Non-technical summary
The sense of body ownership tells us that our body belongs to us, and other bodies do not. That our body belongs to us is fundamental to self-awareness. It is known that synchronous touch and vision can be used to induce an illusion of ownership over an artificial rubber hand. Like the skin receptors used for touch, sensory receptors in the muscles only provide information about events occurring to the body. Whether muscle receptors contribute to our sense of body ownership is not known. This study developed a technique to induce an illusion of ownership over a plastic finger using movement, which excites muscle receptors. This sense of ownership still occurred when the contribution of skin and joint receptors was removed using local anaesthetic. The results clearly show that muscle receptors can contribute to the sense of body ownership.
Abstract
Abstract
The sense of body ownership, knowledge that parts of our body ‘belong’ to us, is presumably developed using sensory information. Cutaneous signals seem ideal for this and can modify the sense of ownership. For example, an illusion of ownership over an artificial rubber hand can be induced by synchronously stroking both the subject's hidden hand and a visible artificial hand. Like cutaneous signals, proprioceptive signals (e.g. from muscle receptors) exclusively signal events occurring in the body, but the influence of proprioceptors on the sense of body ownership is not known. We developed a technique to generate an illusion of ownership over an artificial plastic finger, using movement at the proximal interphalangeal joint as the stimulus. We then examined this illusion in 20 subjects when their index finger was intact and when the cutaneous and joint afferents from the finger had been blocked by local anaesthesia of the digital nerves. Subjects still experienced an illusion of ownership, induced by movement, over the plastic finger when the digital nerves were blocked. This shows that local cutaneous signals are not essential for the illusion and that inputs arising proximally, presumably from receptors in muscles which move the finger, can influence the sense of body ownership. Contrary to other studies, we found no evidence that voluntary movements induce stronger illusions of body ownership than those induced by passive movement. It seems that the congruence of sensory stimuli is more important to establish body ownership than the presence of multiple sensory signals.
Introduction
We know that our body parts ‘belong’ to us without having to move, contract or otherwise test the body part in question. Presumably, the brain develops the map of what belongs to it by using sensory information. However, not all sensory channels are appropriate to do this. For example, we can use vision to see the parts of the body, but we can also see the parts of other bodies, so vision alone cannot differentiate foreign body parts from those we own. By contrast, touch seems ideal for identification of ownership as any tactile stimuli that are perceived must, by definition, be occurring against the brain's own body – we do not usually perceive tactile stimuli on anything that is not part of our body.
Although this sense of body ownership seems robust, it can be disrupted in clinical conditions, for example stroke (Feinberg et al. 2010) and epilepsy (Boesebeck, 2004). Furthermore, it can be easily modified by manipulation of sensory input. Perhaps the most well-known example of such manipulation is the ‘rubber hand illusion’, first described by Botvinick & Cohen (1998). This illusion can be generated by synchronously stroking the subject's hand (out of view) and a rubber hand (in view), with the stroking applied to a similar anatomical position. This illusion can also be induced using somatic signals only, that is without visual cues, by moving a blindfolded subject's index finger so that it touches a rubber hand while the experimenter simultaneously touches the subject's real other hand (Ehrsson et al. 2005). However, the illusion is more vivid if the rubber hand is placed in a posture that the subject's real hand could occupy (Pavani et al. 2000; Austen et al. 2004) and the stimuli are spatially congruent (Costantini & Haggard, 2007). One proposed mechanism for the illusion is the detection of multisensory signals by the premotor, intraparietal and cerebellar regions of the brain (Ehrsson et al. 2004, 2005). Neural activity in primary somatosensory cortex has been linked to body ownership (Schaefer et al. 2006), as well as activity in frontal cortex and the insula (Tsakiris et al. 2007). Once this illusion of ownership of the hand is established, subjects have physiological responses to threats made against the rubber hand (e.g. Armel & Ramachandran, 2003; Ehrsson et al. 2007). The illusion is not broken by subjective reasoning or explanation by the experimenter. Furthermore, there are physiological changes, such as cooling, in the real hand that is ‘replaced’ by the rubber hand (Moseley et al. 2008).
Production of the rubber hand illusion by cutaneous stroking shows that cutaneous inputs can provide a signal of body ownership. However, touch is not the only sense that reports exclusively about events acting on the body. The other proprioceptive cues from muscle receptors, joint receptors and central command signals also provide information only about what is happening to the body. Could these sensory channels be as important as cutaneous channels in the development of the brain's sense of body ownership? Is cutaneous information essential? A combination of visual and joint movement stimuli have been used previously to investigate the induction of the rubber hand illusion (Dummer et al. 2009), but cutaneous stimuli were not excluded in that study. It is well established that movement of the hand excites input from specialized skin, joint and muscle receptors (e.g. Hulliger et al. 1979; Burke et al. 1988). Although the results of Dummer et al. (2009) show that joint movements, in place of tactile stroking, can induce the rubber hand illusion, their results do not reveal whether signals from muscle receptors, joint receptors or central motor command signals have any role in the sense of body ownership. In the study of Dummer et al. (2009), signals from cutaneous receptors around the joints were available and may have been the critical input which induced the illusion. Cutaneous signals not only provide information about objects and surfaces touched by the skin, but cutaneous stretch receptors signal movement of the joints (Edin & Johansson, 1995; Collins et al. 2005).
The present study was designed to investigate whether the non-cutaneous proprioceptive signals contribute to the generation of the sense of body ownership and to determine whether these non-cutaneous signals were as influential as the cutaneous signals. As a tool to measure the influence of cutaneous and non-cutaneous proprioceptive signals on the perception of body ownership, we developed a ‘plastic finger’ illusion. The finger was used because it is feasible to block the digital nerves with local anaesthesia and remove all input from local cutaneous and joint receptors. As the muscles which flex and extend the fingers are proximal in the hand and forearm, proprioceptive signals from muscle receptors remain intact. We hypothesized that proprioceptive cues would be sufficient to induce an illusion of ownership of a finger in the absence of cutaneous information.
Methods
Thirty naive healthy subjects (twelve male) participated in this study. Twenty subjects (nine male) aged 21–56 years participated in experiment one. Sixteen of these subjects performed the experiment in all conditions. Four subjects did not perform two of the control conditions, which used incongruent movement and were introduced after these subjects had been tested. Ten subjects (three male) aged 23–33 years participated in experiment two. All subjects gave informed consent, and the experimental procedures were carried out in accordance with the Declaration of Helsinki. The University of New South Wales Human Research Ethics Committee approved the study. All subjects were informed about the experimental procedures but were unaware of the exact experimental hypothesis.
Experimental set-up
Subjects sat with their right arm resting in a semi-pronated position on the lower of two tables (Fig. 1). The upper table had a rotatable shaft running through it with an axis of rotation that was collinear with the proximal interphalangeal joint of the subject's right index finger. The distal and middle segment of the subject's index finger was wrapped in a piece of neoprene and pushed into a piece of pipe. The pipe was attached to the rotatable shaft via a coupling. The coupling allowed the rotation of the shaft to be either locked to the subject's proximal interphalangeal joint or to move independently. The apparatus prevented movement at the other joints of the finger and the wrist.
Figure 1. Diagram of the experimental set-up.
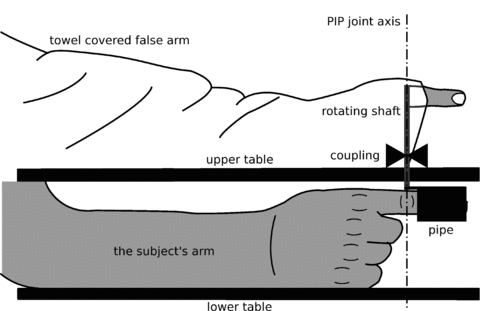
The subject sat with their right arm resting on the lower table in front of them. The upper table covered the subject's arm from the elbow down, and a towel covered a false arm placed on the upper table, made from appropriately shaped blocks, and the subject's arm up to and including the shoulder. Thus, the subject's right arm was not visible from the shoulder down. The towel and false arm were arranged so that it looked as if the towel was simply covering the subject's own arm. A plastic finger protruded from the end of the towel so that the distal and intermediate segments were visible to the subject. This plastic finger was fixed to a rotating shaft though its proximal interphalangeal (PIP) joint. This shaft was aligned with the proximal interphalangeal joint of the subject's right index finger and connected to a piece of pipe that held the distal and intermediate segments of the subject's index finger. In this set-up, the subject's proximal interphalangeal joint was aligned with that of the plastic finger and both could move freely around that axis. The pipe and the positioning of the subject's arm relative to it ensured that the subject's distal interphalangeal joint and metacarpophalangeal joint could not move. The coupling could be released so that the subject's index finger and the plastic finger could move independently, or it could be locked so that the movement of the subject's index finger and the plastic finger were congruent. The subject's other fingers were kept in a relaxed, curled position. For the two conditions where touch was used as a stimulus instead of movement, the pipe was removed to allow access to the skin of the subject's index finger.
A plastic finger, of the type used by magicians, was attached to the top of the rotatable shaft through its proximal interphalangeal joint so that when the shaft coupling was engaged, movement of the plastic finger was synchronized with that of the subject's finger. A set of blocks and pipes were placed in a line ‘proximal’ to the plastic finger and covered with a towel to provide the visual impression that there was an arm covered by a towel attached to the plastic finger. The same towel also covered the subject's arm to the shoulder and occluded visual input of the rotatable shaft that connected the plastic finger to their own finger. This false arm was placed in a position so that it was directly over the subject's arm, which was resting on the lower table. The subject's view was limited to the plastic finger and the towel over the false arm.
Experiment one
This experiment tested whether proprioceptive cues, in the absence of tactile cues, could be used to induce an illusion of body ownership over the plastic finger. Furthermore, it tested whether the illusion was as strong when proprioceptive cues were used as when tactile cues were used. In order to do this, we stimulated the subject's index finger at the same time as the plastic finger in eight different conditions.
Basic condition
The basic condition was intended to ensure that an illusion of ownership could be induced over an artificial plastic finger using a similar experimental approach to that established for the rubber hand illusion (Botvinick & Cohen, 1998). Using commercially available 12 mm paintbrushes, the experimenter synchronously stroked the subject's finger and the plastic finger in a congruent direction for 3 min. The subject could see the stroking of the plastic finger, but not the stroking of their own finger.
Test conditions
There were four test conditions. During all test conditions, the coupling on the rotatable shaft that connected the subject's finger to the plastic finger was locked so that the movements of the two fingers were congruent. For the first test condition, the subject's index finger digital nerves were intact. The subject was instructed to keep the hand relaxed while the experimenter held the distal segment of the plastic finger and moved it continuously into flexion and extension though an arc of about 30 deg for 3 min. The subject saw the experimenter moving the plastic finger and also felt (but could not see) their own finger performing exactly the same movement at exactly the same time. The second test condition was similar to the first, except that the subject was instructed to flex and extend the proximal interphalangeal joint of their finger voluntarily through an arc of ∼30 deg continuously for 3 min. Here, the subjects voluntarily moved their finger and felt it moving, but could not see it moving. What they saw was the plastic finger moving in a manner that was congruent to their own finger movements. The third and fourth test conditions were the same as the first and second conditions, respectively, except that these conditions were performed after a digital nerve block of the right index finger (see ‘Digital nerve block of the index finger’ below).
Control conditions
Three control conditions were used to ensure that the illusions reported by subjects in the test conditions were due to the congruence of the visual and proprioceptive information. The first of the control conditions repeated the synchronous stroking of the basic condition, but was done after both digital nerves of the subject's index finger had been blocked with local anaesthetic (see ‘Digital nerve block of the index finger’ below). The second control condition used movement of the proximal interphalangeal joint as the stimulus; however, this stimulus was not delivered in a congruent manner. The coupling on the rotatable shaft that connected the subject's finger to the plastic finger (Fig. 1) was disengaged so that the two fingers could move independently of one another. The subject was instructed to keep the hand relaxed, and the experimenter flexed and extended the subject's right index proximal interphalangeal joint through an arc of ∼30 deg for 3 min. At the same time, the experimenter controlled the movement of the plastic finger to make movements that were similar in velocity and magnitude to those applied to the subject's finger, but were otherwise unrelated. The final control condition was the same as the second control condition, except that the subject was instructed to flex and extend their right index proximal interphalangeal joint voluntarily through an arc of ∼30 deg. Once again, the experimenter controlled the movement of the plastic finger to make movements that were similar in velocity and magnitude to the subject's voluntary movement, but were otherwise unrelated. The subject could not see the experimenter's hand controlling the movements of the plastic finger.
Digital nerve block of the index finger
A total of 3–4 ml of 1% lignocaine was injected into the medial and lateral side of the index finger 10 mm distal to the metacarpophalangeal joint in order to block both digital nerves. A piece of tape was placed around the index finger immediately distal to the metacarpophalangeal joint to impede slightly the venous return from the finger and thus prolong the block. The block was clinically complete in 5–10 min, with complete loss of light touch sensation. Light touch was tested intermittently to ensure that the block remained complete. After the experiment, the tape was removed and the subject recovered completely within a few hours.
Measurements
To evaluate the strength of the illusion of ownership over the plastic finger, the subject was asked to complete a questionnaire. The established nine-item questionnaire (Botvinick & Cohen, 1998) was modified to apply to a finger illusion instead of a hand/arm illusion and to incorporate the provision of non-tactile instead of solely tactile stimuli (Table 1). Others have used a version with only five items (e.g. Dummer et al. 2009), but we opted to use the whole item set because we were establishing the illusion in novel conditions and we needed to understand what the subjects were experiencing. For the conditions involving passive or active movement, the items were altered to use the term ‘movement’ instead of the term ‘touch’, but were otherwise the same. The order of questionnaire items was randomized between conditions. Each item had a discrete seven-point scale. Subjects were instructed to circle the ‘correct’ answer. The conditions in which the digital nerves were blocked were always undertaken after the other conditions. However, the order of both the blocked conditions and the intact conditions was randomized.
Table 1.
The set of items used in the study
| Number | Condition | Item |
|---|---|---|
| 1 | Touch | It seemed as if I were feeling the touch of the paintbrush at the location where I saw the plastic finger touched. |
| Movement | It seemed as if I were feeling the movement at the location where I saw the plastic finger move. | |
| 2 | Touch | It seemed as though the touch I felt was caused by the paintbrush touching the plastic finger. |
| Movement | It seemed as though the movement I felt was caused by the movement of the plastic finger. | |
| 3 | Common | I felt as if the plastic finger were my finger. |
| 4 | Common | I felt as if my (real) finger were drifting up (towards the plastic finger). |
| 5 | Common | It seemed as if I might have more than one right index finger, hand or arm. |
| 6 | Touch | It seemed as if the touch I was feeling came from somewhere between my own finger and the plastic finger. |
| Movement | It seemed as if the movement I was feeling came from somewhere between my own finger and the plastic finger. | |
| 7 | Common | It felt as if my (real) finger were turning ‘plasticy’. |
| 8 | Common | It appeared (visually) as if the plastic finger were drifting down (towards my finger). |
| 9 | Common | The plastic finger began to resemble my own (real) finger, in terms of shape, skin tone, freckles or some other visual feature. |
The nine items used by Botvinick & Cohen (1998) were adapted to refer to the finger, rather than the hand, and to plastic, rather than rubber. Items 1, 2 and 6 were further modified to create a second version of the item that related to movement rather than touch. Note that after the modification to refer to movement, the meaning of item 2 became ambiguous (see main text). The items labelled as being a ‘touch’ condition were used for the two synchronous touch conditions. ‘Movement’ items were used for all six movement conditions. Items labelled ‘common’ were used for all conditions.
Experiment two
The order of conditions was such that all the intact conditions were presented to subjects before any of the blocked conditions. This design does not exclude a possible order effect that could occur because subjects were exposed to the illusion of body ownership before they experienced the blocked congruent movement condition. Thus, a second experiment was designed to test whether the blocked congruent passive movement condition could induce the illusion in completely naive subjects; that is, to remove any order effect of the blocked conditions in experiment one. In addition, this experiment used an objective measure of the illusion.
Subjects underwent a digital nerve block of the index finger as described for experiment one. After the block was clinically complete, subjects were set up in the experimental equipment, and the coupling on the rotatable shaft was locked so that movement between the plastic finger and the subject's finger was congruent. The subject was then instructed to keep the hand relaxed, while the experimenter held the distal segment of the plastic finger and moved it continuously into flexion and extension though an arc of about 30 deg for 3 min. The subject saw the experimenter moving the plastic finger and also felt (but could not see) their own finger performing exactly the same movement at exactly the same time.
After 3 min, the presence of an illusion of ownership over the plastic finger was measured objectively using a test similar to that used by Tsakiris & Haggard (2005). The subject was presented with a vertically aligned ruler marked with centimetre graduations. Each graduation line was numbered. The base of the ruler was placed on the table to the left of the subject's arm but at a distance the same as the distance to the tip of the subject's index finger. The subject was instructed to report “the number of the line that is level with the tip of your index finger”. When set up for the experiment, the plastic finger was in fact located 120 mm above the subject's finger. After the subject made a judgement, the ruler was removed and the subject filled out the questionnaire that was used for experiment one.
Twenty four hours later, after the subject had recovered completely from the nerve block, he or she underwent a second control condition. During this condition, the coupling on the rotatable shaft was disengaged so that the subject's finger and the plastic finger could move independently of each other. The subject was instructed to keep the hand relaxed, and the experimenter flexed and extended the subject's right index proximal interphalangeal joint through an arc of ∼30 deg for 3 min. At the same time, the experimenter also controlled the movement of the plastic finger to make movements that were similar in velocity and magnitude to the movements applied to the subject's finger, but were otherwise unrelated. After 3 min, the subject made a judgement of the elevation of their index finger using the ruler, as described above. However, the numbering system on the ruler was unrelated to the one used for the test condition the previous day.
Data and statistical analysis
For the responses taken from the questionnaire, each of the seven possible responses to the questions, ranging from ‘Disagree strongly’ to ‘Agree strongly’, was given an integer value that ranged from −3 to +3, respectively. A zero value corresponded to a response of ‘Unsure’. The data from each subject were pooled within conditions. The data were not normally distributed, so the median and interquartile ranges (IQRs) were calculated. Where questions were tested to determine whether the median answer was greater than zero (see Results, Figs 2 and 3) a Wilcoxon signed-rank test was used. When comparing passive movement with active movement, data for the blocked and intact conditions were pooled and when comparing a blocked finger with an intact finger, the data for passive movements and active movements were pooled. Differences between these integer values were used to indicate whether subjects had a more positive response to one condition than they did to the other (Fig. 4). Where the median responses to questions for experimental conditions were compared with each other (see Results, Fig. 4) a Wilcoxon paired sample test was used to determine whether there was a difference between conditions. The same test was used to determine whether the median response to questions for each of the test conditions were significantly different from each other (e.g. intact active versus blocked active) and to determine whether the test and control ruler judgements were significantly different. The threshold for significance was always P < 0.05. Data were analysed using Igor Pro version 6.12 (Wavemetrics, Lake Oswego, OR, USA).
Figure 2. Questionnaire responses for the group during the ‘intact synchronous touch’ condition, shown as medians (± IQR; n = 20).
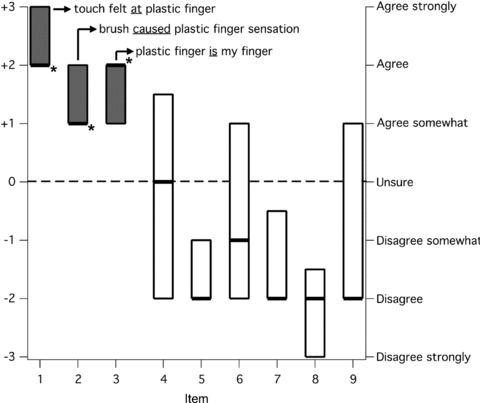
The items are the same as those used by Botvinick & Cohen (1998). Item 1: it seemed as if I were feeling the touch of the paintbrush at the location where I saw the plastic finger touched. Item 2: it seemed as though the touch I felt was caused by the paintbrush touching the plastic finger. Item 3: I felt as if the plastic finger were my finger. Item 4: I felt as if my (real) finger were drifting up (towards the plastic finger). Item 5: it seemed as if I might have more than one right index finger, hand or arm. Item 6: it seemed as if the touch I was feeling came from somewhere between my own finger and the plastic finger. Item 7: it felt as if my (real) finger were turning ‘plasticy’. Item 8: it appeared (visually) as if the plastic finger were drifting down (towards my finger). Item 9: the plastic finger began to resemble my own (real) finger, in terms of shape, skin tone, freckles or some other visual feature. The boxes show the interquartile ranges of the group data for the responses to each item, and the thick black line indicates the median response. The grey boxes show the three items that showed a positive response (i.e. > 0). These positive responses were significantly greater than zero (*P < 0.05) for each of these three items. Items 1 and 3 were used in the other conditions to measure the presence of an illusion of body ownership. Item 2 was not used because its meaning is ambiguous when directly translated to ‘movement’. Items 5, 7, 8 and 9 showed median responses that were significantly less than zero.
Figure 3. Median (± IQR) responses to items 1 and 3 for all control and congruent movement conditions.
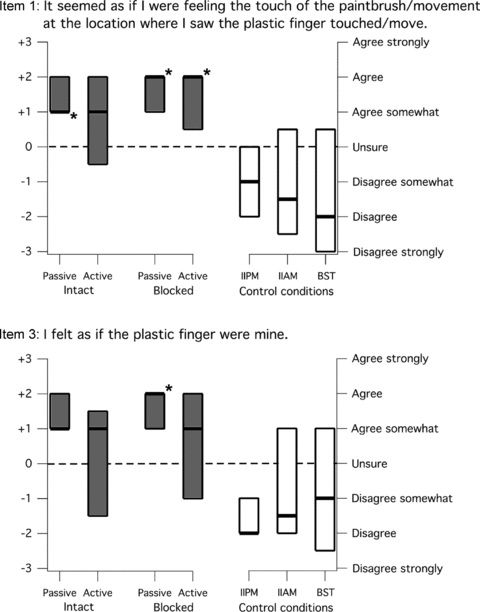
The boxes show the interquartile range of the group data for each item response, and the thick black line indicates the median response. Grey boxes show data for the experimental conditions where the movements of the plastic finger and the subject's finger were congruent. Passive and active congruent movements were tested for the intact index finger and the index finger after it had both its digital nerves blocked with local anaesthetic. An asterisk indicates a response that was significantly greater than zero (i.e. a response of ‘Agree somewhat’ or higher; P < 0.05). The open boxes show the data for the three control conditions: IIPM, intact incongruent passive movement; IIAM, intact incongruent active movement; and BST, blocked synchronous touch. No control condition showed a median positive response for either item. Item 1 had a median response that was significantly positive for all congruent movement conditions except active movement in an intact finger. The only significant positive response for item 3 was for congruent movement of a blocked and passive finger. Thus, subjects adopted the plastic finger into the body schema to some degree for the experimental conditions except for active congruent movements of the intact index finger.
Figure 4. Histograms of the difference between response of subjects for passive movement versus active movement, and a blocked finger versus an intact finger.
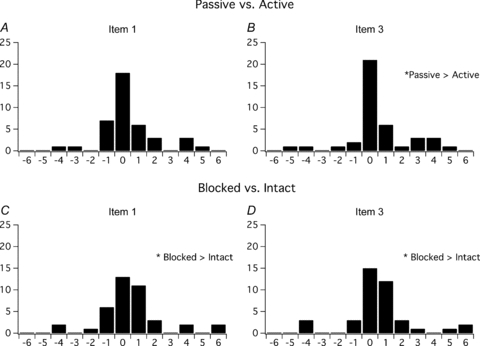
A and B show how many subjects had a given difference between responses for passive congruent movement and active congruent movement. Each subject provided two answers because there were two active and two passive conditions. A positive difference indicates that the subject responded with a more positive response for the passive task than the active task. Passive versus active differences for item 3 show that significantly more subjects gave a more positive response to the passive test conditions versus active test conditions (P < 0.05). C and D show how many subjects had a given difference between responses in conditions with a blocked finger and an intact finger. Each subject provided two answers because there were two blocked and two intact conditions. A positive difference shows that subjects provided a more positive response to the item during the digital nerve block. For both item 1 and item 3, significantly more subjects gave a more positive response for blocked test conditions versus intact test conditions (P < 0.05).
Results
We investigated whether non-tactile proprioceptive cues could reliably induce an illusion of ownership over a plastic finger. We coupled the subject's index finger to an artificial plastic finger so that the two moved in unison. We then flexed and extended the proximal interphalangeal joint passively or had subjects make the same movements voluntarily to induce an illusion of ownership of the artificial finger. Both of these conditions were performed with an intact finger, as well as after the digital nerves had been blocked. In addition, we tested key control conditions. During two of these, the movements of the subject's finger and the plastic finger were unrelated and during the third, a touch stimulus was used, but the subject's index finger was anaesthetized.
Experiment one
Basic condition
This basic condition produced a vivid illusion of ownership of the plastic finger in all 20 subjects. This condition involved tactile stimulation by stroking synchronously both the subject's finger and the plastic finger with the same movement. This was then chosen as the benchmark for induction of an illusion of ownership of the finger. This general method reliably induces an illusion of ownership of a rubber hand (Tsakiris & Haggard, 2005). Of the nine questionnaire items, three items showed a median response that was significantly greater than zero (P < 0.05; Fig. 2). The same three items have already been seen to indicate the presence of a rubber hand illusion (Giummarra et al. 2010). We excluded item 2 because a simple rewording of this item to make it relevant to movement also gave it ambiguous meaning (see Table 1). The item was ambiguous because the subjects knew that they or the experimenter was causing the movement. Many reported this ambiguity, whereas no subject questioned the meaning of any of the other items. We used subjects’ responses in the remaining two items to determine the presence of an illusion of finger ownership in the subsequent conditions.
Test conditions
The median (± IQR) responses to items 1 and 3 are shown for the four test conditions in Fig. 3. During all of these conditions, the plastic finger was coupled to the subject's right index finger so that both proximal interphalangeal joints moved in unison. When the subject's finger was intact and the movements were passive, 19 (of 20) subjects gave a positive response to item 1 or item 3, with 14 of those giving a positive response to both questions. When the movement was made actively by the subject, only 14 subjects gave a positive response to item 1 or 3, with 10 of those subjects giving a positive response to both items. When the subject's finger was blocked, 17 subjects gave a positive response to item 1 or item 3. If the movements were controlled by the experimenter, 16 of the 17 subjects gave a positive answer to both items, whereas only 12 gave a positive response to both items when the movements were made actively by the subject.
For item 1, significantly positive median responses (P < 0.05) occurred when the movements were passive, that is controlled by the experimenter (Fig. 3). A significantly positive group response was also found for active movements, but only when the finger was blocked. For item 3, we found one significant positive response (P < 0.05), which occurred when the subject's index finger was blocked and the movements were imposed on a passive finger. These results are consistent with comments from the subjects, which suggested that an illusion of ownership of the plastic finger was easier to induce and more vivid with passive movements and when the finger was blocked. Figure 3 shows a trend towards a positive response to item 3 when the finger was intact and the movements were passive, but the result was not significant. There was no significant difference between the responses to either item 1 or item 3 when comparing intact passive versus intact active, blocked passive versus blocked active, intact passive versus blocked passive or intact active versus blocked active. There were also no significant differences between the responses to items 1 and 3 for any of the test conditions when compared with the basic tactile condition.
An illusion of ownership of the plastic finger was induced in more subjects and was more vivid for passive movements than active movements and with a blocked finger rather than an intact finger. We calculated the difference between subjects’ responses both for passive movement versus active movement and for a blocked finger versus an intact finger (Fig. 4). These differences showed that significantly more subjects gave a more positive response to both items 1 and 3 when their finger was blocked compared with when it was not (P < 0.5). For passive movement compared with active movement, significantly more subjects gave a more positive response to item 3 (P < 0.05).
Control conditions
No more than six subjects gave a positive response to item 1 or item 3 for any of the control conditions. The first control condition used the same tactile cues as the basic condition except that the digital nerves of the subject's index finger were blocked, which eliminated tactile cues. This condition did not produce a significant median positive response to items 1 and 3 (Fig. 3), which means that no illusion of finger ownership was experienced. While some subjects reported a ‘strange feeling’, they did not report any feelings of the basic plastic finger illusion. The remaining two control conditions used movement as the stimulus, but this movement was not congruent between the subject's finger and the plastic finger. For these conditions, there was not a significant median positive response to items 1 and 3 (Fig. 3). In addition, the subjects gave no indication that they experienced a plastic finger illusion or any other strange perceptions during these two conditions.
Experiment two
The median responses to items 1 and 3 for both the test and control condition are shown in Fig. 5A. When their finger was blocked, eight (of 10) subjects gave a positive response to item 1 and nine (of 10) subjects gave a positive response to item 3. These subjects were completely naive to the illusion and the congruent passive movement stimulus. After the control condition that used incongruent passive movement, only one subject (of 10) gave a positive response to item 1 and only two subjects gave a positive response to item 3. For both items 1 and 3, a significantly positive median response (P < 0.001) was only found after congruent passive movement was applied to the blocked index finger (Fig. 5A). These median responses were also significantly different from the responses after the control stimulus (P < 0.001). These results show that the results of experiment one are not due to an effect of the order of conditions.
Figure 5. Median (± IQR) responses to items 1 and 2 and the median (± IQR) perceived elevation of the index finger.
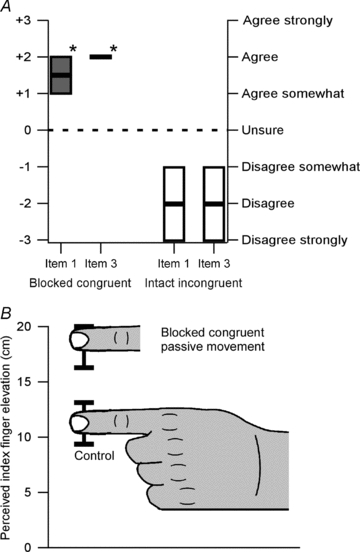
A shows the median responses to items 1 and 3 of the questionnaire after blocked congruent passive movement and intact incongruent passive moment. The thick black lines show the median and the boxes show the IQR. An asterisk indicates that the median response is significantly greater than zero (P < 0.001) and significantly different from the same item after the incongruent stimulus (P < 0.001). B shows the perceived elevation of the index finger above the table on which the subject's hand was resting. Zero represents the level of the table. The hand shows the perceived position after the control condition, and the disembodied finger shows the perceived position of the finger after the blocked congruent passive movement condition. The black bars show the interquartile ranges. The two conditions are significantly different from each other (P < 0.005).
After the test condition, subjects reported, on average, their index finger to be 19 cm above the table top (Fig. 5B). This was significantly more than the 11.5 cm reported after the control condition (P < 0.005). For individual subjects, nine (of 10) reported a greater elevation of their index finger after the test condition than after the control condition. For seven subjects, this difference was ≥7 cm. This objective test demonstrates that subjects experienced a proprioceptive displacement of their index finger during the illusion of body ownership.
Discussion
This study provides new insight into the physiological mechanisms underlying the sense of body ownership and the generation of the body schema. We used congruent movements of the proximal interphalangeal joint of the subject's right index finger and an artificial index finger. The combination of visual and proprioceptive stimuli caused the subjects to incorporate the plastic finger into their body schema and report that they felt as if the plastic finger was their finger, consistent with our hypothesis. This study produced one novel and indisputable result. When the digital nerves of the subject's finger were blocked with local anaesthetic, removing sensory input from skin and joint receptors, the visuo-proprioceptive stimuli still induced an illusion of ownership over the plastic finger. Furthermore, the illusion produced by congruent passive movement of the anaesthetized finger was associated with a perceived elevation of the real finger towards the location of the plastic finger. This was true even for a set of naive subjects who had not previously experienced the illusion. These results show that visuo-tactile cues are not critical for manipulation of the sense of body ownership and thus suggest that they would not be critical for establishing it. Furthermore, non-cutaneous proprioceptive cues, coupled with vision, are sufficient to establish body ownership. These results support our main hypothesis. The remainder of the Discussion considers other novel findings of the study.
Congruent movements performed under digital nerve block induced an illusion of finger ownership that was significantly stronger than the illusion of ownership that was induced by congruent movements performed with an intact finger. This result might not be predicted because joint and skin afferents in the digital nerves contribute to the perception of joint movement (Browne et al. 1954; Gandevia & McCloskey, 1976), so that anaesthesia of the finger reduces the proprioceptive information which is congruent with the visual information but strengthens the illusion. However, different classes of skin receptor differ in their contribution to proprioception. While some slowly adapting stretch receptors provide signals of joint movements (Edin & Johansson, 1995; Collins et al. 2005), some rapidly adapting skin receptors interfere with proprioceptive judgements. Vibration that excites Pacinian corpuscles reduces proprioceptive ability in the finger (Weerakkody et al. 2007, 2009). Thus, it may be that blocking the digital nerves removed a component of the finger's cutaneous input that interfered with the proprioceptive input used to establish the illusion of body ownership. However, it is also likely that, despite our best efforts, the signals from skin receptors in the subject's finger were not perfectly congruent with the visual stimulus. The pipe that held the subject's index finger (Fig. 1) was designed to mimic the way the experimenter held the plastic finger, but the tactile input, which the subject expected on the basis of visual input, and the actual tactile input from the pipe were almost certainly not identical. This slight mismatch in the passive conditions (greater mismatch in active conditions, see below) may impair the adoption of the plastic finger into the body schema. If this is the reason for the less vivid illusion with an intact finger than with a blocked finger, it shows that what is critical to manipulate the sense of body ownership is congruence between sensory stimuli. That is, fewer channels of perfectly congruent sensory information exert a stronger effect than more channels of imperfectly congruent sensory information.
Which of the peripheral signals arising proximal to the finger are likely to be contributing to the illusion of ownership of the artificial finger? The most obvious signals are derived from muscle spindle afferents that arise in the extrinsic and intrinsic hand muscles. They encode changes in joint position and movements (Matthews, 1972; Edin & Vallbo, 1990) and their population discharge produces illusory changes in these parameters (e.g. Goodwin et al. 1972; Gandevia, 1985; Macefield et al. 1990; Wise et al. 1996). However, while Golgi tendon organ afferents are unlikely to be driven powerfully by passive movement (Houk & Henneman, 1967; Stephens et al. 1975), a role for them and other proximal mechanoreceptors cannot be ruled out.
It has been shown that when subjects voluntarily control the movements used to induce an illusion of body ownership over a rubber hand, the illusion was ∼23% stronger than when the movements were passively imposed by the experimenter (Dummer et al. 2009). This is not surprising because the subject had ‘agency’ over the rubber hand. That is, the subject had a sense of intending and executing their own actions. This agency may be expected to strengthen the sense of body ownership because we normally have agency over our own body and things in contact with it, for example tools. Previous studies support this position (Tsakiris et al. 2006), but our results do not; active congruent movements (i.e. voluntary movements) produced an illusion that was the same or weaker than that produced by passive congruent movements (Fig. 4). Perhaps this was due to the greater incongruence between the tactile and visual information in the active conditions. In the active conditions, the subject's finger was still held by the apparatus, but the plastic finger was not held by the experimenter. However, if this was the only reason for a weaker illusion, then it would be expected that blocking the digital nerves of the finger and removing all tactile information would make the illusion induced by active movements stronger than that induced by passive movements. This did not occur. While anaesthesia of the finger significantly increased the strength of the illusion of ownership over the plastic finger, we found no significant difference between the illusions induced by intact active movements and those induced by blocked active movements. Furthermore, the data suggest that during the nerve block the active movements still induced a similar, or weaker, illusion of ownership over the plastic finger than the passive movements did (Fig. 3). An alternative explanation is that agency may not be critical to establish body ownership because agency is not unique to our body. We can exert agency over tools and other external objects (e.g. Maravita & Iriki, 2004). In contrast, congruence between vision and tactile or proprioceptive input is unique to our body parts, because no external object can provide the brain with tactile or proprioceptive signals. Psychology studies have shown that agency and body ownership are dissociable (e.g. Longo et al. 2008). A dissociation between agency and body ownership has also been shown with neuroimaging (Tsakiris et al. 2010), although this study also suggested that questionnaire data may not reflect the dissociation. Another point to consider in the comparison of passive movements versus active movements is that there is fusimotor activation of muscle spindles in active movements (e.g. Vallbo, 1971; Burke et al. 1976). This makes the processing of spindle signals more complex and it may change the way in which their population discharge is interpreted (Dimitriou & Edin, 2008a,b, 2010). It is possible that coherent input from populations of spindles in passive muscles is more easily decoded as a useful signal that can influence body ownership (Prochazka & Gorassini, 1998).
Some subjects reported a strange feeling after they had been set up in the apparatus, but before any stimuli had been presented. In this situation, the only information about the plastic finger is from the visual system, signalling that the plastic finger is in a position and posture that could be adopted by the subject's own finger. Importantly, despite this feeling, these subjects did not report that they felt any ownership over the plastic finger. In fact, these anecdotal reports and the results from the control conditions show that visual stimuli alone were not enough to establish the sense of body ownership over the finger. Vision must be coupled with congruent proprioceptive or tactile signals for subjects to adopt the plastic finger as their own. Of course, that the rubber hand illusion can be induced without visual input (Ehrsson et al. 2005) emphasizes the importance of cross-modal congruence rather than visual input.
In summary, we have shown that non-tactile proprioceptive cues contribute to the sense of body ownership and that signals from skin receptors are not essential. The quality of the congruence between vision and tactile or proprioceptive cues is more important than having multiple congruent sensory modes, and we find no evidence that voluntarily controlled stimuli can induce stronger illusions of body ownership than externally imposed stimuli.
Acknowledgments
This work is funded by the National Health and Medical Research Council of Australia.
Author contributions
Each author contributed to all aspects of the study. All experiments were performed at Neuroscience Research Australia (formerly Prince of Wales Medical Research Institute) in Sydney, NSW, Australia.
References
- Armel KC, Ramachandran VS. Projecting sensations to external objects: evidence from skin conductance response. Proc Biol Sci. 2003;270:1499–1506. doi: 10.1098/rspb.2003.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austen EL, Soto-Faraco S, Enns JT, Kingstone A. Mislocalizations of touch to a fake hand. Cogn Affect Behav Neurosci. 2004;4:170–181. doi: 10.3758/cabn.4.2.170. [DOI] [PubMed] [Google Scholar]
- Boesebeck F. Paroxysmal alien limb phenomena due to epileptic seizures and electrical cortical stimulation. Neurology. 2004;63:1725–1727. doi: 10.1212/01.wnl.0000143064.81746.e9. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Cohen J. Rubber hands ‘feel’ touch that eyes see. Nature. 1998;391:756. doi: 10.1038/35784. [DOI] [PubMed] [Google Scholar]
- Browne K, Lee J, Ring PA. The sensation of passive movement at the metatarso-phalangeal joint of the great toe in man. J Physiol. 1954;126:448–458. doi: 10.1113/jphysiol.1954.sp005221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Gandevia S, Macefield G. Response to passive movement of receptors in joint, skin and muscle of the human hand. J Physiol. 1988;402:347–361. doi: 10.1113/jphysiol.1988.sp017208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Hagbarth KE, Lofstedt L, Wallin BG. The responses of human muscle spindle endings to vibration during isometric contraction. J Physiol. 1976;261:695–711. doi: 10.1113/jphysiol.1976.sp011581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Refshauge KM, Todd G, Gandevia SC. Cutaneous receptors contribute to kinesthesia at the index finger, elbow, and knee. J Neurophysiol. 2005;94:1699–1706. doi: 10.1152/jn.00191.2005. [DOI] [PubMed] [Google Scholar]
- Costantini M, Haggard P. The rubber hand illusion: sensitivity and reference frame for body ownership. Conscious Cogn. 2007;16:229–240. doi: 10.1016/j.concog.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Dimitriou M, Edin BB. Discharges in human muscle receptor afferents during block grasping. J Neurosci. 2008a;28:12,632–12,642. doi: 10.1523/JNEUROSCI.3357-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriou M, Edin BB. Discharges in human muscle spindle afferents during a key-pressing task. J Physiol. 2008b;586:5455–5470. doi: 10.1113/jphysiol.2008.160036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriou M, Edin BB. Human muscle spindles act as forward sensory models. Curr Biol. 2010;20:1763–1767. doi: 10.1016/j.cub.2010.08.049. [DOI] [PubMed] [Google Scholar]
- Dummer T, Picot-Annand A, Neal T, Moore C. Movement and the rubber hand illusion. Perception. 2009;38:271–280. doi: 10.1068/p5921. [DOI] [PubMed] [Google Scholar]
- Edin BB, Johansson N. Skin strain patterns provide kinesthetic information to the human central nervous system. J Physiol. 1995;487:243–251. doi: 10.1113/jphysiol.1995.sp020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin BB, Vallbo AB. Dynamic response of human muscle spindle afferents to stretch. J Neurophysiol. 1990;63:1297–1306. doi: 10.1152/jn.1990.63.6.1297. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Holmes NP, Passingham RE. Touching a rubber hand: feeling of body ownership is associated with activity in multisensory brain areas. J Neurosci. 2005;25:10,564–10,573. doi: 10.1523/JNEUROSCI.0800-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrsson HH, Spence C, Passingham RE. That's my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science. 2004;305:875–877. doi: 10.1126/science.1097011. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Wiech K, Weiskopf N, Dolan RJ, Passingham RE. Threatening a rubber hand that you feel is yours elicits a cortical anxiety response. Proc Natl Acad Sci USA. 2007;104:9828–9833. doi: 10.1073/pnas.0610011104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg TE, Venneri A, Simone AM, Fan Y, Northoff G. The neuroanatomy of asomatognosia and somatoparaphrenia. J Neurol Neurosurg Psychiatry. 2010;81:276–281. doi: 10.1136/jnnp.2009.188946. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Illusory movements produced by electrical stimualtion of low-threshold muscle afferents from the hand. Brain. 1985;108:965–981. doi: 10.1093/brain/108.4.965. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, McCloskey DI. Joint sense, muscle sense, and their combination as position sense, measured at the distal interphalangeal joint of the middle finger. J Physiol. 1976;260:387–407. doi: 10.1113/jphysiol.1976.sp011521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giummarra MJ, Georgiou-Karistianis N, Nicholls MER, Gibson SJ, Bradshaw JL. The phantom in the mirror: a modified rubber-hand illusion in amputees and normals. Perception. 2010;39:103–118. doi: 10.1068/p6519. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Matthews PBC. The contribution of muscle afferents to kinæsthesia shown by vibration induced illusion of movement and by the effects of paralysing joint afferents. Brain. 1972;95:705–748. doi: 10.1093/brain/95.4.705. [DOI] [PubMed] [Google Scholar]
- Houk J, Henneman E. Responses of Golgi tendon organs to active contractions of the soleus muscle of the cat. J Neurophysiol. 1967;30:466–481. doi: 10.1152/jn.1967.30.3.466. [DOI] [PubMed] [Google Scholar]
- Hulliger M, Nordh E, Thelin AE, Vallbo AB. The responses of afferent fibres from the glabrous skin of the hand during voluntary finger movements in man. J Physiol. 1979;291:233–249. doi: 10.1113/jphysiol.1979.sp012809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo MR, Schüür F, Kammers MPM, Tsakiris M, Haggard P. What is embodiment? A psychometric approach. Cognition. 2008;107:978–998. doi: 10.1016/j.cognition.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Macefield G, Gandevia SC, Burke D. Perceptual responses to microstimulation of single afferents innervating joints, muscles and skin of the human hand. J Physiol. 1990;429:113–129. doi: 10.1113/jphysiol.1990.sp018247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maravita A, Iriki A. Tools for the body (schema) Trends Cogn Sci. 2004;8:79–86. doi: 10.1016/j.tics.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Matthews PBC. Mammalian Muscle Receptors and their Central Actions. London: Edward Arnold Ltd; 1972. [Google Scholar]
- Moseley GL, Olthof N, Venema A, Don S, Wijers M, Gallace A, Spence C. Psychologically induced cooling of a specific body part caused by the illusory ownership of an artificial counterpart. Proc Natl Acad Sci USA. 2008;105:13,169–13,173. doi: 10.1073/pnas.0803768105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavani F, Spence C, Jon D. Visual capture of touch: out-of-the-body experiences with rubber gloves. Psychol Sci. 2000;11:353–359. doi: 10.1111/1467-9280.00270. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Gorassini M. Ensemble firing of muscle afferents recorded during normal locomotion in cats. J Physiol. 1998;507:293–304. doi: 10.1111/j.1469-7793.1998.293bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M, Flor H, Heinze H-J, Rotte M. Dynamic modulation of the primary somatosensory cortex during seeing and feeling a touched hand. Neuroimage. 2006;29:587–592. doi: 10.1016/j.neuroimage.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Stephens JA, Reinking RM, Stuart DG. Tendon organs of cat medial gastrocnemius: responses to active and passive forces as a function of muscle length. J Neurophysiol. 1975;38:1217–1231. doi: 10.1152/jn.1975.38.5.1217. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Haggard P. The rubber hand illusion revisited: visuotactile integration and self-attribution. J Exp Psychol Hum Percept Perform. 2005;31:80–91. doi: 10.1037/0096-1523.31.1.80. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Hesse MD, Boy C, Haggard P, Fink GR. Neural signatures of body ownership: a sensory network for bodily self-consciousness. Cereb Cortex. 2007;17:2235–2244. doi: 10.1093/cercor/bhl131. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Longo MR, Haggard P. Having a body versus moving your body: neural signatures of agency and body-ownership. Neuropsychologia. 2010;48:2740–2749. doi: 10.1016/j.neuropsychologia.2010.05.021. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Prabhu G, Haggard P. Having a body versus moving your body: how agency structures body-ownership. Conscious Cogn. 2006;15:423–432. doi: 10.1016/j.concog.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Vallbo ÅB. Muscle spindle response at the onset of isometric voluntary contractions in man. Time difference between fusimotor and skeletomotor effects. J Physiol. 1971;218:405–431. doi: 10.1113/jphysiol.1971.sp009625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerakkody NS, Mahns DA, Taylor JL, Gandevia SC. Impairment of human proprioception by high-frequency cutaneous vibration. J Physiol. 2007;581:971–980. doi: 10.1113/jphysiol.2006.126854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerakkody NS, Taylor J, Gandevia S. The effect of high-frequency cutaneous vibration on different inputs subserving detection of joint movement. Exp Brain Res. 2009;197:347–355. doi: 10.1007/s00221-009-1921-3. [DOI] [PubMed] [Google Scholar]
- Wise A, Gregory J, Proske U. The effects of muscle conditioning on movement detection thresholds at the human forearm. Brain Res. 1996;735:125–130. doi: 10.1016/0006-8993(96)00603-8. [DOI] [PubMed] [Google Scholar]


