Non-technical summary
Skeletal muscle comprises ∼40% of total body mass, and the control of muscle mass has significant effects on overall health. Skeletal muscle mass is determined by the balance of protein synthesis and degradation within muscle cells. We sought to determine which cellular proteins that control protein synthesis within muscle cells are associated with muscle growth after resistance exercise, a potent growth stimulus. We identified two proteins that were associated with muscle growth in humans: p70S6K and eIF2Bɛ. Follow up studies determined that eIF2Bɛ alone is sufficient to induce muscle growth. This is the first study to determine that this protein can induce skeletal muscle growth. These results further our understanding of how skeletal muscle responds to resistance exercise.
Abstract
Abstract
The purpose of this study was to identify signalling components known to control mRNA translation initiation in skeletal muscle that are responsive to mechanical load and may be partly responsible for myofibre hypertrophy. To accomplish this, we first utilized a human cluster model in which skeletal muscle samples from subjects with widely divergent hypertrophic responses to resistance training were used for the identification of signalling proteins associated with the degree myofibre hypertrophy. We found that of 11 translational signalling molecules examined, the response of p(T421/S424)-p70S6K phosphorylation and total eukaryotic initiation factor 2Bɛ (eIF2Bɛ) protein abundance after a single bout of unaccustomed resistance exercise was associated with myofibre hypertrophy following 16 weeks of training. Follow up studies revealed that overexpression of eIF2Bɛ alone was sufficient to induce an 87% increase in cap-dependent translation in L6 myoblasts in vitro and 21% hypertrophy of myofibres in mouse skeletal muscle in vivo (P < 0.05). However, genetically altering p70S6K activity had no impact on eIF2Bɛ protein abundance in mouse skeletal muscle in vivo or multiple cell lines in vitro (P > 0.05), suggesting that the two phenomena were not directly related. These are the first data that mechanistically link eIF2Bɛ abundance to skeletal myofibre hypertrophy, and indicate that eIF2Bɛ abundance may at least partially underlie the widely divergent hypertrophic phenotypes in human skeletal muscle exposed to mechanical stimuli.
Introduction
Translation initiation pathways, including PI3K/ Akt/mTOR and eIF2B, are key determinates of cell size regulation in a number of model systems including skeletal muscle (Bodine et al. 2001; Rommel et al. 2001; Hardt et al. 2004; Lai et al. 2004). Furthermore, mechanical load, a stimulus that induces skeletal muscle cell (i.e. myofibre) hypertrophy, increases the activity of translation initiation pathways in both animal (Baar & Esser, 1999; Parkington et al. 2003) and human (Blomstrand et al. 2006; Coffey et al. 2006; Dreyer et al. 2006; Mayhew et al. 2009) muscle leading to increased functional and metabolic reserves of the tissue. It is therefore important to identify which endogenous proteins controlling mRNA translation initiation are most influential in determining myofibre hypertrophy in response to mechanical load.
Translation initiation is thought to be the rate limiting step in protein synthesis (Augert et al. 1986). An extensively studied signalling pathway that has been shown to increase translation initiation rates of 5′-capped mRNA is that involving the serine/threonine kinase mammalian target of rapamycin (mTOR). As a component of the mTOR complex 1 (mTORC1), mTOR phosphorylates at least two downstream targets that control translation: eIF4E binding protein 1 (4E-BP1) and the 70 kDa isoform of ribosomal protein S6 kinase 1 (p70S6K). Together, mTORC1-dependent signalling aids in the creation of a mature, translation-competent ribonucleoprotein complex.
Another component of the translational machinery that exerts rate-limiting influence over translation rates in some scenarios is eIF2 (Kimball et al. 1998). The GTP-bound form of this initiation factor aids in initiator methionine tRNA (Met-tRNAi) binding to the AUG start codon, thereby supplying the initial amino acid in a nascent polypeptide chain. Recycling of GDP for GTP on eIF2 is performed by eIF2B, which is composed of α–ɛ subunits (Webb & Proud, 1997). Importantly, the cellular abundance of the catalytic ɛ subunit was shown to be regulated by mechanical load in skeletal muscle in vivo at the level of mRNA translation (Kubica et al. 2005), while exogenous overexpression of eIF2Bɛ was sufficient to increase eIF2B guanine nucleotide exchange factor (GEF) activity (Tuckow et al. 2010) and overall protein synthesis (Balachandran & Barber, 2004; Hardt et al. 2004). Phosphorylation of eIF2Bɛ can occur at numerous sites and by multiple kinases, and is generally considered to be inhibitory. Most well-characterized is the GSK3β-directed inhibition of eIF2Bɛ by S539 (corresponding to S535 in mouse) phosphorylation. To date, the specific contribution of increased eIF2Bɛ abundance or activity to the overall phenotypic outcome of myofibre hypertrophy remains unclear.
The specific signalling proteins altered by an acute bout of unaccustomed mechanical load have been interpreted as implicit drivers of myofibre hypertrophy after a period of repeated exposures to mechanical stimuli (i.e. training) in humans (Dreyer et al. 2008; Wilkinson et al. 2008; Burd et al. 2010). However, the multifaceted nature of the signalling response of mechanically loaded skeletal muscle makes it particularly difficult to segregate mechanisms that are instructive for, as opposed to simply permissive of or coincident with, myofibre hypertrophy in vivo. We have previously applied K-means cluster analysis to delineate humans that experience robust (extreme responders, XR), moderate (moderate responders, MR), or no hypertrophy (non-responders, NR) in response to a standardized lower extremity resistance training programme (Bamman et al. 2007; Kim et al. 2007; Petrella et al. 2008). Given the limitations of human research, this cluster-centred model may be the most robust analytical approach available to reveal the regulatory mechanisms mediating mechanical load-induced hypertrophy as opposed to those induced by mechanical load per se but not instructive for hypertrophy.
The purpose of this study was to identify translational signalling components in skeletal muscle that are responsive to mechanical load and may be partly responsible for myofibre hypertrophy in vivo. To accomplish this, we employed a multilayered approach beginning with a human cluster model, followed by targeted studies in vitro and in mice in vivo. We found that of 11 translational signalling molecules examined in humans, only the response of p(T421/S424)-p70S6K phosphorylation and eIF2Bɛ protein abundance 24 h after a single bout of unaccustomed resistance exercise was coincident with myofibre hypertrophy following 16 weeks of resistance training. Overexpression of eIF2Bɛ alone was sufficient to induce cap-dependent translation in vitro and hypertrophy in mouse skeletal muscle in vivo. However, genetically altering p70S6K activity had no influence over eIF2Bɛ protein abundance. These are the first data that mechanistically link eIF2Bɛ abundance to skeletal myofibre hypertrophy, and that this may at least partially underlie the widely divergent hypertrophic phenotypes in human muscle exposed to mechanical stimuli.
Methods
Ethical approval
The project was conducted in accordance with the standards set forth by the Declaration of Helsinki and was approved by the Institutional Review Boards of both the University of Alabama at Birmingham and the Birmingham Veterans’ Affairs Medical Center. Written informed consent was obtained from each volunteer prior to participation. All animal experiments in this study followed protocols approved by the Animal Care and Use Committee at the University of Wisconsin – Madison, and were in accordance with the guidelines of The Journal of Physiology (Drummond, 2009).
Plasmid construction
Plasmid DNA containing mouse eIF2Bɛ (IMAGE clone ID 4211776) was purchased from Invitrogen (Carlsbad, CA, USA). Both the full-length and C-terminal deletion mutant (deleted after amino acid 529) were subcloned, each with the addition of a C-terminal HA-tag, into the pRK7 vector using SalI/EcoRI sites. Green fluorescent protein (GFP) was cloned into the same vector using identical sites. Myc-tagged p70S6K mutants (T389E and T389A) were kindly provided by Dr. George Thomas (University of Cincinnati) and have been described previously (Pearson et al. 1995). The pΔEMCV luciferase reporter (Carter & Sarnow, 2000) was a gift from Dr. Sunnie Thompson (University of Alabama at Birmingham). The plasmids encoding GFP, HA-tagged Ras homologue enriched in the brain (Rheb), and glutathione S-transferase (GST)-tagged p70S6K used in mouse overexpression studies have been previously described (Goodman et al. 2010).
Human subjects
Sixty-six adults recruited from the Birmingham, Alabama metropolitan area completed the 16 week resistance training study. All participants were screened by a health history questionnaire and older subjects also passed a physical exam and graded exercise stress test. Subjects were not obese (BMI <30) and free of any musculoskeletal or other disorders that might have affected their ability to complete testing and/or resistance training. Subjects had not experienced any leg resistance training in the 5 years prior to initiating the study protocol. None of the subjects were being treated with pharmacological interventions that are known to influence muscle mass or that may have interacted with the exercise stimulus (such as testosterone, GH, IGF-1, or immunosuppressive therapy).
Using statistical (K-means) cluster analysis, subjects were classified post hoc into three clusters based on changes in vastus lateralis mean myofibre cross sectional area (MFA, μm2) across 16 weeks training as described previously (Bamman et al. 2007). The resulting three clusters – XR (n = 17; change in MFA ± SEM =+2,475 ± 140 μm2), MR (n = 32; +1,111 ± 46 μm2), and NR (n = 17; −16 ± 99 μm2) – were characterized in detail elsewhere (Bamman et al. 2007). It is noteworthy for this report that the clusters did not differ in training intensity, volume, or weekly adherence to the programme. A random subset of subjects (n = 8–10 XR, 13–15 MR, and 8 NR) was analysed for translation initiation markers in the current study.
Resistance training
A more extensive description of the resistance training programme performed by these subjects is provided elsewhere (Bamman et al. 2007). Briefly, participants completed a 16 week progressive training regimen consisting of three sets of 8–12 repetitions to volitional fatigue with 90 s recovery between sets on the squat, machine leg press, and knee extension exercises performed 3 times per week. All participants were supervised by a Certified Strength and Conditioning Specialist (National Strength and Conditioning Association) or Certified Health Fitness Instructor (American College of Sports Medicine).
Human tissue collection and processing
Fasted morning muscle biopsy samples were collected using routine methods (Evans et al. 1982) at baseline, 24 h after the first full bout of resistance exercise at week 1, and 24 h after a bout of resistance exercise at week 16 of the training programme. We chose to sample at 24 h post-exercise since muscle protein synthesis has been found to be elevated at this time point (Phillips et al. 1997), and indeed we have found this to be the case in a subset of the subjects examined here (Mayhew et al. 2009). Although many transient signalling events are likely to return to baseline levels by 24 h, we postulated that the 24 h time point would allow us to capture key events that drive protein synthesis during recovery without interference from the myriad processes activated during and shortly after resistance exercise. Approximately 70 mg of tissue were mounted cross-sectionally in liquid nitrogen-cooled isopentane for subsequent histological analysis and the remainder was weighed, divided, and snap frozen (30–35 mg per tube) in liquid nitrogen. Snap frozen samples were separated into cytosolic and membrane fractions as described previously (Mayhew et al. 2009), with Western blotting performed on the cytosolic fraction only.
Animals
Eighteen female FVB mice 8–10 weeks of age were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and randomly assigned to one of three experimental groups: GFP (n = 7), eIF2Bɛ (n = 5), and eIF2Bɛ-S535A (n = 6). Mice used for expression of GFP (n = 3), GFP + GST-p70S6K (n = 3), or HA-Rheb + GST-p70S6K (n = 4) were described previously (Goodman et al. 2010).
Skeletal muscle transfection
Mice were anaesthetized with 100 mg kg−1 ketamine plus 10 mg kg−1 xylazine and the tibialis anterior (TA) muscle was transfected using electroporation as previously described (Goodman et al. 2010) with 2.5 μg μl−1 for GFP, 4.0 μg μl−1 for eIF2Bɛ-HA, or 4.0 μg μl−1 eIF2Bɛ-S535A-HA dissolved in 12 μl sterile phosphate buffered saline (PBS). Similar methods were used for the injection of GFP, HA-Rheb, and GST-p70S6K (Goodman et al. 2010). Mice were allowed to recover for 2 or 7 days before muscle harvest, depending upon the experiment.
Measurements of transfected muscle fibre cross sectional area
Mice were killed by cervical dislocation, after which muscles were excised and fixed in ice cold PBS containing 4% paraformaldehyde with gentle rocking at 4°C for 30 min. The fixed muscles were submerged in optimal cutting temperature compound and frozen in liquid nitrogen chilled isopentane. Cross sections (10 μm thick) from the mid-belly of the muscle were obtained with a cryostat and fixed in −20°C acetone for 10 min. Sections were warmed to room temperature for 5 min and then rehydrated with cool steam vapours. Under gentle rocking, the rehydrated sections were incubated in PBS for 15 min followed by a 20 min incubation in solution A (PBS containing 0.5% bovine serum albumin (BSA) and 0.5% Triton X-100). Sections were then incubated with rabbit anti-laminin (Sigma-Aldrich, St Louis, MO, USA) and rat anti-HA (Roche, Madison, WI, USA) antibodies dissolved in solution A for 1 h at room temperature. Sections were washed with PBS and then incubated with anti-rabbit TRITC-conjugated and anti-rat FITC conjugated secondary antibodies (Santa Cruz Biotechnologies, Santa Cruz, CA, USA) dissolved in solution A for 1 h at room temperature. Finally, the sections were washed with PBS and mounted with Vectashield mounting media (Vector Laboratories, Burlingame, CA, USA). Transfected fibres (HA or GFP positive) and laminin were identified in dual fluorescence images captured through FITC and TRITC cubes on a Nikon 80i epi-fluorescence microscope. The images were merged with Nikon NIS Elements D image analysis software and the cross-sectional area (CSA) of 30–90 randomly selected transfected and non-transfected fibres per sample were measured by tracing the laminin stain of individual fibres. All CSA analyses were performed by investigators blinded to the treatment. The CSA of the transfected and non-transfected fibres were plotted on a histogram and the average CSA of each was calculated for each sample.
Cell culture
C2C12, L6 (ATCC, Manassas, VA, USA), and HEK293T cells (a gift from Dr. Sunnie Thompson, University of Alabama at Birmingham) were propagated in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mm Glutamax (Invitrogen), 50 U ml−1 penicillin, and 50 μg ml−1 streptomycin. All cells were grown in a 37°C humidified incubator at 5% CO2.
Transient transfections
C2C12 and L6 cells were grown to 80–90% confluence on six-well plastic plates. Cells were trypsinized, re-plated onto collagen-I (6.7 μg cm−2) coated plates, and immediately transfected with 2 μg of either constitutively active (T389E) or dominant negative (T389A) myc-tagged p70S6K, or pRK5 empty vector control using Lipofectamine 2000 (Invitrogen) per the manufacturer's instructions. HEK293T cells were transfected while adherent. The day following transfection C2C12 and HEK293T were exposed to serum-free medium for 24 h prior to harvest, while L6 cells were exposed to 0.5% FBS-containing medium (complete serum starvation results in L6 cell death in our hands). Cells were harvested in modified RIPA buffer (50 mm Tris-HCl pH 7.4, 150 mm NaCl, 1 mm EDTA, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% SDS), to which was added 1:100 each Sigma P2714 (4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF), N-(trans-epoxysuccinyl)-l-leucine-4-guanidinobutylamide (E-64), bestatin, leupeptin, aprotinin, and Na-EDTA), P2850 (microcystin LR, cantharidin, and bromotetramisole), and P5726 (sodium vanadate, sodium molybdate, sodium tartrate, and imidazole) and homogenized with repeated pipette tip flushing. Lysates were centrifuged at 5000 g for 5 min and equal protein loads of the soluble fraction, as determined by the BCA (bicinchoninic acid) protein assay, were subjected to Western blotting as described below. Luciferase reporter experiments were carried out on 12-well plates of L6 myoblasts transfected with 20 ng of pΔEMCV luciferase reporter and 800 ng of either wild-type mouse eIF2Bɛ-HA, eIF2Bɛ-ΔC-HA, or GFP. Twenty-four hours after transfection cells were cultured in 0.5% FBS-containing medium for an additional 24 h before determination of Renilla luciferase activity. All experiments were carried out in quadruplicate.
Immunoblotting
Immunoblotting was performed on lysates using established methods in our laboratory (Bamman et al. 2004). Samples were run on 4–15% Tris-HCl SDS-PAGE (Bio-Rad) or 4–12% Bis-Tris SDS-PAGE (Invitrogen) gel matrices with 35 μg (in vivo human and mouse lysates) or 20 μg (in vitro cell lysates) total protein loaded into each well and transferred to PVDF membranes. Primary antibodies used included p(S473)- (no. 4051) and total (no. 9272) Akt, Rheb (no. 4935), p(S2448)- (no. 2971) and total (no. 2972) mTOR, p(T421/S424)- (no. 9204), p(T389)- (no. 9206), and total (no. 9206) p70S6K, p(S21/S9)-GSK3α/β (no. 9331), total GSK3β (no. 9315), p(T37/46)- (no. 9459) and total (no. 9452) 4E-BP1, p(S209)- (no. 9741) and total (no. 9742) eIF4E, p(S1108)- (no. 2441) and total (no. 2498) eIF4G, total eIF2Bɛ (no. 3595), p(S240/244)- (no. 4838) and total (no. 2217) rpS6, GFP (no. 2555), α-tubulin (no. 2125), myc-tag (no. 2278), mouse HA-tag (no. 2367), and p(T197/202)-Mnk1 (no. 2111), which were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Antibodies specific for the p85 subunit of PI3K (no. 06–497) were purchased from Upstate Biotechnology, Inc. (Lake Placid, NY, USA), total Mnk1 (no. sc-28780) was from Santa Cruz Biotechnology, and rat HA-tag (no. 3F10) was from Roche Diagnostics Corp (Indianapolis, IN, USA). Ideal primary antibody concentrations were determined in preliminary experiments and were 1:2000 (v/v) for PI3K(p85) and GFP, 1:2500 for p(S21/S9)-GSK3α/β, GSK3β, p(S240/244)-rpS6, rpS6, p70S6K, and Rheb, 1:3000 for myc-tag, and rat HA-tag, 1:5000 for mouse HA-tag and α-tubulin, and 1:1000 for all other antibodies. HRP-conjugated secondary antibody was used at 1:50,000 (w/v) followed by chemiluminescent detection with SuperSignal West Dura or Femto substrate (Thermo Scientific, Rockford, IL, USA) in a Bio-Rad ChemiDoc imaging system with band densitometry performed using Bio-Rad Quantity One (software package 4.5.1). Parameters for image development in the ChemiDoc were consistent across all membranes using predefined saturation criteria for the CCD camera.
RT-PCR
Transfected L6 myoblasts were harvested in 1 ml Tri-reagent (Molecular Research Center, Inc., Cincinnati, OH, USA) to which was added 100 ul BCP (Molecular Research Center, Inc.), vortexed for 1 min, and centrifuged at 13,000 g for 10 min at 4°C. An equal volume of isopropanol was added to the supernatant, pelleted at 13,000 g for 15 min, and washed twice with 75% ethanol in nuclease free water. The remaining pellet was air dried and resuspended in nuclease free water. One or three micrograms of RNA was reverse transcribed using SuperScript III (Invitrogen) and oligo-dT primers per the manufacturer's instructions. PCR of the cDNA product was carried out as described (Bamman et al. 2007) using Renilla luciferase primers published elsewhere (Butcher et al. 2005) and the following β-actin primers: forward 5′-GAGAGGGAAATCGTGCGTGAC-3′, reverse 5′-CATCTGCTGGAAGGTGGACA-3′.
Luciferase activity
Transfected L6 cells plated on 12-well plates were lysed in 250 μl Passive Lysis Buffer (Promega, Madison, WI, USA). Renilla luciferase activity was determined with the Dual Luciferase Reporter assay kit (Promega) using a Berthold Lumat LB9507 luminometer.
Data analysis
Human response cluster data were subjected to 3 × 3 (response cluster × time) repeated measures ANOVA for each protein to test differences in total protein expression levels and phosphorylation state among clusters and across time. Tukey's HSD test was performed post hoc to localize main effects, while Fisher's LSD test was used to localize interaction effects. For the in vivo mouse experiments, myofibre CSA data for transfected vs. non-transfected fibres in each condition were analysed using Student's t test. All other data were analysed by one-way ANOVA and, when appropriate, Tukey's HSD test performed post hoc. For all statistics, significance was set at the P = 0.05 level.
Results
Translation initiation signalling proteins not differentially regulated by human response cluster
We have previously reported on three distinct phenotypic groups of humans that differ in their hypertrophic responsiveness to 16 weeks of resistance training (Bamman et al. 2007). Further, it is established that mechanical load-induced myofibre hypertrophy is regulated in large part at the level of mRNA translation initiation (Augert et al. 1986; Kimball et al. 2002). We thus chose to investigate which signalling components that control translation initiation were differentially regulated among response clusters and may account for the observed phenotypic disparities.
Repeated measures ANOVA revealed main time effects (P < 0.05) for p(S473)-Akt, which increased 51% at week 1 (all percentage increases are with respect to baseline unless otherwise noted), and total Akt, which increased 37% at week 16 (Fig. 1A). These changes were noted without any change in the total content of the p85 regulatory subunit of PI3K, an upstream regulator of Akt (data not shown). GSK3 is a known downstream target of Akt and negative regulator of eIF2Bɛ catalytic activity (Bolster et al. 2004). Phosphorylation (inhibition) of the α isoform of this protein increased 15% at week 16 (P < 0.05); however it did so equally in all response clusters (Fig. 1B). Despite the increase in p(S473)-Akt at week 1, neither an mTOR phosphorylation site downstream of Akt, S2448, nor total mTOR levels were altered at any time point (Fig. 1C). Downstream of mTOR, the phosphorylation state of the eIF4E binding protein, 4E-BP1, trended toward significance (P = 0.052), with a 23% increase occurring from baseline to week 1, while the total expression level of 4E-BP1 was significantly decreased by 25% at week 16 (P < 0.05) (Fig. 1D). Thus, while the levels of p(S473)-Akt, total Akt, p(S21)-GSK3α, and total 4E-BP1 were altered significantly by mechanical load, these alterations occurred to an equal degree in XR, MR, and NR and could therefore not explain the differences in hypertrophy noted among these groups.
Figure 1. Resistance exercise-induced changes in translational signalling components not altered differentially by hypertrophy response cluster.
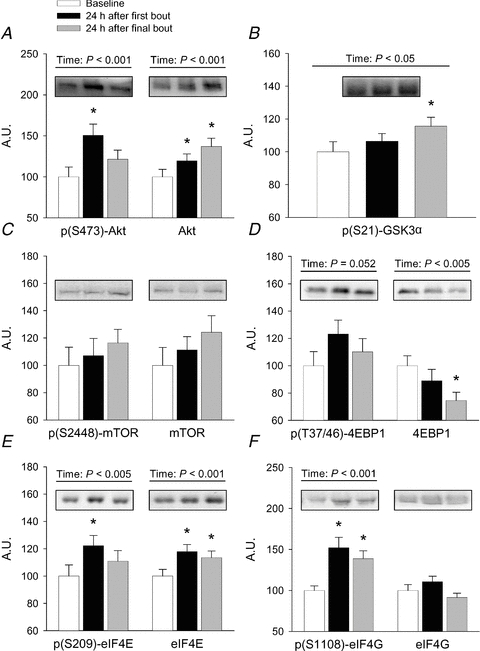
Human subjects completed a 16 week resistance training study as described in the text, with skeletal muscle biopsies taken at baseline and 24 h after the first and last loading bouts. Western blotting was used to determine the phosphorylation and/or abundance of each protein. Since the data were not different among response clusters (non-significant response cluster × time interaction term), the data are presented collapsed across all subjects. Values are mean ± SEM arbitrary units based on optical density of immunoblotted protein bands, relative to baseline levels within each cluster, n = 27–34 / group. *Different from baseline, P < 0.05; †different from first bout response, P < 0.05.
The phosphorylation of eIF4E on S209 increased 22% at week 1 in all clusters (P < 0.05) and returned to baseline levels by week 16 (Fig. 1E). Total protein expression of eIF4E increased 18% at week 1 and remained 14% elevated at week 16 (P < 0.05). eIF4G, a protein that complexes with eIF4E and eIF4A to form eIF4F (Vary & Lynch, 2006), associates with both the cytosolic and membrane fractions in our hands; it is important to note that we report only the cytosolic compartment here. Phosphorylation (S1108) of cytosolic eIF4G increased 52% at week 1 and 39% at week 16 (P < 0.05) without any change in the total cytosolic expression level (P > 0.05) (Fig. 1F). Therefore the regulation of initiation factors 4E and 4G, while being responsive to mechanical load, were equally responsive in all response clusters at the 24 h time point and could not predict differential myofibre hypertrophy among clusters.
Translation initiation signalling proteins differentially regulated by human response cluster
Mnk1 has been shown to phosphorylate eIF4E and regulate eIF4E activity during stressed conditions (Chen et al. 2010). A significant time × cluster interaction revealed that p(T197/202)-Mnk1 phosphorylation increased 95% at week 16 with respect to week 1 in NR only (Fig. 2A). Total Mnk1 expression tended to increase 39% at 16 week in all clusters combined (P = 0.051, data not shown).
Figure 2. Resistance exercise-induced changes translational signalling components that were altered differentially by hypertrophy response cluster.
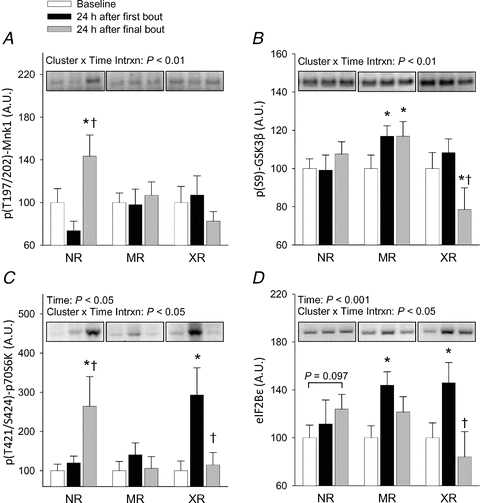
Human subjects completed a 16 week resistance training study as described in the text, with skeletal muscle biopsies taken at baseline and 24 h after the first and last loading bouts. Western blotting was used to determine the phosphorylation and/or abundance of each protein. Each variable presented here demonstrated a significant response cluster × time interaction term, in contrast to the data presented in Fig. 1, and were thus expressed by cluster. Values are mean ± SEM arbitrary units based on optical density of immunoblotted protein bands, relative to baseline levels within each cluster, n = 8–10 XR, 13–15 MR, 8 NR. *Different from baseline within cluster, P < 0.05; †different from first bout response within cluster, P < 0.05.
The regulation of GSK3β seemed to occur by a different mechanism than the α isoform. Phosphorylation of GSK3β on S9 showed a significant cluster × time interaction; it increased slightly (17%), although significantly, at both week 1 and week 16 in MR (P < 0.05), and decreased 28% at week 16 with respect to week 1 in XR only (P < 0.05, Fig. 2B). The expression level of total GSK3β was not altered at any time point (P > 0.05, data not shown). A clear trend between GSK3β phosphorylation and hypertrophy was not evident. Therefore, while GSK3β-dependent signalling may have been slightly altered by response cluster across time, the degree to which this modest change may have been physiologically relevant remains unclear at this time.
A downstream effector of mTOR, p70S6K, is regulated by phosphorylation at numerous sites including T421/S424 in the autoinhibitory domain, and the putative mTOR-specific phosphorylation site T389 in the linker domain (Han et al. 1995; Pearson et al. 1995). We were unable to reliably measure T389 phosphorylation using three commercially available phospho-specific antibodies, most likely due to the fasted state of the subjects. On the other hand, T421/S424 phosphorylation was increased by 193% in XR at week 1 (P < 0.05) and returned to baseline by week 16, while in NR a 164% increase was not evident until week 16 (P < 0.05, Fig. 2C). The response of MR did not change at any time point. These observations occurred without a significant change in the expression level of total p70S6K protein in any group (P > 0.05, data not shown). Therefore, the response of T421/S424 phosphorylation appeared to effectively distinguish the three response clusters. This suggests that either activation of p70S6K signalling or a kinase responsible for T421/S424 phosphorylation early in a resistance training programme may partially underlie the hypertrophic phenotype seen in XR vs. MR and NR after 16 weeks training. p70S6K is a well characterized regulator of mRNA translation and cell size in various cell types, including skeletal muscle (Fingar et al. 2002; Ohanna et al. 2005). We thus chose not to further pursue the role of p70S6K in this response.
eIF2Bɛ is the catalytic component of the eIF2B holoenzyme, and its activity is required to exchange GDP for GTP on eIF2, thereby allowing Met-tRNAi binding to the AUG start codon to initiate translation (Webb & Proud, 1997). Total eIF2Bɛ protein increased 45% and 44% at week 1 in XR and MR (P < 0.05), respectively, with XR fully and MR partially returning to baseline levels by week 16 (Fig. 2D). While eIF2Bɛ expression in NR did not significantly change at any time point, a trend toward an increase from baseline to week 16 was noted (P = 0.09). Therefore, of the initiation factors studied here, eIF2Bɛ was regulated differentially among response clusters, while eIF4E and eIF4G were regulated similarly among all clusters.
Since protein, and particularly leucine, ingestion has been shown to stimulate mTORC1-dependent translation initiation irrespective of mechanical load (Anthony et al. 2000; Norton & Layman, 2006), we previously performed dietary analysis on the full human subjects cohort, and found that all response clusters had similar intakes of total carbohydrate, total fat, total protein and leucine (Thalacker-Mercer et al. 2009). Importantly, this finding is likely to rule out total protein or leucine intake as a causal mechanism for the differential hypertrophy and translation initiation signalling between response clusters.
eIF2Bɛ overexpression increases in vitro translation and in vivo myofibre hypertrophy
Since we observed a mechanical load-induced increase in eIF2Bɛ among only those subjects who experienced subsequent myofibre hypertrophy (XR and MR), we sought to determine if increased eIF2Bɛ was sufficient to induce this response. We first transiently transfected L6 myoblasts with a Renilla luciferase reporter and either GFP, wild-type eIF2Bɛ, or an eIF2Bɛ mutant lacking the C-terminal catalytic domain (eIF2Bɛ-ΔC). The translation of the luciferase reporter is driven exclusively by a cap-dependent mechanism, and this technique has been shown previously to be reflective of overall protein synthesis rates (Balachandran & Barber, 2004). Overexpression of eIF2Bɛ resulted in an 87% increase in luciferase activity relative to GFP transfected controls (P < 0.05, Fig. 3), which is similar to what has been found previously in other cell types (Balachandran & Barber, 2004). This response was dependent upon the catalytic activity of the protein, as a mutant lacking the catalytic domain failed to alter luciferase activity from control levels (P > 0.05). Critically, the altered luciferase activity occurred at the post-transcriptional level, most likely due to increased translation rates, as the abundance of the reporter mRNA did not differ between conditions (Fig. 3). Thus, we interpret these results to indicate that increased eIF2Bɛ catalytic activity was sufficient to increase cap-dependent translation rates in L6 myoblasts in vitro.
Figure 3. eIF2Bɛ activity is sufficient to increase cap-dependent translation in vitro.
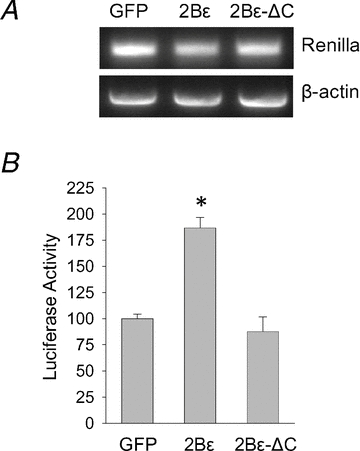
L6 myoblast cells were transfected with a CMV-driven Renilla luciferase reporter and either GFP, or wild-type eIF2Bɛ (2Bɛ), or a mutant lacking the C-terminal catalytic domain (2Bɛ-ΔC). Translation of the luciferase reporter is driven exclusively by a cap-dependent mechanism, and this technique has been shown previously to be reflective of overall protein synthesis rates (Balachandran & Barber, 2004). Twenty-four hours after transfection cells were cultured in 0.5% FBS for an additional 24 h and harvested for the assays indicated. A, RT-PCR showing similar mRNA expression of the luciferase reporter among conditions. B, Renilla luciferase activity was determined and expressed relative to GFP control. Values are means ± SEM, n = 4. *Different from GFP control, P < 0.05.
We next wished to determine if the increased cap-dependent translation rates induced by eIF2Bɛ overexpression would result in myofibre hypertrophy in the intact animal. Overexpression of eIF2Bɛvia electroporation of plasmid DNA into mouse tibialis anterior muscle resulted in a 21% increase in cross-sectional area of transfected fibres 7 days after electroporation (P < 0.05), while expression of GFP had no effect relative to non-transfected fibres (Fig. 4). An eIF2Bɛ mutant (S535A) that has previously been reported to act in a constitutively active manner in cardiac myocytes (Hardt et al. 2004) had an effect equal to that of the wild-type protein (P < 0.05 vs. non-transfected fibres). The mutated residue is a phosphorylation target of GSK3, which could indicate that GSK3-dependent inhibition of eIF2Bɛ may play less of a role in skeletal muscle than in cardiac muscle (Hardt et al. 2004), or that overexpressed wild-type eIF2Bɛ may have been present in a quantity too great to be inhibited by endogenous levels of GSK3. In either case, the degree of overexpression of either wild-type or the S535A mutant was evidently sufficient to saturate the cellular need for eIF2B GEF activity, since we saw equal hypertrophy when either was overexpressed. These data demonstrate that increased abundance of eIF2Bɛ was sufficient to induce significant myofibre hypertrophy in mouse skeletal muscle in a short time (7 days), making it likely that the hypertrophy seen in XR and MR humans over 16 weeks was at least partially due to augmented protein synthesis secondary to increased abundance of eIF2Bɛ.
Figure 4. Overexpression of eIF2Bɛ or eIF2Bɛ-S535A induce skeletal muscle fibre hypertrophy.
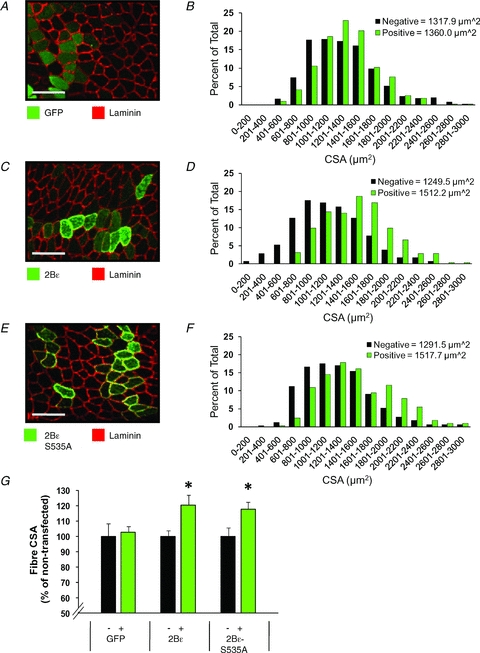
Mouse TA muscles were transfected with GFP (A and B), eIF2Bɛ (2Bɛ) (C and D), or constitutively active eIF2Bɛ (2Bɛ-S535A) (E and F). At 7 days post-transfection, the muscles were collected and cross-sections from the mid-belly of the muscle were subjected to immunohistochemistry for GFP and laminin (A), 2Bɛ and laminin (C), or 2Bɛ-S535A and laminin (E). Scale bar = 100 μm. The CSA of transfected fibres (green bars) and non-transfected fibres (black bars) from GFP (B) 2Bɛ, (D), or 2Bɛ-S535A (F) transfected muscles was determined and plotted on a histogram (n = 285–510 transfected and 285–510 non-transfected fibres/group). H, average CSA of the transfected (+) and non-transfected (−) fibres per muscle from samples transfected with GFP, 2Bɛ, or 2Bɛ-S535A. Values are means ± SEM, n = 5–7 muscles/group. *Different from the non-transfected fibres within a given condition, P < 0.05.
p70S6K signalling does not induce increased eIF2Bɛ protein abundance in vitro or in vivo
It is known that p70S6K signalling is necessary for proper regulation of cell size (Fingar et al. 2002; Holz et al. 2005; Ohanna et al. 2005); however, the downstream effectors that mediate this process are not well defined. We observed increases in both p(T421/S424)-p70S6K phosphorylation and eIF2Bɛ abundance among XR (Fig. 2). Furthermore, Kubica et al. (2008) found that mTORC1 signalling was sufficient to induce increased translation of eIF2Bɛ, which was blocked by rapamycin administration. Together these data suggested that p70S6K may be the rapamycin-sensitive component downstream of mTORC1 that mediates this response. In order to test this hypothesis, we transfected multiple cell lines with constitutively active (T389E) or dominant negative (T389A) p70S6K in order to determine the effects of p70S6K signalling on eIF2Bɛ protein abundance. In C2C12 mouse myoblasts, alterations in p70S6K activity failed to exert an effect on eIF2Bɛ protein abundance (P > 0.05) despite the expected effects on rpS6 phosphorylation (a consequence of p70S6K signalling) relative to pRK5-transfected control cells (Fig. 5). Similar results were obtained in L6 myoblasts. Since C2C12 and L6 myoblasts have a propensity to differentiate under serum withdrawal, we also performed these experiments in the non-myogenic HEK293T cells. Although we obtained nearly 100% transfection efficiency and greater subsequent effects on rpS6 phosphorylation in HEK293T cells, there remained no effect on total eIF2Bɛ abundance (P > 0.05, Fig. 5). This lack of effect occurred despite the ability of each of these cell lines to increase eIF2Bɛ in response to other stimuli in our hands (data not shown), indicating that eIF2Bɛ levels are in fact malleable in these cell lines. Thus, in three different cell lines of myogenic and non-myogenic origins, p70S6K activity had no influence over the cellular steady state abundance, and therefore presumably the synthesis, of eIF2Bɛ.
Figure 5. p70S6K signalling does not alter total eIF2Bɛ cellular abundance in vitro.
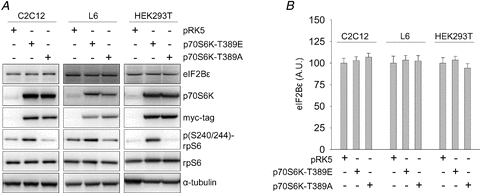
A, C2C12, L6 and HEK293T cells were transfected with myc-tagged constitutively active (T389E) or dominant negative (T389A) p70S6K, or empty vector control (pRK5). Twenty-four hours later cells were exposed to serum free (C2C12 and HEK293T) or 0.5% FBS medium (L6) for an additional 24 h, harvested and analysed by Western blotting with the indicated antibodies. B, values are means ± SEM for the eIF2Bɛ data shown in A, n = 4/group.
Although we found no change in eIF2Bɛ abundance with altered p70S6K activity in vitro, the possibility existed that the behaviour of the cell lines chosen did not reflect the in vivo condition. Therefore, we chose to investigate whether increased p70S6K activity was sufficient to induce an increase in eIF2Bɛ abundance in mouse skeletal muscle in vivo. To do this, we overexpressed wild-type p70S6K with or without Rheb, an upstream activator of mTORC1 (Yang et al. 2006), via electroporation of plasmid DNA into mouse tibialis anterior muscle. Co-expression of Rheb with p70S6K caused significantly increased T389 phosphorylation of both endogenous and GST-tagged p70S6K (Fig. 6), an event that is synonymous with increased activity of the protein (Pearson et al. 1995; Weng et al. 1998). Increased p70S6K activity had no effect on eIF2Bɛ abundance relative to GFP-transfected negative controls (P > 0.05, Fig. 6). Thus, increased p70S6K activity was not sufficient to induce increased eIF2Bɛ abundance under these conditions. Although the mechanical load-induced increase in eIF2Bɛ translation has been shown to be rapamycin sensitive (Kubica et al. 2005), we have shown here that p70S6K signalling alone is not sufficient to increase eIF2Bɛ abundance in vitro or in vivo. Combined, these data strongly suggest that p70S6K signalling is not involved in eIF2Bɛ translation.
Figure 6. p70S6K signalling does not alter total eIF2Bɛ cellular abundance in vivo.
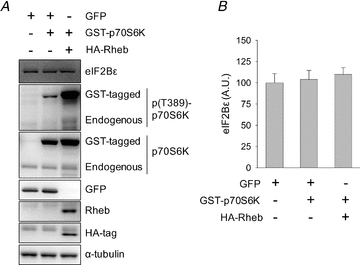
A, mouse TA muscles were transfected with GFP, GST-tagged p70S6K and/or HA-tagged Rheb. Mice recovered for 48 h at which time muscles were harvested and analysed by Western blotting with the indicated antibodies. B, values are means ± SEM for the eIF2Bɛ data shown in A, n = 3–4 muscles/group.
Discussion
The main findings presented here are that mechanical load-induced increases in p(421/S424)-p70S6K phosphorylation and eIF2Bɛ abundance were associated with myofibre hypertrophy after 16 weeks training in humans (Fig. 2). We additionally demonstrated that ectopic expression of eIF2Bɛ, a factor required for all translation initiating at an AUG codon, was sufficient to induce skeletal muscle hypertrophy in vivo (Fig. 4). Together these data strongly indicate that eIF2Bɛ abundance, and by extension eIF2B activity, are key determinants of cellular hypertrophy in vivo. However, although we observed concurrent p70S6K phosphorylation and increased eIF2Bɛ abundance in the humans clustered as XR, p70S6K signalling was not sufficient to increase eIF2Bɛ abundance as confirmed both in vitro and in vivo.
Given the data presented here, we suggest that eIF2Bɛ exerted a greater influence over myofibre hypertrophy than eIF4E or eIF4G in response to mechanical load under these conditions, since neither the phosphorylation state nor the total protein abundance of eIF4E, 4E-BP1, or eIF4G responded differently by cluster at the 24 h post-exercise time points analysed (Fig. 1). While our human results are admittedly limited to this single post-exercise time point, this interpretation of our data is supported by previous work, where neither the phosphorylation state nor the association of eIF4E and eIF4G was altered in rat muscle in response to resistance exercise (Farrell et al. 1999; Farrell et al. 2000) while eIF2B activity was increased (Farrell et al. 1999). A similar dissociation of eIF4F regulation and protein synthesis was observed under a variety of conditions in cardiomyocytes (Huang et al. 2009), L6 myoblasts (Kimball et al. 1998), and mouse muscle in vivo (Anthony et al. 2002). The importance of eIF2Bɛ in translation and cell size regulation can be further emphasized by the dramatic impact of a dominant negative upstream regulator of eIF2Bɛ, GSK3β, on C2C12 myotube diameter (Rommel et al. 2001), and a constitutively active eIF2Bɛ on rat neonatal cardiomyocyte surface area (Hardt et al. 2004). This is consistent with our data in which overexpression of eIF2Bɛ was sufficient to significantly increase both cap-dependent translation in vitro (Fig. 3) and skeletal myofibre size in mice (Fig. 4). Therefore, it seems that eIF2 GTP recycling and Met-tRNAi binding (as indexed by eIF2Bɛ abundance), but not 5′-capped mRNA binding (as indexed by eIF4G, eIF4E and 4E-BP1 phosphorylation or abundance), may be more critical at physiological levels to the in vivo response to mechanical load, and may implicate eIF2B as a rate limiting factor. Indeed, the relative abundance of eIF2B compared to other initiation factors suggests that it may be rate limiting (Oldfield et al. 1994; Kimball et al. 1998; Singh et al. 2007). Based on the putative inhibition of eIF2Bɛ by GSK3β-mediated phosphorylation, and on prior acute resistance exercise findings showing decreased phosphorylation (Glover et al. 2008), we attempted to assess eIF2Bɛ S539 phosphorylation status in our human clusters. However, immunoblotting experiments with two separate primary phospho-antibodies failed to yield satisfactory results (see Supplemental Material).
Tuckow and colleagues recently found that eIF2Bɛ overexpressed in rat skeletal muscle resulted in no change in basal protein synthesis rates; however, it blunted the sepsis-induced decrease in eIF2B activity and overall protein synthesis (Tuckow et al. 2010). In a similar manner, the increased eIF2Bɛ abundance that resulted from cellular transformation also prevented the stress-induced suppression in both eIF2B GEF activity and protein synthesis (Balachandran & Barber, 2004). Others have found that mutations within the catalytic domain of eIF2Bɛ yielded no observable phenotype in yeast under normal conditions, but conferred cold sensitivity (Mohammad-Qureshi et al. 2007). Additionally, we found that differences in luciferase activity (i.e. cap-dependent translation) between myoblasts of differing eIF2B activity were only evident after serum reduction, a significant cell stress (Fig. 3 and D. L. Mayhew, unpublished observations). Taken together these data are consistent with a model in which eIF2B has the greatest impact on overall protein synthesis rates under stressed conditions. As basal protein synthesis rates generally appear unaltered by increased eIF2Bɛ abundance (Balachandran & Barber, 2004; Tuckow et al. 2010) (D. L. Mayhew, unpublished observations), we hypothesize eIF2Bɛ is not rate limiting for protein synthesis under basal conditions but becomes rate limiting in response to stress. A greater abundance of eIF2Bɛ may therefore blunt the suppression of protein synthesis during frequent transient stresses (e.g., temperature stresses, fasted state between feedings) in the free living animal in vivo. This hypothesis is consistent with the well characterized model of stress-induced eIF2B suppression by phosphorylated eIF2α (Wek & Cavener, 2007), in which a greater abundance of eIF2Bɛ would require greater eIF2α phosphorylation in order to be inhibited to a similar extent (i.e. greater stress resistance). Over time, greater eIF2B activity would result in greater muscle mass when compared to animals with less eIF2B activity in response to similar stresses, which may explain the hypertrophy we observed in myofibres overexpressing eIF2Bɛ for 7 days.
Nearly identical regulation of p70S6K to what we have observed here has been noted in a similar model of chickens that were pre-selected for divergent growth phenotypes (Duchene et al. 2008a). In that study, insulin stimulated an increase in T421/S424 phosphorylation only in skeletal muscle of fast growing chickens (similar to XR here), which increased p70S6K activity, while Akt and 4E-BP1 phosphorylation were increased to similar degrees in both fast and slow growing chickens (Duchene et al. 2008a). These data and our data together indicate that the regulation of p(T421/S424)-p70S6K phosphorylation may be a phylogenetically conserved mechanism that results in increased p70S6K activity, translation initiation, and myofibre size in only a subset of animals that are prone to myofibre hypertrophy. Although this same group found that ERK2 signalling was indispensible for insulin induced p70S6K phosphorylation at T421/S424 (Duchene et al. 2008b), further work will be needed to determine the mechanism responsible for the variable sensitivity in mechanical load-induced p70S6K phosphorylation in humans.
In summary, we have observed that many proteins in translation initiation signalling pathways are responsive to mechanical load in humans. However, the regulation of the majority of the proteins examined here, including proteins previously shown to be important for hypertrophy in other models, were not sufficient to induce myofibre hypertrophy in NR. It is probable that many pathways are required for hypertrophy to occur in vivo, with some pathways providing a permissive environment while others provide directive influence over the multitude of cellular processes that result in mRNA translation and hypertrophy (Tang et al. 2001; Bolster et al. 2004). To our knowledge this is the first report to provide in vitro and in vivo evidence that eIF2Bɛ abundance is an important determinant driving protein synthesis and cellular hypertrophy in skeletal muscle, and that increased eIF2Bɛ abundance is not a functional consequence of p70S6K signalling. It is currently unknown whether p(T421/S424)-p70S6K phosphorylation and eIF2Bɛ abundance result from a common upstream event or independently regulated processes. Our results suggest that both p70S6K phosphorylation and eIF2Bɛ abundance may be used as potential signalling biomarkers for myofibre hypertrophy. These are the first data that mechanistically link eIF2Bɛ abundance to skeletal myofibre hypertrophy, and indicate that both eIF2Bɛ abundance and p70S6K phosphorylation may at least partially underlie the widely divergent hypertrophic phenotypes in human skeletal muscle exposed to mechanical stimuli.
Acknowledgments
We would like to sincerely thank Shane Kelly (UAB) for technical assistance. This work was supported by NIH grants AG017896 to M.M.B., AG031623 to D.L.M., AR057347 to T.A.H.
Glossary
Abreviations
- 4E-BP1
eIF4E binding protein 1
- eIF
eukaryotic initiation factor
- FITC
fluorescein isothiocyanate
- GEF
guanine nucleotide exchange factor
- GFP
green fluorescent protein
- GSK3
glycogen synthase kinase 3
- GST
glutathione S-transferase
- HA
haemagglutinin
- MFA
mean fibre area
- MR
modest responder
- mTOR
mammalian target of rapamycin
- mTORC1
mTOR complex 1
- NR
non-responder
- p70S6K
p70 ribosomal protein S6 kinase
- PI3K
phosphatidylinositol 3-kinase
- rpS6
ribosomal protein S6
- Rheb
Ras homologue enriched in the brain
- TRITC
tetramethylrhodamine isothiocyanate
- XR
extreme responder
Author contributions
D.L.M., T.A.H. and H.C.L. contributed to study conception and design, and data collection, analysis and interpretation. M.M.B. contributed to study conception and design, and data analysis and interpretation. All human and in vitro work was conducted in the lab of M.M.B., while all mouse work was conducted in the lab of T.A.H.. D.L.M., M.M.B. and T.A.H. contributed to the initial drafting the article, and all authors revised the article for intellectual content and approved the final version. The authors have no conflicts of interest to disclose.
Supplemental Materials
Figure 1.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Anthony JC, Lang CH, Crozier SJ, Anthony TG, MacLean DA, Kimball SR, Jefferson LS. Contribution of insulin to the translational control of protein synthesis in skeletal muscle by leucine. Am J Physiol Endocrinol Metab. 2002;282:E1092–E1101. doi: 10.1152/ajpendo.00208.2001. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413–2419. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- Augert G, Monier S, Le Marchand-Brustel Y. Effect of exercise on protein turnover in muscles of lean and obese mice. Diabetologia. 1986;29:248–253. doi: 10.1007/BF00454885. [DOI] [PubMed] [Google Scholar]
- Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol. 1999;276:C120–C127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- Balachandran S, Barber GN. Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell. 2004;5:51–65. doi: 10.1016/s1535-6108(03)00330-1. [DOI] [PubMed] [Google Scholar]
- Bamman MM, Petrella JK, Kim JS, Mayhew DL, Cross JM. Cluster analysis tests the importance of myogenic gene expression during myofiber hypertrophy in humans. J Appl Physiol. 2007;102:2232–2239. doi: 10.1152/japplphysiol.00024.2007. [DOI] [PubMed] [Google Scholar]
- Bamman MM, Ragan RC, Kim JS, Cross JM, Hill VJ, Tuggle SC, Allman RM. Myogenic protein expression before and after resistance loading in 26- and 64-yr-old men and women. J Appl Physiol. 2004;97:1329–1337. doi: 10.1152/japplphysiol.01387.2003. [DOI] [PubMed] [Google Scholar]
- Blomstrand E, Eliasson J, Karlsson HK, Kohnke R. Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise. J Nutr. 2006;136:269S–273S. doi: 10.1093/jn/136.1.269S. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- Bolster DR, Jefferson LS, Kimball SR. Regulation of protein synthesis associated with skeletal muscle hypertrophy by insulin-, amino acid- and exercise-induced signalling. Proc Nutr Soc. 2004;63:351–356. doi: 10.1079/PNS2004355. [DOI] [PubMed] [Google Scholar]
- Burd NA, Holwerda AM, Selby KC, West DW, Staples AW, Cain NE, Cashaback JG, Potvin JR, Baker SK, Phillips SM. Resistance exercise volume affects myofibrillar protein synthesis and anabolic signalling molecule phosphorylation in young men. J Physiol. 2010;588:3119–3130. doi: 10.1113/jphysiol.2010.192856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher NJ, Arulpragasam A, Goh HL, Davey T, Minchin RF. Genomic organization of human arylamine N-acetyltransferase Type I reveals alternative promoters that generate different 5′-UTR splice variants with altered translational activities. Biochem J. 2005;387:119–127. doi: 10.1042/BJ20040903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter MS, Sarnow P. Distinct mRNAs that encode La autoantigen are differentially expressed and contain internal ribosome entry sites. J Biol Chem. 2000;275:28301–28307. doi: 10.1074/jbc.M004657200. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Tan BC, Cheng YY, Chen JS, Lee SC. Differential regulation of CHOP translation by phosphorylated eIF4E under stress conditions. Nucleic Acids Res. 2010;38:764–777. doi: 10.1093/nar/gkp1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey VG, Zhong Z, Shield A, Canny BJ, Chibalin AV, Zierath JR, Hawley JA. Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. FASEB J. 2006;20:190–192. doi: 10.1096/fj.05-4809fje. [DOI] [PubMed] [Google Scholar]
- Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab. 2008;294:E392–E400. doi: 10.1152/ajpendo.00582.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576:613–624. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchene S, Audouin E, Berri C, Dupont J, Tesseraud S. Tissue-specific regulation of S6K1 by insulin in chickens divergently selected for growth. Gen Comp Endocrinol. 2008a;156:190–198. doi: 10.1016/j.ygcen.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Duchene S, Audouin E, Crochet S, Duclos MJ, Dupont J, Tesseraud S. Involvement of the ERK1/2 MAPK pathway in insulin-induced S6K1 activation in avian cells. Domest Anim Endocrinol. 2008b;34:63–73. doi: 10.1016/j.domaniend.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Evans W, Phinney S, Young V. Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc. 1982;14:101–102. [PubMed] [Google Scholar]
- Farrell PA, Fedele MJ, Vary TC, Kimball SR, Lang CH, Jefferson LS. Regulation of protein synthesis after acute resistance exercise in diabetic rats. Am J Physiol Endocrinol Metab. 1999;276:E721–E727. doi: 10.1152/ajpendo.1999.276.4.E721. [DOI] [PubMed] [Google Scholar]
- Farrell PA, Hernandez JM, Fedele MJ, Vary TC, Kimball SR, Jefferson LS. Eukaryotic initiation factors and protein synthesis after resistance exercise in rats. J Appl Physiol. 2000;88:1036–1042. doi: 10.1152/jappl.2000.88.3.1036. [DOI] [PubMed] [Google Scholar]
- Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002;16:1472–1487. doi: 10.1101/gad.995802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EI, Oates BR, Tang JE, Moore DR, Tarnopolsky MA, Phillips SM. Resistance exercise decreases eIF2Bɛ phosphorylation and potentiates the feeding-induced stimulation of p70S6K1 and rpS6 in young men. Am J Physiol Regul Integr Comp Physiol. 2008;295:R604–R610. doi: 10.1152/ajpregu.00097.2008. [DOI] [PubMed] [Google Scholar]
- Goodman CA, Miu MH, Frey JW, Mabrey DM, Lincoln HC, Ge Y, Chen J, Hornberger TA. A PI3K/PKB-independent activation of mTOR signaling is sufficient to induce skeletal muscle hypertrophy. Mol Biol Cell. 2010;21:3258–3268. doi: 10.1091/mbc.E10-05-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JW, Pearson RB, Dennis PB, Thomas G. Rapamycin, wortmannin, and the methylxanthine SQ20006 inactivate p70s6k by inducing dephosphorylation of the same subset of sites. J Biol Chem. 1995;270:21 396–21 403. doi: 10.1074/jbc.270.36.21396. [DOI] [PubMed] [Google Scholar]
- Hardt SE, Tomita H, Katus HA, Sadoshima J. Phosphorylation of eukaryotic translation initiation factor 2Bɛ by glycogen synthase kinase-3β regulates β-adrenergic cardiac myocyte hypertrophy. Circ Res. 2004;94:926–935. doi: 10.1161/01.RES.0000124977.59827.80. [DOI] [PubMed] [Google Scholar]
- Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005;123:569–580. doi: 10.1016/j.cell.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Huang BP, Wang Y, Wang X, Wang Z, Proud CG. Blocking eukaryotic initiation factor 4F complex formation does not inhibit the mTORC1-dependent activation of protein synthesis in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2009;296:H505–H514. doi: 10.1152/ajpheart.01105.2008. [DOI] [PubMed] [Google Scholar]
- Kim JS, Petrella JK, Cross JM, Bamman MM. Load-mediated down-regulation fo myostatin mRNA is not sufficient to promote myofiber hypertrophy in humans: a cluster analysis. J Appl Physiol. 2007;103:1488–1495. doi: 10.1152/japplphysiol.01194.2006. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Farrell PA, Jefferson LS. Invited Review: Role of insulin in translational control of protein synthesis in skeletal muscle by amino acids or exercise. J Appl Physiol. 2002;93:1168–1180. doi: 10.1152/japplphysiol.00221.2002. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Horetsky RL, Jefferson LS. Implication of eIF2B rather than eIF4E in the regulation of global protein synthesis by amino acids in L6 myoblasts. J Biol Chem. 1998;273:30945–30953. doi: 10.1074/jbc.273.47.30945. [DOI] [PubMed] [Google Scholar]
- Kubica N, Bolster DR, Farrell PA, Kimball SR, Jefferson LS. Resistance exercise increases muscle protein synthesis and translation of eukaryotic initiation factor 2Bɛ mRNA in a mammalian target of rapamycin-dependent manner. J Biol Chem. 2005;280:7570–7580. doi: 10.1074/jbc.M413732200. [DOI] [PubMed] [Google Scholar]
- Kubica N, Crispino JL, Gallagher JW, Kimball SR, Jefferson LS. Activation of the mammalian target of rapamycin complex 1 is both necessary and sufficient to stimulate eukaryotic initiation factor 2Bɛ mRNA translation and protein synthesis. Int J Biochem Cell Biol. 2008;40:2522–2533. doi: 10.1016/j.biocel.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai KM, Gonzalez M, Poueymirou WT, Kline WO, Na E, Zlotchenko E, Stitt TN, Economides AN, Yancopoulos GD, Glass DJ. Conditional activation of akt in adult skeletal muscle induces rapid hypertrophy. Mol Cell Biol. 2004;24:9295–9304. doi: 10.1128/MCB.24.21.9295-9304.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew DL, Kim JS, Cross JM, Ferrando AA, Bamman MM. Translational signaling responses preceding resistance training-mediated myofiber hypertrophy in young and old humans. J Appl Physiol. 2009;107:1655–1662. doi: 10.1152/japplphysiol.91234.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad-Qureshi SS, Haddad R, Hemingway EJ, Richardson JP, Pavitt GD. Critical contacts between the eukaryotic initiation factor 2B (eIF2B) catalytic domain and both eIF2β and -2γ mediate guanine nucleotide exchange. Mol Cell Biol. 2007;27:5225–5234. doi: 10.1128/MCB.00495-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton LE, Layman DK. Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise. J Nutr. 2006;136:533S–537S. doi: 10.1093/jn/136.2.533S. [DOI] [PubMed] [Google Scholar]
- Ohanna M, Sobering AK, Lapointe T, Lorenzo L, Praud C, Petroulakis E, Sonenberg N, Kelly PA, Sotiropoulos A, Pende M. Atrophy of S6K1(–/–) skeletal muscle cells reveals distinct mTOR effectors for cell cycle and size control. Nat Cell Biol. 2005;7:286–294. doi: 10.1038/ncb1231. [DOI] [PubMed] [Google Scholar]
- Oldfield S, Jones BL, Tanton D, Proud CG. Use of monoclonal antibodies to study the structure and function of eukaryotic protein synthesis initiation factor eIF-2B. Eur J Biochem. 1994;221:399–410. doi: 10.1111/j.1432-1033.1994.tb18752.x. [DOI] [PubMed] [Google Scholar]
- Parkington JD, Siebert AP, LeBrasseur NK, Fielding RA. Differential activation of mTOR signaling by contractile activity in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1086–R1090. doi: 10.1152/ajpregu.00324.2003. [DOI] [PubMed] [Google Scholar]
- Pearson RB, Dennis PB, Han JW, Williamson NA, Kozma SC, Wettenhall RE, Thomas G. The principal target of rapamycin-induced p70s6k inactivation is a novel phosphorylation site within a conserved hydrophobic domain. EMBO J. 1995;14:5279–5287. doi: 10.1002/j.1460-2075.1995.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol. 2008;104:1736–1742. doi: 10.1152/japplphysiol.01215.2007. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab. 1997;273:E99–E107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- Singh CR, Udagawa T, Lee B, Wassink S, He H, Yamamoto Y, Anderson JT, Pavitt GD, Asano K. Change in nutritional status modulates the abundance of critical pre-initiation intermediate complexes during translation initiation in vivo. J Mol Biol. 2007;370:315–330. doi: 10.1016/j.jmb.2007.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Hornstein E, Stolovich M, Levy G, Livingstone M, Templeton D, Avruch J, Meyuhas O. Amino acid-induced translation of TOP mRNAs is fully dependent on phosphatidylinositol 3-kinase-mediated signaling, is partially inhibited by rapamycin, and is independent of S6K1 and rpS6 phosphorylation. Mol Cell Biol. 2001;21:8671–8683. doi: 10.1128/MCB.21.24.8671-8683.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalacker-Mercer AE, Petrella JK, Bamman MM. Does habitual dietary intake influence myofiber hypertrophy in response to resistance training? A cluster analysis. Appl Physiol Nutr Metab. 2009;34:632–639. doi: 10.1139/H09-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckow AP, Vary TC, Kimball SR, Jefferson LS. Ectopic expression of eIF2Bɛ in rat skeletal muscle rescues the sepsis-induced reduction in guanine nucleotide exchange activity and protein synthesis. Am J Physiol Endocrinol Metab. 2010;299:E241–E248. doi: 10.1152/ajpendo.00151.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary TC, Lynch CJ. Meal feeding enhances formation of eIF4F in skeletal muscle: role of increased eIF4E availability and eIF4G phosphorylation. Am J Physiol Endocrinol Metab. 2006;290:E631–E642. doi: 10.1152/ajpendo.00460.2005. [DOI] [PubMed] [Google Scholar]
- Webb BL, Proud CG. Eukaryotic initiation factor 2B (eIF2B) Int J Biochem Cell Biol. 1997;29:1127–1131. doi: 10.1016/s1357-2725(97)00039-3. [DOI] [PubMed] [Google Scholar]
- Wek RC, Cavener DR. Translational control and the unfolded protein response. Antioxid Redox Signal. 2007;9:2357–2371. doi: 10.1089/ars.2007.1764. [DOI] [PubMed] [Google Scholar]
- Weng QP, Kozlowski M, Belham C, Zhang A, Comb MJ, Avruch J. Regulation of the p70 S6 kinase by phosphorylation in vivo. Analysis using site-specific anti-phosphopeptide antibodies. J Biol Chem. 1998;273:16621–16629. doi: 10.1074/jbc.273.26.16621. [DOI] [PubMed] [Google Scholar]
- Wilkinson SB, Phillips SM, Atherton PJ, Patel R, Yarasheski KE, Tarnopolsky MA, Rennie MJ. Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J Physiol. 2008;586:3701–3717. doi: 10.1113/jphysiol.2008.153916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Inoki K, Kim E, Guan KL. TSC1/TSC2 and Rheb have different effects on TORC1 and TORC2 activity. Proc Natl Acad Sci U S A. 2006;103:6811–6816. doi: 10.1073/pnas.0602282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


