Abstract
Chondrocyte CD44 receptors anchor hyaluronan to the cell surface, enabling the assembly and retention of proteoglycan aggregates in the pericellular matrix. Hyaluronan–CD44 interactions also provide signaling important for maintaining cartilage homeostasis. Disruption of chondrocyte–hyaluronan contact alters CD44 occupancy, initiating alternative signaling cascades. Treatment with hyaluronan oligosaccharides is one approach to uncouple CD44 receptors from its native ligand, hyaluronan. In bovine articular chondrocytes, treatment with hyaluronan oligosaccharides or purified hyaluronan hexasaccharides induced the production of nitric oxide that mirrored nitric oxide production following interleukin-1 treatment. In contrast, 120 and 1260 kDa hyaluronan did not induce production of nitric oxide. Human chondrocytes responded similarly to treatment with hyaluronan or hyaluronan oligosaccharides. Nitric oxide production from chondrocytes was mediated by activation of the inducible nitric oxide synthase, as confirmed by mRNA expression and inhibition of nitric oxide production by diphenyleneiodonium. Co-treatment of chondrocytes with hyaluronan oligosaccharides and interleukin-1 did not demonstrate additive effects. Blocking interleukin-1 receptors with an antagonist did not abolish the production of nitric oxide induced by treatment with hyaluronan oligosaccharides. Moreover, only COS-7 following transfection with a pCD44, not the CD44-null parental cells, responded to treatment with hyaluronan oligosaccharides by releasing nitric oxide. This study demonstrates a novel signaling potential by hyaluronan fragments, in lieu of endogenous hyaluronan–chondrocyte interactions, resulting in the activation of inducible nitric oxide synthase.
Keywords: Chondrocytes, Hyaluronan, Interleukin-1 (IL-1), Nitric oxide (NO), CD44
1. Introduction
Articular cartilage is sparsely populated by chondrocytes surrounded by an extensive extracellular matrix (ECM). Chondrocyte–ECM interactions are essential for cartilage homeostasis. The glycosaminoglycan hyaluronan (HA) is a scaffold in the organization of a highly hydrated ECM yet also has the capacity to interact directly with cells (Knudson & Knudson, 1993). The most widely expressed HA receptor is CD44. Chondrocyte CD44 receptors facilitate the retention of HA and proteoglycan aggregates to the cell surface and within the pericellular matrix (Knudson & Knudson, 2004). Compartmentalized structural elements may enable chondrocytes to acquire information from the ECM and transmit it to cytoplasmic domains via CD44 receptors. During pathologic states such as osteoarthritis (OA), an increase in the fragmentation of ECM macromolecules is observed, concurrent with the disruption of chondrocyte–ECM interactions. HA oligosaccharides have the capacity to displace the binding of macro-molecular HA from CD44 receptors (Hua, Knudson, & Knudson, 1993; Knudson, Aguiar, Hua, & Knudson, 1996). Moreover, in vitro studies have demonstrated that HA oligosaccharides alter chondrocyte metabolism and induce chondrocytic chondrolysis. Enhanced MMP activity was observed after HA oligosaccharide treatment of both cartilage explants and chondrocytes in culture balanced by increased synthesis of both HA and aggrecan, but no change in expression of CD44 receptors (Nishida, Knudson, & Knudson, 2003; Ohno, Im, Knudson, & Knudson, 2005). Thus, disruption of HA-chondrocyte interactions by HA oligosaccharides initiates both anabolic and catabolic responses. In addition, treatment of articular chondrocytes with CD44-directed antisense oligonucleotides enhanced catabolism with depletion of proteoglycans within the matrix (Chow, Nietfeld, Knudson, & Knudson, 1998). Therefore, uncoupling of CD44 receptors from the native ligand, HA, activates alternative intracellular signaling cascades that initiate matrix remodeling. Outside-in signaling via CD44 receptors might be consequential for repair processes in early stages of OA.
Nitric oxide (NO) is a potent mediator produced by the inducible nitric oxide synthase (iNOS, NOS2) during inflammatory reactions involved in infection, disease or tissue damage (Galea & Feinstein, 1999). IL-1 and TNF-α stimulate iNOS and NO production by articular chondrocytes (Abramson, Attur, Amin, & Clancy, 2001; Evans, Watkins, & Stefanovic-Racic, 1996; Goodstone & Hardingham, 2002; Goldring & Berenbaum, 2004; Kuhn, Shikhman, & Lotz, 2003). However, in OA cartilage other mediators might be required to overturn the metabolic balance and propagate a continuous imbalance between catabolic or anabolic activities. Although chondrocytes have the capacity to produce NO subsequent to IL-1 treatment, this effect was counteracted by addition of sodium hyaluronate (Fukuda et al., 2001). Consequently, HA might play a modulatory role during inflammatory processes as well as OA, with the caveat that effects of HA are size- and tissue type-specific. The present study elucidates the role of HA oligosaccharides in activating CD44 receptors, followed by the induction of the iNOS gene, resulting in NO production from articular chondrocytes.
2. Materials and methods
2.1. Materials and reagents
Dulbecco’s modified Eagle’s medium (DMEM), Ham’s F12 medium and Hank’s balanced salt solution (HBSS) were purchased from Mediatech Inc. (Herndon, VA) and fetal bovine serum (FBS) from HyClone (Logan, UT). Streptomyces hyaluronidase, testicular hyaluronidase, sodium hyaluronate (Grade I), chondroitin-4-sulfate sodium salt, chondroitin-6-sulfate sodium salt, potassium nitrate, NADH, diphenyleneiodonium (DPI), sulfanilamide, NED and L-glutamine were purchased from Sigma–Aldrich (St. Louis, MO). High molecular weight HA (1260 kDa) and intermediate molecular weight HA (120 kDa) were obtained from Genzyme (Cambridge, MA). Purified HA4 and HA6 were obtained from the Seikagaku Corporation (Japan) (Tawada et al., 2002). TrizolR reagent, AmpliTaq DNA polymerase and gentamicin were purchased from Invitrogen (Carlsbad, CA). Zea mays nitrate reductase was purchased from NECi (Lake Linden, MI) and hrIL-1α and hrIL-1β from R&D Systems (Minneapolis, MN). The GeneAmp RNA PCR kit was purchased from Perkin-Elmer (Norwalk, CT) and primers from DNA Technologies (Coralville, IA). Anakinra, an IL-1 receptor antagonist protein (IRAP), was provided by Amgen (Thousand Oaks, CA).
2.2. Preparation of HA oligosaccharides
Additionally, HA oligosaccharides (HAoligos) were prepared from HA (Sigma, grade I) by digestion with testicular hyaluronidase (Type I-S) at a ratio of 320 U/mg HA in 0.1 M Na acetate buffer pH 5 for 16 h at 37 °C. This protocol generates a mixture of oligosaccharides containing primarily HA tetrasaccharides (HA4), HA hexasaccharides (HA6) and HA octasaccharides (HA8), with some HA8+ (Knudson et al., 2000). HAoligos in suspension were vacuum-dried and reconstituted at 4 mg/ml in PBS.
2.3. Chondrocyte isolation and culture
Human cartilage was obtained from donors through the Gift of Hope Organ and Tissue Donor Network, Chicago, IL. The ages of the donors ranged from 54 to 83 years. The ankle joint samples were obtained by the Core Facility pathologist in accordance with institutional guidelines within 24 h post mortem and cartilage was dissected from the talar dome. All articular cartilage samples showed either no signs of cartilage lesions (Collins grade 0) or very limited disruptions of the articular surface (Collins grade 1) (Muehleman, Bareither, Huch, Cole, & Kuettner, 1997). Bovine articular cartilage was dissected from the metacarpophalangeal joint of animals of about 18 months of age obtained from a local slaughterhouse. Explant cultures were established from 1 mm × 5 mm × 5 mm articular cartilage slices.
Chondrocytes were isolated from cartilage by sequential enzymatic digestion with 0.2% pronase (Calbiochem, San Diego, CA), followed by 0.025% collagenase-P (Roche Diagnostics, Indianapolis, IN) in DMEM containing 5% FBS at 37°C and encapsulated in 1.2% alginate gel (Chow, Knudson, Homandberg, & Knudson, 1995). Cultures of chondrocytes in alginate gel beads and cartilage explants were maintained in DMEM/F12 supplemented with 10% FBS, gentamicin, and 25 μg/ml ascorbic acid, at 37 °C in a humidified 95% air/5% CO2 atmosphere.
Following equilibration for 5 days, chondrocytes in alginate beads or cartilage explants were transferred to DMEM/F12 media (without phenol red) containing 10% heat-inactivated FBS and gentamicin. Thereafter, cultures were treated without (control group) or with HA fragments, intact glycosaminoglycans or IL-1 for the required time periods. When indicated, chondrocytes in alginate beads were pre-treated with 5 U/ml Streptomyces hyaluronidase overnight at 37 °C in media with 10% FBS. Following treatments, chondrocytes were released from alginate beads with 55 mM Na citrate in 0.15 M NaCl (Chow et al., 1995). Total RNA and protein from chondrocytes were isolated with TrizolR reagent according to the manufacturer’s specifications.
As alternative approaches, following isolation chondrocytes were cultured in suspension in spin flasks at 37 °C, in a humidified 95% air/5% CO2 atmosphere (Sommarin & Heinegard, 1986) or plated as high density monolayers at 4.2 × 105 cells/100 mm2 culture surface area.
2.4. COS-7 cell culture
COS-7 cells (ATCC, Rockville, MD) were transiently transfected using LipofectAMINE 2000 (Invitrogen) with pCD44H, containing the full-length construct of the human hematopoietic isoform CD44H subcloned into the GFP co-expression vector pTracer-SV40 (Invitrogen) (Jiang et al., 2002). Successful uptake and expression of the CD44H construct was confirmed by fluorescence microscopy (Nikon TE2000) with cells expressing GFP and positive staining for cell-surface CD44, as detected by immunocytochemistry with BU52 antibodies (The Binding Site Ltd., San Diego, CA), determined a transfection efficiency of approximately 40%. Two days following transfection, cells were incubated without or with 250 μg/ml HAoligos for 24 or 48 h.
2.5. Nitrite determination
The culture media was analyzed for NO by the colorimetric assay based on the Griess reaction (Granger, Taintor, Boockvar, & Hibbs, 1996). Conditioned media was filtered through 10,000 mwco filter units (Millipore; Bedford, MA) and aliquots were incubated with Zea mays nitrate reductase in 50 mM MOPS buffer and NADH for 25 min at room temperature. Following enzymatic conversion, samples were reacted with the Griess reagent (equal parts of 1% sulfanilamide in 3N HCl and 0.1% NED in H2O) and absorbance was detected at 530 nm. The concentration of total nitrites was calculated from a potassium nitrate standard curve and expressed per microgram total cellular protein isolated from each cell culture with TrizolR reagent or per mg weight for each cartilage explant culture. Values represent the average ± range of duplicate analyses of replicate cultures as indicated in the figure legends and were compared by an unpaired student’s t-test.
2.6. RT-PCR and real-time PCR analysis
Total RNA was isolated from bovine and human articular chondrocytes with TrizolR reagent according to the manufacturer’s instructions. Aliquots (0.5 μg) of the RNA were reverse transcribed with MuLV reverse transcriptase in the presence of 0.15 μM iNOS-specific downstream primers (bovine iNOS: 5′-ATG GCC GAC CTG ATG TTG CC-3′, human iNOS: 5′-TTT AGC TCC AGT TCC CGA A-3′) using the PTC-100TM Programmable Thermal Controller (MJ Research Inc., Watertown, MA). GAPDH primers used were as previously reported (Chow et al., 1995; Ohno et al., 2005). The resultant cDNA were amplified in the presence of 0.15 μM upstream primers for iNOS (bovine iNOS: 5′-CGG AGT GAC TTT CCA AGA CAC GC-3′, human iNOS: 5′-AAC ATC AGG TCG GCC ATC ACC G-3′), in a PCR mixture consisting of 2 mM MgCl2, 200 μM of each deoxyribonucleotide, and AmpliTaq DNA polymerase. The bovine cDNA was denatured by heating at 95°C for 2 min, followed 30 cycles of 95 °C for 30 s, 58 °C for 1 min and 72 °C for 2 min and, a final extension step at 72 °C for 7 min. Human cDNA was amplified under the same conditions except the annealing temperature was 56 °C. The amplified products were separated by electrophoresis on 1% (bovine iNOS) or 1.5% (human iNOS) agarose gels and stained with ethidium bromide. Bands were scanned using a Fluor-S MultiImager (Bio-Rad Laboratories, Hercules, CA) and mean densities were quantified using Quantity One 4.1.1 software (Bio-Rad). Real-time RT-PCR was performed with a SmartCycler II (Cepheid, CA). Copy number values were calculated by comparison of crossing point (CP) values. CP is the PCR cycle number that represents the peak of the second derivative of change in SYBRR Green fluorescence intensity (Molecular Probes, Eugene, OR). Copy numbers for iNOS mRNA (expressed as CP values) were determined by comparison to an iNOS standard curve prepared using quantified dilutions of reverse-transcribed single-stranded cDNA of iNOS, plotting the log of the cDNA concentration versus the CP values. Values were also normalized in response to small changes in GAPDH copy numbers.
3. Results
3.1. NO production by chondrocytes
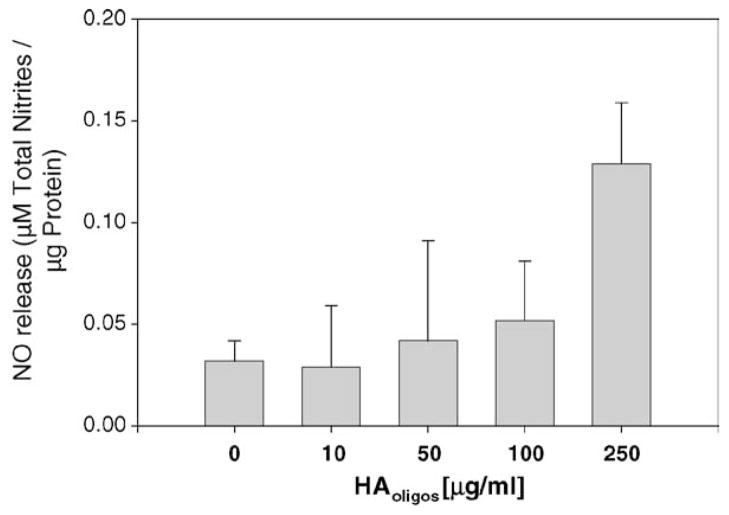
Bovine articular chondrocytes within alginate bead cultures showed an increased production of NO after treatment with HAoligos that became significant at a concentration of 250 μg/ml (Fig. 1). NO production increased ~4.3-fold as compared to basal levels observed in the control cultures. Production of NO was further enhanced following treatment with 400 μg/ml HAoligos (data not shown). Next, the temporal production of NO from bovine articular chondrocytes following treatment with 250 μg/ml HAoligos or 1 ng/ml IL-1α; was determined. As shown in Fig. 2, the increase in NO production by bovine chondrocytes following treatment with HAoligos mirrored NO release induced by IL-1α at 3, 6 and 24 h. An increased level of NO production was also detected following 48 h of HAoligos treatment (data not shown).
Fig. 1.
Production of NO from bovine articular chondrocytes following treatment with HA oligosaccharides. Bovine articular chondrocytes in alginate beads were treated without (0), or with increasing doses of 10, 50, 100 and 250 μg/ml HAoligos for 24 h. NO production was quantified and expressed as μM total nitrites/μg total cellular protein. Values represent the average ± range S.E.M. of two cultures (10–100 μg/ml) or eight cultures (0 or 250 μg/ml), analyzed in duplicate.
Fig. 2.
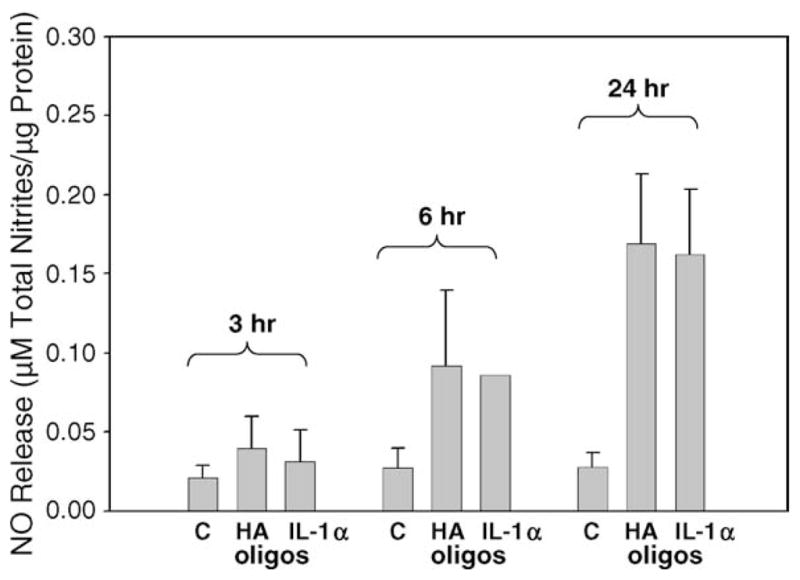
Time-dependent production of NO by articular chondrocytes induced by treatment with HAoligos and IL-1. Bovine articular chondrocytes in alginate beads were treated without (C) or with 250 μg/ml HAoligos or 1 ng/ml hrIL-1α for 3, 6 and 24 h. NO production is expressed as μM total nitrites/μg total cellular protein. Values represent the average ± range of four cultures, analyzed in duplicate.
To further investigate the specificity of the response, NO production in response to HAoligos was compared to chondrocytes treated with purified preparations of HA6 (Tawada et al., 2002), with high molecular weight glycosaminoglycans or IL-1α. Treatment of chondrocytes in high-density monolayer cultures with HAoligos or with highly purified HA6 (Seikagaku) at concentrations of 100 or 250 μg/ml resulted in similar NO production, but somewhat lower than the response to IL-1α (Fig. 3). Incubation of chondrocytes with 250 μg/ml of high molecular weight HA (the HA used to prepare the HAoligos) or 1260 kDa HA resulted in a slight decrease in NO release as compared with untreated controls as did incubation with the intact glycosaminoglycans chondroitin-4-sulfate or chondroitin-6-sulfate (Fig. 3). Enhanced production of NO was also detected in explant cultures of bovine articular cartilage slices treated with HAoligos or with purified HA6 for 48 h (Fig. 4) with a similar dose response as shown in Fig. 1.
Fig. 3.
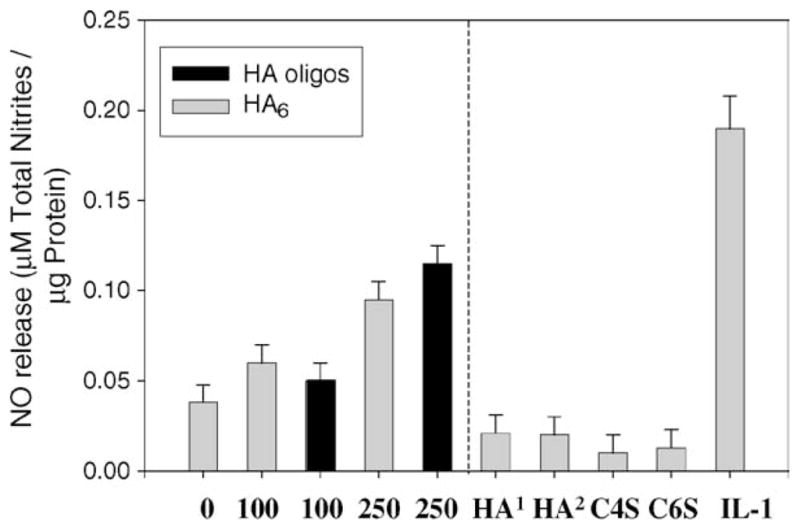
Production of NO from bovine articular chondrocytes in high density monolayer cultures. Chondrocytes were incubated with 0, 100 or 250 μg/ml HA6 (Seikagaku; gray bars) or HAoligos (black bars); bars to the left of the dotted line represent NO production by chondrocytes incubated with 250 μg/ml of HA1 (Grade I HA; Sigma), or HA2 (Genzyme; 1260 kDa), chondroitin-4-sulfate (C4S), chondroitin-6-sulfate (C6S) or with 1 ng/ml hrIL-1α for 24 h. NO production is expressed as μM total nitrites/μg total cellular protein. Values represent the average ± range of two cultures, analyzed in duplicate.
Fig. 4.
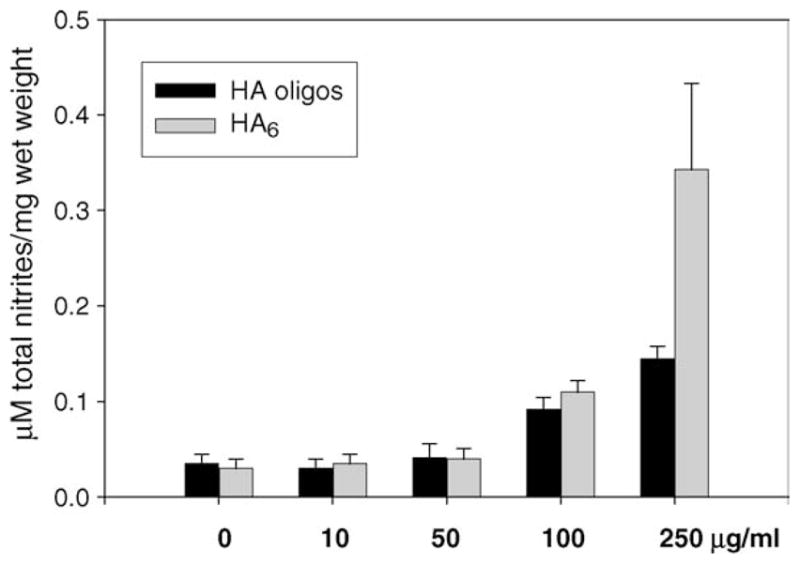
NO production in bovine articular cartilage explant cultures. Cartilage explants were treated without (0), or with increasing doses of 10, 50, 100 and 250 μg/ml of HAoligos or purified HA6 for 48 h. NO production was quantified and values represent average ± range of μM total nitrites/mg wet weight of two experiments analyzed in duplicate.
In aging cartilage, HA fragmentation is increased generating intermediate-sized HA (Holmes, Bayliss, & Muir, 1988). To determine whether chondrocytes also respond to intermediate-sized HA, bovine and human articular chondrocytes treated with HA of several sizes. Whereas HAoligos stimulated NO production from both bovine articular chondrocytes (4.3-fold) and human articular chondrocytes (12.8-fold), neither the 1260 kDa HA (hmwHA) nor the 120 kDa HA fragments (imwHA) induced the production of NO above basal levels observed in control cultures (Table 1). To confirm the lack of response by chondrocytes to treatment with hmwHA, and to eliminate the issue of penetration of HA through the pericellular matrix or alginate, spin cultures of freshly isolated bovine chondrocytes were established. Chondrocytes in suspension responded similarly to chondrocytes in alginate beads; treatment for 24 h with HAoligos but not treatment with hmwHA stimulated NO production (Table 1). Enhanced production of NO was detected in explant cultures of bovine and human articular cartilage slices treated with HAoligos or IL-1α for 24 h (Table 1). IL-1β also stimulated the production of NO in bovine articular cartilage explant and alginate bead cultures (Table 1). In parallel, explant and alginate bead cultures were treated with 250 μg/ml of the purified preparations of HA6 and HA4 for 24 h (Tawada et al., 2002). Enhanced production of NO was found in bovine articular cartilage explant and chondrocyte cultures treated with HA6 but not with HA4 (Table 1). This suggests that HA6—the minimum size HA fragment that occupies the HA binding site of CD44 (Teriete et al., 2004)—is also the minimum size for stimulation of NO production. The similarities in responses to the HA6 and HAoligos and the lack of response to the high molecular weight HA suggests that the stimulation in response to HAoligos is not due to a contaminant. In summary, small HA oligosaccharides of at least the size of a hexasaccharides, but not ≥120 kDa, stimulated the production of NO from chondrocytes to a similar extent as stimulation with IL-1, in cartilage explant or chondrocyte cultures derived from human or bovine articular cartilage.
Table 1.
Production of NO by bovine and human articular chondrocytes
| Culture condition | Bovine
|
Human
|
|||
|---|---|---|---|---|---|
| Alginatea | Spin culturea | Explantsb | Alginatea | Explantsb | |
| Control | 0.030 ± 0.01 | 0.032 ± 0.009 | 0.018 ± 0.003 | 0.013 ± 0.01 | <0.001 |
| HAoligos | 0.129 ± 0.03 | 0.098 ± 0.030 | 0.145 ± 0.01 | 0.167 ± 0.04 | 0.418 |
| IL-1α | 0.162 ± 0.03 | 0.095 ± 0.003 | 0.220 ± 0.00 | 0.206 ± 0.02 | 0.347 |
| IL-1β | 0.280 ± 0.04 | nd | 0.398 ± 0.02 | nd | nd |
| HA6 | 0.150 ± 0.03 | nd | 0.476 ± 0.03 | nd | nd |
| HA4 | 0.053 ± 0.01 | nd | 0.004 ± 0.01 | nd | nd |
| imw HA | 0.031 ± 0.02 | nd | nd | 0.016 ± 0.003 | nd |
| hmw HA | 0.020 ± 0.01 | 0.038 ± 0.012 | nd | nd | nd |
Chondrocytes maintained in alginate beads or spin cultures, and cartilage explant cultures were treated for 24 h with 250 μg/ml HAoligos, HA6, HA4, imwHA (120 kDa), hmwHA (1260 kDa), 1 ng/ml hrIL-1α or 10 ng/ml hrIL-1β. NO production was quantified and values represent average ± range of 3 to 8 experiments analyzed in duplicate.
Notes: nd, not determined.
μM total nitrites/μg cellular protein.
μM total nitrites/mg wet weight.
3.2. Induction of iNOS
Previous studies have demonstrated that enhanced NO production is due, in part, to the induction of the iNOS isozyme in activated cells. Hence, the effect of HAoligos on chondrocyte iNOS mRNA was examined. As shown in Fig. 5, semi-quantitative RT-PCR analysis demonstrated a substantial increase in iNOS mRNA expression in both bovine (panel A, lane 3) and human (panel B, lane 3) articular chondrocytes incubated with HAoligos. On the other hand, incubation of bovine chondrocytes with imwHA (Fig. 5A, lane 4) resulted in little change in iNOS mRNA as compared to control cells (lane 2). Parallel increases in iNOS mRNA were observed in bovine and human chondrocytes treated with IL-1α used as positive control. Treatment of human articular chondrocytes with HAoligos and 1 ng/ml IL-1α combined had no additive effect on iNOS mRNA expression (Fig. 5B, lane 5). To obtain more precise quantification at lower cycle numbers, real time RT-PCR was performed. After 24 h of treatment with HAoligos bovine articular chondrocytes exhibited a 45-fold increase in iNOS mRNA copy number as compared to control, untreated cells and a 39-fold increase due to treatment with 1 ng/ml hrIL-1α. The enhanced response to HAoligos as compared to IL-1α is consistent with the standard RT-PCR results shown in Fig. 5A (lane 3 versus lane 5). Furthermore, iNOS mRNA copy numbers were increased by 128-fold over that of control cells after 3 h of treatment with HAoligos.
Fig. 5.
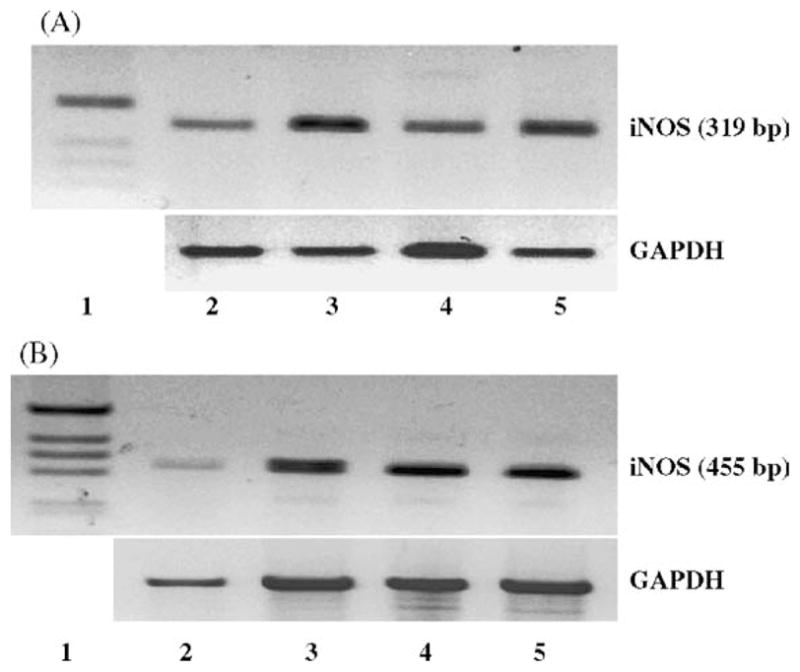
Identification of enhanced iNOS expression in bovine and human articular chondrocytes following treatment with HAoligos and IL-1. iNOS mRNA was detected by RT-PCR amplification of total RNA isolated from bovine (5A) and human (5B) articular chondrocytes following 24-h treatment without (lane 2) or with 250 μg/ml HAoligos (lane 3), 1.0 ng/ml hrIL-1α (A, lane 5; B, lane 4), 250 μg/ml imwHA (A, lane 4), or co-treatment with IL-1α and HAoligos (B, lane 5). Products were separated by agarose gel electrophoresis, visualized by ethidium bromide staining and compared with φX174 DNA/Hae III markers or the 100 bp DNA ladder (lane 1). Insets below: GAPDH primers were used to assess loading of total RNA for individual samples.
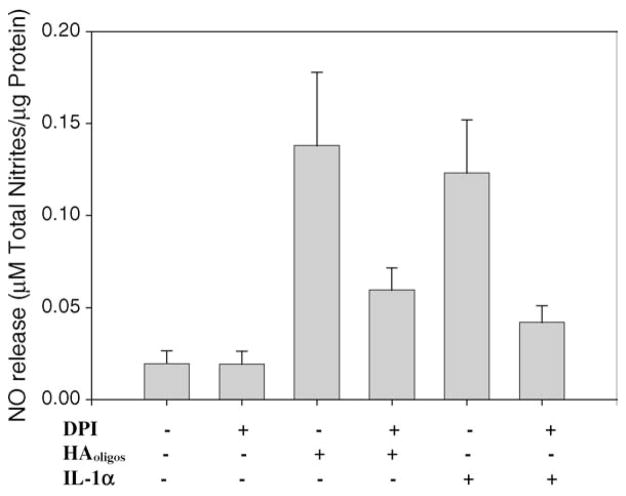
To further explore the role of the iNOS in the NO signaling pathway, bovine chondrocytes in alginate beads were stimulated with IL-1α or HAoligos in the presence or absence of DPI, a flavin-dependent inhibitor of iNOS enzymatic activity (Mendes, Carvalho, Caramona, & Lopes, 2001). As shown in Fig. 6, DPI treatment abolished NO production induced by both HAoligos (P < 0.05) and IL-1α (P < 0.02).
Fig. 6.
Inhibition of iNOS enzymatic activity affects production of NO from articular chondrocytes activated by treatment with HAoligos. Bovine chondrocytes in alginate beads were treated without (−) or with 250 μg/ml HAoligos, or 1 ng/ml hrIL-1α, in the presence or absence of DPI for 24 h. NO production is expressed as μM total nitrites/μg total cellular protein. Values represent the average ± range of three experiments analyzed in duplicate.
3.3. CD44 receptors in the induction of NO
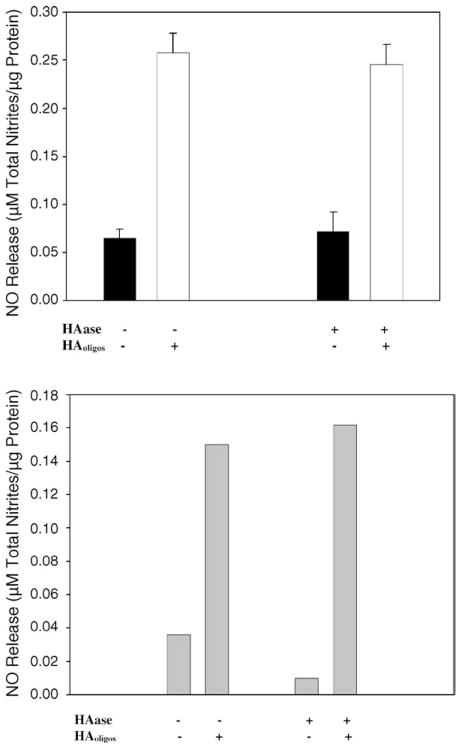
Chondrocyte CD44 receptors function primarily as adhesion molecules for HA. As such, HAoligos can displace the native HA-dependent pericellular matrix of chondrocytes (Knudson, 1993). To determine whether binding of HAoligos alone or, the displacement of a pericellular matrix was required to initiate signaling, the effect of HAoligos on chondrocytes possessing an intact pericellular matrix was compared to chondrocytes depleted of matrix by pre-treatment with Streptomyces hyaluronidase (Knudson, 1993). As shown in Fig. 7, the magnitude of NO production from bovine (Fig. 7A) or human (Fig. 7B) chondrocytes was not influenced by removal of pericellular matrices. Therefore, it would appear that the displacement of pericellular matrix is not required for induction of NO signaling. However, new synthesis of receptor-bound HA may have occurred during the treatment period (Knudson, 1998).
Fig. 7.
HAoligos treatment of articular chondrocytes induces NO production autonomous of an intact pericellular matrix. Bovine chondrocytes (panel A) and human articular chondrocytes (panel B) in alginate beads were incubated without (−), or pre-treated with Streptomyces hyaluronidase (+ HAase) at 37 °C, overnight. The beads were washed and treated without (−) or with (+) 250 μg/ml HAoligos for 24 h, and the conditioned media were processed for NO production. Values represent the average ± range of 3 experiments (panel A) or one representative experiment (panel B) analyzed in duplicate.
To further validate the participation of CD44-mediated signaling in NO production, COS-7 cells transfected with pCD44 were used as a model. As shown in Fig. 8, parental, CD44-null COS-7 cells did not exhibit augmented NO release when treated with HAoligos. However, for the COS-7 transfectants, 40% of the cells in the cultures being positive for CD44 expression, there was enhanced NO production subsequent to activation by HAoligos (Fig. 8), with a P < 0.05 as determined with an unpaired Student’s t test as compared with transfectants cultured in the absence of HAoligos. Therefore, NO release from COS-7 cells stimulated by HAoligos requires the expression of CD44 and thus implicates the role of chondrocyte CD44 receptors in the induction of signaling pathways that activate the iNOS gene resulting in NO production.
Fig. 8.
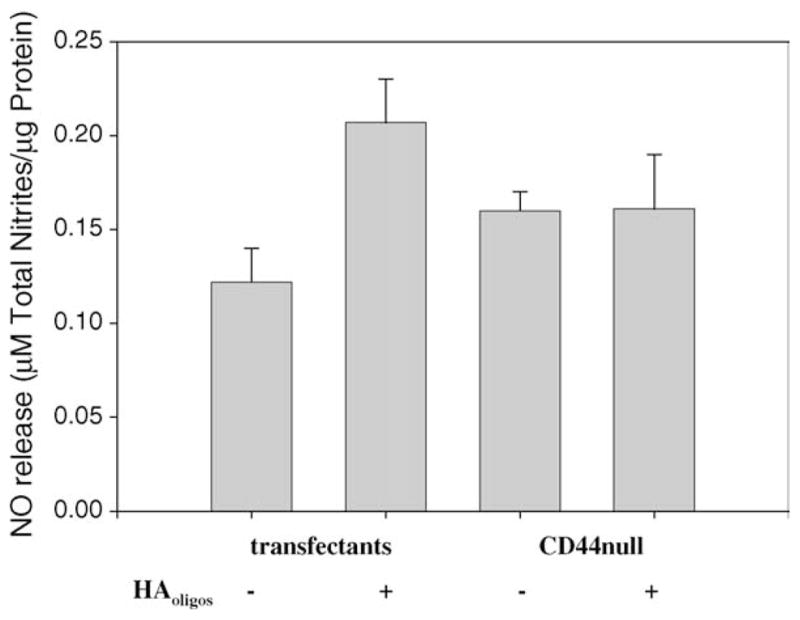
Production of NO induced by treatment with HAoligos occurs via a CD44-dependent mechanism. COS-7 cells (CD44-null) were transfected with pCD44H. Parental COS-7 cells and COS-7 transfectants were treated without (−) or with (+) 250 μg/ml HAoligos. NO production is expressed as μM total nitrites/μg total cellular protein. Each bar represents the average ± range of six individual cultures analyzed in duplicate.
3.4. NO production in response to HAoligos does not require IL-1 activity
Inflammatory episodes associated with OA may also include autocrine or paracrine release of mediators such as IL-1. To explore possible relationships between HAoligos-mediated and IL-1-mediated signaling pathways associated with NO release, assays with combinatorial additions were performed. Treatment of human or bovine articular chondrocytes with 1 ng/ml IL-1α plus HAoligos combined had no additive effect on NO production (data not shown) confirming the iNOS mRNA data (Fig. 5B). Treatment of bovine chondrocytes in alginate beads with 10 ng/ml IL-1β for 24 h resulted in enhanced NO release as expected (Fig. 9), but sub-maximal release resulted from 24 h treatment with 1 ng/ml IL-1β. The addition of 250 μg/ml HAoligos to cells primed with 1 ng/ml IL-1β concentration resulted in a NO response that was additive but not synergistic. Therefore, the signaling pathways induced by IL-1 and HAoligos may have some elements in common, or the HAoligos could induce autocrine expression of IL-1. Thus, whether the effect of HAoligos-mediated NO production is dependent on the initial induction of IL-1 and its subsequent stimulation of NO production was then investigated. Chondrocytes in alginate beads were pre-treated with IRAP and then treated with HAoligos or IL-1β. Blockade of IL-1 receptors by IRAP completely abolished NO production induced by 10 ng/ml IL-1β. In contrast to this significant inhibition of NO production, pre-treatment with IRAP resulted in only ~20% reduction in NO production sub-sequent to HAoligos treatment of articular chondrocytes (Fig. 10).
Fig. 9.
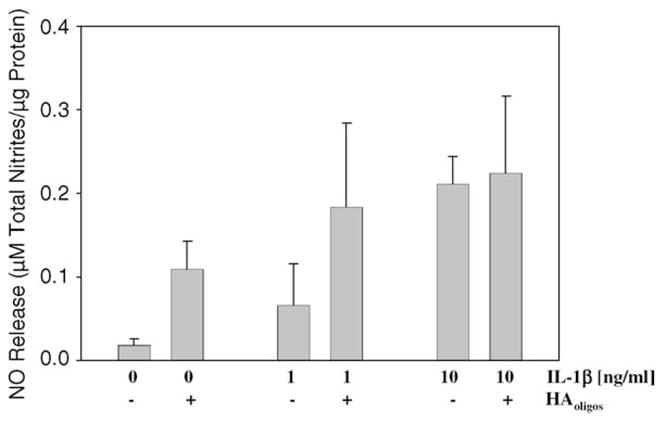
Modulation of NO production from articular chondrocytes following treatment with HAoligos and IL-1β. Bovine articular chondrocytes in alginate beads were incubated without (0) or pre-treated with 1 or 10 ng/ml hrIL-1β for 24 h. Thereafter, chondrocyte cultures were treated for an additional 24 h without (−) or with (+) 250 μg/ml HAoligos. Production of NO is expressed as μM total nitrites/μg total cellular protein. Values represent average ± range of four cultures in each treatment group analyzed in duplicate.
Fig. 10.
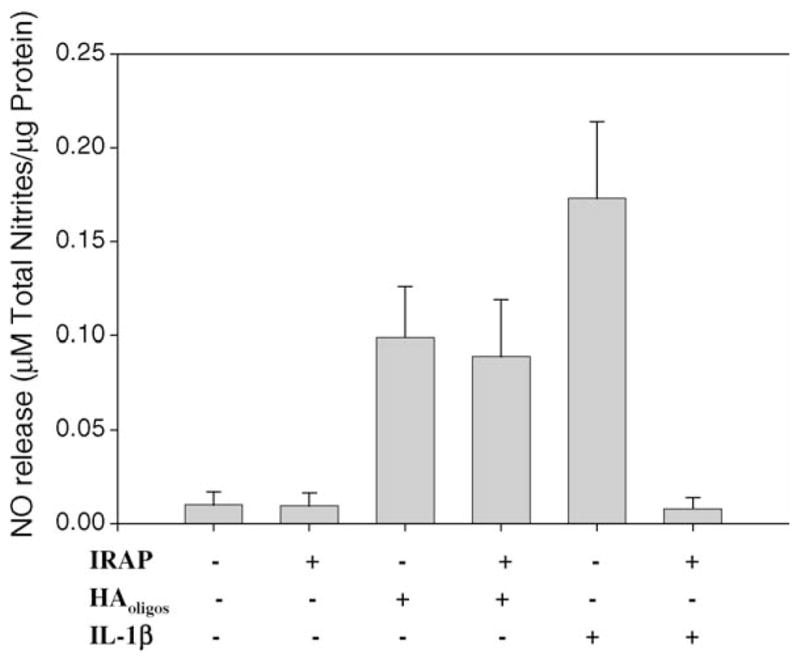
Inhibition by IRAP does not abolish production of NO induced by treatment with HAoligos. Bovine articular chondrocytes in alginate beads were pre-treated with 100 nM IRAP for 30 min; thereafter, chondrocytes were treated with 250 μg/ml HAoligos or 10 ng/ml hrIL-1β for 24 h. Production of NO is expressed as μM total nitrites/μg total cellular protein. Values represent the average ± range of three experiments analyzed in duplicate.
4. Discussion
In cartilage, cell–matrix interactions are critical to maintaining tissue homeostasis. These interactions are the primary means for chondrocytes to sense changes in the extracellular environment, such as when to mount a reparative response. HA binding to CD44 represents one such interaction—providing a structural link between the ECM, the plasma membrane and the cortical cytoplasm of chondrocytes (Nofal & Knudson, 2002). The present study elucidates the potential activation of CD44 resulting in stimulated NO production and induction of iNOS gene expression in response to HAoligos.
Previously, we have shown that small HA oligosaccharides, such as HA6 act as antagonists to high molecular mass HA, interfering with normal chondrocyte cell–matrix interactions such as the assembly of peri-cellular matrix (Maleski & Knudson, 1996; Ohno et al., 2005). HA6 compete with high molecular mass HA for CD44 binding (Nemec, Toole, & Knudson, 1987; Teriete et al., 2004) yet, are too small in size to interfere with HA-aggrecan or, HA-link protein interactions (Hardingham & Muir, 1973; Hascall & Heinegard, 1974). The consequences of this loss of chondrocyte HA interactions are dramatic. In previous studies we demonstrated that the addition of HAoligos to human or bovine articular cartilage slices resulted in the activation of enhanced catabolism including elevated production and activity of MMPs, increases in aggrecanase neoepitope expression and concomitant loss of proteoglycan from the tissue (Knudson et al., 2000; Ohno et al., 2005). This enhanced catabolic state mimicked experiments wherein cartilage slices were incubated in the presence of IL-1 (Ratcliffe, Tyler, & Hardingham, 1986). However, unlike IL-1, chondrocytes treated with HAoligos displayed an increase in aggrecan and type II collagen but no change in CD44 (Nishida, D’Souza, Thonar, & Knudson, 2000). These results suggested that whatever mechanism was responsible for the activation, the pathway differed significantly from pathways activated by IL-1. A similar catabolic response was also obtained in bovine articular cartilage explants when HA-CD44 interactions were perturbed through the use of phosphorothioate CD44 antisense oligonucleotides (Chow et al., 1998). Thus, the loss of CD44-HA interactions gives rise to a cellular phenotype similar to early stage OA, enhanced degradation coupled with attempted repair. In this study we have shown that the same model includes an enhanced production of NO. It remains to be determined whether cartilage damage or attempted repair is being facilitated by the HAoligos-mediated stimulation of NO production. Human chondrocytes from OA patients express high levels of NO and iNOS (Melchiorri et al., 1998). Several studies highlight the metabolic significance of NO production in articular cartilage (Evans et al., 1996). NO decreases the sensitivity of chondrocytes to IGF-1, especially with respect to proteoglycan synthesis (Studer et al., 2000). Perhaps NO is responsible in part for the insensitivity of OA or aging chondrocytes to IGF-1, making that cartilage more susceptible to damage or lack of repair. However, the protective effect of NO with respect to catabolism of proteoglycan in articular cartilage has also been proposed (Stefanovic-Racic, Morales, Taskiran, McIntyre, & Evans, 1996).
The activation of NO production by HAoligos has been shown in other cell types. Binding of HAoligos to CD44 initiated the nuclear translocation of NF-κB resulting in the enzymatic activation of iNOS and stimulation of NO production in macrophages (Noble, McKee, Cowman, & Shin, 1996). Subsequent studies with rat liver endothelial and Kupffer cells confirmed that only HAoligos but not macromolecular HA induced iNOS expression (Rockey, Chung, McKee, & Noble, 1998). The activation of the NF-κB pathway by small HAoligos in T-24, HeLa, MCF7 and J774 cell lines has also been demonstrated (Fitzgerald, Bowie, Skeffington, & O’Neill, 2000). Using a protein-DNA array screen and EMSA analyses we have also observed the induction of NF-κB in bovine articular chondrocytes treated with HAoligos as well as purified HA6 (Ohno et al., 2005). Given that increased activity of NF-κB is closely associated with iNOS induction (Clancy, Gomez, & Abramson, 2004) these results suggest a possible mechanism for the increases in the iNOS and NO production following HAoligos stimulation. As well, HAoligos induced iNOS expression in BV-2 microglia mediated by a p38 MAP kinase-dependent pathway (Wang et al., 2004).
The activation of p38 MAP kinase and NF-κB nuclear translocation by HAoligos are in common with the stimulation of NO due to treatment of cells with IL-1 (Badger et al., 1998; Clancy et al., 2004; Mendes et al., 2001). In this study we demonstrated that this similarity was not due to HAoligos-induced autocrine release of endogenous IL-1 (Fig. 10), but may share common signaling pathways. However, as discussed above, exposure of chondrocytes to IL-1α or IL-1β results in the stimulation of NO, MMP-13, HA synthase-2 (HAS-2) and CD44 but, a dramatic inhibition of aggrecan and type II collagen. HAoligos affect a stimulation of NO, MMP-13 and HAS-2 but, little change in CD44 and a stimulation of aggrecan and type II collagen (Knudson et al., 2000; Nishida et al., 2003; Ohno et al., 2005). In addition, whereas IL-1 mediated signaling terminates in an activation of AP-1 transcription factors, HAoligos affect no activation of AP-1 (Ohno et al., 2005). That multiple pathways are responsible for NO stimulation is borne out in animal models of OA; for example, inhibition of iNOS attenuated, but did not completely abolish, the effects of IL-1 (Pelletier, Mineau, Fernandes, Duval, & Martel-Pelletier, 1998). Future studies will determine where these two pathways have commonality and where they diverge.
Although it has been demonstrated that the molecular weight of the HA in whole tissue extracts of cartilage shows considerable decrease in size with age (Holmes et al., 1988) HA oligosaccharides as small as HA6 have not been conclusively demonstrated. The average molecular weight of HA in normal synovial fluid is ~7 million, but is decreased in rheumatoid synovial fluid to 4.8 million (Al-Assaf, Navaratnam, Parsons, & Phillips, 2003; Pitsillides, Worrall, Wilkinson, Bayliss, & Edwards, 1994). Superoxide radical anions from neutrophils in the synovial fluid are key candidates for the initiation of damage to HA and the consequent reduction in molecular weight (Baker, Green, & Lowther, 1989). Released NO may also generate peroxynitrite—a compound that has been proposed as an alternative reactive species that could cause the degradation of HA (Al-Assaf et al., 2003). Nonetheless, in this study we have utilized HAoligos as an HA antagonist. As such, these studies may reflect cartilage physiology in which HA retention is deficient; possibly due to defective HAS-2 regulation, defective CD44 retention, enhanced CD44-mediated HA catabolism, etc. This suggests one potential mechanism of action to explain the effects of intra-articular injection of HA, in clinical use for the purpose of viscosupplementation. Besides the possible biomechanical effects, investigators are attempting to determine whether HA exerts chondroprotective effects and, if so, the mechanism for this protection. In vitro experiments showed that HA inhibits cartilage matrix degradation induced by inflammatory cytokines (Julovi, Yasuda, Shimizu, Hiramitsu, & Nakamura, 2004; Stove et al., 2002). Intra-articular injection of HA was correlated with a significant decrease in levels of NO in the synovial fluid of patients with knee OA (Kobayashi et al., 2004). In all, these studies support the suggestion that HA interactions with CD44 promote cartilage homeostasis.
Acknowledgments
We thank the Gift of Hope Organ and Tissue Donor Network of Chicago, IL for providing donor samples. The generosity and support of the donor families is greatly appreciated. The authors thank Drs. Geraldine Chow and Warren Knudson for helpful discussions during this project and Ankit Desai for technical assistance. Our study was supported by NIH grants P50-AR39239, R01-AR39507 and T32-AR0759.
References
- Abramson S, Attur M, Amin A, Clancy R. Nitric oxide and inflammatory mediators in the perpetuation of osteoarthritis. Current Rheumatology Reports. 2001;3:535–541. doi: 10.1007/s11926-001-0069-3. [DOI] [PubMed] [Google Scholar]
- Al-Assaf S, Navaratnam S, Parsons BJ, Phillips GO. Chain scission of hyaluronan by peroxynitrite. Archives of Biochemistry and Biophysics. 2003;411:73–82. doi: 10.1016/s0003-9861(02)00724-5. [DOI] [PubMed] [Google Scholar]
- Badger AM, Cook MN, Lark MW, Newman-Tarr TM, Swift BA, Nelson AH, et al. SB 203580 inhibits p38 mitogen-activated protein kinase, nitric oxide production, and inducible nitric oxide synthase in bovine cartilage-derived chondrocytes. Journal of Immunology. 1998;161:467–473. [PubMed] [Google Scholar]
- Baker MS, Green SP, Lowther DA. Changes in the viscosity of hyaluronic acid after exposure to a myeloperoxidase-derived oxidant. Arthritis & Rheumatism. 1989;32:461–467. doi: 10.1002/anr.1780320416. [DOI] [PubMed] [Google Scholar]
- Chow G, Knudson CB, Homandberg G, Knudson W. Increased CD44 expression in bovine articular chondrocytes by catabolic cellular mediators. Journal of Biological Chemistry. 1995;270:27734–27741. doi: 10.1074/jbc.270.46.27734. [DOI] [PubMed] [Google Scholar]
- Chow G, Nietfeld J, Knudson CB, Knudson W. Anti-sense inhibition of chondrocyte CD44 expression results in cartilage chondrolysis. Arthritis & Rheumatism. 1998;41:1411–1419. doi: 10.1002/1529-0131(199808)41:8<1411::AID-ART10>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Clancy RM, Gomez PF, Abramson SB. Nitric oxide sustains nuclear factor kappa B activation in cytokine-stimulated chondrocytes. Osteoarthritis & Cartilage. 2004;12:552–558. doi: 10.1016/j.joca.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Evans CH, Watkins SC, Stefanovic-Racic M. Nitric oxide and cartilage metabolism. Methods in Enzymology. 1996;269:75–88. doi: 10.1016/s0076-6879(96)69011-9. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, Bowie AG, Skeffington BS, O’Neill LA. Ras, protein kinase C zeta and I kappa B kinases 1 and 2 are downstream effectors of CD44 during the activation of NF-kappa B by hyaluronic acid fragments in T-24 carcinoma cells. Journal of Immunology. 2000;164:2053–2063. doi: 10.4049/jimmunol.164.4.2053. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Oh M, Asada S, Hara F, Matsukawa M, Otani K, et al. Sodium hyaluronate inhibits interleukin-1-evoked reactive oxygen species of bovine articular chondrocytes. Osteoarthritis & Cartilage. 2001;9:390–392. doi: 10.1053/joca.2000.0400. [DOI] [PubMed] [Google Scholar]
- Galea E, Feinstein DL. Regulation of the expression of the inflammatory nitric oxide synthase (NOS2) by cyclic AMP. The FASEB Journal. 1999;13:2125–2137. doi: 10.1096/fasebj.13.15.2125. [DOI] [PubMed] [Google Scholar]
- Goldring MB, Berenbaum F. The regulation of chondrocyte function by proinflammatory mediators: Prostaglandins and nitric oxide. Clinical Orthopaedics and Related Research. 2004;427S:S37–S46. doi: 10.1097/01.blo.0000144484.69656.e4. [DOI] [PubMed] [Google Scholar]
- Goodstone NJ, Hardingham TE. Tumour necrosis factor alpha stimulates nitric oxide production more potently than interleukin-1beta in porcine articular chondrocytes. Rheumatology. 2002;41:883–891. doi: 10.1093/rheumatology/41.8.883. [DOI] [PubMed] [Google Scholar]
- Granger DL, Taintor RR, Boockvar KS, Hibbs JB., Jr Measurement of nitrate and nitrite in biological samples using nitrate reductase and Griess reaction. Methods in Enzymology. 1996;268:142–151. doi: 10.1016/s0076-6879(96)68016-1. [DOI] [PubMed] [Google Scholar]
- Hardingham TE, Muir H. Binding of oligosaccharides of hyaluronic acid to proteoglycans. Biochemistry Journal. 1973;135:905–908. doi: 10.1042/bj1350905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall VC, Heinegard D. Aggregation of cartilage proteoglycans. II. Oligosaccharide competitors of the proteoglycan–hyaluronic acid interaction. Journal of Biological Chemistry. 1974;249:4242–4249. [PubMed] [Google Scholar]
- Hauselmann HJ, Flechtenmacher J, Michal L, Thonar EJ, Shinmei M, Kuettner KE, et al. The superficial layer of human articular cartilage is more susceptible to interleukin-1-induced damage than the deeper layers. Arthritis & Rheumatism. 1996;39:478–488. doi: 10.1002/art.1780390316. [DOI] [PubMed] [Google Scholar]
- Holmes MWA, Bayliss MT, Muir H. Hyaluronic acid in human articular cartilage: Age-related changes in content and size. Biochemical Journal. 1988;250:435–441. doi: 10.1042/bj2500435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Q, Knudson CB, Knudson W. Internalization of hyaluronan by chondrocytes occurs via receptor-mediated endocytosis. Journal of Cell Science. 1993;106:365–375. doi: 10.1242/jcs.106.1.365. [DOI] [PubMed] [Google Scholar]
- Jiang H, Peterson RS, Wang W, Bartnik E, Knudson CB, Knudson W. A requirement for the CD44 cytoplasmic domain for hyaluronan binding, pericellular matrix assembly and receptor mediated endocytosis in COS-7 cells. Journal of Biological Chemistry. 2002;277:10531–10538. doi: 10.1074/jbc.M108654200. [DOI] [PubMed] [Google Scholar]
- Julovi SM, Yasuda T, Shimizu M, Hiramitsu T, Nakamura T. Inhibition of interleukin-1β-stimulated production of matrix metalloproteinases by hyaluronan via CD44 in human articular cartilage. Arthritis & Rheumatism. 2004;50:516–525. doi: 10.1002/art.20004. [DOI] [PubMed] [Google Scholar]
- Knudson CB. Hyaluronan receptor-directed assembly of chondrocyte pericellular matrix. The Journal of Cell Biology. 1993;120:825–834. doi: 10.1083/jcb.120.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson CB. Hyaluronan in embryonic cell adhesion and matrix assembly. In: Laurent TC, editor. The chemistry, biology and medical applications of Hyaluronan and its derivatives. London: Portland Press; 1998. pp. 161–168. [Google Scholar]
- Knudson CB, Knudson W. Hyaluronan-binding proteins in development, tissue homeostasis and disease. The FASEB Journal. 1993;7:1233–1241. [PubMed] [Google Scholar]
- Knudson W, Aguiar DJ, Hua Q, Knudson CB. CD44-anchored hyaluronan-rich pericellular matrices: An ultra-structural and biochemical analysis. Experimental Cell Research. 1996;228:216–228. doi: 10.1006/excr.1996.0320. [DOI] [PubMed] [Google Scholar]
- Knudson W, Casey B, Nishida Y, Eger W, Kuettner KE, Knudson CB. Hyaluronan oligosaccharides perturb cartilage matrix homeostasis and induce chondrocytic chondrolysis. Arthritis & Rheumatism. 2000;43:1165–1174. doi: 10.1002/1529-0131(200005)43:5<1165::AID-ANR27>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Knudson W, Knudson C. An update on hyaluronan and CD44 in cartilage. Current Opinion in Orthopaedics. 2004;15:369–375. [Google Scholar]
- Kobayashi K, Matsuzaka S, Yoshida Y, Miyauchi S, Wada Y, Moriya H. The effects of intraarticularly injected sodium hyaluronate on levels of intact aggrecan and nitric oxide in the joint fluid of patients with knee osteoarthritis. Osteoarthritis & Cartilage. 2004;12:536–542. doi: 10.1016/j.joca.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Kuhn K, Shikhman A, Lotz M. Role of nitric oxide, reactive oxygen species, and p38 MAP kinase in the regulation of human chondrocyte apoptosis. Journal of Cellular Physiology. 2003;197:379–387. doi: 10.1002/jcp.10372. [DOI] [PubMed] [Google Scholar]
- Maleski MP, Knudson CB. Matrix accumulation and retention in embryonic cartilage and in vitro chondrogenesis. Connective Tissue Research. 1996;34:75–86. doi: 10.3109/03008209609028895. [DOI] [PubMed] [Google Scholar]
- McKee CM, Lowenstein CJ, Horton MR, Wu J, Bao C, Chin BY, et al. Hyaluronan fragments induce nitric-oxide synthase in murine macrophages through a nuclear kB-dependent mechanism. Journal of Biological Chemistry. 1997;272:8013–8018. doi: 10.1074/jbc.272.12.8013. [DOI] [PubMed] [Google Scholar]
- Melchiorri C, Meliconi R, Frizziero L, Silvestri T, Pulsatelli L, Mazzetti I, et al. Enhanced and coordinated in vivo expression of inflammatory cytokines and nitric oxide synthase by chondrocytes from patients with osteoarthritis. Arthritis & Rheumatism. 1998;41:2165–2174. doi: 10.1002/1529-0131(199812)41:12<2165::AID-ART11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Mendes AF, Carvalho AP, Caramona MM, Lopes MC. Diphenyleneiodonium inhibits NF-kappa B activation and iNOS expression induced by IL-1beta: Involvement of reactive oxygen species. Mediators of Inflammation. 2001;10:209–215. doi: 10.1080/09629350120080401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehleman C, Bareither D, Huch K, Cole AA, Kuettner KE. Prevalence of degenerative morphological changes in the joints of the lower extremity. Osteoarthritis & Cartilage. 1997;5:23–37. doi: 10.1016/s1063-4584(97)80029-5. [DOI] [PubMed] [Google Scholar]
- Nemec RE, Toole BP, Knudson W. The cell surface hyaluronate binding sites of invasive human bladder carcinoma cells. Biochemical and Biophysical Research Communications. 1987;149:249–257. doi: 10.1016/0006-291x(87)91632-9. [DOI] [PubMed] [Google Scholar]
- Nishida N, Knudson CB, Knudson W. Extracellular matrix recovery by human articular chondrocytes after treatment with hyaluronan hexasaccharides or Streptomyces hyaluronidase. Modern Rheumatology. 2003;13:62–68. doi: 10.3109/s101650300009. [DOI] [PubMed] [Google Scholar]
- Nishida Y, D’Souza AL, Thonar JMA, Knudson W. IL-1α stimulates hyaluronan metabolism in human articular cartilage. Arthritis & Rheumatism. 2000;43:1315–1326. doi: 10.1002/1529-0131(200006)43:6<1315::AID-ANR14>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Noble PW, McKee CM, Cowman M, Shin HS. Hyaluronan fragments activate an NF-κB/I-κBα autoregulatory loop in murine macrophages. Journal of Experimental Medicine. 1996;183:2373–2378. doi: 10.1084/jem.183.5.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nofal GA, Knudson CB. Latrunculin and cytochalasin decrease chondrocyte matrix retention. Journal of Histochemistry and Cytochemistry. 2002;50:1313–1324. doi: 10.1177/002215540205001004. [DOI] [PubMed] [Google Scholar]
- Ohno S, Im HJ, Knudson CB, Knudson W. Hyaluronan oligosaccharide-induced activation of transcription factors in bovine articular chondrocytes. Arthritis & Rheumatism. 2005;52:800–809. doi: 10.1002/art.20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier JP, Mineau F, Fernandes JC, Duval N, Martel-Pelletier J. Diacerhein and rhein reduce the inter-leukin 1beta stimulated inducible nitric oxide synthesis level and activity while stimulating cyclooxygenase-2 synthesis in human osteoarthritic chondrocytes. The Journal of Rheumatology. 1998;25:2417–2424. [PubMed] [Google Scholar]
- Pitsillides AA, Worrall JG, Wilkinson LS, Bayliss MT, Edwards JCW. Hyaluronan concentration in non-inflamed and rheumatoid synovium. British Journal of Rheumatology. 1994;33:5–10. doi: 10.1093/rheumatology/33.1.5. [DOI] [PubMed] [Google Scholar]
- Ratcliffe A, Tyler JA, Hardingham TE. Articular cartilage cultured with interleukin 1. Biochemistry Journal. 1986;238:571–580. doi: 10.1042/bj2380571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockey DC, Chung JJ, McKee CM, Noble PW. Stimulation of inducible nitric oxide synthase in rat liver by hyaluronan fragments. Hepatology. 1998;27:86–92. doi: 10.1002/hep.510270115. [DOI] [PubMed] [Google Scholar]
- Sommarin Y, Heinegard D. Four classes of cell-associated proteoglycans in suspension cultures of articular-cartilage chondrocytes. Biochemistry Journal. 1986;233:809–818. doi: 10.1042/bj2330809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanovic-Racic M, Morales TI, Taskiran D, McIntyre LA, Evans CH. The role of nitric oxide in proteoglycan turnover by bovine articular cartilage organ cultures. Journal of Immunology. 1996;156:1213–1220. [PubMed] [Google Scholar]
- Stove J, Gerlach C, Huch K, Gunther KP, Puhl W, Scharf HP. Effects of hyaluronan on proteoglycan content of osteoarthritic chondrocytes in vitro. Journal of Orthopaedic Research. 2002;20:551–555. doi: 10.1016/S0736-0266(01)00141-3. [DOI] [PubMed] [Google Scholar]
- Studer RK, Levicoff E, Georgescu H, Miller L, Jaffurs D, Evans CH. Nitric oxide inhibits chondrocyte response to IGF-I: Inhibition of IGF-IRbeta tyrosine phosphorylation. American Journal of Physiology—Cell Physiology. 2000;279:C961–C969. doi: 10.1152/ajpcell.2000.279.4.C961. [DOI] [PubMed] [Google Scholar]
- Tawada A, Masa T, Oonuki Y, Watanabe A, Matsuzaki Y, Asari A. Large-scale preparation, purification, and characterization of hyaluronan oligosaccharides from 4-mers to 52-mers. Glycobiology. 2002;12:421–426. doi: 10.1093/glycob/cwf048. [DOI] [PubMed] [Google Scholar]
- Teriete P, Banerji S, Noble M, Blundell CD, Wright AJ, Pickford AR, et al. Structure of the regulatory hyaluronan binding domain in the inflammatory leukocyte homing receptor CD44. Molecular Cell. 2004;13:483–496. doi: 10.1016/s1097-2765(04)00080-2. [DOI] [PubMed] [Google Scholar]
- Wang MJ, Jeng KC, Kuo JS, Chen HL, Huang HY, Chen WF, et al. c-Jun N-terminal kinase and, to a lesser extent, p38 mitogen-activated protein kinase regulate inducible nitric oxide synthase expression in hyaluronan fragments-stimulated BV-2 microglia. Journal of Neuroimmunology. 2004;146:50–62. doi: 10.1016/j.jneuroim.2003.10.034. [DOI] [PubMed] [Google Scholar]