Abstract
Accurate diagnosis of cerebral malaria (CM) is important for patient management, epidemiological and end point surveillance, and enrolling patients with CM in studies of pathogenesis or therapeutic trials. In malaria-endemic areas, where asymptomatic Plasmodium falciparum parasitemia is common, a positive blood film in a comatose individual does not prove that the coma is due to malaria. A retinopathy consisting of two unique features – patchy retinal whitening and focal changes of vessel color – is highly specific for encephalopathy of malarial etiology. White-centered retinal hemorrhages are a common but less specific feature. Either indirect or direct ophthalmoscopy can be used to identify the changes, and both procedures can be learned and practiced by nonspecialist clinicians. In view of its important contributions to both clinical care and research, examination of the retina should become a routine component of the assessment of a comatose child or adult when CM is a possible diagnosis.
Keywords: cerebral malaria, papilledema, retinopathy
According to WHO estimates, there were over 850,000 malaria deaths in 2008; 90% of these were in Africa and 85% were in children [101]. Reductions in malaria have occurred in other regions and in some localized areas in Africa, but severe malaria continues to be an important contributor to child mortality. In many African countries with good access to anti-malarial drugs and insecticide-treated bednets, expected reductions in mortality and morbidity have yet to occur. When malaria control improves, the burden of disease may shift to older age groups [1].
Accurate identification of severe malaria syndromes is essential for patient management, for enrollment of patients in studies of therapy or pathogenesis, and for monitoring the progress of control programs. A syndrome that significantly contributes to malaria mortality, especially in African children, is the diffuse encephalopathy known as cerebral malaria (CM). Interventions aimed at treating CM are unlikely to be successful if the diagnosis is wrong. Diagnosis is problematic and the disease is frequently misdiagnosed. In symptomatic travelers, military personnel or other malaria-naive individuals, the presence of malaria parasites on a blood film is generally sufficient to establish the diagnosis of a malaria illness. Antidisease immunity develops over time in malaria-endemic areas, and in those settings, the presence of parasitemia may be incidental to the cause of a severe illness – or parasitemia may be one of several factors contributing to a life-threatening syndrome.
Diagnostic difficulty in severe malaria
The clinical diagnosis of severe malaria based on WHO criteria requires Plasmodium falciparum parasitemia and a measure of severe disease, such as impaired consciousness or severe anemia, with the exclusion of other potential causes of the disease. This is an inclusive definition that relies on accurate assessment of blood films, and the reliable exclusion of other causes of severe disease. In malaria-endemic areas both of these facets of the definition are difficult to achieve.
Patients who present in coma with parasitemia may have another unrecognized cause for their coma with an incidental parasitemia. A prospective autopsy study in Malawi of children dying with clinically defined CM found that 23% did not have the cerebral histopathological hallmarks of CM, and had other causes of death [2]. This finding was made in a research center where blood films are accurate and clinical care is intense. Unfortunately, a study of this nature has not been replicated in other settings, largely because of financial, logistical and ethical barriers. However, there is no reason, to expect that the situation in Malawi is unique.
Misdiagnosis occurs because the predominant manifestations of severe malaria in children (coma, seizures, hypertonia, hyperventilation and anemia) are not specific to malaria. Higher-level diagnostic laboratory and radiological investigations are rarely available in hospitals in malaria-endemic regions. In the Malawi autopsy study, the other causes of death (e.g., intracranial hemorrhage, Reye’s syndrome, poisoning and rabies) were unexpected and difficult to diagnose with the investigations that are routinely available. When these patients present in coma, with seizures and parasitemia, it is not surprising that they are diagnosed with CM, as they conform to the WHO definition.
In fact, misdiagnosis may be more common in patients who die with presumed CM than in those who survive. This is owing to the fact that mortality rates in other diseases with profound coma are probably even higher than the mortality rate in CM (evidence for this comes from our funduscopy results in children with CM over 6 years). The mortality and neurological sequele are significantly higher in patients with papilledema and no malaria retinopathy (45%) than in those with retinopathy (20%). It is likely that patients with papilledema and no malaria retinopathy have other causes of coma and death, with an unrelated parasitemia. Only two such cases have undergone autopsy in the Malawi study; neither had evidence of sequestration of parasitized red blood cells in the cerebral microvasculature. One died of hepatic necrosis and the other of left ventricular failure and pulmonary edema.
Along the whole spectrum of disease associated with malaria infection, misdiagnosis is not unusual. A large study in ten Tanzanian district hospitals found that the majority (54%) of adults and children treated for severe malaria had no parasitemia [3]. In people over 5 years of age, living at an altitude of over 600 m, 69% treated for severe malaria had no parasitemia; this proportion dropped to 31% in subjects under 5 years in high transmission areas. Of the slide-negative patients, 66% were not treated with antibiotics and the case fatality was higher than in the slide-positive group. Respiratory distress and reduced consciousness were the strongest predictors of death in both parasitemic and nonparasitemic patients, underlining the nonspecific nature of signs in severe malaria and other severe disease.
It is increasingly recognized that presumptive diagnosis of uncomplicated malaria is no longer appropriate in this era in which first-line therapy is more expensive and less readily available than in previous decades, and that diagnostic tools can reduce overprescription at the primary healthcare level [4]. It is less well recognized that diagnosis of those at risk of dying from severe malaria in secondary and tertiary hospitals is commonly inadequate. Rapid diagnostic tests do not solve this problem because, as already noted, parasitemia alone does not indicate that malaria infection is the cause of the acute illness. There is a clinical need for an improved diagnostic test that is specific to severe malaria.
We present the evidence here for why we think it is essential to include an assessment for malaria retinopathy in the diagnosis of CM, and why this is important for clinical care, for research studies and for malaria control and elimination efforts.
Malaria retinopathy as a diagnostic test
The first published report of an ocular abnormality in malaria was retinal hemorrhage, described by Poncet in 1878 [5], who noted that nearly all cases of ‘pernicious malaria’ were accompanied by retinal hemorrhages. This report was corroborated by others early in the 20th century [6,7], and surfaced again in the context of a seminal study of adult malaria in Thailand [8] and a largely pediatric study in Zaire [9]. In the adult series, retinal hemorrhages were observed in 14.6% of adult patients, and were associated with several measures of disease severity (parasitemia, schizontemia, anemia and high serum concentrations of creatinine). The prevalence of retinal hemorrhages in the Zaire study was 31%; in this study, 70% of patients were under the age of 6 years.
The first description of a constellation of funduscopic findings, now known as malaria retinopathy, emerged from studies of children in Malawi in 1993 [10], and was futher investigated by fundus photography and angiography in Kenyan children in 1997 [11], and by pediatric case–control studies in Mali in 2002 [12], and in The Gambia in 2004 [13]. Adult Bangladeshis with severe malaria also demonstrate features of malaria retinopathy [14,15], but one of these features, vessel changes (see below), was observed much less commonly than in African children. Several published studies have compared funduscopic findings in patients with CM to those with less severe malaria [7,12,13,15,16] and in each report the prevalence and intensity of malaria retinopathy was proportional to the intensity of the clinical syndrome.
Malaria retinopathy has three main components, the first two being unique to malaria: retinal whitening, vessel changes and retinal hemorrhages. Papilledema can co-exist with any or all of the three features, but papilledema is not specific for malaria; papilledema alone is not diagnostic of CM. Malaria retinopathy is most readily appreciated through fully dilated pupils, and with the use of both direct and indirect ophthalmoscopy.
Retinal whitening consists of patches of opacification, and is most commonly seen within the macula (Figure 1). It is common around the fovea (center of the macula) and temporal macula; the central fovea is spared. When the whitening is present outside the macula, it is termed peripheral whitening. Patches of peripheral whitening may form a mosaic pattern; they are less well demarcated, less bright and more widely distributed than cotton wool spots, which may also occur. Retinal whitening closely resembles the patchy ischemic retinal changes in central vein occlusion, but has a different retinal distribution [17]. Fluorescein angiographic studies from Malawi subsequently demonstrated that retinal whitening closely matches areas of retinal hypo-perfusion, likely related to the presence of parasitized erythrocytes, sequestered in the retinal microvasculature [18].
Figure 1. Central and temporal macula showing patchy retinal whitening.
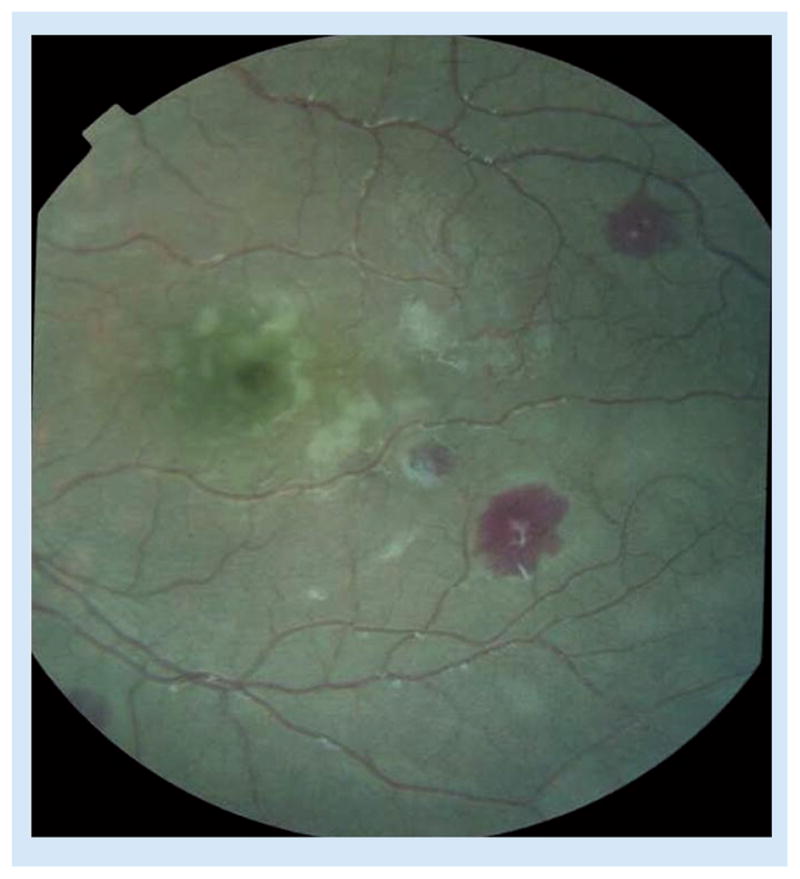
Three white-centered hemorrhages are also present.
Vessel changes are most commonly seen in the peripheral fundus, and are generally seen in discrete sections of vessels, often originating at a branch point (Figure 2). Instead of the normal red color, affected retinal vessels are orange or white. Any retinal vessel can be affected but these changes in capillaries are more easily seen at higher magnification. The histological correlate of these vessel changes is the presence of mature parasites within de-hemoglobinized red cells, sequestered within and filling the lumen of the vessels that have an altered appearance [19].
Figure 2. Retinal vessel whitening.
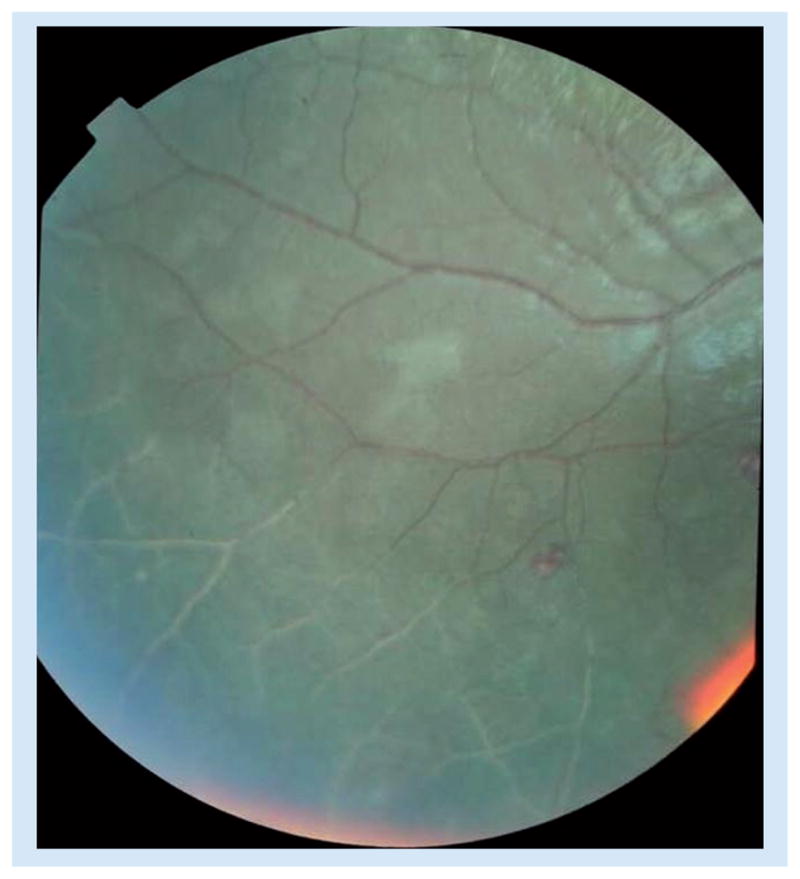
Shown in the lower section of the photograph.
Retinal hemorrhages in malaria are predominantly white-centered, intra-retinal blot hemorrhages, and are similar to Roth spots, retinal hemorrhages with a white or pale center consisting of coagulated fibrin (Figure 3).
Figure 3. Multiple white-centered blot hemorrhages to an extent typical of malaria retinopathy.
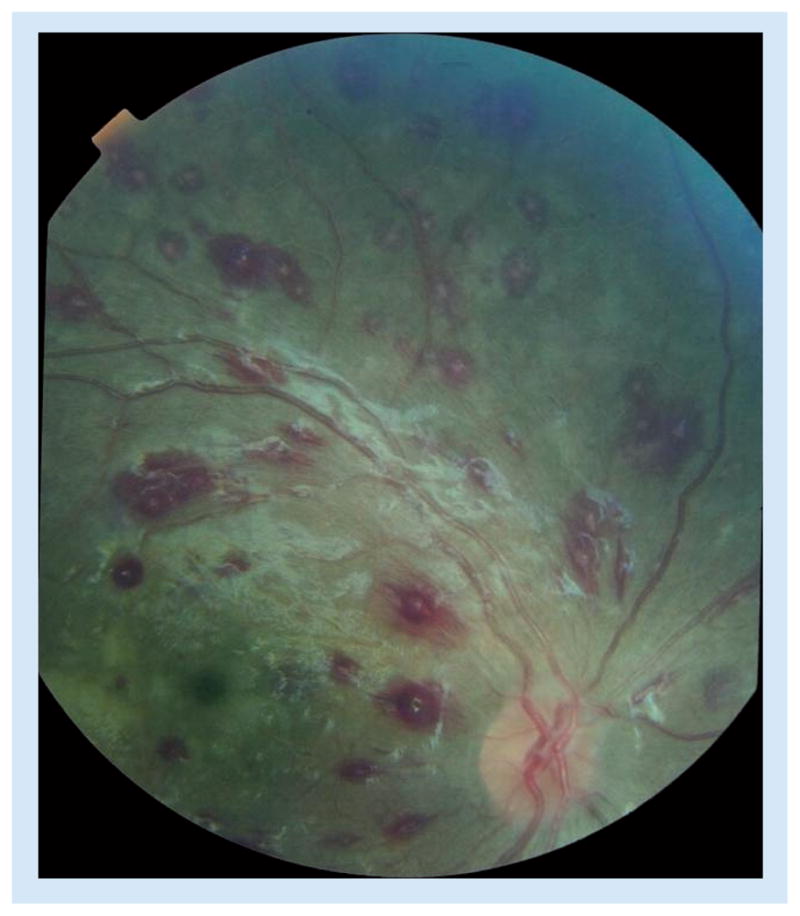
Retinal whitening and small vessel changes are also present. There is artefactual glare along and below the vascular arcade.
In the Malawi autopsy study of children with WHO-defined CM, the only clinical or laboratory feature that distinguished malarial coma from nonmalarial coma was the presence or absence of malaria retinopathy [2]. At present, malaria retinopathy is the most sensitive and specific clinical indicator of cerebral sequestration, the histopathologic hallmark of CM. Retinopathy is present in two-thirds of children with clinically defined CM. In a population in which asymptomatic parasitemia is common, identification of malaria retinopathy is the only test currently available that can strengthen confidence in the malarial cause of a syndrome – such as coma – that could have other etiologies. An assessment for malaria retinopathy provides prognostic as well as diagnostic information. Funduscopic evidence supports a growing recognition that there is considerable clinical diversity among patients with the syndrome known as CM.
Identifying subsets within CM
Data from 6 years of standardized funduscopy on children with clinically defined CM in Blantyre, Malawi, has demonstrated three distinct groups: those with normal fundi (37%), those with malaria retinopathy (60%) and those with papilledema alone (3%). These groups have very different case-fatality rates: 7% (95% CI: 4–10%) in those with normal fundi, 18% (95% CI: 16–22%) for patients with malaria retinopathy and 44% (95% CI: 25–63%) in those with papilledema alone. These three groups also differ significantly in the prevalance of hypotension and respiratory distress, and in the mean values of hematocrit, platelet concentration, parasite density and coma resolution time. Papilledema can occur in conjunction with malaria retinopathy, and the case-fatality rate in the subgroup of patients with malaria retinopathy who have papilledema is 36% (27–46%), still not has high as in the group with papilledema alone. We have previously demonstrated that papilledema in CM is independent of the other signs of malaria retinopathy in predicting death.
There are plausible explanations for these different mortality rates within the group of children with clinically defined CM [20]. Among children with retinopathy, those with papilledema have an increased risk of dying, but the case-fatality rate is even higher among those with papilledema alone. Papilledema is a nonspecific sign of intracranial disease with raised intracranial pressure. In the context of malaria retinopathy, papilledema indicates that the malarial pathology is particularly severe and when papilledema occurs without retinopathy, there is likely to be a nonmalarial cause of cerebral disease and raised intracranial pressure.
Further work has shed light on the group with the lowest mortality, those with normal fundi. A prospective case–control study of seizures after CM in Malawi included children meeting the standard clinical case definition of CM, but who lacked malaria retinopathy. Compared with age-matched controls with no history of CM, the retinopathy-negative CM children had a higher prevalence of pre-existing neurodisabilities and a family history of epilepsy suggesting that they may have a pre-existing propensity toward adverse neurologic symptoms and outcomes [21].
To summarize the subsets of children with clinically defined CM, the majority have malaria retinopathy, and can be described as ‘true CM’ – normal children who have an approximately 20% risk of death from the episode of CM. A significant minority have normal fundi and this comprises patients who are predisposed to seizures and some patients who may have an intercurrent nonmalaria illness and with falciparum coinfection – distinguishing between these options and identifying a treatable cause of coma in the latter group is, at present, a diagnostic challenge. Treated for malaria and any apparent bacterial infection, their case-fatality rate is between 5 and 10%. Patients with coma, parasitemia and papilledema alone are an uncommon group, but an important one because they have a case-fatality rate of nearly 50%. It is likely that they are suffering from an alternative cause of coma, which carries a high mortality whether accompanied by malaria infection or not.
We believe that the results of funduscopy should be included in the diagnosis of CM in children, and that funduscopic findings can inform a new classification of the CM syndrome.
Barriers to uptake of funduscopy in CM
The most significant barrier to inclusion of retinal findings in the diagnosis of CM seems to be at the clinician level: lack of training, experience, and/or confidence to perform funduscopy. There is little question that indirect ophthalmoscopy, which provides a wider field of view and a 2D image, is faster and more reliable than direct ophthalmoscopy, which provides more magnification at the cost of a very restricted field of view. The indirect ophthalmoscope is expensive compared with the direct and it takes more time to master, but any clinician can learn to use it. If cost and availability are insurmountable obstacles, then a thorough direct ophthalmoscopic examination will suffice to identify approximately 90% of malaria retinopathy. The pupils must be fully dilated, and time must be taken to study the retina as far peripherally as it can be seen by direct ophthalmoscopy. In comatose African children, it takes approximately 45 min for pupils to dilate adequately after drops have been instilled, so the procedure requires planning and coordination if it is to be accomplished properly. Practice is essential; the clinician who only views a fundus once a week or once a month will not become skilled in funduscopy. During the learning period, it is necessary to examine several patients each day. Once mastered, however, skills can be maintained by examining one or two patients every week.
Implications for patient management, clinical research & assessing malaria control & elimination efforts Patient management
The basis of medicine is an understanding of disease. Funduscopic findings further our understanding of severe malaria, and syndromes that may masquerade as severe malaria in the presence of parasitemia. Obviously any child who is in coma with parasitemia should be treated for CM as an emergency. The presence of malaria retinopathy reassures us that the malarial infection is the prime cause of their illness.
The absence of retinopathy on dilated funduscopy, on the other hand, should alert the physician to the possibility that there is another cause for coma. Unfortunately, the other causes that have become apparent in Malawi from autopsy and virology work are difficult to identify and treat, especially in a developing world setting. Trypanosomiasis should be carefully looked for. In a child with focal neurological signs and a lymphocytosis in the cerebrospinal fluid, Herpes simplex encephalitis should be considered and treated with acyclovir if available. Conditions such as intracranial hemorrhage from a vascular malformation, Reye’s syndrome from salicylate use, poisoning, rabies [22] and other encephalopathies are disparate and only treatable with supportive measures. Bacterial infections should be looked for and must be appropriately treated on the basis of a definitive or presumptive diagnosis.
Risk factors for epilepsy should be sought. The presence of these risk factors indicate that the patient may have a predisposition to seizures and an extended postictal period when challenged with P. falciparum infection.
The presence of pre-existing developmental delay will not change management of the acute illness, but it would alter the expectations for final recovery. The presence of papilledema without retinopathy in a comatose, parasitemic child has a very poor prognosis. Everything should be done to investigate other possible causes of coma while treating malaria and providing intensive supportive care. Unfortunately, if a searching history does point the finger of suspicion towards another cause, there may not be any feasible specific therapy.
Clinical research
Without a stringent clinical case definition of CM, research studies of association are hindered. True associations may be obscured because the sample size is inadequate and spurious associations may emerge when patients with severe nonmalaria disease are misclassified as having CM. Clinical trials of interventions targeted at CM would be more efficient if a diagnosis that included the presence of at least one feature of malaria retinopathy were used.
Malaria control & elimination efforts
These also rely on accurate case definitions. It is possible that the apparent impact of a highly effective intervention would be diminished if comatose, parasitemic children without malaria retinopathy were included as ‘severe malaria’ outcomes. Misclassification here has implications at all levels, from the communities grappling with the disease up to policy makers who are trying to ascertain the relative value of various interventions.
Conclusion
The evidence for including an assessment for malaria retinopathy in the diagnosis of CM is very strong and is based on over two decades of study of clinical, laboratory, angiographic and histopathologic findings. Including the signs of malaria retinopathy in the definition of CM in African children clearly benefits individual patients; it would also result in more useful data and a much stronger clinical case definition for studies of this serious clinical syndrome associated with the major pathogen P. falciparum.
Future perspective
Increasing use of bedside ophthalmoscopy will improve the accuracy of diagnosis of CM, a syndrome that is frequently misdiagnosed, and will prompt greater care in the search for other possible causes of encephalopathy in patients without retinal changes. We hope that health services will invest in ophthalmoscopes and in the training of staff in their use.
A large majority of children who die of malaria do so without ever reaching a formal health facility. Many health services are working to improve the access of communities to competent clinical care. Improved access is likely to increase the number of cases reaching medical attention and needing accurate diagnosis to guide appropriate therapy.
There is a theoretical possibility that as the intensity of transmission of P. falciparum falls as a result of successful control measures, communities will acquire antimalarial immunity more slowly, with an increasing risk of malaria infections progressing to severe disease. This may contribute a further increase to the number of individuals requiring competent diagnosis of CM.
Retinopathy results from pathologic changes within and around vessels occupied by parasitized erythrocytes. In research centers, improvements in magnification and imaging, with the added possibility of fluorescent labeling of component tissues, may lead to further opportunities to investigate pathogenic mechanisms in severe malaria, and thus to improved and novel methods of treatment and prevention.
Over the next decade, efforts will increase to improve malaria control in many countries and to eliminate it where possible. Accurate identification of cerebral and other forms of severe malaria will be important in the monitoring of progress towards these targets, and towards the more distant goal of the eventual eradication of malaria.
Executive summary.
Diagnostic difficulty in severe malaria
Misdiagnosis of individuals with coma and parasitemia is common, because of a high prevalence of asymptomatic parasitemia in tropical communities.
Malaria retinopathy as a diagnostic test
The presence of malaria retinopathy increases the likelihood that a clinical diagnosis of cerebral malaria is correct.
Retinopathy occurs in both adults and children with cerebral malaria, although it has been more fully described in children.
Of four funduscopic abnormalities that can be observed in patients with cerebral malaria – retinal whitening, vessel changes, white-centered hemorrhages and papilledema – the first two are malaria specific, and the third is highly suggestive of a malarial etiology.
Retinal angiographic studies indicate that retinal whitening coincides with areas of nonperfusion, while vessel changes are associated with vascular filling-defects (vessels appear orange) or vascular occlusion (vessels appear white).
Ocular histopathology suggests that such vessels are partially or totally occupied by sequestered parasitized erythrocytes.
Barriers to uptake of funduscopy in cerebral malaria
Lack of clinician training, experience and confidence.
Expenses associated with the required equipment (direct and indirect ophthalmoscope).
Time required to carry out the examination properly.
Implications for patient management, clinical research & assessing malaria control & elimination efforts
Ophthalmoscopy in the comatose individual can contribute to good clinical management, to accurate enrollment in studies of pathogenesis or treatment, and to correct monitoring of end points during trials or disease surveillance.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪ ▪ of considerable interest
- 1.O’Meara WP, Bejon P, Mwangi TW, et al. Effect of a fall in malaria transmission on morbidity and mortality in Kilifi, Kenya. Lancet. 2008;474(9649):1555–1564. doi: 10.1016/S0140-6736(08)61655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2▪▪.Taylor TE, Fu WJ, Carr RA, et al. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. 2004;10(2):143–145. doi: 10.1038/nm986. Demonstrates that retinopathy is the best in vivo predictor of cerebral sequestration of parasitized red blood cells. [DOI] [PubMed] [Google Scholar]
- 3.Reyburn H, Mbatia R, Drakeley C, et al. Overdiagnosis of malaria in patients with severe febrile illness in Tanzania: a prospective study. BMJ. 2004;329(7476):1212. doi: 10.1136/bmj.38251.658229.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyabayinze DJ, Asiimwe C, Nakanjako D, Nabakooza J, Counihan H, Tibenderana JK. Use of RDTs to improve malaria diagnosis and fever case management at primary health care facilities in Uganda. Malar J. 2010;9:200. doi: 10.1186/1475-2875-9-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poncet F. De la retino-coroidiste palustre. Ann d’Oculistique. 1878;79:201–218. cited in: Looareesuwan S, Warrell DA, White NJ et al Am J Trop Med Hyg 32, 911–915, (1983) [Google Scholar]
- 6.Pereyra G. Emmorhagie retiniche da malaria. Arch Ottalmol Napoli. 1922;29:49–70. [Google Scholar]
- 7.Werner H. Uber Netzhautb lutungun bei Malaria. Archiv Schiffs-u Tropen Hyg. 1911;15:431–435. [Google Scholar]
- 8.Looareesuwan S, Warrell DA, White NJ, et al. Retinal hemorrhage, a common sign of prognostic significance in cerebral malaria. Am J Trop Med Hyg. 1983;32(5):911–915. doi: 10.4269/ajtmh.1983.32.911. [DOI] [PubMed] [Google Scholar]
- 9.Kayembe D, Maertens K, De Laey JJ. Ocular complications of cerebral malaria. Bull Soc Belge Ophtalmol. 1980;190:53–60. [PubMed] [Google Scholar]
- 10▪▪.Lewallen S, Taylor TE, Molyneux ME, Wills BA, Courtright P. Ocular fundus findings in Malawian children with cerebral malaria. Ophthalmology. 1993;100(6):857–861. doi: 10.1016/s0161-6420(93)31563-0. Describes the whole spectrum of findings in malaria retinopathy and demonstration of their association with outcome. [DOI] [PubMed] [Google Scholar]
- 11.Hero M, Harding SP, Riva CE, Winstanley PA, Peshu N, Marsh K. Photographic and angiographic characterization of the retina of Kenyan children with severe malaria. Arch Ophthalmol. 1997;115(8):997–1003. doi: 10.1001/archopht.1997.01100160167005. [DOI] [PubMed] [Google Scholar]
- 12.Schemann JF, Doumbo O, Malvy D, et al. Ocular lesions associated with malaria in children in Mali. Am J Trop Med Hyg. 2002;67(1):61–63. doi: 10.4269/ajtmh.2002.67.61. [DOI] [PubMed] [Google Scholar]
- 13.Burton M, Nyong’o O, Burton K, et al. Retinopathy in Gambian children admitted to hospital with malaria. Trop Doct. 2004;34(4):214–218. doi: 10.1177/004947550403400409. [DOI] [PubMed] [Google Scholar]
- 14.Sattar MA, Hoque HW, Amin MR, Faiz MA, Rahman MR. Neurological findings and outcome in adult cerebral malaria. Bangladesh Med Res Counc Bull. 2009;35(1):15–17. doi: 10.3329/bmrcb.v35i1.2313. [DOI] [PubMed] [Google Scholar]
- 15▪.Maude RJ, Beare NA, Abu Sayeed A, et al. The spectrum of retinopathy in adults with Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 2009;103(7):665–671. doi: 10.1016/j.trstmh.2009.03.001. Describes retinopathy in adults, compared with well-recognized findings in children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewallen S, Harding SP, Ajewole J, et al. A review of the spectrum of clinical ocular fundus findings in P falciparum malaria in African children with a proposed classification and grading system. Trans R Soc Trop Med Hyg. 1999;93(6):619–622. doi: 10.1016/s0035-9203(99)90071-8. [DOI] [PubMed] [Google Scholar]
- 17.Browning DJ. Patchy ischemic retinal whitening. Ophthalmology. 2004;111(3):606–607. doi: 10.1016/j.ophtha.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 18▪▪.Beare NA, Harding SP, Taylor TE, Lewallen S, Molyneux ME. Perfusion abnormalities in children with cerebral malaria and malarial retinopathy. J Infect Dis. 2009;199(2):263–271. doi: 10.1086/595735. Decribes the angiographic correlates of retinal whitening. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewallen S, White VA, Whitten RO, et al. Clinical-histopathological correlation of the abnormal retinal vessels in cerebral malaria. Arch Ophthalmol. 2000;118(7):924–928. [PubMed] [Google Scholar]
- 20▪.Lewallen S, Bronzan RN, Beare NA, Harding SP, Molyneux ME, Taylor TE. Using malarial retinopathy to improve the classification of children with cerebral malaria. Trans R Soc Trop Med Hyg. 2008;102(11):1089–1094. doi: 10.1016/j.trstmh.2008.06.014. Suggested categorization of children with clinical cerebral malaria according to retinal findings. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birbeck GL, Beare N, Lewallen S, et al. Identification of malaria retinopathy improves the specificity of the clinical diagnosis of cerebral malaria: findings from a prospective cohort study. Am J Trop Med Hyg. 2010;82(2):231–234. doi: 10.4269/ajtmh.2010.09-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mallewa M, Fooks AR, Banda D, et al. Rabies encephalitis in malaria-endemic area, Malawi, Africa. Emerg Infect Dis. 2007;13(1):136–139. doi: 10.3201/eid1301.060810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Website
- 101.WHO. World Malaria Report. Geneva: WHO; 2009. [Accessed 24 February 2011]. www.who.int/malaria/publications/atoz/9789241563901/en/index.html. [Google Scholar]


