Abstract
The BRCA-1 protein is a tumor suppressor involved in repair of DNA damage. Epigenetic mechanisms contribute to its reduced expression in sporadic breast tumors. Through diet, humans are exposed to a complex mixture of xenobiotics and natural ligands of the aromatic hydrocarbon receptor (AhR), which contributes to the etiology of various types of cancers. The AhR binds xenobiotics, endogenous ligands, and many natural dietary bioactive compounds, including the phytoalexin resveratrol (Res). In estrogen receptor- alpha (ER alpha )-positive and BRCA-1 wild-type MCF-7 breast cancer cells, we investigated the influence of AhR activation with the agonist 2,3,7,8 tetrachlorobenzo(p)dioxin (TCDD) on epigenetic regulation of the BRCA-1 gene and the preventative effects of Res. We report that activation and recruitment of the AhR to the BRCA-1 promoter hampers 17 beta -estradiol (E2)–dependent stimulation of BRCA-1 transcription and protein levels. These inhibitory effects are paralleled by reduced occupancy of ER alpha , acetylated histone (AcH)-4, and AcH3K9. Conversely, the treatment with TCDD increases the association of mono-methylated-H3K9, DNA-methyltransferase-1 (DNMT1), and methyl-binding domain protein-2 with the BRCA-1 promoter and stimulates the accumulation of DNA strand breaks. The AhR-dependent repression of BRCA-1 expression is reversed by small interference for the AhR and DNMT1 or pretreatment with Res, which reduces TCDD-induced DNA strand breaks. These results support the hypothesis that epigenetic silencing of the BRCA-1 gene by the AhR is preventable with Res and provide the molecular basis for the development of dietary strategies based on natural AhR antagonists.
Introduction
Changes in histone codes and DNA methylation are epigenetic events mediated by the dynamic interplay among various factors, including histone acetyl transferases, histone deacetylases (HDAC),5 and DNA methyltransferases (DNMT) (1, 2). Resetting of epigenetic modifications with bioactive food components is important in cancer prevention and control (3). Examples of epigenetic regulation by food constituents include reduction of acetylated histone (AcH)4 at the cyclooxygenase-2 (COX-2) promoter by the metabolite 3,3′-diindolylmethane in breast cancer cells (4), accumulation of AcH3 by sodium butyrate in a mouse model of colorectal cancer (5), inhibition of DNMT and reactivation of methylation-silenced genes by polyphenol(-)-epigallocatechin-3-gallate (6), and inhibition of HDAC activity by sulforaphane in preclinical models (7) and humans (8).
The BRCA-1 gene encodes for a tumor suppressor protein involved in repair of DNA damage. In sporadic breast tumors, which represent the vast majority of breast cancer cases, epigenetic mechanisms contribute to its reduced expression in the absence of mutations in the BRCA-1 gene (9–13). In women who carry a mutated BRCA-1 allele, silencing of the wild-type allele further increases the susceptibility to breast cancer. Therefore, knowledge of the mechanisms that contribute to epigenetic silencing of BRCA-1 is important in the prevention of hereditary and sporadic breast cancers.
Through diet, humans are exposed to a complex mixture of ligands of the aromatic hydrocarbon receptor (AhR). Prototypical AhR agonists include the polycyclic aromatic hydrocarbon (PAH) benzo[a]pyrene (B[a]P), and the dioxin-like compound 2,3,7,8 tetrachlorodibenzene(p)dioxin (TCDD). Increased incidence of breast cancer is documented in human populations of industrialized areas where high levels of dioxins are found in the air, soil, drinking water, and cow milk (14). Unlike PAH, TCDD is not metabolized and it promotes tumor development (15). Population studies reported the presence of TCDD in breast milk (16, 17), suggesting this agent may accumulate in breast tissue and be a potential risk factor in mammary neoplasia. The in-utero activation of the AhR with TCDD increased the susceptibility to mammary carcinogens in rat female offspring (18). The activation of the AhR pathway may increase the susceptibility to breast cancer through epigenetic silencing of tumor suppressor genes, including p16 and p53 (19), while inducing transcription of the proinflammatory COX-2 gene (4).
The AhR influences transcriptional activity through binding to xenobiotic responsive element (XRE = 5′-GCGTG-3′) and other response elements. Most studies investigated the role of the AhR in the context of environmental exposure (14). However, XRE-reporter gene assays in humans reported higher blood AhR-inducing equivalents than those accounted for by the predicted exposure to dioxin-like compounds. The latter have a high binding affinity for the AhR, as defined by their half-maximal effective concentration, which for TCDD is #126 1 × 10−8 mol/L. Bioactive compounds with AhR-binding activity are found in cruciferous vegetables, bread, hamburgers, potatoes, and grapefruit juice (20). Compounds present in Western-style diets have been shown to induce AhR-dependent genes in a mouse model of sporadic colon cancer (21). The AhR contains a promiscuous ligand-binding site, which is targeted by numerous dietary bioactive compounds (22) with various half-maximal inhibitory concentrations, including indol-3-carbinol (half-maximal inhibitory concentration 2.5 × 10−3 mol/L), 3,3′-diindolylmethane (5 × 10−5 mol/L), resveratrol (Res) ( #126 2 ×10−6 mol/L), and the isoflavones biochanin A and formononetin ( #126 2 × 10−7 mol/L) (23). Interestingly, the binding affinity for the latter group approximates that of the mammary carcinogen 7,12-dimethylbenz[a]anthracene (DMBA; #126 3.5 × 10−7 mol/L) (24, 25). Therefore, food components with AhR-binding activity may influence to various degrees the expression of genes targeted by the AhR.
We previously reported that activation of the AhR interfered with regulation of BRCA-1 transcription by 17β-estradiol (E2) (26). In this study, we investigated the influence of AhR activation on epigenetic regulation of the BRCA-1 gene in estrogen receptor-α (ERα)-positive and BRCA-1 wild-type MCF-7 breast cancer cells. We also tested the preventative effects of Res based on the information it antagonizes the AhR (27) and reduces DMBA-initiated mammary tumors in rodent models (28).
Materials and Methods
Cell culture and reagents.
MCF-7 breast cancer cells were obtained from the American Type Culture Collection and maintained as described previously (29). TCDD was supplied by the National Cancer Institute, Division of Cancer Biology, Chemical and Physical Carcinogenesis Branch, and distributed by Midwest Research Institute under contract (64 CFR 72090, 64 CFR 28205). E2 and Res were purchased from Sigma. Treatments were carried out in phenol red-free medium containing 5% charcoal-stripped fetal bovine serum.
Western blotting and transfection experiments.
Western-blot analysis was performed as previously described (29) with antibodies against DNMT1 (Upstate Cell Signaling Solutions), HDAC1 and ERα (Millipore), BRCA-1 and human glyceraldehyde-3-phosphate (Cell Signaling), and AhR and actin (Santa Cruz Biotechnologies). Reporter plasmids were transiently transfected using the Lipofect-AMINE Plus (Invitrogen) procedure as previously described (30). Plasmids encoding for renilla were cotransfected to account for variations in transfection efficiency. Luciferarase reporter activity was expressed as relative luciferase units corrected for renilla.
DNA protein-binding (pull-down) assay.
The binding of AhR to BRCA-1 oligonucleotides is described elsewhere (29). Following dissociation, bound nuclear proteins were separated by SDS-PAGE and analyzed by western-blot analysis with an antibody against the AhR (Santa Cruz Biotechnology). Sequences of biotin-labeled oligonucleotides (Sigma) were: BRCA-1/XRE2, forward: 5′-7GGGTACTGGCGTGGGAGAGTG-3′; reverse: 5′-7CACTCTCCCACGCCAGTACCC-3′.
Small inhibitory RNA for AhR and DNMT1.
Small inhibitory RNA (siRNA) duplexes for the AhR (siAhR) and DNMT1 (siDNMT1) were synthesized by Dharmacon. The AhR ON-TARGETplus SMART pool siRNA sequences used were: 5′-GCAAGUUAAUGGCAUGUUU-3′, 5′-GAACUCAAGCUGUAUGGUA-3′, 5′-GCACGAGAGGCUCAGGUUA-3′, and 5′-GCAACAAGAUGAGUCUAUU-3′ (4). The DNMT1 ON-TARGETplus SMART pool siRNA sequences used were: 5′-GCACCUCAUUUGCCGAAUA-3′, 5′-AUAAAUGAAUGGUGGAUCA-3′, 5′-CCUGAGCCCUACCGAAUUG-3′, and 5′-GGACGACCCUGACCUCAAA-3′. The siRNA duplexes were transfected into MCF-7 cells using Lipofectamine 2000 (Invitrogen). Nontargeting siRNA (siCon) and siRNA for glyceraldehyde-3-phosphate (GAPDH) (siGAPDH) were purchased from Dharmacon.
Chromatin immunoprecipitation assay and quantitative real-time PCR.
Chromatin immunoprecipitation (ChIP) assays were performed using the EZ ChIP kit (Millipore) as previously described (31). Chromatin was immunoprecipitated with antibodies against methyl-binding protein-2 (MBD2), AcH4, AcH3K9, mono-methylated H3K9 (mMH3K9), and ERα (Millipore); AhR (Santa Cruz Biotechnologies); and DNMT1 (Upstate Cell Signaling Solutions). DNA were amplified by quantitative real-time PCR using the SYBR Green PCR Reagents kit (Applied Biosystems). Briefly, reactions were done at a final volume of 25 μL consisting of the following master mix: 12.5 μL of 2× SybrGreen buffer, 1 μL each of forward (5′-CTGACAGATGGGTATTCTTTGACG -3′) and reverse (5′-GCATATTCCAGTTCCTATCACGAG -3′) primers that flank the activator protein-1 (AP-1) binding site in the BRCA-1 gene, 8.5 μL nuclease free water, and 2 μL DNA purified from the ChIP assay. The standard curve was generated using a plasmid containing the BRCA-1 promoter. Bound DNA was normalized to input DNA.
Comet assay (alkaline single cell electrophoresis).
The procedure for alkaline COMET assay was performed on MCF-7 cells according to the manufacturer's instructions (Trevigen) and is described elsewhere (32). At least 200 tail moments for each group were analyzed to calculate the mean ± SE for each group. Untreated cells were used as a negative control group.
Statistical analysis.
Densitometry after western blotting was performed using Kodak ID Image Analysis Software. Statistical analysis was performed using GraphPad Prism5. Data for AhR-binding assays were analyzed by 1-way ANOVA. Data from factorial experiments were analyzed by 2-way ANOVA. Post hoc multiple comparisons among all means were conducted using Tukey's Test after main effects and interactions were significant at P ≤ 0.05.
Results
The activated AhR antagonizes E2 induction of BRCA-1 expression.
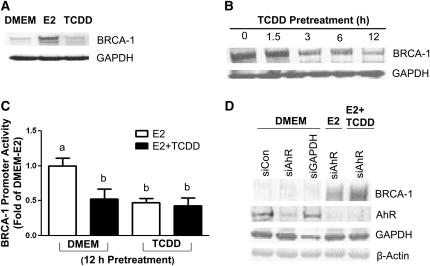
Previous studies by our group (31) and other laboratories (33) documented activation of BRCA-1 expression by E2 in epithelial cells expressing the ERα. Western-blot analysis of cell lysates confirmed that E2 induced BRCA-1 protein levels in breast cancer MCF-7 cells, whereas culture of cells in basal DMEM or treatment with the AhR agonist TCDD did not alter BRCA-1 protein levels (Fig. 1A). Conversely, washout time course experiments with TCDD reduced from 30%, at 3 and 6 h, to 60% at 12 h, the subsequent E2 induction of BRCA-1 protein expression (Fig. 1B). Transient transfection experiments with a BRCA-1-luciferase promoter construct (pGL3-BRCA-1) revealed that the pretreatment with TCDD reduced by #126 60% the subsequent stimulation of BRCA-1 promoter activity by E2 (Fig. 1C).
FIGURE 1.
TCDD disrupts E2-dependent activation of BRCA-1 expression. (A) Breast cancer MCF-7 cells were cultured in control DMEM or DMEM plus 100 nmol/L TCDD or 10 nmol/L E2 for 24 h. Bands are immunocomplexes for BRCA-1 and GAPDH. (B) MCF-7 cells were pretreated for various periods of time with 100 nmol/L TCDD and then treated for an additional 24 h with E2. (C) Values represent means ± SD, n = 3 (means of quadruplicates) from DMEM and TCDD-pretreated (100 nmol/L, 12 h) MCF-7 cells transfected with pGL3-BRCA-1. Treatments were with E2 (10 nmol/L) or E2 plus TCDD (100 nmol/L) for 24 h. Means without a common letter differ, P < 0.05. (D) BRCA-1 protein expression in MCF-7 cells transfected with siRNA and treated for 24 h with E2 (10 nmol/L) or E2 plus TCDD (100 nmol/L). Bands are immunocomplexes for BRCA-1, AhR, GAPDH, and β-actin. siCon and siGAPDH were used as controls.
To investigate the role of the AhR in the TCDD-dependent repression of BRCA-1 protein, we carried out siRNA experiments (Fig. 1D) with control (siCon), AhR (siAhR), or GAPDH (siGAPDH)-specific duplexes (34). Following transfection of specific siRNA, MCF-7 cells were cultured for 24 h in DMEM, DMEM supplemented with E2, or E2 plus TCDD. The transfection with siAhR or siGAPDH, but not siCon, reduced endogenous AhR or GAPDH protein levels, respectively. More importantly, transfection of siAhR into MCF-7 cells antagonized the repressive effects of TCDD on BRCA-1 protein expression.
Res modulates the recruitment of the AhR and ERalpha to the BRCA-1 promoter.
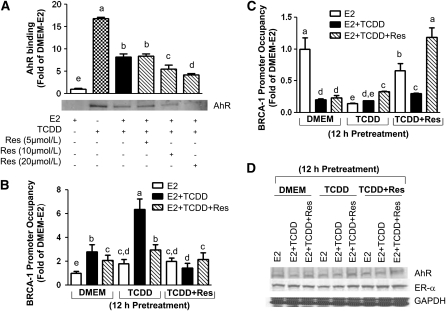
Previously, we documented that E2 activation of BRCA-1 transcription required the assembly of an ERα/p300 complex at an AP-1 site located in the proximal BRCA-1 promoter (31). The AP-1 site is flanked on the 5′ region by an XRE, which is a consensus binding sequence for the AhR (26). Therefore, we tested whether the treatment with TCDD stimulated the interaction of the AhR with a BRCA-1 promoter segment harboring the XRE. Results of western blots after DNA pull-down with nuclear extracts obtained from MCF-7 cells indicated that the treatment with TCDD induced the rapid (90 min) association of the AhR with a BRCA-1/XRE oligonucleotide (Fig. 2A). Conversely, the binding of the AhR was reduced ( #126 50%) by the cotreatment with E2 (10 nmol/L) or E2 plus Res at concentrations of 10 ( #126 70%) and 20 ( #126 80%) μmol/L. These data corroborated previous reports by our laboratory (4) and other groups (35) documenting the antagonistic properties of Res toward the AhR.
FIGURE 2.
TCDD stimulates the association of the AhR to BRCA-1 promoter. (A) Breast cancer MCF-7 cells were cultured in DMEM plus E2 (10 nmol/L), TCDD (100 nmol/L), or E2 and TCDD plus various concentrations of Res (5, 10, and 20 μmol/L) for 90 min. Bands represent AhR immunocomplexes after immunoprecipitation of DNA:nuclear protein complexes with an AhR antibody. Bars are means ± SD, n = 2 (means of triplicates) independent experiments with CV < 5%. (B,C) Pretreatments with TCDD (100 nmol/L) or TCDD plus Res for 12 h were followed by cotreatment for 24 h with E2, E2 plus TCDD, or E2 plus TCDD plus Res. Bars are means ± SD, n = 3 (means of triplicates) independent experiments. Means without a common letter differ, P < 0.05. (D) Bands depict AhR, ERα, and GAPDH protein.
Using a ChIP assay, we found that the occupancy of the AhR at the BRCA-1 promoter (Fig. 2B) was augmented in cells receiving both the pretreatment and cotreatment with TCDD. Conversely, the presence of Res in the pre- and cotreatment media reduced ( #126 60%) the recruitment of the AhR. Both pre- and cotreatment with TCDD reduced the recruitment of ERα to (Fig. 2C). The latter effect was reversed by the addition of Res to the preculture medium for 12 h (Fig. 2C, column 7) and cotreatment with Res (Fig. 2C, column 9). Fluctuations in the recruitment of the AhR and ERα were not accounted for by changes in the expression levels of endogenous AhR and ERα protein (Fig. 2D).
Res antagonizes TCDD-induced histone modifications at the BRCA-1 gene.
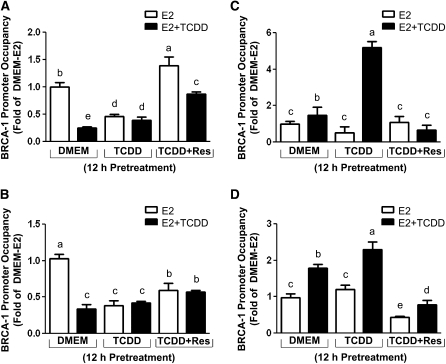
We previously reported the activation of the AhR led to increased recruitment of HDAC1 at the BRCA-1 gene (26). Therefore, we tested whether TCDD induced specific histone modifications and the effects of Res. MCF-7 cells were precultured for 12 h in DMEM, DMEM supplemented with TCDD, or TCDD plus Res. After washout of preculture media, MCF-7 cells were cultured for an additional 24 h in the presence of E2 or E2 plus TCDD. The cotreatment or combination of pretreatment plus cotreatment with TCDD reduced the levels of AcH4 (Fig. 3A) and AcH3K9 (Fig. 3B) associated with the BRCA-1 promoter by #126 50–75%, whereas the pretreatment with Res increased AcH4 and AcH3K9 levels. In MCF-7 cells cotreated with TCDD, we observed a 0.5-fold increase in the occupancy of mMH3K9, which was further augmented ( #126 4.0-fold) in MCF-7 cells pretreated with TCDD (Fig. 3C). Conversely, the addition of Res to the pretreatment medium reduced to DMEM-E2 levels the occupancy of mMH3K9. The increased association of mMH3K9 was paralleled by an increase in the occupancy of MBD2 ( #126 0.8- to 1.3-fold) (Fig. 3D). In contrast, the preexposure to Res antagonized the recruitment of MBD2.
FIGURE 3.
Res modulates the recruitment of (A) AcH4, (B) AcH3K9, (C) mMH3k9, and (D) MBD2 to the BRCA-1 promoter in breast cancer MCF-7 cells. Pretreatments with TCDD (100 nmol/L) or TCDD plus Res for 12 h were followed by cotreatment for 24 h with E2 or E2 plus TCDD. Bars are means ± SD, n = 3 (means of triplicates) independent experiments. Means without a common letter differ, P < 0.05.
TCDD induces the recruitment of DNMT1 at the BRCA-1 promoter.
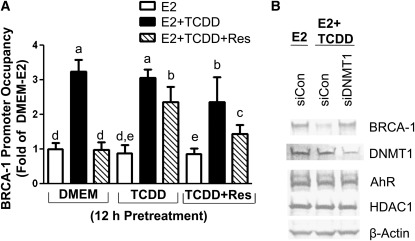
The increased association of MBD2 suggested the participation of DNMT in repression of BRCA-1 promoter activity by TCDD. In fact, we found the cotreatment with TCDD induced a 2.2-fold increase in the recruitment of DNMT1 (Fig. 4A), which is an enzyme involved in the maintenance of DNA methylation (36), whereas no additional association was observed when TCDD was included in the pretreatment medium. In contrast, the cotreatment (column 3) or pretreatment (column 9) with Res reduced DNMT1 levels at the BRCA-1 promoter. Therefore, we tested whether DNMT1 contributed to silencing of the BRCA-1 gene. MCF-7 cells were transiently transfected with siCon- or siDNMT1-specific siRNA. After transfection, MCF-7 cells were treated for 24 h with E2 or TCDD plus E2. Results of western blots (Fig. 4B) illustrated that the knockdown of DNMT1 restored BRCA-1 protein expression while reducing the endogenous levels of DNMT1 protein. The transfection with siRNA for DNMT1 did not alter the expression of AhR and HDAC1 protein, which were used as internal controls for the siRNA studies.
FIGURE 4.
Res prevents the recruitment of DNMT1 to the BRCA-1 promoter. (A) Pretreatment of breast cancer MCF-7 cells with TCDD (100 nmol/L) or TCDD plus Res for 12 h was followed by cotreatment for 24 h with E2, E2 plus TCDD, or E2 plus TCDD plus Res. Bars are means ± SD, n = 3 (means of triplicates) independent experiments. Means without a common letter differ, P < 0.05. (B) Transfection of siDNMT1 antagonizes TCDD-dependent repression of BRCA-1 protein expression. Bands are immunocomplexes for BRCA-1, DNMT1, AhR, HDAC1, and β-actin. siCon was used as internal control.
Res attenuates TCDD-dependent repression of BRCA-1 protein expression and induction of DNA damage.
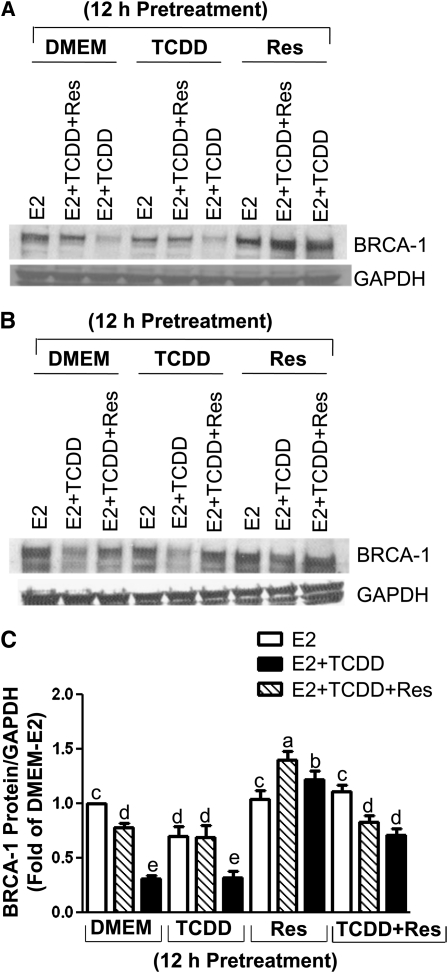
To assess the effects of TCDD and Res on BRCA-1 protein expression, MCF-7 cells were precultured for 12 h in DMEM or DMEM supplemented with TCDD, Res, or their combination. After washout of pretreatment medium, MCF-7 cells were cultured for an additional 24 h in the presence of E2, E2 plus TCDD, or E2 plus TCDD and Res. Quantitation of BRCA-1 immunocomplexes illustrated that (Fig. 5A) the treatment with TCDD (lanes 3 and 6) reduced ( #126 75%) BRCA-1 protein levels compared with those measured in cells treated with E2 (lane 1). However, BRCA-1 protein was restored to near DMEM-E2 levels by the cotreatment with Res (lane 2). The pretreatment (lane 9) and cotreatment (lane 8) with Res increased BRCA-1 protein by #126 25–30% compared with DMEM-E2. The addition of Res in the pretreatment medium (Fig. 5B, lanes 7, 8, and 9) attenuated the repressive effects of TCDD on BRCA-1 protein expression (Fig. 5C).
FIGURE 5.
Res antagonizes TCDD-dependent repression of BRCA-1 protein expression. (A) Breast cancer MCF-7 cells precultured in DMEM or DMEM with 100 nmol/L TCDD, 20 μmol/L Res, (B) or TCDD plus Res for 12 h. Then, cells were cultured for an additional 24 h in the presence of E2 (10 nmol/L), E2 plus TCDD (100 nmol/L), or E2 plus TCDD plus Res (20 μmol/L). Bands are immunocomplexes for BRCA-1 and GAPDH. In C, bars are means ± SD, n = 2 (means of duplicates) independent experiments (CV < 5%). Means without a common letter differ, P < 0.05.
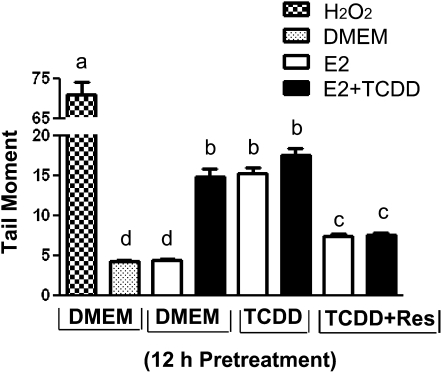
The exposure to TCDD induces oxidative stress and DNA strand breaks in ERα-positive breast cancer cells exposed to E2 (37). Because BRCA-1 is involved in repair of DNA damage, we examined the effects of TCDD, Res, and their combination on accumulation of DNA strand breaks by alkaline single cell electrophoresis (comet) assay. As a positive control, MCF-7 cells were treated with H2O2, which induces DNA damage (32). Both the preexposure and cotreatment with TCDD increased DNA damage as evidenced by a 2.0-fold increase in the mean tail moment. Conversely, the addition of Res to the pretreatment medium counteracted the effects of TCDD and reduced to near DMEM-E2 levels the formation of nuclear comets (Fig. 6).
FIGURE 6.
Res antagonizes TCDD-induced DNA damage. Breast cancer MCF-7 cells were precultured in DMEM or DMEM supplemented with 100 nmol/L TCDD or TCDD plus Res (20 μmol/L) for 12 h. Then, cells were cultured for an additional 24 h in the presence of E2 (10 nmol/L) or E2 plus TCDD (100 nmol/L). Bars represent means ± SE, n = 2 independent experiments of DNA strand breaks from at least 200 tail moments (CV < 5%). H2O2 was used as positive control. Means without a common letter differ, P < 0.05.
Taken together, these data illustrate that activation of the AhR interferes with E2-dependent activation of BRCA-1 transcription and reduces BRCA-1 protein expression. These silencing effects are accompanied by reduced association at the BRCA-1 gene of AcH4 and AcH3K9; increased association of mMH3K9, DNMT1, and MBD2; and accumulation of DNA strand breaks. These epigenetic changes are attenuated most effectively by the pretreatment with Res.
Discussion
Unlike mutations, epigenetic events are reversible and represent viable targets for intervention using dietary compounds (3). Previous studies advocate a role for the AhR in the etiology of mammary (18, 38) and intestinal (39) tumors. The occupancy of the activated AhR at an XRE harbored in the BRCA-1 gene disrupts the formation of an ERα/p300 complex and activation of BRCA-1 transcription by E2 (26). In this study, we investigated the epigenetic regulation of the BRCA-1 gene by the AhR and the preventative effects of Res, which possesses antagonistic properties toward the AhR (27). We used the dioxin compound TCDD as a prototype AhR agonist, because, unlike PAH, it is not metabolized and the interpretation of the results is not confounded by effects due to reactive metabolites.
The recruitment of the AhR to the BRCA-1 promoter and reduction of BRCA-1 expression were antagonized most effectively by pretreatment with Res. These results are in agreement with those of previous investigations reporting inhibition by Res of TCDD- and B[a]P/DMBA-dependent stimulation of CYP1A1 and CYCP1B1 expression (27, 40). The preexposure and treatment with Res alone amplified the stimulatory effects of E2 on BRCA-1 expression. This effect was attributed to the partial agonistic properties of Res for the ERα (41). Previous studies have documented accumulation of BRCA-1 mRNA in MCF-7 cells treated with Res (42, 43).
The increased association of the AhR with the BRCA-1 promoter was paralleled by reduced AcH4 and AcH3K9 and increased levels of mMH3K9. These epigenetic marks are linked to transcriptional repression of tumor suppressor genes (44). Methylation at H3K9 by histone methyl transferases is linked to the recruitment of histone protein-1, which recruits factors with methylating activity (45) and facilitates the assembly of heterochromatin (46). Increases in AcH3 and AcH4 coupled to a reduction in mH3K9 are reported in human leukemia cells following treatment with isothiocyanates (47). Acetylated H3 is increased by sodium butyrate supplementation in a mouse model of colorectal cancer (5) and in response to diallyl sulfate (48). Finally, p21 gene-associated regions of chromatin had hyper-AcH3 in colon cancer cells treated with sodium butyrate (49).
Methyl-binding proteins bind to methylated CpG sequences and complex with HDAC (50). We observed that TCDD induced the association of MBD2 at the BRCA-1 gene. The MBD2 is 1 of 5 proteins that bind methylated cytosines within CpG dinucleotides. The recruitment of MBD2 was reversed by pretreatment with Res. Moreover, the cotreatment as well as preexposure with Res antagonized the recruitment of DNMT1, a DNA methyl-transferase that functions as maintenance methylase during DNA synthesis and propagates DNA methylation patterns (36). The transfection of siRNA for DNMT1 into MCF-7 cells reduced endogenous levels of DNMT1 protein while increasing BRCA-1 protein expression. The increased recruitment of DNMT1 and reduced association of AcH3 and AcH4 on the BRCA-1 gene are correlated to repression of BRCA-1 expression in sporadic breast cancers (51). The prevalence of DNA methylation of the BRCA-1 gene is also correlated to increased expression of DNMT1 in ductal carcinoma of the pancreas (52).
Previous studies reported that preexposure to TCDD increased reactive oxygen species production and DNA damage in MCF-7 cells subsequently treated with E2 (37, 53). In addition, TCDD is reported to increase the frequency of homologous recombination (54). In contrast, Res has been shown to reduce the accumulation of reactive oxygen species (55). Using the comet assay, we observed that TCDD induced DNA strand breaks, which were reduced by pretreatment with Res. A working model (Fig. 7) that emerges from the current study is one in which AhR agonists may increase cancer risk by inducing the frequency of homologous recombination and DNA damage while causing epigenetic silencing of BRCA-1, which participates in DNA repair during homologous recombination (56). Results with Res highlight the potential for developing prevention strategies based on dietary AhR-antagonists.
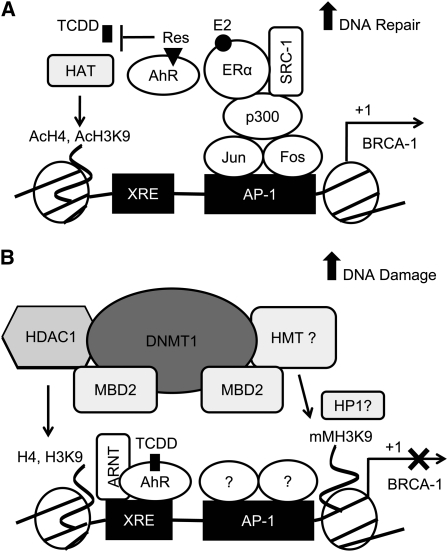
FIGURE 7.
Proposed model of epigenetic regulation of BRCA-1 by AhR agonists. In A, E2 stimulates the assembly of a p300/ERα/SRC-1 heterocomplex at an AP-1 site (31), the association of AcH4 and AcH3K9 leading to activation of BRCA-1 transcription and participation of BRCA-1 in DNA repair. (B) The exposure to AhR agonists such as TCDD induces silencing of BRCA-1 through the recruitment of an AhR/ARNT heterocomplex to an adjacent XRE disrupting the formation of the p300/ERα/SRC-1 complex. The recruitment of HDAC1 (26), DNMT1, MBD2, and mMH3K9 orchestrate the epigenetic silencing of BRCA-1 by AhR agonists, which may be antagonized by dietary AhR antagonists, including Res.
In summary, the current studies provide proof-of-principle dietary AhR-antagonists may be useful in preventing AhR-dependent epigenetic silencing of BRCA-1 in mammary epithelial cells. Future studies should investigate whether the in vitro effects of TCDD and Res on BRCA-1 expression illustrated in this study mimic the mechanisms of action and cumulative exposure to dietary AhR ligands in humans. The doses of Res (10 and 20 μmol/L) used in this study were lower than those (30–50 μmol/L) used in previous BRCA-1 investigations (42, 43) but higher than concentrations (2.4 μmol/L) achieved in pharmacokinetic studies with human participants using a single dose of Res (57). Interestingly, recent reports documented that certain natural isoflavones may be more powerful agonists of the AhR compared with indol-3-carbinol and 3,3′-diindolylmethane (23) and that their binding affinities for the AhR approximate that of the carcinogens DMBA and B[a]P (24). Moreover, Western-style diets are reported to contain AhR-inducing compounds (21). Because the AhR has a promiscuous binding site for many bioactive food components, combination diets with AhR antagonists may offer the advantage of higher cancer prevention efficacies while reducing the problem of using pharmacological and potentially toxic levels of single bioactives. Kinetic studies are currently in progress in our laboratory to investigate the effects of Res metabolites and combinations of dietary AhR ligands on epigenetic regulation of BRCA-1 in mammary epithelial cells.
Acknowledgments
A.J.P., S.D.L., O.I.S., G.T.W., and D.F.R. designed the research and contributed to interpretation of data; A.J.P. had primary responsibility for conducting the research and analyzing the data; S.D.L. conducted comet experiments and analyzed data; and A.J.P. and D.F.R. wrote the manuscript. All authors read and approved the manuscript.
Footnotes
Supported by grants from the Arizona Biomedical Research Commission (0819, D.F.R.), Susan G. Komen Breast Cancer Foundation (BCTR0707643, D.F.R.), Arizona Cancer Center Support grant P30CA23074, and SWEHSC grant ES006694. TCDD was supplied by the National Cancer Institute, Division of Cancer Biology, Chemical and Physical Carcinogenesis Branch and distributed by Midwest Research Institute under a contract to NCI (64 CFR 72090, 64 CFR 28205).
Abbreviations used: AcH, acetylated histone; AhR, aromatic hydrocarbon receptor; AP-1, activator protein-1; B[a]P, benzo[a]pyrene; DMBA, 7,12-dimethylbenz[a]anthracene; ChIP, chromatin immunoprecipitation assay; DNMT1, DNA methyltransferase; E2, 17 beta -estradiol; ER alpha , estrogen receptor- alpha ; GAPDH, glyceraldehyde-3-phosphate; HDAC, histone deacetylase; MBD2, methyl-binding domain protein-2; mMH3, monomethylated histone-3; PAH, polycyclic aromatic hydrocarbon; Res, resveratrol; siAhR, small interference RNA duplexes for aromatic hydrocarbon receptor; siCon, nontargeting small interference RNA; siDNMT1, small interference RNA duplexes for DNA methyltransferase; siGAPDH, small interference RNA duplexes for glyceraldehyde-3-phosphate; siRNA, small interference RNA; TCDD, 2,3,7,8 tetrachlorobenzo(p)dioxin; XRE, xenobiotic responsive element.
Literature Cited
- 1.Guil S, Esteller M. DNA methylomes, histone codes and miRNAs: tying it all together. Int J Biochem Cell Biol. 2009;41:87–95 [DOI] [PubMed] [Google Scholar]
- 2.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross SA, Dwyer J, Umar A, Kagan J, Verma M, Van Bemmel DM, Dunn BK. Introduction: diet, epigenetic events and cancer prevention. Nutr Rev. 2008;66 Suppl 1:S1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Degner SC, Papoutsis AJ, Selmin O, Romagnolo DF. Targeting of aryl hydrocarbon receptor-mediated activation of cyclooxygenase-2 expression by the indole-3-carbinol metabolite 3,3′-diindolylmethane in breast cancer cells. J Nutr. 2009;139:26–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu R, Wang X, Sun DF, Tian XQ, Zhao SL, Chen YX, Fang JY. Folic acid and sodium butyrate prevent tumorigenesis in a mouse model of colorectal cancer. Epigenetics. 2008;3:330–5 [DOI] [PubMed] [Google Scholar]
- 6.Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, Welsh W, Yang CS. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63:7563–70 [PubMed] [Google Scholar]
- 7.Myzak MC, Dashwood WM, Orner GA, Ho E, Dashwood RH. Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apc-minus mice. FASEB J. 2006;20:506–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myzak MC, Tong P, Dashwood WM, Dashwood RH, Ho E. Sulforaphane retards the growth of human PC-3 xenografts and inhibits HDAC activity in human subjects. Exp Biol Med (Maywood). 2007;232:227–34 [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson CA, Ramos L, Villaseñor MR, Anders KH, Press MF, Clarke K, Karlan B, Chen JJ, Scully R, et al. Localization of human BRCA1 and its loss in high-grade, non-inherited breast carcinomas. Nat Genet. 1999;21:236–40 [DOI] [PubMed] [Google Scholar]
- 10.Birgisdottir V, Stefansson OA, Bodvarsdottir SK, Hilmarsdottir H, Jonasson JG, Eyfjord JE. Epigenetic silencing and deletion of the BRCA1 gene in sporadic breast cancer. Breast Cancer Res. 2006;8:R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rice JC, Futscher BW. Transcriptional repression of BRCA1 by aberrant cytosine methylation, histone hypoacetylation and chromatin condensation of the BRCA1 promoter. Nucleic Acids Res. 2000;28:3233–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyamoto K, Fukutomi T, Asada K, Wakazono K, Tsuda H, Asahara T, Sugimura T, Ushijima T. Promoter hypermethylation and post-transcriptional mechanisms for reduced BRCA-1 immunoreactivity in sporadic human breast cancers. Jpn J Clin Oncol. 2002;32:79–84 [DOI] [PubMed] [Google Scholar]
- 13.Butcher DT, Rodenhiser DI. Epigenetic inactivation of BRCA1 is associated with aberrant expression of CTCF and DNA methyltransferase (DNMT3B) in some sporadic breast tumours. Eur J Cancer. 2007;43:210–9 [DOI] [PubMed] [Google Scholar]
- 14.Revich B, Aksel E, Ushakova T, Ivanova I, Zhuchenko N, Klyuev N, Brodsky B, Sotskov Y. Dioxin exposure and public health in Chapaevsk, Russia. Chemosphere. 2001;43:951–66 [DOI] [PubMed] [Google Scholar]
- 15.Dragan YP, Schrenk D. Animal studies addressing the carcinogenicity of TCDD (or related compounds) with an emphasis on tumour promotion. Food Addit Contam. 2000;17:289–302 [DOI] [PubMed] [Google Scholar]
- 16.Hooper K, Petreas MX, Chuvakova T, Kazbekova G, Druz N, Seminova G, Sharmanov T, Hayward D, She J, et al. Analysis of breast milk to assess exposure to chlorinated contaminants in Kazakstan: high levels of 2,3,7, 8-tetrachlorodibenzo-p-dioxin (TCDD) in agricultural villages of southern Kazakstan. Environ Health Perspect. 1998;106:797–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiss J, Päpke O, Bignert A, Jensen S, Greyerz E, Agostoni C, Besana R, Riva E, Giovannini M, et al. Concentrations of dioxin and other organochlorines (PCBs, DDTs, HCHs) in human milk from Seveso, Milan and a Lombardian rural area in Italy: a study performed 25 years after the heavy dioxin exposure in Seveso. Acta Paediatr. 2003;92:467–72 [DOI] [PubMed] [Google Scholar]
- 18.Jenkins S, Rowell C, Wang J, Lamartiniere CA. Prenatal TCDD exposure predisposes for mammary cancer in rats. Reprod Toxicol. 2007;23:391–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ray SS, Swanson HI. Dioxin-induced immortalization of normal human keratinocytes and silencing of p53 and p16INK4a. J Biol Chem. 2004;279:27187–93 [DOI] [PubMed] [Google Scholar]
- 20.Connor KT, Harris MA, Edwards MR, Budinsky RA, Clark GC, Chu AC, Finley BL, Rowlands JC. AH receptor agonist activity in human blood measured with a cell-based bioassay: evidence for naturally occurring AH receptor ligands in vivo. J Expo Sci Environ Epidemiol. 2008;18:369–80 [DOI] [PubMed] [Google Scholar]
- 21.Erdelyi I, Levenkova N, Lin EY, Pinto JT, Lipkin M, Quimby FW, Holt PR. Western-style diets induce oxidative stress and dysregulate immune responses in the colon in a mouse model of sporadic colon cancer. J Nutr. 2009;139:2072–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–34 [DOI] [PubMed] [Google Scholar]
- 23.Medjakovic S, Jungbauer A. Red clover isoflavones biochanin A and formononetin are potent ligands of the human aryl hydrocarbon receptor. J Steroid Biochem Mol Biol. 2008;108:171–7 [DOI] [PubMed] [Google Scholar]
- 24.Piskorska-Pliszczynska J, Keys B, Safe S, Newman MS. The cytosolic receptor binding affinities and AHH induction potencies of 29 polynuclear aromatic hydrocarbons. Toxicol Lett. 1986;34:67–74 [DOI] [PubMed] [Google Scholar]
- 25.Russo J, Tahin Q, Lareef MH, Hu YF, Russo IH. Neoplastic transformation of human breast epithelial cells by estrogens and chemical carcinogens. Environ Mol Mutagen. 2002;39:254–63 [DOI] [PubMed] [Google Scholar]
- 26.Hockings JK, Thorne PA, Kemp MQ, Morgan SS, Selmin O, Romagnolo DF. The ligand status of the aromatic hydrocarbon receptor modulates transcriptional activation of BRCA-1 promoter by estrogen. Cancer Res. 2006;66:2224–32 [DOI] [PubMed] [Google Scholar]
- 27.Casper RF, Quesne M, Rogers IM, Shirota T, Jolivet A, Milgrom E, Savouret JF. Resveratrol has antagonist activity on the aryl hydrocarbon receptor: implications for prevention of dioxin toxicity. Mol Pharmacol. 1999;56:784–90 [PubMed] [Google Scholar]
- 28.Banerjee S, Bueso-Ramos C, Aggarwal BB. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002;62:4945–54 [PubMed] [Google Scholar]
- 29.Degner SC, Kemp MQ, Hockings JK, Romagnolo DF. Cyclooxygenase-2 promoter activation by the aromatic hydrocarbon receptor in breast cancer mcf-7 cells: repressive effects of conjugated linoleic acid. Nutr Cancer. 2007;59:248–57 [DOI] [PubMed] [Google Scholar]
- 30.Jeffy BD, Chirnomas RB, Chen EJ, Gudas JM, Romagnolo DF. Activation of the aromatic hydrocarbon receptor pathway is not sufficient for transcriptional repression of BRCA-1: requirements for metabolism of benzo[a]pyrene to 7r,8t-dihydroxy-9t,10-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene. Cancer Res. 2002;62:113–21 [PubMed] [Google Scholar]
- 31.Jeffy BD, Hockings JK, Kemp MQ, Morgan SS, Hager JA, Beliakoff J, Whitesell LJ, Bowden GT, Romagnolo DF. An estrogen receptor-alpha/p300 complex activates the BRCA-1 promoter at an AP-1 site that binds Jun/Fos transcription factors: repressive effects of p53 on BRCA-1 transcription. Neoplasia. 2005;7:873–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamore SD, Cabello CM, Wondrak GT. The topical antimicrobial zinc pyrithione is a heat shock response inducer that causes DNA damage and PARP-dependent energy crisis in human skin cells. Cell Stress Chaperones. 2010:15:309–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gudas JM, Nguyen H, Li T, Cowan KH. Hormone-dependent regulation of BRCA1 in human breast cancer cells. Cancer Res. 1995;55:4561–5 [PubMed] [Google Scholar]
- 34.Abdelrahim M, Smith R III, Safe S. Aryl hydrocarbon receptor gene silencing with small inhibitory RNA differentially modulates Ah-responsiveness in MCF-7 and HepG2 cancer cells. Mol Pharmacol. 2003;63:1373–81 [DOI] [PubMed] [Google Scholar]
- 35.de Medina P, Casper R, Savouret JF, Poirot M. Synthesis and biological properties of new stilbene derivatives of resveratrol as new selective aryl hydrocarbon modulators. J Med Chem. 2005;48:287–91 [DOI] [PubMed] [Google Scholar]
- 36.Reid G, Gallais R, Métivier R. Marking time: the dynamic role of chromatin and covalent modification in transcription. Int J Biochem Cell Biol. 2009;41:155–63 [DOI] [PubMed] [Google Scholar]
- 37.Lin PH, Lin CH, Huang CC, Chuang MC, Lin P. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces oxidative stress, DNA strand breaks, and poly(ADP-ribose) polymerase-1 activation in human breast carcinoma cell lines. Toxicol Lett. 2007;172:146–58 [DOI] [PubMed] [Google Scholar]
- 38.Schlezinger JJ, Liu D, Farago M, Seldin DC, Belguise K, Sonenshein GE, Sherr DH. A role for the aryl hydrocarbon receptor in mammary gland tumorigenesis. Biol Chem. 2006;387:1175–87 [DOI] [PubMed] [Google Scholar]
- 39.Kawajiri K, Kobayashi Y, Ohtake F, Ikuta T, Matsushima Y, Mimura J, Pettersson S, Pollenz RS, Sakaki T, et al. Aryl hydrocarbon receptor suppresses intestinal carcinogenesis in ApcMin/+ mice with natural ligands. Proc Natl Acad Sci USA. 2009;106:13481–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beedanagari SR, Bebenek I, Bui P, Hankinson O. Resveratrol inhibits dioxin-induced expression of human CYP1A1 and CYP1B1 by inhibiting recruitment of the aryl hydrocarbon receptor complex and RNA polymerase II to the regulatory regions of the corresponding genes. Toxicol Sci. 2009;110:61–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gehm BD, McAndrews JM, Chien PY, Jamison JL. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the oestrogen receptor. Proc Natl Acad Sci USA. 1997;94:14138–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fustier P, Le Corre L, Chalabi N, Vissac-Sabatier C, Communal Y, Bignon YJ, Bernard-Gallon DJ. Resveratrol increases BRCA1 and BRCA2 mRNA expression in breast tumour cell lines. Br J Cancer. 2003;89:168–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le Corre L, Fustier P, Chalabi N, Bignon YJ, Bernard-Gallon D. Effects of resveratrol on the expression of a panel of genes interacting with the BRCA1 oncosuppressor in human breast cell lines. Clin Chim Acta. 2004;344:115–21 [DOI] [PubMed] [Google Scholar]
- 44.Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–12 [DOI] [PubMed] [Google Scholar]
- 45.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705 [DOI] [PubMed] [Google Scholar]
- 46.Nakayama J, Rice JC, Strahl BD, Allis CD, Grewal SI. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science. 2001;292:110–3 [DOI] [PubMed] [Google Scholar]
- 47.Ma X, Fang Y, Beklemisheva A, Dai W, Feng J, Ahmed T, Liu D, Chiao JW. Phenylhexyl isothiocyanate inhibits histone deacetylases and remodels chromatins to induce growth arrest in human leukemia cells. Int J Oncol. 2006;28:1287–93 [PubMed] [Google Scholar]
- 48.Zhao J, Huang WG, He J, Tan H, Liao QJ, Su Q. Diallyl disulfide suppresses growth of HL-60 cell through increasing histone acetylation and p21WAF1 expression in vivo and in vitro. Acta Pharmacol Sin. 2006;27:1459–66 [DOI] [PubMed] [Google Scholar]
- 49.Chen YX, Fang JY, Zhu HY, Lu R, Cheng ZH, Qiu DK. Histone acetylation regulates p21WAF1 expression in human colon cancer cell lines. World J Gastroenterol. 2004;10:2643–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ellis L, Atadja PW, Johnstone RW. Epigenetics in cancer: targeting chromatin modifications. Mol Cancer Ther. 2009;8:1409–20 [DOI] [PubMed] [Google Scholar]
- 51.Jin W, Chen L, Chen Y, Xu SG, Di GH, Yin WJ, Wu J, Shao ZM. UHRF1 is associated with epigenetic silencing of BRCA1 in sporadic breast cancer. Breast Cancer Res Treat. Epub 2009 Nov 27 [DOI] [PubMed] [Google Scholar]
- 52.Peng DF, Kanai Y, Sawada M, Ushijima S, Hiraoka N, Kitazawa S, Hirohashi S. DNA methylation of multiple tumor-related genes in association with overexpression of DNA methyltransferase 1 (DNMT1) during multistage carcinogenesis of the pancreas. Carcinogenesis. 2006;27:1160–8 [DOI] [PubMed] [Google Scholar]
- 53.Knerr S, Schrenk D. Carcinogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in experimental models. Mol Nutr Food Res. 2006;50:897–907 [DOI] [PubMed] [Google Scholar]
- 54.Chan CY, Kim PM, Winn LM. TCDD affects DNA double strand-break repair. Toxicol Sci. 2004;81:133–8 [DOI] [PubMed] [Google Scholar]
- 55.Martinez J, Moreno JJ. Effect of resveratrol, a natural polyphenolic compound, on reactive oxygen species and prostaglandin production. Biochem Pharmacol. 2000;59:865–70 [DOI] [PubMed] [Google Scholar]
- 56.Moynahan ME, Chiu JW, Koller BH, Jasin M. Brca1 controls homology-directed DNA repair. Mol Cell. 1999;4:511–8 [DOI] [PubMed] [Google Scholar]
- 57.Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246–52 [DOI] [PubMed] [Google Scholar]