Summary
Eosinophils are leukocytes resident in mucosal tissues. During Th2-type inflammation, eosinophils are recruited from bone marrow and blood to the sites of immune response. While eosinophils have been considered end-stage cells involved in host protection against parasite infection and immunopathology in hypersensitivity disease, recent studies changed this perspective. Eosinophils are now considered multifunctional leukocytes involved in tissue homeostasis, modulation of adaptive immune responses, and innate immunity to certain microbes. Eosinophils are capable of producing immunoregulatory cytokines and are actively involved in regulation of Th2-type immune responses. However, such new information does not preclude earlier observations showing that eosinophils, in particular human eosinophils, are also effector cells with pro-inflammatory and destructive capabilities. Eosinophils with activation phenotypes are observed in biological specimens from patients with disease, and deposition of eosinophil products is readily seen in the affected tissues from these patients. Therefore, it would be reasonable to consider the eosinophil a multifaceted leukocyte that contributes to various physiological and pathological processes depending on their location and activation status. This review summarizes the emerging concept of the multifaceted immunobiology of eosinophils and discusses the roles of eosinophils in health and disease and the challenges and perspectives in the field.
Keywords: eosinophils, humans, mice, inflammation, hypersensitivity
1. Introduction
The eosinophil granulocyte, although likely first observed by Wharton Jones in 1846 in unstained preparations of peripheral blood, was so named by Paul Ehrlich in 1879 because of the intense staining of its granules with the acidic dye eosin (1). Since that time the eosinophil has been the subject of extensive investigation. Its occurrence in such disparate conditions as parasitic infections (presumably for the benefit of the human host) and hypersensitivity diseases (perhaps to the detriment of the patient) has become better understood as a consequence of newer information. Eosinophils are resident in various organs such as the gastrointestinal tract, mammary glands and bone marrow, and they may play roles in tissue and immune homeostasis of these organs. In Th2-type immune response, eosinophils are recruited into sites of inflammation where they produce an array of cytokines and lipid mediators and release toxic granule proteins. These molecules may regulate immune responses, cause tissue damage, and facilitate tissue repair. Eosinophils can also present antigens to naïve and memory T cells and initiate/amplify antigen-specific immune responses. This review summarizes the biological and immunological properties of eosinophils and discusses the roles of eosinophils in tissue homeostasis, immune regulation, and innate and adaptive immunity. This review is not intended to cover the entire field of eosinophil biology but to provide insights into and conceptual understandings of eosinophils in various stages of activation and to discuss their roles in health and disease. Outstanding recent reviews provide detailed information regarding the basic biology of eosinophils and their products (1–3).
2. Eosinophils at baseline condition
2.1. Eosinophils are resident in several tissues at baseline condition
The life cycle of the eosinophil is divided into bone marrow, blood, and tissue phases. Eosinophils are produced in bone marrow from pluripotential stem cells. The stem cells differentiate into a progenitor, which is capable of giving rise to mixed colonies of basophils and eosinophils, pure basophil colonies, or pure eosinophil colonies. Production of eosinophils involves a cascade of interdependent regulatory events of at least three classes of transcription factors, including GATA-1 (a zinc family finger member), PU.1 (an ETS family member), and C/EBP members (CCAAT/enhancer-binding protein family) (4). Specifically, PU.1 determines distinct cell lineage fates, with low levels inducing lymphocytic cells and high levels myeloid differentiation (5). GATA-1 and PU.1 synergistically induce eosinophil lineage differentiation (6). Of these transcription factors, GATA-1 is likely the most important for eosinophil lineage because mice with a targeted deletion of the high-affinity GATA-binding site show a specific loss of eosinophils (7). Among various hematopoietic factors, those important for eosinophil proliferation and differentiation are interleukin (IL)-3, granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-5. IL-3 and GM-CSF are relatively nonspecific and stimulate proliferation of neutrophils, basophils, and eosinophils. In contrast, IL-5 potently and specifically stimulates eosinophil production (8).
Although the eosinophil is a formed element of the peripheral circulation, it is primarily a tissue-dwelling cell. In healthy individuals, most eosinophils are found in the gut (but not the esophagus), mammary gland, uterus, thymus and bone marrow; the gastrointestinal eosinophil is the predominant population of eosinophils (9). At baseline condition, eosinophils are present in the gastrointestinal tract independent of adaptive immunity and enteric flora, and the eosinophil levels are regulated by the constitutive expression of eotaxin-1 and eosinophil chemokine receptor, CCR3 (10, 11). Eosinophils also home into the thymus, mammary gland, and uterus, as controlled by eotaxin-1 (12).
2.2. Potential homeostatic roles for eosinophils
Although the roles of eosinophils in these organs at baseline condition are not fully understood, eotaxin-1-deficient mice show a two-week delay in the onset of estrus, along with a delay in the first age of parturition, suggesting a role for eosinophils in preparing the mature uterus for pregnancy and in blastocyst implantation (13). Deletion of the eotaxin-1 gene also resulted in reduction in terminal end bud formation and reduced branching complexity of the ductal tree, suggesting a role for eosinophils in postnatal mammary gland development (12). In addition, eosinophils may have evolved to maintain epithelial barrier integrity (14) and/or innate host defense in the gastrointestinal tract (15) as discussed more in detail below.
Another role for eosinophils at baseline condition may include maintenance of immune homeostasis. Thymic eosinophils express major histocompatibility complex (MHC) class I and costimulatory molecules such as CD86 and CD30L (16). Thymic eosinophils are also positive for CD11b/CD11c and show decreased expression of CD62L and increased expression of CD25 and CD69, suggesting that they have an activated phenotype. These eosinophils are postulated to be involved in MHC class I-restricted thymocyte deletion because their anatomical localization within discrete compartments of the thymus coincides with negative selection of double-positive thymocytes. Indeed, increased numbers of thymic eosinophils associated with apoptotic bodies were found in MHC class I-restricted male (H–Y) antigen T cell receptor (TCR) transgenic mice following cognate antigen treatment. Eosinophils also likely play roles in long-term maintenance of plasma cells in bone marrow in mice (17). In bone marrow, eosinophils localize together with plasma cells. Eosinophils support survival of plasma cells by secreting a proliferation inducing ligand (APRIL) and IL-6, and depletion of eosinophils induces apoptosis in long-lived bone marrow plasma cells. Plasma cell numbers were decreased in eosinophil-deficient mice and this defect was restored by eosinophil reconstitution. Altogether, these results suggest that a steady-state presence of eosinophils at baseline condition may play an important role in the morphogenesis and maintenance of mucosal organs as well as in immune homeostasis of the thymus and bone marrow.
3. Immunoregulatory roles of eosinophils
Previously, eosinophils have been considered as end-stage effector cells. However, accumulating evidence suggests that eosinophils can perform various immune regulatory functions likely through presentation of antigens and production and release of a range of cytokines and other immunomodulatory molecules.
3.1. Eosinophils present antigens
Eosinophils possess the ability to internalize, process and present antigenic peptides within the context of surface-expressed major MHC II, the capacity to provide co-stimulatory signals to T cells through surface expression of molecules such as CD80, CD86 and CD40 and the ability to physically interact with CD4+ T cells (18). Antigen-pulsed eosinophils administered intratracheally into mice migrate to draining lymph nodes and localize primarily within T cell zones (19, 20). Similarly, following airway allergen challenge of mice, eosinophils traffic to and accumulate within draining lymph nodes, where they upregulate MHC II, CD86, and CD54 (21). Murine eosinophils process and present antigen to T cell clones and hybridomas (22) and to antigen-primed and naïve CD4+ T cells in vitro (23). Furthermore, antigen-pulsed eosinophils instilled intratracheally into recipient mice were sufficient for the expansion of Th2 cells within draining lymph nodes in vivo (24). In humans, although circulating eosinophils from healthy donors are generally devoid of surface MHC II expression, they are induced to express MHC II (25) and costimulatory molecules (26, 27) with appropriate cytokine stimulation and after transmigration through endothelial cell monolayers (28). Human eosinophils also constitutively express a Notch ligand, Jagged1 (29), suggesting a capacity of eosinophils to provide a polarization signal to naïve CD4+ T cells (30).
3.2. Production of cytokines and other immunomodulatory molecules by eosinophils
Eosinophils are a source of a number of regulatory or pro-inflammatory cytokines and chemokines (3, 31). For example, eosinophils produce cytokines which are able to act on eosinophils themselves, the so-called "autocrine" cytokines, including IL-3 and GM-CSF (32, 33). Other eosinophil-derived cytokines can be divided into two major groups, those affecting tissue cells and those influencing other immune cells. Human eosinophils infiltrating intestinal tissues and eosinophils from patients with eosinophilia express transforming growth factor-α (TGF-α) and TGF-β1 (34, 35). Eosinophils from nasal polyps also express TGF-β1, suggesting that TGF-β1 synthesis by eosinophils may contribute to the structural abnormalities of nasal polyps, such as stromal fibrosis and basement thickening (36). Indeed, eosinophil-derived TGF-β enhances proliferation and collagen synthesis of lung and dermal fibroblasts (37). TGF-α produced by cytokine-activated eosinophils increases mucin production by airway epithelial cells (38). Other profibrotic and angiogenic factors, such as osteopontin (39), vascular endothelial cell growth factor (VEGF), and metalloproteinases (MMPs) are produced by human eosinophils (40) (41). Furthermore, eosinophils produce and release nerve growth factor (NGF) and promote the extension of neurites in nerve cells (42). Thus, a growing body of evidence demonstrates the ability of eosinophils to influence tissue cells, leading to remodeling of tissues and changes in their physiological properties (e.g. hyperreactivity to exogenous stimuli).
By producing cytokines and chemokines, eosinophils may modulate the functions of other immune cells. Human eosinophils can produce IL-4 (43, 44), and IL-4 protein has been localized to eosinophils in airway (45) and skin (43) tissue specimens from patients with allergic diseases. Furthermore, when stimulated with eotaxin (CCL11) or RANTES (CCL5), human eosinophils rapidly release stored IL-4 by vesicular transport to the local milieu (46). Several other cytokines and chemokines are also transcribed and/or produced by eosinophils (3, 31); for example, by RT-PCR, constitutive expression of IL-6 and IL-10 mRNA was detected in blood eosinophils (44, 45, 47, 48). Eosinophils also express a proinflammatory cytokine, TNF-α, and chemokines, such as MIP-1α (CCL3) and RANTES (CCL5) (49, 50). Murine eosinophils were shown to produce APRIL, IL-6 (17) and TGF-β1 (51). Thus, eosinophils can provide a strikingly wide variety of cytokines and chemokines, suggesting that eosinophils are potentially involved in diverse biological responses, from tissue remodeling to activation of resident and infiltrating immune cells.
In addition to producing these cytokines, eosinophils secrete mediators with the potential to promote Th2-type immune responses. Indoleamine 2,3-dioxygenase (IDO) is an enzyme that catalyzes the oxidative catabolism of tryptophan to kynurenines. Kynurenines inhibit proliferation and promote preferential apoptosis of Th1 cells (52). Human eosinophils from the blood of allergic donors constitutively express IDO (53). Another immunomodulatory factor generated by human eosinophils is one of their granule proteins, namely eosinophil-derived neurotoxin (EDN) (see below for more details). EDN is an RNase A superfamily member and, in addition to its antiviral properties, EDN is a chemoattractant (54) and activator (55) of dendritic cells (DCs). As a consequence, EDN enhances Th2 responses through a TLR2-dependent mechanism (56).
3.3. Immunoregulatory functions of eosinophils in vivo
The immunomodulatory functions of eosinophils in vivo are demonstrated in murine models of allergen sensitization and challenge with ovalbumin (OVA) and helminth infection. Eosinophil recruitment into the sites of Th2-type inflammation was considered previously a result of activation of adaptive immune responses that produced IL-5 and eotaxins (2). However, in vivo studies with helminth infection models revealed that an early influx of eosinophils into inflammation sites precedes that of lymphocytes (57–59) and that it occurs even in mice deficient in adaptive immunity (60, 61). Notably, in IL-5/eotaxin double-knockout mice, in which eosinophil numbers in both blood and tissues are severely decreased, IL-13 production of Th2 cells in response to OVA challenge is attenuated. This defect in Th2 cells was restored by eosinophil reconstitution (62), suggesting regulation of an adaptive Th2-type immune response by eosinophils.
The roles of eosinophils in Th2-type airway inflammation were subsequently addressed directly by using eosinophil-deficient animals. Lee et al (63) depleted eosinophils by using an eosinophil-specific promoter to drive expression of a cytocidal protein, diphtheria toxin A chain (PHIL mice). In comparison, Yu et al (7) developed mice harboring a deletion of a high-affinity GATA-binding site in the GATA-1 promoter (Δdbl-GATA), which led to the specific ablation of the eosinophil lineage. When sensitized and challenged with OVA, Th2-type airway inflammation and asthma-like pathology (e.g. airway hyperreactivity and mucus production) were attenuated in both PHIL mice and Δdbl-GATA mice, and these responses were restored by reconstitution of eosinophils alone (64) or a combination of eosinophils and antigen-specific T cells (65). Likewise, airway production of Th2 cytokines and asthma-like pathology were diminished in Δdbl-GATA mice exposed intranasally to a product of the fungus Aspergillus fumigatus (66). Although the mechanisms to explain how eosinophils modulate Th2 responses are not fully understood, the deficiency in eosinophils resulted in attenuated airway production of chemokine, such as CCL7, CCL11, CCL17, CCL22, and CCL24 (64, 65), and decreased expression of genes regulating the coagulation cascade (66), suggesting that eosinophils may be involved in priming of the tissue environment for effective mobilization of Th2 cells. It will be important to dissect whether these chemokines are derived from eosinophils themselves or from other immune cells or tissue cells interacting with eosinophils.
4. Effector functions of eosinophils
As summarized in the reviews (1–3), eosinophils contain numerous highly basic and cytotoxic granule proteins that are released upon activation. They also produce an arsenal of enzymes and lipid mediators, which are implicated in effector functions of eosinophils.
4.1. Granule Proteins
Human eosinophil granules contain major basic protein (MBP), MBP2, eosinophil cationic protein (ECP), eosinophil peroxidase (EPO), EDN, and β-glucuronidase. Human MBP is a 13.8kD single polypeptide rich in arginine and five unpaired cysteines with a calculated pI of 11.4 (67). It has weak sequence identity (23–28%) with C-type lectin domains of mannose-binding protein and the lectin domain of the low-affinity IgE receptor, FcεRII (68). MBP is translated as a 23- to 25.2-kD proMBP with a calculated pI of 6 to 6.2. The 9.9-kD propiece of proMBP might protect the cell during transport of cytotoxic MBP from the Golgi apparatus to the eosinophil granule (69). Because human MBP binds to and disrupts the schistomula's membrane, it directly damages S. mansoni (1). MBP is cytotoxic against other helminths, including T. spiralis newborn larvae, Brugia pahangi, Brugia malayi microfilariae, and Trypanosoma cruzi, and certain bacteria, such as Staphylococcus aureus and Escherichia coli. Human MBP is also toxic to tumor cells and other mammalian cells by disrupting the integrity of lipid bilayers (70). MBP2, the recently discovered MBP homolog, has a calculated pI of 8.7 and is about 100 times less basic than MBP (71). MBP and MBP2 have identity in 42 of the approximately 117 total amino acids. MBP2 has effects similar to MBP in cell killing and neutrophil (superoxide anion production and IL-8 release) and basophil (histamine and leukotriene C4 release) stimulation assays, but likely with reduced potency.
Human ECP is a basic protein, pI 10.8, and consists of a single polypeptide chain of 15.5 kD (1, 72). The ECP amino acid sequence has 32% identity to human pancreatic ribonuclease (RNase) A. ECP possesses RNase activity and is also known as RNase-3. In general, the RNase activity of ECP is required for the neurotoxic and antiviral properties of the protein, but not for the antibacterial and antihelminthic activities. ECP is also a potent toxin for parasites including schistosomula of S. mansoni, newborn larvae of T. spiralis, and microfilariae of B. pahangi and B. malayi. Importantly, both EDN and ECP show ribonuclease-dependent antiviral activity (73, 74).
Human EDN is a powerful neurotoxin that can severely damage myelinated neurons in experimental animals (1). With ECP, EDN belongs to the pancreatic RNase A superfamily and is also known as RNase-2 or RNase Us ; it is also found in human liver, lung and spleen (75). EDN shows 36% amino acid sequence homology with RNase A and marked amino acid sequence homology (67%) with ECP. However, EDN has about 100 times more RNase activity than ECP. EDN shows significant toxicity against newborn larvae of T. spiralis. Interestingly, pancreatic RNase by itself does not produce neurotoxicity when injected intrathecally into rabbits (76), nor is it toxic to newborn larvae of T. spiralis. EDN and ECP have antiviral activities and decrease the infectivity in RSV group B suspensions (74, 77). When purified eosinophils were added to RSV viral suspensions, the viral titers were reduced; addition of EDN or ECP had a similar effect and was dependent upon the ribonuclease activity in the EDN and ECP preparations (73, 77). Interestingly, ribonuclease A lacked this antiviral activity, suggesting that ribonuclease activity is necessary but not sufficient for the anti-viral effects of EDN and ECP. Furthermore, in guinea pigs infected with parainfluenza, pretreatment with anti-IL-5 and reduction of eosinophils strikingly increased the viral content in the airways (78), suggesting a potential role for eosinophils in viral immunity.
EPO is a member of a mammalian peroxidase family. EPO consists of two subunits, a heavy chain of 50 to 58 kD and a light chain of 10.5 to 15.5 kD in a 1:1 stoichiometry, and it has an isoelectric point greater than 11 (76). Myeloperoxidase (from neutrophils and monocytes) in the presence of H2O2 and halide kills bacteria, viruses, Mycoplasma, and fungi. EPO shows similar antimicrobial activity, but it prefers bromide over chloride. Furthermore, at plasma concentrations of bromide (20–120 µM) and thiocyanate (20–100 µM), both hypobromous acid and oxidation products of thiocyanate are produced by EPO (79). EPO is a central participant in generating reactive oxidants and radical species by activated eosinophils (80). Eosinophil activation in vivo shows oxidative damage of proteins through bromination of tyrosine residues (81). Furthermore, eosinophils are a major source of nitric oxide-derived oxidants in specimens from patients with severe asthma (82). EPO plus H2O2 and halide (Cl−, Br−, or I−) kills not only a variety of microorganisms, such as E. coli, schistosomula, microfilariae of B. pahangi and B. malayi, trypanosoma, toxoplasma, and mycobacteria, but also mast cells and tumor cells. Furthermore, binding of EPO to microbes such as S. aureus, T. cruzi, and Toxoplasma gondii markedly potentiates their killing by mononuclear phagocytes.
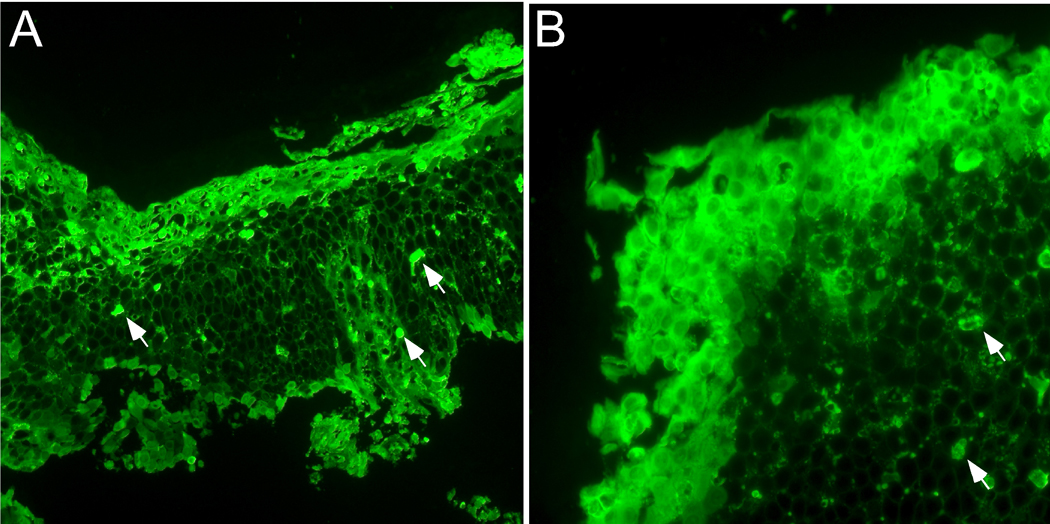
Considerable evidence exists to link these eosinophil granule proteins and human diseases. For example, the concentrations of MBP in the bronchial alveolar lavage (BAL) fluids from patients with asthma and from monkeys are correlated with the severity of bronchial hyperreactivity (83, 84). MBP has been localized to damaged sites of bronchial epithelium in patients with asthma and chronic rhinosinusitis (85, 86). Instillation of human MBP and human EPO provokes bronchoconstriction, and MBP increases airway responsiveness to inhaled methacholine (83). Interestingly, polyglutamic acid antagonizes MBP’s ability to increase respiratory resistance and bronchial hyperreactivity in cynomolgus monkeys (87), suggesting that the cationic nature of MBP contributes to the damage and physiologic changes. In vitro, MBP acts as an antagonist for M2 muscarinic receptors. Many eosinophils localized close to nerves with extracellular MBP adhering to the nerves in human airway tissues (88). Finally, neutralization of endogenously secreted MBP, either with a polyanionic peptide or with antibodies to MBP, can prevent antigen-induced bronchial hyperreactivity in guinea pigs (89). Marked deposition of free EDN is also observed in affected tissues from patients with eosinophilic esophagitis (EoE) (90) (Figure 1). Deposition of EDN is reduced in certain patients with EoE who are treated with anti-IL-5 antibody (91). Future studies are necessary to dissect whether localization of these eosinophil granule proteins in mucosal organs simply reflects eosinophil infiltration, whether they play important roles in the pathophysiology of disease, and if that is the case, how they cause immunopathology.
Figure 1.
Deposition of EDN in esophageal tissue from patients with eosinophilic esophagitis (EoE). Esophageal biopsy specimens from two separate EoE patients (A and B) were stained with rabbit anti-human EDN antibody followed by FITC-conjugated goat anti-rabbit IgG. Wide spread diffuse extracellular EDN is observed throughout the esophageal epithelium, in particular on the luminal surface and in the superficial mucosa. Arrows indicate intact eosinophils. Original magnification; ×160 (A) and ×400 (B).
4.2. Lipid mediators and other inflammatory molecules
In general, PLA2 hydrolyzes membrane phospholipids, phosphatidylcholine (PC), and/or phosphatidylethanolamine (PE) to produce arachidonic acid (AA) and lyso-PC and/or lyso-PE. AA may be metabolized by cyclooxygenase or 5-lipoxygenase/5-lipoxygenase-activating protein (FLAP) into prostaglandins or leukotrienes, as well as lipoxins and 5-hydroxyeicosatetraenoic acid (5-HETE). Lyso-PC is acetylated to form platelet activating factor (PAF) (92). In eosinophils, the predominant metabolite via the 5-lipoxygenase pathway is LTC4, which, in turn, is metabolized to LTD4 and the less active LTE4 (1). This finding is in contrast to neutrophils, which can produce large amounts of LTB4 but little, if any, LTC4. These mediators contract airway smooth muscle, promote secretion of mucus, alter vascular permeability, and elicit eosinophil and neutrophil infiltration. Eosinophils contain high levels of ether phospholipids (the stored precursor to PAF), about fourfold more than neutrophils, suggesting that the eosinophil is a good PAF producer (93). PAF has a number of important pharmacologic activities, including the activation of platelets and neutrophils and induction of bronchoconstriction.
Several proinflammatory enzymes have been associated with the eosinophil (1). Arylsulfatase B is located predominantly in the small granules of the eosinophil. β-glucuronidase activity in eosinophils is about twice that in neutrophils, and exposure of eosinophils to opsonized zymosan particles releases up to 24% of the total cellular β-glucuronidase. Eosinophils are a major source of the 92-kD MMP-9 (gelatinase B) (94, 95). This gelatinase is also localized on eosinophils infiltrating into the lesions of patients with bullous pemphigoid (96), and it cleaves type XVII collagen, a transmembrane molecule of the epidermal hemidesmosome. Furthermore, MMP-9 is likely required during eosinophil migration (41, 97).
4.3. Activation of human eosinophils takes multiple stages
In early 1980’s, increased numbers of unusual human eosinophils with a specific gravity <1.085 g/ml (98) were reported in peripheral blood of patients with eosinophilic disorders, such as hypereosinophilic syndrome (99) and asthma (100). These eosinophils, called “hypodense eosinophils”, were highly reactive to stimuli and showed increased survival, adhesion, leukotriene synthesis, superoxide production and antibody-dependent cytotoxicity as compared to “normodense eosinophils” (101, 102). Thus, eosinophils in human blood are not a homogenous population but represent various magnitudes of activation. Subsequent studies showed that exposure of eosinophils to activating cytokines, such as IL-3, IL-5, and GM-CSF, leads to the development of hypodense eosinophils.
IL-3, IL-5, and GM-CSF, besides being growth and maturation factors for eosinophils, stimulate several functions of mature human eosinophils. Among human peripheral blood leukocytes, eosinophils are the only cells having detectable levels of IL-5 receptors, consistent with the specific action of IL-5 on human eosinophils (103, 104). Importantly, IL-5 synergistically enhances the chemotactic response of eosinophils toward chemokines or lipid mediators (105, 106). IL-5 also activates LTC4 and superoxide generation, phagocytosis, and helminthotoxic activity, as well as immunoglobulin (Ig)-induced degranulation (107). GM-CSF and IL-3 also activate and enhance eosinophil functions, such as cytotoxic killing, superoxide production, leukotriene production, phagocytosis of serum-opsonized zymosan, and Ig-induced degranulation (108). Other Th2 cytokines, such as IL-4 and IL-13, also activate eosinophils. IL-4 upregulates the binding of eosinophils to IgA (109). IL-4 or IL-13 act synergistically with TNF-α or IL-5 for increased expression of CD69 (110). Another Th2 cytokine, IL-9, may act synergistically with these eosinophil-active cytokines during cellular development. The addition of IL-9 to CD34+ cells cultured in IL-3 and IL-5 enhances eosinophil development (111), likely through upregulation of the expression of IL-5R alpha chain on human CD34+ cord blood progenitor cells (112). A prototypic Th1 cytokine, IFN-γ, activates eosinophils, but does so in a delayed manner (113). For example, in short-term cytotoxicity assays, eosinophils are maximally activated by GM-CSF followed in order of potency by IL-3, IL-5, TNF-α, and IL-4; whereas, after a 24-hour incubation, maximal eosinophil activation is caused by IFN-γ followed by GM-CSF, IL-3, and IL-5. The proinflammatory cytokines, TNF-α, IL-33 and TSLP, also modulate eosinophil functions. TNF-α prolongs eosinophil survival in vitro, enhances eosinophil synthesis of LTC4, increases eosinophil toxicity towards S. mansoni larvae, and increases eosinophil adhesion for endothelial cells (108). Although TNF-α itself has only modest activity toward eosinophils compared with the eosinophil hemopoietins (e.g., IL-5), the activity of TNF-α synergizes with that of IL-5 (114). IL-33 and TSLP are cytokines produced by tissue cells, such as airway epithelium, and they are implicated in Th2-type immune responses (115). Both TSLP (116) and IL-33 (117) activate eosinophil effector functions such as adhesion to matrix proteins, cytokine production and degranulation. Although TSLP enhances survival of mature eosinophils, IL-33 is devoid of this activity. Interestingly, IL-33 does not affect neutrophil functions (117), suggesting that IL-33 may regulate functions of eosinophils specifically in airway mucosa.
Little information is available regarding cytokines and molecules that inhibit eosinophils. TGF-β, an anti-inflammatory cytokine (108), decreases the number of eosinophils in human bone marrow suspension cultures (118). TGF-β also inhibits eosinophil survival induced by the eosinophil hemopoietins in vitro (119). Furthermore, IFN-α and IFN-γ suppresses antigen-induced eosinophilia and CD4+ T cell recruitment into airway tissues in mice (120, 121). IFN-α has been successfully used to treat certain subgroups of patients with eosinophilia (122). More recently, a selective mechanism for the induction of human eosinophil apoptosis by cross-linking of surface Siglec-8 (sialic acid-binding immunoglobulin-like lectin 8) has been identified (123). Importantly, the presence of eosinophil activating factors, such as IL-5 and GM-CSF, enhances eosinophil apoptosis induced by Siglec-8-crosslinking (124). Furthermore, glycan ligands for the mouse orthologue of Siglec-8, namely Siglec-F, have been localized in mouse lungs (125), suggesting that Siglec-8/Siglec-F may play a role in the disposal of activated eosinophils in the airways.
Mature eosinophils change their morphology and phenotype as they are exposed to cytokines in vitro and in vivo. For example, as discussed above, after culture with GM-CSF alone, IL-5 plus TNF-α, or IL-3 plus IFN-γ, eosinophils express HLA-DR and increased amounts of ICAM-1, giving these eosinophils the capacity to process and present antigens to T cells (114, 126). In vitro stimulation of blood eosinophils with IL-3, IL-5, or GM-CSF induces the expression of an early activation antigen, CD69 (127), and the IL-2 receptor α-chain (CD25) (128); these cytokines also upregulate the expression of the β2 integrin, CD11b(129). Peripheral blood eosinophils from patients with helminth infections and BAL eosinophils from patients with asthma show increased expression of ICAM-1, HLA-DR, CD11b, CD11c, CD44, CD66, CD66b, CD69, and CD81 (130–134). Importantly, expression of αMβ2 integrin (i.e. CD11b/CD18) and an activation epitope of αMβ2 are increased in airway eosinophils from the patients with asthma who are exposed to airway allergens (135); the changes correlated with clinical deterioration of respiratory function after antigen exposure. Thus, the activation status of eosinophils is unlikely to be the same among different individuals and in different organs, and this status is regulated actively by the immunological milieu surrounding the eosinophils.
In vivo-activated human eosinophils are highly reactive to various agonists. For example, eosinophils in the BAL after allergen challenge show a greater respiratory burst and adherence response to fMLP (132). Eosinophils isolated from the blood of patients with asthma also demonstrate many enhanced proinflammatory properties compared to those from normal individuals, including enhanced adhesion, chemotaxis, transendothelial migration, and LTC4 production (134). Furthermore, the treatment of asthma patients with a combination of inhaled glucocorticoids and β-adrenergic agonists not only improves lung function, but also inhibits markers of eosinophil priming, such as the respiratory burst and PAF releasability (136). These phenotypic and functional changes in human eosinophils are likely a “priming” step while eosinophils are recruited from bone marrow into the tissues, and these primed eosinophils can be promptly and fully activated in response to secretagogues with a lower threshold than that for normal or resting eosinophils.
4.4. Eosinophil activation in innate immunity
Fully activated human eosinophils appear to defend against large, nonphagocytosable organisms, most notably the multicellular helminthic parasites and fungi. Some of the mechanisms used by eosinophils in host defense against these organisms may also produce detrimental effects on the host. Several lines of evidence have indicated that bacterial and/or viral infections may exacerbate allergic inflammation. Direct activation of eosinophils by microbe-derived molecules may explain the mechanism.
Functionally important receptors for recognition of conserved motifs in pathogens, the Toll-like receptors (TLRs), have been identified in mammals (137). Expression of TLR4 in eosinophils has been reported (138, 139). An extensive analysis of eosinophil expression of TLRs and their responses to TLR ligands showed that eosinophils constitutively expressed TLR1, TLR4, TLR7, TLR9, and TLR10 mRNAs and that the TLR7 ligand, R-848, can activate eosinophils (140). Thus, although the natural ligands have not yet been identified, eosinophils’ TLR7 system may represent an important mechanism for host defense against viral infections.
Proteolytic enzymes, produced by various microbes and allergens, such as house dust mites, fungi, and cockroaches, potently induce activation and non-cytotoxic degranulation of eosinophils. Proteases, especially serine proteases, activate hematologic and interstitial cells through a family of G-protein-coupled protease-activated receptors (PAR) and induce production of several pro-inflammatory mediators (141, 142). Four members of this receptor family have been cloned and are designated PAR1, PAR2, PAR3, and PAR4. Protease cleavage of these receptors creates a neo-NH2 terminus, which acts as a tethered ligand and activates the seven-transmembrane segment of the PAR. Human PAR1, PAR3, and PAR4 are activated by thrombin, whereas PAR2 is activated by trypsin but not by thrombin. Human eosinophils constitutively transcribe mRNA for PAR2 and PAR3, but not for PAR1 and PAR4 (143). Trypsin, an authentic agonist for PAR2, potently induces superoxide anion production and degranulation of eosinophils; 5 nM trypsin induces responses that were 50~70 % of those induced by 100 nM PAF, a positive control. Similarly, a cysteine protease, papain, potently induces isolated human eosinophils to degranulate and to produce superoxide anion (144). Importantly, eosinophils are activated by a natural cysteine protease from mite allergens, Der f 1, and release their granule proteins (144). Eosinophils also recognize aspartate protease activity and cysteine protease activity produced by fungus Alternaria alternata (145) and cockroaches (146), respectively, through PAR-2, and they release granule proteins and cytokines. Thus, human eosinophils are likely equipped with mechanisms that recognize and respond to proteases, such as those found in microbes and at allergic response sites, resulting in active release of proinflammatory mediators.
An association between fungal exposure and asthma has been widely recognized (147). Moreover, exposure to Alternaria is a risk factor for respiratory arrest in patients with asthma (148). These airborne fungi and their products may contribute to the development and exacerbation of allergic airway diseases. For example, fungal products, e.g., proteases, induce immunologic and inflammatory reactions, resulting in a Th2-like cytokine response and the destruction of mucosal barrier functions (149). Extracts of Alternaria potently induces eosinophil degranulation (150). Alternaria also strongly induces other activation events in eosinophils, including increases in intracellular calcium concentration, cell surface expression of CD63 and CD11b, and production of IL-8. Interestingly, Alternaria does not induce neutrophil activation, suggesting specificity for fungal species and cell type. In addition, when human eosinophils are exposed to live Alternaria alternata fungus, eosinophils release their cytotoxic granule proteins into the extracellular milieu and onto the surface of fungal organisms and kill the fungus in a contact-dependent manner (151). Eosinophils do not express common fungus receptors, such as dectin-1, but use their versatile β2 integrin molecule, CD11b (see below for more details), to recognize and to adhere to a major cell wall component, β-glucan.
A unique antibacterial activity of human eosinophils is also recognized. Human eosinophils rapidly release mitochondrial DNA in response to exposure to bacteria. The traps contain the granule proteins ECP and MBP and display antimicrobial activity (15). The mitochondrial DNA and the granule proteins bind and kill bacteria in the extracellular space in vitro and in vivo. Indeed, after cecal ligation and puncture, eosinophil-rich IL-5 transgenic, but not wild-type, mice are protected from microbial sepsis. These data suggest previously unrecognized mechanisms for eosinophil-mediated innate immune responses that may be crucial for maintaining the mucosal barrier function. Alternatively, if these eosinophil responses are targeted to commensal bacteria or environmental fungi, they may trigger exacerbation of inflammation leading to pathological outcomes.
Eosinophil activation can be induced by other biological molecules. For example, important mediators in the nervous and cardiovascular system, adenosine triphosphate (ATP) and other nucleotides, induce Ca2+ mobilization, oxygen radical production and CD11b upregulation of human eosinophils through P2X and P2Y purinoreceptors (152, 153). PGD2 is the major prostanoid released by mast cells during an allergic response. Exposure of eosinophils to PGD2 induces rapid morphological changes, chemokinesis, and cellular degranulation of human eosinophils through a newly discovered seven-transmembrane receptor, the chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2) (154). During tissue injury, endogenous molecules are released by stressed or damaged tissues, including uric acid, ATP, high mobility group box (HMGB)-1 protein, and S100 calcium-binding protein family members (155, 156). Eosinophils are attracted to uric acid crystals and produce a large quantities and various kinds of cytokines and chemokines (157); minimal levels of granule proteins are released in response to uric acid. Furthermore, the endogenous ATP produced by eosinophils themselves likely provides a positive feedback signal to eosinophils through a P2Y purinoreceptor, P2Y2. Similarly, HMGB-1 attracts eosinophils and enhances their survival in vitro (158). Aluminum-containing compounds (e.g. alum) are routinely used to elicit an effective immune response to vaccines. After intraperitoneal injection of alum, eosinophils are quickly recruited to the spleen and are involved in early priming of B cells and antigen-specific IgM production (159). Thus, eosinophils may recognize damaged tissues/cells and contribute to tissue homeostasis or modulate the inflammatory and immunological processes.
4.5. Eosinophil activation in adaptive immunity
The ability of eosinophils to recognize the products of adaptive immunity was known before investigations of their activities in innate immunity were performed. Initial studies of eosinophil degranulation in vitro used parasites as a model. Eosinophils incubated with antiserum-coated schistosomula of S. mansoni degranulate and release MBP (1, 108). However, investigation of eosinophil degranulation in this system was complicated by the presence of the viable worm. Subsequently, artificial but more defined models for “parasites” have been used to investigate the mechanisms of eosinophil degranulation. Sepharose beads coated with IgG, IgA, and sIgA stimulated eosinophil degranulation (160); sIgA was the most effective among these Igs. IgA2 is a highly potent stimulus for eosinophil killing of S. mansoni (161). Comparison of neutrophils and eosinophils suggests that neutrophils express much higher levels of FcαR (CD89), detected by the binding of IgA and by anti-FcαR antibody (162). The FcαR on eosinophils has a higher molecular mass (range 70 to 100 kD) than FcαR on neutrophils (range 55 to 75 kD), suggesting that the FcαR on eosinophils and neutrophils is likely highly glycosylated with a major protein core of 32 kD for both cell types. Interestingly, eosinophils from allergic individuals display enhanced FcαR expression, whereas neutrophils do not (162). While the exact mechanism to explain why sIgA is a more potent stimulus than IgA is unknown, eosinophils have been shown to possess a binding site(s) for the secretory component (163, 164). Furthermore, the interaction with the secretory component greatly enhanced pro-inflammatory functions of eosinophils, but not of neutrophils (164). IgG may also be involved in eosinophil activation. Human eosinophils killed both an anti-FcγRII-bearing hybridoma cell line and chicken erythrocytes coated with antibody to chicken erythrocyte covalently coupled to Fab fragments of anti-FcγRII. Flow cytometric analyses showed that freshly isolated human eosinophils possess FcγRII (CD32), but not FcγRI (CD64) or FcγRIII (CD16) (165). Collectively, these findings along with the localization of eosinophils at mucosal organs suggest an important role for sIgA and maybe IgG in mediating the eosinophil's effector functions in vivo.
The role of IgE in mediating eosinophil activation is controversial. Eosinophils isolated from patients with eosinophilia degranulated in response to anti-IgE antibody or IgE-coated parasites (108, 166). Eosinophils can potentially express three types of IgE receptors, the low-affinity IgE receptor, the lectin-type IgE-binding molecule (167), and the high-affinity IgE receptor. It has been claimed that the high-affinity IgE receptor, FcεRI, is present on eosinophils from patients with eosinophilia and that various functions of eosinophils, including degranulation and parasite cytotoxicity, are mediated through this receptor (168). On the other hand, the number of high-affinity receptors expressed on the surfaces of eosinophils from patients with allergic diseases or airway eosinophilia was minimal or undetectable (169); ligation of FcεRI did not result in detectable eosinophil degranulation (170). Furthermore, eosinophils make protein for the α chain of FcεRI, but this protein is released extracellularly instead of being expressed on the cellular surface (169).
Eosinophil degranulation can be induced by soluble stimuli themselves. Eosinophil-chemotactic cytokines, such as RANTES (CCL5) and MIP-lα (CCL3), also induce eosinophil degranulation, although the effects are less pronounced than those of IL-5 or GM-CSF (171, 172). Interestingly, eosinophil granule proteins themselves, including MBP and EPO, stimulate eosinophils and cause degranulation in a noncytotoxic manner, suggesting an autocrine mechanism of eosinophil degranulation (173). The other stimuli for eosinophil degranulation include serum-opsonized zymosan; fMLP; the lipid mediator, PAF; the complement fragments C5a and C3a; naturally occurring peptides, such as substance P and mellitin; calcium ionophore A23187; and PMA (174, 175). For example, PAF potently evokes the release of granule proteins, reactive oxygen species, and LTC4 from eosinophils (176, 177) . Furthermore, eosinophils and neutrophils react differently to PAF, and PAF activates two separate and distinct effector pathways in human eosinophils (178).
Notably, an integrin, Mac-1 (CD11b/CD18, αMβ2), likely plays a critical role for regulating eosinophil degranulation; this molecule is not only important for eosinophil recruitment but is also crucial for eosinophil effector functions. Historically, as described above, receptor ligands immobilized to relatively large surfaces (such as IgG-coated Sepharose beads and parasites) but not particulate ligands (such as aggregated IgG and bacteria) are effective stimuli for eosinophil degranulation (108). Later, it was found that β2 integrins, especially Mac-1, play a crucial role in the activation of eosinophils stimulated by IgG, when IgG is immobilized onto tissue culture plates or Sepharose beads (179). Similarly, the eosinophil functional response to PAF or GM-CSF is greatly influenced by the availability of cellular adhesion (180). When eosinophils are stimulated with these soluble mediators, eosinophils adhere to the tissue culture wells through β2 integrins, and this adhesion process provides critical signals to induce cellular activation and degranulation (176). Conversely, eosinophils activated with sIgA but kept in suspension (i.e. without Sepharose beads) produce cytokines such as GM-CSF and IL-8 but do not release granule proteins. Similar findings are observed when eosinophils are stimulated through FcγRII (175). As described above, Mac-1 is also used to recognize β-glucan on the surface of fungi and to release granule proteins (151). Indeed, eosinophil granule protein release can be induced by the direct ligation of integrins by antibodies or ligands to Mac-1 (181) or VLA-4 (182) in the absence of additional stimuli. Some in vivo experiments also suggest that these adhesion molecules may be important in bronchial asthma. For example, in cynomolgus monkeys that had been allergen challenged several times, anti-Mac-1 mAb inhibited the development of bronchial hyperreactivity and reduced the levels of ECP in the BAL fluid, but did not inhibit the airway eosinophilia (183). Thus, β2 integrins, in particular CD11b/CD18, may play a pivotal role in determining the functional outcomes and cell survival of human eosinophils that are exposed to immunological and inflammatory stimuli. The ability of human eosinophils to effectively engage the integrins and to respond to microbes/molecules with a large surface likely makes this leukocyte distinct from other granulocytes. While the molecular mechanism to explain the pronounced activation of eosinophils by cellular adhesion through β2 integrin is not fully understood, it likely involves other cell surface molecules that are physically associated with the integrin such as the GPI-anchored protein CD66b and signaling molecules localized in lipid rafts (184).
5. Conundrum in mouse and human eosinophils
Mice in many respects mirror human biology well and have been used extensively and successfully to study the mechanisms of Th2-type inflammation in asthma and parasite infection and the roles of eosinophils in these disease models. The sequencing of mouse and human genomes revealed that only 300 or so genes appear to be unique to one species or the other (185). Despite this conservation significant differences exist between mice and humans in immune system development, activation, and response to challenge, in both the innate and adaptive arms (186). Such difference may not be surprising as the two species diverged somewhere between 65 and 75 million years ago, differ hugely in both size and lifespan, and have evolved in quite different ecological niches where widely different pathologic challenges need to be met. We run the risk of overlooking aspects of human immunology that do not occur, or cannot be modeled, in mice. Unfortunately, eosinophils are not exempt from these challenges. For example, mouse eosinophil-associated ribonucleases are remarkably divergent (>50%) orthologues of human EDN and ECP (187). Among various molecules involved in activation and effector functions of human eosinophils as discussed above, FcαRI (IgA receptor), FcγRIIA and C (activating IgG receptor), and CD66b cannot be identified in the mouse genome. Biologically, mouse eosinophils show distinct responses to cytokines and other agonists and have a limited propensity to degranulate or produce inflammatory mediators in vitro (176, 188) (Figure 2). Lack of eosinophil degranulation in mouse models of asthma and Th2-type airway inflammation is reported by several investigators (189, 190). While it is difficult to determine whether the features of the mouse asthma model are the result of an inherent property of mouse eosinophils, a limitation of the stimuli used to provoke eosinophils, or both, it is crucial to keep in mind the species divergence and to consider such differences in applying mouse models to human disease.
Figure 2.
Differential responses of mouse and human eosinophils to stimuli. Panels A and B show superoxide production induced by immobilized IgGs, PAF, IL-5 or eotaxin in human and IL-5 transgenic mouse eosinophils, respectively. Human eosinophils were stimulated with immobilized IgGs [wells precoated with 100 µg/ml human (h)IgG1, mouse (m)IgG1, mIgG2a or mIgG2b], 1 µM PAF, 5 ng/ml recombinant hIL-5, 50 ng/ml hEotaxin, or 10 ng/ml PMA. Peritoneal lavage mouse eosinophils were stimulated similarly except recombinant mIL-5 and mEotaxin were used at the same concentrations. Superoxide production was measured by reduction of cytochrome c. Results are means ± SEM of three experiments. Panels C and D depict the morphology of human and mouse eosinophils stimulated with immobilized hIgG1 and mIgG2a, respectively. Original magnification; ×400.
The roles of eosinophils in mouse models of helminth infection are also controversial (191, 192). Although treatment with anti-IL-5 reduced the number of circulating and tissue eosinophils, there was no evidence for any change in the nature or extent of helminth infection (193, 194). Similar conclusions were reached in several studies with genetically altered mice, including eosinophil-less mice (IL-5 or IL-5Rα gene deletion) or eosinophil-deficient mice (Δdbl-GATA and PHIL) (195–197). On the other hand, eosinophils appear to play a role in reducing the parasite burden in mice infected with the nematodes Strongyloides and Angiostrongylus (198, 199). There are several reasons to be considered for explaining why it is difficult to discern a role for eosinophils in mouse models. Most of all, many of these experiments are performed with human pathogens that do not naturally infect rodents. Therefore, the mouse immune system may not have been exposed to evolutionary pressure to develop the responses to these pathogens. Different immune responses to parasites and other antigens observed among different mouse strains add to this complexity (64, 200).
6. Summary and future directions
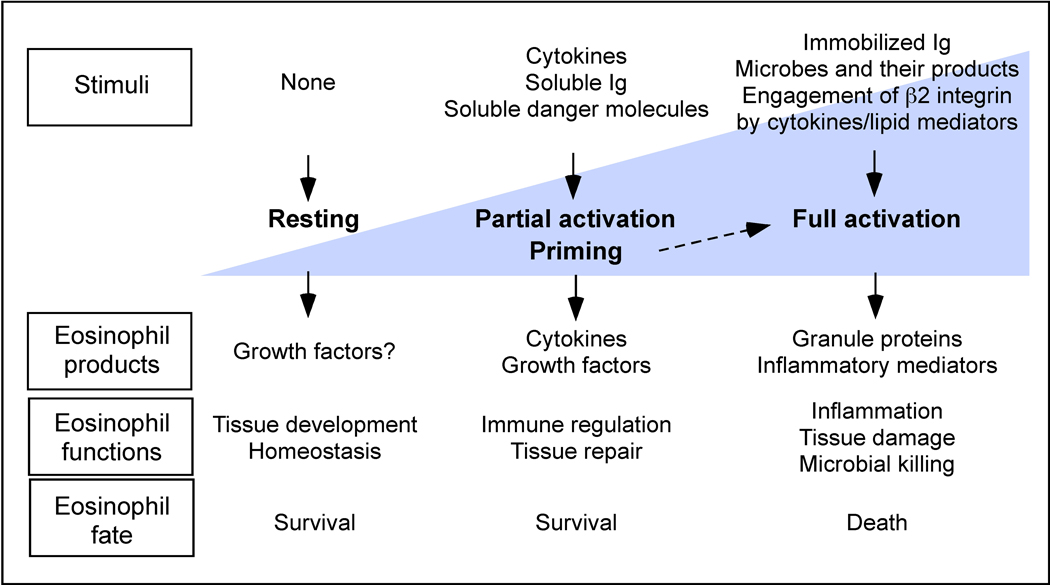
Eosinophils have been considered end-stage cells involved in host protection against parasite infection and immunopathology in Th2-type inflammation. However, recent studies changed this perspectives and eosinophils are now considered multifunctional leukocytes involved in tissue homeostasis, modulation of adaptive immune responses, and innate immunity to certain microbes. As normal constituents of the gastrointestinal tract, mammary glands and bone marrow, eosinophils likely play roles in development and homeostasis of these organs. Numerous lines of evidence suggest that eosinophils are capable of producing immunoregulatory cytokines and are actively involved in regulation of Th2-type immune responses in vivo. However, such new information does not preclude data from earlier studied showing that eosinophils, in particular human eosinophils, are also effector cells with pro-inflammatory and destructive capabilities. The damaging characteristics of eosinophil lipid mediators and granule proteins were well-established in the 1990s. Eosinophils with activation phenotypes are observed in biological specimens from patients with disease, and deposition of eosinophil products is readily seen in the affected tissues from these patients. Therefore, it would be reasonable to consider the eosinophils as multifaceted leukocytes that contributes to various physiological and pathological processes depending on their location and activation status (Figure 3).
Figure 3.
Multifaceted functions of eosinophils. Functional properties of eosinophil can be divided into at least three stages including resting, partial activation and full activation. Different types of immunological stimuli (or absence of stimuli) trigger production and release of different molecules by eosinophils. Accordingly, eosinophils may affect tissue development, homeostasis and repair, regulate the immune responses and exert pro-inflammatory effector functions. Eosinophil survival and death is controlled similarly by stimuli and tissue envi
The challenge is that these functions of eosinophils most likely overlap with the effects of other immune cells, such as CD4+ T cells, and the role of eosinophils alone may be difficult to demonstrate in physiological settings including patients with real disease. Furthermore, inflammation is a complex event induced by various triggers, such as infection and tissue injury and stress, resulting in not only tissue damage but also tissue repair and homeostasis (201). Eosinophils can be involved in any of these processes or their combinations in a given disease or disease model. It would be difficult to discern the role of eosinophils if eosinophils are involved in both damaging and repair processes. Thus, further and careful analysis of recent genetically-engineered eosinophil-deficient mice in vivo and further characterization of the immunobiology of eosinophils in vitro will be necessary to answer critical questions concerning the true involvement of this leukocyte in health and a variety of disease processes. The relevance of experimental models in human disease and the potential biological differences in mouse and human eosinophils also need to be taken into account. Although this is a challenging task, future studies have a promise to reveal the true importance of eosinophils in human health and disease.
References
- 1.Gleich GJ, Adolphson CR. The eosinophilic leukocyte: structure and function. Adv Immunol. 1986;39:177–253. doi: 10.1016/s0065-2776(08)60351-x. [DOI] [PubMed] [Google Scholar]
- 2.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 3.Hogan SP, et al. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38:709–750. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- 4.McNagny K, Graf T. Making eosinophils through subtle shifts in transcription factor expression. J Exp Med. 2002;195:F43–F47. doi: 10.1084/jem.20020636. PMC:2193544M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh JC, et al. Cooperative and antagonistic interplay between PU.1 and GATA-2 in the specification of myeloid cell fates. Immunity. 2002;17:665–676. doi: 10.1016/s1074-7613(02)00452-1. [DOI] [PubMed] [Google Scholar]
- 6.Du J, et al. Novel combinatorial interactions of GATA-1, PU.1, and C/EBPepsilon isoforms regulate transcription of the gene encoding eosinophil granule major basic protein. J Biol Chem. 2002;277:43481–43494. doi: 10.1074/jbc.M204777200. [DOI] [PubMed] [Google Scholar]
- 7.Yu C, et al. Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J Exp Med. 2002;195:1387–1395. doi: 10.1084/jem.20020656. PMC:2193547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanderson CJ. Interleukin-5, eosinophils, and disease. Blood. 1992;79:3101–3109. [PubMed] [Google Scholar]
- 9.Mishra A, Hogan SP, Lee JJ, Foster PS, Rothenberg ME. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J Clin Invest. 1999;103:1719–1727. doi: 10.1172/JCI6560. PMC:408388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Humbles AA, et al. The murine CCR3 receptor regulates both the role of eosinophils and mast cells in allergen-induced airway inflammation and hyperresponsiveness. Proc Natl Acad Sci U S A. 2002;99:1479–1484. doi: 10.1073/pnas.261462598. PMC:122216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pope SM, et al. Identification of a cooperative mechanism involving interleukin-13 and eotaxin-2 in experimental allergic lung inflammation. J Biol Chem. 2005;280:13952–13961. doi: 10.1074/jbc.M406037200. [DOI] [PubMed] [Google Scholar]
- 12.Gouon-Evans V, Rothenberg ME, Pollard JW. Postnatal mammary gland development requires macrophages and eosinophils. Development. 2000;127:2269–2282. doi: 10.1242/dev.127.11.2269. [DOI] [PubMed] [Google Scholar]
- 13.Robertson SA, Mau VJ, Hudson SN, Tremellen KP. Cytokine-leukocyte networks and the establishment of pregnancy. Am J Reprod Immunol. 1997;37:438–442. doi: 10.1111/j.1600-0897.1997.tb00257.x. [DOI] [PubMed] [Google Scholar]
- 14.Furuta GT, et al. Eosinophils alter colonic epithelial barrier function: role for major basic protein. Am J Physiol Gastrointest Liver Physiol. 2005;289:G890–G897. doi: 10.1152/ajpgi.00015.2005. [DOI] [PubMed] [Google Scholar]
- 15.Yousefi S, et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14:949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 16.Throsby M, Herbelin A, Pleau JM, Dardenne M. CD11c+ eosinophils in the murine thymus: developmental regulation and recruitment upon MHC class I-restricted thymocyte deletion. J Immunol. 2000;165:1965–1975. doi: 10.4049/jimmunol.165.4.1965. [DOI] [PubMed] [Google Scholar]
- 17.Chu VT, et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat Immunol. 2011;12:151–159. doi: 10.1038/ni.1981. [DOI] [PubMed] [Google Scholar]
- 18.Spencer LA, Weller PF. Eosinophils and Th2 immunity: contemporary insights. Immunol Cell Biol. 2010;88:250–256. doi: 10.1038/icb.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi HZ, Humbles A, Gerard C, Jin Z, Weller PF. Lymph node trafficking and antigen presentation by endobronchial eosinophils. J Clin Invest. 2000;105:945–953. doi: 10.1172/JCI8945. PMC:377484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Rijt LS, Vos N, Hijdra D, de Vries VC, Hoogsteden HC, Lambrecht BN. Airway eosinophils accumulate in the mediastinal lymph nodes but lack antigen-presenting potential for naive T cells. J Immunol. 2003;171:3372–3378. doi: 10.4049/jimmunol.171.7.3372. [DOI] [PubMed] [Google Scholar]
- 21.Duez C, et al. Migration and accumulation of eosinophils toward regional lymph nodes after airway allergen challenge. J Allergy Clin Immunol. 2004;114:820–825. doi: 10.1016/j.jaci.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Del Pozo V, et al. Eosinophil as antigen-presenting cell: activation of T cell clones and T cell hybridoma by eosinophils after antigen processing. Eur J Immunol. 1992;22:1919–1925. doi: 10.1002/eji.1830220736. [DOI] [PubMed] [Google Scholar]
- 23.Padigel UM, Lee JJ, Nolan TJ, Schad GA, Abraham D. Eosinophils can function as antigen-presenting cells to induce primary and secondary immune responses to Strongyloides stercoralis. Infect Immun. 2006;74:3232–3238. doi: 10.1128/IAI.02067-05. PMC:1479274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi HZ, et al. Endobronchial eosinophils preferentially stimulate T helper cell type 2 responses. Allergy. 2004;59:428–435. doi: 10.1046/j.1398-9995.2003.00405.x. [DOI] [PubMed] [Google Scholar]
- 25.Lucey DR, Nicholson-Weller A, Weller PF. Mature human eosinophils have the capacity to express HLA-DR. Proc Natl Acad Sci U S A. 1989;86:1348–1351. doi: 10.1073/pnas.86.4.1348. PMC:286687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Celestin J, et al. IL-3 induces B7.2 (CD86) expression and costimulatory activity in human eosinophils. J Immunol. 2001;167:6097–6104. doi: 10.4049/jimmunol.167.11.6097. [DOI] [PubMed] [Google Scholar]
- 27.Ohkawara Y, et al. CD40 expression by human peripheral blood eosinophils. J Clin Invest. 1996;97:1761–1766. doi: 10.1172/JCI118603. PMC:507241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto H, Sedgwick JB, Vrtis RF, Busse WW. The effect of transendothelial migration on eosinophil function. Am J Respir Cell Mol Biol. 2000;23:379–388. doi: 10.1165/ajrcmb.23.3.3707. [DOI] [PubMed] [Google Scholar]
- 29.Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 30.Radke AL, Reynolds LE, Melo RC, Dvorak AM, Weller PF, Spencer LA. Mature human eosinophils express functional Notch ligands mediating eosinophil autocrine regulation. Blood. 2009;113:3092–3101. doi: 10.1182/blood-2008-05-155937. PMC:2662649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moqbel R, Lacy P. New concepts in effector functions of eosinophil cytokines. Clin Exp Allergy. 2000;30:1667–1671. doi: 10.1111/j.1365-2222.2000.00991.x. [DOI] [PubMed] [Google Scholar]
- 32.Kita H, Ohnishi T, Okubo Y, Weiler D, Abrams JS, Gleich GJ. Granulocyte/macrophage colony-stimulating factor and interleukin 3 release from human peripheral blood eosinophils and neutrophils. J Exp Med. 1991;174:745–748. doi: 10.1084/jem.174.3.745. PMC:2118930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moqbel R, et al. Expression of mRNA and immunoreactivity for the granulocyte/macrophage colony-stimulating factor in activated human eosinophils. J Exp Med. 1991;174:749–752. doi: 10.1084/jem.174.3.749. PMC:2118946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong DT, et al. Human eosinophils express transforming growth factor alpha. J Exp Med. 1990;172:673–681. doi: 10.1084/jem.172.3.673. PMC:2188564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong DT, et al. Eosinophils from patients with blood eosinophilia express transforming growth factor beta 1. Blood. 1991;78:2702–2707. [PubMed] [Google Scholar]
- 36.Ohno I, et al. Eosinophils in chronically inflamed human upper airway tissues express transforming growth factor beta 1 gene (TGF beta 1) J Clin Invest. 1992;89:1662–1668. doi: 10.1172/JCI115764. PMC:443044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levi-Schaffer F, et al. Human eosinophils regulate human lung- and skin-derived fibroblast properties in vitro: a role for transforming growth factor beta (TGF-beta) Proc Natl Acad Sci U S A. 1999;96:9660–9665. doi: 10.1073/pnas.96.17.9660. PMC:22266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burgel PR, et al. Human eosinophils induce mucin production in airway epithelial cells via epidermal growth factor receptor activation. J Immunol. 2001;167:5948–5954. doi: 10.4049/jimmunol.167.10.5948. [DOI] [PubMed] [Google Scholar]
- 39.Puxeddu I, et al. Osteopontin is expressed and functional in human eosinophils. Allergy. 2010;65:168–174. doi: 10.1111/j.1398-9995.2009.02148.x. [DOI] [PubMed] [Google Scholar]
- 40.Puxeddu I, Alian A, Piliponsky AM, Ribatti D, Panet A, Levi-Schaffer F. Human peripheral blood eosinophils induce angiogenesis. Int J Biochem Cell Biol. 2005;37:628–636. doi: 10.1016/j.biocel.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Okada S, Kita H, George TJ, Gleich GJ, Leiferman KM. Migration of eosinophils through basement membrane components in vitro: role of matrix metalloproteinase-9. Am J Respir Cell Mol Biol. 1997;17:519–528. doi: 10.1165/ajrcmb.17.4.2877. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi H, Gleich GJ, Butterfield JH, Kita H. Human eosinophils produce neurotrophins and secrete nerve growth factor on immunologic stimuli. Blood. 2002;99:2214–2220. doi: 10.1182/blood.v99.6.2214. [DOI] [PubMed] [Google Scholar]
- 43.Moqbel R, et al. Identification of messenger RNA for IL-4 in human eosinophils with granule localization and release of the translated product. J Immunol. 1995;155:4939–4947. [PubMed] [Google Scholar]
- 44.Nakajima H, Gleich GJ, Kita H. Constitutive production of IL-4 and IL-10 and stimulated production of IL-8 by normal peripheral blood eosinophils. J Immunol. 1996;156:4859–4866. [PubMed] [Google Scholar]
- 45.Nonaka M, et al. Distinct immunohistochemical localization of IL-4 in human inflamed airway tissues. IL-4 is localized to eosinophils in vivo and is released by peripheral blood eosinophils. J Immunol. 1995;155:3234–3244. [PubMed] [Google Scholar]
- 46.Bandeira-Melo C, Sugiyama K, Woods LJ, Weller PF. Cutting edge: eotaxin elicits rapid vesicular transport-mediated release of preformed IL-4 from human eosinophils. J Immunol. 2001;166:4813–4817. doi: 10.4049/jimmunol.166.8.4813. [DOI] [PubMed] [Google Scholar]
- 47.Melani C, et al. Interleukin-6 expression in human neutrophil and eosinophil peripheral blood granulocytes. Blood. 1993;81:2744–2749. [PubMed] [Google Scholar]
- 48.Hamid Q, et al. Human eosinophils synthesize and secrete interleukin-6, in vitro. Blood. 1992;80:1496–1501. [PubMed] [Google Scholar]
- 49.Costa JJ, et al. Human eosinophils can express the cytokines tumor necrosis factor-alpha and macrophage inflammatory protein-1 alpha. J Clin Invest. 1993;91:2673–2684. doi: 10.1172/JCI116506. PMC:443331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lacy P, et al. Rapid mobilization of intracellularly stored RANTES in response to interferon-gamma in human eosinophils. Blood. 1999;94:23–32. [PubMed] [Google Scholar]
- 51.Kobayashi T, Iijima K, Kita H. Marked airway eosinophilia prevents development of airway hyper-responsiveness during an allergic response in IL-5 transgenic mice. J Immunol. 2003;170:5756–5763. doi: 10.4049/jimmunol.170.11.5756. [DOI] [PubMed] [Google Scholar]
- 52.Terness P, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196:447–457. doi: 10.1084/jem.20020052. PMC:2196057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Odemuyiwa SO, et al. Cutting edge: human eosinophils regulate T cell subset selection through indoleamine 2,3-dioxygenase. J Immunol. 2004;173:5909–5913. doi: 10.4049/jimmunol.173.10.5909. [DOI] [PubMed] [Google Scholar]
- 54.Yang D, Rosenberg HF, Chen Q, Dyer KD, Kurosaka K, Oppenheim JJ. Eosinophil-derived neurotoxin (EDN), an antimicrobial protein with chemotactic activities for dendritic cells. Blood. 2003;102:3396–3403. doi: 10.1182/blood-2003-01-0151. [DOI] [PubMed] [Google Scholar]
- 55.Yang D, et al. Human ribonuclease A superfamily members, eosinophil-derived neurotoxin and pancreatic ribonuclease, induce dendritic cell maturation and activation. J Immunol. 2004;173:6134–6142. doi: 10.4049/jimmunol.173.10.6134. PMC:2847482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang D, et al. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J Exp Med. 2008;205:79–90. doi: 10.1084/jem.20062027. PMC:2234357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sabin EA, Kopf MA, Pearce EJ. Schistosoma mansoni egg-induced early IL-4 production is dependent upon IL-5 and eosinophils. J Exp Med. 1996;184:1871–1878. doi: 10.1084/jem.184.5.1871. PMC:2192874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267–277. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- 59.Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006;203:1435–1446. doi: 10.1084/jem.20052448. PMC:2118302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shinkai K, Mohrs M, Locksley RM. Helper T cells regulate type-2 innate immunity in vivo. Nature. 2002;420:825–829. doi: 10.1038/nature01202. [DOI] [PubMed] [Google Scholar]
- 61.Sabin EA, Pearce EJ. Early IL-4 production by non-CD4+ cells at the site of antigen deposition predicts the development of a T helper 2 cell response to Schistosoma mansoni eggs. J Immunol. 1995;155:4844–4853. [PubMed] [Google Scholar]
- 62.Mattes J, et al. Intrinsic defect in T cell production of interleukin (IL)-13 in the absence of both IL-5 and eotaxin precludes the development of eosinophilia and airways hyperreactivity in experimental asthma. J Exp Med. 2002;195:1433–1444. doi: 10.1084/jem.20020009. PMC:2193548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee JJ, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 64.Walsh ER, et al. Strain-specific requirement for eosinophils in the recruitment of T cells to the lung during the development of allergic asthma. J Exp Med. 2008;205:1285–1292. doi: 10.1084/jem.20071836. PMC:2413027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jacobsen EA, et al. Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells. J Exp Med. 2008;205:699–710. doi: 10.1084/jem.20071840. PMC:2275390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fulkerson PC, Fischetti CA, McBride ML, Hassman LM, Hogan SP, Rothenberg ME. A central regulatory role for eosinophils and the eotaxin/CCR3 axis in chronic experimental allergic airway inflammation. Proc Natl Acad Sci U S A. 2006;103:16418–16423. doi: 10.1073/pnas.0607863103. PMC:1637597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oxvig C, Gleich GJ, Sottrup-Jensen L. Localization of disulfide bridges and free sulfhydryl groups in human eosinophil granule major basic protein. FEBS Lett. 1994;341:213–217. doi: 10.1016/0014-5793(94)80459-1. [DOI] [PubMed] [Google Scholar]
- 68.Swaminathan GJ, et al. Crystal structure of the eosinophil major basic protein at 1.8 A. An atypical lectin with a paradigm shift in specificity. J Biol Chem. 2001;276:26197–26203. doi: 10.1074/jbc.M100848200. [DOI] [PubMed] [Google Scholar]
- 69.Popken-Harris P, et al. Expression, purification, and characterization of the recombinant proform of eosinophil granule major basic protein. J Immunol. 1995;155:1472–1480. [PubMed] [Google Scholar]
- 70.Abu-Ghazaleh RI, Gleich GJ, Prendergast FG. Interaction of eosinophil granule major basic protein with synthetic lipid bilayers: a mechanism for toxicity. J Membr Biol. 1992;128:153–164. doi: 10.1007/BF00231888. [DOI] [PubMed] [Google Scholar]
- 71.Plager DA, et al. A novel and highly divergent homolog of human eosinophil granule major basic protein. J Biol Chem. 1999;274:14464–14473. doi: 10.1074/jbc.274.20.14464. [DOI] [PubMed] [Google Scholar]
- 72.Boix E. Eosinophil cationic protein. Methods Enzymol. 2001;341:287–305. doi: 10.1016/s0076-6879(01)41159-1. [DOI] [PubMed] [Google Scholar]
- 73.Domachowske JB, Dyer KD, Adams AG, Leto TL, Rosenberg HF. Eosinophil cationic protein/RNase 3 is another RNase A-family ribonuclease with direct antiviral activity. Nucleic Acids Res. 1998;26:3358–3363. doi: 10.1093/nar/26.14.3358. PMC:147714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rosenberg HF, Domachowske JB. Eosinophils, eosinophil ribonucleases, and their role in host defense against respiratory virus pathogens. J Leukoc Biol. 2001;70:691–698. [PubMed] [Google Scholar]
- 75.Rosenberg HF, Domachowske JB. Eosinophil-derived neurotoxin. Methods Enzymol. 2001;341:273–286. doi: 10.1016/s0076-6879(01)41158-x. [DOI] [PubMed] [Google Scholar]
- 76.Gleich GJ, Loegering DA, Bell MP, Checkel JL, Ackerman SJ, McKean DJ. Biochemical and functional similarities between human eosinophil-derived neurotoxin and eosinophil cationic protein: homology with ribonuclease. Proc Natl Acad Sci U S A. 1986;83:3146–3150. doi: 10.1073/pnas.83.10.3146. PMC:323469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Domachowske JB, Dyer KD, Bonville CA, Rosenberg HF. Recombinant human eosinophil-derived neurotoxin/RNase 2 functions as an effective antiviral agent against respiratory syncytial virus. J Infect Dis. 1998;177:1458–1464. doi: 10.1086/515322. [DOI] [PubMed] [Google Scholar]
- 78.Adamko DJ, Yost BL, Gleich GJ, Fryer AD, Jacoby DB. Ovalbumin sensitization changes the inflammatory response to subsequent parainfluenza infection. Eosinophils mediate airway hyperresponsiveness, m(2) muscarinic receptor dysfunction, and antiviral effects. J Exp Med. 1999;190:1465–1478. doi: 10.1084/jem.190.10.1465. PMC:2195693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Dalen CJ, Kettle AJ. Substrates and products of eosinophil peroxidase. Biochem J. 2001;358:233–239. doi: 10.1042/0264-6021:3580233. PMC:1222052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mitra SN, Slungaard A, Hazen SL. Role of eosinophil peroxidase in the origins of protein oxidation in asthma. Redox Rep. 2000;5:215–224. doi: 10.1179/135100000101535771. [DOI] [PubMed] [Google Scholar]
- 81.Wu W, et al. Eosinophils generate brominating oxidants in allergen-induced asthma. J Clin Invest. 2000;105:1455–1463. doi: 10.1172/JCI9702. PMC:315470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.MacPherson JC, et al. Eosinophils are a major source of nitric oxide-derived oxidants in severe asthma: characterization of pathways available to eosinophils for generating reactive nitrogen species. J Immunol. 2001;166:5763–5772. doi: 10.4049/jimmunol.166.9.5763. [DOI] [PubMed] [Google Scholar]
- 83.Gleich GJ, Adolphson CR, Kita H. The eosinophil and asthma. In: Busse WW, Holgate ST, editors. Asthma and rhinitis. 2nd ed. Oxford, UK: Blackwell Science; 2000. pp. 429–479. [Google Scholar]
- 84.Gleich GJ, Fryer AD, Jacoby DB. Eosinophil granule proteins and bronchial hyperreactivity. In: Holgate ST, et al., editors. Asthma: Physiology, immunopharmacology, and treatment. London: Academic Press; 1993. pp. 119–129. [Google Scholar]
- 85.Gleich GJ. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol. 2000;105:651–663. doi: 10.1067/mai.2000.105712. [DOI] [PubMed] [Google Scholar]
- 86.Ponikau JU, et al. Striking deposition of toxic eosinophil major basic protein in mucus: implications for chronic rhinosinusitis. J Allergy Clin Immunol. 2005;116:362–369. doi: 10.1016/j.jaci.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 87.Gundel RH, Letts LG, Gleich GJ. Human eosinophil major basic protein induces airway constriction and airway hyperresponsiveness in primates. J Clin Invest. 1991;87:1470–1473. doi: 10.1172/JCI115155. PMC:295201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Costello RW, Schofield BH, Kephart GM, Gleich GJ, Jacoby DB, Fryer AD. Localization of eosinophils to airway nerves and effect on neuronal M2 muscarinic receptor function. Am J Physiol. 1997;273:L93–L103. doi: 10.1152/ajplung.1997.273.1.L93. [DOI] [PubMed] [Google Scholar]
- 89.Costello RW, et al. Antigen-induced hyperreactivity to histamine: role of the vagus nerves and eosinophils. Am J Physiol. 1999;276:L709–L714. doi: 10.1152/ajplung.1999.276.5.L709. [DOI] [PubMed] [Google Scholar]
- 90.Kephart GM, et al. Marked deposition of eosinophil-derived neurotoxin in adult patients with eosinophilic esophagitis. Am J Gastroenterol. 2010;105:298–307. doi: 10.1038/ajg.2009.584. PMC:2824254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Straumann A, et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut. 2010;59:21–30. doi: 10.1136/gut.2009.178558. [DOI] [PubMed] [Google Scholar]
- 92.Dennis EA, Rhee SG, Billah MM, Hannun YA. Role of phospholipase in generating lipid second messengers in signal transduction. FASEB J. 1991;5:2068–2077. doi: 10.1096/fasebj.5.7.1901288. [DOI] [PubMed] [Google Scholar]
- 93.Sugiura T, et al. Synthesis and action of PAF in human eosinophils. J Lipid Mediat. 1992;5:151–153. [PubMed] [Google Scholar]
- 94.Stahle-Backdahl M, Sudbeck BD, Eisen AZ, Welgus HG, Parks WC. Expression of 92-kDa type IV collagenase mRNA by eosinophils associated with basal cell carcinoma. J Invest Dermatol. 1992;99:497–503. doi: 10.1111/1523-1747.ep12616171. [DOI] [PubMed] [Google Scholar]
- 95.Stahle-Backdahl M, Parks WC. 92-kd gelatinase is actively expressed by eosinophils and stored by neutrophils in squamous cell carcinoma. Am J Pathol. 1993;142:995–1000. PMC:1886874. [PMC free article] [PubMed] [Google Scholar]
- 96.Stahle-Backdahl M, Inoue M, Guidice GJ, Parks WC. 92-kD gelatinase is produced by eosinophils at the site of blister formation in bullous pemphigoid and cleaves the extracellular domain of recombinant 180-kD bullous pemphigoid autoantigen. J Clin Invest. 1994;93:2022–2030. doi: 10.1172/JCI117196. PMC:294314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kumagai K, et al. Inhibition of matrix metalloproteinases prevents allergen-induced airway inflammation in a murine model of asthma. J Immunol. 1999;162:4212–4219. [PubMed] [Google Scholar]
- 98.Rothenberg ME, et al. Human eosinophils have prolonged survival, enhanced functional properties, and become hypodense when exposed to human interleukin 3. J Clin Invest. 1988;81:1986–1992. doi: 10.1172/JCI113547. PMC:442652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Winqvist I, Olofsson T, Olsson I, Persson AM, Hallberg T. Altered density, metabolism and surface receptors of eosinophils in eosinophilia. Immunology. 1982;47:531–539. PMC:1555549. [PMC free article] [PubMed] [Google Scholar]
- 100.Fukuda T, Dunnette SL, Reed CE, Ackerman SJ, Peters MS, Gleich GJ. Increased numbers of hypodense eosinophils in the blood of patients with bronchial asthma. Am Rev Respir Dis. 1985;132:981–985. doi: 10.1164/arrd.1985.132.5.981. [DOI] [PubMed] [Google Scholar]
- 101.Lopez AF, et al. Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survival. J Clin Invest. 1986;78:1220–1228. doi: 10.1172/JCI112705. PMC:423807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lopez AF, Sanderson CJ, Gamble JR, Campbell HD, Young IG, Vadas MA. Recombinant human interleukin 5 is a selective activator of human eosinophil function. J Exp Med. 1988;167:219–224. doi: 10.1084/jem.167.1.219. PMC:2188822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ingley E, Young IG. Characterization of a receptor for interleukin-5 on human eosinophils and the myeloid leukemia line HL-60. Blood. 1991;78:339–344. [PubMed] [Google Scholar]
- 104.Migita M, et al. Characterization of the human IL-5 receptors on eosinophils. Cell Immunol. 1991;133:484–497. doi: 10.1016/0008-8749(91)90120-z. [DOI] [PubMed] [Google Scholar]
- 105.Warringa RA, Schweizer RC, Maikoe T, Kuijper PH, Bruijnzeel PL, Koendermann L. Modulation of eosinophil chemotaxis by interleukin-5. Am J Respir Cell Mol Biol. 1992;7:631–636. doi: 10.1165/ajrcmb/7.6.631. [DOI] [PubMed] [Google Scholar]
- 106.Schweizer RC, Welmers BA, Raaijmakers JA, Zanen P, Lammers JW, Koenderman L. RANTES- and interleukin-8-induced responses in normal human eosinophils: effects of priming with interleukin-5. Blood. 1994;83:3697–3704. [PubMed] [Google Scholar]
- 107.Kita H, Weiler DA, Abu-Ghazaleh R, Sanderson CJ, Gleich GJ. Release of granule proteins from eosinophils cultured with IL-5. J Immunol. 1992;149:629–635. [PubMed] [Google Scholar]
- 108.Kita H, Adolphson CR, Gleich GJ. Biology of eosinophils. In: Adkinson NF, et al., editors. Middleton's Allergy: Principles and Practice. 6th ed. Mosby: St. Louis; 2003. pp. 305–332. [Google Scholar]
- 109.Bracke M, et al. Differential effects of the T helper cell type 2-derived cytokines IL-4 and IL-5 on ligand binding to IgG and IgA receptors expressed by human eosinophils. J Immunol. 1997;159:1459–1465. [PubMed] [Google Scholar]
- 110.Luttmann W, Matthiesen T, Matthys H, Virchow JC., Jr Synergistic effects of interleukin-4 or interleukin-13 and tumor necrosis factor-alpha on eosinophil activation in vitro. Am J Respir Cell Mol Biol. 1999;20:474–480. doi: 10.1165/ajrcmb.20.3.3326. [DOI] [PubMed] [Google Scholar]
- 111.Temann UA, Geba GP, Rankin JA, Flavell RA. Expression of interleukin 9 in the lungs of transgenic mice causes airway inflammation, mast cell hyperplasia, and bronchial hyperresponsiveness. J Exp Med. 1998;188:1307–1320. doi: 10.1084/jem.188.7.1307. PMC:2212487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gounni AS, et al. Interleukin-9 enhances interleukin-5 receptor expression, differentiation, and survival of human eosinophils. Blood. 2000;96:2163–2171. [PubMed] [Google Scholar]
- 113.Valerius T, Repp R, Kalden JR, Platzer E. Effects of IFN on human eosinophils in comparison with other cytokines. A novel class of eosinophil activators with delayed onset of action. J Immunol. 1990;145:2950–2958. [PubMed] [Google Scholar]
- 114.Hansel TT, et al. Induction and function of eosinophil intercellular adhesion molecule-1 and HLA-DR. J Immunol. 1992;149:2130–2136. [PubMed] [Google Scholar]
- 115.Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008;226:172–190. doi: 10.1111/j.1600-065X.2008.00713.x. PMC:2683382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wong CK, Hu S, Cheung PF, Lam CW. Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: implications in allergic inflammation. Am J Respir Cell Mol Biol. 2010;43:305–315. doi: 10.1165/rcmb.2009-0168OC. [DOI] [PubMed] [Google Scholar]
- 117.Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J Allergy Clin Immunol. 2008;121:1484–1490. doi: 10.1016/j.jaci.2008.04.005. PMC:2821937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sillaber C, et al. Type beta transforming growth factors promote interleukin-3 (IL-3)-dependent differentiation of human basophils but inhibit IL-3-dependent differentiation of human eosinophils. Blood. 1992;80:634–641. [PubMed] [Google Scholar]
- 119.Alam R, Forsythe P, Stafford S, Fukuda Y. Transforming growth factor beta abrogates the effects of hematopoietins on eosinophils and induces their apoptosis. J Exp Med. 1994;179:1041–1045. doi: 10.1084/jem.179.3.1041. PMC:2191410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nakajima H, Sano H, Nishimura T, Yoshida S, Iwamoto I. Role of vascular cell adhesion molecule 1/very late activation antigen 4 and intercellular adhesion molecule 1/lymphocyte function-associated antigen 1 interactions in antigen-induced eosinophil and T cell recruitment into the tissue. J Exp Med. 1994;179:1145–1154. doi: 10.1084/jem.179.4.1145. PMC:2191449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fulkerson PC, et al. Negative regulation of eosinophil recruitment to the lung by the chemokine monokine induced by IFN-gamma (Mig, CXCL9) Proc Natl Acad Sci U S A. 2004;101:1987–1992. doi: 10.1073/pnas.0308544100. PMC:357039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zielinski RM, Lawrence WD. Interferon-alpha for the hypereosinophilic syndrome. Ann Intern Med. 1990;113:716–718. doi: 10.7326/0003-4819-113-9-716. [DOI] [PubMed] [Google Scholar]
- 123.Nutku E, Aizawa H, Hudson SA, Bochner BS. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101:5014–5020. doi: 10.1182/blood-2002-10-3058. [DOI] [PubMed] [Google Scholar]
- 124.Nutku-Bilir E, Hudson SA, Bochner BS. Interleukin-5 priming of human eosinophils alters siglec-8 mediated apoptosis pathways. Am J Respir Cell Mol Biol. 2008;38:121–124. doi: 10.1165/rcmb.2007-0154OC. PMC:2176128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Guo JP, et al. Characterization of expression of glycan ligands for siglec-f in normal mouse lungs. Am J Respir Cell Mol Biol. 2011;44:238–243. doi: 10.1165/rcmb.2010-0007OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Weller PF, Rand TH, Barrett T, Elovic A, Wong DT, Finberg RW. Accessory cell function of human eosinophils. HLA-DR-dependent, MHC-restricted antigen-presentation and IL-1 alpha expression. J Immunol. 1993;150:2554–2562. [PubMed] [Google Scholar]
- 127.Hartnell A, Robinson DS, Kay AB, Wardlaw AJ. CD69 is expressed by human eosinophils activated in vivo in asthma and in vitro by cytokines. Immunology. 1993;80:281–286. PMC:1422202. [PMC free article] [PubMed] [Google Scholar]
- 128.Rand TH, Silberstein DS, Kornfeld H, Weller PF. Human eosinophils express functional interleukin 2 receptors. J Clin Invest. 1991;88:825–832. doi: 10.1172/JCI115383. PMC:295468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hartnell A, Kay AB, Wardlaw AJ. Interleukin-3-induced up-regulation of CR3 expression on human eosinophils is inhibited by dexamethasone. Immunology. 1992;77:488–493. PMC:1421652. [PMC free article] [PubMed] [Google Scholar]
- 130.Mawhorter SD, Stephany DA, Ottesen EA, Nutman TB. Identification of surface molecules associated with physiologic activation of eosinophils. Application of whole-blood flow cytometry to eosinophils. J Immunol. 1996;156:4851–4858. [PubMed] [Google Scholar]
- 131.Hansel TT, et al. Sputum eosinophils from asthmatics express ICAM-1 and HLA-DR. Clin Exp Immunol. 1991;86:271–277. doi: 10.1111/j.1365-2249.1991.tb05809.x. PMC:1554121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sedgwick JB, Calhoun WJ, Vrtis RF, Bates ME, McAllister PK, Busse WW. Comparison of airway and blood eosinophil function after in vivo antigen challenge. J Immunol. 1992;149:3710–3718. [PubMed] [Google Scholar]
- 133.Mengelers HJ, Maikoe T, Brinkman L, Hooibrink B, Lammers JW, Koenderman L. Immunophenotyping of eosinophils recovered from blood and BAL of allergic asthmatics. Am J Respir Crit Care Med. 1994;149:345–351. doi: 10.1164/ajrccm.149.2.8306028. [DOI] [PubMed] [Google Scholar]
- 134.Bochner BS. Systemic activation of basophils and eosinophils: markers and consequences. J Allergy Clin Immunol. 2000;106:S292–S302. doi: 10.1067/mai.2000.110164. [DOI] [PubMed] [Google Scholar]
- 135.Johansson MW, Kelly EA, Busse WW, Jarjour NN, Mosher DF. Up-regulation and activation of eosinophil integrins in blood and airway after segmental lung antigen challenge. J Immunol. 2008;180:7622–7635. doi: 10.4049/jimmunol.180.11.7622. PMC:2585992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Grutters JC, Brinkman L, Aslander MM, van den Bosch JM, Koenderman L, Lammers JW. Asthma therapy modulates priming-associated blood eosinophil responsiveness in allergic asthmatics. Eur Respir J. 1999;14:915–922. doi: 10.1034/j.1399-3003.1999.14d31.x. [DOI] [PubMed] [Google Scholar]
- 137.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 138.Plotz SG, et al. The interaction of human peripheral blood eosinophils with bacterial lipopolysaccharide is CD14 dependent. Blood. 2001;97:235–241. doi: 10.1182/blood.v97.1.235. [DOI] [PubMed] [Google Scholar]
- 139.Sabroe I, Jones EC, Usher LR, Whyte MK, Dower SK. Toll-like receptor (TLR)2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J Immunol. 2002;168:4701–4710. doi: 10.4049/jimmunol.168.9.4701. [DOI] [PubMed] [Google Scholar]
- 140.Nagase H, et al. Expression and function of Toll-like receptors in eosinophils: activation by Toll-like receptor 7 ligand. J Immunol. 2003;171:3977–3982. doi: 10.4049/jimmunol.171.8.3977. [DOI] [PubMed] [Google Scholar]
- 141.Cirino G, Vergnolle N. Proteinase-activated receptors (PARs): crossroads between innate immunity and coagulation. Curr Opin Pharmacol. 2006;6:428–434. doi: 10.1016/j.coph.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 142.Shpacovitch V, Feld M, Bunnett NW, Steinhoff M. Protease-activated receptors: novel PARtners in innate immunity. Trends Immunol. 2007;28:541–550. doi: 10.1016/j.it.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 143.Miike S, McWilliam AS, Kita H. Trypsin induces activation and inflammatory mediator release from human eosinophils through protease-activated receptor-2. J Immunol. 2001;167:6615–6622. doi: 10.4049/jimmunol.167.11.6615. [DOI] [PubMed] [Google Scholar]
- 144.Miike S, Kita H. Human eosinophils are activated by cysteine proteases and release inflammatory mediators. J Allergy Clin Immunol. 2003;111:704–713. doi: 10.1067/mai.2003.1332. [DOI] [PubMed] [Google Scholar]
- 145.Matsuwaki Y, et al. Recognition of fungal protease activities induces cellular activation and eosinophil-derived neurotoxin release in human eosinophils. J Immunol. 2009;183:6708–6716. doi: 10.4049/jimmunol.0901220. PMC:2843542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wada K, et al. Inflammatory responses of human eosinophils to cockroach are mediated through protease-dependent pathways. J Allergy Clin Immunol. 2010;126:169–172. doi: 10.1016/j.jaci.2010.04.007. e162. PMC:2902556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bush RK, Prochnau JJ. Alternaria-induced asthma. J Allergy Clin Immunol. 2004;113:227–234. doi: 10.1016/j.jaci.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 148.O'Hollaren MT, et al. Exposure to an aeroallergen as a possible precipitating factor in respiratory arrest in young patients with asthma. N Engl J Med. 1991;324:359–363. doi: 10.1056/NEJM199102073240602. [DOI] [PubMed] [Google Scholar]
- 149.Kheradmand F, Kiss A, Xu J, Lee SH, Kolattukudy PE, Corry DB. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J Immunol. 2002;169:5904–5911. doi: 10.4049/jimmunol.169.10.5904. [DOI] [PubMed] [Google Scholar]
- 150.Inoue Y, Matsuwaki Y, Shin SH, Ponikau JU, Kita H. Nonpathogenic, environmental fungi induce activation and degranulation of human eosinophils. J Immunol. 2005;175:5439–5447. doi: 10.4049/jimmunol.175.8.5439. [DOI] [PubMed] [Google Scholar]
- 151.Yoon J, Ponikau JU, Lawrence CB, Kita H. Innate antifungal immunity of human eosinophils mediated by a beta 2 integrin, CD11b. J Immunol. 2008;181:2907–2915. doi: 10.4049/jimmunol.181.4.2907. PMC:2614918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dichmann S, et al. Adenosine triphosphate-induced oxygen radical production and CD11b up-regulation: Ca(++) mobilization and actin reorganization in human eosinophils. Blood. 2000;95:973–978. [PubMed] [Google Scholar]
- 153.Idzko M, et al. Functional characterization of P2Y and P2X receptors in human eosinophils. J Cell Physiol. 2001;188:329–336. doi: 10.1002/jcp.1129. [DOI] [PubMed] [Google Scholar]
- 154.Hirai H, et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med. 2001;193:255–261. doi: 10.1084/jem.193.2.255. PMC:2193345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 156.Rock KL, Kono H. The inflammatory response to cell death. Annu Rev Pathol. 2008;3:99–126. doi: 10.1146/annurev.pathmechdis.3.121806.151456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kobayashi T, Kouzaki H, Kita H. Human eosinophils recognize endogenous danger signal crystalline uric acid and produce proinflammatory cytokines mediated by autocrine ATP. J Immunol. 2010;184:6350–6358. doi: 10.4049/jimmunol.0902673. PMC:2874987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lotfi R, et al. Eosinophils oxidize damage-associated molecular pattern molecules derived from stressed cells. J Immunol. 2009;183:5023–5031. doi: 10.4049/jimmunol.0900504. [DOI] [PubMed] [Google Scholar]
- 159.Wang HB, Weller PF. Pivotal advance: eosinophils mediate early alum adjuvant-elicited B cell priming and IgM production. J Leukoc Biol. 2008;83:817–821. doi: 10.1189/jlb.0607392. PMC:2735468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Abu-Ghazaleh RI, Fujisawa T, Mestecky J, Kyle RA, Gleich GJ. IgA-induced eosinophil degranulation. J Immunol. 1989;142:2393–2400. [PubMed] [Google Scholar]
- 161.Dunne DW, Richardson BA, Jones FM, Clark M, Thorne KJ, Butterworth AE. The use of mouse/human chimaeric antibodies to investigate the roles of different antibody isotypes, including IgA2, in the killing of Schistosoma mansoni schistosomula by eosinophils. Parasite Immunol. 1993;15:181–185. doi: 10.1111/j.1365-3024.1993.tb00598.x. [DOI] [PubMed] [Google Scholar]
- 162.Monteiro RC, Hostoffer RW, Cooper MD, Bonner JR, Gartland GL, Kubagawa H. Definition of immunoglobulin A receptors on eosinophils and their enhanced expression in allergic individuals. J Clin Invest. 1993;92:1681–1685. doi: 10.1172/JCI116754. PMC:288327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lamkhioued B, Gounni AS, Gruart V, Pierce A, Capron A, Capron M. Human eosinophils express a receptor for secretory component. Role in secretory IgA-dependent activation. Eur J Immunol. 1995;25:117–125. doi: 10.1002/eji.1830250121. [DOI] [PubMed] [Google Scholar]
- 164.Motegi Y, Kita H. Interaction with secretory component stimulates effector functions of human eosinophils but not of neutrophils. J Immunol. 1998;161:4340–4346. [PubMed] [Google Scholar]
- 165.van de Winkel JG, Capel PJ. Human IgG Fc receptor heterogeneity: molecular aspects and clinical implications. Immunol Today. 1993;14:215–221. doi: 10.1016/0167-5699(93)90166-I. [DOI] [PubMed] [Google Scholar]
- 166.Moqbel R, et al. The effect of platelet-activating factor on IgE binding to, and IgE-dependent biological properties of, human eosinophils. Immunology. 1990;70:251–257. PMC:1384202. [PMC free article] [PubMed] [Google Scholar]
- 167.Truong MJ, Gruart V, Liu FT, Prin L, Capron A, Capron M. IgE-binding molecules (Mac-2/epsilon BP) expressed by human eosinophils. Implication in IgE-dependent eosinophil cytotoxicity. Eur J Immunol. 1993;23:3230–3235. doi: 10.1002/eji.1830231228. [DOI] [PubMed] [Google Scholar]
- 168.Gounni AS, et al. High-affinity IgE receptor on eosinophils is involved in defence against parasites. Nature. 1994;367:183–186. doi: 10.1038/367183a0. [DOI] [PubMed] [Google Scholar]
- 169.Seminario MC, Saini SS, MacGlashan DW, Jr, Bochner BS. Intracellular expression and release of Fc epsilon RI alpha by human eosinophils. J Immunol. 1999;162:6893–6900. [PubMed] [Google Scholar]
- 170.Kita H, et al. Does IgE bind to and activate eosinophils from patients with allergy? J Immunol. 1999;162:6901–6911. [PubMed] [Google Scholar]
- 171.Rot A, Krieger M, Brunner T, Bischoff SC, Schall TJ, Dahinden CA. RANTES and macrophage inflammatory protein 1 alpha induce the migration and activation of normal human eosinophil granulocytes. J Exp Med. 1992;176:1489–1495. doi: 10.1084/jem.176.6.1489. PMC:2119467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Alam R, et al. RANTES is a chemotactic and activating factor for human eosinophils. J Immunol. 1993;150:3442–3448. [PubMed] [Google Scholar]
- 173.Kita H, Abu-Ghazaleh RI, Sur S, Gleich GJ. Eosinophil major basic protein induces degranulation and IL-8 production by human eosinophils. J Immunol. 1995;154:4749–4758. [PubMed] [Google Scholar]
- 174.Daffern PJ, Pfeifer PH, Ember JA, Hugli TE. C3a is a chemotaxin for human eosinophils but not for neutrophils. I. C3a stimulation of neutrophils is secondary to eosinophil activation. J Exp Med. 1995;181:2119–2127. doi: 10.1084/jem.181.6.2119. PMC:2192052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kernen P, et al. Shape changes, exocytosis, and cytosolic free calcium changes in stimulated human eosinophils. J Clin Invest. 1991;87:2012–2017. doi: 10.1172/JCI115230. PMC:296956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dyer KD, et al. Mouse and human eosinophils degranulate in response to platelet-activating factor (PAF) and lysoPAF via a PAF-receptor-independent mechanism: evidence for a novel receptor. J Immunol. 2010;184:6327–6334. doi: 10.4049/jimmunol.0904043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bartemes KR, McKinney S, Gleich GJ, Kita H. Endogenous platelet-activating factor is critically involved in effector functions of eosinophils stimulated with IL-5 or IgG. J Immunol. 1999;162:2982–2989. [PubMed] [Google Scholar]
- 178.Kato M, et al. Platelet-activating factor activates two distinct effector pathways in human eosinophils. J Immunol. 2002;169:5252–5259. doi: 10.4049/jimmunol.169.9.5252. [DOI] [PubMed] [Google Scholar]
- 179.Kaneko M, Horie S, Kato M, Gleich GJ, Kita H. A crucial role for beta 2 integrin in the activation of eosinophils stimulated by IgG. J Immunol. 1995;155:2631–2641. [PubMed] [Google Scholar]
- 180.Horie S, Kita H. CD11b/CD18 (Mac-1) is required for degranulation of human eosinophils induced by human recombinant granulocyte-macrophage colony-stimulating factor and platelet-activating factor. J Immunol. 1994;152:5457–5467. [PubMed] [Google Scholar]
- 181.Kato M, Abraham RT, Okada S, Kita H. Ligation of the beta2 integrin triggers activation and degranulation of human eosinophils. Am J Respir Cell Mol Biol. 1998;18:675–686. doi: 10.1165/ajrcmb.18.5.2885. [DOI] [PubMed] [Google Scholar]
- 182.Nagata M, Sedgwick JB, Kita H, Busse WW. Granulocyte macrophage colony-stimulating factor augments ICAM-1 and VCAM-1 activation of eosinophil function. Am J Respir Cell Mol Biol. 1998;19:158–166. doi: 10.1165/ajrcmb.19.1.3001. [DOI] [PubMed] [Google Scholar]
- 183.Wegner CD, Gundel RH, Churchill L. Adhesion glycoproteins as regulators of airway inflammation: Emphasis on the role of ICAM-1. London: Academic Press; 1993. [Google Scholar]
- 184.Yoon J, Terada A, Kita H. CD66b regulates adhesion and activation of human eosinophils. J Immunol. 2007;179:8454–8462. doi: 10.4049/jimmunol.179.12.8454. [DOI] [PubMed] [Google Scholar]
- 185.Waterston RH, et al. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- 186.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 187.Rosenberg HF. The eosinophil ribonucleases. Cell Mol Life Sci. 1998;54:795–803. doi: 10.1007/s000180050208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Borchers MT, et al. In vitro assessment of chemokine receptor-ligand interactions mediating mouse eosinophil migration. J Leukoc Biol. 2002;71:1033–1041. [PubMed] [Google Scholar]
- 189.Stelts D, et al. Eosinophils retain their granule major basic protein in a murine model of allergic pulmonary inflammation. Am J Respir Cell Mol Biol. 1998;18:463–470. doi: 10.1165/ajrcmb.18.4.2957. [DOI] [PubMed] [Google Scholar]
- 190.Denzler KL, et al. Extensive eosinophil degranulation and peroxidase-mediated oxidation of airway proteins do not occur in a mouse ovalbumin-challenge model of pulmonary inflammation. J Immunol. 2001;167:1672–1682. doi: 10.4049/jimmunol.167.3.1672. [DOI] [PubMed] [Google Scholar]
- 191.Behm CA, Ovington KS. The role of eosinophils in parasitic helminth infections: insights from genetically modified mice. Parasitol Today. 2000;16:202–209. doi: 10.1016/s0169-4758(99)01620-8. [DOI] [PubMed] [Google Scholar]
- 192.Meeusen EN, Balic A. Do eosinophils have a role in the killing of helminth parasites? Parasitol Today. 2000;16:95–101. doi: 10.1016/s0169-4758(99)01607-5. [DOI] [PubMed] [Google Scholar]
- 193.Sher A, Coffman RL, Hieny S, Cheever AW. Ablation of eosinophil and IgE responses with anti-IL-5 or anti-IL-4 antibodies fails to affect immunity against Schistosoma mansoni in the mouse. J Immunol. 1990;145:3911–3916. [PubMed] [Google Scholar]
- 194.Herndon FJ, Kayes SG. Depletion of eosinophils by anti-IL-5 monoclonal antibody treatment of mice infected with Trichinella spiralis does not alter parasite burden or immunologic resistance to reinfection. J Immunol. 1992;149:3642–3647. [PubMed] [Google Scholar]
- 195.Takamoto M, Ovington KS, Behm CA, Sugane K, Young IG, Matthaei KI. Eosinophilia, parasite burden and lung damage in Toxocara canis infection in C57Bl/6 mice genetically deficient in IL-5. Immunology. 1997;90:511–517. doi: 10.1046/j.1365-2567.1997.00208.x. PMC:1456701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Le Goff L, Loke P, Ali HF, Taylor DW, Allen JE. Interleukin-5 is essential for vaccine-mediated immunity but not innate resistance to a filarial parasite. Infect Immun. 2000;68:2513–2517. doi: 10.1128/iai.68.5.2513-2517.2000. PMC:97453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Swartz JM, et al. Schistosoma mansoni infection in eosinophil lineage-ablated mice. Blood. 2006;108:2420–2427. doi: 10.1182/blood-2006-04-015933. PMC:1895572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Korenaga M, Hitoshi Y, Yamaguchi N, Sato Y, Takatsu K, Tada I. The role of interleukin-5 in protective immunity to Strongyloides venezuelensis infection in mice. Immunology. 1991;72:502–507. PMC:1384368. [PMC free article] [PubMed] [Google Scholar]
- 199.Yoshida T, et al. Defective B-1 cell development and impaired immunity against Angiostrongylus cantonensis in IL-5R alpha-deficient mice. Immunity. 1996;4:483–494. doi: 10.1016/s1074-7613(00)80414-8. [DOI] [PubMed] [Google Scholar]
- 200.Pearce EJ, Sher A. Functional dichotomy in the CD4+ T cell response to Schistosoma mansoni. Exp Parasitol. 1991;73:110–116. doi: 10.1016/0014-4894(91)90014-n. [DOI] [PubMed] [Google Scholar]
- 201.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]