Abstract
We recently reported that activation of the inositol 1,4,5-triphosphate receptor (IP3R) is required for efficient HIV-1 Gag trafficking and viral particle release (Ehrlich 2010). IP3R activation requires phospholipase C (PLC)-catalyzed hydrolysis of PI(4,5)P2 to IP3 and diacylglycerol. Here we show that Sprouty2 (Spry2), which binds PI(4,5)P2 and PLCγ, interfered with PI(4,5)P2 in a manner similar to U73122, an inhibitor of PI(4,5)P2 hydrolysis, suggesting that Spry2 may negatively regulate IP3R by preventing formation of its activating ligand, IP3. Mutation to Asp of R252, a critical determinant of PI(4,5)P2 binding in the C-terminal domain of Spry2, prevented the interference completely, indicating that binding to the phospholipid is required. In contrast, deletion of the PLCγ binding region or mutation of a critical Tyr residue in the region did not prevent the interference but Spry2-PI(4,5)P2 colocalization was not detected, suggesting that PLC binding is required for their stable association. Like U73122, Spry2 over-expression inhibited WT Gag release as virus-like particles. The inhibition was relieved by disrupting either binding determinant. IP3R-mediated Ca2+ signaling, in turn, was found to influence Spry2 subcellular distribution and ERK, a Spry2 regulator. Our findings suggest that Spry2 influences IP3R function through control of PI(4,5)P2 and IP3R influences Spry2 function by controlling its distribution and ERK activation.
Keywords: inositol 1,4,5-triphosphate receptor (IP3R); Human Immunodeficiency Virus type 1 (HIV-1); virus-like particles (VLPs); Sprouty2 (Spry2); phosphatidylinositol (4,5) biphosphate (PI(4,5)P2)
INTRODUCTION
HIV-1 release from the plasma membrane of infected cells is directed by determinants in the matrix (MA) and Late (L) domains of the structural precursor polyprotein, Gag 1. These determinants respectively bind the phospholipid phosphatidylinositol (4,5) biphosphate (PI(4,5)P2) and the Endosomal Sorting Complex Required for Transport (ESCRT) factor Tsg101 or ESCRT adaptor Alix 2. Most of PI(4,5)P2 is in the plasma membrane and Gag release requires both intact PI(4,5)P2 and PI(4,5)P2 hydrolysis. The former targets Gag to the plasma membrane from the site of synthesis in the cell interior 3. The latter results in activation of the inositol (1,4,5)-triphosphate receptor (IP3R) which we recently showed to be required for stable plasma membrane localization and efficient release of Gag 4. IP3R proteins form oligomeric Ca2+ channels in plasma membrane, ER, and nuclear membranes and are the major regulator of the concentration of free calcium (Ca2+) in the cytosol 5. Directly or indirectly through the resulting Ca2+ signaling, phosphoinositides, particularly PI(4,5)P2, regulate a broad range of cellular processes 6.
The identification of Spry2 in a proteomic search for cellular proteins associated with Gag on membranes in which IP3R had also been detected prompted us to hypothesize that Spry2 control of Ca2+ signaling was a component of HIV’s utilization of IP3R-regulated Ca2+ signaling machinery. Targeted depletion of Spry2 7 or targeted depletion of IP3R 4 leads to inhibition of HIV release supporting the notion that the functions of both proteins are required for high efficiency budding. There are four Spry genes in higher vertebrates. They exhibit partial overlap in expression pattern 8 and biochemical properties 9; 10; 11. They have been shown to function as general inhibitors of receptor tyrosine kinases (RTKs) 12; 13; 14 and are induced in response to RTK signaling through both MAP kinase 15 and calcium signaling pathways 16. The molecular mechanisms by which Spry2 might inhibit RTK or Ca2+ signaling are uncertain but it is generally believed to be through protein-protein interactions with its multiple binding partners 10; 11 Spry2 interacts specifically with PI(4,5)P2 and PLC-γ 11; 17; 18 ; both factors play critical roles in Gag trafficking and release 3; 4 as well as IP3R activation 19.
Here we show that overexpression of Spry2 in COS-1 cells expressing Gag interfered with PI(4,5)P2, exhibiting the same effect as inhibitors of PLC hydrolysis. The interference required Spry2 interaction with PI(4,5)P2, since it was prevented by mutation of a previously identified determinant of PI(4,5)P2 binding. As predicted by the hypothesis, Gag release as virus-like particles (VLPs) was inhibited by overexpression of the wild-type (WT), but not the mutated Spry2 protein. We also show that inhibition of Ca2+ signaling by siRNA-targeted depletion of IP3R interfered with Spry2 subcellular distribution and ERK activation. These observations support the hypothesis that Spry2 and IP3R functions in Gag release are linked and reveal a novel aspect of HIV-1 Gag assembly.
RESULTS
Spry2 interferes with PI(4,5)P2 dynamics
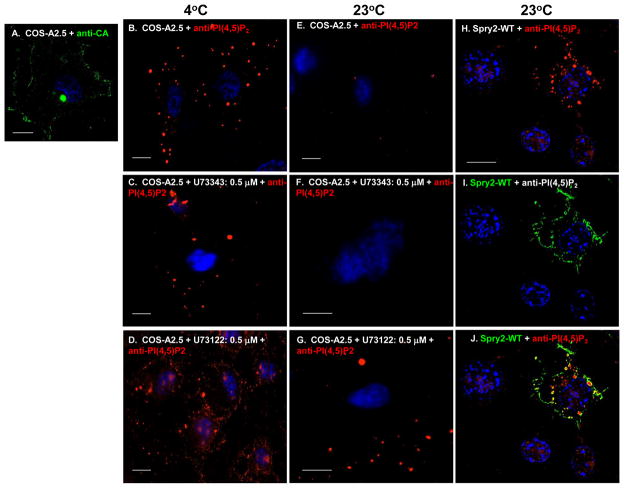
Activation of IP3R releases Ca2+ required for efficient budding of infectious virions or the virus-like particles (VLPs) assembled in cells expressing Gag alone 4. Activation requires phospholipase C (PLC)-catalyzed hydrolysis of plasma membrane-bound PI(4,5)P2 to form IP3 and DAG 19. Blocking formation of IP3 with the widely used agent U73122, that mainly inhibits PLC activity 20; 21 but which can also activate the nuclear estrogen receptors 22, inhibits VLP release 4. FIGURE 1 shows experiments conducted in COS cells that express HIV-1 Gag constitutively 23 (shown in panel A). To determine whether expression of Spry2 influences the fate of the lipid, we used previously identified conditions (0.075% saponin/0.1% glutaraldehyde/3.7% formaldehyde, 37°C, 20 min) where PI(4,5)P2 is not normally preserved unless its hydrolysis is pharmacologically blocked to examine for the phospholipid by confocal microscopy: Detection of PI(4,5)P2 in samples to be viewed by confocal microscopy requires storage at 4 degrees to minimize the activity of hydrolytic enzymes; when done at 37 degrees, plasma membrane PI(4,5)P2 is not preserved 24, 25. For this study, we employed U73122, a widely used inhibitor of PI(4,5)P2 hydrolysis 21 that we and others have used previously to inhibit PLC activity in HIV-infected or gag-transfected cells 4, 20. U73343 is an inactive form of the drug that must be used as a control to ensure that observed effects are specific to inactivation of the PLC enzyme activity 21. Antibody that recognized PI(4,5)P2 specifically was used to detect the phospholipid. As shown in panel B, PI(4,5)P2 was detected in mock-treated samples prepared at 4°C but not in samples exposed to 37 °C (panel E). Similar results were obtained with samples treated with the control agent U73343 (compare panels C and F). At both 4°C and 23°C, the samples treated with the PLC inhibitor U73122 exhibited significantly more PI(4,5)P2 than the mock- or U73343-treated counterparts (compare panel D to panels B and C; compare panel G to panels E and F). This indicated that PI(4,5)P2 hydrolysis was largely responsible for failure to detect the lipid in the mock- and U73343-treated preparations. Strikingly, a similar interference with PI(4,5)P2 was detected in samples incubated at 23°C if they expressed HA-tagged Spry2 (panels H–J). Like the samples derived from the U73122-treated cells, the phospholipid was detected in punctate structures. These results suggested that, like the PLC inhibitor, Spry2 possessed the ability to interfere with PI(4,5)P2 dynamics.
FIGURE 1. Spry2 inhibits PI(4,5)P2 hydrolysis.
Panels A–D. COS-12A2 cells were mock-treated with PBS (panel A) or incubated for 24 hr in media containing either DMSO alone (panel B) or 0.5 μM U73122 or U73343 in DMSO (panels C and D). Samples in panels B–D were prepared at 4 °C; samples in panels E–J were prepared at 23 °C. The mock-treated sample in panel A was stained for Gag with anti-capsid (CA) antibody to demonstrate that the cell line expresses Gag constitutively. Cells in panels B-J were stained with anti-PI(4,5)P2 antibody. Panels H–J. COS-12A2 cells were transfected with DNA encoding HA-tagged Spry2. HA-Spry2 (green) and PI(4,5)P2 (red) were visualized by indirect immunofluorescence using FITC- or TRITC-labeled secondary antibodies targeted to primary antibodies against the HA tag on Spry2 or the phospholipid, respectively. The bar measures 10 μm.
Interference requires PI(4,5)P2 binding
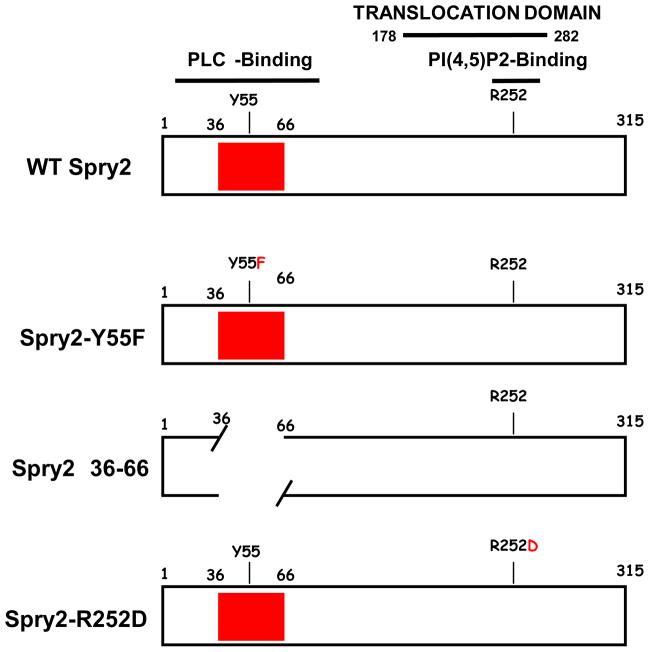
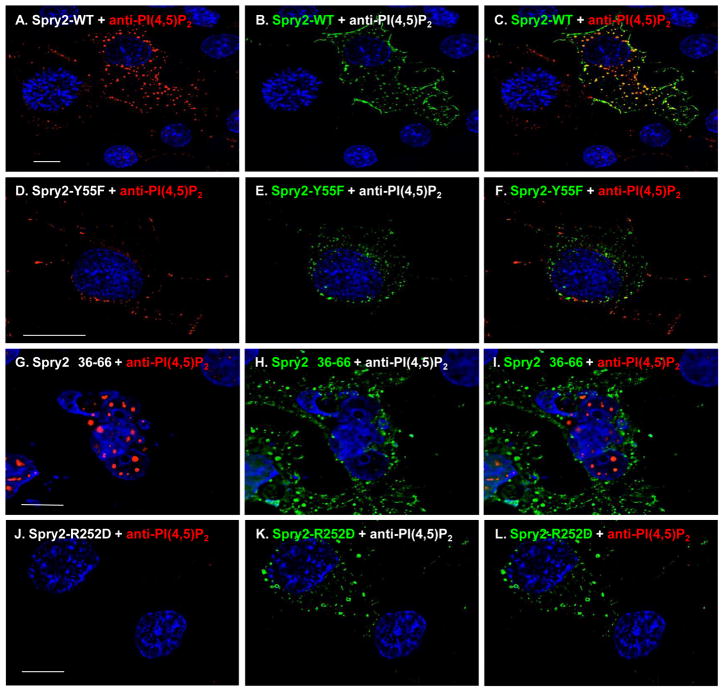
Spry2 has been reported to bind PI(4,5)P2 and PLCγ 17; 18. Binding to phospholipid is mediated through a determinant, R252, located in the translocation domain in the highly conserved C-terminal domain (CTD) of the protein 18. Binding to PLCγ is reported to require Y55 within the most conserved region of the otherwise highly divergent N-terminal domain (NTD) 17. We therefore used constructs that encoded variants with disruptions in these regions of the Spry2 protein schematically shown in FIGURE 2 and determined whether the alterations prevented the effect on PI(4,5)P2 exhibited by the WT protein. As shown in FIGURE 3 and consistent with the results above, PI(4,5)P2 in punctate structures was detected by the anti-PI(4,5)P2 antibody only in cells that expressed the Spry2 protein (panels A–C). Also consistent, Spry2 exhibited significant co-localization with PI(4,5)P2 as indicated by the Pearson’s coefficient of correlation26 = 0.6 (c.f., Materials & Methods). The substitution of Phe for Tyr at aa55 (Y55F, panels D–F) did not prevent the Spry2-mediated interference nor did deletion of the region flanking this residue in the NTD (Δ36–66, panels G–I). However, the appearance of PI(4,5)P2 was qualitatively different in cells expressing Y55F compared to cells expressing the WT protein or the Δ36–66 mutant: In cells expressing Y55F, the PI(4,5)P2-containing puncta were smaller than the structures in cells expressing WT Spry2 or Spry2-Δ36–66. However, both mutants failed to co-localize with the phospholipid suggesting that their effects were weaker than WT. Pearson’s coefficients of correlation were <0.4. In contrast to the Y55F and the Δ36–66 mutations, the substitution of Asp for Arg at residue 252 (R252D) completely prevented the Spry2-mediated interference, i.e., no PI(4,5)P2 was detected (panels J–L). Identical results were obtained using two independently prepared commercial anti-PI(4,5)P2 antibodies in four independent experiments. We conclude that full interference requires the PI(4,5)P2 binding determinant in the C-terminal region of the Spry2 protein.
FIGURE 2. Spry2 mutants used in this study.
The location of the PLCγ-binding determinant Y55 and its flanking region in the Spry2 N-terminal domain (NTD) and the PI(4,5)P2-binding determinant R252 in the C-terminal domain (CTD) are indicated. The filled box represents the most conserved region in the otherwise highly divergent NTD.
FIGURE 3. Mapping determinants of Spry2-mediated preservation of PI(4,5)P2.
COS-12A2 cells were transfected with DNA encoding WT (panels A–C) or mutated forms of the Spry2 protein (panels D–L). Spry2 (green) and PI(4,5)P2 (red) were visualized as described in the legend to figure 1. The bar measures 10 μm.
Inhibition of VLP release correlates with Spry2-mediated interference and is relieved by disruption of PI(4,5)P2- and PLC-binding
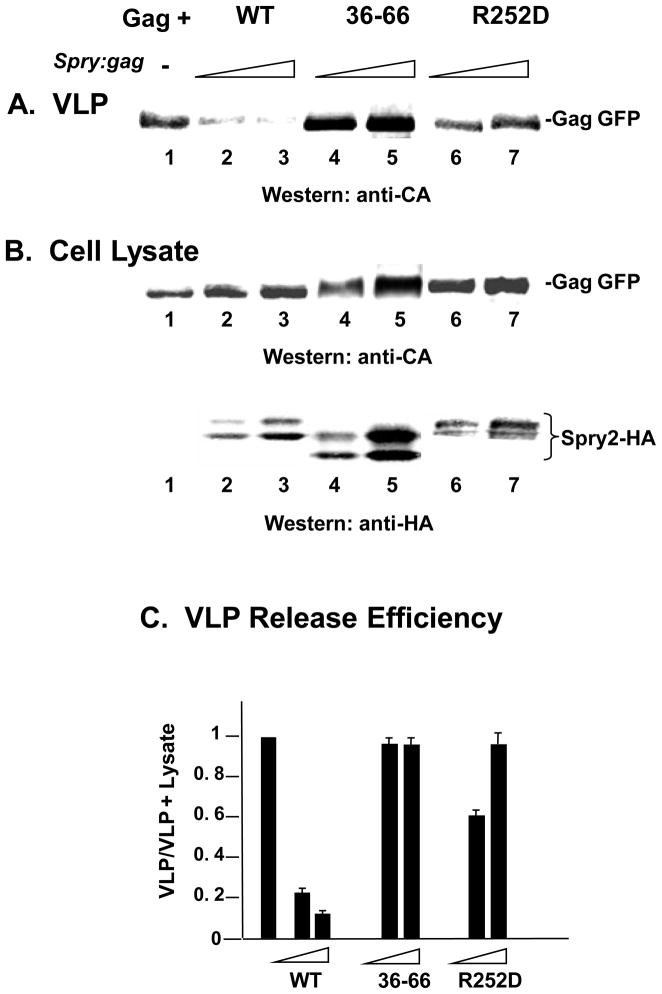
As PI(4,5)P2 hydrolysis is required for viral particle release 4, Spry2 interference is predicted to inhibit Gag release. To test this, COS-1 cells were co-transfected with DNA encoding Gag and WT Spry2 and examined after 48 hr for VLP release into the media. Consistent with this expectation, co-expression of Gag alone (FIGURE 4, panel A, lane 1) or Gag with WT Spry2 (lanes 2 and 3) reduced the amount of VLP in the media compared to expression of Gag alone. We then determined whether loss of the ability to interfere with PI(4,5)P2 correlated with reduced inhibition of VLP release. Examination of cell lysates indicated that the mutants (panel B, lanes 4–7) and WT (lanes 2 and 3) Spry2 proteins were expressed and that Gag accumulated to a similar extent in the samples. Interestingly, although only the R252D mutation completely prevented the Spry2-mediated interference (c.f., Fig. 3J), both mutations relieved the inhibition to VLP release imposed by expression of the WT Spry2 protein (lanes 4–7). Quantitative analysis of VLP release efficiency (Gag signal intensity in [VLP/VLP + Lysate]) indicated that VLP release efficiency was reduced 5- to 10-fold by the WT Spry2 protein but to a significantly lesser degree by either mutant (panel C). We conclude that Spry2 interactions with PI(4,5)P2 and PLCγ permit it to influence Gag release through interference with PI(4,5)P2 dynamics.
FIGURE 4. Co-expression of Gag with WT Spry2 inhibits VLP release; inhibition is relieved by mutation of the PI(4,5)P2 and PLCγ-binding regions.
COS-1 cells were transfected with DNA encoding WT HIV-1 Gag alone (lane 1) or with DNA encoding Gag and WT Spry2 (lanes 2 and 3), Spry2-Δ36–66 (lanes 4 and 5) or Spry2-R252D (lanes 6 and 7) at spry2-gag ratios of 1:1 or 2:1. Forty eight hours after transfection, the cells were harvested and media analyzed. Media and cell lysates were examined for VLP production (panel A) and expression of Gag-HA in the cytoplasm (panel B) by Western analysis. Panel C, Quantitative analysis of VLP release efficiency.
IP3R influences Spry2
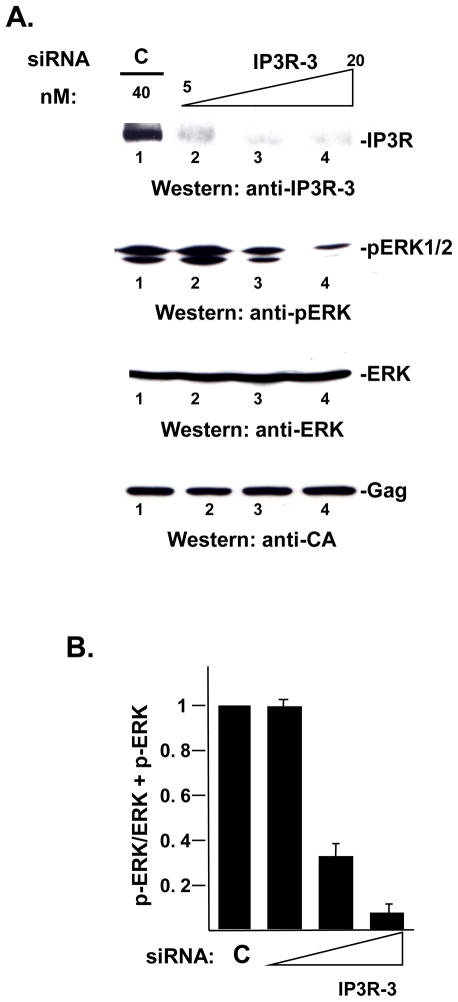
If Spry2 exerts negative control on Ca2+ signaling through its effect on PI(4,5)P2 dynamics, the block to IP3R activation might signal cells to terminate the Spry2 function. It is well-established that ligand binding to RTKs initiates ERK activation and that ERK activation signals induction of Spry2 gene expression 10; 11. We therefore examined cells for changes in ERK activation and, more directly, for loss of Spry2 from the plasma membrane following disruption of IP3R function. siRNA targeted to IP3R-3, the major isoform of the protein in COS-1 cells 27 was used to deplete the endogenous pool of the protein in gag-transfected COS-1 cells. First, the effect on ERK activation, as indicated by its phosphorylation, was measured in Western analysis using anti-phospho ERK antibody. As shown in FIGURE 5, specific siRNA-targeted depletion reduced the pool of endogenous IP3R-3 protein by more than 95% at the highest concentration tested (panel A, top, lanes 1–4). Under these conditions, the pool of phospho-ERK was reduced in a dose-dependent manner (panel A, bottom, lanes 1–4 and panel B), indicating that ERK activation had been disrupted. The effect on ERK activation was specific, as no change was detected in the level of Gag or ERK that accumulated in the cytoplasm (panel A, bottom, lanes 1–4). The results indicate that the steady-state level of IP3R-3 is required for ERK activation, supporting the possibility that IP3R may influence Spry2 function.
FIGURE 5. Depletion of the endogenous pool of IP3R-3 inhibits ERK activation.
Panel A, Analysis of IP3R-3 depletion by Western analysis. Cell lysates prepared from cells transfected with DNA encoding HIV-1 Gag as described in Materials and Methods were analyzed by Western blotting for expression of IP3R-3. The blot was re-probed sequentially for p-ERK, ERK and Gag using specific antibodies directed to the proteins. Panel B, Quantitative analysis of p-ERK activation. The panel shows the ratio of the p-ERK signal in the cell lysate to the sum of the pERK plus ERK signals in the lysate.
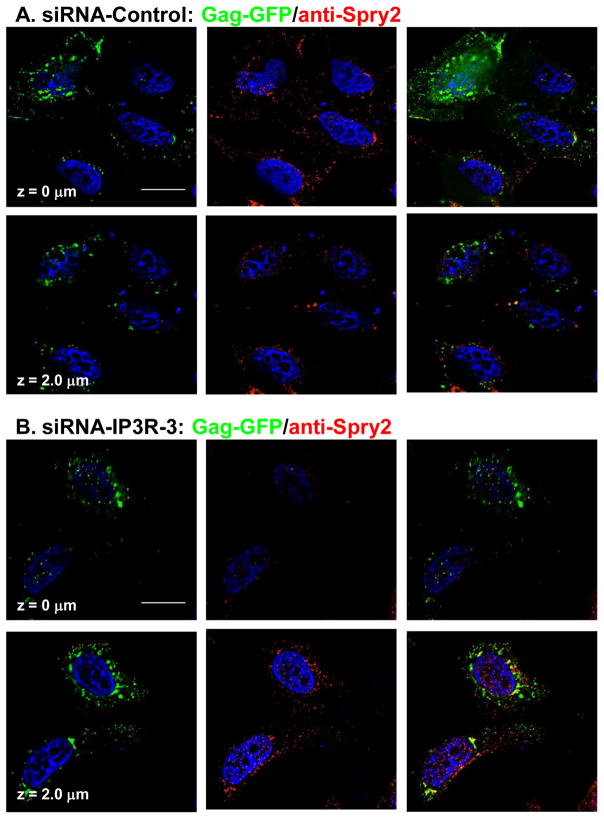
To obtain more direct support for the hypothesis, cells in the cultures treated with control or targeted siRNA were examined by confocal microscopy for changes in Spry2 distribution (FIGURE 6). In previous studies, we established that a fluorescent siRNA that localizes in the cytoplasm was delivered into 75% of cells under the transfection conditions used for the experiment, indicating that the majority of the cells examined contained the desired siRNA molecules 4. Moreover, we demonstrated that, under these conditions, Gag accumulated in the cell interior in cells receiving siRNA targeted to IP3R but at the cell periphery in cells treated with the control siRNA 4. Consistent with our previous findings 4, Gag was detected mainly at the cell periphery (panel A, top left) but also in the cell interior (bottom left) in cells treated with control siRNA. The endogenous Spry2 protein exhibited a similar distribution, i.e., most of the protein was detected in the plasma membrane-proximal region of the cell (center panel top versus bottom). No co-localization was detected (right panels). As expected, based on our previous studies, examination of cells in cultures transfected with siRNA targeted to IP3R mRNA (panel B) showed relatively little Gag at the cell periphery (top). Rather, the protein was detected mainly in the cell interior (bottom). The Spry2 protein similarly accumulated in the cell interior rather than at the periphery (center, bottom and top panels, respectively). The distribution patterns of the two proteins overlapped in the perinuclear region, however, there was no clear indication they co-localized. The results indicate that the steady-state level of IP3R, which we previously demonstrated is necessary to maintain localization of Gag at the plasma membrane and prevent its accumulation in the cell interior, is also important for Spry2 localization. Together, these findings support the possibility that IP3R and Spry2 influence each others’ functions in Gag assembly.
FIGURE 6. IP3R-3 depletion alters Spry2 distribution.
HeLa cells expressing Gag-GFP in cultures treated with non-targeting control siRNA (panel A) or siRNA targeted to IP3R-3 (panel B) were examined by confocal microscopy. Spry2 was detected by indirect immunofluorescence using TRITC-tagged secondary antibody. z = section through z plane of cell. Bars indicate 10 μm.
DISCUSSION
In this study, we tested the hypothesis that Spry2 control of Ca2+ signaling is a component of HIV’s utilization of IP3R-regulated Ca2+ signaling machinery. Our results indicate that Spry2 exerts a level of control over PI(4,5)P2 such that the phospholipid is preserved at 23 °C in the presence of the protein under conditions where it was not detected at this temperature unless PLC hydrolysis was blocked. The observation that Spry2 expression had the same effect has the inhibitor suggests that interference with PI(4,5)P2 hydrolysis accounts for at least part of the Spry2-mediated preservation. There are potentially two Spry2 activities that might enable Spry2 to inhibit PI(4,5)P2 hydrolysis: (i) binding to PI(4,5)P2 18 and (ii) sequestering of PLCγ 17. Our results suggest that both Spry2 functions are important but play distinct roles: R252 in the CTD of the Spry2 protein plays a critical role in the Spry2-mediated preservation of PI(4,5)P2, as the effect was completely lost by mutation of R25D to Asp. In contrast, the Spry2-mediated effect was not blocked by mutations in the region required for PLCγ binding 17, however, the Spry2 mutant and PI(4,5)P2 failed to co-localize.
Previous studies have suggested that Spry can affect and be affected by Ca2+ signaling and MAP/ERK kinase signaling. It was shown that in Xenopus oocytes, overexpression of Xenopus Spry2 (Xspry2) inhibited Ca2+ signaling but not MAP/ERK kinase signaling 28. Subsequent studies showed that Spry2 mediated inhibition of Ca2+ signaling while a related protein, Spred2, inhibited MAP kinase signaling 29. Drosophila spry was shown to inhibit MAP/ERK kinase but not Ca2+ signaling 15. Overall, these studies suggest that Spry2 proteins can inhibit either Ca2+ signaling or MAP/ERK kinase signaling but not both signaling pathways in the same system. Our results suggest that IP3R also can affect and be affected by Ca2+ signaling and MAP/ERK kinase signaling: IP3R activation is necessary to initiate Ca2+ signaling and, under conditions of IP3R depletion, both Ca2+ and ERK signaling are negatively impacted. Taken together, our results suggest that the Spry2 and IP3R functions in the two signaling circuits are linked.
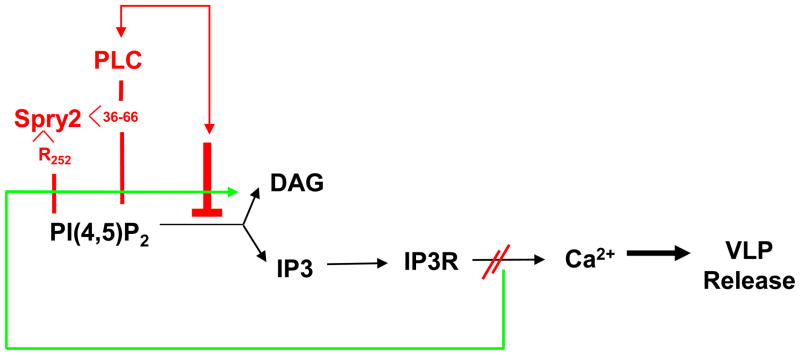
A model for the possible interplay between control of Ca2+ and ERK signaling in Gag release from the plasma membrane is shown in FIGURE 7. According to this model, IP3R plays a central role in Gag trafficking to the budding site, providing the Ca2+ required for activation of enzymes involved in PI(4,5)P2 synthesis and PLC activation. Our previous studies support this notion, as we demonstrated that depletion of IP3R and inhibition of PLC activity resulted in accumulation of Gag in the cell interior rather than at the plasma membrane to which it is targeted by PI(4,5)P2 3; 4; 30; 31. IP3-mediated activation of IP3R would result in the release of Ca2+ from the stores and promote vesicular trafficking and cytoskeletal rearrangements that may facilitate ESCRT-III function during viral particle release. We propose that sustained IP3R activation would lead, at some point, to a high cytosolic concentration of Ca2+, which activates ERK, and induce Spry2 translocation to PI(4,5)P2 sites on the plasma membrane. There, Spry2 interaction with PI(4,5)P2 would be promoted by its intrinsic ability to bind the phospholipid and augmented by its binding to PLCγ. While deletion of the region in Spry2 required for PLC binding did not prevent preservation of PI(4,5)P2, the mutant failed to co-localize with the phospholipid, suggesting that the region enhances Spry2-PI(4,5)P2 association. This augmenting role must be important, as the deletion was as effective as mutation of the PI(4,5)P2-binding determinant in relieving the block to VLP release. Support for linking IP3R to ERK activation is provided by our finding that depletion of IP3R was accompanied by reduction in the level of phospho-ERK in depleted cells. Support for linking IP3R function to Spry2 localization is provided by our finding that IP3R depletion led to a reduction in Spry2 accumulation on the plasma membrane. Loss of Spry2 from the plasma membrane is expected to result in reduced interference with PI(4,5)P2 hydrolysis, generation of the IP3R activating ligand and re-initiation of the Ca2+ signaling circuit.
FIGURE 7. A model linking Spry2 control of Ca2+ release from IP3R-gated stores to IP3R control of Spry2 at the plasma membrane.
The blockade to release of Ca2+ from the IP3R-gated stores due to the Spry2-mediated inhibition of PI(4,5)P2 hydrolysis (red lines and font) may signal disruption of Spry2-PI(4,5)P2 binding at the plasma membrane (green line).
An alternative possibility is that the inhibitory effect of Spry2 on VLP release is unrelated to a blockade to IP3R activation and instead reflects competition between Spry2 and Gag for binding to PI(4,5)P2 at the plasma membrane. In fact, this possibility is supported by our observation that under conditions where the full-length Spry2 protein inhibits VLP release, inhibition maps to the CTD. In these experiments, Gag is detected in the cell interior, consistent with the notion that the two proteins compete. However, consistent with the lack of impact of the CTD on PI(4,5)P2 described in this study, the Spry2-CTD fragment accumulates in the cell interior rather than at the plasma membrane whether or not its expression inhibits VLP release, indicating that the inhibitory effect, when observed, is not due to an interaction with plasma membrane-bound PI(4,5)P2. When co-expressed with the CTD, Gag was detected inside the cell only when the fragment was inhibitory. This suggests (i) that the PI(4,5)P2-binding determinant is not exposed in the CTD fragment and (ii) that the CTD inhibits VLP release and interferes with Gag accumulation on the plasma membrane for reasons other than straightforward competition. The possibility of Spry2-Gag competition also was tested by co-expressing Gag with the Δ36–66 mutant, which is very stable and accumulates to higher than WT levels (c.f., figure 4). As shown in the figure, this mutant does not inhibit VLP release. These lines of experimentation argue against the notion that Spry2 simply competes with Gag for binding to PI(4,5)P2 (although this may occur under some conditions).
The potential linkage of IP3R and Spry2 functions in Gag release provides an explanation for the otherwise apparently conflicting observation that PI(4,5)P2 in both intact and hydrolyzed forms are requirements of HIV-1 budding. Disruption of this control may provide new opportunities for design of novel anti-viral interventions.
MATERIALS AND METHODS
Plasmids and reagents
Plasmid pCMV-HIV-gag encoding wild-type HIV-1 Gag C-terminally tagged with GFP was described previously 32. Plasmids encoding the full length human Spry2 N-terminally tagged with HA (pCGN-HASpry2) and all mutants were generous gifts of D. Bar-Sagi (NYU, NY) and were previously described 33. The COS-12A2.5 cell line that expresses HIV-1 Gag constitutively 23 was a generous gift of L. Babe and C. Craik. Spry2 was detected by using a polyclonal antibody (Sigma-Aldrich) that recognizes an antigenic site in the CTD. HA-tagged proteins were detected by using a monoclonal antibody directed at the tag (Sigma-Aldrich). Gag tagged with GFP were detected with a monoclonal antibody against GFP (Clontech Laboratories) or a polyclonal antibody against the native form of the capsid protein 34. Actin levels were detected by using a monoclonal antibody against actin (Sigma-Aldrich). Phosphorylated-ERK (p-ERK) was detected using rabbit polyclonal antibody purchased from Cell Signaling. Mouse monoclonal antibodies 2C11 and 2335 raised against PI(4,5)P2 were obtained from Abcam.
Cell culture and transfection
COS-1 or COS-12A2.5 cells were cultured in Dulbecco’s modified Eagle medium supplemented with fetal bovine serum (5%) and antibiotics (1%) to 60% confluency at 37 °C. The cells were transfected using Fugene6 reagent (Roche) for DNA or Lipofectamine for siRNA (Invitrogen). At 48 h post-transfection, the culture medium was separated and cells were scraped in lysis buffer [50 mM Tris, pH 7.4, 137 mM NaCl, 1.5 mM MgCl2, 1 mM EDTA, 1% Triton X-100, 10% glycerol] with a rubber policeman and collected by centrifugation. VLPs particles were passed through a 0.45 um pore size filter and isolated by ultracentrifugation through a cushion of 20% sucrose at 36,000 rpm for 90 min at 4°C using a Beckman SW41 rotor.
Protein analysis
Cells were lysed in buffer containing protease and phosphatase inhibitors. Cell lysates were passed through 26G 3/8 needles four times and clarified by centrifugation at 10,000xg for 15 min at 4°C. Proteins were separated in 10 to 12.5% SDS polyacrylamide gels and identified by Western blotting using Alexa Fluor 680 goat anti-mouse IgG (Molecular Probes, 1:10,000) and IRDyeTM800-conjugated affinity purified goat anti-rabbit IgG (Rockland, 1:10,000). Proteins were visualized using an infrared-based imaging system (Odyssey, LI-COR Biosciences).
RNA interference
siRNA targeting IP3R was purchased from Dharmacon and transfected as described previously 4.
Confocal microscopy
Cos-1 cells were grown to 40% confluency on square coverslips (22 × 22mm) in six well plates in DMEM supplemented with fetal bovine serum and antibiotics as described previously 32. At 24 hours post-transfection, cells were washed twice with PBS and fixed in 3.7% formaldehyde in PBS for 20 min. Samples were washed three times with PBS for 5 min, permeabilized with 0.1% Triton X-100 for 4 min and washed three times with PBS. After blocking for 10 min in PBS containing 1% bovine serum albumin (BSA), cells were incubated with primary antibody for 1 hour at 37° C, rinsed three times with PBS and incubated with the secondary antibody conjugated with the fluorophore Texas Red for 30 min. The nuclear stain Hoechst (Molecular Probes) was added in the last 10 min. Cells were mounted using Vectashield hard set medium (Vector Laboratories). Confocal images were captured with an inverted fluorescent/dic Zeiss Axiovert 200M microscope equipped with an AxioCam HRm camera (Zeiss) and mercury arc lamp light source using a 63x Plan-Apochromat (NA 1.40) oil objective and operated using Axiovision version 4.5 software. Figures show images captured at the center z plane. Where noted, serial sections through the z plane of the cell were examined at 0.4 μm intervals. Also where indicated, protein co-localization was assessed in 15 cells by determination of Pearson correlation coefficient 26 and regarded as significant when values of 0.6 or higher (equivalent to a 95% level of confidence for that number of cells) were observed.
Acknowledgments
We thank Drs. H.J. Kim and D. Bar-Sagi for reagents. G.M. was supported by W. Burghardt Turner Postdoctoral Fellowship (NSF-HRD-funded SUNY AGEP, Grant #35583). This study was supported by NIH R01 award AI068463 (to CC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Freed EO. Viral Late Domains. J Virol. 2002;76:4679–4687. doi: 10.1128/JVI.76.10.4679-4687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bieniasz PD. The cell biology of HIV-1 virion genesis. Cell Host Microbe. 2009;5:550–558. doi: 10.1016/j.chom.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ono A, Ablan SD, Lockett SJ, Nagashima K, Freed EO. Phosphatidylinositol (4,5) biphosphate regulates HIV-1 Gag targeting to the plasma membrane. Proc Natl Acad Sci USA. 2004;101:14889–14894. doi: 10.1073/pnas.0405596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehrlich LS, Medina GN, Khan MB, Powell MD, Mikoshiba K, Carter CA. Activation of the Inositol (1,4,5)-Triphosphate Calcium Gate Receptor is Required for HIV Gag Release. J Virol. 2010;84:6438–6451. doi: 10.1128/JVI.01588-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mikoshiba K. IP3 receptor/Ca2+ channel: from discovery to new signaling concepts. J Neurochem. 2007;102:1426–1446. doi: 10.1111/j.1471-4159.2007.04825.x. [DOI] [PubMed] [Google Scholar]
- 6.Balla T, Szentpetery Z, Kim YJ. Phosphoinositide signaling: new tools and insights. Physiology. 2009;24:231–244. doi: 10.1152/physiol.00014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medina GN, Ehrlich LS, Chen MH, Khan MB, Powell MD, Carter CA. Sprouty2 binds ESCRT-2 factor Eap20 and facilitates HIV-1 Gag release. J Virol. 2011 doi: 10.1128/JVI.00141-11. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minowada G, Jarvis LA, Chi CL, Neubuser A, Sun X, Hacohen N, Krasnow MA, Martin GR. Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development. 1999;126:4465–4475. doi: 10.1242/dev.126.20.4465. [DOI] [PubMed] [Google Scholar]
- 9.Mason JM, Morrison DJ, Basson MA, Licht JD. Sprouty proteins: multifaceted negative-feedback regulators of receptor tyrosine kinase signaling. Trends Cell Biol. 2006;16:45–54. doi: 10.1016/j.tcb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Edwin F, Anderson K, Ying C, Patel TB. Intermolecular interactions of Sprouty proteins and their implications in development and disease. Mol Pharmacol. 2009;76:679–691. doi: 10.1124/mol.109.055848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guy GR, Jackson RA, Yusoff P, Chow SY. Sprouty proteins: modified modulators, matchmakers or missing links? J Endocrinol. 2009;203:191–202. doi: 10.1677/JOE-09-0110. [DOI] [PubMed] [Google Scholar]
- 12.Casci T, Vinos J, Freeman M. Sprouty, an intracellular inhibitor of Ras signaling. Cell Calcium. 1999;96:655–665. doi: 10.1016/s0092-8674(00)80576-0. [DOI] [PubMed] [Google Scholar]
- 13.Kramer S, Okabe M, Hacohen N, Krasnow MA, Hiromi Y. Sprouty: a common antagonist of FGF and EGF signaling pathways in Drosophila. Development. 1999;126:2515–2525. doi: 10.1242/dev.126.11.2515. [DOI] [PubMed] [Google Scholar]
- 14.Reich A, Sapir A, Shilo B. Sprouty is a general inhibitor of receptor tyrosine kinase signaling. Development. 1999;126:4139–4147. doi: 10.1242/dev.126.18.4139. [DOI] [PubMed] [Google Scholar]
- 15.Ozaki K, Kadomoto R, Asato K, Tanimura S, Itoh N, Kohno M. ERK pathway positively regulates the expression of Sprouty genes. BBRC. 2001;285:1084–1088. doi: 10.1006/bbrc.2001.5295. [DOI] [PubMed] [Google Scholar]
- 16.Abe M, Naski M. Regulation of sprouty expression by PLCgamma and calcium-dependent signals. BBRC. 2004;323:1040–1047. doi: 10.1016/j.bbrc.2004.08.198. [DOI] [PubMed] [Google Scholar]
- 17.Akbulut S, Reddi RA, Aggarwal P, Ambardekar C, Cancian B, Kim MK, Hix L, Vilimas T, Mason J, Basson MA, Lovatt M, Powell J, Collins S, Quatela S, Phillips M, Licht JD. Sprouty proteins inhibit receptor-mediated activation of phosphatidylinositol-specific phospholipase C. Mol Biol Cell. 2010;21:3487–3496. doi: 10.1091/mbc.E10-02-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim J, Yusoff P, Wong ES, Chandramouli S, Lao DH, Fong CW, Guy GR. The cysteine-rich sprouty translocation domain targets mitogen-activated protein kinase inhibitory proteins to phosphatidylinositol 4,5-bisphosphate in plasma membranes. Mol Cell Biol. 2002;22:7953–7966. doi: 10.1128/MCB.22.22.7953-7966.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harmon B, Ratner L. Induction of the Galpha(q) signaling cascade by the human immunodeficiency virus envelope is required for virus entry. J Virol. 2008;82:9191–9205. doi: 10.1128/JVI.00424-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horowitz LF, Hirdes W, Suh BC, Hilgemann DW, Mackie K, Hille B. Phospholipase C in living cells: activation, inhibition, Ca2+ requirement, and regulation of M current. J Gen Physiol. 2005;126:243–262. doi: 10.1085/jgp.200509309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cenni B, Picard D. Two compounds commonly used for phospholipase C inhibition activate the nuclear estrogen receptors. BBRC. 1999;261:340–344. doi: 10.1006/bbrc.1999.1017. [DOI] [PubMed] [Google Scholar]
- 23.Babe LM, Rose J, Craik CS. Trans-dominant inhibitory human immunodeficiency virus type 1 protease monomers prevent protease activation and virion maturation. Proc Natl Acad Sci USA. 1995;92:10069–10073. doi: 10.1073/pnas.92.22.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammond GR, Dove SK, Nicol A, Pinxteren JA, Zicha D, Schiavo G. Elimination of plasma membrane phosphatidylinositol (4,5)-bisphosphate is required for exocytosis from mast cells. J Cell Sci. 2006;119:2084–2094. doi: 10.1242/jcs.02912. [DOI] [PubMed] [Google Scholar]
- 25.Hammond GRV, Schiavo G, Irvine RF. Immunocytochemical techniques reveal multiple, distinct cellular pools of PtdIns4P and PtdIns(4,5)P2. Biochem J. 2009;422:23–35. doi: 10.1042/BJ20090428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manders EMM, Verbeek JF, Aten JA. Measurement of colocalization of objects in dual color confocal images. J Microsc. 1993;169:375–82. doi: 10.1111/j.1365-2818.1993.tb03313.x. [DOI] [PubMed] [Google Scholar]
- 27.Hattori M, Suzuki AZ, Higo T, Miyauchi H, Michikawa T, Nakamura T, Inoue T, Mikoshiba K. Distinct roles of inositol 1,4,5-triphosphate receptor types 1 and 3 in Ca2+ signaling. J Biol Chem. 2004;279:11967–11975. doi: 10.1074/jbc.M311456200. [DOI] [PubMed] [Google Scholar]
- 28.Nutt SL, Dingwell KS, Holt CE, Amaya E. Xenopus Sprouty2 inhibits FGF-mediated gastrulation movements but does not affect mesoderm induction and patterning. Genes Dev. 2001;15:1152–1166. doi: 10.1101/gad.191301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sivak JM, Petersen LF, Amaya E. FGF signal interpretation is directed by Sprouty and Spred proteins during mesoderm formation. Dev Cell. 2005;8:689–701. doi: 10.1016/j.devcel.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 30.Fernandes F, Chen K, Ehrlich LS, Jin J, Chen MH, Medina GN, Symons M, Montelaro R, Donaldson J, Tjandra N, Carter CA. Phosphoinositides Direct Equine Infectious Anemia Virus Gag Trafficking and Release. Traffic. 2011;4:438–451. doi: 10.1111/j.1600-0854.2010.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saad JS, Miller J, Tai J, Kim A, Ghanam RH, Summers MF. Structural basis for targeting HIV-1 Gag proteins to the plasma membrane for virus assembly. Proc Natl Acad Sci U S A. 2006;103:11364–11369. doi: 10.1073/pnas.0602818103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medina G, Pincetic A, Ehrlich LS, Zhang Y, Tang Y, Leis J, Carter CA. Tsg101 can replace Nedd4 function in ASV Gag release but not membrane targeting. Virology. 2008;377:30–38. doi: 10.1016/j.virol.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall AB, Jura N, DaSilva J, Jang YJ, Gong D, Bar-Sagi D. hSpry2 is targeted to the ubiquitin-dependent proteasome pathway by c-Cbl. Curr Biol. 2003;13:308–314. doi: 10.1016/s0960-9822(03)00086-1. [DOI] [PubMed] [Google Scholar]
- 34.Ehrlich LS, Krausslich HG, Wimmer E, Carter C. Expression in Escherichia coli and purification of human immunodeficiency virus type 1 capsid protein (p24) AIDS Res Hum Retroviruses. 1990;6:1169–1175. doi: 10.1089/aid.1990.6.1169. [DOI] [PubMed] [Google Scholar]