Abstract
HIV-1 assembly is a multi-step process that occurs at the plasma membrane. Targeting and binding of Gag to the plasma membrane are the first steps in this assembly process and are mediated by the matrix domain of Gag. This review highlights our current knowledge on viral and cellular determinants that affect specific interactions between Gag and the plasma membrane. We will discuss potential mechanisms by which the matrix domain might integrate three regulatory components, myristate, phosphatidylinositol-(4,5)-bisphosphate, and RNA to ensure that HIV-1 assembly occurs at the plasma membrane.
HIV-1 particle assembly is mediated by the viral structural protein Gag, which is synthesized as the precursor polyprotein Pr55Gag. As defined by sites of the viral protease-dependent cleavage that occurs during or after virus release, this polyprotein has four major structural domains: matrix (MA), capsid (CA), nucleocapsid (NC) and p6 1,2. In addition, it also contains spacer peptides 1 and 2 (SP1 and 2). Each of these structural domains performs a crucial role during the assembly process. MA mediates the targeting of Gag to the site of assembly and facilitates membrane binding. The C-terminal domain of CA forms the Gag dimerization interface, whereas NC promotes higher order multimerization of Gag through binding to RNA, which is thought to serve as a scaffold. In addition, NC contains two zinc finger/knuckle structures that are required for specific encapsidation of viral genomic RNA. The late domain motifs in p6 recruit cellular protein complexes called endosomal-sorting complex required for transport (ESCRT) that aid in the release of virus particles from the cell membrane. During formation of infectious HIV-1 particles, several components need to be at the assembly site for their packaging into the virion, including Gag, GagPol, which contains all the viral enzymes, Env, viral RNA, and host factors that are required for virus infectivity, such as tRNALys3 and cyclophilin A.
HIV-1 assembly occurs at the plasma membrane (PM) 3(Fig. 1) or, in the case of macrophages, in deep invaginations of the PM, which are connected to the cell surface via membrane-lined tubular conduits 4–6. Endosomes were also proposed previously to serve as native sites for assembly of HIV-1 particles as Gag was frequently observed in these compartments. However, most of the WT virus particles localized in these endosomal compartments are now thought to originate from endocytosis of particles already assembled at the PM 7(for reviews, see 8,9). Consistent with the notion that endosomes do not constitute a native virus release pathway, redirecting Gag to endosomal compartments severely inhibits release of HIV-1 in HeLa, COS, and 293T cells perhaps because assembled particles are trapped in these compartments 7,10–13. It is therefore possible that the PM serves as the preferred site of HIV-1 assembly because it allows nascent particles to readily exit to the extracellular space. However, recent evidence suggests that at least in T cells, mutant Gag proteins targeted to the intracellular compartments can still be released as extracellular particles and are not necessarily trapped in these compartments 14. Therefore, easy access to the extracellular space is unlikely to be the only reason that necessitates specific localization of HIV-1 Gag to the PM. While it remains to be determined what other advantages are associated with virus assembly at the PM, the molecular mechanisms by which Gag is specifically targeted to the PM are becoming clearer recently. In this review, we will summarize recent findings on the molecular determinants that regulate Gag-PM interaction, one of the earliest events during HIV-1 assembly, and discuss potential relationships between this event and other steps in virus assembly and post assembly processes.
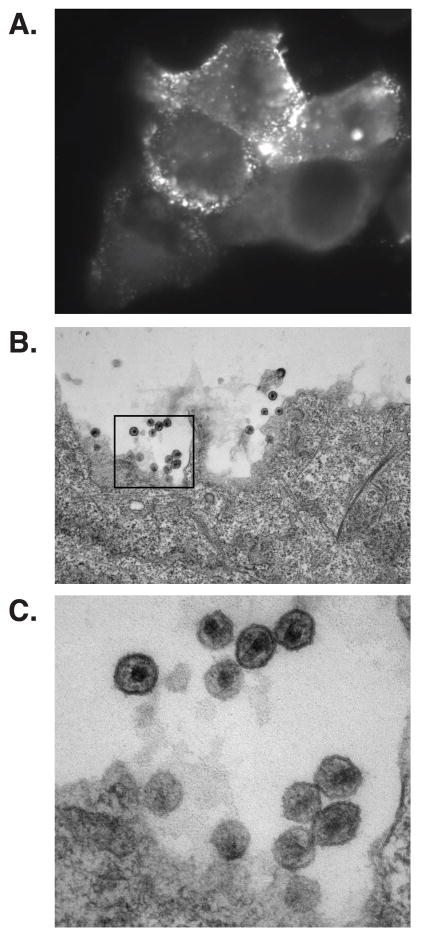
Figure 1. HIV-1 assembly on the plasma membrane.
HeLa cells were transfected with HIV-1 molecular clones that encode Gag tagged with Venus fluorescent protein (A) or WT Gag (B and C), fixed, and analyzed by epifluorescence microscopy (A) or electron microscopy (B and C), respectively. Higher magnification image of the area corresponding to the black rectangle in B is shown in C. Note that GagVenus puncta and virus particle formation are seen on the plasma membrane.
Gag membrane binding is driven by bipartite signal in MA
MA is the membrane proximal domain of Pr55Gag. The first 104 amino acids of the MA form a compact globular domain consisting of five major α-helices that are capped by a mixed three-stranded β-sheet 15,16. MA is myristoylated at the amino-terminus and facilitates membrane binding of Gag 17,18. In addition, a highly basic region (HBR) spanning residues 15–31 (Fig. 2A) is important for efficient membrane binding and proper targeting of Gag to the PM. These basic residues are clustered around the mixed β-sheet on the surface of MA and are thought to form interface with acidic phospholipids 10–12,16,19–21. Mutagenesis studies suggest that the region spanning residues 84–88 is also important for PM localization of Gag 12,22. However, this region is buried within MA and hence it is likely that the mutations in this region indirectly alter the HBR and affect Gag localization. Consistent with this hypothesis, a revertant of an MA mutant with a substitution in this region had a compensatory second site mutation in the MA HBR, suggesting that these regions are functionally linked 23. Besides having determinants for membrane binding and proper targeting, MA is also important for other processes in viral life cycle, such as envelope glycoprotein incorporation and post-entry processes [for review see 24].
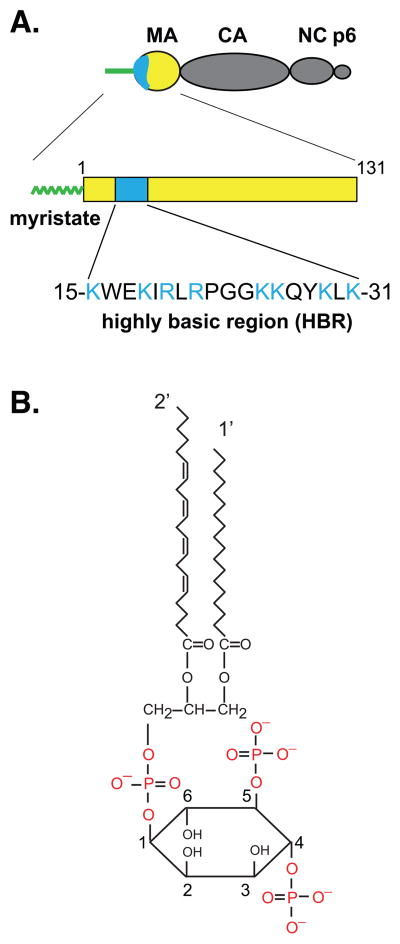
Figure 2. The MA domain and PI(4,5)P2.
A) Schematic representation of the bipartite membrane binding signal within the MA domain (yellow) of Gag. The N-terminal myristoyl moiety (green) and the highly basic region (blue), both of which are important for membrane binding of Gag, are shown. The amino acid sequence of the highly basic region corresponds to that of HIV-1NL4-3 MA. B) Structure of PI(4,5)P2. PI(4,5)P2 has a polar inositol head group, which is phosphorylated at D-4 and D-5 on the inositol ring. The acyl chains of PI(4,5)P2 found commonly in cells are saturated stearic acid at position 1′ and unsaturated arachidonic acid at position 2′.
The N-terminal myristoyl moiety is sequestered in the globular domain of MA
Myristoylation is a process that occurs co-translationally, where a 14-carbon saturated fatty acid, myristic acid, is attached to the N-terminal glycine that is exposed after the first methionine is removed. The enzyme that catalyzes this reaction is the myristoyl-CoA: protein N-myristoyltransferase (NMT) [for detailed review see 25]. Mutating the N-terminal glycine to alanine severely reduces membrane binding of Gag and inhibits virus particle release 10,17,18,22,26. In addition, mutating MA residues 2–5 (numbering of MA residues in this paper is based on the fact that glycine will be the first residue after the first methionine is removed) also reduces membrane binding and virus particle production, which is consistent with the idea that these residues are required for NMT recognition 23,26.
Differential membrane binding of the MA domain alone and of full-length Gag led to the hypothesis that a structural change in MA alters its myristate-mediated membrane binding 27–30. According to the original hypothesis of myristoyl switch, the myristate moiety is sequestered in the MA globular domain, and upon Gag multimerization, a structural change exposes the acyl chain facilitating efficient membrane binding. Consistent with this model, mutations of MA residues 6 and 7 were shown to inhibit myristate exposure 26,31,32, and certain other mutations or deletions in the MA globular domain were observed to enhance Gag membrane binding, presumably through inhibition of myristate sequestration 21,26,27,29,32,33. The presence of myristoyl switch was eventually demonstrated by NMR analysis 34. In this study, MA was shown to exist as an equilibrium mixture of myristate-sequestered monomer and myristate-exposed trimer. Notably, this study observed that the myristoyl moiety moves into or out of a hydrophobic cavity of MA in a manner coupled to MA trimerization without involving a drastic structural change of MA. Interestingly, C-terminal addition of the CA sequence, which contains a dimerization domain, shifted the equilibrium toward the myristate-exposed trimeric state by increasing the self-association of otherwise low-affinity molecules. These results suggest a model in which Gag multimerization stabilizes MA trimerization thereby facilitating myristate exposure and membrane binding.
Consistent with the multimerization-coupled myristoyl switch model, liposome or lipid monolayer binding studies showed that addition of the CA-derived dimerization domain to HIV-1 MA enhances its affinity for membranes 35,36. However, membrane binding of these Gag constructs was dependent on the presence of acidic phospholipids. Furthermore, similar observation was also obtained for RSV MA that lacks myristate 37. Therefore, these studies suggest that multimerization-dependent enhancement of membrane binding is explained at least partly by the increased avidity of the whole Gag complex to the membranes via clustering of multiple HBRs and myristate moieties on MA. In addition to the avidity, virus particle formation also could partly account for enhanced membrane binding of full-length Gag in cells, since Gag molecules within virus particles that remain on the cell surface are in effect irreversibly membrane-bound in fractionation experiments. Altogether, it is likely that the myristate exposure is not the sole reason for the difference between membrane binding of MA and that of full-length Gag 27–30. Further in vivo and in vitro studies are required to sort out mechanisms contributing to the difference between membrane affinities of MA and full-length Gag.
Gag multimerization is not the sole trigger for exposure of the myristoyl moiety. As detailed later, the interaction of the HBR with a PM-specific acidic lipid, phosphatidylinositol-(4,5)-bisphosphate [PI(4,5)P2] was also shown to trigger the myristate exposure for efficient membrane binding 38. Interestingly, non-myristoylated Gag that exists in a monomer-dimer equilibrium shifts to a monomer-trimer equilibrium when inositol hexakisphosphate (IP6) is added to the solution 39. As IP6 is structurally similar to the head group of PI(4,5)P2, it is conceivable that PI(4,5)P2 may also modulate trimerization of Gag, which is coupled to myristate exposure. In addition to PI(4,5)P2, binding of calmodulin to MA and pH changes were shown to cause structural changes that modulate the status of myristate exposure 40,41. The role of calmodulin and pH changes in Gag membrane binding in cells has yet to be elucidated.
The MA HBR binds acidic lipids
Even though myristoylation is necessary, it is thought to be insufficient for efficient membrane binding of proteins 42,43. Myristoylation only provides reversible membrane binding, and a second signal is thought to be required for strong localization of the myristoylated protein to the membrane 43. This second signal can be a polybasic cluster, a second acylation such as palmitoylation, or a protein-protein interaction that increases avidity of the protein complex. In the case of HIV-1 Gag, the interaction of the HBR with acidic lipids can provide such second signal10.
The PM is one of the cellular membranes that are rich in anionic lipids such as phosphatidylserine (PS) and phosphoinositides that preferentially localize on the cytoplasmic leaflet 44. This distinctive localization makes the cytoplasmic surface of the PM acidic. Proteins or protein domains with polybasic clusters on the surface, such as pleckstrin homology (PH) domains, myristoylated alanine-rich protein kinase C substrate (MARCKS), and k-ras, are targeted to this negatively charged surface where they interact with acidic lipids either in a lipid-head-group-specific manner or simply via electrostatic interactions 45–47. Mutations within the polybasic cluster of these proteins significantly alter their PM binding and localization 48,49. Similarly, mutations in the MA HBR mislocalize Gag to intracellular compartments and reduce membrane binding of Gag 10–12,19–21. Structural and mutagenesis studies suggest that the HBR is exposed on the surface of the MA domain and interacts with acidic lipids 10,15,16,21,35,50. Notably, however, Gag mutants lacking the MA globular domain except the N-terminal myristoylation signal can still bind membranes and release virus, suggesting that the HBR is important for efficient membrane binding only in the context of full-length MA 33,51–54.
Interaction between the MA HBR and phosphatidylinositol-(4,5)-bisphosphate is important for PM targeting and efficient membrane binding of Gag
As alluded to earlier, recent studies have shown that the basic residues in the HBR interact with the acidic phospholipid, PI(4,5)P2 21,38,55,56. PI(4,5)P2 belongs to a family of phospholipids called phosphoinositides. These lipids are derivatives of phosphatidylinositol and have a hydrophobic diacylglycerol backbone esterified to a polar inositol head group that can be phosphorylated at three of the five hydroxyl residues (Fig. 2B). Thus, seven different phosphatidylinositol phosphates (PIPs) are formed, which vary in the number and position of phosphates on the inositol ring 57. Various cellular proteins with basic residues on the molecule surface have different affinity and specificity to the head groups on phosphoinositides 46. Through the recruitment of these proteins in either a head-group-specific or simply a charge-dependent manner, PIPs control several important cellular processes including membrane fusion, budding, and actin dynamics 58,59. Notably, PIPs themselves are spatially and temporally regulated by the action of kinases and phosphatases that add or remove phosphate on the inositol ring, respectively. Among the PIPs, PI(4,5)P2 is the most abundant and is specifically localized on the cytoplasmic leaflet of the PM.
The first clue for the role of PIPs in HIV-1 assembly came from an in vitro assembly study where both RNA and inositol pentakisphosphate (IP5) were required for Gag to form a proper virus-like structure 60. IP5 is an inositol derivative structurally related to the head group of PI(4,5)P2. The requirement for PI(4,5)P2 in HIV-1 assembly became evident from the study in which cellular PI(4,5)P2 was depleted or mislocalized in HeLa cells, using either phosphatidylinositol polyphosphate 5 phosphatase type IV (5ptaseIV), which removes D-5 phosphate from the inositol head group 61 or constitutively active Arf 6, which induces PI(4,5)P2-enriched intracellular vesicles 62,63. Both methods of PI(4,5)P2 perturbation mislocalized Gag to internal compartments and significantly reduced virus particle release 13. Subsequently, several in vitro structural and biochemical studies showed that Gag interacts with PI(4,5)P2 via basic residues in the HBR 38,55,56,64. Shkriabai, et al. examined the accessibility of lysines in non-myristoylated full-length Gag proteins after Gag was mixed with a water-soluble analogue of PI(4,5)P2 that has short acyl chains and found two lysines in the MA HBR are masked from solvent by PI(4,5)P2 56. Saad, et al. determined the structure of the complex between myristoylated MA and a water-soluble PI(4,5)P2 analogue using NMR and identified binding of PI(4,5)P2 to MA as a trigger for myristate exposure 38. Notably, in addition to interactions between the acidic head group of PI(4,5)P2 and basic amino acids in the HBR, this study revealed the interaction between 2′ acyl chain and a hydrophobic pocket that is normally occupied by the myristoyl moiety (Fig 3A). Consistent with this finding, a study based on a surface plasmon resonance assay later showed that the presence of short acyl chains increased affinity of inositol phosphates to MA and Gag 65. While these studies utilized the water-soluble analogues of PI(4,5)P2, studies using liposomes demonstrated that full-length myristoylated Gag or purified MA binds to membrane-associated PI(4,5)P2 that contains native-length acyl chains 55,64,66. Gaining structural details of MA interaction with membrane-associated PI(4,5)P2 will require the use of new techniques such as a recently developed NMR method that utilizes reverse micelle encapsulation 67. The notion that specific Gag-PI(4,5)P2 interaction occurs in cells was supported by the detailed lipidomics study performed on HIV-1 particles. This study demonstrated that PI(4,5)P2 is enriched in the HIV-1 membrane compared to the PM of virus-producing cells in an MA-dependent manner 68. Consistent with the role for PI(4,5)P2 in efficient Gag-membrane binding observed in liposome-based experiments, ectopic expression of 5ptaseIV not only mislocalized Gag population to intracellular compartments but also increased non-membrane-bound Gag population in HeLa cells 55. In addition to HeLa cells, depletion of PI(4,5)P2 in the natural host cells of HIV-1, T cells and macrophages, significantly reduced Gag-membrane association and virus release emphasizing the physiological relevance of the Gag-PI(4,5)P2 interaction 69(VC and AO unpublished data).
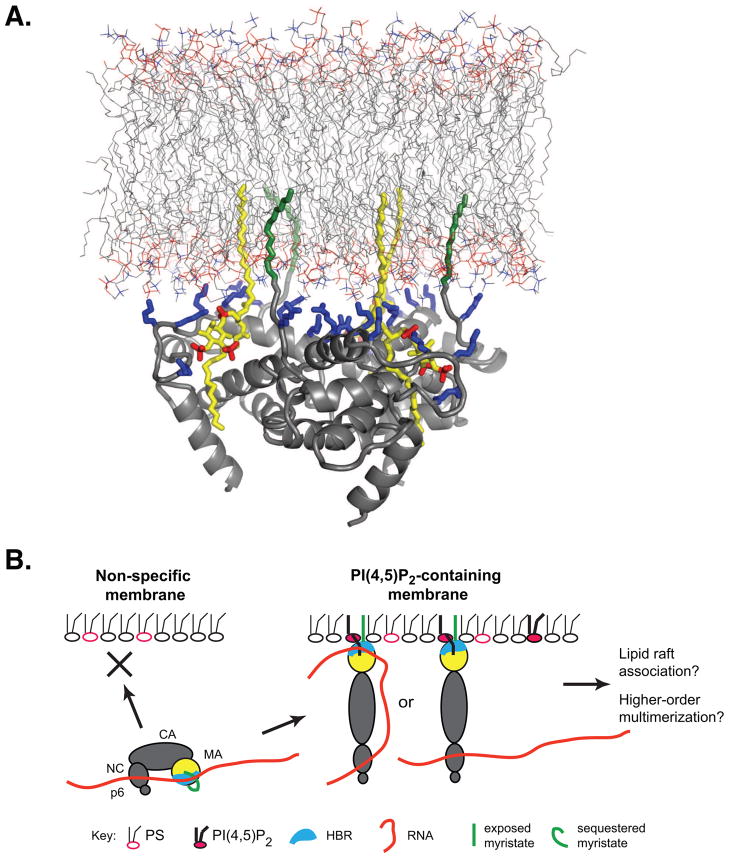
Figure 3. Regulation of Gag-plasma membrane binding via MA-PI(4,5)P2 and MA-RNA interactions.
A) A model of MA-PI(4,5)P2-membrane interaction reprinted from reference (38) is shown (Copyright, Michael F. Summers, used with permission). Upon PI(4,5)P2 (yellow with red phosphates) binding to the MA HBR (lysine and arginine side chains shown in blue), the myristate (green) is exposed, and instead 2′ acyl chain of PI(4,5)P2 occupies the hydrophobic pocket of MA. The exposed myristate moieties and 1′ acyl chains of PI(4,5)P2 projected from MA are expected to synergistically promote membrane binding. B) A possible mechanism by which PI(4,5)P2 and RNA regulate Gag binding to the plasma membrane is shown. In this schematic representation, both sequestration of the N-terminal myristoyl moiety and binding of RNA to the HBR inhibit non-specific membrane binding of MA via hydrophobic and electrostatic interactions, respectively. Once Gag reaches the plasma membrane, PI(4,5)P2 binds to the MA HBR thereby serving as a membrane anchor for Gag even when RNA is bound to the HBR. In addition, PI(4,5)P2 binding to MA exposes myristate, further enhancing membrane binding. Altogether, these positive and negative regulatory mechanisms ensure the plasma membrane specificity of Gag. Upon PI(4,5)P2 binding, sequestration of unsaturated 2′ acyl chain of PI(4,5)P2 into a hydrophobic pocket vacated by the myristoyl moiety may result in Gag association with membrane via two saturated acyl chains as shown in panel A. This is likely to increase propensity of Gag to partition into lipid rafts. In addition, MA-PI(4,5)P2 interaction might induce dissociation of RNA from MA, which in turn may facilitate NC-mediated functions, such as higher-order multimerization. Therefore, it is possible that MA-PI(4,5)P2 interaction coordinates Gag membrane binding with other assembly processes.
A recent study suggested that PI(4,5)P2 has an additional function in HIV-1 assembly in which activation of inositol trisphosphate receptor by PI(4,5)P2 hydrolysis and subsequent Ca2+ mobilization modulate endocytosis of Gag 70. Relationships between this indirect role of PI(4,5)P2 and its role as a Gag binding partner remain to be elucidated.
Not only HIV-1, but other retroviruses including HIV-2, MLV and MPMV also require PI(4,5)P2 for efficient virus particle production 68,71–73. However, not all retroviruses require PI(4,5)P2. For example, although EIAV Gag that localizes both to the PM and intracellular compartments can bind PI(4,5)P2 74, this Gag seems to depend on PI(3,5)P2 rather than PI(4,5)P2 for efficient virus release and membrane binding 75. Similarly, HTLV-1, which also localizes at the PM and intracellular compartments, is less sensitive to PI(4,5)P2 depletion than HIV-1 and is able to bind membranes efficiently even in the absence of PI(4,5)P2 76. Thus, different classes of retroviruses may have different requirements in terms of targeting to the site of assembly and virus particle production.
RNA bound to the MA HBR inhibits PI(4,5)P2-independent membrane binding of Gag in vitro
In addition to binding acidic lipids, several in vitro studies have shown that the MA HBR can bind RNA 56,77–83. We hypothesize that RNA binding to MA could be a factor that necessitates the involvement of PI(4,5)P2 for efficient membrane binding of Gag. We have recently shown that RNase treatment of Gag synthesized in vitro using rabbit reticulocyte lysate significantly increases binding of Gag to control liposomes that lack PI(4,5)P2 but still contain another acidic lipid, phosphatidylserine (PS)21. These data suggest that RNA inhibits membrane binding of Gag, especially in the absence of PI(4,5)P2. Consistent with these data, PI(4,5)P2-containing liposomes, but not liposomes containing PS, out-competed oligonucleotides for binding to beads coated with purified MA domain 66. In addition to increased binding to acidic liposomes, RNase treatment of WT Gag also caused a modest increase in myristate-dependent binding to liposomes containing only a neutral lipid, PC 21. Thus, at least in in vitro experiments, RNA is likely to act as a negative regulator of non-specific membrane binding by blocking both electrostatic interactions of Gag with acidic lipids and to a lesser extent hydrophobic interactions determined by myristate exposure. Unlike PI(4,5)P2, however, there is little data that provide insights into the role of MA-bound RNA in Gag- membrane binding in cells. Ongoing studies in our laboratory suggest that not only Gag proteins synthesized in reticulocyte lysates but also Gag derived from cells are susceptible to similar RNA-mediated inhibition of membrane binding (VC and AO, unpublished data). Currently, however, the identity of the RNA involved in this inhibition and the molecular determinants for the MA-RNA interaction that causes this inhibition remain unknown. What would be the implication of such MA-RNA interaction in the viral life cycle? MA-viral RNA interaction has been shown to be important for genomic RNA dimerization, encapsidation, or nucleocytoplasmic transport of Gag in other retroviruses 84–87, but there is no report indicating a similar role for this interaction in HIV-1 life cycle. Nevertheless, there are several recent studies functionally linking nuclear export of HIV-1 gRNA and MA-mediated membrane binding. It has been known that HIV-1 assembly is blocked in murine cells 88–91. However, altering the RNA export pathway or introducing MA mutations that enhance Gag membrane binding 31–33,92 was sufficient for alleviating the block in HIV-1 assembly in murine cells 93. Similar MA-dependent inhibition of assembly was also observed in human cells, when the classical Rev response element (RRE) was replaced with Hepatitis B virus post-transcriptional regulatory element (PRE) 94. Thus, these studies suggest that the membrane binding of Gag mediated by MA is functionally linked to the RNA export pathway used by HIV-1. The nature of the link between RNA export and membrane binding has yet to be elucidated.
MA-PI(4,5)P2 binding may be important for coordinating Gag membrane binding with other assembly and post-assembly events
As mentioned earlier, both myristate and HBR are necessary for efficient membrane binding of Gag. Either one alone is not sufficient in the context of full-length Gag in cells, as supported by observations that both non-myristoylated Gag and Gag derivatives with substitutions of all basic residues in the HBR do not bind membranes efficiently 10,17,19,21,26. However, in contrast to amino acid substitutions in the HBR, deletion of the entire MA globular head except for the myristoylation signal enhances Gag membrane binding 33,51–54. Treating non-myristoylated Gag with RNase also rescues binding of this otherwise defective Gag to membranes that contain PS, an abundant acidic phospholipid in the inner leaflet of cell membranes 21. These observations suggest that membrane binding is inhibited by the globular-head-dependent sequestration of myristate and potentially by RNA bound to the HBR. In this context, it is intriguing that PI(4,5)P2 appears to be capable of reversing both modes of inhibition. Unlike PS, PI(4,5)P2 is able to bind MA HBR even in the presence of RNA 21,66, perhaps because it binds to a site in MA not blocked by RNA or has a higher affinity for MA than the RNA. NMR studies on MA-PI(4,5)P2 interaction suggest that binding of Gag to PI(4,5)P2 causes a structural change that can expose myristate 38. Thus, both targeting of Gag to PI(4,5)P2-enriched membranes, i.e. PM, and efficient membrane binding are tightly linked.
NMR data further suggest that once the myristoyl moiety is exposed, the hydrophobic pocket of Gag is then occupied by the 2′ acyl chain of PI(4,5)P2 that is usually unsaturated 38. This would leave two saturated acyl chains that mediate MA-membrane interaction; the remaining saturated 1′ acyl chain of PI(4,5)P2 and the N-terminal myristoyl moiety of Gag (Fig. 3A). The intriguing implication of this association is that it could enhance the affinity of Gag for PM microdomains known as lipid rafts, where saturated but not unsaturated acyl chains are preferred. Consistent with this model, replacing N-terminal myristate with an unsaturated analog significantly reduces Gag in detergent resistant membrane fractions that are thought to originate from lipid rafts 95. Lipid rafts have been previously shown to play a crucial role in virus assembly and infectivity of progeny virus particles 95–99 (for review see 100,101). Therefore, interaction with PI(4,5)P2 not only promotes specific Gag localization to the PM and stable membrane binding, but may also confer additional advantage to HIV-1 by associating assembly events to lipid rafts. The latter role for PI(4,5)P2 has not been tested experimentally.
As mentioned earlier, in vitro studies indicate that RNA bound to MA HBR can block interactions with PS but not with PI(4,5)P2 21,66. Therefore, it is possible that the requirement for PI(4,5)P2 could be a mere consequence of RNA-mediated inhibition of MA interaction with other acidic lipids. Conversely, RNA may be recruited to ensure specificity of the MA HBR for PI(4,5)P2 and membranes containing this lipid (i.e., the PM). Indeed, HTLV-1 Gag membrane binding that is less dependent on PI(4,5)P2 is also not inhibited by RNA, suggesting a correlation between RNA binding and PI(4,5)P2-dependent membrane interaction76. Interestingly, a neutron scattering study showed that in solution, monomeric Gag adapts a conformation in which Gag is folded over, and MA and NC are in a close proximity 102. When bound to either acidic membrane or oligonucleotides, Gag is still in a compact shape. However, when both acidic membrane and oligonucleotides are present, Gag forms the extended rod-like structure similar to what is observed in immature virions 103 (Fig. 3B). An intriguing model proposed in these studies is that when bound to either the PM or RNA, Gag can do so via both basic regions in MA and NC simultaneously. When both the PM and RNA are present, however, MA-PM and NC-RNA interactions may occur in parallel. Thus, it is conceivable that once RNA-bound Gag reaches the PM, a high affinity of the MA HBR to PI(4,5)P2 may help strip off RNA from MA, but not NC (Fig. 3B). Consistent with this possibility, in vitro RNA chaperone activity (annealing of tRNA to the primer-binding site of viral RNA) of NC in the context of Gag increased several fold when IP6 was added to Gag or when MA was deleted 104. These data suggest that RNA binding of MA hinders NC activities in the absence of inositol phosphates. Based on the above data, we speculate that RNA-mediated regulation of membrane binding may be a way of coordinating several major assembly steps for HIV-1. Once the MA HBR is bound to PI(4,5)P2 and releases RNA, NC may become capable of freely performing its functions, such as tRNA annealing and RNA-mediated Gag multimerization. However, it is important to note that this model assumes that the same RNA binds both MA and NC, an assumption that has to be tested experimentally.
Interestingly, NC-mediated multimerization has been shown to be important for localization of Gag to the rear-end protrusions known as uropods in polarized T cells 105. Therefore, binding of PI(4,5)P2 to MA might represent the transition from the MA-driven PM-targeting phase to the NC-dependent uropod localization phase. Notably, these Gag-containing uropods form frequent contacts with other T cells and participate in virological synapses 105,106 at which cell-to-cell virus transmission is thought to take place efficiently107–110(for review see 111–115). Therefore, this polarized localization of Gag on the PM likely facilitates efficient cell-to-cell transfer of the virus at the contact sites, which might represent another advantage associated with targeting of Gag to the PM. Further studies are required to better understand the connection between the MA-mediated regulation of targeting and membrane binding of Gag and the NC- mediated Gag multimerization step that drive Gag to specific sites within the plane of the PM.
Conclusions
Binding of Gag to the PM is the essential early step in HIV-1 assembly. These processes are likely modulated by MA in three different but coordinated ways: positively by interacting with PI(4,5)P2, negatively by suppressing myristate-mediated hydrophobic interaction, and negatively by interacting with RNA. With the present knowledge on Gag-membrane binding, several types of drugs can be developed for inhibiting this process during HIV-1 assembly. Blocking N-terminal myristoylation of Gag or supplementing cells with unsaturated analogues of myristate has already been shown to significantly reduce infectious virus particle production 95,116–119. Similarly, as Gag- PI(4,5)P2 interaction plays an essential role in virus assembly, small molecule inhibitors that inhibit this interaction can be developed. Lately, RNA aptamers have been emerging as a new class of therapeutics for several different diseases 120. With the recent evidence for the role of RNA in the inhibition of membrane binding of Gag, RNA aptamers that bind MA with higher affinity than PI(4,5)P2 might prove useful as inhibitors of HIV assembly (for example, see reference 83).
Mechanisms regulating both MA-PI(4,5)P2 and MA-RNA interactions, as well as their potential roles in coordinating Gag membrane binding and other steps in assembly and post-assembly processes remain to be elucidated. In addition, several events that precede Gag-membrane binding continue to pose outstanding questions: Where is Gag synthesized? How does Gag traffic to the PM at which PI(4,5)P2 stabilizes its membrane binding? When and where does Gag encounter the RNA that inhibits MA-dependent membrane binding? How and why are RNA-export pathway and membrane binding linked? Addressing these questions will provide new strategies to inhibit HIV-1 replication at early stages of virus assembly.
Acknowledgments
We thank members of our lab for helpful discussions and critical review of this manuscript. Our work related to the topics discussed in this review is supported by National Institute of Allergy and Infectious Diseases (R01 AI071727 and R56 AI089282), American Heart Association (0850133Z), and amfAR (107449-45-RGHF).
Abbreviations
- HIV-1
HIV type 1
- MA
matrix
- HBR
highly basic region
- PI(4,5)P2
phosphatidylinositol-(4,5)-bisphosphate
- CA
capsid
- NC
nucleocapsid
- PM
plasma membrane
- WT
wildtype
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adamson CS, Jones IM. The molecular basis of HIV capsid assembly--five years of progress. Rev Med Virol. 2004;14:107–21. doi: 10.1002/rmv.418. [DOI] [PubMed] [Google Scholar]
- 2.Adamson CS, Freed EO. Human immunodeficiency virus type 1 assembly, release, and maturation. Adv Pharmacol. 2007;55:347–87. doi: 10.1016/S1054-3589(07)55010-6. [DOI] [PubMed] [Google Scholar]
- 3.Gelderblom HR, Hausmann EH, Ozel M, Pauli G, Koch MA. Fine structure of human immunodeficiency virus (HIV) and immunolocalization of structural proteins. Virology. 1987;156:171–6. doi: 10.1016/0042-6822(87)90449-1. [DOI] [PubMed] [Google Scholar]
- 4.Deneka M, Pelchen-Matthews A, Byland R, Ruiz-Mateos E, Marsh M. In macrophages, HIV-1 assembles into an intracellular plasma membrane domain containing the tetraspanins CD81, CD9, and CD53. J Cell Biol. 2007;177:329–41. doi: 10.1083/jcb.200609050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welsch S, Keppler OT, Habermann A, Allespach I, Krijnse-Locker J, Krausslich HG. HIV-1 Buds Predominantly at the Plasma Membrane of Primary Human Macrophages. PLoS Pathog. 2007;3:e36. doi: 10.1371/journal.ppat.0030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett AE, Narayan K, Shi D, Hartnell LM, Gousset K, He H, Lowekamp BC, Yoo TS, Bliss D, Freed EO, Subramaniam S. Ion-abrasion scanning electron microscopy reveals surface-connected tubular conduits in HIV-infected macrophages. PLoS Pathog. 2009;5:e1000591. doi: 10.1371/journal.ppat.1000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jouvenet N, Neil SJ, Bess C, Johnson MC, Virgen CA, Simon SM, Bieniasz PD. Plasma membrane is the site of productive HIV-1 particle assembly. PLoS Biol. 2006;4:e435. doi: 10.1371/journal.pbio.0040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu H, Wang JJ, Spearman P. Human immunodeficiency virus type-1 gag and host vesicular trafficking pathways. Curr Top Microbiol Immunol. 2009;339:67–84. doi: 10.1007/978-3-642-02175-6_4. [DOI] [PubMed] [Google Scholar]
- 9.Ono A. HIV-1 Assembly at the Plasma Membrane: Gag Trafficking and Localization. Future Virol. 2009;4:241–257. doi: 10.2217/fvl.09.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou W, Parent LJ, Wills JW, Resh MD. Identification of a membrane-binding domain within the amino-terminal region of human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J Virol. 1994;68:2556–69. doi: 10.1128/jvi.68.4.2556-2569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan X, Yu X, Lee TH, Essex M. Mutations in the N-terminal region of human immunodeficiency virus type 1 matrix protein block intracellular transport of the Gag precursor. J Virol. 1993;67:6387–6394. doi: 10.1128/jvi.67.11.6387-6394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ono A, Orenstein JM, Freed EO. Role of the Gag matrix domain in targeting human immunodeficiency virus type 1 assembly. J Virol. 2000;74:2855–66. doi: 10.1128/jvi.74.6.2855-2866.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ono A, Ablan SD, Lockett SJ, Nagashima K, Freed EO. Phosphatidylinositol (4,5) bisphosphate regulates HIV-1 Gag targeting to the plasma membrane. Proc Natl Acad Sci U S A. 2004;101:14889–14894. doi: 10.1073/pnas.0405596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joshi A, Ablan SD, Soheilian F, Nagashima K, Freed EO. Evidence that productive human immunodeficiency virus type 1 assembly can occur in an intracellular compartment. J Virol. 2009;83:5375–87. doi: 10.1128/JVI.00109-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massiah MA, Starich MR, Paschall C, Summers MF, Christensen AM, Sundquist WI. Three-dimensional structure of the human immunodeficiency virus type 1 matrix protein. J Mol Biol. 1994;244:198–223. doi: 10.1006/jmbi.1994.1719. [DOI] [PubMed] [Google Scholar]
- 16.Hill CP, Worthylake D, Bancroft DP, Christensen AM, Sundquist WI. Crystal structures of the trimeric human immunodeficiency virus type 1 matrix protein: implications for membrane association and assembly. Proc Natl Acad Sci U S A. 1996;93:3099–3104. doi: 10.1073/pnas.93.7.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bryant M, Ratner L. Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proc Natl Acad Sci U S A. 1990;87:523–7. doi: 10.1073/pnas.87.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottlinger HG, Sodroski JG, Haseltine WA. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989;86:5781–5. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hermida-Matsumoto L, Resh MD. Localization of human immunodeficiency virus type 1 Gag and Env at the plasma membrane by confocal imaging. J Virol. 2000;74:8670–8679. doi: 10.1128/jvi.74.18.8670-8679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ono A, Freed EO. Cell-type-dependent targeting of human immunodeficiency virus type 1 assembly to the plasma membrane and the multivesicular body. J Virol. 2004;78:1552–1563. doi: 10.1128/JVI.78.3.1552-1563.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chukkapalli V, Oh SJ, Ono A. Opposing mechanisms involving RNA and lipids regulate HIV-1 Gag membrane binding through the highly basic region of the matrix domain. Proc Natl Acad Sci U S A. 2010;107:1600–5. doi: 10.1073/pnas.0908661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freed EO, Orenstein JM, Buckler-White AJ, Martin MA. Single amino acid changes in the human immunodeficiency virus type 1 matrix protein block virus particle production. J Virol. 1994;68:5311–5320. doi: 10.1128/jvi.68.8.5311-5320.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ono A, Huang M, Freed EO. Characterization of human immunodeficiency virus type 1 matrix revertants: effects on virus assembly, Gag processing, and Env incorporation into virions. J Virol. 1997;71:4409–18. doi: 10.1128/jvi.71.6.4409-4418.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hearps AC, Jans DA. Regulating the functions of the HIV-1 matrix protein. AIDS Res Hum Retroviruses. 2007;23:341–6. doi: 10.1089/aid.2006.0108. [DOI] [PubMed] [Google Scholar]
- 25.Wright MH, Heal WP, Mann DJ, Tate EW. Protein myristoylation in health and disease. J Chem Biol. 2009 doi: 10.1007/s12154-009-0032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ono A, Freed EO. Binding of human immunodeficiency virus type 1 Gag to membrane: role of the matrix amino terminus. J Virol. 1999;73:4136–4144. doi: 10.1128/jvi.73.5.4136-4144.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spearman P, Horton R, Ratner L, Kuli-Zade I. Membrane binding of human immunodeficiency virus type 1 matrix protein in vivo supports a conformational myristyl switch mechanism. J Virol. 1997;71:6582–92. doi: 10.1128/jvi.71.9.6582-6592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hermida-Matsumoto L, Resh MD. Human immunodeficiency virus type 1 protease triggers a myristoyl switch that modulates membrane binding of Pr55(gag) and p17MA. J Virol. 1999;73:1902–8. doi: 10.1128/jvi.73.3.1902-1908.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou W, Resh MD. Differential membrane binding of the human immunodeficiency virus type 1 matrix protein. J Virol. 1996;70:8540–8. doi: 10.1128/jvi.70.12.8540-8548.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandefur S, Varthakavi V, Spearman P. The I domain is required for efficient plasma membrane binding of human immunodeficiency virus type 1 Pr55Gag. J Virol. 1998;72:2723–32. doi: 10.1128/jvi.72.4.2723-2732.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saad JS, Loeliger E, Luncsford P, Liriano M, Tai J, Kim A, Miller J, Joshi A, Freed EO, Summers MF. Point mutations in the HIV-1 matrix protein turn off the myristyl switch. J Mol Biol. 2007;366:574–85. doi: 10.1016/j.jmb.2006.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paillart JC, Gottlinger HG. Opposing effects of human immunodeficiency virus type 1 matrix mutations support a myristyl switch model of gag membrane targeting. J Virol. 1999;73:2604–12. doi: 10.1128/jvi.73.4.2604-2612.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perez-Caballero D, Hatziioannou T, Martin-Serrano J, Bieniasz PD. Human immunodeficiency virus type 1 matrix inhibits and confers cooperativity on gag precursor-membrane interactions. J Virol. 2004;78:9560–3. doi: 10.1128/JVI.78.17.9560-9563.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang C, Loeliger E, Luncsford P, Kinde I, Beckett D, Summers MF. Entropic switch regulates myristate exposure in the HIV-1 matrix protein. Proc Natl Acad Sci U S A. 2004;101:517–22. doi: 10.1073/pnas.0305665101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dalton AK, Ako-Adjei D, Murray PS, Murray D, Vogt VM. Electrostatic interactions drive membrane association of the human immunodeficiency virus type 1 Gag MA domain. J Virol. 2007;81:6434–45. doi: 10.1128/JVI.02757-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alfadhli A, Huseby D, Kapit E, Colman D, Barklis E. Human immunodeficiency virus type 1 matrix protein assembles on membranes as a hexamer. J Virol. 2007;81:1472–8. doi: 10.1128/JVI.02122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dalton AK, Murray PS, Murray D, Vogt VM. Biochemical characterization of rous sarcoma virus MA protein interaction with membranes. J Virol. 2005;79:6227–38. doi: 10.1128/JVI.79.10.6227-6238.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saad JS, Miller J, Tai J, Kim A, Ghanam RH, Summers MF. Structural basis for targeting HIV-1 Gag proteins to the plasma membrane for virus assembly. Proc Natl Acad Sci U S A. 2006;103:11364–9. doi: 10.1073/pnas.0602818103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Datta SA, Zhao Z, Clark PK, Tarasov S, Alexandratos JN, Campbell SJ, Kvaratskhelia M, Lebowitz J, Rein A. Interactions between HIV-1 Gag molecules in solution: an inositol phosphate-mediated switch. J Mol Biol. 2007;365:799–811. doi: 10.1016/j.jmb.2006.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghanam RH, Fernandez TF, Fledderman EL, Saad JS. Binding of calmodulin to the HIV-1 matrix protein triggers myristate exposure. J Biol Chem. 2010;285:41911–20. doi: 10.1074/jbc.M110.179093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fledderman EL, Fujii K, Ghanam RH, Waki K, Prevelige PE, Freed EO, Saad JS. Myristate Exposure in the Human Immunodeficiency Virus Type 1 Matrix Protein Is Modulated by pH. Biochemistry. 2010 doi: 10.1021/bi101245j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McLaughlin S, Aderem A. The myristoyl-electrostatic switch: a modulator of reversible protein-membrane interactions. Trends Biochem Sci. 1995;20:272–6. doi: 10.1016/s0968-0004(00)89042-8. [DOI] [PubMed] [Google Scholar]
- 43.Resh MD. Trafficking and signaling by fatty-acylated and prenylated proteins. Nat Chem Biol. 2006;2:584–90. doi: 10.1038/nchembio834. [DOI] [PubMed] [Google Scholar]
- 44.Quinn PJ. Plasma membrane phospholipid asymmetry. Subcell Biochem. 2002;36:39–60. doi: 10.1007/0-306-47931-1_3. [DOI] [PubMed] [Google Scholar]
- 45.McLaughlin S, Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 2005;438:605–11. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- 46.Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol. 2008;9:99–111. doi: 10.1038/nrm2328. [DOI] [PubMed] [Google Scholar]
- 47.Leventis R, Silvius JR. Lipid-binding characteristics of the polybasic carboxy-terminal sequence of K-ras4B. Biochemistry. 1998;37:7640–8. doi: 10.1021/bi973077h. [DOI] [PubMed] [Google Scholar]
- 48.Yagisawa H, Sakuma K, Paterson HF, Cheung R, Allen V, Hirata H, Watanabe Y, Hirata M, Williams RL, Katan M. Replacements of single basic amino acids in the pleckstrin homology domain of phospholipase C-delta1 alter the ligand binding, phospholipase activity, and interaction with the plasma membrane. J Biol Chem. 1998;273:417–24. doi: 10.1074/jbc.273.1.417. [DOI] [PubMed] [Google Scholar]
- 49.Prior IA, Hancock JF. Compartmentalization of Ras proteins. J Cell Sci. 2001;114:1603–8. doi: 10.1242/jcs.114.9.1603. [DOI] [PubMed] [Google Scholar]
- 50.Murray PS, Li Z, Wang J, Tang CL, Honig B, Murray D. Retroviral matrix domains share electrostatic homology: models for membrane binding function throughout the viral life cycle. Structure (Camb) 2005;13:1521–1531. doi: 10.1016/j.str.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 51.Reil H, Bukovsky AA, Gelderblom HR, Gottlinger HG. Efficient HIV-1 replication can occur in the absence of the viral matrix protein. EMBO J. 1998;17:2699–708. doi: 10.1093/emboj/17.9.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee PP, Linial ML. Efficient particle formation can occur if the matrix domain of human immunodeficiency virus type 1 Gag is substituted by a myristylation signal. J Virol. 1994;68:6644–54. doi: 10.1128/jvi.68.10.6644-6654.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang CT, Zhang Y, McDermott J, Barklis E. Conditional infectivity of a human immunodeficiency virus matrix domain deletion mutant. J Virol. 1993;67:7067–76. doi: 10.1128/jvi.67.12.7067-7076.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang CT, Lai HY, Li JJ. Analysis of minimal human immunodeficiency virus type 1 gag coding sequences capable of virus-like particle assembly and release. J Virol. 1998;72:7950–9. doi: 10.1128/jvi.72.10.7950-7959.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chukkapalli V, Hogue IB, Boyko V, Hu WS, Ono A. Interaction between the human immunodeficiency virus type 1 Gag matrix domain and phosphatidylinositol-(4,5)-bisphosphate is essential for efficient gag membrane binding. J Virol. 2008;82:2405–17. doi: 10.1128/JVI.01614-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shkriabai N, Datta SA, Zhao Z, Hess S, Rein A, Kvaratskhelia M. Interactions of HIV-1 Gag with assembly cofactors. Biochemistry. 2006;45:4077–83. doi: 10.1021/bi052308e. [DOI] [PubMed] [Google Scholar]
- 57.Downes CP, Gray A, Lucocq JM. Probing phosphoinositide functions in signaling and membrane trafficking. Trends Cell Biol. 2005;15:259–68. doi: 10.1016/j.tcb.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 58.Balla T, Szentpetery Z, Kim YJ. Phosphoinositide signaling: new tools and insights. Physiology (Bethesda) 2009;24:231–44. doi: 10.1152/physiol.00014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–7. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 60.Campbell S, Fisher RJ, Towler EM, Fox S, Issaq HJ, Wolfe T, Phillips LR, Rein A. Modulation of HIV-like particle assembly in vitro by inositol phosphates. Proc Natl Acad Sci U S A. 2001;98:10875–10879. doi: 10.1073/pnas.191224698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kisseleva MV, Wilson MP, Majerus PW. The isolation and characterization of a cDNA encoding phospholipid-specific inositol polyphosphate 5-phosphatase. J Biol Chem. 2000;275:20110–20116. doi: 10.1074/jbc.M910119199. [DOI] [PubMed] [Google Scholar]
- 62.Brown FD, Rozelle AL, Yin HL, Balla T, Donaldson JG. Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J Cell Biol. 2001;154:1007–1017. doi: 10.1083/jcb.200103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aikawa Y, Martin TF. ARF6 regulates a plasma membrane pool of phosphatidylinositol(4,5)bisphosphate required for regulated exocytosis. J Cell Biol. 2003;162:647–659. doi: 10.1083/jcb.200212142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alfadhli A, Barklis RL, Barklis E. HIV-1 matrix organizes as a hexamer of trimers on membranes containing phosphatidylinositol-(4,5)-bisphosphate. Virology. 2009;387:466–72. doi: 10.1016/j.virol.2009.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anraku K, Fukuda R, Takamune N, Misumi S, Okamoto Y, Otsuka M, Fujita M. Highly sensitive analysis of the interaction between HIV-1 Gag and phosphoinositide derivatives based on surface plasmon resonance. Biochemistry. 2010;49:5109–16. doi: 10.1021/bi9019274. [DOI] [PubMed] [Google Scholar]
- 66.Alfadhli A, Still A, Barklis E. Analysis of human immunodeficiency virus type 1 matrix binding to membranes and nucleic acids. J Virol. 2009;83:12196–203. doi: 10.1128/JVI.01197-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Valentine KG, Peterson RW, Saad JS, Summers MF, Xu X, Ames JB, Wand AJ. Reverse micelle encapsulation of membrane-anchored proteins for solution NMR studies. Structure. 2010;18:9–16. doi: 10.1016/j.str.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chan R, Uchil PD, Jin J, Shui G, Ott DE, Mothes W, Wenk MR. Retroviruses human immunodeficiency virus and murine leukemia virus are enriched in phosphoinositides. J Virol. 2008;82:11228–38. doi: 10.1128/JVI.00981-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Monde K, Chukkapalli V, Ono A. Assembly and Replication of HIV-1 in T Cells with Low Levels of Phosphatidylinositol-(4,5)-Bisphosphate. J Virol. 2011;85:3584–95. doi: 10.1128/JVI.02266-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ehrlich LS, Medina GN, Khan MB, Powell MD, Mikoshiba K, Carter CA. Activation of the inositol (1,4,5)-triphosphate calcium gate receptor is required for HIV-1 Gag release. J Virol. 2010;84:6438–51. doi: 10.1128/JVI.01588-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stansell E, Apkarian R, Haubova S, Diehl WE, Tytler EM, Hunter E. Basic residues in the Mason-Pfizer monkey virus gag matrix domain regulate intracellular trafficking and capsid-membrane interactions. J Virol. 2007;81:8977–88. doi: 10.1128/JVI.00657-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saad JS, Ablan SD, Ghanam RH, Kim A, Andrews K, Nagashima K, Soheilian F, Freed EO, Summers MF. Structure of the myristylated human immunodeficiency virus type 2 matrix protein and the role of phosphatidylinositol-(4,5)-bisphosphate in membrane targeting. J Mol Biol. 2008;382:434–47. doi: 10.1016/j.jmb.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hamard-Peron E, Juillard F, Saad JS, Roy C, Roingeard P, Summers MF, Darlix JL, Picart C, Muriaux D. Targeting of murine leukemia virus gag to the plasma membrane is mediated by PI(4,5)P2/PS and a polybasic region in the matrix. J Virol. 2010;84:503–15. doi: 10.1128/JVI.01134-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen K, Bachtiar I, Piszczek G, Bouamr F, Carter C, Tjandra N. Solution NMR characterizations of oligomerization and dynamics of equine infectious anemia virus matrix protein and its interaction with PIP2. Biochemistry. 2008;47:1928–37. doi: 10.1021/bi701984h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fernandes F, Chen K, Ehrlich LS, Jin J, Chen MH, Medina GN, Symons M, Montelaro R, Donaldson J, Tjandra N, Carter CA. Phosphoinositides Direct Equine Infectious Anemia Virus Gag Trafficking and Release. Traffic. 2010 doi: 10.1111/j.1600-0854.2010.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Inlora J, Chukkapalli V, Derse D, Ono A. Gag Localization and Virus-Like Particle Release Mediated by the Matrix Domain of Human T-Lymphotropic Virus Type 1 Gag Are Less Dependent on Phosphatidylinositol-(4,5)-Bisphosphate than Those Mediated by the Matrix Domain of HIV-1 Gag. J Virol. 2011;85:3802–10. doi: 10.1128/JVI.02383-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Purohit P, Dupont S, Stevenson M, Green MR. Sequence-specific interaction between HIV-1 matrix protein and viral genomic RNA revealed by in vitro genetic selection. Rna. 2001;7:576–84. doi: 10.1017/s1355838201002023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lochrie MA, Waugh S, Pratt DG, Jr, Clever J, Parslow TG, Polisky B. In vitro selection of RNAs that bind to the human immunodeficiency virus type-1 gag polyprotein. Nucleic Acids Res. 1997;25:2902–10. doi: 10.1093/nar/25.14.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang CY, Chang YF, Wang SM, Tseng YT, Huang KJ, Wang CT. HIV-1 matrix protein repositioning in nucleocapsid region fails to confer virus-like particle assembly. Virology. 2008;378:97–104. doi: 10.1016/j.virol.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cimarelli A, Luban J. Translation elongation factor 1-alpha interacts specifically with the human immunodeficiency virus type 1 Gag polyprotein. J Virol. 1999;73:5388–401. doi: 10.1128/jvi.73.7.5388-5401.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hearps AC, Wagstaff KM, Piller SC, Jans DA. The N-terminal basic domain of the HIV-1 matrix protein does not contain a conventional nuclear localization sequence but is required for DNA binding and protein self-association. Biochemistry. 2008;47:2199–210. doi: 10.1021/bi701360j. [DOI] [PubMed] [Google Scholar]
- 82.Ott DE, Coren LV, Gagliardi TD. Redundant roles for nucleocapsid and matrix RNA-binding sequences in human immunodeficiency virus type 1 assembly. J Virol. 2005;79:13839–47. doi: 10.1128/JVI.79.22.13839-13847.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramalingam D, Duclair S, Datta SA, Ellington A, Rein A, Prasad VR. RNA aptamers directed to human immunodeficiency virus type 1 Gag polyprotein bind to the matrix and nucleocapsid domains and inhibit virus production. J Virol. 2011;85:305–14. doi: 10.1128/JVI.02626-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garbitt RA, Albert JA, Kessler MD, Parent LJ. trans-acting inhibition of genomic RNA dimerization by Rous sarcoma virus matrix mutants. J Virol. 2001;75:260–8. doi: 10.1128/JVI.75.1.260-268.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Parent LJ, Cairns TM, Albert JA, Wilson CB, Wills JW, Craven RC. RNA dimerization defect in a Rous sarcoma virus matrix mutant. J Virol. 2000;74:164–72. doi: 10.1128/jvi.74.1.164-172.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang H, Norris KM, Mansky LM. Involvement of the matrix and nucleocapsid domains of the bovine leukemia virus Gag polyprotein precursor in viral RNA packaging. J Virol. 2003;77:9431–8. doi: 10.1128/JVI.77.17.9431-9438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gudleski N, Flanagan JM, Ryan EP, Bewley MC, Parent LJ. Directionality of nucleocytoplasmic transport of the retroviral gag protein depends on sequential binding of karyopherins and viral RNA. Proc Natl Acad Sci U S A. 2010;107:9358–63. doi: 10.1073/pnas.1000304107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mariani R, Rutter G, Harris ME, Hope TJ, Krausslich HG, Landau NR. A block to human immunodeficiency virus type 1 assembly in murine cells. J Virol. 2000;74:3859–70. doi: 10.1128/jvi.74.8.3859-3870.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bieniasz PD, Cullen BR. Multiple blocks to human immunodeficiency virus type 1 replication in rodent cells. J Virol. 2000;74:9868–77. doi: 10.1128/jvi.74.21.9868-9877.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reed M, Mariani R, Sheppard L, Pekrun K, Landau NR, Soong NW. Chimeric human immunodeficiency virus type 1 containing murine leukemia virus matrix assembles in murine cells. J Virol. 2002;76:436–43. doi: 10.1128/JVI.76.1.436-443.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen BK, Rousso I, Shim S, Kim PS. Efficient assembly of an HIV-1/MLV Gag-chimeric virus in murine cells. Proc Natl Acad Sci U S A. 2001;98:15239–44. doi: 10.1073/pnas.261563198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kiernan RE, Ono A, Englund G, Freed EO. Role of matrix in an early postentry step in the human immunodeficiency virus type 1 life cycle. J Virol. 1998;72:4116–26. doi: 10.1128/jvi.72.5.4116-4126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sherer NM, Swanson CM, Papaioannou S, Malim MH. Matrix Mediates the Functional Link between Hiv-1 Rna Nuclear Export Elements and Gag Assembly Competency in Murine Cells. J Virol. 2009 doi: 10.1128/JVI.00699-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jin J, Sturgeon T, Weisz OA, Mothes W, Montelaro RC. HIV-1 matrix dependent membrane targeting is regulated by Gag mRNA trafficking. PLoS One. 2009;4:e6551. doi: 10.1371/journal.pone.0006551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lindwasser OW, Resh MD. Myristoylation as a target for inhibiting HIV assembly: unsaturated fatty acids block viral budding. Proc Natl Acad Sci U S A. 2002;99:13037–42. doi: 10.1073/pnas.212409999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ono A, Freed EO. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc Natl Acad Sci U S A. 2001;98:13925–30. doi: 10.1073/pnas.241320298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pickl WF, Pimentel-Muinos FX, Seed B. Lipid rafts and pseudotyping. J Virol. 2001;75:7175–83. doi: 10.1128/JVI.75.15.7175-7183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ono A, Waheed AA, Freed EO. Depletion of cellular cholesterol inhibits membrane binding and higher-order multimerization of human immunodeficiency virus type 1 Gag. Virology. 2007;360:27–35. doi: 10.1016/j.virol.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liao Z, Graham DR, Hildreth JE. Lipid rafts and HIV pathogenesis: virion-associated cholesterol is required for fusion and infection of susceptible cells. AIDS Res Hum Retroviruses. 2003;19:675–87. doi: 10.1089/088922203322280900. [DOI] [PubMed] [Google Scholar]
- 100.Ono A. Relationships between plasma membrane microdomains and HIV-1 assembly. Biol Cell. 2010;102:335–50. doi: 10.1042/BC20090165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Waheed AA, Freed EO. Lipids and membrane microdomains in HIV-1 replication. Virus Res. 2009;143:162–76. doi: 10.1016/j.virusres.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Datta SA, Curtis JE, Ratcliff W, Clark PK, Crist RM, Lebowitz J, Krueger S, Rein A. Conformation of the HIV-1 Gag protein in solution. J Mol Biol. 2007;365:812–24. doi: 10.1016/j.jmb.2006.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Datta SA, Heinrich F, Raghunandan S, Krueger S, Curtis JE, Rein A, Nanda H. HIV-1 Gag Extension: Conformational Changes Require Simultaneous Interaction with Membrane and Nucleic Acid. J Mol Biol. 2010 doi: 10.1016/j.jmb.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jones CP, Datta SA, Rein A, Rouzina I, Musier-Forsyth K. Matrix Domain Modulates Hiv-1 Gag’s Nucleic Acid Chaperone Activity Via Inositol Phosphate Binding. J Virol. 2010 doi: 10.1128/JVI.01809-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Llewellyn GN, Hogue IB, Grover JR, Ono A. Nucleocapsid promotes localization of HIV-1 gag to uropods that participate in virological synapses between T cells. PLoS Pathog. 2010;6:e1001167. doi: 10.1371/journal.ppat.1001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen P, Hubner W, Spinelli MA, Chen BK. Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained Env-dependent neutralization-resistant virological synapses. J Virol. 2007;81:12582–95. doi: 10.1128/JVI.00381-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jolly C, Kashefi K, Hollinshead M, Sattentau QJ. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J Exp Med. 2004;199:283–93. doi: 10.1084/jem.20030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Igakura T, Stinchcombe JC, Goon PK, Taylor GP, Weber JN, Griffiths GM, Tanaka Y, Osame M, Bangham CR. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science. 2003;299:1713–6. doi: 10.1126/science.1080115. [DOI] [PubMed] [Google Scholar]
- 109.Hubner W, McNerney GP, Chen P, Dale BM, Gordon RE, Chuang FY, Li XD, Asmuth DM, Huser T, Chen BK. Quantitative 3D video microscopy of HIV transfer across T cell virological synapses. Science. 2009;323:1743–7. doi: 10.1126/science.1167525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rudnicka D, Feldmann J, Porrot F, Wietgrefe S, Guadagnini S, Prevost MC, Estaquier J, Haase AT, Sol-Foulon N, Schwartz O. Simultaneous cell-to-cell transmission of human immunodeficiency virus to multiple targets through polysynapses. J Virol. 2009;83:6234–46. doi: 10.1128/JVI.00282-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sattentau Q. Avoiding the void: cell-to-cell spread of human viruses. Nat Rev Microbiol. 2008;6:815–26. doi: 10.1038/nrmicro1972. [DOI] [PubMed] [Google Scholar]
- 112.Feldmann JS, Olivier HIV-1 Virological Synapse: Live Imaging of Transmission. Viruses. 2010;2:1666–1680. doi: 10.3390/v2081666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jolly C. T Cell Polarization at the Virological Synapse. Viruses. 2010;2:1261–1278. doi: 10.3390/v2061261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sattentau QJ. Cell-to-Cell Spread of Retroviruses. Viruses. 2010;2:1306–1321. doi: 10.3390/v2061306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mothes W, Sherer NM, Jin J, Zhong P. Virus cell-to-cell transmission. J Virol. 2010;84:8360–8. doi: 10.1128/JVI.00443-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bryant ML, Heuckeroth RO, Kimata JT, Ratner L, Gordon JI. Replication of human immunodeficiency virus 1 and Moloney murine leukemia virus is inhibited by different heteroatom-containing analogs of myristic acid. Proc Natl Acad Sci U S A. 1989;86:8655–9. doi: 10.1073/pnas.86.22.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bryant ML, Ratner L, Duronio RJ, Kishore NS, Devadas B, Adams SP, Gordon JI. Incorporation of 12-methoxydodecanoate into the human immunodeficiency virus 1 gag polyprotein precursor inhibits its proteolytic processing and virus production in a chronically infected human lymphoid cell line. Proc Natl Acad Sci U S A. 1991;88:2055–9. doi: 10.1073/pnas.88.6.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Morikawa Y, Hinata S, Tomoda H, Goto T, Nakai M, Aizawa C, Tanaka H, Omura S. Complete inhibition of human immunodeficiency virus Gag myristoylation is necessary for inhibition of particle budding. J Biol Chem. 1996;271:2868–73. doi: 10.1074/jbc.271.5.2868. [DOI] [PubMed] [Google Scholar]
- 119.Furuishi K, Matsuoka H, Takama M, Takahashi I, Misumi S, Shoji S. Blockage of N-myristoylation of HIV-1 gag induces the production of impotent progeny virus. Biochem Biophys Res Commun. 1997;237:504–11. doi: 10.1006/bbrc.1997.7178. [DOI] [PubMed] [Google Scholar]
- 120.Bunka DH, Platonova O, Stockley PG. Development of aptamer therapeutics. Curr Opin Pharmacol. 2010;10:557–62. doi: 10.1016/j.coph.2010.06.009. [DOI] [PubMed] [Google Scholar]