Abstract
Fibronectin (FN) is required for embryogenesis, morphogenesis, and wound repair, and its Arg–Gly–Asp-containing central cell-binding domain (CCBD) is essential for mesenchymal cell survival and growth. Here, we demonstrate that FN contains three growth factor-binding domains (FN-GFBDs) that bind platelet-derived growth factor-BB (PDGF-BB), a potent fibroblast survival and mitogenic factor. These sites bind PDGF-BB with dissociation constants of 10–100 nm. FN-null cells cultured on recombinant CCBD (FNIII8–11) without a FN-GFBD demonstrated minimal metabolism and underwent autophagy at 24 hours, followed by apoptosis at 72 hours, even in the presence of PDGF-BB. In contrast, FN-null cells plated on FNIII8–11 contiguous with FN-GFBD survived without, and proliferated with, PDGF-BB. FN-null cell survival on FNIII8–11 and noncontiguous arrays of FN-GFBDs required these domains to be adsorbed on the same surface, suggesting the existence of a mesenchymal cell-extracellular matrix synapse. Thus, fibroblast survival required GF stimulation in the presence of a FN-GFBD, as well as adhesion to FN through the CCBD. The findings that fibroblast survival is dependent on FN-GFBD underscore the critical importance of pericellular matrix for cell survival and have significant implications for cutaneous wound healing and regeneration.
INTRODUCTION
Fibroblasts are responsible for connective tissue formation, homeostasis, and repair, and thereby for the overall architecture of many organs. During cutaneous wound healing, fibroblast ingrowth is the rate-limiting step in new connective tissue production (McClain et al., 1996). Injury progression and failure to heal are associated with fibroblast dysfunction, often leading to apoptosis (Clark, 2008a). A better understanding of the requirements for fibroblast survival and growth under both normal and pathological conditions would facilitate the design of new therapies for cutaneous wound healing. Fibronectin (FN) and platelet-derived growth factor-BB (PDGF-BB) have been identified as two major survival factors for fibroblasts (Zhang et al., 1995; Ilic et al., 1998; Romashkova and Makarov, 1999), but whether there is interdependency between these fibroblast survival factors has not been addressed.
FN is a 500-kDa multidomain, multifunctional cell adhesion glycoprotein (Figure 1a) found in blood and deposited with fibrin in wounds as a provisional matrix (Yamada and Clark, 1996; Pankov and Yamada, 2002). Additional FN, derived from wound fibroblasts and other local tissue cells, appears in concert with reepithelialization and granulation tissue formation (Clark et al., 1983). As FN ablation is embryonically lethal (George et al., 1993), no definitive studies have been performed to demonstrate the requirement of FN during cutaneous injury. However, as the brain lacks macrophages and fibroblasts, and thus cannot produce local FN, Sakai et al. (2001) ablated hepatic FN in a mouse and determined that plasma FN supports neuronal survival and reduces brain injury. Importantly, chronic nonhealing cutaneous wounds and burns demonstrate an absence of FN in the wound bed (Grinnell and Zhu, 1994; Herrick et al., 1996). That FN may be required for wound healing comes from several lines of evidence: FN promotes fibroblast survival (Zhang et al., 1995; Ilic et al., 1998), proliferation (Renshaw et al., 1997), and migration through three-dimensional extracellular matrix (ECM) (Greiling and Clark, 1997). These activities require fibroblast attachment to FN at the Arg–Gly–Asp (RGD) sequence in the tenth FN type III repeat (see numbered rectangles in Figure 1a) through cell surface membrane integrin receptors (Ruoslahti, 1991). Of all integrins, α5β1 mediates the strongest binding to FN, as well as fibroblast survival signals (Zhang et al., 1995). Optimal α5β1-binding activity requires a synergy site that includes the Pro–His–Ser–Arg–Asn sequence in the ninth FN type III repeat (Aota et al., 1994; Redick et al., 2000), as well as the RGD peptide in the tenth FN type III repeat (Pierschbacher and Ruoslahti, 1987). Thus, together the ninth and tenth FN type III repeats compose the critical core of the FN-central cell-binding domain (CCBD) (Redick et al., 2000; Feng and Mrksich, 2004). Although the ninth and tenth FN type III repeats when expressed as a recombinant protein are conformationally unstable (Litvinovich et al., 1998), flanking the core with FN ninth and eleventh type III repeats confers stability.
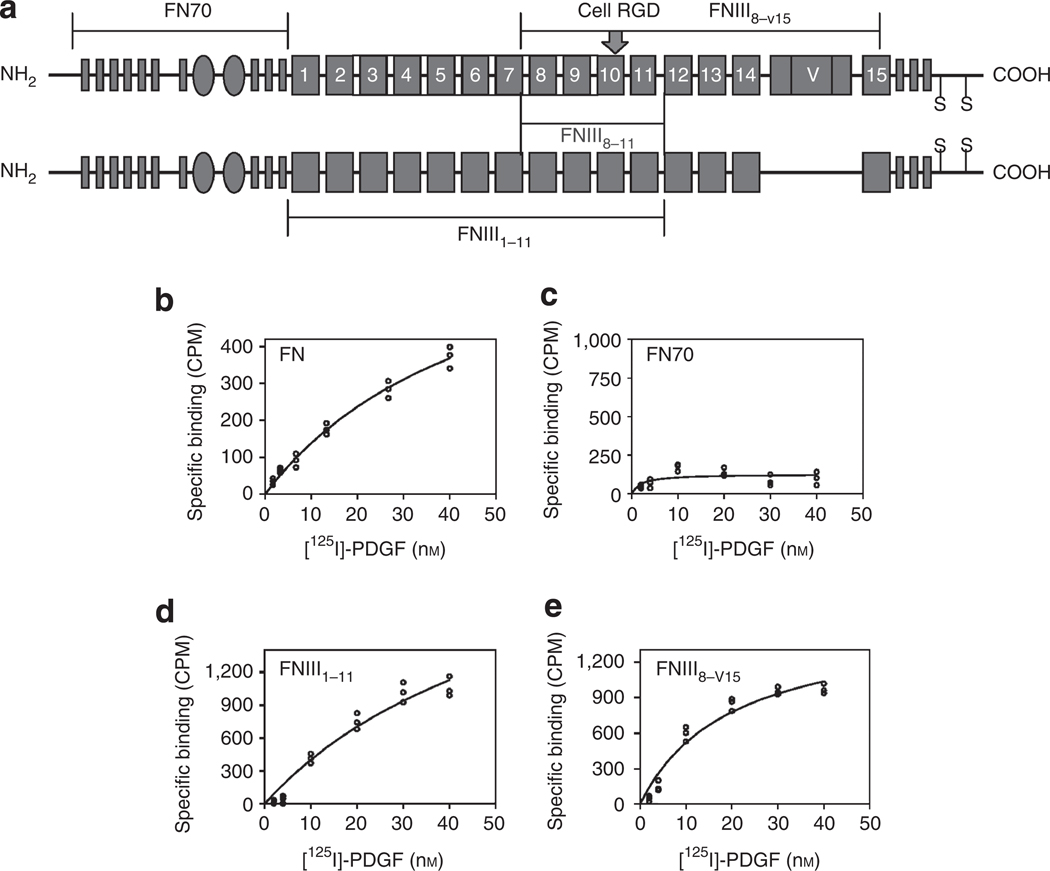
Figure 1. Platelet-derived growth factor-BB (PDGF-BB) association with intact fibronectin (FN), FN 70-kDa terminal fragment (FN70), and fibronectin type III repeats: FNIII1–11 and FNIII8–v15.
(a) Schematic of human plasma FN. FN type I repeats are shown as thin rectangles, FN type II repeats as ovals, and FN type III repeats as large rectangles. The large functional domains of FN assayed for PDGF-BB binding are noted by brackets above and below the schematic. The Arg–Gly–Asp (RGD)-containing (arrow) FN-central cell-binding domain (CCBD) (FNIII8–11) is indicated by the bracket between the two chains of FN. V indicates the entire 120-kDa FN variable domain that is also called IIICS. (b–e) Intact human plasma FN, FN70, or an extended type III repeat domain (either FNIII1–11 or FNIII8–v15) was adsorbed to plastic tissue culture dishes at 0.125 µm, and then incubated with 125I-radiolabeled PDGF-BB in DMEM containing 1% BSA for 90 minutes at room temperature. After thorough washing, radioactivity of bound PDGF-BB was determined by a γ-counter. (b) PDGF-BB association with intact human plasma FN. (c) Lack of PDGF-BB association with FN70 fragment. (d) PDGF-BB association with FNIII1–11 . (e) PDGF-BB association with FNIII8–v15. Figures are representative of at least three independent experiments. CPM, counts per minute.
PDGFs are major mitogens and survival factors for many mesenchymal cell types including fibroblasts (Romashkova and Makarov, 1999). PDGF occurs as five isoforms: classic PDGF-AA, AB, and BB isoforms, and more recently described PDGF-CC and PDGF-DD isoforms (Fredriksson et al., 2004). The most potent isoforms, PDGF-AB and -BB, are secreted by platelets (PDGF-AB), macrophages (PDGF-BB), and epidermal cells (PDGF-BB) in response to injury. PDGFB knockout mice are invariably embryonically lethal, whereas loss of other isoforms is not lethal (Betsholtz, 2004).
In fibroblasts, FN and growth factor (GF) signals converge at the level of the focal contact (Plopper et al., 1995) and are costimulatory (Miyamoto et al., 1996) for progression through the cell cycle (Assoian and Schwartz, 2001). Here, we demonstrate for the first time that FN and PDGF-BB survival signals for fibroblasts require FN-growth factor-binding domains (FN-GFBDs) and we map the operational sites. We believe that the FN-GFBDs delineated here have a potential use in therapies that facilitate and accelerate wound healing and possibly induce tissue regeneration.
RESULTS
FN-bound PDGF-BB
PDGF-BB associated with human plasma FN when it was incubated with reduced FN monomers that were attached to SulfoLink Beads (Pierce, Rockford, IL) (Figure 1b). To determine the site(s) of this association, three large fragments of the human plasma FN molecule that span from its amino-terminus through the fifteenth type III repeat were tested (Figure 1a). PDGF-BB had no affinity for the FN 70-kDa terminal fragment (FN70), which includes the fibrin and the collagen/gelatin-binding domains, and is critical for FN fibrillogenesis (Mao and Schwarzbauer, 2005) (Figure 1c). In contrast, PDGF-BB associated with FN domains consisting of the FN-CCBD and either the amino- or carboxy-terminal thirds of FN type III repeats (FNIII1–11 and FNIII8–v15) (Figure 1d and e). To confirm and expand these results, additional recombinant FN domains were cloned and expressed as previously described (Wang et al., 2005) and assayed by plasmon surface resonance to determine dissociation constants (KDs) (Figure 2, Table 1).
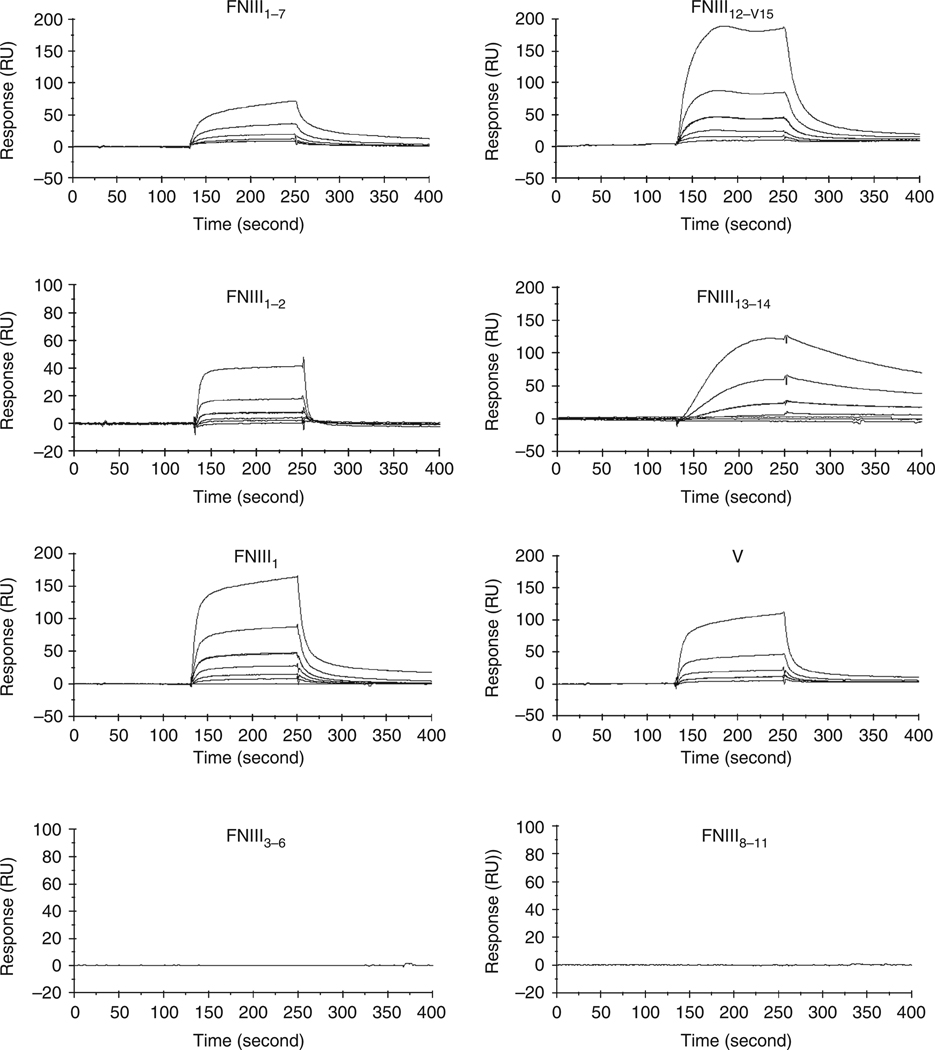
Figure 2. Platelet-derived growth factor-BB (PDGF-BB)-bound fibronectin (FN) recombinant domains that included fibronectin type III repeats: FNIII1, FNIII13–14, or fibronectin variable domain (V).
Real-time interaction of PDGF-BB with FN domains was determined by plasmon surface resonance (BiaCore 2000). FN domains were injected (6.25–200 nm, 30 µl minute−1) across a biosensor chip to which PDGF-BB had been conjugated. Chip cell without PDGF-BB was used as a reference. Sensorgrams are representative of three different experiments. Dissociation constants (KDs) were derived from the ratio of kinetic dissociation constants (kd) divided by kinetic association constants (ka). RU, resonance unit.
Table 1.
KDs for PDGF-BB interactions with FN domains derived from ka and kd
| PDGF-BB ka (m−1 s−1) |
PDGF-BB kd (s−1) |
PDGF-BB KD (nm)1 |
EGF KD (nm) |
|
|---|---|---|---|---|
| FN70 | NBB2 | NBB | NBB | NBB |
| rFNIII1–113 | 3.0 × 105 | 2.8 × 10−2 | 93 | NBB |
| rFNIII1–7 | 5.1 × 105 | 4.2 × 10−2 | 82 | NBB |
| rFNIII1–2 | 7.6 × 105 | 8.2 × 10−2 | 108 | NBB |
| rFNIII1 | 3.5 × 105 | 3.6 × 10−2 | 103 | NBB |
| FNIII2–114 (FN120) |
NBB | NBB | NBB | NBB |
| rFNIII3–6 | NBB | NBB | NBB | NBB |
| rFNIII8–11 | NBB | NBB | NBB | NBB |
| rFNIII 12–V155 | 8.0 × 105 | 2.3 × 10−2 | 29 | NBB |
| rFNIII12–15 | 4.5 × 105 | 1.2 × 10−2 | 27 | NBB |
| rFNIII12–14 | 5.6 × 105 | 2.7 × 10−2 | 48 | NBB |
| rFNIII13–14 | 4.5 × 104 | 4.1 × 10−4 | 9 | NBB |
| rV5 | 2.9 × 105 | 4.1 × 10−2 | 141 | NBB |
Abbreviations: FN, fibronectin; rFNIII, recombinant fibronectin type III repeat; ka, kinetic association constant; kd, kinetic dissociation constant; KD, dissociation constant; PDGF-BB, platelet-derived growth factor-BB
KD (nm) derived from surface plasmon resonance determinations.
NBB, no binding observed as determined by surface plasmon resonance determinations.
rFNIII, recombinant FN domain produced as previously described (Wang et al., 2005).
FNIII2–11 by QStar pulsar I quadrupole time-of-flight mass spectroscopy sequence identification of FN120 fragment.
rFNIII12–V15, recombinant FN domain in which the variable domain (V) contains 120 residues.
PDGF-BB binding for FN and rFNIII8–V15 could not be determined secondary to high noise.
As determined by plasmon surface resonance, PDGF-BB bound FN recombinant domains that included FNIII1, FNIII13–14, or the entire FN variable domain (V) with KDs of 103, 9, and 141 nm, respectively (Figure 2, Table 1). In contrast, PDGF-BB did not bind FN120—a chymotrypic fragment of FN (Ginsberg et al., 1985) that contained the second to eleventh FN type III repeats as determined by QStar pulser (Applied Biosystems, Foster City, CA) quadrupole time-of-flight mass spectroscopy sequencing—FNIII3–6, or the FN-CCBD (FNIII8–11) (Figure 2, Table 1). Importantly, PDGF-BB binding did not demonstrate any diminution when up to 2.5 m NaCl was added or when the pH was lowered to 2, indicating that electrostatic charge was not important for the interactions (data not shown).
FN-CCBD was not sufficient for FN-null cell survival
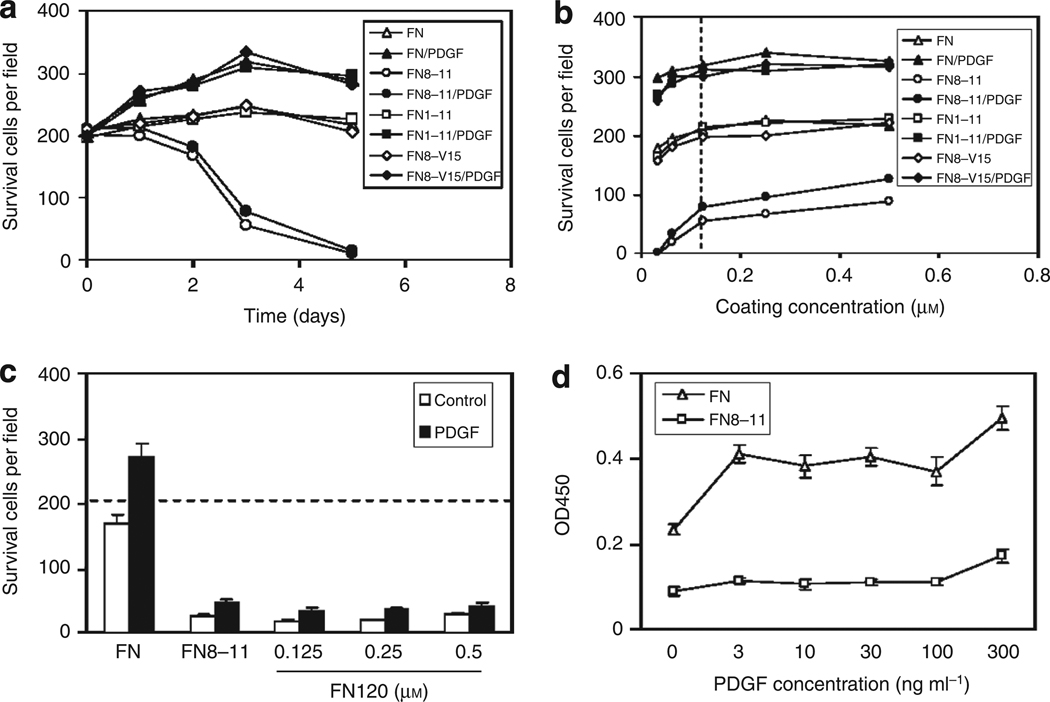
FN is secreted and deposited in the pericellular matrix by all mesenchymal cells (Mosher, 1995). Therefore, it was necessary to perform survival studies with mouse FN-null fibroblasts (Saoncella et al., 1999). When these cells were plated on intact FN in the absence of serum or exogenous GFs, they survived for 5 days, and proliferated in response to a single dose of 30 ng ml−1 PDGF-BB (Figure 3a). In contrast, FN-null cells failed to proliferate or even survive in the presence of exogenous PDGF-BB when plated on the FN central cell-binding domain (FNIII8–11) fused to glutathione S-transferase (GST) to improve surface adsorption as previously described (Wang et al., 2005). FNIII8–11, not fused with GST, also failed to support FN-null cell survival (data not shown). Remarkably, contiguous constructs of the CCBD with all type III repeats on its amino terminal side (FNIII1–11) or with all type III repeats on its carboxy-terminal side (FNIII8–V15) supported FN-null cell survival and proliferation equivalent to intact FN (Figure 3a). Increasing GST-FNIII8–11 concentration to 5-fold in these studies improved survival marginally at 3 days (Figure 3b) and not appreciably at 5 days (data not shown). Furthermore, FN120 (FNIII2–11) also failed to sustain FN-null cell survival (Figure 3c). Supraphysiological concentrations of PDGF-BB, up to 300 ng ml−1, which is > 10-fold higher than normal serum levels (Heldin and Westermark, 1999), failed to improve survival when cells were cultured on GST-FNIII8–11 (Figure 3d). Endogenous PDGF appeared to contribute little, if any, to these results as FN-null cells produced only 0.34 ng PDGF-AA per 106 cells and 0.28 ng PDGF-BB per 106 cells over a 48-hour period as judged by ELISA. Addition of 30 ng ml−1 PDGF-BB repeatedly at 4, 8 and 24 hours (Jones and Kazlauskas, 2001) also failed to promote survival (data not shown). The difference in cell survival was not attributable to differential attachment, as numbers of FN-null cells that attached to GST-FNIII8–11 were indistinguishable from the numbers of cells that attached to intact FN, FNIII1–11, or FNIII8–v15 after 4 hours (data not shown). Furthermore, FN-GFBDs do not interact with fibroblast surface membranes in the absence of PDGF-BB as judged by confocal spectroscopy (data not shown).
Figure 3. Surface-bound intact fibronectin (FN) or FN-growth factor-binding domains (GFBDs), in contiguous arrays with the central cell-binding domain (FNIII8–11), supported FN-null fibroblast survival and responsiveness to platelet-derived growth factor-BB (PDGF-BB).
Mouse FN-null cells were plated at 10,000 cells per well in 48-well tissue culture plates precoated (a) with 0.125 µm intact FN, FNIII1–11, FNIII8–V15, or glutathione S-transferase (GST)-tagged FNIII8–11; or (b) with intact FN, FNIII1–11, FNIII8–V15, or GST-tagged FNIII8–11 at the concentrations indicated. Cells were first incubated in DMEM for 4 hours and then with DMEM plus 1% BSA with or without 30 ng ml−1 PDGF-BB at 37°C. Viable spread cells in five × 10 fields were visually counted in each of the three replicate wells at the times shown (a) or at 3 days (b) for quantification of mean ± SD (not shown for clarity but < 10% of qmean, n=15). Approximately 200 cells per field were observed at 4 hours (baseline) in all conditions represented in a and b. All cells in all conditions were well spread at 4 and 24 hours. In panel b, the precoating concentration of 0.125 µm is denoted by the vertical dotted line. (c and d) FN-null cells were plated as above in wells precoated with 0.125 µm intact FN or GST-tagged FNIII8–11, or with FN120 at the given concentrations (c), incubated in DMEM for 4 hours, and then stimulated with PDGF-BB at 30 ng ml−1 (c) or at the dose indicated (d) for 3 days at 37 °C. Cell viability in c was quantified as in a and b and in d was determined by cell mitochondrial metabolism using the XTT assay. OD, optical density.
Thus, FN-null cells failed to survive on GST-FNIII8–11 that had been preadsorbed at any concentration tested even when the cells were cultured in the presence of exogenous PDGF-BB.
Initial spreading and FA formation did not require FN-GFBDs
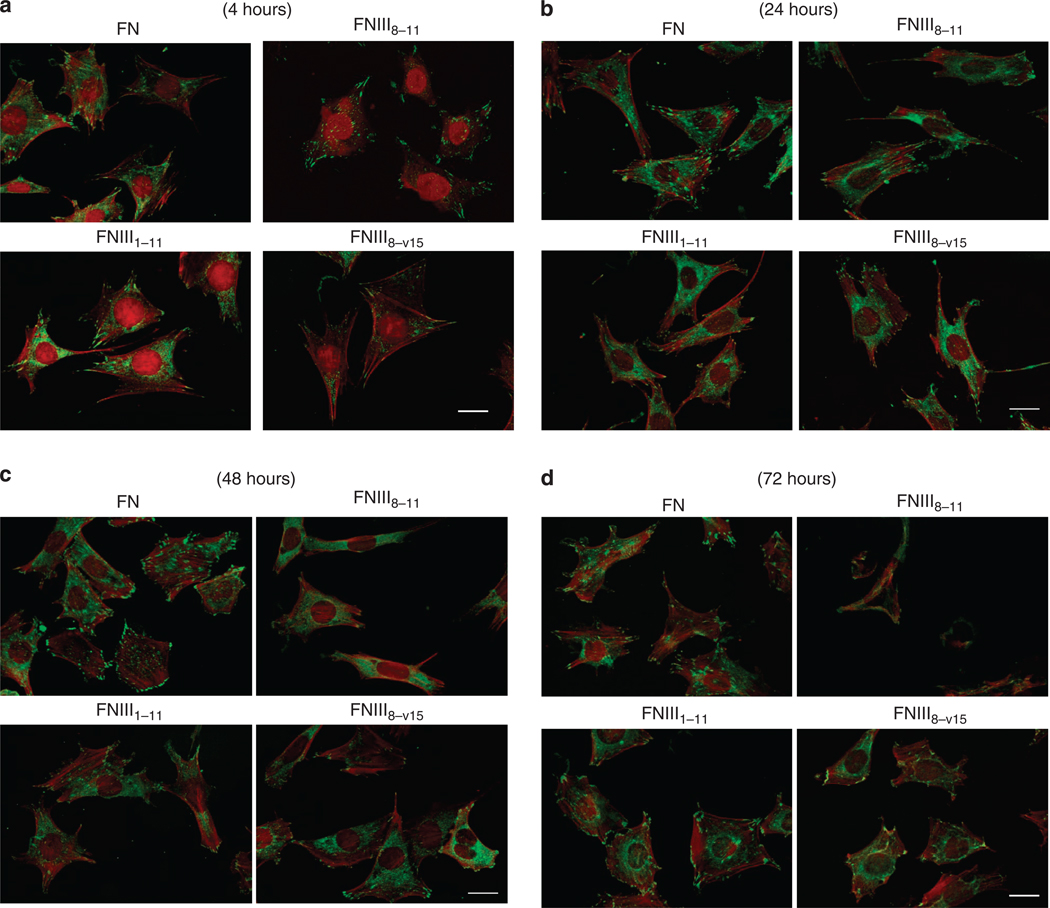
Previously, we reported that GST-tagged CCBD (FNIII8–11), unlike FNIII8–11 without GST, appeared similar to intact FN in its support of fibroblast spreading, actin stress fiber assembly, and focal adhesion (FA) formation for as long as 2 hours, using both adult human dermal fibroblasts and mouse FN-null cells (Wang et al., 2005). Here, we extend the observations on FN-null cell responses to intact FN for time periods from 4 to 72 hours and responses to domains of FN that include the GST-tagged central cell-binding domain (GST-FNIII8–11) in context of a FN-GFBD (FNIII1–11 or FNIII8–v15) for the same time periods. FN-null cells plated on GST-FNIII8–11 displayed vinculin-positive FAs and actin stress fibers at 4 hours that were morphologically equivalent to those formed when cells were plated on FNIII1–11, or FNIII8–v15, although the spreading parameters were always best on intact FN (Figure 4a). At 24 hours, FN-null cells on all surfaces continued to demonstrate good spreading, vinculin-positive FAs, and actin stress fibers (Figure 4b). These cellular attributes appeared essentially equivalent among cells plated on GST-FNIII8–11, FNIII1–11, or FNIII8–v15, although cells plated on intact FN appeared to have more and larger FAs than cells plated on the recombinant FN domains. By 48 hours, FN-null cells cultured on GST-FNIII8–11 were less spread and demonstrated a loss of vinculin-positive FAs compared to the other surface conditions (Figure 4c). By 72 hours, FN-null cells cultured on GST-FNIII8–11 had become sparse. Although many microscopic fields of cultures where FN-null cells had been plated on GST-FNIII8–11 for 72 hours had no cells, the field shown in Figure 4d was selected for the presence of several cells. This was distinctly unusual. The few cells remaining attached to GST-FNIII8–11 coated plates were rounded and contained fragmented nuclei (Figure 4d). Cells plated on intact FN, FNIII1–11, or FNIII8–v15 continued to be mostly spread and have vinculin-positive FAs and well-formed actin stress fibers at 72 hours. Given that FN-null cells were well spread with good vinculin-positive FAs and actin stress fibers for at least 24 hours on FNIII8–11, the question arose as to whether a cellular abnormality could be identified that preceded the loss of FAs and ability to remain spread and attached to FNIII8–11-coated surfaces.
Figure 4. Fibronectin (FN)-null cells spread and formed focal contacts on FN-central cell-binding domain (FNIII8–11) similar to cells on intact FN or contiguous arrays of FN-CCBD and FN-growth factor-binding domains (GFBDs) (FNIII1–11 or FNIII8–v15) for at least 24 hours.
FN-null fibroblasts were cultured at 8,000 cells per 35-mm dish that had been precoated with 0.125 µm intact FN or glutathione S-transferase (GST)-tagged FNIII8–11, FNIII1–11, or FNIII8–v15 in DMEM for 4, 24, 48, or 72 hours at 37 °C. After the appropriate incubation times, cells were fixed, permeabilized, and stained for vinculin and actin as described in Materials and Methods. (a) FN-null cells had formed vinculin-positive focal contacts (green) and actin stress fibers (red) on all precoated surfaces by 4 hours. (b) Vinculin-positive focal contacts and actin stress fibers persisted in all cells at 24 hours. (c) By 48 hours, FN-null cells plated on GST-FNIII8–11 were less well spread and had only a few vinculin-positive focal contacts, whereas cells on intact FN, FNIII1–11, or FNIII8–v15 remained well spread with prominent actin stress fibers and many focal contacts. (d) By 72 hours, FN-null cells on dishes coated with GST-FNIII8–11 had become sparse and those that remained were mostly rounded with minimal evidence of actin bundles or focal contacts, whereas cells on intact FN, FNIII1–11, or FNIII8–v15 continued to be spread with prominent actin stress fibers and focal contacts. Bar = 20 µm.
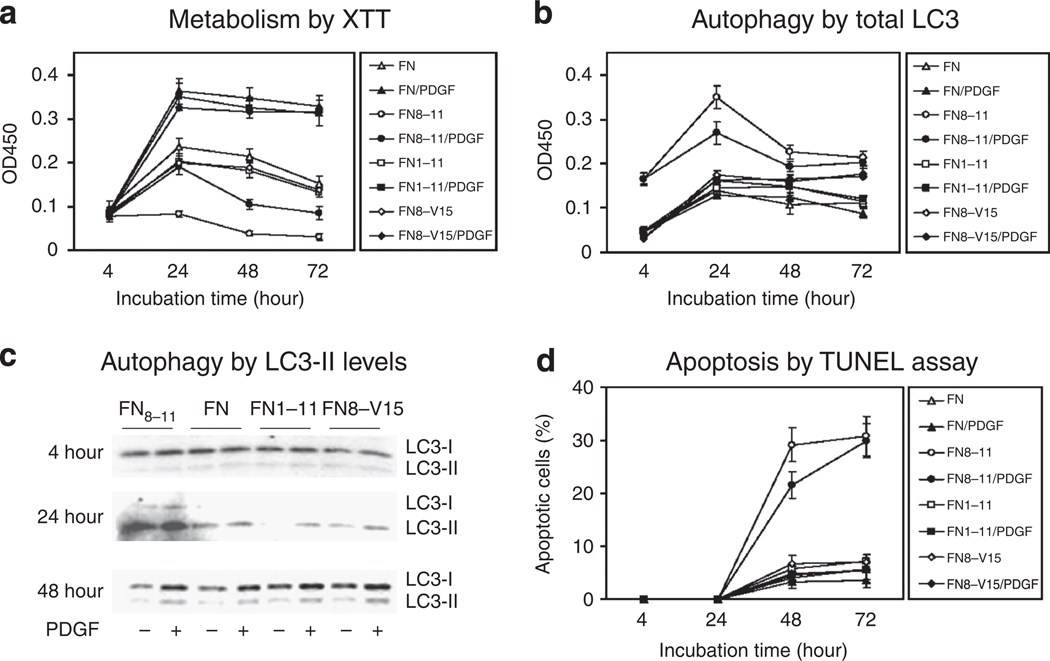
Fibroblasts undergo metabolic arrest, autophagy, and apoptosis in the absence of FN-GFBDs
To understand better why FN-null cells died in the absence of FN-GFBDs, cells were plated on GST-FNIII8–11 in the presence or absence of exogenous PDGF-BB and assayed for metabolic activity, autophagy, and apoptosis over a 72-hour time course. Although cell numbers at 24 hours were nearly identical among FN-null cells cultured on plates precoated with GST- FNIII8–11 and those cultured on intact FN, FNIII1–11, or FNIII8–V15, cells cultured on GST-FNIII8–11 in the absence of exogenous PDGF demonstrated no rise in metabolic activity at any time point, whereas cells cultured on the other substrates did (Figure 5a). Although absolute metabolic activity was increased in cells under all conditions by the addition of PDGF-BB, FN-null cells on GST-FNIII8–11 still demonstrated relatively low metabolic activity compared with other substrates. By 48 hours, the metabolism of PDGF-treated FN-null cells on GST-FNIII8–11 was 30% of PDGF-treated cells on intact FN. Although about one-third of this metabolic reduction could be attributed to decreased cell numbers (Figure 3a), the major deficiency could not. As PDGF-BB-stimulated proliferation requires an initial signaling event followed by a second signaling event 6–8 hours later (Jones and Kazlauskas, 2001), 30 ng ml−1 PDGF-BB was added at 4 hours after plating and again at 10 hours after plating to cells on GST-FNIII8–11. This maneuver, however, did not correct the poor metabolic response to PDGF-BB, nor improve FN-null cell survival (data not shown). Importantly, cells plated on either FNIII1–11 or FNIII8–V15 had essentially the same metabolic activity as cells plated on FN, in the absence and presence of exogenous PDGF-BB (Figure 5a).
Figure 5. Surface-bound fibronectin (FN)-growth factor-binding domains (GFBDs) contiguous with FN-central cell-binding domain (FNIII8–11) prevented metabolic arrest, autophagy, and apoptosis of FN-null cells even in the absence of platelet-derived growth factor (PDGF).
FN-null fibroblasts were cultured at 4,000 cells per well in 96-well plates precoated with 0.125 µm intact FN or glutathione S-transferase (GST)-tagged FNIII8–11, FNIII1–11, or FNIII8–v15 in DMEM for 4 hours, and then incubated with DMEM and 1% BSA with or without 30 ng ml−1 PDGF-BB at 37 °C for times indicated. (a) Metabolic response over 4 hours as judged by XTT assay. (b) Total cellular light chain 3 (LC3), a component of autophagosomes, was detected by rabbit polyclonal antibodies using ELISA (Jager et al., 2004). (c) LC3-II, a translationally modified product of LC3 that binds to autophagosomes, was detected by a size shift on western blot analysis using a polyclonal antibody specific for LC3 (Zhang et al., 2007). Alpha-actin was probed as a housekeeping protein in all gels and showed no substantial difference among samples within a given gel. (d) Apoptosis was determined by the TUNEL assay for fragmented DNA manifested by bright yellow fluorescent spots on a background of red 4′,6-diamidino-2-phenylindole-stained nuclei. Percent apoptosis was determined by counting those cells positive for DNA fragmentation, dividing by total cell number, and multiplying by 100. Fifty cells were counted in each of three replicate plates. Data points indicate mean ±SD. In panels a and b, each data point was performed in quadruplicate and each panel is representative of at least three different experiments. The western blot in panel c is representative of two different experiments. OD, optical density.
As FN-null cells cultured on GST-FNIII8–11 lacked metabolic activity in basal medium (DMEM) and had a poor metabolic response to PDGF-BB, we examined whether FN-null cells under these conditions developed signs of increased autophagy, a homeostatic self-phagocytic process that increases during times of cell stress such as nutrient or GF deprivation (Gozuacik and Kimchi, 2007). In fact, FN-null cells cultured on GST-FNIII8–11 showed signs of increased autophagy by 4 hours even in the presence of PDGF-BB, as judged by ELISA assay of total cellular light chain 3 (LC3-I plus LC3-II), a marker for autophagosome formation (Kabeya et al., 2000; Jager et al., 2004) (Figure 5b). Total LC3 peaked at 24 hours regardless of whether PDGF-BB had been added at 4 hours. By comparison, cells plated on FN, FNIII1–11, or FNIII8–V15 showed much less accumulation of LC3 at 24 hours and significantly less accumulation at all time points. The relatively low level of LC3 expression observed in these latter conditions was expected as autophagy is a physiological phenomenon that facilitates homeostatic processes such as protein degradation and organelle turnover (Gozuacik and Kimchi, 2007). Finally, western blots demonstrated increased LC3-II, a cleaved LC3-I product that is conjugated with phosphatidylethanolamine and bound to phagolysomes (Kabeya et al., 2000, 2004), in cells on FNIII8–11 at 24 hours (Figure 5c).
By 48 hours, substantial numbers of FN-null cells on FNIII8–11 were undergoing apoptosis (29 ± 3.2% without PDGF, 22 ± 2.5% with PDGF) (Figure 5d) as judged by the TUNEL assay. Clearly, cells cultured on GST-tagged FNIII8–11 for 2 days or longer were refractory to exogenous PDGF-BB rescue from apoptosis.
FN-null cell survival required at least one FN-GFBD
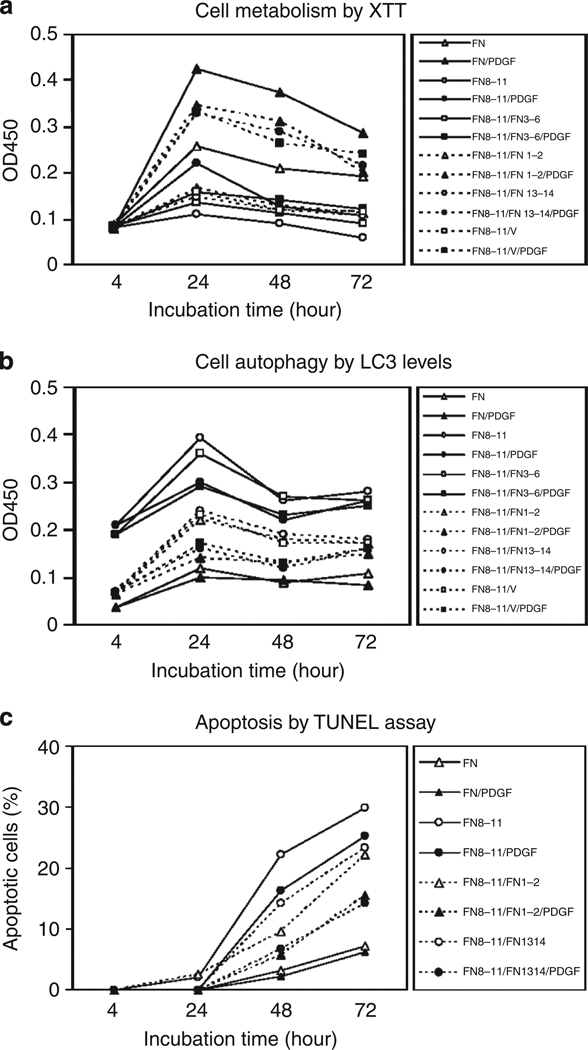
To determine whether FN-null cell survival and response to PDGF required contiguous FN-CCBD and -GFBDs, FN-null cells were plated on plastic surfaces precoated with 0.125 µm GST-tagged FNIII8–11 with or without 0.125 µm GST-tagged FN-GFBDs. DMEM was changed 4 hours later to DMEM + 1% BSA ± PDGF-BB and then incubated for up to 3 days. FN-null cells survived in the presence of PDGF-BB when cultured on GST-tagged FNIII8–11 with GST-tagged FN domains with PDGF-BB-binding activity, i.e., FNIII1–7, FNIII1–2, FNIII1, FNIII12–V15, FNIII12–15, or V, but did not survive on FNIII8–11 alone or on FNIII8–11 and FNIII3–6, a domain without PDGF-binding activity (Figure 6a). The surface density of FNIII3–6 adsorbed to plastic dishes was as high or higher than the FN-GFBDs as judged by ELISA (data not shown). Therefore, the negative results with FNIII3–6 were not attributable to poor surface adsorption. Hence, FN-null cell survival response to PDGF did not require contiguous FN-CCBD and -GFBDs. However, in the absence of PDGF-BB, FN-null cells on noncontiguous arrays of FN-CCBD (FNIII8–11) and FN-GFBDs did not survive, although these cells had survived on contiguous arrays of FN-CCBD and -GFBD (FNIII1–11 or FNIII8–V15) (Figure 3a). Importantly, differential response of FN-null cells plated on different FN domains to exogenous PDGF-BB was not secondary to differences in cell attachment as judged by cell counts 4 hours after plating (data not shown).
Figure 6. Surface-bound fibronectin (FN)-growth factor-binding domains (GFBDs) in noncontiguous arrays with the FN-central cell-binding domain (FNIII8–11) supported FN-null fibroblast responsiveness to platelet-derived growth factor-BB (PDGF-BB).
FN-null fibroblasts were cultured at 4,000 cells per well in 96-well plates precoated with 0.125 µm intact FN or glutathione S-transferase (GST)-tagged FN domains in DMEM for 4 hours, and then incubated with DMEM and 1% BSA with or without 30 ng ml−1 PDGF-BB at 37 °C for times indicated. (a) Survival as judged by the XTT assay. (b) Autophagy as judged by ELISA for light chain 3 (LC3). (c) Apoptosis as determined by the TUNEL assay. Percent apoptosis was determined by counting those cells positive for DNA fragmentation, dividing by total cell number, and multiplying by 100. Fifty cells were counted in three replicate plates. Data points indicate mean ± SD. The data shown in each of these panels are representative of at least three different experiments. All recombinant FN domains were GST-tagged but the prefix is omitted here for brevity. OD, optical density.
The findings above led us to investigate whether noncontiguous arrays of FN-GFBDs with FNIII8–11 required PDGF to prevent autophagy and apoptosis of FN-null cells. FN-null cells on GST-FNIII8–11 alone, or on noncontiguous arrays of GST-FNIII8–11 and GST-FNIII3–6, demonstrated increased levels of the autophagy marker LC3 by 4 hours, which increased further at 24 hours before returning to the 4-hour level by 48 hours, in the presence or absence of exogenous PDGF-BB (Figure 6b, solid lines, closed circles, and squares compared with open circles or squares, respectively). In contrast, cells on noncontiguous arrays of GST-GFBDs and GST-FNIII8–11 displayed little or no LC3 from 4 to 72 hours in the presence of exogenous PDGF-BB (Figure 5c, dotted lines, closed symbols), although modest increases in LC3 that peaked at 24 hours were observed in the absence of PDGF-BB (Figure 6b, dotted lines, open symbols). In concordance with the data in Figure 5b, cells on intact FN showed basal levels of autophagy whether PDGF-BB was present or not (Figure 6b, solid lines, closed-, and open triangles, respectively).
FN-null cells on GST-FNIII8–11 alone demonstrated increasing apoptosis over 3 days (30% at 3 days) (Figure 6c, solid line, open circles) and exogenous PDGF-BB weakly protected against this (25% at 3 days) (Figure 6c, solid line, closed circles). In the absence of PDGF-BB, FN-null cells on noncontiguous arrays of GST-FNIII8–11 and GST-GFBDs demonstrated less apoptosis (Figure 6c, dotted lines, open circles, and triangles). More striking, however, was the protection against apoptosis afforded to FN-null cells on noncontiguous arrays of GST-FNIII8–11 and GST-GF-binding domains with exogenous PDGF-BB (FNIII1–2, 15%; FNIII13–14, 14%, respectively, at 3 days) (Figure 6c, dotted lines, closed circles, and triangles).
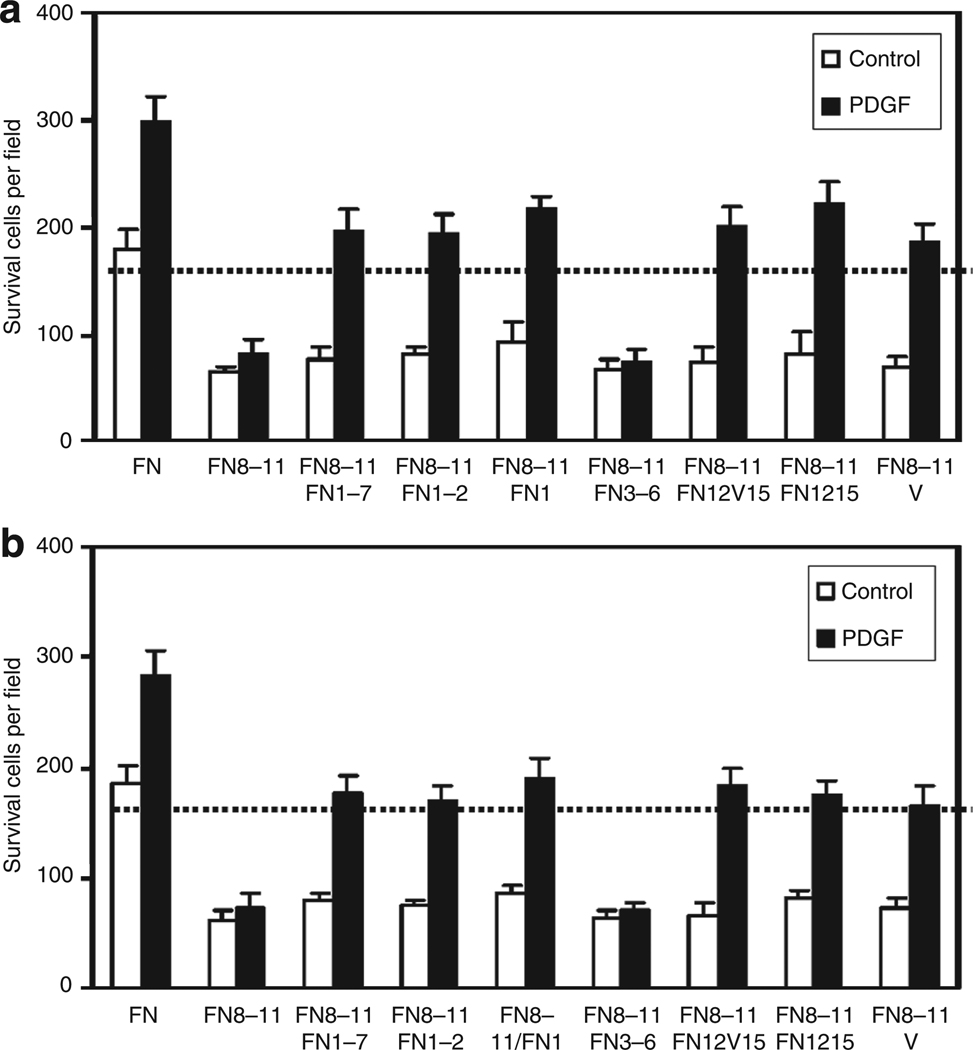
PDGF-BB preloaded on surface-bound FN-GFBD supported FN-null fibroblast survival
PDGF-BB in media supported FN-null fibroblast survival on GST-tagged FNIII8–11 and FN-GFBDs, i.e., FNIII1–7, FNIII1–2, FNIII1, FNIII12–V15, FNIII12–15, or V, but not on FNIII8–11 alone, nor on FNIII8–11 and FNIII3–6, after culture for 3 days (Figure 7a). Importantly, PDGF-BB also supported FN-null fibroblast survival after culture for 3 days when preloaded on FNIII8–11 and FN-GFBDs, but not when preincubated on FNIII8–11 alone, nor on FNIII8–11 and FNIII3–6 (Figure 7b).
Figure 7. Surface-bound fibronectin (FN)-growth factor-binding domains (GFBDs) supported FN-null fibroblast responsiveness to platelet-derived growth factor-BB (PDGF-BB) whether the PDGF-BB was present in the medium or preloaded on the FN-GFBD.
FN-null fibroblasts were cultured at 4,000 cells per well in 96-well plates precoated with 0.125 µm intact FN or glutathione S-transferase (GST)-tagged FN domains in DMEM for 4 hours, and then incubated in DMEM at 37°C for 3 days under the following conditions. (a) PDGF-BB was present in the medium during 3-day culture and then cells were counted. (b) PDGF-BB was preloaded on FN domain-coated dishes for 2 hours, washed 10 times, cells cultured as above, and then counted. Cells in three × 10 fields were counted in each of the four wells (mean ±SD, n = 12).
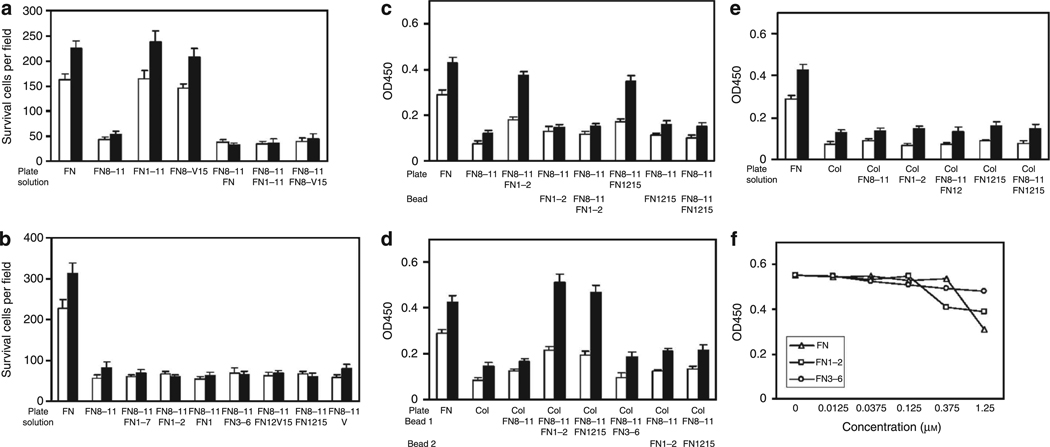
Intact FN and FN-GFBDs must be surface-bound to support FN-null cell survival and to promote PDGF-BB responsiveness
To determine whether FN-GFBD activity required their presence on a surface, we investigated whether intact FN or recombinant FN-GFBDs that were contiguous with the FN-CCBD (FNIII1–11 or FNIII8–v15) could support FN-null cell survival and/or promote PDGF-BB responsiveness when added to cultures in solution (DMEM). At 4 hours after FN-null cells had been plated on GST-FNIII8–11-coated wells, cells were treated with intact FN, FNIII1–11, or FNIII8–v15 in DMEM with or without 30 µg ml−1 PDGF-BB. All these conditions failed to promote survival of FN-null cells cultured on FNIII8–11 for 3 days (Figure 8a). As controls, FN-null cells were plated on intact FN, FNIII1–11, or FNIII8–v15 and 4 hours later exposed to exogenous PDGF-BB in DMEM or fresh DMEM alone. FN-null cells survived on these surfaces without PDGF-BB and proliferated in its presence (Figure 8a) in concert with results shown in Figure 3a and b. Likewise, shorter recombinant GST-GFBDs added in DMEM (solution) with or without PDGF-BB failed to promote FN-null cell survival on FNIII8–11 for 3 days (Figure 8b).
Figure 8. Fibronectin (FN)-null fibroblast response to platelet-derived growth factor-BB (PDGF-BB) required surface-bound FN or FN-growth factor-binding domains (GFBDs).
(a) FN-null fibroblasts were cultured at 10,000 cells per well in 48-well tissue culture plates precoated with 0.125 µm of intact FN or glutathione S-transferase (GST)-tagged fibronectin type III repeats (FNIII8–11), FNIII1–11, or FNIII8–V15, in DMEM for 4 hours, and then incubated in DMEM along with 1% BSA with or without 30 ng ml−1 PDGF-BB ±0.125 µm intact FN or FN domains in solution as indicated at 37 °C for 3 days. Cells in five × 10 fields were visually counted in each of the three wells at times indicated for mean ± SD (n=15). (b) FN-null fibroblasts were cultured at 4,000 cells per well in 96-well plates precoated with 0.125 µm intact FN or GST-FNIII8–11 in DMEM for 4 hours, and then incubated with DMEM and 1% BSA with or without 30 ng ml−1 PDGF-BB ± 0.125 µm GST-tagged FN domains in solution as indicated at 37 °C for 3 days. Cells were counted as described above. (c) FN-null cells were cultured at 4,000 cells per well in 96-well plates precoated with 0.125 µm intact FN or GST-FNIII8–11. Media were changed at 4 hours to DMEM and 1% BSA with or without 30 ng ml−1 PDGF-BB ± 0.125 µm GST-tagged FN domains on microbeads as indicated, and then cultures were incubated at 37°C for 3 days. Viability was measured by the XTT assay. (d) FN-null cells were cultured at 4,000 cells per well in 96-well plates precoated with 0.125 µm intact FN or type I collagen. Media were changed at 4 hours to DMEM and 1% BSA with or without 30 ng ml−1 PDGF-BB ± 0.125 µm GST-tagged FN domains on microbeads as indicated, and then cultures were incubated at 37°C for 3 days. Viability was measured by the XTT assay. (e) FN-null cells were cultured at 4,000 cells per well in 96-well plates precoated with 0.125 µm intact FN or type I collagen. Media were changed at 4 hours to DMEM and 1% BSA with or without 30 ng ml−1 PDGF-BB ± 0.125 µm GST-tagged FN domains in solution as indicated, then cultures were incubated at 37 °C for 3 days. Viability was measured by the XTT assay. (f) FN-null fibroblasts were cultured at 4,000 cells per well in 96-well plates precoated with 0.125 µm intact FN in DMEM for 4 hours and then incubated with DMEM and 1% BSA with or without intact FN, GST-FNIII1–2, or GST-FNIII3–6 in solution at the concentrations indicated with or without 30 ng ml−1 PDGF-BB at 37°C for 3 days. Viability was measured by XTT assay. SD are not shown here for clarity, but were <10% of mean (n= 4). Open bars = DMEM alone. Closed bars = DMEM plus 30 ng ml−1 PDGF-BB. OD, optical density.
Next, we investigated whether FN-GFBDs on the surface of beads added to culture medium could support the viability of FN-null cells plated on FNIII8–11. For these experiments, 0.125 µm cys-tagged FNIII1–2 or FNIII12–15 with or without cys-tagged FNIII8–11 were linked to agarose beads as described in Materials and Methods. Surprisingly, bead-bound recombinant FN-GFBDs, in the presence or absence of FNIII8–11, failed to promote FN-null cell viability, despite the fact that the same FN-GFDBs when used with FNIII8–11 to precoat plates did support cell viability (Figure 8c). It was possible, however, that insufficient numbers of cell surface receptors for the FN-CCBD (FNIII8–11) were available on the upper surface of cells plated on FNIII8–11, i.e., all such receptors may have already been ligated to FNIII8–11 on the plates. To examine whether this possibility was correct, FN-null cells were plated in tissue culture wells precoated with type I collagen and then incubated in the presence of PDGF with or without microbeads coated with either FNIII8–11 alone, GFBDs (FNIII1–2 or FNIII12–15) alone, or the combination of FNIII8–11 plus either FNIII1–2 or FNIII12–15 (Figure 8d). When FNIII8–11 and FN-GFBDs (either FNIII1–2 or FNIII12–15) were present on the same bead, FN-null cell viability was sustained in the presence of exogenous PDGF-BB. If beads were precoated with only one domain, cells died even in the presence of PDGF-BB. Importantly, FN-null cells also failed to survive on collagen if two sets of beads, one set precoated with FNIII8–11 and another set precoated with either FNIII1–2 or FNIII12–15, were added to cells simultaneously (Figure 8d). Given the data in Figure 8d, it was not surprising that FN-null cells cultured on plates precoated with type I collagen failed to survive when FNIII8–11 and GFBDs (either FNIII1–2 or FNIII12–15) in solution were added to the culture medium either individually or together (Figure 8e). These findings were not simply secondary to fluid-phase FN or FN-GFBDs inhibiting exogenous PDGF-BB activity on cultured cells, as FN-null cells plated on surface-bound intact FN responded similarly to PDGF-BB whether or not FN or FN-GFBDs were present in the medium until supraphysiological concentrations of intact FN were used (Figure 8f).
DISCUSSION
Here, for the first time, FN domains flanking the CCBD were found to bind PDGF-BB with KDs between 10 and 100 nm. Together, the FN-CCBD and FN-GFBDs were demonstrated to be necessary and sufficient for FN-null cell survival, and when contiguous, also necessary and sufficient for FN-null cell proliferation. Furthermore, cell survival activity required that FN-CCBD and FN-GFBDs be in proximity on the same surface.
FN and fibroblast survival
Previous studies have demonstrated that for survival some mesenchymal cells, e.g., Chinese Hamster Ovary cells, require integrin α5β1 binding to FN (Zhang et al., 1995), and by extrapolation, ligation of the Pro–His–Ser–Arg–Asn and RGD-binding sites within the ninth and tenth type III repeats of FN, respectively (Pankov and Yamada, 2002). Thus, it was initially surprising that the FN-CCBD (FNIII8–11) did not support FN-null cell survival. Even more surprising was the observation that exogenous PDGF-BB could not rescue FN-null cells cultured on FNIII8–11 from apoptosis, as PDGF-BB is known to be an important survival factor for fibroblasts (Franke et al., 1997; Romashkova and Makarov, 1999). That FN-null cell survival and responsiveness to exogenous PDGF required surface-bound FN-GFBDs suggested that an intimate interaction of FN and PDGF-BB with the appropriate fibroblast surface receptors may be requisite for the generation of appropriate survival signals (Figure 9).
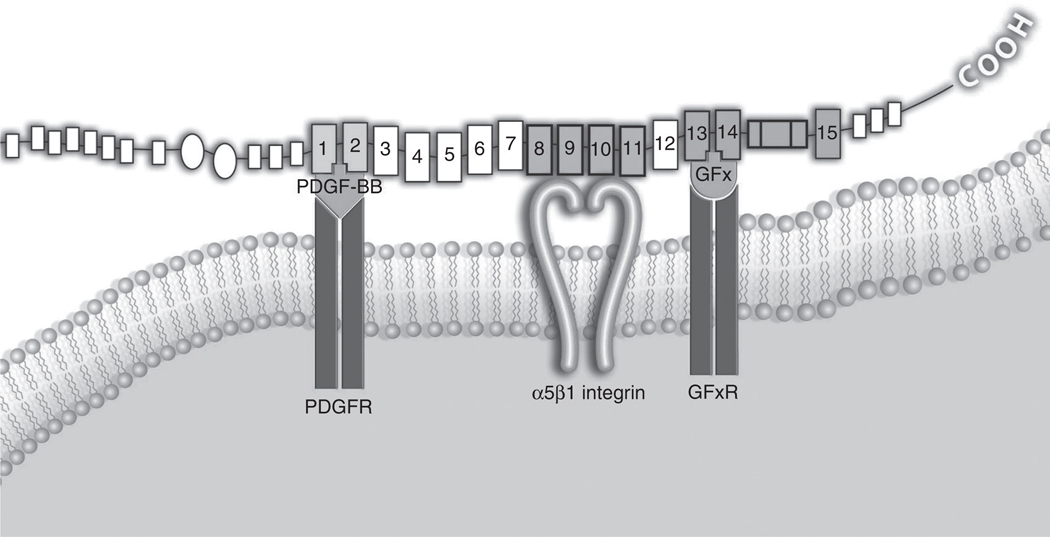
Figure 9. Proposed mesenchymal cell-extracellular matrix (ECM) synapse.
Transmembrane α5β1 integrin binds to the fibronectin (FN) central cell-binding domain (fibronectin type III repeats (FNIII8–11) and is flanked by a platelet-derived growth factor (PDGF) receptor and a putative growth factor x receptor (GFxR), which are binding their respective ligands that in turn are bound to two of the GF-binding sites of FN, herein indicated arbitrarily by the carboxy-terminal portion of FNIII1 and FNIII13. PDGF-BB, platelet-derived growth factor-BB; PDGFR, platelet-derived growth factor receptor.
Although it is known that integrins and GFs can be costimulatory for fibroblasts (Plopper et al., 1995; Miyamoto et al., 1996), it has not been previously reported that fibroblast survival requires a coordinate interaction of FN and PDGF-BB, or other GFs. On further consideration, the fact that mesenchymal cell survival requires coordinate interaction of FN with integrins and PDGF-BB with PDGF receptors in proximity is not dissimilar to the concept of T-cell multidimensional signaling network stimulation through the immunological synapse and costimulatory pairs in the same nanospace (Tseng and Dustin, 2002). As signal transduction pathways are often under strict, solid-state control inside the cell (Saltiel and Pessin, 2002), initiators of signaling outside the cell may be under the same kind of spatial control, rather than the result of simple diffusion. Future studies, using immunocytological techniques, such as fluorescence resonance energy transfer analysis, are needed to determine whether the proximity of FN-CCBD and its ligand α5β1 with PDGD-β receptors and its ligand PDGF-BB is at the nanospace level.
The data presented here clearly demonstrate that FN-GFBDs, in addition to the FN-CCBD (Renshaw et al., 1997), were required for FN-null cell survival. Nevertheless, FN-null cells plated on FNIII8–11 could and did respond to exogenous PDGF-BB, although suboptimally, as demonstrated by increased metabolism and decreased autophagy. In preliminary studies not shown in this paper, PDGF-β receptor was hypophosphorylated in response to PDGF-BB when FN-null cells were cultured on FN-CCBD (FNIII8–11) without FN-GFBDs. The dependence on FN-GFBD for FN-null cell survival or PDGF-BB responsiveness was further constrained by the requirement of the FN-GFBDs to be surface bound.
Although FN-GFBDs in noncontiguous arrays with FNIII8–11 required exogenous PDGF-BB for their prosurvival activity, contiguous arrays of FNIII8–11 and FN-GFBDs did not require the presence of PDGF-BB to promote survival. In the latter situation, however, PDGF-BB induced a growth response, whereas in the former situation it did not. These differential levels of PDGF-BB responsiveness were not simply a manifestation of the need for two distinct phases of PDGF-BB signaling (Jones and Kazlauskas, 2001), as 30 ng ml−1 PDGF-BB added at 4 and 10 hours to cells on FNIII8–11 did not correct the poor metabolic response to PDGF-BB nor improve FN-null cell survival. Thus, FN-null cell responsiveness to PDGF-BB was a gradient function of surface-bound FN domains (FNIII8–11 < noncontiguous arrays of FNIII8–11 and FN-GFBDs < contiguous arrays of FNIII8–11 and FN-GFBDs). Exogenous PDGF-BB could partially protect FN-null cells plated on the FN-CCBD (FNIII8–11) against cytolysis and autophagy and promote a weak metabolic response; however, these cells eventually underwent apoptosis. When FN-null cells were cultured on noncontiguous arrays of FNIII8–11 and FN-GFBDs, exogenous PDGF-BB had a greater protective affect against cytolysis and autophagy, and promoted a stronger metabolic response. More importantly, and perhaps as a consequence, FN-null cells on noncontiguous arrays of FNIII8–11 and FN-GFBDs responded to PDGF-BB with increased survival and decreased apoptosis. Finally, when FN-null cells were cultured on intact FN or on contiguous arrays of FNIII8–11 and FN-GFBDs, they survived without PDGF-BB and proliferated in its presence. These findings suggested that there are either GF-independent survival sequences within the larger recombinant FN polypeptides, or perhaps loading of minute, but strategically localized, amounts of endogenous PDGF can occur on contiguous arrays of FN cell- and GF-binding domains, but not on noncontiguous arrays of these domains.
FN and fibroblast FA formations
Previous studies have indicated that the FN-CCBD is insufficient for FA formation and that the major heparin-binding domain (HepII) facilitates FA formation (Woods et al., 1986; Morgan et al., 2007) in a RhoA-dependent manner (Ridley and Hall, 1992; Ren et al., 1999). More recently, however, we demonstrated that the HepII requirement for FA formation can be overcome by increased surface density of the CCBD (FNIII8–11) (Wang et al., 2005). This was accomplished by GST-tagging FNIII8–11, which greatly improved surface adsorption of the recombinant protein. When plated on GST-FNIII8–11, both normal human dermal fibroblasts and FN-null cells attached, spread, and formed FA, in a manner indistinguishable from cells plated on intact human plasma FN, for up to 2 hours (Wang et al., 2005). Here, we have expanded these observations to 3 days. At 4 hours, FN-null cells formed FA on GST-FNIII8–11 that continued to be indistinguishable from cells plated on intact human plasma FN. At 24 hours, the FA formation on GST-FNIII8–11 was clearly evident, although less robust than on intact FN. FA formation on recombinant FNIII1–11 and FNIII8–v15, which included the FN-GFBDs, appeared similar to FA formation on GST-FNIII8–11 alone. However, by 48 hours, the FN-null cell FA formation on GST-FNIII8–11 had dissipated, whereas FA in FN-null cells plated on intact FN, or on either recombinant FNIII1–11 or FNIII8–v15, were sustained. This should not be surprising as FA depends on the activation of RhoA (Wang et al., 2005), which, unlike Rac1 and Cdc42, is exquisitely sensitive to GTP and ATP depletion (Hallett et al., 2003), and FN-null cells cultured on GST-FNIII8–11 demonstrated a metabolic block.
FN and fibroblast metabolism and autophagy
Cells require a constant supply of oxidizable nutrients for survival. Cells in multicellular organisms meet this need through two fundamental mechanisms (Lum et al., 2005). GF stimulation induces uptake of glucose and amino acids to support ATP generation and biosynthesis. If this fails, cells lower their biosynthetic demand, but still need sufficient nutrients to support basic functions such as adhesion and survival. To sustain themselves such cells begin to degrade intracellular molecules. An extreme form of this catabolic degradation is autophagy, a process in which cells sequester macromolecules and cell organelles into acidic vacuoles for bulk digestion. These vacuoles, or autophagolysosomes, arise from the entrapment of cell components within a double-membrane structure that forms around them and then fuses with lysosomes. The molecular processes involved in autophagolysosome formation are evolutionarily conserved from yeast to man, and require an orchestrated recruitment of specific proteins and lipoproteins to the double membrane as it expands and engulfs cell constituents (Xie and Klionsky, 2007). If autophagy fails to satisfy the cell’s need for nutrients, then autophagy can segue to apoptosis (Yoshimori, 2007). This is the first report to demonstrate that surface-bound FN was required for GF-stimulated metabolism. Furthermore, this activity was attributable to FN-GFBDs that prevent induction of autophagy and subsequent apoptosis.
Multiple FN-GFBDs
Given the apparent importance of FN-GFBDs to fibroblast survival, it is reasonable that multiple FN-GFBDs might exist. However, the possibility of redundant binding sites raises the questions of their explicit molecular localization and of possible similarities in their sequence pattern. If fact, using bioinformatics screening techniques to search for such patterns, we have discovered two peptides within FNIII1, one peptide within FNIII13, and one peptide within V that have similar sequence patterns and binds PDGF-BB with similar KD as their parent domains. The complete elucidation of these sites, their PDGF-BB-binding activities, and their bioactivities are the current focus of our laboratory.
GF binding to FN and other ECM molecules
PDGF-BB is one of an expanding number of GFs that are bound and retained by FN, either directly or indirectly. GFs that directly bind FN include VEGF (Wijelath et al., 2002, 2004, 2006), hepatocyte growth factor (Rahman et al., 2005), tumor necrosis factor-α (Alon et al., 1994), and connective tissue growth factor (Yoshida and Munakata, 2007). Those that bind indirectly include IGF via IGF-binding proteins (Gui and Murphy, 2001), and transforming growth factor-β via latent transforming growth factor-β-binding protein-1 (Dallas et al., 2005). As FN is a key component of the provisional matrix of wounds (Welch et al., 1990), its ability to bind and localize GFs helps it to serve as a vital modulator of biological activity during repair and regeneration. Although these previous studies have shown that GFs bound to FN retain activity, GF–FN interactions have not previously been demonstrated to be required for cell survival.
GFs also can bind to ECM molecules other than FN, as previously reviewed (Macri et al., 2007). Fibrin(ogen), heparan sulfate, decorin, FN, and vitronectin are just a few of the many molecules located in the provisional matrix and/or ECM that can bind and modulate the activity of various GFs. However, binding of any of these GFs to ECM molecules has not been previously shown to be essential for cell survival (Macri et al., 2007).
Conclusion
Here, we demonstrated for the first time that FN-GFBDs were critical for fibroblast survival and were required for fibroblast proliferative response to PDGF-BB. Furthermore, we found that the FN-CCBD (FNIII8–11) and FN-GFBDs must be affixed to the same surface to promote cell survival in the presence of exogenous PDGF-BB. That cell survival and responsiveness to PDGF required surface-bound FN-GFBD in proximity to the FN-CCBD suggested that an intimate interaction of FN and PDGF-BB with the appropriate cell surface receptors may be requisite for the generation of appropriate survival signals (Figure 9). In fact, the absence of FN in nonhealing chronic wounds and burns (Grinnell and Zhu, 1994; Herrick et al., 1996) may be the reason why they fail to heal and/or demonstrate progressive tissue destruction, respectively. Although FN itself is exquisitely sensitive to proteolytic digestion, its functional domains are relatively resistant to such degradation (Yamada and Clark, 1996; Pankov and Yamada, 2002). Thus, a tissue-engineered construct composed of FN domains that are necessary and sufficient for fibroblast survival and growth and a relatively proteolytically resistant matrix to which they are tethered may be of great benefit for chronic nonhealing wounds, acute wounds that are too large to close, or extensive burns (Clark, 2008b). In addition, these finding are important to our further understanding of cell-matrix interactions, i.e., perhaps FN signaling, apart from that generated directly by integrin receptor binding, is mediated indirectly through its ability to localize GFs to the matrix and trigger GF-mediated signaling concurrently.
MATERIALS AND METHODS
Materials
Recombinant PDGF-BB was obtained from Biosource (Camarillo, CA), and 125I-PDGF-BB from Amersham Biosciences (Piscataway, NJ). Human plasma FN, purchased from Chemicon (Temecula, CA), was 99% pure and intact as determined by SDS-PAGE. FN fragment (70 kDa) and DMEM was obtained from Sigma (St Louis, MO). SulfoLink coupling gel was acquired from Pierce. Fatty acid-free BSA was purchased from ICN Biomedicals (Aurora, OH). Ni-NTA agarose beads were acquired from Qiagen (Valencia, CA). Isopropyl-beta-d-thiogalactopyranoside and L-broth were purchased from Fisher Scientific (Fair Lawn, NJ) and fetal bovine serum from Hyclone (Logan, UT). Culture plates were from BD Bioscience (Billerica, MA). Cell Proliferation kit II (XTT) and In Situ Cell Death Detection kit (TUNEL reaction mixture) were from Roche Diagnostics (Indianapolis, IN). The BIAcore 2000, CM5 sensor chips, N-hydroxysuccinimide, N-ethyl-N′-(3-diethylaminopropyl)carbodiimide hydrochloride, and 1 m ethanolamine (pH 8.5) were purchased from BIAcore (Piscataway, NJ). Surface plasmon resonance experiments were performed using a BIAcore 2000 at the University Proteomics Center at Stony Brook University. Mouse monoclonal anti-vinculin antibody (V9131) was purchased from Sigma. Oregon Green 488 goat anti-mouse IgG(H + L) (O11033), Alexa Fluor 488 phalloidin, and 4′,6-diamidino-2-phenylindole were acquired from Molecular Probes, Invitrogen (Carlsbad, CA).
Expression and purification of FN functional domains
Methods for cloning, expression, and purification of recombinant human FN domains and fusion products of FN domains with GST were previously published (Wang et al., 2005). A GST tag on the amino-terminus of the recombinant proteins facilitated FN domain adsorption onto tissue culture plastic for cell function studies. We also previously described cloning, expression, and purification of cys-tagged FN domains (Ghosh et al., 2006). Cys-tagged FN domains allowed linkage to SulfoLink agarose beads with minimal impact on protein conformation. Briefly, FN domains were generated by PCR using the human cDNA clones pFH1, pFH111, and pFH154 (ATCC) as templates or by subcloning the restriction enzyme fragments from each of the aforesaid plasmids. FN domains were cloned in pETCH, a modified pCal-n vector (Stratagene, La Jolla, CA), with a carboxy-terminal six-histidine affinity tag with or without a carboxy-terminal cysteine, and with or without a amino-terminal GST tag. The inserts were cloned at the BamHI and HindIII sites, and confirmed by DNA sequencing to rule out possible synthesis errors during PCR. Protein expression was induced in the BL21DE3LysS strain of E. coli by the addition of 0.5 mm isopropyl-beta-d-thiogalactopyranoside and affinity purified using Ni-NTA agarose beads. After elution with a buffer containing 300 mm imidazole and 200 mm NaCl, the proteins were desalted by passing over a Sephadex G25 column (Amersham Pharmacia Biotech AB, Uppsala, Sweden) in the presence of phosphate buffered saline (PBS). In recombinant constructs that contained the FN variable domain, all 120 residues were included (V120).
Equilibrium binding of PDGF-BB with FN or FN domains
FN domains were conjugated to agarose beads via –SH groups according to the manufacturer’s protocol (SulfoLink Coupling Gel; Pierce). To block nonspecific binding, agarose beads conjugated with or without FN or FN domains were incubated with 2% BSA at room temperature for 2 hours. For equilibrium binding, 20 µl of conjugated-agarose beads were incubated with varying concentrations of 125I-labeled PDGF-BB in 100 µl of binding buffer (DMEM+1% BSA) at room temperature for 2 hours with rotation. The beads were then washed six times with PBS, and the radioactivity bound to the agarose beads was quantified using a γ-counter. The binding constant of PDGF-BB with FN or FN domains was determined using Prism 4 nonlinear regression software (GraphPad, San Diego, CA).
Immobilization of PDGF-BB on sensor chips
PDGF-BB was coupled to CM5 sensor chip surfaces using a standard amine coupling procedure and a flow rate set at 5 µl minute−1 . Sequential injections consisted of a 0.05 m N-hydroxysuccinimide and 0.2 m N-ethyl-N′-(3-diethylaminopropyl) carbodiimide hydrochloride mixture (10 µl), followed by PDGF-BB (10 µgm−1) in 10mm acetic acid (pH 4.0) until the desired amount of coupled PDGF was reached. A solution of 0.1m ethanolamine HCl (35 µl, pH 8.5) was then used to block the remaining activated carboxyl groups. Dextran-coated chips were used as controls.
Kinetic binding assay of PDGF-BB with FN domains
In the BIAcore 2000 system, plasmon resonance system determines real-time protein–protein interaction allowing determination of koff and kon, the ratio of which is KD. The kinetic binding constants were determined by passing varying concentrations of FN functional domains (analytes) dissolved in 20 mm Hepes, 150 mm NaCl, 3.4 mm EDTA, and 0.05% Tween-20, pH 7.4, over chip surfaces that had been coupled with PDGF. All kinetic experiments were carried out at 20°C at a flow rate of 30µl minute−1 . For mass transport experiments, each analyte was injected at a fixed concentration and run at flow rates ranging from 5 to 75 µl minute−1 . All analytes were injected over a PDGF-coupled surface, as well as over a control surface for 120 seconds, followed by 300 seconds of dissociation in running buffer. Regeneration of the sensor chip for subsequent injections was accomplished by one pulse of 0.05% SDS (30 seconds).
Data preparation and analysis for kinetic binding assay
Sensorgrams were prepared and globally fit using nonlinear least-squares analysis and numerical integration of the differential rate equations using the BIAcore manual. Briefly, each sensorgram generated using a control surface was subtracted from the corresponding experimental sensorgram, and the resulting curves were transformed to concentration units using the molecular mass of the injected species, the equivalence of 1,000 resonance units per 1 ng mm−2, and a matrix thickness of 100 nm. Each data set, which consisted of a series of sensorgrams from injections of different concentrations of analyte over the same surface type, was then analyzed using kinetic models that are available in the BIAevaluation software (GE Healthcare Bio-Science Biacore, Piscataway, NJ).
Cell culture
Generation of mouse FN-null fibroblasts was described previously (Saoncella et al., 1999). The cells were maintained in DMEM supplemented with 100 Uml−1 penicillin, 100 µg ml−1 streptomycin, and 10% fetal bovine serum at 37°C and 5% CO2/95% air in a humidified atmosphere. For the cell functional assays, the FN-null cells were grown to ~80% confluence, harvested, and centrifuged to obtain a pellet that was rinsed once with DMEM. After resuspension in DMEM, cells were counted and transferred to tissue culture plates in the appropriate numbers for a given assay.
Cell survival assays
Cell survival experiments were carried out on 48- or 96-well plates that had been coated with 0.125 µm of FN, other ECM protein, or FN domains in PBS overnight at room temperature (Wang et al., 2005). After blocking with 2% BSA for 2 hours at room temperature to exclude further protein binding, the plates were washed three times with PBS. For each 48- or 96- well plate, 10,000 or 4,000 FN-null cells per well, respectively, were seeded in DMEM and incubated at 37°C for 4 hours. Then, BSA to a final concentration of 1% was added to each well, with or without PDGF-BB or FN domains, and the wells were incubated for varying time periods. Cell survival was measured by counting cells and by determining cell mitochondrial metabolism using the XTT assay (Roche Diagnostics).
FN-null cell endogenous PDGF
To establish whether FN-null cells were producing and secreting appreciable amounts of either PDGF-AA or PDGF-BB, the levels of these GFs were determined in FN-null cell lysates and in cell culture medium by ELISA using anti-mouse PDGF-AA and anti-mouse PDGF-BB antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). The detection limits for both PDGF-AA and PDGF-BB was 0.1 ng ml−1. To determine cellular PDGF-AA and PDGF-BB, 1 × 106 cells per ml FN-null cells were dissolved in lysis buffer containing 1% Triton X-100, 0.1% SDS, 1% BSA, and proteinase inhibitors. After removing particulates by centrifugation, supernatants were tested by ELISA. Results showed that lysates of cells, which had been incubated in serum-free DMEM for 48 hours, contained 0.34 ng per 106 cells of PDGF-AA and 0.28 ng per 106 cells of PDGF-BB. Negligible PDGF-AA and PDGF-BB had accumulated in the culture medium during this time period.
TUNEL assay
Apoptotic cells were identified by nick-end labeling using In Situ Cell Death Detection kit and TMR red as per the manufacturer’s protocol. Briefly, eight-well cell culture chambers were coated with 0.125 µm of FN or FN domains in PBS overnight at room temperature. The chambers were then blocked with 2% BSA for 2 hours, followed by a triple wash with PBS. For each well, 8,000 FN-null cells were seeded in DMEM and incubated at 37°C for 4 hours, followed by the addition of BSA to a final concentration of 1% in the presence or absence of 30 ng ml−1 PDGF-BB, unless otherwise stated. At the end of the various incubations, cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, probed with TUNEL reaction mixture, and cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole. Specimens were imaged in PBS with a × 20, aperture 0.4 objective on an inverted Diaphot-TMD Nikon (Nikon, Melville, NY) fluorescent microscope using a CCD camera and Metamorph software (Molecular Devices, MDS Analytical Technologies, Sunnyvale, CA).
Analysis of the autophagosome protein LC3
The assay for total LC3 followed the manufacturer’s protocol for Cellular Activation of Signaling ELISA (CASE) kits from Superarray (Fredrick, MD). All reagents were from the Superarray CASE kit except the polyclonal anti-LC3 antibody, which was purchased from Santa Cruz Biotechnology.
For western blots of LC3I and LC3II, cells were harvested and homogenized in 10 mm Tris buffer, pH 8.0, containing 1% Triton X-100, 0.1% SDS, 140 mm NaCl,1% sodium acetate, 10 µg ml−1 leupeptin, 10 µg ml−1 aprotinin, and 0.1 mm phenylmethylsulfonyl fluoride. Nuclei were removed by low-speed centrifugation. Post-nuclear fraction protein/lane (10 µg) was subjected to 15% SDS-PAGE. Separated proteins were transferred onto nitrocellulose membrane, blocked with 5% (w/v) dry milk, and incubated with primary polyclonal antibodies specific for LC3 (Novus Biologicals, Littleton, CO) overnight at 4°C. After washing, the blots were incubated with a secondary anti-rabbit IgG antibody labeled with peroxidase. The blots were developed by chemiluminescence and images were collected using Chemiimager 4400 system (Imgen Technologies, Alexandria, VA).
Cell staining and imaging
Eight 35-mm-well plate (Becton Dickinson, Franklin Lakes, NJ) surfaces were precoated using 0.125 µm FN or FN domains, maintained overnight at room temperature, and then blocked using 2% (w/v) BSA at room temperature for 2 hours. Each well was rinsed three times with PBS, and then FN-null fibroblasts were seeded at 40,000 cells per ml (8,000 cells in 200 µl serum-free DMEM for each well), and incubated at 37 °C. After 4, 24, 48, or 72 hours incubation time periods, cells were fixed with 4% paraformaldehyde in PBS for 15 minutes, permeabilized with 0.4% Triton X-100 in PBS for 5 minutes, and blocked with 2% BSA in PBS for 30 minutes at room temperature. FAs were stained at room temperature with anti-vinculin antibody for 1 hour, followed by Oregan Green 488 goat anti-mouse IgG for 1 hour. Actin was counterstained with Alexa Fluor 546 phalloidin. After washing, cells in residual PBS were immediately overlaid with a coverslip and imaged for fluorescence with a × 40, aperture 0.9, oil objective on a Leica TCS SP2 laser scanning confocal microscope (Leica Microsystems, Wetzlar, Germany). All images were recorded with a CCD camera using the same resolution (1024 × 1024 pixel).
ACKNOWLEDGMENTS
This work was support by the following grants: NA10143 Merit Award and Armed Forces Institute of Regenerative Medicine (RAFC) and NSF MRSEC (MR). We thank Deane F. Mosher for invaluable advice and providing the FN-null cells used in this paper.
Abbreviations
- ECM
extracellular matrix
- FN
fibronectin
- FN-CCBD
fibronectin-central cell-binding domain
- FN-GFBD
fibronectin-growth factor-binding domain
- FNIII
fibronectin type III repeats
- FNIII8–11
fibronectin central cell-binding domain
- GF-binding
growth factor-binding
- GST
glutathione S-transferase
- KD
dissociation constant
- RGD
Arg–Gly–Asp
- LC3
light chain 3
- V
fibronectin variable domain
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Alon R, Cahalon L, Hershkoviz R, et al. TNF-alpha binds to the N-terminal domain of fibronectin and augments the beta 1-integrin-mediated adhesion of CD4+ T lymphocytes to the glycoprotein. J Immunol. 1994;152:1304–1313. [PubMed] [Google Scholar]
- Aota K, Nomizu M, Yamada KM. The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J Biol Chem. 1994;269:24756–24761. [PubMed] [Google Scholar]
- Assoian RK, Schwartz MA. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr Opin Genet Dev. 2001;11:48–53. doi: 10.1016/s0959-437x(00)00155-6. [DOI] [PubMed] [Google Scholar]
- Betsholtz C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 2004;15:215–228. doi: 10.1016/j.cytogfr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Clark RA. Oxidative stress and “Senescent” fibroblasts in non-healing wounds as potential therapeutic targets. J Invest Dermatol. 2008a;128:2361–2364. doi: 10.1038/jid.2008.257. [DOI] [PubMed] [Google Scholar]
- Clark RA. Synergistic signaling from extracellular matrix-growth factor complexes. J Invest Dermatol. 2008b;128:1354–1355. doi: 10.1038/jid.2008.75. [DOI] [PubMed] [Google Scholar]
- Clark RAF, Winn HJ, Dvorak HF, et al. Fibronectin beneath reepithelializing epidermis in vivo: sources and significance. J Invest Dermatol. 1983;80 suppl:26S–30S. [PubMed] [Google Scholar]
- Dallas SL, Sivakumar P, Jones CJ, et al. Fibronectin regulates latent transforming growth factor-beta (TGF beta) by controlling matrix assembly of latent TGF beta-binding protein-1. J Biol Chem. 2005;280:18871–18880. doi: 10.1074/jbc.M410762200. [DOI] [PubMed] [Google Scholar]
- Feng Y, Mrksich M. The synergy peptide PHSRN and the adhesion peptide RGD mediate cell adhesion through a common mechanism. Biochemistry. 2004;43:15811–15821. doi: 10.1021/bi049174+. [DOI] [PubMed] [Google Scholar]
- Franke TF, Kaplan DR, Cantley LC, et al. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- Fredriksson L, Li H, Eriksson U. The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 2004;15:197–204. doi: 10.1016/j.cytogfr.2004.03.007. [DOI] [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, et al. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Ghosh K, Ren XD, Shu XZ, et al. Fibronectin functional domains coupled to hyaluronan stimulate adult human dermal fibroblast responses critical for wound healing. Tissue Eng. 2006;12:601–613. doi: 10.1089/ten.2006.12.601. [DOI] [PubMed] [Google Scholar]
- Ginsberg M, Pierschbacher MD, Ruoslahti E, et al. Inhibition of fibronectin binding to platelets by proteolytic fragments and synthetic peptides which support fibroblast adhesion. J Biol Chem. 1985;260:3931–3936. [PubMed] [Google Scholar]
- Gozuacik D, Kimchi A. Autophagy and cell death. Curr Top Dev Biol. 2007;78:217–245. doi: 10.1016/S0070-2153(06)78006-1. [DOI] [PubMed] [Google Scholar]
- Greiling D, Clark RAF. Fibronectin provides a conduit for fibroblast transmigration from a collagen gel into a fibrin gel. J Cell Sci. 1997;110(Part 7):861–870. doi: 10.1242/jcs.110.7.861. [DOI] [PubMed] [Google Scholar]
- Grinnell F, Zhu M. Identification of neutrophil elastase as the proteinase in burn wound fluid responsible for degradation of fibronectin. J Invest Dermatol. 1994;103:155–161. doi: 10.1111/1523-1747.ep12392625. [DOI] [PubMed] [Google Scholar]
- Gui Y, Murphy LJ. Insulin-like growth factor (IGF)-binding protein-3 (IGFBP-3) binds to fibronectin (FN): demonstration of IGF-I/IGFBP-3/fn ternary complexes in human plasma. J Clin Endocrinol Metab. 2001;86:2104–2110. doi: 10.1210/jcem.86.5.7472. [DOI] [PubMed] [Google Scholar]
- Hallett MA, Dagher PC, Atkinson SJ. Rho GTPases show differential sensitivity to nucleotide triphosphate depletion in a model of ischemic cell injury. Am J Physiol Cell Physiol. 2003;285:C129–C138. doi: 10.1152/ajpcell.00007.2003. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- Herrick SE, Ireland G, Simon D, et al. Venous ulcer fibroblasts compared with normal fibroblasts show differences in collagen but not fibronectin production under both normal and hypoxic conditions. J Invest Dermatol. 1996;106:187–193. doi: 10.1111/1523-1747.ep12329920. [DOI] [PubMed] [Google Scholar]
- Ilic D, Almeida EA, Schlaepfer DD, et al. Extracellular matrix survival signals transduced by focal adhesion kinase suppress p53-mediated apoptosis. J Cell Biol. 1998;143:547–560. doi: 10.1083/jcb.143.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager S, Bucci C, Tanida I, et al. Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci. 2004;117:4837–4848. doi: 10.1242/jcs.01370. [DOI] [PubMed] [Google Scholar]
- Jones SM, Kazlauskas A. Growth-factor-dependent mitogenesis requires two distinct phases of signalling. Nat Cell Biol. 2001;3:165–172. doi: 10.1038/35055073. [DOI] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Yamamoto A, et al. LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J Cell Sci. 2004;117:2805–2812. doi: 10.1242/jcs.01131. [DOI] [PubMed] [Google Scholar]
- Litvinovich SV, Brew SA, Aota S, et al. Formation of amyloid-like fibrils by self-association of a partially unfolded fibronectin type III module. J Mol Biol. 1998;280:245–258. doi: 10.1006/jmbi.1998.1863. [DOI] [PubMed] [Google Scholar]
- Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nat Rev Mol Cell Biol. 2005;6:439–448. doi: 10.1038/nrm1660. [DOI] [PubMed] [Google Scholar]
- Macri L, Silverstein D, Clark RAF. Growth factor binding to the pericellular matrix and its importance in tissue engineering. Adv Drug Deliv Rev. 2007;59:1366–1381. doi: 10.1016/j.addr.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Mao Y, Schwarzbauer JE. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 2005;24:389–399. doi: 10.1016/j.matbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- McClain SA, Simon M, Jones E, et al. Mesenchymal cell activation is the rate limiting step of granulation tissue induction. Am J Pathol. 1996;149:1257–1270. [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Gutkind JS, et al. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J Cell Biol. 1996;135:1633–1642. doi: 10.1083/jcb.135.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MR, Humphries MJ, Bass MD. Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol. 2007;8:957–969. doi: 10.1038/nrm2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher DF. Organization of the provisional fibronectin matrix: control by products of blood coagulation. Thromb Haemost. 1995;74:529–533. [PubMed] [Google Scholar]
- Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- Pierschbacher MD, Ruoslahti E. Influence of stereochemistry of the sequence Arg-Gly-Asp-Xaa on binding specificity in cell adhesion. J Biol Chem. 1987;262:17294–17298. [PubMed] [Google Scholar]
- Plopper GE, McNamee HP, Dike LE, et al. Convergence of integrin and growth factor receptor signaling pathways within the focal adhesion complex. Mol Biol Cell. 1995;6:1349–1365. doi: 10.1091/mbc.6.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Patel Y, Murray J, et al. Novel hepatocyte growth factor (HGF) binding domains on fibronectin and vitronectin coordinate a distinct and amplified Met-integrin induced signalling pathway in endothelial cells. BMC Cell Biol. 2005;6:8. doi: 10.1186/1471-2121-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redick SD, Settles DL, Briscoe G, et al. Defining fibronectin’s cell adhesion synergy site by site-directed mutagenesis. J Cell Biol. 2000;149:521–527. doi: 10.1083/jcb.149.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw MW, Ren XD, Schwartz MA. Growth factor activation of MAP kinase requires cell adhesion. EMBO J. 1997;16:5592–5599. doi: 10.1093/emboj/16.18.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991;87:1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Johnson KJ, Murozono M, et al. Plasma fibronectin supports neuronal survival and reduces brain injury following transient focal cerebral ischemia but is not essential for skin-wound healing and hemostasis. Nat Med. 2001;7:324–330. doi: 10.1038/85471. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Pessin JE. Insulin signaling pathways in time and space. Trends Cell Biol. 2002;12:65–71. doi: 10.1016/s0962-8924(01)02207-3. [DOI] [PubMed] [Google Scholar]
- Saoncella S, Echtermeyer F, Denhez F, et al. Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and actin stress fibers. Proc Natl Acad Sci USA. 1999;96:2805–2810. doi: 10.1073/pnas.96.6.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng SY, Dustin ML. T-cell activation: a multidimensional signaling network. Curr Opin Cell Biol. 2002;14:575–580. doi: 10.1016/s0955-0674(02)00370-8. [DOI] [PubMed] [Google Scholar]
- Wang R, Clark RA, Mosher DF, et al. Fibronectin’s central cell-binding domain supports focal adhesion formation and Rho signal transduction. J Biol Chem. 2005;280:28803–28810. doi: 10.1074/jbc.M501421200. [DOI] [PubMed] [Google Scholar]
- Welch MP, Odland GF, Clark RAF. Temporal relationships of F-actin bundle formation, collagen and fibronectin matrix assembly, and fibronectin receptor expression to wound contraction. J Cell Biol. 1990;110:133–145. doi: 10.1083/jcb.110.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijelath ES, Murray J, Rahman S, et al. Novel vascular endothelial growth factor binding domains of fibronectin enhance vascular endothelial growth factor biological activity. Circ Res. 2002;91:25–31. doi: 10.1161/01.res.0000026420.22406.79. [DOI] [PubMed] [Google Scholar]
- Wijelath ES, Rahman S, Murray J, et al. Fibronectin promotes VEGF-induced CD34 cell differentiation into endothelial cells. J Vasc Surg. 2004;39:655–660. doi: 10.1016/j.jvs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- Wijelath ES, Rahman S, Namekata M, et al. Heparin-II domain of fibronectin is a vascular endothelial growth factor-binding domain: enhancement of VEGF biological activity by a singular growth factor/matrix protein synergism. Circ Res. 2006;99:853–860. doi: 10.1161/01.RES.0000246849.17887.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Couchman JR, Johansson S, et al. Adhesion and cytoskeletal organization of fibroblasts in response to fibronectin fragments. EMBO J. 1986;5:665–670. doi: 10.1002/j.1460-2075.1986.tb04265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- Yamada KM, Clark RAF. Provisional matrix. In: Clark RAF, editor. Molecular and Cellular Biology of Wound Repair. 2nd edn. New York: Plenum; 1996. pp. 51–93. [Google Scholar]
- Yoshida K, Munakata H. Connective tissue growth factor binds to fibronectin through the type I repeat modules and enhances the affinity of fibronectin to fibrin. Biochim Biophys Acta. 2007;1770:672–680. doi: 10.1016/j.bbagen.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Yoshimori T. Autophagy: paying Charon’s toll. Cell. 2007;128:833–836. doi: 10.1016/j.cell.2007.02.023. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yu J, Pan H, et al. Small molecule regulators of autophagy identified by an image-based high-throughput screen. Proc Natl Acad Sci USA. 2007;104:19023–19028. doi: 10.1073/pnas.0709695104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Vuori K, Reed JC, et al. The alpha 5 beta 1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc Natl Acad Sci USA. 1995;92:6161–6165. doi: 10.1073/pnas.92.13.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]