Abstract
Experts in the field of carotenoids met at the Hohenheim consensus conference in July 2009 to elucidate the current status of β-carotene research and to summarize the current knowledge with respect to the chemical properties, physiological function, and intake of β-carotene. The experts discussed 17 questions and reached an agreement formulated in a consensus answer in each case. These consensus answers are based on published valid data, which were carefully reviewed by the individual experts and are justified here by background statements. Ascertaining the impact of β-carotene on the total dietary intake of vitamin A is complicated, because the efficiency of conversion of β-carotene to retinol is not a single ratio and different conversion factors have been used in various surveys and following governmental recommendations within different countries. However, a role of β-carotene in fulfilling the recommended intake for vitamin A is apparent from a variety of studies. Thus, besides elucidating the various functions, distribution, and uptake of β-carotene, the consensus conference placed special emphasis on the provitamin A function of β-carotene and the role of β-carotene in the realization of the required/recommended total vitamin A intake in both developed and developing countries. There was consensus that β-carotene is a safe source of vitamin A and that the provitamin A function of β-carotene contributes to vitamin A intake.
Based on the Chemical Data, What Is the Major Function of β-Carotene in the Human Diet?
Consensus
Based on chemical data, the major function of β-carotene is as an optimal, naturally occurring, provitamin A. β-Carotene is structurally and functionally different from other carotenoids. There is no difference between naturally occurring or chemically synthesized β-carotene.
Furthermore, β-carotene can also act as a lipid radical scavenger and as a singlet oxygen quencher, as demonstrated in vitro.
Is There Scientific Evidence That Isolated β-Carotene Is Deleterious While Natural Food-Derived β-Carotene Is Not?
Consensus
No, when comparing food-derived all-trans-β-carotene with synthetic all-trans-β-carotene, there is no evidence that the latter is deleterious.
Background
Structure of β-carotene.
β-Carotene (β,β-carotene) is the most prominent member of the group of carotenoids, natural colorants that occur in the human diet (1). As a tetraterpenoid it consists of 40 carbon atoms in a core structure of conjugated double bonds substituted with 2 β-ionone rings. Due to its extended system of 9 fully conjugated double bonds, β-carotene shows a major absorption peak in the visible spectrum with a maximum at ∼450 nm, responsible for the orange to red color of the compound (2). In biological systems, the predominant isomer is all-trans β-carotene (E-isomer). However, cis-isomers have been found in living organisms and food samples (3, 4); among them are 9-cis-, 13-cis-, and 15-cis-β-carotene (Z-isomers), in addition to several di- and poly-cis analogs.
β-Carotene structure and vitamin A activity.
All-trans-β-carotene is the most suitable and important precursor for vitamin A (5). This is primarily due to its symmetrical structure, because all-trans-β-carotene is the only carotenoid capable of yielding 2 molecules of all-trans-retinal upon oxidative cleavage of the central 15,15´ carbon-carbon bond, which is catalyzed by the β-carotene monooxygenase. There is evidence from in vitro studies that other geometrical isomers are less efficiently cleaved (6).
All-trans retinoic acid is synthesized enzymatically from all-trans retinal and binds as a ligand to the retinoic acid receptor family involved in the regulation of transcription (7). 9-cis-retinoic acid binds to both retinoic acid receptor and retinoic acid-X receptors, although it has not yet been proven that it is the natural ligand of the retinoic acid-X receptors (8, 9). Inadequate levels of this regulatory molecule are likely to disturb important cellular signaling pathways. Therefore, retinoic acid homeostasis requires tight regulation via control of retinoic acid synthesis and catabolism. Thus, the fate of the 9-cis-β-carotene geometrical isomers is of considerable interest, because studies with rats and other species have provided evidence that 9-cis-β-carotene is an isomeric precursor of 9-cis retinal or 9-cis retinoic acid. However, the cleavage rates are distinctly lower than with the all-trans form (6, 10, 11). Furthermore, 9-cis-β-carotene is not found in human plasma even when the isomer is consumed in considerable amounts (12). It might be speculated that the organism takes advantage of the structure of all-trans-β-carotene and already controls the formation of ultimately active compounds at the precursor level. In the case of retinoids, the symmetric all-trans-β-carotene is apparently an optimal structure. Formation of all-trans- or 9-cis- retinoic acid can be triggered at the level of cleavage, oxidation, and isomerization, providing optimal tools for fine tuning.
Antioxidant activity.
For structural reasons and based on experimental data, β-carotene has been designated an antioxidant (1, 5, 13, 14). However, this has been challenged and even prooxidant properties have been assigned to the compound, at least in vitro (14–19). There are numerous in vitro data both demonstrating and disputing antioxidant properties of the compound (19). Based on the analyses of biomarkers for oxidative damage (e.g. malondialdehyde, isoprostanes, 8-oxo-guanosine), antioxidant properties of β-carotene have also been studied in humans, although with conflicting results (20–22).
Singlet oxygen quenching.
Due to the core system of conjugated carbon-carbon bonds, β-carotene is an efficient singlet oxygen quencher and prevents the formation of singlet oxygen by quenching excited triplet sensitizers (23).
Most carotenoids with 9 or more conjugated double bonds, including lutein and zeaxanthin, are comparably efficient quenchers of singlet oxygen, with reaction rate constants in homogenous systems of 5–10 × 109 mol−1 sec−1. Such rate constants are close to diffusion control and thus cannot be improved further (24). In the light-harvesting system of plants, carotenoids prevent photooxidative damage by inhibiting singlet oxygen generation (23). A key question addresses the point of whether such antioxidant processes attributable to β-carotene are important in humans. Studies with patients suffering from erythropoietic protoporphyria support the idea that β-carotene quenches excited molecules (25, 26). Due to a genetic defect, nonphysiologically high levels of the photosensitizer protoporphyrin IX circulate in the organism, generating singlet oxygen in light-exposed tissues. Symptoms are ameliorated by supplementation with high doses of β-carotene (27). It is not known if this mechanism of singlet oxygen generation is relevant in healthy people.
Direct photosensitizing occurs only in the eye or the skin and requires the presence of sensitizing molecules. It has been proposed that such reaction sequences play a major role in the pathogenesis of age-related macular degeneration and cataracts. The major carotenoids of the macula lutea are lutein, zeaxanthin, and meso-zeaxanthin; β-carotene is not present in this tissue (28). The concentration of β-carotene in the lens is low compared with other carotenoids (i.e. lutein and zeaxanthin) or antioxidants such as hydrophilic ascorbate and glutathione, indicating that β-carotene is of limited importance as a direct photoprotective carotenoid in the eye (29).
β-Carotene is a major carotenoid in skin and is enriched in this tissue upon supplementation (30). Human intervention studies show moderate UV protective effects of β-carotene in the skin (31, 32). In most of these studies, an elevated intake of β-carotene partially ameliorated sunburn or UV-induced erythema (erythema solare), the primary reaction of the skin following UV exposure. Whether this was dependent upon primary protection against photooxidation (singlet oxygen quenching), antioxidant activity against secondary reactive oxygen species, or interference with inflammatory signaling pathways is not known.
The efficacy of β-carotene in systemic photo-protection depends on both the duration of treatment and the dose given. In studies documenting protection against UV-induced erythema, supplementation with carotenoids lasted for at least 7 wk, with doses > 12 mg/d of carotenoids (33–36). In studies reporting no protective effects, the treatment period was only 3–4 wk (37).
Several light-independent mechanisms for the generation of singlet oxygen in biological systems have been proposed (38). Excited singlet oxygen can be formed when hypochlorite reacts with hydrogen peroxide. Both substances are products of preceding enzymatic processes (myeloperoxidase, catalase) related to oxidative burst (39). Singlet oxygen may also be generated upon spontaneous dismutation of superoxide. Furthermore, peroxyradicals, e.g. formed following lipid peroxidation, may decompose releasing singlet oxygen according to the Russell mechanism (40). It is not yet known if significant amounts of singlet oxygen are produced via such reaction sequences in non-light–exposed tissues.
Radical scavenging.
Under conditions of oxidative stress, radicals and nonradical reactive species are generated (41). Lipid peroxidation is a continuous chain reaction in membranes, affecting PUFA, which finally destroys lipophilic compartments if not inhibited by a radical scavenger. In addition to vitamin E, β-carotene has been postulated to be an important chain-breaking antioxidant, scavenging lipid oxide and lipid peroxide radicals (5).
The basic chemistry of a β-carotene-radical interaction is still not well understood (1). Radical scavenging processes involve the donation of a hydrogen atom or an electron, which leads to the formation of a carotenoid radical (Car⋅) or a carotenoid radical cation (Car+⋅). Upon electron acceptance, the carotenoid radical anion is formed (Car−⋅). All these species are thought to be stabilized via electron delocalization over the entire system of double bonds (5, 42–44). However, β-carotene rapidly decomposes when exposed to radicals in further reaction sequences. Thus, the compound is consumed and cannot be regenerated. Reactions with radical intermediates of lipid peroxidation are likely to lead to the formation of carotenoid radical adducts according to:
In a second scavenging process, a further lipid radical may be added to form a neutral molecule:
Again, the radical is scavenged, but β-carotene is chemically modified and cannot be regenerated (45).
The prooxidant properties of β-carotene especially have been investigated in the context of lipid peroxidation (15–18, 42). Several in vitro studies showed that, under conditions of high oxygen tension, prooxidant activities can be measured. Increased markers of lipid peroxidation (e.g. malondialdehyde) were measured following exposure of β-carotene to oxidizing conditions in high levels of oxygen. Reactions of the first carotenoid radical adduct with molecular oxygen are thought to be responsible for prooxidant properties under these conditions. A carotenoid peroxylradical is generated according to:
Possible prooxidant reactions involve the abstraction of a hydrogen atom from an unsaturated fatty acid, resulting in propagation of lipid peroxidation. Carotenoid epoxides and cyclic peroxides may be produced, the latter decomposing upon cleavage of the carotenoid molecule. Oxidative cleavage products, such as apo-carotenals, retain the structure of biologically active signaling molecules (e.g. retinoic acid) and may interfere with or trigger signaling pathways.
Conclusion.
Due to its unique structure and cleavage efficacy, β-carotene is the most efficient provitamin A carotenoid. As an antioxidant, the compound quenches singlet molecular oxygen and scavenges reactive oxygen species, especially peroxyl radicals. Singlet oxygen quenching is likely to be restricted to the skin as the only light-exposed tissue that contains higher levels of β-carotene; other carotenoids demonstrate similar activity. Upon radical scavenging, β-carotene decomposes and cannot be regenerated. Thus, it is suggested that the major function of β-carotene in human nutrition is that of a provitamin A.
Are There Specific Non-Vitamin A#x2013Related Effects in Humans?
Consensus
Based on in vitro data, there is evidence that β-carotene has effects that go beyond the established provitamin A function. However, such effects have not yet been unequivocally proven in humans.
Background
The physiological relevance of effects such as protection against photosensitivity and UV-induced erythema has not yet been clearly proven in humans.
In particular, β-carotene has been prescribed and used against photosensitivity in erythropoietic protoporphyria, but its beneficial potential in normal skin remains uncertain (27, 46). After ∼10–12 wk of dietary intervention, a decrease in the sensitivity toward UV-induced erythema was observed in volunteers and a number of experimental studies have indicated protective effects of β-carotene against acute and chronic manifestations of skin photodamage, but there is a lack of controlled clinical studies demonstrating its beneficial effects (15, 47, 48).
A role for vitamin A in regulating adipose tissue size and function has been proposed following studies in rodents in which thermogenic capacity was increased by β-carotene and retinoic acid (49–52). However, conflicting results recently have been obtained in ferrets in which mechanisms apparently distinctive to vitamin A may operate, possibly related to the fact that this animal model has a limited intestinal cleavage of β-carotene compared with rodents (53). On the other hand, β-carotene-monooxygenase-1 (BCMO1)12 activity has been found in many mammalian tissues, including adipose tissue. BCMO1−/− knockout mice develop various signs of adiposity, i.e. high levels of triglycerides and fatty acids, an increased adipose tissue mass, and liver steatosis. This phenotype was shown to be independent of the vitamin A content in the diet, suggesting a specific role of BCMO1 and potentially extending the function of carotenoids as regulators of lipid metabolism (54). However, the potential role of β-carotene in controlling thermogenesis and/or adipose tissue depot size and function has not been explored in controlled human studies.
Are There Special Effects of β-Carotene Compared with Other Carotenoids?
Consensus
Compared with other carotenoids, the primary role of β-carotene is its provitamin A activity.
Background
Provitamin A activity is the main function of β-carotene known in humans. The uniqueness of β-carotene is that, compared with other carotenoids, it has a β-ionone structure as the terminal ring system on each end of the polyene chain, and its central oxidative cleavage in the intestine allows for its conversion to 2 molecules of vitamin A in a physiologically regulated manner. The oxidative cleavage of β-carotene, the major carotenoid of human diets, is achieved by BCMO1, which cleaves β-carotene into 2 molecules of all-trans-retinal (retinal-aldehyde). This is the first step in the pathway for the biosynthesis of vitamin A (all-trans-retinol and retinyl esters) (55, 56).
Approximately 50 of the naturally occurring carotenoids (containing at least 1 unsubstituted β-ionone ring and a polyene chain) can potentially yield vitamin A and are thus referred to as provitamin A carotenoids (57). However, the different carotenoids, although similar in structure, could well have quite different activities at the cellular level and the specific products of their metabolism vary depending on the particular carotenoid. It should be mentioned that of the 50 carotenoids present in the human diet, only 5 or 6 are detectable in human plasma (α- and β-carotene, β-cryptoxanthin, lycopene, lutein, zeaxanthin). Only α- and β-carotene and β-cryptoxanthin have provitamin A activity. Therefore, the importance of the others for human nutrition is rather limited. Numerous studies have shown that a substantial amount of the absorbed carotenoids are not cleaved by the intestinal BCMO1 enzyme. A proportion of the carotenoids (up to 60% of dietary intake) that escape BCMO1 enzyme activity are incorporated in chylomicrons together with lipids and circulate in association with VLDL, LDL, and HDL and hence can be taken up by the respective receptors for these lipoproteins (58). Interestingly, the recent demonstration that the responsible β-carotene–cleaving enzyme BCMO1 is also present in tissues other than intestine opens new questions concerning the tissue-specific function of this enzyme and the physiological potential of vitamin A production in tissues other than intestine, including the retinal pigment epithelial cells or other specific targets for β-carotene [reviewed in (59)].
Vitamin A is important for many functions in the human body; in particular, it is essential for normal growth and development, immune function, and vision. Preformed vitamin A is present only in animal products (e.g. liver, eggs, and milk products); thus, in countries where the intake of animal products is low, vitamin A requirements are mostly met by carotenoids (i.e. by 80% or more in Asia and Africa). During pregnancy and lactation, vitamin A has a particularly important role in the healthy development of the child, and an increase in vitamin A intake has been recommended. However, it is currently recommended that women who are planning to become pregnant or who are pregnant should not consume cooked animal liver or other organ meats that are rich sources of vitamin A. Because current dietary intake may exceed the tolerable upper intake levels (UL), careful consideration should be given to the appropriateness of enrichment of human foods with preformed vitamin A (60). The UL for preformed vitamin A (retinol and retinyl esters) has been set at 3000 μg retinol equivalents (RE)/d. However, in addressing the possible risk of bone fracture in postmenopausal women, who are at greater risk of osteoporosis and fracture, restricting intake to 1500 μg RE/d of preformed vitamin A has been recommended (60, 61). The toxicity of pharmacological doses of vitamin A does not occur with the intake of high doses of β-carotene; the cleavage of β-carotene is regulated through feedback mechanisms; therefore, only the required amount is metabolized to retinol (60). All in all, it can be recommended that a proportion of vitamin A dietary requirements should be consumed in the form of β-carotene. Determining the optimal proportions of provitamin A and preformed vitamin A is currently not possible, but any proposal has to take into account the narrow margin between the population reference intake of vitamin A and adverse effects associated with preformed vitamin A. In addition, possible adverse effects of β-carotene have to be taken into account (60, 61).
The recent demonstration that BCMO1 is also present in tissues other than the intestine led to numerous investigations of the molecular structure and function of this enzyme in several species, including humans (59). For example, retinal pigment epithelial cells were shown to contain BCMO1 and to be able to cleave β-carotene into retinal in vitro, indicating a new pathway for vitamin A production in a tissue other than the intestine and possibly explaining the milder vitamin A deficiency symptoms of 2 human siblings lacking the retinol-binding protein for the transport of hepatic vitamin A to target tissues [reviewed in (59)].
What Is the Maximum β-Carotene Uptake Achieved via Diet (Fruit and Vegetables)?
Consensus
Dietary β-carotene intake varies widely and is not normally distributed in the population. The majority of people consume 1–2 mg/d (reported for the USA and the UK), although, in rare cases, an intake of 10 mg/d has been reported.
Background
β-Carotene intake helps to balance inadequate retinol supply in significant parts of the world. The highest intake of provitamin A is achieved in vegans and vegetarians (62). Based on calculated RE and using a conversion factor of 6:1, the equivalent intake of β-carotene would be as shown in Table 1. These data show that high provitamin A intake is possible under certain conditions, but the data also show that the intake of preformed vitamin A is sometimes critically low and does not reach the recommended levels.13
TABLE 1.
Intake of total RE and β-carotene depending on the diet1
| Men |
Women |
|||
| Diet | Total RE | β-Carotene | Total RE | β-Carotene |
| mg | ||||
| Vegan | 1.2 | 7.2 | 1.5 | 9.0 |
| Vegetarian | 0.7 | 4.2 | 0.9 | 5.4 |
| Omnivore | 0.5 | 3.0 | 0.8 | 4.8 |
The conversion ratio mentioned here is referring to a mg:mg conversion, not to a mol:mol. Conversion factors for dietary β-carotene to vitamin A have changed over time and have not always been the same in different studies and surveys.
The Institute of Medicine (IOM) dietary reference intake report states that a strict vegetarian can obtain his or her recommended daily allowance (RDA) of vitamin A from β-carotene in foods by carefully selecting his or her diet (61).
Mean daily intake of total vitamin A and carotenoids in Germany was determined in the NVS (1985–1988) on food and nutrient intake (63). Based on these data, 15% of 10- to 25-y olds do not meet the recommendations for total vitamin A intake. However, according to this survey, recommended vitamin A intake levels will be met by <25% of the population (depending on age group) by preformed vitamin A only, thus demonstrating the importance of provitamin A in ensuring adequate vitamin A intake levels. Daily vitamin A intake is particularly insufficient in children and adolescents when the recommended intake of β-carotene is not being met. The German National Nutrition Survey (Nationale Verzehrsstudie; NVS) II showed different results from the first. Based on the calculation of RE, the NVS showed that ∼33% of vitamin A comes from preformed retinol (meat, meat products, dairy products, fat); 48% from β-carotene sources, such as vegetables, vegetable soups, and nonalcoholic beverages; and 19% from mixed carotenoids. These sources contribute to a total of 1.7 mg/d RE (median). If 48% of RE comes from β-carotene, the mean intake of 4.5 mg/d of β-carotene can be calculated based on the conversion factor for RE to β-carotene (6:1) as used in the NVS II. This, however, is in strong contrast to other studies from Germany and Europe showing a mean intake of 1.5–1.8 mg/d β-carotene (64). If the data are taken as realistic, ∼15% of the female survey participants and 10.3% of male participants do not meet the recommendations for total vitamin A intake. Compared with other countries, β-carotene intake in Germany is not higher than, e.g., in Austria (0.8–2.1 mg/d of β-carotene from vegetables and 0.3 mg/d from fruits), Ireland (0.5 mg/d), or Spain (0.3 mg/d) (62).
Food intake in Spain was investigated using a 24-h recall questionnaire employing photographic food models to estimate portion sizes. The survey included 4728 males and 5480 females aged 25–60 y, and the prevalence of inadequate vitamin A intake [less than two-thirds the recommended dietary intake (RDI) of vitamin A based on RE] was 60.5% in men and 48.5% in women (65). The major reason for these findings seems to be the unexpectedly low intake of preformed vitamin A (mean levels of 293 μg/d in men and 276 μg/d in women). Mean β-carotene intake was 1.7 mg/d in men and 2 mg/d in women.
A nutrition survey in the UK comprising a 7-d weighed intake dietary record of 1724 respondents showed a vitamin A intake below the lower reference nutrient intake (LRNI) in 16% of men and 30% of women aged 19–34 y (66). The LRNI is the amount of a nutrient that is sufficient for only a small proportion of the population (2.5%). The vast majority of the population, therefore, has much greater requirements. It is particularly significant to note that, based on the results of the above-mentioned survey, 30% of women of child-bearing age do not achieve an adequate vitamin A intake; such a low intake may adversely affect fetal development in pregnancy.
In addition to recommended and actual daily intake of vitamin A, the potential risks of excessive intake (hypervitaminosis A) must be addressed. The question arises as to whether Western populations with a wide variety of sources of vitamin A, or developing countries with a higher intake of fortified food, are at greater risk. Based on national surveys (e.g. the NVS), it appears that these concerns may be justified. However, vitamin A intake is calculated from RE from all sources of vitamin A and provitamin A based on a conversion rate of 6:1, resulting in an apparently high overall intake. If, however, intake is calculated purely on the basis of preformed vitamin A, derived from liver, liver products, or eggs, e.g., it becomes clear that the intake is rather low. Recently, vitamin A intake from food, fortified food, and supplements was estimated in 9 European countries (67). Total intakes of retinol in adults vary considerably between countries, mainly due to different intakes from base diet and supplements. However, 95th percentile (P95) intake does not exceed the UL. For 4- to 10-y-old children (but not in 11- to 17-y-old children), P95 of total intake approaches or exceeds the UL in Poland due to high intakes from the base diet. The limited available data on intake from fortified foods indicate that intake of retinol from voluntarily fortified foods is low and has little effect on the P95 intake of total diet. It should be noted that foods that are (semi) mandatorily fortified with retinol, e.g., margarine and fat spreads, were included in the base diet data. A study performed in children in Zambia, where sugar was fortified, demonstrated that vitamin A status improved, but intake levels did not exceed the UL (68). In Western populations, food fortification with preformed vitamin A is marginal and may not contribute to levels exceeding the UL. Provitamin A intake is <3 mg/d in most European countries and consequently does not significantly contribute to intake levels approaching the UL for retinol, even if based on a cleavage ratio of 6:1.
Is There a Proportion of Dietary Provitamin A To Be Recommended That Will Ensure a Sufficient Vitamin A Supply?
Consensus
The number of people at risk for poor vitamin A status depends on the intake of total vitamin A, which is defined as preformed vitamin plus provitamin A.
Based on numerous studies, it is evident that parts of the world’s population do not meet the recommendation for vitamin A intake with dietary sources of preformed vitamin A. To fill the gap between the low intake from sources containing preformed vitamin A, adequate amounts of provitamin A must additionally be supplied. In Germany, 2–4 mg/d of β-carotene is recommended. However, if the intake of preformed vitamin A is low, at least 6 mg/d of β-carotene should be consumed (69).
Background
Provitamin A is a major source of vitamin A. Based on the intake data as discussed above, it is suggested that, if the intake of provitamin A falls below 2 mg/d (equivalent to 0.3 mg RE), the gap between the inadequate vitamin A intake and the RDI of 1 mg/d cannot be bridged. This is of special importance for pregnant women for 2 reasons: daily intake should be higher (1.1 mg/d) due to a higher demand and the majority of women of child-bearing age avoid eating liver as the major source for preformed vitamin A.
Provitamin A found in the typical vegetarian diet plays an important role in meeting vitamin A requirements, especially in a vegan diet that excludes the consumption of meat, particularly the visceral portions (organ meats), milk, and eggs. According to the First NVS and the reference intake values of Germany, Austria, and Switzerland (DACH), men obtain 25% and women 30% of their vitamin A intake from the provitamin β-carotene (70).
According to the DACH reference values, the recommended daily intake of vitamin A is 0.6–1.1 mg for children, between 0.8 mg and 1 mg (for pregnant women) for adults, and 1.5 mg for breastfeeding women (70). In the US, the IOM recommends 0.3–0.4 mg/d for children aged 1–8 y and 0.6–0.9 mg/d for boys and 0.6–0.7 mg/d for girls aged 9–18 y. The recommended daily vitamin A intake for adult men is 0.9 mg and for women is 0.7 mg, and the RDA for pregnant women is 0.77 mg and for lactating women is 1.3 mg (61). According to Müller (71), the β-carotene intake of almost one-half of the German population is <1 mg/d whereas 64.1% have intakes less than the recommended value of 2 mg/d. The β-carotene intake of 75% of the population is <3 mg/d, even when nutritional supplements and fortified foods are included. Policies to increase the bioavailability of β-carotene could include food fortification, which is of utmost importance, because β-carotene is a major source of vitamin A. However, β-carotene alone might not be enough to meet the increased need for vitamin A during pregnancy and lactation. Because vitamin A is also important for fetal lung development and maturation, sufficient intake should be ensured during the second and third trimesters of pregnancy (72). The best source of preformed vitamin A is liver. However, depending on animal feeding practices, liver may contain excessively high concentrations of retinyl esters. This has led the German Federal Institute for Consumer Protection and Veterinary Medicine to advise pregnant women to avoid consuming liver, but this frequently results in an insufficient supply of vitamin A in pregnant and breastfeeding women and they are therefore reliant on β-carotene as a source of vitamin A (73). However, the same institute recently claimed that the higher requirement for vitamin A during pregnancy can be achieved only via consumption of liver (74). The low intakes of vitamin A and β-carotene are also of concern in young women, especially those with multiple births, of low socioeconomic status, and breastfeeding women. In a recent study, it was documented that a low vitamin A intake from preformed vitamin A resulted in low plasma retinol, low cord blood retinol levels in newborns, and low colostrum and breast milk retinol levels in 28% of the participants (10 of 36) (75). Based on preformed vitamin A sources, 75% of participants did not reach the recommended intake level for pregnant women (1.1 mg/d) and 90% did not reach the recommended level for breastfeeding women (1.5 mg/d). Considering carotenoid intake in terms of RE, using the accepted conversion factor of 12, 67.8% of women still do not reach the vitamin A requirement during the breastfeeding period (61).
As mentioned above, there are 2 main causes for this finding. Young women, particularly those considering pregnancy, have been repeatedly advised to avoid consumption of liver due to the excessively high vitamin A content (73). However, following consumption of 100 g of liver, only ∼40% of its vitamin A content is absorbed. Furthermore, the vitamin A in liver is absorbed more slowly than from capsules, with very little formation of retinoic acid, a formation that is tightly controlled (76). Thus, the likelihood that critically high vitamin A levels are reached after dietary intake of liver is minimal. The potential teratogenic metabolite of vitamin A, retinoic acid, does not exist in food and under normal circumstances will not be formed beyond the physiological limit, because the metabolism of vitamin A to retinoic acid is strictly controlled in several ways. Even in cases of a regular high intake (e.g. liver consumption more often than once per week), the plasma level of retinol and, consequently, its delivery to target cells will not increase. This is due to the controlled hepatic synthesis of retinol binding protein (RBP). If the supply of preformed vitamin A is low, RBP accumulates as a result of either a continued synthesis or nondelivery to the bloodstream. This enables all vitamin A to be immediately delivered to the target cells. If, however, intake is high, RBP synthesis remains constant, ensuring a constant release of vitamin A from liver stores. This homoeostatic control can be easily determined using the relative dose-response test. In cases of a postprandial increase in circulating retinyl esters, delivery to target cells may occur before the chylomicrons are taken up by the liver and stored there (77).
However, retinyl esters entering the cells are either stored there or metabolized to retinol, a step that is strictly controlled by the intracellular level of retinol and cytoplasmic RBP (78). Therefore, the warning against the consumption of normal portions of liver (e.g. 100 g once/wk) is scientifically questionable and might cause the already low level of liver consumption to decrease further, especially among young women. The amount of liver consumed in Germany is ∼500 g/capita/y and for young women, liver consumption has halted almost completely (79). Nevertheless, to exclude individual uncertainties with respect to vitamin A and pregnancy, liver should be avoided in the first trimester of pregnancy and in cases without contraception. In the second and third trimesters, there is no risk if liver is consumed on a regular basis.
How Much Do Fortified Food and Food Supplements Contribute To the Daily Supply?
Consensus
According to NVS II, foods fortified with β-carotene often are important contributors (up to 30%) to the daily supply of vitamin A. The extent (roughly estimated as 3–12%) differs in various countries and depends on different food sources (fortified nonalcoholic beverages, cheese, butter, etc.)
Background
β-Carotene is used as a food colorant and for food fortification. It can be roughly calculated that up to 5 mg of β-carotene is present in 1 kg of either colored or fortified food. Based on data from the Dortmund Nutritional and Anthropometric Longitudinally Designed Study and the Austrian nutrition survey, it can be calculated that the daily intake of β-carotene from fortified food is between 0.5 and 1 mg, or 0.08 and 0.16 mg RE, respectively (80, 81).
Considering the higher bioavailability of β-carotene from dietary supplements compared with fruit and vegetables and the purposeful addition of supplemental amounts of β-carotene to juices and other foods, specifically those containing adequate fats and oils, fortified foods have a potentially important role in supplying vitamin A to the population. Studies conducted in Germany show that β-carotene makes up 25–30% of vitamin A intake (82). This implies that ∼10% of the total vitamin A supply comes from nonalcoholic beverages and other fortified food (cheese, butter, etc.). This is particularly important for young women who avoid meat and offal. Although the β-carotene in fruit juices is an important source of vitamin A for young women, the amounts present in food do not pose any health risk, such as that associated with excessive intake, because the absolute amount in drinks is small. This amount contributes only marginally to ensure sufficient vitamin A intake. The Dortmund Nutritional and Anthropometric Longitudinally Designed Study examined children aged 2–15 y (80). Boys aged between 13 and 15 y had the highest average intake of 0.88 mg/d vitamin A. Increased vitamin A intake was obtained through fortified food. During the 1996–2000 period of the study, intake of vitamin A from fortified food increased significantly, whereas vitamin A intake from the basic diet remained nearly unchanged.
Based on different studies estimating mean β-carotene intake, a range between 1.09 and 1.45 mg/d is evident (63, 71, 83). If fortified foods contribute ∼30% to the total vitamin A intake, they can fill the gap between the intake of preformed vitamin A and 100% of the RDA calculated as RE. Studies investigating the use of fortified beverages calculated the maximum amount of β-carotene consumed by heavy users (top 5% drinking ∼30 L in 4 mo or 0.245 mL/d) and reported average daily β-carotene intakes of between 0.575 mg (France) and 0.72 mg (UK). In terms of RE, these intake levels contribute to 96 and 120 μg/d of retinol, respectively (even if using a conversion factor of 6:1 instead of 12:1). Indeed, this constitutes 10% of the total vitamin A intake and is comparable to the results of the National Nutrition Survey II (82).
The percentage of the population with mean daily intakes below the average requirement (AR) is an estimate of the percentage of the population with inadequate intake (61). Supplements of either β-carotene or vitamin A considerably contribute to the total vitamin A supply. Kiely et al. (84) evaluated the impact of supplement use on the adequacy of micronutrient intakes and on the risk of exceeding the upper levels (UL) in an Irish population (662 males and 717 females aged 18–64 y) based on a 7-d food diary. Within the supplement users, 4% of male participants aged 51–64 y were near the UL (3000 μg RE/d). However, use of supplements by males reduced the percentage of participants with intakes below the AR (500 μg/d) from 20 to 5%; in female users, the percentage of participants with intakes below the AR (400 μg/d) was reduced from 18 to 14%. Within the group of participants who never used supplements, 23 and 21% had daily intakes below the AR (males and females, respectively). In these cases, fortified food might play an important role in ensuring sufficient supply. In a second study, the authors documented the nutritional profile of the participants (85). The main contributors of total vitamin A to the diet of the total participant population were vegetables and vegetable dishes (30.7%), milk and yoghurt (14.2%), and meat and meat products (11.5%). A total of 4.5% of males and 3.8% of females were below the lowest threshold intake (300 and 250 μg/d, in males and females, respectively). The lowest threshold intake is the intake level below which nearly all individuals will be unable to maintain metabolic integrity (86).
Based on Nutrition Surveys, Is It Possible To Ensure 100% of the RDA for the Whole Population?
Consensus
Although fruits and vegetables contribute to the daily vitamin A supply, the recommended β-carotene intake of 2–4 mg/d is not achieved in the general population. Even based on a conversion ratio of 6:1, it is not possible to ensure that the whole population consumes the recommended intake levels of vitamin A (including intake of preformed vitamin A).
Background
Interestingly, the minimum requirement levels of vitamin A, established by various regulatory authorities, are somewhat different. This must be taken into account, because nutritional surveys use the level established in the country in which they were performed. Consequently, even if 100% of the required or recommended levels are achieved in a given population, different populations might have different intakes. Because the nutritional habits of various populations are quite different, it is to be expected that the total intake of vitamin A might be met mostly by the intake of preformed vitamin A, or, in other words, by a high intake of animal products. On the other hand, populations or subgroups not having such a high intake of animal products might fill the gap between their actual requirement for vitamin A and their intake of preformed vitamin A by consuming β-carotene. Consequently, the required β-carotene intake strongly depends on the amount of vitamin A consumed in other food sources (Fig. 1). Strict vegans not consuming any animal products therefore need to meet their vitamin A requirement exclusively from provitamin A carotenoids. In persons for whom a high percentage of their vitamin A intake is realized from β-carotene consumption, the question of the conversion factor (β-carotene to retinol) is of huge importance. This is exemplarily demonstrated by the calculations shown in Table 2.
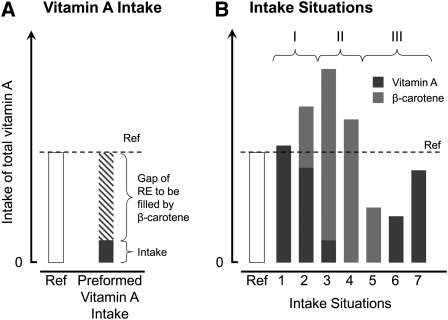
FIGURE 1.
Contribution of preformed vitamin A and β-carotene to the total vitamin A intake. (A) Relation between the required vitamin A intake (Ref = reference value for the given population) and the intake of preformed vitamin A products from food. If this intake is not sufficient, the difference (gap) between the reference intake and the real intake of preformed vitamin A has to be filled by β-carotene. Some theoretical intake situations are shown in (B). Intakes of preformed vitamin A and β-carotene after conversion to RE. Under intake situations 1 and 2 (group I), the reference value is reached largely independently of the conversion factor for β-carotene. Under intake situations 3 and 4 (group II), the reference value is reached if the conversion factor used is adequate. Group III (intake situations 5, 6, and 7) do not reach the reference value. Please note that reference values and conversion factors currently used are different in different countries.
TABLE 2.
Total daily preformed vitamin A and β-carotene intakes depending on the conversion factor1
| Preformed vitamin A intake, mg/d | β-Carotene intake, mg/d | Total vitamin A intake
Conversion factors |
DACH reference, 2mg/d | |||
| Participant | 6:1 | 12:1 | 25:1 | |||
| 13 | 0.50 | 3.00 | 1.00 | 0.75 | 0.62 | 1.0 |
| 24 | 0.60 | 3.00 | 1.10 | 0.85 | 0.72 | 1.0 |
| 35 | 0.50 | 6.00 | 1.50 | 1.00 | 0.74 | 1.0 |
| 46 | 0.50 | 1.00 | 0.66 | 0.58 | 0.54 | 1.0 |
Data given in RE using the indicated conversion factors.
As reference values, the DACH levels were used (73).
Average of a 25- to 51-y-old Austrian male.
Average of a 25- to 51-y-old Austrian female.
Average of an Austrian vegetarian female.
Average of a 13- to 15-y-old Austrian female.
Nutrition surveys as performed in the US, Germany, Austria, UK, Ireland, Spain, and other countries demonstrate that the average intake of total vitamin A falls within the range of the recommended intake levels. The NVS II shows that the median of total vitamin A intake is between 140 and 170% of the DACH reference value and the Austrian nutrition report states a median intake level above 100% for all adult population groups (81, 87). The same is true for Ireland, demonstrated in The North/South Ireland Food Consumption Survey, and the UK, reported in the British National Diet and Nutrition Survey (NDNS), the latter with the exception of 19- to 24-y-old males and 19- to 34-y-old females (66, 85). Interestingly, the eVe study in Spain showed a median RE intake considerably below the Spanish RDA in 25- to 60-y-old adults (65). Because most of the data in these nutrition surveys are reported as median intake levels, it should be taken into consideration that the total RE intake varies considerably. Although in Germany very high total vitamin A intakes are reported, some 10–15% of the population (14- 80-y olds) have an intake below the recommended DACH value (87). The same was also reported in Ireland (85). In the US, a median daily intake of 744–811 μg retinol activity equivalents (RAE) was reported for adult males and 530–716 μg RAE for females (61). However, it is worth noting that the dietary reference intakes for the U.S. population are based on a conversion factor of 12, whereas in Germany and Austria, a conversion factor of 6 is used (61, 81, 87). Other sources report an adequate intake of total vitamin A in the US as measured by RE (88, 89). Thus, the intake of vitamin A in some countries, such as Germany and Austria, might be overestimated due to the use of a low conversion factor, and a significantly higher percentage of the population might not meet their biological requirements for vitamin A (Table 2). In the Austrian nutrition report, it is evident that the median vitamin A intake for the entire female adult population (19–64 y) and for young adult males (18–25 y) will be below the DACH value if a conversion factor of 12 is applied (81). Interestingly, a study of 154 inhabitants of Vienna revealed that vegetarians and vegans take in very high β-carotene levels, enabling them to meet the recommendations for vitamin A intake (81).
Studies in Germany and Austria also investigated the vitamin A and provitamin A intake in children or adolescents (81, 87). In Germany, the situation for adolescents is the same as for adults, where the mean intake is approximately at the level of the recommended intake, revealing that a considerable percentage of this population group is not meeting the recommended intake levels (87). Interestingly, the percentage of the population not reaching the DACH reference level is highest in 14- to 18-y olds (87). This is true also for Austria, where the median intake of the 7- to 15-y-old population (boys and girls) does not meet the reference intake, even using a conversion factor of 6 for β-carotene (81). The same report did not find a difference between ethnic backgrounds (Austrian vs. non-Austrian parents) of the children, but there was a clear difference in the total vitamin A intake regarding educational background (i.e. depending on school type) in adolescent boys and girls (81).
In general, the elderly population successfully meets the recommended total vitamin A intake levels in Germany and Austria (87). Because the intake of preformed vitamin A tends to be high, the conversion factor of β-carotene appears not to be so important for this group (81). Furthermore, Austrian inhabitants of retirement or nursing homes seem to be well nourished with respect to total vitamin A intake (81).
Conclusion.
The intake of total vitamin A differs in various parts of the population and, because the intake of preformed vitamin A is not sufficient in large parts of the population, β-carotene has an important function in providing an adequate supply of total vitamin A. However, this goal is not achieved for large parts of the population and several at-risk groups can be identified, including young and adolescent girls, population groups with low educational background and/or low income, and those with particular nutritional preferences (e.g. vegetarians or vegans) in some parts of the adult population.
What Are the Sources of Vitamin A Supply in Asia and How Much Does β-Carotene Contribute To This?
Consensus
The intake of preformed vitamin A in large parts of the Asian population is very low. β-Carotene is only partially able to ensure an optimal total vitamin A intake and is mostly derived from leafy vegetables and fruits.
Background
Sources of vitamin A supply in Asia.
There is not much scientific information about the sources of vitamin A intake at a country level in Asia and in most studies mentioned below, a conversion ratio of 6:1 was used to convert β-carotene intakes to RE (90–94). Based on the data collected in the National Nutrition and Health Survey of the Chinese People in 2002, the average RE intake was 469.2 μg RE/d per reference men and 469.9 and 453.7 μg RE/d in child-bearing women aged 15–45 y in urban and rural areas, respectively (90). However, the average RE intake has not markedly improved during the past 20 y in China (92). In urban Bangladesh, the food consumption data from a total of 384 girls aged from 10–16 y showed that vitamin A intake was less than the RDA (62%). Leafy vegetables and fruits were the main sources of provitamin A (carotenes) (95). Based on data from a total of 1001 households (771 in rural areas and 230 in urban areas) in the Red River Delta population of northern Vietnam, retinol intakes (mean ± SD) were 101 ± 275 and 201 ± 470 μg/capita∙d−1 in rural and urban areas, respectively (96). Plants were the main source of vitamin A for the local population. From 1996 to 1998, the proportion of pregnant women below the Indonesian RDI for vitamin A (700 μg RE/d) ranged from 83% in the first trimester to 76% in the 3rd, with plant sources contributing to the supply of 64–79% of pregnant women in all 3 trimesters (97). In South India, the daily median intake of total vitamin A, β-carotene, and retinol in children aged 1–3 y (5683 children) was 121, 100, and 21 μg RE, respectively. Taking into account the potential contribution of breast milk, non-breast–fed children met only 60% of the Indian RDA for vitamin A intake (250 μg RE/d), whereas breast-fed children met 90% of the RDA during the second year of life. Dietary vitamin A intakes were mainly from plant sources (98).
In a recent study, we showed that spirulina β-carotene could be efficiently converted to retinol in Chinese adults and the conversion factor of spirulina β-carotene to retinol was 4.5 ± 1.6 (range 2.3–6.9) by weight, which indicated that spirulina could be a good source of vitamin A in developing countries (93).
How much does β-carotene contribute to vitamin A supply in Asia?
Carotenoids from plants are the main source of vitamin A intake in Asian countries. Based on data from the Chinese National Nutrition and Health Survey in 2002, average carotene intake per man from vegetables and tubers accounted for 61.7 and 4.7% of RE, respectively, in rural areas and 53.0% and 2.0% of RE, respectively, in urban areas; carotene intakes of child-bearing women in urban and rural areas contributed 68.8 and 73.4% of RE, respectively (91, 92). A study on the maintenance of the body stores of carotenoids in children aged 3–7 y in a kindergarten in Shandong Province of China showed that the total carotenoid intake from green-yellow vegetables was 8289 μg/d, of which 4670 μg/d was β-carotene, 4200 μg/d was lutein (lacking vitamin A function), and 810 μg/d was other provitamin A carotenoids. The total carotenoid intake from light-colored vegetables was 1340 μg/d, of which 700 μg/d was β-carotene, 600 μg/d was lutein, and 40 μg/d was other provitamin A carotenoids (94). In South India, the median intake of β-carotene in 5683 children aged 1–3 y was 100 μg RE/d (98). However, it should be emphasized that the carotene content in the plant foods in the Chinese Food Composition Table was determined using paper chromatographic methods (90–94).
Are There Any Differences in β-Carotene Bioavailability and Is It Less Effective from Fortified Foods?
Consensus
The bioavailability of β-carotene from natural sources depends on the food matrix and individual response.
Background
The absorption of β-carotene has been reported to range between 10 and 90% (99–104). For pure β-carotene in oil, absorption is generally higher than for β-carotene in plant foods. An early study (in 1966) on β-carotene in oil reported absorption of 8.7% β-carotene (61-y-old man fed 47 μg of [3H]-β-carotene) and 16.76% β-carotene (58-y-old woman fed 46 μg of [3H]-β-carotene) by measuring the radioisotope-labeled β-carotene that was absorbed and recovered in the thoracic lymph duct (99). In another study using 14C-labeled β-carotene, it was reported that 55% of the β-carotene dose (0.27 μg) was absorbed, as measured by accelerator MS (100). A mean of 23% (±42%) of 15 mg β-carotene (in the form of 10% water-soluble beadlets) was detected in triglyceride-rich lipoproteins in 10 adult males (101). A mathematical model study on data collected from a 53-y-old male participant who ingested 40 mg β-carotene in oil suggested that 22% of the β-carotene dose was absorbed (102). A further report on the absorption of 10 mg of all-trans- (84%) and 9-cis- (16%) β-carotene dispersed in oil by 5 ileostomy volunteers showed that 90% (range 74–97%) of the total β-carotene was absorbed (103). A recent study in a healthy 30-y-old man who consumed 0.54 μg of [14C]-β-carotene mixed in a shake showed that 65% of the administered [14C]-β-carotene was absorbed as measured by accelerator MS analysis (104). It was also reported that 20–90% of absorbed β-carotene was converted to vitamin A (99, 101, 102, 105).
By using 1 d as a cutoff time for the intestinal conversion of β-carotene to retinal, Tang et al. (106) calculated the postintestinal absorption conversion of β-carotene, and it accounted for 19.7, 22.7, 26.3, 27.8, 28.6, 29.5, and 30.1% of the total converted retinol at 6, 13, 20, 27, 34, 41, and 53 d after the administration of β-carotene, respectively.
The relative absorption efficiency of supplemental and dietary β-carotene has been reported to range from 5% (spinach) to 26% (raw carrots) and has been reviewed and presented in Table 4–2 of the IOM publication in 2001 (61).
What Factors or Circumstances May Influence Bioavailability?
Consensus
The following food-related factors largely influence the bioavailability of β-carotene: food matrix, food processing, dosage, fat in the meal [including avocado fat and fat replacers such as sucrose polyesters (SPE)], other carotenoids in the meal, and dietary fiber.
Additional consumer-related factors include polymorphisms related to metabolism, vitamin A status, and gut integrity.
Background
It has been observed, and generally accepted, that a number of factors affect the utilization of β-carotene in humans. These factors have been extensively discussed; see representative reviews by Furr and Clark (107), Castenmiller and West (58), van het Hof et al. (108), and Yeum and Russell (109). Dr. Clive West (58) coined the mnemonic SLAMENGHI to summarize the factors involved: species of carotenoid, molecular linkage, amount of carotenoid consumed in a meal, matrix in which the carotenoid is incorporated, effectors of absorption and bioconversion, nutrient status of the host, genetic factors, host-related factors, and mathematical interactions.
Recent publications have provided more information on the factors that affect the bioavailability of β-carotene and its conversion to vitamin A in humans. These factors are: food matrix; food processing (physical size, cooking, etc.); dosage; fat in the meal (including avocado or fat replacers such as SPE); other carotenoids in the meal; and dietary fiber.
Reports suggest that the conversion of β-carotene (in oil) to vitamin A varies from 2 to 1 to 16 to 1, by weight, in humans. Two studies that were conducted in 1949 and 1974 and used a depletion/repletion approach in adults reported conversion factors of 2 to 1 and 3.8 to 1 by weight, respectively (110, 111). In 2001 a study conducted in children with normal or marginal vitamin A status, in which stable isotope-labeled β-carotene and vitamin A were given for 10 wk, a conversion factor of β-carotene to vitamin A of 2 to 1 by weight was determined (112). Two studies that used the stable isotope reference method in adults in the US (22 participants) and China (15 participants) with normal vitamin A status showed that for a 6-mg dose of β-carotene in oil, the conversion factor of β-carotene to vitamin A was 9 to 1 by weight (106, 113). In another study in an adult participant using a dose of 16 mg of [2H6]-β-carotene in oil and a dose of 10 mg of [2H6]-vitamin A, the conversion factor was 15.9 to 1 by weight (100).
The conversion of vegetable and fruit β-carotene to vitamin A is affected by the food matrix and can vary >5-fold. A conversion factor of 12:1 by weight for fruit β-carotene and 26:1 by weight for vegetable β-carotene was established (114). This was confirmed by another study, which observed an apparent conversion factor of green/yellow vegetable β-carotene to be 27:1 by weight (94). Finally, adults who consumed 750 RE daily as either sweet potato β-carotene or Indian spinach β-carotene had a conversion factor for sweet potato β-carotene to retinol of 13:1 by weight and for Indian spinach β-carotene of 10:1 (115).
Plant foods provide β-carotene, but the matrix of plant foods is one of the major factors affecting the efficiency of dietary β-carotene. By using hydroponically grown and intrinsically labeled spinach and carrots (cooked and pureed) and a reference dose of labeled vitamin A, it was found that spinach β-carotene conversion to retinol was 21 to 1 by weight (n = 14) and that carrot β-carotene conversion to retinol was 15 to 1 by weight (n = 7) in healthy adults (116). Recently, hydroponically grown and intrinsically labeled spirulina was given to Chinese men (n = 10), which determined the conversion efficiency of spirulina β-carotene to vitamin A as 4.5 to 1 by weight (93). Following a similar approach, hydroponically grown and intrinsically labeled Golden Rice was fed to a group of 5 healthy adult volunteers in the US. The Golden Rice β-carotene conversion to retinol was 3.8 to 1 by weight (117). It is obvious that due to the relatively greater complexity of the vegetable matrix compared with algae and rice matrices, β-carotene in vegetables is less bioavailable than was previously expected (6:1 by weight).
Processing, such as mechanical homogenization or heat treatment, has the potential to enhance the bioavailability of carotenoids from vegetables (from 18% to a 6-fold increase) (108). The dosage level of dietary β-carotene was reported to affect the bioconversion efficiency to vitamin A (118).
Inclusion of fat in a meal will help the absorption of fat-soluble β-carotene. It has also been demonstrated recently that fat in a meal can be from another meal component, e.g. a meal including an avocado fruit that is rich in fat. The addition of avocado to salsa enhanced β-carotene absorption (P < 0.003) compared with the intake of avocado-free salsa. Adding avocado fruit significantly enhanced carotenoid absorption from salad and salsa, which was attributed primarily to the lipids present in avocado. Thus, the type of lipid source does not affect the dietary fat effect on carotenoid absorption (119). As a dietary fat replacer, SPE are on the market in the United States as a component of certain snack foods. Consumption of SPE spread (10 g SPE/d) significantly decreased plasma carotenoid concentrations by 11–29% and SPE chips (7 g SPE/d) decreased β-carotene by 21% (120).
The simultaneous intake of lutein with β-carotene interfered with β-carotene absorption compared with intake of β-carotene alone. However, simultaneous intake of lutein did not affect the ratio of β-carotene and retinyl esters, suggesting a lack of effect on β-carotene cleavage or conversion to vitamin A (121).
The effect of dietary fiber on β-carotene absorption was studied in 6 young women aged 26–29 y. This study found that absorption of β-carotene that was homogenously distributed in cream was reduced significantly in the presence of water-soluble fiber pectin, guar, and alginate in a range of 33–43% but was not affected by cellulose or wheat bran (122).
IS β-Carotene Conversion a Stable Ratio or Are There Tissue-Specific Differences?
Consensus
In any given population, the conversion rate appears to differ substantially among individuals, resulting in a population mean with a high variance.
Following early intestinal conversion, there is a continuous postabsorptive conversion over time.
There may be tissue-specific differences in the propensity for uptake, storage, and cleavage, but they have not yet been systematically defined.
Background
To address the question “Is β-carotene conversion a stable ratio, or are there tissue-specific differences?”, 3 aspects need to be considered. First, is the ratio (conversion factor or conversion efficiency) consistent within individuals and therefore a predictable ratio for populations? Second, within individuals, is the ratio of conversion stable over time, or does it change as a result of continued metabolism? Third, is there evidence of tissue-specific differences in conversion? Data to address these questions come primarily from studies that have used stable isotopic forms of β-carotene coupled with computer modeling to determine the plasma response to β-carotene and the conversion efficiency of β-carotene to vitamin A (retinol). With respect to inter-individual variation, a number of studies have shown that β-carotene conversion efficiency during absorption varies widely from person to person, even within studies that were conducted in relatively homogeneous groups (e.g. men and women of similar age, similar dietary patterns, and studied following the same protocols). In a study of 12 postmenopausal women and 10 age-matched men, Tang et al. (106) determined that intestinal conversion efficiency, assessed over the first few days after β-carotene administration, averaged 9.1 mg of β-carotene converted to 1 mg of retinol. The range was very wide, from 2.4–20.2 mg, and the CV was nearly 65%. A similarly wide variation in conversion efficiency has been observed in other isotope dilution studies (123, 124). This suggests that, for many individuals, the intake of provitamin A that is needed to meet the RDA for vitamin A is higher than would be calculated based on a traditional conversion factor of 6 mg of β-carotene to 1 mg of retinol. Studies such as this one and those reviewed above led the IOM, in 2001, to increase the conversion factor for β-carotene in foods so that 12 mg of β-carotene in foods is now considered equivalent to 1 mg of preformed vitamin A (retinol) (61).
Whether the conversion factor is stable over time or changes in the course of metabolism has been addressed in only a few studies. To assess conversion after the intestinal phase, a long-term follow-up is required. Novotny et al. (102) used compartmental modeling to evaluate the kinetics of conversion of a dose of deuterated β-carotene to deuterated retinol in a single, healthy, male participant. Results of compartmental modeling indicated that 22% of the β-carotene dose was absorbed, 17.8% as intact β-carotene and 4.2% as retinoid. The model also suggested that both liver and enterocytes are important in converting β-carotene to retinoid, with 43% being converted in liver and 57% in enterocytes. The model attributed both slow and fast kinetic compartments to the liver. It should be noted that liver was not sampled directly and thus the location of the metabolism of β-carotene could only be inferred. The mean residence time (from intake to irreversible disposal) for β-carotene was 51 d. In the study of 12 postmenopausal women and 10 age-matched men described above, participants were followed for 53 d after administration of 6 mg of deuterated β-carotene, and the percentage of total retinol derived from the postintestinal conversion of β-carotene was determined after 6, 14, 21, and 53 d. The percentage converted increased over time, averaging 7.8, 13.6, 16.4, and 19% on d 6, 14, 21, and 53, respectively, after dose administration, suggesting a slow but continual conversion long after intestinal uptake is complete. Postintestinal β-carotene conversion also exhibited a high person-to-person variation. The mechanism of postintestinal conversion of β-carotene to retinol is not well understood. However, indirect evidence based on the distribution of BCMO1 in human tissues would support the idea that β-carotene metabolism takes place in a wide variety of organs, including cells of the stomach, small intestine, colon, liver, pancreas, prostate, uterus, mammary gland, adrenal gland, kidney, skin, and skeletal muscle (125). Additional research on human tissue specimens or in appropriate animal models will be necessary to determine just how β-carotene conversion occurs in nonintestinal tissues and whether diet or other factors influence this process.
Does Vitamin A Status or Other Metabolic Issues Influence β-Carotene Conversion?
Consensus
Conversion efficiency depends at least in part on the individual’s vitamin A status, BMI, and comorbidities. Genetic factors are becoming known that influence bioavailability of β-carotene.
Background
Several studies have suggested that certain factors influence the efficiency of β-carotene absorption and/or conversion to retinol. The conversion efficiency depends at least in part on the individual’s vitamin A status. This has been demonstrated biochemically, first in studies of rat and chicks in which feeding all-trans-retinoic acid resulted in feedback regulation of BCMO1 activity, and subsequently in studies of the cloned and expressed 15,15’-monooxgenase gene (126, 127). Human studies also support a relationship between vitamin A status, or intake, and efficiency of utilization, although no mechanism has yet been identified. An inverse relationship was observed between the bioconversion of plant carotenoids to vitamin A and vitamin A status in Filipino children (128). Thus, low vitamin A status appears to increase the utilization of β-carotene. In contrast, Lemke et al. (129) reported a modest reduction in the cleavage of β-carotene following supplementation with vitamin A (10,000 IU/d vitamin A for 3 wk). However, this study was very small (n = 2 men). Taken together, these reports provide an initial level of support for the idea that the efficiency of absorption and/or cleavage of β-carotene is somewhat influenced by vitamin A nutritional status and may be directly responsive to the level of retinoic acid (130, 131). Additional studies are needed to determine what types of feedback mechanisms exist and how a person’s vitamin A status may affect the provitamin A function of β-carotene.
For other metabolic conditions that may affect β-carotene utilization, data are also limited. However, several factors are potentially important. Adiposity may be a factor. The β-carotene conversion factor was significantly correlated with BMI in postmenopausal women and a similar but nonsignificant relationship was observed in men of a similar age (106). The relationship appears plausible, because adipose tissue is a site of β-carotene accumulation and BCMO1 expression (see above), but further research is needed. Interestingly, BMI was inversely associated with the plasma response to dietary carotenoids in a study of Filipino school-children with low vitamin A status (132). These studies may not be discrepant, because the differences could possibly reflect a partitioning of β-carotene between plasma and adipose tissue, with more β-carotene remaining in plasma when BMI is low and more being stored in adipose tissue, perhaps leading to slow conversion to vitamin A when BMI is high. However, additional studies are needed to confirm and extend these findings.
Metabolic conditions could be determinants of β-carotene uptake or conversion, as suggested by differences in serum carotene concentrations in different metabolic states. Several reports have indicated a negative relationship between β-carotene status and type 2 diabetes. Plasma β-carotene was low, even after correction for confounders, in patients with impaired glucose metabolism or type 2 diabetes (133–135). Patients with certain eating disorders such as anorexia nervosa (AN) have been reported to have an elevated plasma carotene concentration, independent of dietary intake (136). In a case control study, Boland et al. (137) reported a high prevalence (62%) of hypercarotenemia (>3.72 μmol/L) in the AN population, with a mean serum β-carotene concentration higher in AN patients than in controls without AN (4.4 ± 2.05 vs. 3.0 ± 1.45 μmol/L, mean ± SD) (P < 0.0001). Among AN patients, in a subgroup comparison of anorectics (dieters) to bulemics, the level was higher in those who restricted intake than in those who were bulemic (P < 0.005). Additional studies of β-carotene conversion per se are needed to better understand the provitamin A function of β-carotene in different metabolic states.
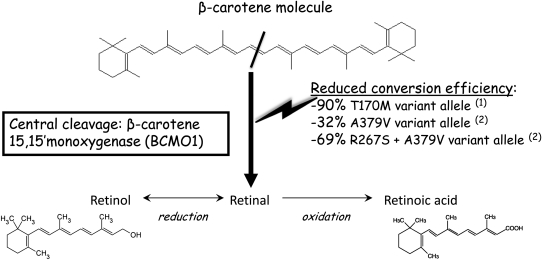
Genetic background is likely a factor in the provitamin A function of β-carotene. As discussed above, single nucleotide polymorphisms (SNP) in the BCMO1 gene may be associated with differences in conversion efficiency and thus may affect the production of vitamin A from β-carotene (Fig. 2). A rare mutation was reported in a case study in which a patient exhibiting hypercarotenemia had a point mutation (T170M) in the BCMO1 gene (138). In vitro, the expressed BCMO1 protein containing this mutation exhibited only ∼10% of normal biochemical activity compared with wild-type BCMO1. Recently, 2 polymorphisms, R267S and A379V, were reported in the human BCMO1 gene, which decreased the intestinal conversion of a high dose of β-carotene and were associated with higher fasting β-carotene concentrations (139). As reviewed by Tourniaire et al. (140), besides mutations in the BCMO1 gene, differences in other genes that are involved in lipid and lipoprotein metabolism might also have an indirect effect on the metabolism of β-carotene. Polymorphisms in hepatic lipase, lipoprotein lipase, and scavenger receptor-B1 may also contribute to variation in the efficiency of β-carotene conversion to vitamin A, and some have been reported to be associated with high- or low- plasma β-carotene concentrations. However, research in this area has just begun and large-scale population genetic studies are needed to define the prevalence of BCMO1 genotypes as well as other polymorphisms in other genes that may affect carotenoid metabolism.
FIGURE 2.
Reduced conversion efficiency of β-carotene to vitamin A via BCMO1 depending on genetic variations. (1) see reference (138). (2) see reference (139).
Is There a Specific Tissue Distribution of β-Carotene?
Consensus
Yes, there is a special distribution of β-carotene in adipose tissue, skin, intestine, adrenal gland, liver, corpus luteum, and the macula. However, the regulation, if any, of β-carotene uptake, retention, and turnover in these tissues is largely unknown.
Background
Despite the certainty that the distribution of β-carotene among tissues is not uniform, very few systematic data have been collected on β-carotene concentrations in human tissues. Even less is known regarding how tissue carotenoid concentrations differ with dietary intake or other factors. Most studies have determined β-carotene concentrations only in plasma, plasma lipoprotein fractions, and a few tissues such as adipose, fatty tissue from breast, or buccal mucosal cells, due to their accessibility, or in tissues collected at autopsy. As reviewed by Bendich and Olson (141), numerous human tissues contain some β-carotene: adipose, skin, intestine, liver, corpus luteum, and eye. Of total body β-carotene, 80–85% was present in adipose, 8–12% in liver, and 2–3% in muscle; however, based on concentration, the order was corpus luteum, 60 μg/g; adrenal gland, 20 μg/g; adipose tissue and liver, 10 μg/g; and other tissues, 0.5–3 μg/g.
The plasma concentration of β-carotene reflects in part recent intake. Micozzi et al. (142) tested the plasma response to supplementation with 12 or 30 mg/d of purified β-carotene in capsule form as well as after consumption of carrots or tomatoes containing equivalent amounts of β-carotene for 6 wk, followed by a 4-wk washout period of self-selected diet without supplementation. The 12- and 30-mg/d supplements increased plasma β-carotene within the first week, which continued to increase up to 6 wk and then gradually declined nearly to baseline during the 4-wk washout period. β-Carotene in supplement form was more effective than an equal amount of β-carotene in foods for increasing plasma β-carotene concentration. In another study, plasma β-carotene increased by ∼50–100% 1–2 wk after participants began consuming a high-carotene diet without additional supplementation (143). Within the plasma compartment, ∼75% of the hydrocarbon carotenoids (β-carotene and lycopene) were present in LDL and the remaining 25% in HDL in both normal and hypercarotenemic participants (144–146). Within tissues, β-carotene and lycopene were the predominant carotenoids in human adipose, averaging 20.2 and 18.5% of total carotenoids, respectively, with a large inter-individual variation (147). In a study of fatty breast tissue from 8 adult women, β-carotene was found in all samples, but the plasma:tissue concentration ratio varied 10-fold (148). In a study of plasma and buccal mucosal cells from young women supplemented with 25 mg/d of β-carotene for 1 wk, plasma β-carotene rose significantly after 1 wk and the buccal mucosal cell β-carotene concentration was significantly higher after 2 wk (149). Overall, the available information supports the conclusion that some tissues accumulate higher concentrations of β-carotene than others. Yet knowledge about β-carotene concentrations in human tissues is still incomplete and much remains to be learned about the regulation, if any, of β-carotene uptake, retention, and turnover in specific human tissues.
Is the Current Recommendation of 2#x20134 mg/d Scientifically Sound?
Consensus
Based on recent data including food composition and data from national surveys, the intake of preformed vitamin A is inadequate in a substantial part of the general population. For many people, the gap between the RDA/recommended nutrient intake (RNI) (or other reference intake) and the quantity of vitamin A consumed as preformed vitamin A cannot be closed by consumption of 2–4 mg/d of β-carotene from a regular diet.
Background
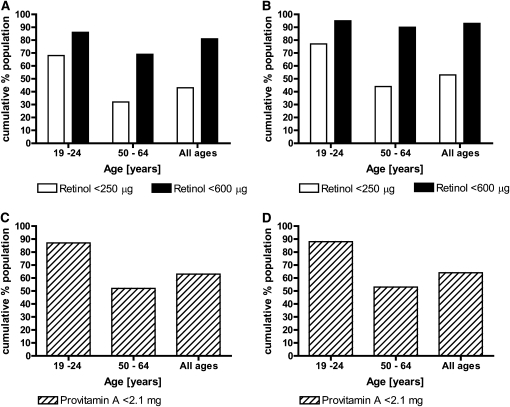
The average intake of preformed retinol in the UK is low: 673 μg (median 363 μg) in men and 472 μg (median 277 μg) in women (150). This intake includes the consumption of dietary supplements mostly comprised of multivitamins and cod and halibut liver oil, which were taken by 34% of women and 18% of men (150). The percentage of men and women with intakes of preformed retinol below the RNI or the LRNI are 81 and 43%, respectively (Fig. 3), indicating that for a majority of the population, vitamin A requirements are not met by dietary intake of preformed retinol. More importantly, median daily intake of preformed retinol was significantly lower in men and women aged 19–24 y (280 and 201 μg, respectively) than in those aged 50–64 y (444 and 331 μg, respectively) (150). The results indicate that there is a tendency for intakes to increase with age, which manifests itself in higher percentages of young men and women below the recommended intakes for vitamin A (Fig. 3). It also highlights the fact that the younger age group relies on 50% of their vitamin A needs to be met through provitamin A sources. This raises the question whether the current recommendation of 2–4 mg/d of β-carotene could close the gap between the low intake of preformed vitamin A and the recommended intake of total RAE. Using a conversion factor of 12:1, 4 mg of β-carotene would produce 333 μg of retinol. Taking the RNI of 0.6 mg/d RE for women in the UK and the RDA of 0.7 mg/d RAE for women in the US, this would mean that a daily intake of at least 270 or 370 μg preformed vitamin A has to be consumed to reach recommended intake levels for the UK or the US, respectively. The median intake of preformed retinol in the UK and the US is 277 and 378 μg, respectively (150, 151). Individuals consuming preformed vitamin A at the median intake levels and the highest recommended dose of β-carotene could therefore achieve the recommended intake of total RE. However, 50% of the female population would not achieve adequate vitamin A intake even with a consumption of 4 mg/d of β-carotene if their preformed vitamin A intake remains below the current median. Furthermore, total daily provitamin A intake in the UK (i.e. the sum of β-carotene and one-half the amount of α-carotene and β-cryptoxanthin) was 2.1 mg for men and 1.9 mg for women of all ages, with the youngest group of men and women having significantly lower mean daily intakes of total provitamin A (1.5 mg/d for both men and women) than all other age groups (Fig. 3) (150). Currently, β-carotene intake in Germany is below 1 mg/d for almost one-half of the population and 64.1% have intakes <2 mg/d (see above).
FIGURE 3.
Preformed retinol and provitamin A intakes in different female age groups within the UK [based on data from (150)]. Proportions of women of different ages in the UK with retinol (A,B) and provitamin A (C,D) intakes from food sources and supplements (A,C) or from food sources alone (B,D) below the RNI or LRNI. The RNI for retinol is 600 μg/d and for LRNI it is 250 μg/d. Provitamin A intake is the sum of β-carotene and one-half the amount of α-carotene and β-cryoptoxanthin consumed. Data are from (150).
Taken together, these data indicate that current preformed vitamin A intake levels are low even when supplements are consumed and only those individuals consuming at least the median intake of preformed vitamin A would reach the recommended intake levels of RE if they would additionally consume the maximum recommended dose of β-carotene given the current conversion factors. In summary, a substantial part of the population will not be able to close the gap between the RNI and the actual daily intake of preformed vitamin A by consuming the recommended dietary levels of 2–4 mg of β-carotene, if current intake levels of preformed vitamin A do not change.
What Is the Basic Need for β-Carotene to Ensure a Sufficient Intake to Meet the Vitamin A Requirement?
Consensus
The basic need for β-carotene in its provitamin A function is defined by the existing gap between preformed vitamin A intake and recommendations for total vitamin A intake.
In cases of a poor vitamin A status due to low intake of preformed vitamin A, an intake of β-carotene in the range of 2–4 mg/d still might not sufficiently correct the individual vitamin A status. Indeed, an appropriate intake of β-carotene from food and/or supplements will safely compensate the gap of vitamin A.
Background
The NDNS data clearly indicate that men aged 19–24 y and women aged 19–34 y were the only age groups in which mean intake of vitamin A from food sources was below the RNI (150). Although a large portion of the UK population seems to consume enough vitamin A, the NDNS data are based on the underlying assumption that 6 μg of β-carotene is equivalent to 1 μg retinol and that provitamin A conversion to vitamin A has no inter-individual variation. These assumptions were recently rectified, because the bioefficacy of provitamin A sources is lower than the assumed conversion efficiency of 6:1 and has recently been altered to 12:1 for β-carotene and 24:1 for both α-carotene and β-cryptoxanthin (152). More importantly, the vitamin A activity of provitamin A sources such as β-carotene, even when measured under controlled conditions, is highly variable and surprisingly low (113, 123, 124, 153, 154). Approximately 27–45% of volunteers in double-tracer studies have been classified as poor converters (113, 123, 124). These individuals have a capacity to form only 9% vitamin A from β-carotene compared with those who are classified as normal converters (113). Genetic variability in β-carotene metabolism may provide an explanation for the molecular basis of the poor converter phenotype within the population, because 2 nonsynonymous SNP (R267S and A379V) in the human BCMO1 gene with variant allele frequencies of 42 and 24%, respectively, have recently been identified (139). Responsiveness to a pharmacological dose of β-carotene in a human intervention study revealed that these SNP were associated with significant alterations in β-carotene metabolism in female volunteers with reduced β-carotene conversion efficiency of 32 and 69% for the A379V and R267S/A379V variant carriers, respectively (139).
Even if the current recommendation of 4 mg/d of β-carotene would be adhered to, female volunteers with either the A379V and R267S/A379V variant would obtain only 72–51% of the recommended intake, respectively, given the conversion efficiency of 12:1 for β-carotene and assuming an intake of 201 μg preformed retinol.
If we assume no inter-individual differences in the ability to convert β-carotene to retinol and no changes in current preformed retinol intakes, the amount of β-carotene needed to cover between 95 and 97.5% of the US or UK population with recommended RE would be 7 mg/d, based on a conversion efficiency of 12:1 (152). If, however, we correct the conversion efficiency factor for reduced conversion efficiencies of 69% for the R267S/A379V variant carriers, the recommended intake of β-carotene would need to be 22 mg/d to cover between 95 and 97.5% of the US or UK population with recommended RE. Due to the possible health risks associated with high intakes of β-carotene in smokers, it might be advisable to screen these individuals and provide personalized nutritional advice in these circumstances (155). On the other hand, if preformed retinol intakes could be increased to a level of 430 μg/d at the 5th percentile, a daily intake of 7 mg of β-carotene would be enough for those individuals who have the double SNP to enable a coverage of 95–97.5% of the US or UK population with recommended RE intake.
Supplements and fortified foods contribute substantially to achieving the RNI and RDA for total vitamin A intake, because, e.g., 25.9% of U.S. adults take vitamin and mineral supplements, and fortified foods themselves contributed 26% of the RDA for vitamin A (156).
In summary, the current recommended amounts of consumed β-carotene should be increased to 7 mg/d to ensure that at least 95% of the population consumes the recommended intake of total vitamin A. Individuals with reduced conversion efficiencies due to a genetic variability in β-carotene metabolism might need to increase their daily β-carotene or preformed vitamin A intake. However, much more research is needed to define new recommended intake levels if the large inter-individual variation in β-carotene conversion efficiency is taken into account.
Is There a Special Need in Specific Groups (Pregnant Women, Elderly, Disease Related)?
Consensus
There are indeed various groups at risk for an inadequate vitamin A supply, especially young individuals as well as pregnant and lactating women.
Young individuals are at increased risk of developing at least a mild form of vitamin A deficiency due to their very low intakes of total vitamin A (see above) (150). More importantly, physiological needs for vitamin A increase during pregnancy and lactation to 770 and 1300 μg/d, respectively (61). In Germany, Strobel et al. (157) stressed the fact that risk groups for low vitamin A supply exist in the Western world and that pregnant and breastfeeding women are especially advised to achieve adequate intake. The Feskanich (158) study further indicated that one-half of American women in NHANES III consumed multivitamins, which were the primary contributor to total retinol intake. Finally, individuals who are classified as poor converters will have higher needs for provitamin A sources to cover their recommended total vitamin A intakes (113, 123, 124). More research is needed, however, to correctly define adequate recommendations for these risk groups.
In conclusion, the provitamin A function of β-carotene is well established. Nutritional surveys from various countries consistently report β-carotene intake to be essential to meet vitamin A requirements.
Acknowledgments
We thank Jana Tinz for the organization of the consensus conference as well as assistance in publishing the manuscript as supplement coordinator. We also thank Dr. Claudia Wicke and Tabea Frey for their organizational and technical assistance before as well as during the conference. H.K.B. and T.G. designed the questions for the consensus talk and had primary responsibility for their final content; the questions were circulated to all members prior to the meeting; W.S. was responsible for writing the text related to consensus answers 1 and 2; A.P. was responsible for writing the text related to consensus answers 3 and 4; H.K.B. was responsible for writing the text related to consensus answers 5, 6, and 7; S.Y. was responsible for writing the text related to consensus answer 9; G.T. was responsible for writing the text related to consensus answers 10 and 11; C.R. was responsible for writing the text related to consensus answers 12, 13, and 14; G.L. was responsible for writing the text related to consensus answers 15, 16, and 17; H.K.B., T.G., and D.T. had primary responsibility for the final content. All authors read and approved the final manuscript and all authors contributed equally to the paper.
Footnotes
Published in a supplement to The Journal of Nutrition. Presented at the Hohenheim Consensus Conference “Beta-Carotene as an Important Vitamin A Source for Humans,” held in Stuttgart-Hohenheim, Germany, July 11, 2009. The conference was organized by the Institute of Biological Chemistry and Nutrition, University of Hohenheim, Stuttgart, and was cosponsored by DSM Nutritional Products Ltd., Basel, Switzerland. The supplement contents are solely the responsibility of the authors and do not necessarily represent the official views of DSM Nutritional Products Ltd. The supplement coordinators for this supplement are Hans Konrad Biesalski and Jana Tinz, Institute of Biological Chemistry and Nutrition, University of Hohenheim. Supplement Coordinator disclosures: Hans Biesalski and Jana Tinz declare no conflicts of interest. Supplement Guest Editor disclosure: Jesse Gregory declares no conflict of interest. Publication costs for this supplement were defrayed in part by the payment of page charges. This publication must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact. The opinions expressed in this publication are those of the authors and are not attributable to the sponsors or the publisher, editor, or editorial board of The Journal of Nutrition.
The meeting was supported by DSM, Switzerland. DSM employees had no access to the questions and were not involved in either the consensus meeting or the formulation of the final text.
Abbreviations used: AN, anorexia nervosa; AR, average requirement; BCMO1, b-carotene-monooxygenase-1 or β-carotene-15,15’-monooxygenase; DACH, reference intake values of Germany-Austria-Switzerland; IOM, Institute of Medicine; LRNI, lower reference nutrient intake; NDNS, National Diet and Nutrition Survey (UK); NVS, German National Nutrition Survey; P95, 95th percentile; RAE, retinol activity equivalent; RBP, retinol binding protein; RDA, recommended daily allowance; RDI, recommended dietary intake; RE, retinol equivalent; RNI, recommended nutrient intake; SNP, single nucleotide polymorphism; SPE, sucrose polyester; UL, upper level.
Recommendations for populations may be greater than, and are typically set to exceed, an individual’s actual physiological requirements. A margin of safety is added to the estimated physiological requirement to ensure that the recommended amount will provide an adequate intake of the nutrient for essentially the entire population. In discussing the requirement for β-carotene, we have focused on meeting the recommendations or reference values that have been stated in different terms as NRI, RDA, dietary reference intakes, AR, etc., in different countries and at different times.
Literature Cited
- 1.Britton G, Liaaen-Jensen S, Pfander H. Carotenoids. Basel: Birkhäuser; 2008 [Google Scholar]
- 2.Britton G. UV/visible spectroscopy. : Britton G, Liaaen-Jensen S, Pfander H, Carotenoids, spectroscopy. Volume 1B Basel: Birkhäuser; 1995. p. 13–62 [Google Scholar]
- 3.O'Neil CA, Schwartz SJ. Chromatographic analysis of cis/trans carotenoid isomers. J Chromatogr A. 1992;624:235–52 [DOI] [PubMed] [Google Scholar]
- 4.Stahl W, Schwarz W, Sundquist AR, Sies H. cis-trans Isomers of lycopene and beta-carotene in human serum and tissues. Arch Biochem Biophys. 1992;294:173–7 [DOI] [PubMed] [Google Scholar]
- 5.Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med. 2005;26:459–516 [DOI] [PubMed] [Google Scholar]
- 6.Nagao A, Olson JA. Enzymatic information of 9-cis, 13-cis, and all-trans retinals from isomers of beta-carotene. FASEB J. 1994;8:968–73 [DOI] [PubMed] [Google Scholar]
- 7.Germain P, Chambon P, Eichele G, Evans RM, Lazar MA, Leid M, De Lera AR, Lotan R, Mangelsdorf DJ, et al. International Union of Pharmacology. LX. Retinoic acid receptors. Pharmacol Rev. 2006;58:712–25 [DOI] [PubMed] [Google Scholar]
- 8.Wolf G. Is 9-cis-retinoic acid the endogenous ligand for the retinoic acid-X receptor? Nutr Rev. 2006;64:532–8 [DOI] [PubMed] [Google Scholar]
- 9.Germain P, Chambon P, Eichele G, Evans RM, Lazar MA, Leid M, De Lera AR, Lotan R, Mangelsdorf DJ, et al. International Union of Pharmacology. LXIII. Retinoid X receptors. Pharmacol Rev. 2006;58:760–72 PubMed [DOI] [PubMed] [Google Scholar]
- 10.Hébuterne X, Wang XD, Johnson EJ, Krinsky NI, Russell RM. Intestinal absorption and metabolism of 9-cis-beta-carotene in vivo: biosynthesis of 9-cis-retinoic acid. J Lipid Res. 1995;36:1264–73 [PubMed] [Google Scholar]
- 11.Wang XD, Krinsky NI, Benotti PN, Russell RM. Biosynthesis of 9-cis-retinoic acid from 9-cis-beta-carotene in human intestinal mucosa in vitro. Arch Biochem Biophys. 1994;313:150–5 [DOI] [PubMed] [Google Scholar]
- 12.Stahl W, Schwarz W, von Laar J, Sies H. All-trans beta-carotene preferentially accumulates in human chylomicrons and very low density lipoproteins compared with the 9-cis geometrical isomer. J Nutr. 1995;125:2128–33 [DOI] [PubMed] [Google Scholar]
- 13.Stahl W, Sies H. Bioactivity and protective effects of natural carotenoids. Biochim Biophys Acta. 2005;1740:101–7 [DOI] [PubMed] [Google Scholar]
- 14.Burton GW, Ingold KU. beta-Carotene: an unusual type of lipid antioxidant. Science. 1984;224:569–73 [DOI] [PubMed] [Google Scholar]
- 15.Biesalski HK, Obermueller-Jevic UC. UV light, beta-carotene and human skin: beneficial and potentially harmful effects. Arch Biochem Biophys. 2001;389:1–6 [DOI] [PubMed] [Google Scholar]
- 16.Eichler O, Sies H, Stahl W. Divergent optimum levels of lycopene, beta-carotene and lutein protecting against UVB irradiation in human fibroblastst. Photochem Photobiol. 2002;75:503–6 [DOI] [PubMed] [Google Scholar]
- 17.Palozza P. Prooxidant actions of carotenoids in biologic systems. Nutr Rev. 1998;56:257–65 [DOI] [PubMed] [Google Scholar]
- 18.Palozza P, Serini S, Di Nicuolo F, Piccioni E, Calviello G. Prooxidant effects of beta-carotene in cultured cells. Mol Aspects Med. 2003;24:353–62 [DOI] [PubMed] [Google Scholar]
- 19.Young AJ, Lowe GM. Antioxidant and prooxidant properties of carotenoids. Arch Biochem Biophys. 2001;385:20–7 [DOI] [PubMed] [Google Scholar]
- 20.Dragsted LO. Biomarkers of exposure to vitamins A, C, and E and their relation to lipid and protein oxidation markers. Eur J Nutr. 2008;47 Suppl 2:3–18 [DOI] [PubMed] [Google Scholar]
- 21.Loft S, Møller P, Cooke MS, Rozalski R, Olinski R. Antioxidant vitamins and cancer risk: is oxidative damage to DNA a relevant biomarker? Eur J Nutr. 2008;47 Suppl 2:19–28 [DOI] [PubMed] [Google Scholar]
- 22.Mayne ST. Antioxidant nutrients and chronic disease: use of biomarkers of exposure and oxidative stress status in epidemiologic research. J Nutr. 2003;133 Suppl 3:S933–40 [DOI] [PubMed] [Google Scholar]
- 23.Demmig-Adams B, Adams WW III. Antioxidants in photosynthesis and human nutrition. Science. 2002;298:2149–53 [DOI] [PubMed] [Google Scholar]
- 24.Baltschun D, Beutner S, Briviba K, Martin HD, Paust J, Peters M, Röver S, Sies H, Stahl W, et al. Singlet oxygen quenching abilities of carotenoids. Liebigs Ann. 1997. p. 1887–93 [Google Scholar]
- 25.Mathews-Roth MM. Carotenoids in erythropoietic protoporphyria and other photosensitivity diseases. Ann N Y Acad Sci. 1993;691:127–38 [DOI] [PubMed] [Google Scholar]
- 26.von Laar J, Stahl W, Bolsen K, Goerz G, Sies H. Beta-carotene serum levels in patients with erythropoietic protoporphyria on treatment with the synthetic all-trans isomer or a natural isomeric mixture of beta-carotene. J Photochem Photobiol B. 1996;33:157–62 [DOI] [PubMed] [Google Scholar]
- 27.Mathews-Roth MM, Pathak UA, Fitzpatrick TB, Harber LC, Kass EH. Beta-carotene as an oral photoprotective agent in erythropoietic protoporphyria. JAMA. 1974;228:1004–8 [PubMed] [Google Scholar]
- 28.Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr. 2003;23:171–201 [DOI] [PubMed] [Google Scholar]
- 29.Chiu CJ, Taylor A. Nutritional antioxidants and age-related cataract and maculopathy. Exp Eye Res. 2007;84:229–45 [DOI] [PubMed] [Google Scholar]
- 30.Alaluf S, Heinrich U, Stahl W, Tronnier H, Wiseman S. Dietary carotenoids contribute to normal human skin color and UV photosensitivity. J Nutr. 2002;132:399–403 [DOI] [PubMed] [Google Scholar]
- 31.Köpcke W, Krutmann J. Protection from sunburn with beta-carotene: a meta-analysis. Photochem Photobiol. 2008;84:284–8 [DOI] [PubMed] [Google Scholar]
- 32.Sies H, Stahl W. Nutritional protection against skin damage from sunlight. Annu Rev Nutr. 2004;24:173–200 [DOI] [PubMed] [Google Scholar]
- 33.Heinrich U, Gärtner C, Wiebusch M, Eichler O, Sies H, Tronnier H, Stahl W. Supplementation with beta-carotene or a similar amount of mixed carotenoids protects humans from UV-induced erythema. J Nutr. 2003;133:98–101 [DOI] [PubMed] [Google Scholar]
- 34.Lee J, Jiang S, Levine N, Watson RR. Carotenoid supplementation reduces erythema in human skin after simulated solar radiation exposure. Proc Soc Exp Biol Med. 2000;223:170–4 [DOI] [PubMed] [Google Scholar]
- 35.Mathews-Roth MM, Pathak MA, Parrish J, Fitzpatrick TB, Kass EH, Toda K, Clemens W. A clinical trial of the effects of oral beta-carotene on the responses of human skin to solar radiation. J Invest Dermatol. 1972;59:349–53 [DOI] [PubMed] [Google Scholar]
- 36.Stahl W, Heinrich U, Jungmann H, Sies H, Tronnier H. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light-induced erythema in humans. Am J Clin Nutr. 2000;71:795–8 [DOI] [PubMed] [Google Scholar]
- 37.Wolf C, Steiner A, Hönigsmann H. Do oral carotenoids protect human skin against ultraviolet erythema, psoralen phototoxicity, and ultraviolet-induced DNA damage? J Invest Dermatol. 1988;90:55–7 [DOI] [PubMed] [Google Scholar]
- 38.Kanofsky JR. Singlet oxygen in biological systems: a comparison of biochemical and photochemical mechanisms for singlet oxygen generation. : Tarrv M, Samson F, Oxygen free radicals in tissue damage. 4th ed Boston: Birkhäuser; 1993. p. 77–92 [Google Scholar]
- 39.Redmond RW, Kochevar IE. Spatially resolved cellular responses to singlet oxygen. Photochem Photobiol. 2006;82:1178–86 [DOI] [PubMed] [Google Scholar]
- 40.Sun S, Bao Z, Ma H, Zhang D, Zheng X. Singlet oxygen generation from the decomposition of alpha-linolenic acid hydroperoxide by cytochrome c and lactoperoxidase. Biochemistry. 2007;46:6668–73 [DOI] [PubMed] [Google Scholar]
- 41.Sies H, Stahl W, Sevanian A. Nutritional, dietary and postprandial oxidative stress. J Nutr. 2005;135:969–72 [DOI] [PubMed] [Google Scholar]
- 42.El-Agamey A, Lowe GM, McGarvey DJ, Mortensen A, Phillip DM, Truscott TG, Young AJ. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch Biochem Biophys. 2004;430:37–48 [DOI] [PubMed] [Google Scholar]
- 43.El-Agamey A, Cantrell A, Land EJ, McGarvey DJ, Truscott TG. Are dietary carotenoids beneficial? Reactions of carotenoids with oxy-radicals and singlet oxygen. Photochem Photobiol Sci. 2004;3:802–11 [DOI] [PubMed] [Google Scholar]
- 44.Truscott TG. The photophysics and photochemistry of the carotenoids. J Photochem Photobiol B Biol. 1990;6:359–71 [Google Scholar]
- 45.Mortensen A, Skibsted LH, Truscott TG. The interaction of dietary carotenoids with radical species. Arch Biochem Biophys. 2001;385:13–9 [DOI] [PubMed] [Google Scholar]
- 46.Harper P, Wahlin S. Treatment options in acute porphyria, porphyria cutanea tarda, and erythropoietic protoporphyria. Curr Treat Options Gastroenterol. 2007;10:444–55 [DOI] [PubMed] [Google Scholar]
- 47.Stahl W, Sies H. Carotenoids and flavonoids contribute to nutritional protection against skin damage from sunlight. Mol Biotechnol. 2007;37:26–30 [DOI] [PubMed] [Google Scholar]
- 48.Minder EI, Schneider-Yin X, Steurer J, Bachmann LM. A systematic review of treatment options for dermal photosensitivity in erythropoietic protoporphyria. Cell Mol Biol (Noisy-le-grand). 2009;55:84–97 [PubMed] [Google Scholar]
- 49.Felipe F, Bonet ML, Ribot J, Palou A. Modulation of resistin expression by retinoic acid and vitamin A status. Diabetes. 2004;53:882–9 [DOI] [PubMed] [Google Scholar]
- 50.Mercader J, Madsen L, Felipe F, Palou A, Kristiansen K, Bonet ML. All-trans retinoic acid increases oxidative metabolism in mature adipocytes. Cell Physiol Biochem. 2007;20:1061–72 [DOI] [PubMed] [Google Scholar]
- 51.Bonet ML, Oliver J, Picó C, Felipe F, Ribot J, Cinti S, Palou A. Opposite effects of feeding a vitamin A-deficient diet and retinoic acid treatment on brown adipose tissue uncoupling protein 1 (UCP1), UCP2 and leptin expression. J Endocrinol. 2000;166:511–7 [DOI] [PubMed] [Google Scholar]
- 52.Serra F, Bonet ML, Puigserver P, Oliver J, Palou A. Stimulation of uncoupling protein 1 expression in brown adipocytes by naturally occurring carotenoids. Int J Obes Relat Metab Disord. 1999;23:650–5 [DOI] [PubMed] [Google Scholar]
- 53.Fuster A, Oliver P, Sánchez J, Picó C, Palou A. UCP1 and oxidative capacity of adipose tissue in adult ferrets (Mustela putorius furo). Comp Biochem Physiol A Mol Integr Physiol. 2009;153:106–12 [DOI] [PubMed] [Google Scholar]
- 54.Hessel S, Eichinger A, Isken A, Amengual J, Hunzelmann S, Hoeller U, Elste V, Hunziker W, Goralczyk R, et al. CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice. J Biol Chem. 2007;282:33553–61 [DOI] [PubMed] [Google Scholar]
- 55.von Lintig J, Vogt K. Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving beta-carotene to retinal. J Biol Chem. 2000;275:11915–20 [DOI] [PubMed] [Google Scholar]
- 56.Fierce Y, de Morais Vieira M, Piantedosi R, Wyss A, Blaner WS, Paik J. In vitro and in vivo characterization of retinoid synthesis from beta-carotene. Arch Biochem Biophys. 2008;472:126–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olson JA. Provitamin A function of carotenoids: the conversion of beta-carotene into vitamin A. J Nutr. 1989;119:105–8 [DOI] [PubMed] [Google Scholar]
- 58.Castenmiller JJ, West CE. Bioavailability and bioconversion of carotenoids. Annu Rev Nutr. 1998;18:19–38 [DOI] [PubMed] [Google Scholar]
- 59.Biesalski HK, Chichili GR, Frank J, von Lintig J, Nohr D. Conversion of beta-carotene to retinal pigment. Vitam Horm. 2007;75:117–30 [DOI] [PubMed] [Google Scholar]
- 60.EFSA Scientific Panel on Dietetic Products, Nutrition and Allergies and Scientific Committee on Food Tolerable upper intake levels for vitamins and minerals; 2006. [cited December 2006]. Available from: http://www.efsa.eu.int
- 61.Institute of Medicine Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academies Press; 2001 [PubMed] [Google Scholar]
- 62.Elmadfa I, Freisling H. Austrian Nutrition Report 2003. University of Vienna: Institute of Nutritional Sciences; 2004 [Google Scholar]
- 63.Schneider R, Eberhardt W, Heseker H, Kübler W. Vitamin intake and vitamin status in Germany. Bibl Nutr Dieta. 1995;116–27 [DOI] [PubMed] [Google Scholar]
- 64.Elmadfa I, Weichselbaum E, König J, Remaut de Winter A, Trolle E, Haapala I, Uusitalo U, Mennen L, Hercberg S, et al. European Nutrition and Health Report 2004. Forum of Nutrition. Basel: Karger; 2005 [PubMed] [Google Scholar]
- 65.Aranceta J, Serra-Majem L, Pérez-Rodrigo C, Llopis J, Mataix J, Ribas L, Tojo R, Tur JA. Vitamins in Spanish food patterns: the eVe Study. Public Health Nutr. 2001;4:1317–23 [DOI] [PubMed] [Google Scholar]
- 66.Swan G. Findings from the latest National Diet and Nutrition Survey. Proc Nutr Soc. 2004;63:505–12 [DOI] [PubMed] [Google Scholar]
- 67.Flynn A, Hirvonen T, Mensink GB, Ocké MC, Serra-Majem L, Stos K, Szponar L, Tetens I, Turrini A, et al. Intake of selected nutrients from foods, from fortification and from supplements in various European countries. Food Nutr Res. 2009;53:doi: 10.3402/fnr.v53i0.2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kafwembe EM, Chipipa J, Njunju E, Chilengi R. The vitamin A status of Zambian children in a community of vitamin A supplementation and sugar fortification strategies as measured by the modified relative dose response (MRDR) test. Int J Vitam Nutr Res. 2009;79:40–7 [DOI] [PubMed] [Google Scholar]
- 69.Lachance PA. "Natural" cancer prevention. Science. 1996;272:1860–1 [DOI] [PubMed] [Google Scholar]
- 70.DACH Deutsche Gesellschaft für Ernährung, Österreichische Gesellschaft für Ernährung, Schweizerische Gesellschaft für Ernährungsforschung, Schweizerische Vereinigung für Ernährung: Referenzwerte für die Nährstoffzufuhr. 1. Auflage, 3. korrigierter Nachdruck, Frankfurt: Umschau/Braus; 2008 [Google Scholar]
- 71.Müller H. [Daily intake of carotenoids (carotenes and xanthophylls) from total diet and the carotenoid content of selected vegetables and fruit]. Z Ernahrungswiss. 1996;35:45–50 [DOI] [PubMed] [Google Scholar]
- 72.Biesalski HK, Nohr D. Importance of vitamin A for lung function and development. Mol Aspects Med. 2003;24:431–40 [DOI] [PubMed] [Google Scholar]
- 73. Bundesinstitut für gesundheitlichen Verbraucherschutz und Veterinärmedizin. Schwangere sollten weiterhin aif den Verzehr von Leber verzichten. Pressedienst 20/1995, [cited 1995 23 Oct]. Available from: http://www.bfr.bund.de/cms5w/sixcms/detail.php/775.
- 74.Domke A, Großklaus R, Niemann B, Przyrembel H, Richter K, Schmidt E, Weißdorn A, Wörner B, Ziegenhagen R. Verwendung von Vitaminen in Lebensmitteln. Toxikologische und ernährungsphysiologische Aspekte. Berlin: BfR Wissenschaft; 2004 [Google Scholar]
- 75.Schulz C, Engel U, Kreienberg R, Biesalski HK. Vitamin A and beta-carotene supply of women with gemini or short birth intervals: a pilot study. Eur J Nutr. 2007;46:12–20 [DOI] [PubMed] [Google Scholar]
- 76.Buss NE, Tembe EA, Prendergast BD, Renwick AG, George CF. The teratogenic metabolites of vitamin A in women following supplements and liver. Hum Exp Toxicol. 1994;13:33–43 [DOI] [PubMed] [Google Scholar]
- 77.Biesalski HK, Nohr D. New aspects in vitamin A metabolism: the role of retinyl esters as systemic and local sources for retinol in mucous epithelia. J Nutr. 2004;134:S3453–7 [DOI] [PubMed] [Google Scholar]
- 78.Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66:606–30 [DOI] [PubMed] [Google Scholar]
- 79.Mensink GB, Beitz R. Food and nutrient intake in East and West Germany, 8 years after the reunification: The German Nutrition Survey 1998. Eur J Clin Nutr. 2004;58:1000–10 [DOI] [PubMed] [Google Scholar]
- 80.Sichert-Hellert W, Kersting M, Manz F. Changes in time-trends of nutrient intake from fortified and non-fortified food in German children and adolescents–15 year results of the DONALD study. Dortmund Nutritional and Anthropometric Longitudinally Designed Study. Eur J Nutr. 2001;40:49–55 [DOI] [PubMed] [Google Scholar]
- 81.Elmadfa I, Freisling H, Nowak V, Hofstädter D, Hasenegger V, Ferge M, Fröhler M, Fritz K, Meyer AL, et al. Österreichischer Ernährungsbericht 2008. 1. Auflage, Institut für Ernährungswissenschaft, Universität Wien; 2009 [Google Scholar]
- 82.Deutsche Gesellschaft für Ernährung Ernährungsbericht 2008. Bonn: Deutsche Gesellschaft für Ernährung e.V; 2008 [Google Scholar]
- 83.Pelz R, Schmidt-Faber B, Heseker H. [Carotenoid intake in the German National Food Consumption Survey]. Z Ernahrungswiss. 1998;37:319–27 [DOI] [PubMed] [Google Scholar]
- 84.Kiely M, Flynn A, Harrington KE, Robson PJ, O'Connor N, Hannon EM, O'Brien MM, Bell S, Strain JJ. The efficacy and safety of nutritional supplement use in a representative sample of adults in the North/South Ireland Food Consumption Survey. Public Health Nutr. 2001;4:1089–97 [DOI] [PubMed] [Google Scholar]
- 85.O'Brien MM, Kiely M, Harrington KE, Robson PJ, Strain JJ, Flynn A. The North/South Ireland Food Consumption Survey: vitamin intakes in 18–64-year-old adults. Public Health Nutr. 2001;4:1069–79 [DOI] [PubMed] [Google Scholar]
- 86.Scientific Committee for Food Nutrient and energy intakes for the European Community. Reports of the Scientific Committee for Food, Thirty First Series. Luxembourg: European Commission; 1993 [cited 1992 11 Dec]. Available from: http://ec.europa.eu/food/fs/sc/scf/out89.pdf
- 87.Max-Rubner-Institut Nationale Verzehrsstudie. Ergebnisbericht Teil II. Karlsruhe: Bundesforschungsinstitut für Ernährung und Lebensmittel; 2008 [Google Scholar]
- 88.Dixon LB, Winkleby MA, Radimer KL. Dietary intakes and serum nutrients differ between adults from food-insufficient and food-sufficient families: Third National Health and Nutrition Examination Survey, 1988–1994. J Nutr. 2001;131:1232–46 [DOI] [PubMed] [Google Scholar]
- 89.Ervin RB, Wright JD, Wang CY, Kennedy-Stephenson J. Dietary intake of selected vitamins for the United States population: 1999–2000. Adv Data. 2004. Mar 12;1–4 [PubMed] [Google Scholar]
- 90.Li Y, Zhai F, He YN, Yu D. [Survey on the status of dietary vitamin a intakes in Chinese residents]. Wei Sheng Yan Jiu. 2007;36:200–2 [PubMed] [Google Scholar]
- 91.Yin SA, Lai JQ. [The nutritional and health status of child-bearing women aged 15–45 years: 2002 National Nutrition and Health Survey]. Bejing: People’s Medical Publishing House; 2008 [Google Scholar]
- 92.Zai FY, Yang XG. [The status of dietary and nutrient intakes in Nutrition and Health Status of the Chinese People in 2002]. Bejing: People’s Medical Publishing House; 2006 [Google Scholar]
- 93.Wang J, Wang Y, Wang Z, Li L, Qin J, Lai W, Fu Y, Suter PM, Russell RM, et al. Vitamin A equivalence of spirulina beta-carotene in Chinese adults as assessed by using a stable-isotope reference method. Am J Clin Nutr. 2008;87:1730–7 [DOI] [PubMed] [Google Scholar]
- 94.Tang G, Gu X, Hu S, Xu Q, Qin J, Dolnikowski GG, Fjeld CR, Gao X, Russell RM, et al. Green and yellow vegetables can maintain body stores of vitamin A in Chinese children. Am J Clin Nutr. 1999;70:1069–76 [DOI] [PubMed] [Google Scholar]
- 95.Ahmed F, Zareen M, Khan MR, Banu CP, Haq MN, Jackson AA. Dietary pattern, nutrient intake and growth of adolescent school girls in urban Bangladesh. Public Health Nutr. 1998;1:83–92 [DOI] [PubMed] [Google Scholar]
- 96.Khan NC, Mai LB, Minh ND, Do TT, Khoi HH, West CE, Hautvast JG. Intakes of retinol and carotenoids and its determining factors in the Red River Delta population of northern Vietnam. Eur J Clin Nutr. 2008;62:810–6 [DOI] [PubMed] [Google Scholar]
- 97.Persson V, Hartini TN, Greiner T, Hakimi M, Stenlund H, Winkvist A. Vitamin A intake is low among pregnant women in central Java, Indonesia. Int J Vitam Nutr Res. 2002;72:124–32 PubMed [DOI] [PubMed] [Google Scholar]
- 98.Ramakrishnan U, Martorell R, Latham MC, Abel R. Dietary vitamin A intakes of preschool-age children in South India. J Nutr. 1999;129:2021–7 [DOI] [PubMed] [Google Scholar]
- 99.Goodman DS, Blomstrand R, Werner B, Huang HS, Shiratori T. The intestinal absorption and metabolism of vitamin A and beta-carotene in man. J Clin Invest. 1966;45:1615–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hickenbottom SJ, Lemke SL, Dueker SR, Lin Y, Follett JR, Carkeet C, Buchholz BA, Vogel JS, Clifford AJ. Dual isotope test for assessing beta-carotene cleavage to vitamin A in humans. Eur J Nutr. 2002;41:141–7 [DOI] [PubMed] [Google Scholar]
- 101.van Vliet T, Schreurs WH, van den Berg H. Intestinal beta-carotene absorption and cleavage in men: response of beta-carotene and retinyl esters in the triglyceride-rich lipoprotein fraction after a single oral dose of beta-carotene. Am J Clin Nutr. 1995;62:110–6 [DOI] [PubMed] [Google Scholar]
- 102.Novotny JA, Dueker SR, Zech LA, Clifford AJ. Compartmental analysis of the dynamics of beta-carotene metabolism in an adult volunteer. J Lipid Res. 1995;36:1825–38 [PubMed] [Google Scholar]
- 103.Faulks RM, Hart DJ, Wilson PD, Scott KJ, Southon S. Absorption of all-trans and 9-cis beta-carotene in human ileostomy volunteers. Clin Sci (Lond). 1997;93:585–91 [DOI] [PubMed] [Google Scholar]
- 104.Ho CC, de Moura FF, Kim SH, Clifford AJ. Excentral cleavage of beta-carotene in vivo in a healthy man. Am J Clin Nutr. 2007;85:770–7 [DOI] [PubMed] [Google Scholar]
- 105.Blomstrand R, Werner B. Studies on the intestinal absorption of radioactive beta-carotene and vitamin A in man. Conversion of beta-carotene into vitamin A. Scand J Clin Lab Invest. 1967;19:339–45 [DOI] [PubMed] [Google Scholar]
- 106.Tang G, Qin J, Dolnikowski GG, Russell RM. Short-term (intestinal) and long-term (postintestinal) conversion of beta-carotene to retinol in adults as assessed by a stable-isotope reference method. Am J Clin Nutr. 2003;78:259–66 [DOI] [PubMed] [Google Scholar]
- 107.Furr HC, Clark RM. Intestinal absorption and tissue distribution of carotenoids. J Nutr Biochem. 1997;8:364–77 [Google Scholar]
- 108.van Het Hof KH, West CE, Weststrate JA, Hautvast JG. Dietary factors that affect the bioavailability of carotenoids. J Nutr. 2000;130:503–6 [DOI] [PubMed] [Google Scholar]
- 109.Yeum KJ, Russell RM. Carotenoid bioavailability and bioconversion. Annu Rev Nutr. 2002;22:483–504 [DOI] [PubMed] [Google Scholar]
- 110.Sauberlich HE, Hodges RE, Wallace DL, Kolder H, Canham JE, Hood J, Raica N, Jr, Lowry LK. Vitamin A metabolism and requirements in the human studied with the use of labeled retinol. Vitam Horm. 1974;32:251–75 [DOI] [PubMed] [Google Scholar]
- 111.Hume EM, Krebs HA. Vitamin A requirement of human adults. An experimental study of vitamin A deprivation in man. Medical Research Council Special Report Series No. 264. London: His Majesty’s Stationery Office; 1949 [Google Scholar]
- 112.van Lieshout M, West CE. Muhilal, Permaesih D, Wang Y, Xu X, van Breemen RB, Creemers AF, Verhoeven MA, et al. Bioefficacy of beta-carotene dissolved in oil studied in children in Indonesia. Am J Clin Nutr. 2001;73:949–58 [DOI] [PubMed] [Google Scholar]
- 113.Wang Z, Yin S, Zhao X, Russell RM, Tang G. beta-Carotene-vitamin A equivalence in Chinese adults assessed by an isotope dilution technique. Br J Nutr. 2004;91:121–31 [DOI] [PubMed] [Google Scholar]
- 114.de Pee S, West CE, Permaesih D, Martuti S. Muhilal, Hautvast JG. Orange fruit is more effective than are dark-green, leafy vegetables in increasing serum concentrations of retinol and beta-carotene in schoolchildren in Indonesia. Am J Clin Nutr. 1998;68:1058–67 [DOI] [PubMed] [Google Scholar]
- 115.Haskell MJ, Jamil KM, Hassan F, Peerson JM, Hossain MI, Fuchs GJ, Brown KH. Daily consumption of Indian spinach (Basella alba) or sweet potatoes has a positive effect on total-body vitamin A stores in Bangladeshi men. Am J Clin Nutr. 2004;80:705–14 [DOI] [PubMed] [Google Scholar]
- 116.Tang G, Qin J, Dolnikowski GG, Russell RM, Grusak MA. Spinach or carrots can supply significant amounts of vitamin A as assessed by feeding with intrinsically deuterated vegetables. Am J Clin Nutr. 2005;82:821–8 [DOI] [PubMed] [Google Scholar]
- 117.Tang G, Qin J, Dolnikowski GG, Russell RM, Grusak MA. Golden rice is an effective source of vitamin A. Am J Clin Nutr. 2009;89:1776–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tang G, Qin J, Dolnikowski GG, Russell RM. Vitamin A equivalence of beta-carotene in a woman as determined by a stable isotope reference method. Eur J Nutr. 2000;39:7–11 [DOI] [PubMed] [Google Scholar]
- 119.Unlu NZ, Bohn T, Clinton SK, Schwartz SJ. Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. J Nutr. 2005;135:431–6 [DOI] [PubMed] [Google Scholar]
- 120.Broekmans WM, Klöpping-Ketelaars IA, Weststrate JA, Tijburg LB, van Poppel G, Vink AA, Berendschot TT, Bots ML, Castenmiller WA, et al. Decreased carotenoid concentrations due to dietary sucrose polyesters do not affect possible markers of disease risk in humans. J Nutr. 2003;133:720–6 [DOI] [PubMed] [Google Scholar]
- 121.van den Berg H, van Vliet T. Effect of simultaneous, single oral doses of beta-carotene with lutein or lycopene on the beta-carotene and retinyl ester responses in the triacylglycerol-rich lipoprotein fraction of men. Am J Clin Nutr. 1998;68:82–9 [DOI] [PubMed] [Google Scholar]
- 122.Riedl J, Linseisen J, Hoffmann J, Wolfram G. Some dietary fibers reduce the absorption of carotenoids in women. J Nutr. 1999;129:2170–6 [DOI] [PubMed] [Google Scholar]
- 123.Hickenbottom SJ, Follett JR, Lin Y, Dueker SR, Burri BJ, Neidlinger TR, Clifford AJ. Variability in conversion of beta-carotene to vitamin A in men as measured by using a double-tracer study design. Am J Clin Nutr. 2002;75:900–7 [DOI] [PubMed] [Google Scholar]
- 124.Lin Y, Dueker SR, Burri BJ, Neidlinger TR, Clifford AJ. Variability of the conversion of beta-carotene to vitamin A in women measured by using a double-tracer study design. Am J Clin Nutr. 2000;71:1545–54 [DOI] [PubMed] [Google Scholar]
- 125.Lindqvist A, Andersson S. Cell type-specific expression of beta-carotene 15,15'-mono-oxygenase in human tissues. J Histochem Cytochem. 2004;52:491–9 [DOI] [PubMed] [Google Scholar]
- 126.Bachmann H, Desbarats A, Pattison P, Sedgewick M, Riss G, Wyss A, Cardinault N, Duszka C, Goralczyk R, et al. Feedback regulation of beta,beta-carotene 15,15'-monooxygenase by retinoic acid in rats and chickens. J Nutr. 2002;132:3616–22 [DOI] [PubMed] [Google Scholar]
- 127.Takitani K, Zhu CL, Inoue A, Tamai H. Molecular cloning of the rat beta-carotene 15,15'-monooxygenase gene and its regulation by retinoic acid. Eur J Nutr. 2006;45:320–6 [DOI] [PubMed] [Google Scholar]
- 128.Ribaya-Mercado JD, Solon FS, Solon MA, Cabal-Barza MA, Perfecto CS, Tang G, Solon JA, Fjeld CR, Russell RM. Bioconversion of plant carotenoids to vitamin A in Filipino school-aged children varies inversely with vitamin A status. Am J Clin Nutr. 2000;72:455–65 [DOI] [PubMed] [Google Scholar]
- 129.Lemke SL, Dueker SR, Follett JR, Lin Y, Carkeet C, Buchholz BA, Vogel JS, Clifford AJ. Absorption and retinol equivalence of beta-carotene in humans is influenced by dietary vitamin A intake. J Lipid Res. 2003;44:1591–600 [DOI] [PubMed] [Google Scholar]
- 130.Wyss A. Carotene oxygenases: a new family of double bond cleavage enzymes. J Nutr. 2004;134:S246–50 [DOI] [PubMed] [Google Scholar]
- 131.Seino Y, Miki T, Kiyonari H, Abe T, Fujimoto W, Kimura K, Takeuchi A, Takahashi Y, Oiso Y, et al. Isx participates in the maintenance of vitamin A metabolism by regulation of beta-carotene 15,15'-monooxygenase (Bcmo1) expression. J Biol Chem. 2008;283:4905–11 [DOI] [PubMed] [Google Scholar]
- 132.Ribaya-Mercado JD, Maramag CC, Tengco LW, Blumberg JB, Solon FS. Relationships of body mass index with serum carotenoids, tocopherols and retinol at steady-state and in response to a carotenoid-rich vegetable diet intervention in Filipino schoolchildren. Biosci Rep. 2008;28:97–106 [DOI] [PubMed] [Google Scholar]
- 133.Ylönen K, Alfthan G, Groop L, Saloranta C, Aro A, Virtanen SM. Dietary intakes and plasma concentrations of carotenoids and tocopherols in relation to glucose metabolism in participants at high risk of type 2 diabetes: the Botnia Dietary Study. Am J Clin Nutr. 2003;77:1434–41 [DOI] [PubMed] [Google Scholar]
- 134.Coyne T, Ibiebele TI, Baade PD, Dobson A, McClintock C, Dunn S, Leonard D, Shaw J. Diabetes mellitus and serum carotenoids: findings of a population-based study in Queensland, Australia. Am J Clin Nutr. 2005;82:685–93 [DOI] [PubMed] [Google Scholar]
- 135.Ramakrishna V, Jailkhani R. Oxidative stress in non-insulin-dependent diabetes mellitus (NIDDM) patients. Acta Diabetol. 2008;45:41–6 [DOI] [PubMed] [Google Scholar]
- 136.Curran-Celentano J, Erdman JW, Jr, Nelson RA, Grater SJ. Alterations in vitamin A and thyroid hormone status in anorexia nervosa and associated disorders. Am J Clin Nutr. 1985;42:1183–91 [DOI] [PubMed] [Google Scholar]
- 137.Boland B, Beguin C, Zech F, Desager JP, Lambert M. Serum beta-carotene in anorexia nervosa patients: a case-control study. Int J Eat Disord. 2001;30:299–305 [DOI] [PubMed] [Google Scholar]
- 138.Lindqvist A, Sharvill J, Sharvill DE, Andersson S. Loss-of-function mutation in carotenoid 15,15'-monooxygenase identified in a patient with hypercarotenemia and hypovitaminosis A. J Nutr. 2007;137:2346–50 [DOI] [PubMed] [Google Scholar]
- 139.Leung WC, Hessel S, Méplan C, Flint J, Oberhauser V, Tourniaire F, Hesketh JE, von Lintig J, Lietz G. Two common single nucleotide polymorphisms in the gene encoding beta-carotene 15,15'-monoxygenase alter beta-carotene metabolism in female volunteers. FASEB J. 2009;23:1041–53 [DOI] [PubMed] [Google Scholar]
- 140.Tourniaire F, Gouranton E, von Lintig J, Keijer J, Luisa Bonet M, Amengual J, Lietz G, Landrier JF. beta-Carotene conversion products and their effects on adipose tissue. Genes Nutr. 2009;4:179–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bendich A, Olson JA. Biological actions of carotenoids. FASEB J. 1989;3:1927–32 [PubMed] [Google Scholar]
- 142.Micozzi MS, Brown ED, Edwards BK, Bieri JG, Taylor PR, Khachik F, Beecher GR, Smith JC., Jr Plasma carotenoid response to chronic intake of selected foods and beta-carotene supplements in men. Am J Clin Nutr. 1992;55:1120–5 [DOI] [PubMed] [Google Scholar]
- 143.Yeum KJ, Booth SL, Sadowski JA, Liu C, Tang G, Krinsky NI, Russell RM. Human plasma carotenoid response to the ingestion of controlled diets high in fruits and vegetables. Am J Clin Nutr. 1996;64:594–602 [DOI] [PubMed] [Google Scholar]
- 144.Krinsky NI, Cronwell DG, Oncley JL. The transport of vitamin A and carotenoids in human plasma. Arch Biochem Biophys. 1958;73:233–46 [DOI] [PubMed] [Google Scholar]
- 145.Romanchik JE, Morel DW, Harrison EH. Distributions of carotenoids and alpha-tocopherol among lipoproteins do not change when human plasma is incubated in vitro. J Nutr. 1995;125:2610–7 [DOI] [PubMed] [Google Scholar]
- 146.Auletta FJ, Gulbrandsen CL. Transport of beta-carotene in serum in individuals with carotenemia. Clin Chem. 1974;20:1578–9 [PubMed] [Google Scholar]
- 147.Parker RS. Carotenoid and tocopherol composition of human adipose tissue. Am J Clin Nutr. 1988;47:33–6 [DOI] [PubMed] [Google Scholar]
- 148.Parker RS. Carotenoids in human blood and tissues. J Nutr. 1989;119:101–4 [DOI] [PubMed] [Google Scholar]
- 149.Erhardt JG, Mack H, Sobeck U, Biesalski HK. beta-Carotene and alpha-tocopherol concentration and antioxidant status in buccal mucosal cells and plasma after oral supplementation. Br J Nutr. 2002;87:471–5 [DOI] [PubMed] [Google Scholar]
- 150.Henderson L, Irving K, Gregory J, Bates CJ, Prentice A, Perks J, Swan G, Farron M. The National Diet & Nutrition Survey: adults aged 19 to 64 years. Vitamin and mineral intake and urinary analytes. Vitamin and mineral intake and urinary analytes. London: HMSO; 2000 [Google Scholar]
- 151.Allen LH, Haskell M. Estimating the potential for vitamin A toxicity in women and young children. J Nutr. 2002;132:S2907–19 [DOI] [PubMed] [Google Scholar]
- 152.West CE, Eilander A, van Lieshout M. Consequences of revised estimates of carotenoid bioefficacy for dietary control of vitamin A deficiency in developing countries. J Nutr. 2002;132:S2920–6 [DOI] [PubMed] [Google Scholar]
- 153.Borel P, Grolier P, Mekki N, Boirie Y, Rochette Y, LeRoy B. Alexandre Gouabau MC, Lairon D, Azais Braesco V. Low and high responders to pharmacological doses of beta-carotene: proportion in the population, mechanisms involved and consequences on beta-carotene metabolism. J Lipid Res. 1998;39:2250–60 [PubMed] [Google Scholar]
- 154.Edwards AJ, You CS, Swanson JE, Parker RS. A novel extrinsic reference method for assessing the vitamin A value of plant foods. Am J Clin Nutr. 2001;74:348–55 [DOI] [PubMed] [Google Scholar]
- 155.Albanes D, Heinonen OP, Huttunen JK, Taylor PR, Virtamo J, Edwards BK, Haapakoski J, Rautalahti M, Hartman AM, et al. Effects of alpha-tocopherol and beta-carotene supplements on cancer incidence in the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study. Am J Clin Nutr. 1995;62:S1427–30 [DOI] [PubMed] [Google Scholar]
- 156.Berner LA, Clydesdale FM, Douglass JS. Fortification contributed greatly to vitamin and mineral intakes in the United States, 1989–1991. J Nutr. 2001;131:2177–83 [DOI] [PubMed] [Google Scholar]
- 157.Strobel M, Tinz J, Biesalski HK. The importance of beta-carotene as a source of vitamin A with special regard to pregnant and breastfeeding women. Eur J Nutr. 2007;46 Suppl 1:I1–20 [DOI] [PubMed] [Google Scholar]
- 158.Feskanich D, Singh V, Willett WC, Colditz GA. Vitamin A intake and hip fractures among postmenopausal women. JAMA. 2002;287:47–54 [DOI] [PubMed] [Google Scholar]