.1) | (−1.2) | (−0.3) | |
| ICFLICI | 100.2% | 108.7% | 111.5% | 104.0% | 175.5% | 103.0% | 112.0% | 106.0% | 194.7% |
| (0.1) | (0.9) | (1.8) | (0.4) | (3.5*) | (0.5) | (1.7) | (0.9) | (4.2**) | |
Note: Measurements for SICI alone (ICF alone, LICI alone) were calculated as the ratio of CS2–TS (CS10–TS, CS100–TS) generated responses to those generated by TS alone. Measurements for SICI alone' (ICF alone', LICI alone') were calculated as the ratio of CS2–TS′ (CS10–TS′, CS100–TS′) generated responses to those generated by TS′ alone. Measurements for SICILICI and ICFLICI were calculated as the ratio of triple-pulse generated responses to those generated by CS100–TS′ paired-pulse. Values in parentheses show t value.
P < 0.05
P < 0.01
P < 0.001, comparing conditioned response to test response with Bonferroni's correction for multiple comparisons. df = 38 for all comparisons.
In Subject 2, SICI produced by CS2 inhibited I2- and I3-waves generated both by TS alone and adjusted TS intensity (P < 0.001 for all comparisons, Table 2). This led to a reduction in MEP for both experimental conditions (P < 0.001 for both SICI alone and SICI’). LICI elicited by CS100 inhibited I2- and I3-waves for TS alone (P < 0.001 for both comparisons), causing MEP inhibition (P < 0.001). With adjusted TS intensity, I2- and I3-waves (P < 0.001 for both comparisons) were also inhibited by CS100, causing MEP inhibition (P < 0.001). The inhibition ratios for both I-waves and MEP were not significantly different between SICI and SICI’ and between LICI and LICI’. However, similar to the findings for Subject 1, SICI in the presence of LICI (CS100–CS2–TS’ compared to CS100–TS’) did not further inhibit I-waves or MEP amplitudes (Tables 1 and 2).
ICF in the presence of LICI
MEP was facilitated by preceding CS10 in both subjects (P < 0.01 for both subjects, Tables 1 and 2). With adjusted TS intensity, the facilitation was also significant in both subjects (P < 0.05 for both subjects). In the presence of LICI, CS10 still facilitated the test MEP with a similar degree as ICF alone (P < 0.05 for Subject 1; P < 0.01 for Subject 2). However, the MEP facilitation cannot be explained by an increase in I-wave amplitude because there was no significant change in I-wave amplitudes for all ICF conditions (Table 2) except for a significant increase in the I3-wave in Subject 1 for ICF alone condition (P < 0.01).
Discussion
This study investigates how the interaction between two intracortical circuits affects the descending corticospinal waves. We found that: (1) Both LICI and SICI inhibited late I-waves while the I-waves were not changed by ICF; (2) All I-waves increased with adjusted TS intensity. In the presence of LICI with adjusted TS intensity (CS100–TS’), the amplitudes of the late I-waves were comparable to those of TS alone; (3) Neither the descending wave nor the MEP was further inhibited by SICI in the presence of LICI. ICF showed similar degree of MEP facilitation in the presence of LICI compared to ICF alone with little change in I-wave amplitude.
I-wave generation
Posterior–anterior directed current produced multiple descending waves in the present study. The first wave was the I1-wave in both subjects. The number of descending waves differed in the two subjects (I1- to I4-waves for Subject 1 and I1- to I3-waves for Subject 2). The results suggest that a suprathreshold single-pulse TMS probably activates different cortical interneurons with facilitatory connections to the corticospinal neuron, leading to multiple descending waves. In addition, TS with adjusted intensity also generated the D-wave in Subject 1 but not in Subject 2. These results are consistent with previous studies with epidural recordings (Di Lazzaro et al. 1998a, 2001, 2004). TMS at higher intensity may be able to stimulate deeper areas to directly activate corticospinal neurons. As expected, the D-wave was not influenced by SICI, LICI or combined SICI and LICI (Fig. 1). Therefore, it is unlikely that the presence of the D-wave in Subject 1 influenced our findings for the I-waves.
We found that both SICI and LICI inhibited late I-waves but had little effect on early I-waves, consistent with previous studies (Nakamura et al. 1997; Di Lazzaro et al. 1998b, 2002). The results confirmed the notion that both SICI and LICI at an ISI of 100 ms are due to cortical inhibition (Ziemann et al. 1996b; McDonnell et al. 2006). With the adjusted TS intensity (TS’ alone), the amplitudes of all I-waves increased. Importantly, the amplitude of the late I-waves conditioned by CS100 (CS100–TS’) were comparable to those produced by TS alone. Since the early I-waves were less affected by LICI, this resulted in larger amplitudes of early I-waves in the CS100–TS’ condition than those in the TS alone condition. However, MEP amplitudes were similar for CS100–TS’ and TS alone conditions. MEP reflects the global excitability of the motor pathway, including the activity of cortical and spinal motoneurons. After the arrival of early I-waves, some spinal motoneurons achieve the firing threshold and contribute to the MEP. On the other hand, it is likely that more spinal motoneurons are subliminally activated by the early I-waves. When the late I-waves arrive at the spinal motoneuron pool, they activate this subliminal group with temporal (or even spatial) summation. Therefore, late I-waves may play a greater role than early I-waves in producing MEP. This might explain the finding that the early I-waves were of larger amplitudes in the CS100–TS’ than the TS alone condition but the MEP amplitudes were matched for the two conditions.
SICI in the presence of LICI
With surface EMG recordings, it was reported that SICI was largely abolished in the presence of LICI (Sanger et al. 2001). Epidural recordings ensured that the experimental protocol produced comparable and sufficient amount of late I-waves to be inhibited by SICI whether SICI was conditioned by LICI or not. However, late I-waves were not inhibited by SICI in the presence of LICI. These findings suggest that reduction of SICI in the presence of LICI is not due to the absence of late I-waves caused by LICI. Although the present findings do not completely exclude a saturation effect, previous studies suggested that this cannot explain the interaction between SICI and LICI. First, the effect of SICI in the presence of LICI could turn into facilitation both at rest and during voluntary contraction (Sanger et al. 2001; Ni et al. 2007). Such a facilitatory effect on the I3-wave was also found in Subject 1 (Table 2). Second, LICI elicited by weak CS100 that did not produce MEP inhibition also led to reduced SICI (Sanger et al. 2001). Another consideration is that TS’ and TS may activate different groups of neurons responsible for the same late I-wave (Ni et al. 2011a). If this is correct, the late I-waves generated by CS100–TS’ and TS may have different sensitivity to SICI. In addition, with increased TS intensity (TS’) the degree of inhibition in I-waves produced by SICI’ and LICI’ could be different from that of SICI and LICI alone. In Subject 1, the early I-waves were not inhibited by SICI and LICI alone, but the I2-wave was inhibited by SICI’ and LICI’ with adjusted TS intensity (Table 2). These findings suggest that the inhibitory effects on I-waves caused by SICI and LICI may vary with the size of these waves. Therefore, the reduction of SICI in the presence of LICI should be interpreted cautiously although the two measures were matched for both MEP and late I-wave amplitudes.
Our results cannot distinguish between LICI inhibiting SICI and SICI inhibiting LICI. We consider that LICI inhibiting SICI is more likely because animal studies have demonstrated reduction of GABA release caused by presynaptic GABAB-mediated inhibition in both the hippocampus (Pitler & Alger, 1994) and the neocortex (Deisz, 1999). Therefore, the inhibitory interaction between LICI and SICI could be explained by presynaptic inhibition of GABAergic interneurons leading to reduction of GABA release that mediates SICI.
In both subjects we studied the side affected by pain to record more reliable I-waves due to the location of the electrodes. Although peripheral injury can cause plastic changes in the brain (Chen et al. 2003), this is unlikely to affect our conclusions as reliable SICI and LICI were detected in both the I-wave and in the MEP recordings, and the inhibitory interactions between LICI and SICI were similar to those in healthy subjects (Sanger et al. 2001; Cash et al. 2010).
ICF in the presence of LICI
A previous study with a larger sample size (Sanger et al. 2001) that measured MEP with surface EMG showed that ICF alone and ICF in the presence of LICI had similar degrees of facilitation, suggesting that LICI had little effects on ICF. This was confirmed in the present study. Surprisingly, there was little change in I-waves caused by ICF either in the paired-pulse or in the triple-pulse trials. This is similar to a previous study examining ICF alone with epidural recording (Di Lazzaro et al. 2006). ICF may involve cortical facilitatory circuits that are not reflected in the descending waves. An alternative explanation may be that ICF involves facilitatory interactions at subcortical or spinal level.
Conclusion
The inhibitory interactions between LICI and SICI cannot be explained by changes in I-waves caused by LICI or SICI alone, and the results are consistent with presynpatic inhibition at the cortical level.
Acknowledgments
This study was supported by the Canadian Institutes of Health Research operating grant to Robert Chen (MOP 62917). Zhen Ni was funded by a Fellowship Award in the Area of Dystonia by the Canadian Institute of Health Research (DFF 88348) and the Dystonia Medical Research Foundation of Canada.
Glossary
Abbreviations
- CS
conditioning stimulus
- D-wave
direct wave
- EMG
electromyogram
- FDI
first dorsal interosseous
- I-wave
indirect wave
- ICF
intracortical facilitation
- ISI
interstimulus interval
- LICI
long interval intracortical inhibition
- M1
primary motor cortex
- MEP
motor-evoked potential
- SICI
short interval intracortical inhibition
- TMS
transcranial magnetic stimulation
- TS
test stimulus
Author contributions
Z.N. and R.C. conceived and designed the experiment. Data collection and analysis were performed by Z.N., C.G. and A.W. A.M.L. performed the surgical procedure for the patients. Z.N. wrote the paper with scientific inputs from all authors. All authors approved the final version of the manuscript for publication. The experiments were performed in Dr Robert Chen's laboratory at the Toronto Western Research Institute.
References
- Cash RF, Ziemann U, Murray K, Thickbroom GW. Late cortical disinhibition in human motor cortex: a triple-pulse transcranial magnetic stimulation study. J Neurophysiol. 2010;103:511–518. doi: 10.1152/jn.00782.2009. [DOI] [PubMed] [Google Scholar]
- Chen R, Anastakis DJ, Haywood CT, Mikulis DJ, Manktelow RT. Plasticity of the human motor system following muscle reconstruction: a magnetic stimulation and functional magnetic resonance imaging study. Clin Neurophysiol. 2003;114:2434–2446. doi: 10.1016/s1388-2457(03)00283-9. [DOI] [PubMed] [Google Scholar]
- Chen R, Cros D, Curra A, Di Lazzaro V, Lefaucheur JP, Magistris MR, Mills K, Rosler KM, Triggs WJ, Ugawa Y, Ziemann U. The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2008;119:504–532. doi: 10.1016/j.clinph.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Chen R, Lozano AM, Ashby P. Mechanism of the silent period following transcranial magnetic stimulationEvidence from epidural recordings. Exp Brain Res. 1999;128:539–542. doi: 10.1007/s002210050878. [DOI] [PubMed] [Google Scholar]
- Deisz RA. The GABAB receptor antagonist CGP 55845A reduces presynaptic GABAB actions in neocortical neurons of the rat in vitro. Neuroscience. 1999;93:1241–1249. doi: 10.1016/s0306-4522(99)00203-1. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Mazzone P, Pilato F, Saturno E, Insola A, Visocchi M, Colosimo C, Tonali PA, Rothwell JC. Direct demonstration of long latency cortico-cortical inhibition in normal subjects and in a patient with vascular parkinsonism. Clin Neurophysiol. 2002;113:1673–1679. doi: 10.1016/s1388-2457(02)00264-x. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol. 2004;115:255–266. doi: 10.1016/j.clinph.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Saturno E, Pilato F, Insola A, Mazzone P, Tonali P, Rothwell JC. Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalogr Clin Neurophysiol. 1998a;109:397–401. doi: 10.1016/s0924-980x(98)00038-1. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Saturno E, Pilato F, Insola A, Mazzone P, Profice P, Tonali P, Rothwell JC. The effect on corticospinal volleys of reversing the direction of current induced in the motor cortex by transcranial magnetic stimulation. Exp Brain Res. 2001;138:268–273. doi: 10.1007/s002210100722. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Oliviero A, Dileone M, Saturno E, Mazzone P, Insola A, Profice P, Ranieri F, Capone F, Tonali PA, Rothwell JC. Origin of facilitation of motor-evoked potentials after paired magnetic stimulation: direct recording of epidural activity in conscious humans. J Neurophysiol. 2006;96:1765–1771. doi: 10.1152/jn.00360.2006. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates inhibitory circuits. Exp Brain Res. 1998b;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Kaneko K, Kawai S, Fuchigami Y, Morita H, Ofuji A. The effect of current direction induced by transcranial magnetic stimulation on the corticospinal excitability in human brain. Electroencephalogr Clin Neurophysiol. 1996;101:478–482. doi: 10.1016/s0013-4694(96)96021-x. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. The role of GABAB receptors in intracortical inhibition in the human motor cortex. Exp Brain Res. 2006;173:86–93. doi: 10.1007/s00221-006-0365-2. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol. 1997;498:817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Charab S, Gunraj C, Nelson AJ, Udupa K, Yeh IJ, Chen R. Transcranial magnetic stimulation in different current directions activates separate cortical circuits. J Neurophysiol. 2011a;105:749–756. doi: 10.1152/jn.00640.2010. [DOI] [PubMed] [Google Scholar]
- Ni Z, Gunraj C, Chen R. Short interval intracortical inhibition and facilitation during the silent period in human. J Physiol. 2007;583:971–982. doi: 10.1113/jphysiol.2007.135749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Müller-Dahlhaus F, Chen R, Ziemann U. Triple-pulse TMS to study interactions between neural circuits in human cortex. Brain Stimul. 2011b doi: 10.1016/j.brs.2011.01.002. in press. [DOI] [PubMed] [Google Scholar]
- Pitler TA, Alger BE. Differences between presynaptic and postsynaptic GABAB mechanisms in rat hippocampal pyramidal cells. J Neurophysiol. 1994;72:2317–2327. doi: 10.1152/jn.1994.72.5.2317. [DOI] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol. 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenkreis P, Witscher K, Janssen F, Addo A, Dertwinkel R, Zenz M, Malin JP, Tegenthoff M. Influence of the N-methyl-D-aspartate antagonist memantine on human motor cortex excitability. Neurosci Lett. 1999;270:137–140. doi: 10.1016/s0304-3940(99)00492-9. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Fong JK, Meyer BU, Priori A, Rothwell JC, Day BL, Thompson PD. The effect of magnetic coil orientation on the latency of surface EMG and single motor unit responses in the first dorsal interosseous muscle. Electroencephalogr Clin Neurophysiol. 1994;93:138–146. doi: 10.1016/0168-5597(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517:591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Chen R, Cohen LG, Hallett M. Dextromethorphan decreases the excitability of the human motor cortex. Neurology. 1998;51:1320–1324. doi: 10.1212/wnl.51.5.1320. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996a;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996b;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]


