Abstract
It has been suggested that intrasexual competition can be a source of negative frequency-dependent selection, causing agonistic character displacement and facilitating speciation and coexistence of (sibling) species. In this paper we synthesise the evidence that male-male and female-female competition contributes to cichlid diversification, showing that competition is stronger among same-coloured individuals than those with different colours. We argue that intrasexual selection is more complex because there are several examples where males do not bias aggression towards their own type. In addition, sibling species or colour morphs often show asymmetric dominance relationships. We briefly discuss potential mechanisms that might promote the maintenance of covariance between colour and aggression-related traits even in the face of gene-flow. We close by proposing several avenues for future studies that might shed more light on the role of intrasexual competition in cichlid diversification.
1. Introduction
The cichlid fish in East African lakes are emerging as one of the potentially most powerful model systems in speciation and adaptive radiation research [1–4]. The rock-dwelling communities of these lakes comprise several species complexes or genera that can be strongly differentiated in ecology. By contrast, within genera, sibling species tend to be ecologically more similar, yet strikingly different in male nuptial coloration [5–7]. This interspecific colour variation resembles intraspecific colour variation between hybridising incipient species or colour morphs. The inference is that sexual selection by female mate choice on male colour plays a central role in the evolution and maintenance of haplochromine species richness (e.g., [1, 2, 8, 9]). In haplochromines, male-male competition is likely important since territory ownership is a prerequisite to gain access to spawnings [8, 10]. Moreover, territory quality affects mate choice [11, 12]. Hence, aggressive competition over territory sites is intense and is likely to affect sexual selection. In theory, male-male competition can serve as a source of negative frequency-dependent selection in haplochromine cichlid fish due to stronger competition among same-coloured males than those with different colours [13–15]. The resulting disruptive selection may facilitate several evolutionary processes of diversification, including character displacement [16], reinforcement, speciation [15], and the syntopic coexistence of species [14, 17]. In this paper we discuss the relevance of male-male competition in evolutionary diversification. We summarise the empirical evidence that haplochromine cichlid males bias aggression toward similar-coloured rivals. We then show that negative frequency-dependent selection is often not symmetric and that selection arising from male-male competition is often more complex than previously thought.
2. Relevance of Male-Male Competition in Evolutionary Diversification
If territorial males direct more aggression to rivals that phenotypically resemble themselves than to different phenotypes, rare male varieties would enjoy a frequency-dependent advantage because they receive less aggression [13, 14]. This process is akin to negative frequency-dependent resource competition which provides the disruptive selection that is necessary for many evolutionary models of character displacement and speciation (Figure 1; [18] and references herein).
Figure 1.
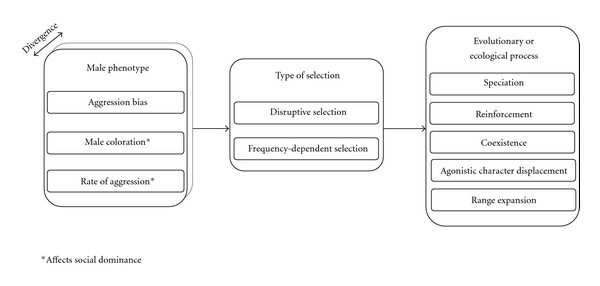
Summary chart of the different components of male-male competition that can generate disruptive and frequency-dependent selection, and the different types of processes it can effect.
2.1. Character Displacement
If species are competing for different resources and/or mates, the cost of accidental interspecific aggression may favour the evolution of divergence in competitor recognition traits (“agonistic character displacement”, [16]). Before discussing this further, we need to define the different forms of character displacement. “Reproductive character displacement” results from selection against maladaptive interspecific hybridisation resulting in enhanced prezygotic reproductive isolation between sympatric species [19]. If speciation is incomplete, this process is known as reinforcement (see below). It may involve a variety of isolating mechanisms, including enhanced mate recognition by virtue of divergence in male sexual traits. “Ecological character displacement” is defined as shifts in traits relevant to ecological resource use resulting from selection against intermediate “ecotypes” [20].
Agonistic character displacement has received limited attention (e.g., [21, 22]), despite the fact that these ideas date back to the 60s [23, 24]. It can take place in a range of different traits that affect the rate, intensity, and outcome of interference competition [16]. Species that evolved in allopatry and are subsequently brought into sympatry can undergo agonistic character displacement as a result of interspecific aggressive interactions ([13, 25–28], reviewed in [16]).
2.2. Speciation
Although male-male competition does not directly contribute to the evolution of assortative mating, it has been implicated as an important factor in the process of speciation by sexual selection since the traits used in mate choice are often also used in intrasexual communication [29]. Male-male competition can contribute to overcoming two important hurdles in models of sympatric speciation by sexual selection (for more details see [15]): (1) considerable genetic variation is required to trigger divergent Fisherian runaway processes [30], but sexual selection may rapidly deplete genetic variability in female mating preferences, hereby constraining sympatric speciation (e.g., [31]); (2) speciation can only occur from a rather narrow range of initial phenotypic distribution of female preference and male display, which must be close to symmetric [15, 32], making the process highly unstable. van Doorn et al. [15] show that speciation can only take place when there is negative frequency-dependent selection on female mating preference assumed possible when males cannot father an unlimited number of offspring, [15, 33, 34] and an additional mechanism that generates negative frequency-dependent selection on either females or males. Since in several taxa male-male competition is a major component of sexual selection [35], aggressive male-male competition for breeding sites could generate this advantage of rarity [15]. Male-male competition may then facilitate the invasion of novel phenotypes, increasing variation in male trait, and stabilising the coexistence of diverging populations and daughter species [15].
2.3. Reinforcement
When species are brought into secondary contact, reproductive isolation can be increased by reinforcement [19]. Speciation by reinforcement is driven directly by selection against maladaptive hybridisation favouring the evolution of nonrandom mating. Hybrids may not be optimally adapted in ecological resource use [19]. Maladaptation can also occur when hybrids incur elevated attacks compared to their nonhybrid counterparts, favouring not only divergence in agonistic signals (agonistic character displacement), but also the evolution of prezygotic reproductive isolation (reinforcement).
2.4. Sympatric Coexistence of Species
Negative frequency-dependent selection arising from male-male competition could also promote species coexistence. Tolerance towards heterospecific neighbours may lead to denser packing of territorial males in mixed species assemblages [14, 36]. In haplochromines, the dense packing of many different species has puzzled many researchers (e.g., [37]), and it has already long been indicated that territoriality can affect the intra- and interspecific interactions that organise spatial patterns of coexistence in communities [36, 38, 39]. In addition, similarity in nuptial dress between resident and immigrant species may determine success or failure of range expansion of the latter in that invading species with dissimilar colour are more likely to be tolerated by the resident species [17, 40, 41].
3. Evidence for Stronger Competition among Same-Coloured Individuals than Those with Different Colours
Several earlier studies indicated that rare male advantages might emerge from intrasexual competition, confirming that same-coloured rival males compete more. Using a large data set of cichlid communities from 47 rocky habitat patches in Lake Victoria, Seehausen and Schluter [13] showed that closely related species with different male nuptial coloration occurred more in sympatry than expected by chance. Also, closely related species of the same colour type were less likely to co-occur than expected by chance. Although disruptive selection on male colour by female mate choice predicts the first pattern [9], it does not predict the negative association between closely related species of the same colour, whereas own-type biases in aggression do. Using abundance data from survey plots of Lake Malawi rock-dwelling cichlids at Thumbi Island, Young et al. [17] found that males of the same body colour had more negative interaction coefficients (derived from a community matrix) than those of different colours. Importantly, they show that male colour influences the abundance and distribution of individuals at the community scale, even among less closelyrelated species. Consistent with this pattern, Pauers et al. [41] provide experimental evidence that males of Metriaclima mbenjii, a rock-dwelling species from Lake Malawi, directed more aggressive behaviours towards similarly coloured opponents, regardless of species. This type of selection against coexistence of similar phenotypes regardless of species is also confirmed by aggression experiments in the Lake Malawi cichlid Metriaclima zebra (Michael Kidd, personal communication). In field observations Genner et al. [42] showed that territorial male cichlids of the Pseudotropheus species complex in Lake Malawi never tolerated males and females of the same species complex in their territories, with a stronger aggression bias for dietary specialists than for dietary generalists, though no colour-based aggression biases were noted. Clearly, aggression can influence the distribution and abundance of haplochromine cichlid species.
There is also evidence for stronger competition among same-coloured rivals at the local (lek) site. First, Lake Victoria and Lake Malawi cichlids have nonoverlapping territories at the intraspecific level, but overlapping territories are more common in males that belong to different species (personal observations, [43]). Second, males of the Lake Victoria cichlid species Neochromis omnicaeruleus and N. rufocaudalis (“red tail”) tend to have territorial neighbours of species that are different in nuptial coloration from themselves [13]. Third, Kohda [36] showed in Lake Tanganyika that the territories of Petrochromis polyodon were separated to a greater degree among conspecific males than among heterospecific males. This suggests that territorial males are more tolerant of heterospecific neighbours. In line with this, mesocosm experiments, using the Lake Malawi cichlids Pseudotropheus emmiltos and P. fainzilberi, showed that the number of territorial males for a given area almost doubled in mixed-species assemblages compared to monospecies assemblages [44]. Not only did heterospecific neighbours receive fewer aggressive interactions than conspecific neighbours, they were also permitted to establish overlapping territories. These findings highlight the potential importance of the increased tolerance for heterospecific males for species coexistence, and it may help explain the syntopic coexistence of different species [42, 45].
Several studies aside from the ones mentioned earlier show that haplochromine males (and females) direct more aggression to conspecifics or same-coloured rivals. Using intruder choice tests, this was demonstrated in several sympatric species pairs from Lake Victoria: Pundamilia pundamilia and P. nyererei [46, 47], P. nyererei and P. “pink anal” (for summary see Figure 2), and finally Mbipia mbipi and M. lutea [48]. The same pattern was demonstrated in two different sympatric species pairs of Lake Malawi cichlids: Pseudotropheus emmiltos and P. fainzilberi; P. zebra and P. “zebra gold” [44]. Light manipulation experiments indicate that these aggression biases are largely based on colour differences [44, 46]. Haplochromine females can also behave aggressively [49], and we showed in the Lake Victoria cichlid species with a female colour polymorphism Neochromis omnicaeruleus that females bias aggression towards their own morph [50]. This could help stabilise the female colour polymorphism in this species.
Figure 2.
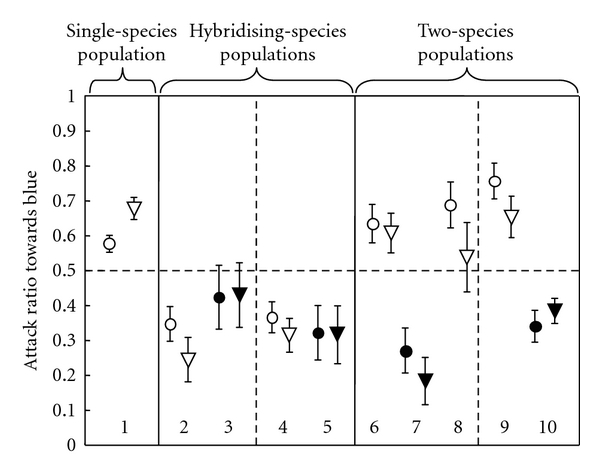
The display and attack ratios of Pundamilia males from five separate locations [4]. The different Pundamilia phenotypes are indicated below with numbers: the single-species population (1) intermediate between blue and red, mostly bluish, Luanso Island, N = 28, the hybridising, incipient species populations (2) blue phenotypes, N = 7; (3) red phenotypes, N = 6, Kissenda Island Island; (4) blue phenotypes, N = 22; (5) red phenotypes, N = 5, Python Island Islands and the two-species populations (reproductively isolated sister species, (6) P. Pundamilia, N = 23; (7) P. nyererei, N = 12, Makobe Island, (8) P. “pink anal”, N = 10, Makobe Island; (9) P. Pundamilia, N = 11; (10) P. nyererei, N = 4, Senga Point. The response ratio is the response to the blue stimulus divided by the sum of the responses to the red and blue stimuli. A response ratio of 0.5 represents identical responses to the two stimuli (- - -). Symbols indicate mean ± SE values. Black symbols denote red males; white symbols denote blue males. Circles denote display behaviour, triangles denote attack behaviour. Data from [46, 51].
If aggression is to facilitate invasion of novel phenotypes (relevant to both speciation and range expansion), it is predicted that males direct less aggression to novel phenotypes. Consistent with this prediction, Pundamilia males from a location where most males display blue nuptial coloration directed more aggression towards blue rivals than towards red-coloured P. nyererei rivals (Figure 2, [51]). Likewise, in Pseudotropheus emmiltos conspecific albino males evoked fewer attacks than “wild type” males [44].
Plenderleith [44] provides evidence for selection against hybrids. In intruder choice tests, hybrid males of P. emmiltos and P. fainzilberi were treated as a conspecific by males of both parental species as well as any other hybrids. This is consistent with the hypothesis that reinforcement can result from intrasexual selection against hybrids.
4. Frequency-Dependent Selection Arising from Male-Male Competition Is Not Always Symmetric between Sibling Species
We have summarised the evidence that cichlid males preferentially attack like coloured rival males, likely causing character displacement both within and between species [13, 16] and facilitating speciation and coexistence of different species [14, 15]. However, in the following we argue that selection by male-male competition on colour is more complex than previously appreciated. We first show that males of certain species do not always bias aggression towards their own species, that learning might be involved, and that social dominance effects should also be considered.
4.1. Lack of Own-Type Aggression Bias and Learning
There are several accounts where males do not bias aggression towards their own colour type. First, males of the sympatric species pair Pundamilia pundamilia (males referred to as blue) and P. nyererei (males referred to as red) from two locations where they hybridise directed more aggression towards red rival males (Figure 2, [46, 51]). The lack of own-type bias in blue males from hybridising species pair in contrast to the behaviour of males where the two species are reproductively isolated can be explained by considering the degree of competition for females (see [46]). Gene-flow between red and blue in the hybridising species pair suggests that males of the red and blue morph compete partly for the same set of females. This may render it less advantageous for blue males to concentrate aggression on males of their own colour than in a situation where red and blue are fully reproductively isolated species. Second, in the Lake Tanganyika cichlid Astatotilapia burtoni, males express bright blue or yellow body colours, and males occasionally change colour. Korzan and Fernald [52] found that males preferentially attack rivals with opposite coloration. The final example is from a nonhaplochromine species, the polychromatic, monogamous Central American cichlid Amphilophus (“Cichlasoma”) citrinellum (Midas cichlid). In this species individuals start as normally coloured cryptic individuals, but some individuals attain a “gold” coloration. Barlow and Siri [53] show that individuals of both colour morphs behave more aggressively to the “normal” morph. Taken together, these findings suggest that own-type aggression biases can be absent in cases where sibling species hybridise (in the Pundamilia system), or where males are conspecific colour morphs (in A. burtoni and C. citrinellum). This is relevant from an evolutionary perspective, because these findings suggest male-male competition is unlikely to stabilise coexistence of different phenotypes in an incipient stage of speciation, or maintain phenotypic bimodality when closely related species hybridise.
Aggression biases may differ between wild-caught and lab-bred individuals. This is an important consideration since the majority of choice tests are conducted with lab-bred individuals, which may not adequately reflect the situation in the wild. Laboratory-bred blue Pundamilia males from a hybridising location biased aggression towards same-coloured rivals [54–56], whereas wild-caught males biased aggression towards red rivals [46, 51]. This may hint at a role of learning in shaping aggression biases in the wild. The occurrence of learning was indeed demonstrated in Pundamilia and another cichlid genus, Mbipia. Blue Pundamilia males raised with only blue males had no preferences for either red or blue rivals, whereas males raised with both blue and red males had a preference for blue rival males, indicating a role of learning [54]. Verzijden et al. [47] found in a crossfostering study that early learning (on the mother's phenotype) does not influence male aggression biases in Pundamilia males, in spite of the fact that females can sexually imprint on their mother's phenotype [57]. Aggression biases of males of two other species, Mbipia mbipi and M. lutea, were affected by the colour of the siblings, but like in Pundamilia, not by the colour of the mother suggesting that colour experience may have to be contingent on aggressive interactions [48].
4.2. Dominance Relationship
Asymmetric dominance relationships between sibling species and colour morphs are common in the animal kingdom [58, 59]. This could in theory facilitate the establishment of the more socially dominant phenotype into a given population, which is relevant to both speciation and range expansion. Moreover, asymmetric dominance relationships may also promote agonistic character displacement in the less dominant species or phenotype to avoid costly interspecific interactions (for an example in damselflies, see [24]). Dominance advantages can come about by colour effects on winning dyadic combats and/or intrinsic differences in the rate of aggression.
In the Lake Victoria cichlid fish Pundamilia red males were more likely to dominate blue males in dyadic combats, but their advantage was significantly reduced under green light. This suggests the intimidating nature of red coloration [60]. A similar intimidating effect of red coloration has been documented in other animal species [61–64] including cichlid fish (Midas cichlids, Amphilophus (“Cichlasoma”) citrinellum [65], firemouth cichlids Cichlasoma meeki: [66]). In Pundamilia the social dominance advantage of red males is likely not only a colour effect since red males are inherently more aggressive than blue males [46, 55]. The dominance of red is consistent with the geographic distribution pattern of red and blue Pundamilia types. Red types always co-occur with blue, whereas purely blue populations are not uncommon [67], indicating repeated invasion of red phenotypes into blue populations by their dominance advantage.
Social dominance is not restricted to the red-blue combination or to males. When Astatotilapia burtoni males were allowed to compete physically for the same territory, yellow males became dominant in the majority of trials [52]. In the Convict cichlids Archocentrus nigrofasciatus the wild-type (WT) black-barred morph dominates the amelanistic barless morph in dyadic interactions [68]. Finally, in the female colour morphs of N. omnicaeruleus white blotched (WB) dominates orange blotched (OB) females, and WB and OB dominate plain colour phenotypes [69].
4.3. Combined Effect of Colour-Related Aggression Bias and Social Dominance
How do colour-related aggression bias and social dominance in dyadic combats jointly influence coexistence between competing species or colour morphs (Figure 1)? We tested this in Pundamilia assemblages with different proportions of red and blue males, bred in captivity [56]. We first showed that males of both colour morphs direct more aggression towards rivals of their own type, which is in contrast to the overall aggression bias to red in wild-caught Pundamilia males ([46, 51], discussed below). We found that red males were indeed socially dominant over blue ones, but only when rare. However, blue males were not socially dominant when rare. We then tried to disentangle the effects of the own-morph attack bias and social dominance of red using computer simulations. The simulation results suggest that an own-morph attack bias reduces the social dominance of red males when they are more abundant. Although these data suggest that dominance is frequency dependent, we found no evidence of symmetric negative frequency-dependent selection acting on social dominance. These data suggest that male-male competition may contribute to coexistence, but cannot always explain it. Likewise, male-male competition may facilitate speciation, but may also constrain it depending on the shape of frequency-dependent selection.
We have discussed two aggression traits that can exert selection on colour: colour-based aggression biases and social dominance. Little is known about the mechanisms underlying these traits, and it is unclear how the association between colour and aggression traits can be maintained in the face of gene-flow. This will be discussed in the following section.
4.4. Pleiotropy between Colour and Aggression?
It is difficult to understand how own-type biases or differences in rate of aggression can evolve during an initial or incipient stage of speciation. Gene-flow would erode linkage disequilibrium between colour and these aggression traits by recombination, unless, for example, learning or a pleiotropic mechanism links these two traits. This is analogous to a major question in speciation research: does reproductive isolation between populations evolve via one- or a two-allele mechanisms (e.g., [70, 71]). For example, a single allele that increases habitat preference when the diverging species inhabit different environments would make speciation in theory easier because they do not require linkage disequilibria to form as is the case when habitat preferences are described by two different alleles. A type of one-allele mechanism is learning, and as discussed earlier, there is some evidence for learning in Pundamilia [54]. Future studies should examine the genetic mechanisms underlying “own-type aggression biases”. Here we tested for pleiotropy (or tight linkage) in two polymorphic haplochromine species, N. omnicaeruleus and a single-species population of Pundamilia (Luanso Island).
We crossed plain with blotched N. omnicaeruleus, yielding broods containing both plain and blotched sisters. We found that own-type biases in aggression in these laboratory-bred sisters were broken down, making pleiotropy or tight linkage unlikely explanations for own-type aggression biases in wild-caught females [50]. However, in dyadic encounters, WB female morphs dominated their plain sisters. Given the largely homogenous genetic background of these full sibs, this finding supports the hypothesis that the social dominance of WB females is a pleiotropic effect of colour or that genes coding for colour and those influencing behavioural dominance are genetically linked. This linkage could explain the maintenance of an association between colour and behavioural dominance despite gene-flow. The causal mechanism underlying the link between colour and dominance is an exciting avenue for future research. While we cannot exclude that the more conspicuous colour patterns of WB and OB have an intimidating effect on opponents (sensu effect of red), we deem it more likely that an endocrine mechanism link dominance and colour in this species, possibly via the melanocortin system that both modulates skin coloration and a suite of other traits including aggressive behaviour [72].
Also in Pundamilia we tested for pleiotropy/tight linkage against the alternative of independently segregating genes. We took advantage of the fact that the Pundamilia population at Luanso Island is a single species or hybrid swarm comprised of males showing a continuous distribution of blue to intermediate (between blue and red) phenotypes. We scored the individuals that we tested in Dijkstra et al. [51] using the phenotype scale in Figure 1 [51], and expressed the aggression preference for the blue morph as a function of phenotype score. There was no significant relationship between aggression preference and phenotype score (one-sided Spearman rank correlation: r = −0.24, P > .1, N = 28, making pleiotropy between colour and aggression bias again unlikely. The lack of pleiotropy in polymorphic Pundamilia and N. omnicaeruleus species suggests that a (strong) buildup of linkage disequilibrium between colour and aggression bias is required for the evolution of own-type biases in aggression. This process is less effective than pleiotropy in causing the expression of own-type biases. Nonetheless, one would predict strong selection favouring individuals that preferentially expel competitors for mates and/or most dangerous usurpers of territorial space, and as suggested earlier, a one-allele mechanism such as learning or other mechanisms during ontogeny might be instrumental in shaping these adaptive aggression biases. Regarding the covariance between colour and intensity of aggression, the possibility that these are pleiotropically linked through a hormonal mechanism deserves more attention, and studies testing the role of the melanocortin system are underway.
4.5. Future Perspectives
We conclude that the dynamics of frequency-dependent selection arising from male-male competition is probably more complex than previously appreciated (Figure 1). Several components of male-male competition should be considered, such as aggression biases and social dominance asymmetries (Figure 1). Their independent effects can be teased apart by using a combination of community studies and simulations. For example, simulations indicated that the dominance of red Pundamilia males over blue males becomes more negative frequency dependent when individuals bias aggression towards their own type [56]. There are several important avenues for future research.
First, to date there is no experimental study in cichlids that actually tested the fitness consequences of frequency-dependent selection arising from male-male competition. Dominant males preferentially occupy high quality territories (size of crevice), and there is some evidence that occupying a high-quality territory translates into reproductive success (e.g., [12]). However, little is known about the long- and short-term fitness consequences of frequency-dependent dominance.
Second, in models of speciation it is usually not taken into account that diverging species may display asymmetries in social dominance. This is an important caveat, since asymmetries in social dominance are common in the animal kingdom, both between conspecific colour morphs and (hybridising) sibling species (e.g., [40, 64, 73]. Differences in success in competition for mates and resources will influence the fitness landscape of diverging phenotypes and will dynamically change the scope and conditions for speciation by disruptive sexual selection. Further, in recent years there is an increase in realisation that natural and sexual selection can jointly drive speciation (e.g., [74, 75], for theoretical paper see [76]) and that in fact sexual traits and preferences are subject to natural selection too (e.g., [75]). Sexual traits may signal local adaptation to females, favouring the evolution of divergent female preferences for these traits. We suggest that frequency-dependent selections arising from male-male competition is also relevant when natural and sexual selection act in concert. Moreover, it is possible that male colour may not signal only local adaptation in ecological traits, but also adaptation in agonistic behaviour tailored to the local “competitive” circumstances.
Third, in competition males do not only pay attention to male body colour, but also other male traits including territory characteristics. Males of rock-dwelling cichlids preferentially occupy larger crevices [12] and more structurally complex territories [77]. In bower-building cichlids bower height may signal dominance over other males, and males placed on shorter bowers were more frequently attacked by neighbouring males [78]. How disruptive selection on colour through male-male competition is influenced by intrasexual selection on other male traits is an exciting topic for future research. For example, rare males may not only be more likely to acquire a high-quality territory, but they may also receive fewer attacks as a result of occupying these high-quality sites, magnifying the rare male effect. By contrast, if high quality territories attract more intense territorial disputes, it would countervail the benefits of occupying a high-quality territory (Machteld Verzijden and Martine Maan pers. comm.).
Finally, both nuptial coloration and aggression have physiological underpinnings. Certain pigments used in nuptial coloration, such as carotenoids, are also required in several health-maintaining functions, posing potential allocation tradeoffs between key life-history traits (e.g., [79]). Agonistic behaviour is regulated by neuroendocrine pathways which impinge on several physiological functions that are important for fitness. A well-known example is the dual effect of androgens in modulating both sexual display and the immune system [80]. This creates a potentially exciting scenario in which colour morphs differ not only in androgen levels, but also in life-history tradeoffs. For example, in Pundamilia red and blue males differed in immunity and oxidative stress (Dijkstra, unpublished). In Astatotilapia burtoni yellow males had significantly higher levels of 11-ketotosterone than blue males [73]. Indirect disruptive selection on hormones (via for example selection on territoriality) may thus facilitate or constrain adaptive evolution in correlated traits (see e.g., [81]). Integrating physiology with evolutionary biology might therefore yield more insight into the mechanisms of phenotypic diversification in haplochromines.
Acknowledgments
The authors would like to acknowledge Ole Seehausen and Martine Maan for introducing them to the Lake Victoria cichlid species. They are also indebted to their collaborators Charlotte Hemelrijk, Hans Hofmann, Jan Lindström, Neil Metcalfe, Michele Pierottie, Inke van der Sluijs, and Machteld Verzijden. Mhoja Kayeba, Mohamed Haluna, John Mrosso, Martine Maan, and Ole Seehausen are acknowledged for fish collection. Roelie Veenstra-Wiegman, Sjoerd Veenstra, Adriana Faber, Monique Huizinga, and Saskia Helder are acknowledged for their assistance with fish care. Many excellent students from the University of Groningen contributed to the work, most of them are formerly acknowledged in the form of coauthorships of articles cites in this paper. Sean Macguire, Martin Genner, and two anonymous reviewers provided useful comments on earlier versions of the paper. The research was financed by the Netherlands Organization for Scientific Research, NWO (SLW) Grant 810.64.013 to TGGG, a Rubicon Grant 825.07.00, and a European Commission Marie Curie Outgoing International Fellowship grant to PDD. The research was carried out with an animal experiment licenses from University of Groningen and complied with current laws in The Netherlands.
References
- 1.Kocher TD. Adaptive evolution and explosive speciation: the cichlid fish model. Nature Reviews Genetics. 2004;5(4):288–298. doi: 10.1038/nrg1316. [DOI] [PubMed] [Google Scholar]
- 2.Genner MJ, Turner GF. The mbuna cichlids of Lake Malawi: a model for rapid speciation and adaptive radiation. Fish and Fisheries. 2005;6(1):1–34. [Google Scholar]
- 3.Salzburger W. The interaction of sexually and naturally selected traits in the adaptive radiations of cichlid fishes. Molecular Ecology. 2009;18(2):169–185. doi: 10.1111/j.1365-294X.2008.03981.x. [DOI] [PubMed] [Google Scholar]
- 4.Seehausen O. The sequence of events along a "speciation transect" in the Lake Victoria cichlid fish Pundamilia. In: Butlin R, Schluter D, Bridle JR, editors. Speciation and Ecology. Cambridge, UK: Cambridge University Press; 2009. pp. 155–176. [Google Scholar]
- 5.Reinthal PN. The feeding habits of a group of herbivorous rock-dwelling cichlid fishes (Cichlidae: Perciformes) from Lake Malawi, Africa. Environmental Biology of Fishes. 1990;27(3):215–233. [Google Scholar]
- 6.Allender JA, Seehausen O, Knight ME, Turner GF, Maclean N. Divergent selection during speciation of Lake Malawi cichlid fishes inferred from parallel radiations in nuptial coloration. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(2):14074–14079. doi: 10.1073/pnas.2332665100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seehausen O. Explosive speciation rates and unusual species richness in haplochromine cichlid fishes: effects of sexual selection. Advances in Ecological Research. 2000;31:237–274. [Google Scholar]
- 8.Maan ME, Seehausen O, Söderberg L, et al. Intraspecific sexual selection on a speciation trait, male coloration, in the Lake Victoria cichlid Pundamilia nyererei. Proceedings of the Royal Society B. 2004;271(1556):2445–2452. doi: 10.1098/rspb.2004.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seehausen O, van Alphen JJM, Witte F. Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science. 1997;277(5333):1808–1811. [Google Scholar]
- 10.Parker A, Kornfield I. Polygynandry in Pseudotropheus zebra, a cichlid fish from Lake Malawi. Environmental Biology of Fishes. 1996;47(4):345–352. [Google Scholar]
- 11.McKaye KR, Louda SM, Stauffer JR., Jr. Bower size and male reproductive success in a cichlid fish lek. American Naturalist. 1990;135(5):597–613. [Google Scholar]
- 12.Dijkstra PD, van der Zee EM, Groothuis TGG. Territory quality affects female preference in a Lake Victoria cichlid fish. Behavioral Ecology and Sociobiology. 2008;62(5):747–755. [Google Scholar]
- 13.Seehausen O, Schluter D. Male-male competition and nuptial-colour displacement as a diversifying force in Lake Victoria cichlid fishes. Proceedings of the Royal Society B. 2004;271(1546):1345–1353. doi: 10.1098/rspb.2004.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mikami OK, Kohda M, Kawata M. A new hypothesis for species coexistence: male-male repulsion promotes coexistence of competing species. Population Ecology. 2004;46(2):213–217. [Google Scholar]
- 15.van Doorn GS, Dieckmann U, Weissing FJ. Sympatric speciation by sexual selection: a critical reevaluation. American Naturalist. 2004;163(5):709–725. doi: 10.1086/383619. [DOI] [PubMed] [Google Scholar]
- 16.Grether GF, Losin N, Anderson CN, Okamoto K. The role of interspecific interference competition in character displacement and the evolution of competitor recognition. Biological Reviews. 2009;84(4):617–635. doi: 10.1111/j.1469-185X.2009.00089.x. [DOI] [PubMed] [Google Scholar]
- 17.Young KA, Whitman JM, Turner GF. Secondary contact during adaptive radiation: a community matrix for Lake Malawi cichlids. Journal of Evolutionary Biology. 2009;22(4):882–889. doi: 10.1111/j.1420-9101.2009.01688.x. [DOI] [PubMed] [Google Scholar]
- 18.Bolnick DI. Can intraspecific competition drive disruptive selection? An experimental test in natural populations of sticklebacks. Evolution. 2004;58(3):608–618. [PubMed] [Google Scholar]
- 19.Coyne JA, Orr HA. Speciation. Sunderland, UK: Sinauer Associates; 2004. [Google Scholar]
- 20.Schluter D. The Ecology of Adaptive Radiation. Oxford, UK: Oxford University Press; 2000. [Google Scholar]
- 21.Adams DC. Character displacement via aggressive interference in appalachian salamanders. Ecology. 2004;85(10):2664–2670. [Google Scholar]
- 22.Peiman KS, Robinson BW. Heterospecific aggression and adaptive divergence in brook stickleback (Culaea inconstans) Evolution. 2007;61(6):1327–1338. doi: 10.1111/j.1558-5646.2007.00113.x. [DOI] [PubMed] [Google Scholar]
- 23.Lorenz K. The function of colour in coral reef fishes. Proceedings of the Royal Institute of Great Britain. 1962;39:282–296. [Google Scholar]
- 24.Lorenz K. On Aggression. New York, NY, USA: Harcourt Brace; 1966. [Google Scholar]
- 25.Alatalo RV, Gustafsson L, Lundberg A. Male coloration and species recognition in sympatric flycatchers. Proceedings of the Royal Society B. 1994;256(1346):113–118. [Google Scholar]
- 26.Tynkkynen K, Rantala MJ, Suhonen J. Interspecific aggression and character displacement in the damselfly Calopterix splendens. Journal of Evolutionary Biology. 2004;17(4):759–767. doi: 10.1111/j.1420-9101.2004.00733.x. [DOI] [PubMed] [Google Scholar]
- 27.Tynkkynen K, Kotiaho JS, Luojumäki M, Suhonen J. Interspecific territoriality in Calopteryx damselflies: the role of secondary sexual characters. Animal Behaviour. 2006;71(2):299–306. [Google Scholar]
- 28.Anderson CN, Grether GF. Interspecific aggression and character displacement of competitor recognition in Hetaerina damselflies. Proceedings of the Royal Society B. 2010;277(1681):549–555. doi: 10.1098/rspb.2009.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berglund A, Bisazza A, Pilastro A. Armaments and ornaments: an evolutionary explanation of traits of dual utility. Biological Journal of the Linnean Society. 1996;58(4):385–399. [Google Scholar]
- 30.Higashi M, Takimoto G, Yamamura N. Sympatric speciation by sexual selection. Nature. 1999;402(6761):523–526. doi: 10.1038/990087. [DOI] [PubMed] [Google Scholar]
- 31.Kirkpatrick M, Nuismer SL. Sexual selection can constrain sympatric speciation. Proceedings of the Royal Society B. 2004;271(1540):687–693. doi: 10.1098/rspb.2003.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnegard ME, Kondrashov AS. Sympatric speciation by sexual selection alone is unlikely. Evolution. 2004;58(2):222–237. [PubMed] [Google Scholar]
- 33.Lande R, Seehausen O, van Alphen JJM. Mechanisms of rapid sympatric speciation by sex reversal and sexual selection in cichlid fish. Genetica. 2001;112-113:435–443. [PubMed] [Google Scholar]
- 34.Almeida CR, de Abreu FV. Dynamical instabilities lead to sympatric speciation. Evolutionary Ecology Research. 2003;5(5):739–757. [Google Scholar]
- 35.Andersson M. Sexual Selection. Princeton, NJ, USA: Princeton University Press; 1994. [Google Scholar]
- 36.Kohda M. Coexistence of permanently territorial cichlids of the genus Petrochromis through male-mating attack. Environmental Biology of Fishes. 1998;52(1–3):231–242. [Google Scholar]
- 37.Turner GF, Seehausen O, Knight ME, Allender CJ, Robinson RL. How many species of cichlid fishes are there in African lakes? Molecular Ecology. 2001;10(3):793–806. doi: 10.1046/j.1365-294x.2001.01200.x. [DOI] [PubMed] [Google Scholar]
- 38.Marsh AC, Ribbink AJ. Feeding-site utilization in three sympatric species of Petrotilapia (Pisces, Cichlidae) from Lake Malawi. Biological Journal of the Linnean Society. 1985;25(4):331–338. [Google Scholar]
- 39.Hert E. Factors in habitat partitioning in Pseudotropheus aurora (Pisces: Cichlidae), an introduced species to a species-rich community of Lake Malawi. Journal of Fish Biology. 1990;36(6):853–865. [Google Scholar]
- 40.Owen-Ashley NH, Butler LK. Androgens, interspecific competition and species replacement in hybridizing warblers. Biology Letters. 2004;271:S498–S500. doi: 10.1098/rsbl.2004.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pauers MJ, Kapfer JM, Fendos CE, Berg CS. Aggressive biases towards similarly coloured males in Lake Malawi cichlid fishes. Biology Letters. 2008;4(2):156–159. doi: 10.1098/rsbl.2007.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Genner MJ, Turner GF, Hawkins SJ. Resource control by territorial male cichlid fish in Lake Malawi. Journal of Animal Ecology. 1999;68(3):522–529. [Google Scholar]
- 43.McKaye KR. Sexual selection and the evolution of the cichlid fishes of Lake Malawi, Africa. In: Keenleyside MHA, editor. Cichlid Fishes: Behaviour, Ecology, and Evolution. New York, NY, USA: Chapman & Hall; 1991. pp. 241–257. [Google Scholar]
- 44.Plenderleith M. Reproductive isolation, mate preference and aggression in Lake Malawi cichlid fish. Hull, UK: University of Hull; 2008. Ph.D. thesis. [Google Scholar]
- 45.Ribbink AJ, Marsh BA, Marsh AC, Ribbink AC, Sharp BJ. A preliminary survey of the cichlid fishes of rocky habitats in Lake Malawi. South African Journal of Zoology. 1983;18(3):149–310. [Google Scholar]
- 46.Dijkstra PD, Seehausen O, Gricar BLA, Maan ME, Groothuis TGG. Can male-male competition stabilize speciation? A test in Lake Victoria haplochromine cichlid fish. Behavioral Ecology and Sociobiology. 2006;59(5):704–713. [Google Scholar]
- 47.Verzijden MN, Zwinkels J, Cate CT. Cross-fostering does not influence the mate preferences and territorial behaviour of males in Lake Victoria cichlids. Ethology. 2009;115(1):39–48. [Google Scholar]
- 48.Verzijden MN, Korthof REM, Cate CT. Females learn from mothers and males learn from others. The effect of mother and siblings on the development of female mate preferences and male aggression biases in Lake Victoria cichlids, genus Mbipia. Behavioral Ecology and Sociobiology. 2008;62(8):1359–1368. [Google Scholar]
- 49.Maan ME, Eshuis B, Haesler MP, Schneider MV, van Alphen JJM, Seehausen O. Color polymorphism and predation in a Lake Victoria cichlid fish. Copeia. 2008;(3):621–629. [Google Scholar]
- 50.Dijkstra PD, Seehausen O, Groothuis TGG. Intrasexual competition among females and the stabilization of a conspicuous colour polymorphism in a Lake Victoria cichlid fish. Proceedings of the Royal Society B. 2008;275(1634):519–526. doi: 10.1098/rspb.2007.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dijkstra PD, Seehausen O, Pierotti MER, Groothuis TGG. Male-male competition and speciation: aggression bias towards differently coloured rivals varies between stages of speciation in a Lake Victoria cichlid species complex. Journal of Evolutionary Biology. 2007;20(2):496–502. doi: 10.1111/j.1420-9101.2006.01266.x. [DOI] [PubMed] [Google Scholar]
- 52.Korzan WJ, Fernald RD. Territorial male color predicts agonistic behavior of conspecifics in a color polymorphic species. Behavioral Ecology. 2007;18(2):318–323. [Google Scholar]
- 53.Barlow GW, Siri P. Consorting among juvenile Midas cichlids (Cichlasoma citrinellum) in relation to own and to parents’ color. Journal of Comparative Psychology. 1987;101(4):312–316. [PubMed] [Google Scholar]
- 54.Dijkstra PD, Seehausen O, Fraterman RE, Groothuis TGG. Learned aggression biases in males of Lake Victoria cichlid fish. Animal Behaviour. 2008;76(3):649–655. [Google Scholar]
- 55.Dijkstra PD, Hemelrijk CK, Seehausen O, Groothuis TGG. Color polymorphism and intrasexual competition in assemblages of cichlid fish. Behavioral Ecology. 2009;20(1):138–144. [Google Scholar]
- 56.Dijkstra PD, Lindström J, Metcalfe NB, et al. Frequency-dependent social dominance in a color polymorphic cichlid fish. Evolution. 2010;64(10):2797–2807. doi: 10.1111/j.1558-5646.2010.01046.x. [DOI] [PubMed] [Google Scholar]
- 57.Verzijden MN, Cate CT. Early learning influences species assortative mating preferences in Lake Victoria cichlid fish. Biology Letters. 2007;3(2):134–136. doi: 10.1098/rsbl.2006.0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Endler JA. Natural Selection in the Wild. Princeton, NJ, USA: Princeton University Press; 1986. [Google Scholar]
- 59.Wong BBM, Candolin U. How is female mate choice affected by male competition? Biological Reviews of the Cambridge Philosophical Society. 2005;80(4):559–571. doi: 10.1017/S1464793105006809. [DOI] [PubMed] [Google Scholar]
- 60.Dijkstra PD, Seehausen O, Groothuis TGG. Direct male-male competition can facilitate invasion of new colour types in Lake Victoria cichlids. Behavioral Ecology and Sociobiology. 2005;58(2):136–143. [Google Scholar]
- 61.Rowland WJ, Bolyard KJ, Halpern AD. The dual effect of stickleback nuptial coloration on rivals: manipulation of a graded signal using video playback. Animal Behaviour. 1995;50(1):267–272. [Google Scholar]
- 62.Pryke SR, Andersson S. Carotenoid-based status signalling in red-shouldered widowbirds (Euplectes axillaris): epaulet size and redness affect captive and territorial competition. Behavioral Ecology and Sociobiology. 2003;53(6):393–401. [Google Scholar]
- 63.Hill RA, Barton RA. Red enhances human performance in contests. Nature. 2005;435(7040):p. 293. doi: 10.1038/435293a. [DOI] [PubMed] [Google Scholar]
- 64.Pryke SR, Griffith SC. Red dominates black: agonistic signalling among head morphs in the colour polymorphic Gouldian finch. Proceedings of the Royal Society B. 2006;273(1589):949–957. doi: 10.1098/rspb.2005.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barlow GW. Do gold Midas cichlid fish win fights because of their color, or because they lack normal coloration? A logistic solution. Behavioral Ecology and Sociobiology. 1983;13(3):197–204. [Google Scholar]
- 66.Evans MR, Norris K. The importance of carotenoids in signaling during aggressive interactions between male firemouth cichlids (Cichlasoma meeki) Behavioral Ecology. 1996;7(1):1–6. [Google Scholar]
- 67.Seehausen O, van Alphen JJM. Can sympatric speciation by disruptive sexual selection explain rapid evolution of cichlid diversity in Lake Victoria? Ecology Letters. 1999;2(4):262–271. [Google Scholar]
- 68.Reddon AR, Hurd PL. Differences in aggressive behavior between convict cichlid color morphs: amelanistic convicts lose even with a size advantage. Acta Ethologica. 2009;12(1):49–53. [Google Scholar]
- 69.Dijkstra PD, van Dijk S, Groothuis TGG, Pierotti MER, Seehausen O. Behavioral dominance between female color morphs of a Lake Victoria cichlid fish. Behavioral Ecology. 2009;20(3):593–600. [Google Scholar]
- 70.Smith JM. Sympatric speciation. American Naturalist. 1966;100:637–650. [Google Scholar]
- 71.Kirkpatrick M, Ravigné V. Speciation by natural and sexual selection: models and experiments. American Naturalist. 2002;159(3):S22–S35. doi: 10.1086/338370. [DOI] [PubMed] [Google Scholar]
- 72.Ducrest AL, Keller L, Roulin A. Pleiotropy in the melanocortin system, coloration and behavioural syndromes. Trends in Ecology and Evolution. 2008;23(9):502–510. doi: 10.1016/j.tree.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 73.Korzan WJ, Robison RR, Zhao S, Fernald RD. Color change as a potential behavioral strategy. Hormones and Behavior. 2008;54(3):463–470. doi: 10.1016/j.yhbeh.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seehausen O, Terai Y, Magalhaes IS, et al. Speciation through sensory drive in cichlid fish. Nature. 2008;455(7213):620–626. doi: 10.1038/nature07285. [DOI] [PubMed] [Google Scholar]
- 75.Maan ME, Seehausen O, van Alphen JJM. Female mating preferences and male coloration covary with water transparency in a Lake Victoria cichlid fish. Biological Journal of the Linnean Society. 2010;99(2):398–406. [Google Scholar]
- 76.van Doorn GS, Edelaar P, Weissing FJ. On the origin of species by natural and sexual selection. Science. 2009;326(5960):1704–1707. doi: 10.1126/science.1181661. [DOI] [PubMed] [Google Scholar]
- 77.Markert JA, Arnegard ME. Size-dependent use of territorial space by a rock-dwelling cichlid fish. Oecologia. 2007;154(3):611–621. doi: 10.1007/s00442-007-0853-5. [DOI] [PubMed] [Google Scholar]
- 78.Martin CH, Genner MJ. A role for male bower size as an intrasexual signal in a Lake Malawi cichlid fish. Behaviour. 2009;146(7):963–978. [Google Scholar]
- 79.Dijkstra PD, Hekman R, Schulz RW, Groothuis TGG. Social stimulation, nuptial colouration, androgens and immunocompetence in a sexual dimorphic cichlid fish. Behavioral Ecology and Sociobiology. 2007;61(4):599–609. [Google Scholar]
- 80.Folstad I, Karter AJ. Parasites, bright males, and the immunocompetence handicap. American Naturalist. 1992;139(3):603–622. [Google Scholar]
- 81.Kitano J, Lema SC, Luckenbach JA, et al. Adaptive divergence in the thyroid hormone signaling pathway in the stickleback radiation. Current Biology. 2010;20(23):2124–2130. doi: 10.1016/j.cub.2010.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]


