Abstract
Dietary supplementation with whole blueberries in a preclinical study resulted in a reduction in glucose concentrations over time. We sought to evaluate the effect of daily dietary supplementation with bioactives from blueberries on whole-body insulin sensitivity in men and women. A double-blinded, randomized, and placebo-controlled clinical study design was used. After screening to resolve study eligibility, baseline (wk 0) insulin sensitivity was measured on 32 obese, nondiabetic, and insulin-resistant subjects using a high-dose hyperinsulinemic-euglycemic clamp (insulin infusion of 120 mU(861 pmol)⋅m−2⋅min−1). Serum inflammatory biomarkers and adiposity were measured at baseline. At the end of the study, insulin sensitivity, inflammatory biomarkers, and adiposity were reassessed. Participants were randomized to consume either a smoothie containing 22.5 g blueberry bioactives (blueberry group, n = 15) or a smoothie of equal nutritional value without added blueberry bioactives (placebo group, n = 17) twice daily for 6 wk. Both groups were instructed to maintain their body weight by reducing ad libitum intake by an amount equal to the energy intake of the smoothies. Participants’ body weights were evaluated weekly and 3-d food records were collected at baseline, the middle, and end of the study. The mean change in insulin sensitivity improved more in the blueberry group (1.7 ± 0.5 mg⋅kg FFM−1⋅min−1) than in the placebo group (0.4 ± 0.4 mg⋅kg FFM−1⋅min−1) (P = 0.04). Insulin sensitivity was enhanced in the blueberry group at the end of the study without significant changes in adiposity, energy intake, and inflammatory biomarkers. In conclusion, daily dietary supplementation with bioactives from whole blueberries improved insulin sensitivity in obese, nondiabetic, and insulin-resistant participants.
Introduction
Increased consumption of berries has been shown to improve cognitive function, risk of cardiovascular disease, and cancer (1, 2). Studies have also reported that specific berries, i.e., blueberries, have antidiabetic effects. Specifically, a study performed in mice (3) found that supplementation with whole blueberries reduced the blood glucose area under the curve (AUC)5 in vivo and cell culture studies (4, 5) demonstrated increased glucose uptake in vitro (6). In addition, inflammatory genes have been reduced in mice after consuming blueberry bioactives, which suggests an antiinflammatory response (3). The purported health benefits from blueberries have been attributed to their phenolic bioactive compounds, such as anthocyanins, which also have antioxidant properties (6–8).
Given the concern regarding the ability to greatly increase and maintain an individual’s fruit and vegetable consumption over a long-term period (9), the role of dietary supplementation with bioactive components in blueberries remains a very attractive and feasible daily dietary intervention. To the best of our knowledge, there is no human research that has reported on the efficacy of increased blueberry bioactive consumption on insulin sensitivity by using the hyperinsulinemic-euglycemic clamp technique (10), which is the gold standard for measuring in vivo insulin action. Therefore, this project’s overall objective was to examine the role of dietary supplementation with bioactives in freeze-dried whole blueberry powder on insulin action in vivo with the use of hyperinsulinemic-euglycemic clamps in individuals who were obese, nondiabetic, and insulin resistant. We hypothesized that increased daily consumption of blueberry bioactives, based on preclinical data, would be effective in increasing insulin action in vivo and ultimately result in improved insulin sensitivity in a human population at high risk for type 2 diabetes.
Subjects and Methods
Subjects.
Participants in the study were recruited from the Greater Baton Rouge area. A total of 32 men and women completed all evaluations (Supplemental Fig. 1). Those included were adults (≥20 y old), obese (BMI between 32 and 45 kg/m2), and insulin resistant (nondiabetic). The exclusion criteria included: 1) diabetes; no diabetes status was confirmed by a 2-h oral glucose tolerance test; 2) medications known to affect glucose metabolism; 3) untreated thyroid or chronic liver, renal, or cardiovascular disease; 4) a history of drug and/or alcohol abuse, or psychiatric disease prohibiting adherence to study protocol; 5) history of allergic reactions to blueberries; 6) consuming berries, grapes, and wine >3 times/wk; and 7) fluctuation in body weight > 5% in the preceding 2 mo. The Institutional Review Board for human subjects at Pennington Biomedical Research Center reviewed and approved the study protocol. All participants gave written consent prior to starting the study.
Study design.
This study design was double blinded, placebo-controlled, and randomized. All study evaluations and measurements were performed on participants that had fasted for 10 h. A week was defined as 7 d (± 2 d).
Clinical intervention and source of whole blueberry bioactives.
The freeze-dried whole blueberry powder was prepared by the United States Highbush Blueberry Council (USDA oversight). The whole blueberry powder was made from a 50/50 mixture of 2 varieties of highbush blueberries, Tifblue (Vaccinium ashei) and Rubel (Vaccinium corymbosum). The whole blueberries were freeze-dried, milled, and stored in aluminum cans under nitrogen. Based on the compositional analysis, the 45 g of blueberry powder contained 1462 mg of total phenolics, 668 mg of anthocyanins, and 16.02 mmol TE of antioxidants (oxygen radical absorbance capacity). Also, the 45 g of blueberry powder that was provided to the participants equated to an amount of bioactives in ∼2 cups of fresh whole blueberries.
After the participants were assessed as being insulin resistant (glucose disposal rate ≤ 650 mg/min), they were randomized to receive twice daily a smoothie with blueberry bioactives added or an identical smoothie without blueberry bioactives (i.e., placebo) (Supplemental Table 1). The participants were instructed to consume 1 smoothie at breakfast meals and the other smoothie at dinner meals (at least 6 h apart). The smoothies were prepared in the metabolic kitchen and a week’s supply of frozen smoothies was provided in a cooler for the participants to pick up at each weekly visit. Participants were instructed to keep the smoothies frozen, thaw them in the refrigerator, avoid exposing them to direct heat, and avoid adding any other ingredients to them. For study compliance, the participants verbally reported their smoothie consumption to the dietitian at each visit. A compliance of >75% was mandatory for continued participation in the study.
Physiologic assessments.
Hyperinsulinemic-euglycemic clamps (10) were performed to assess insulin sensitivity after a 10-h fast. Participants were admitted into the inpatient research unit the evening prior to their insulin sensitivity testing day and consumed a eucaloric standardized meal (50% carbohydrates, 35% fat, and 15% protein). The next morning, an i.v. catheter was placed in an antecubital vein for infusion of insulin and glucose. A second catheter was inserted in a dorsal vein of the contralateral arm for blood withdrawal. The hand was placed between a heating pad for arterialization of venous blood sampling. During the 45 min prior to the clamp, blood samples were collected every 15 min for glucose and insulin. Then insulin was administered at a primed-continuous infusion rate of 120 mU(861 pmol)⋅m−2⋅min−1 for 2 h and blood samples were collected every 5 min for glucose and every 15 min for insulin during this period. Serum insulin was measured by a Siemens Immulite 2000 using immunoassay with chemiluminescent. A variable infusion of dextrose (20% solution) was given to maintain serum glucose concentrations at ~5.6 mmol/L (100 mg/dL). Arterialized serum glucose was measured using a YSI 2300 Stat Plus glucose analyzer (model no. 2300 STAT Plus D) and Beckman Coulter DXC600. During the steady state (last 30 min of clamp), the mean rate of exogenous glucose infusion was corrected for changes in glycemia and divided by fat-free mass to assess insulin sensitivity.
Body weight/fat distribution.
Fat-free mass, fat mass, and body fat percentage were measured by dual-energy X-ray absorptiometry with CV for measurements assessed at 0.6, 1.1, and 1.1%, respectively. Overall, biologic, instrument, and reader variability was assessed at ∼10%.
Serum inflammatory biomarkers and lipids.
During the baseline of the clamp, blood was collected for measuring serum inflammatory biomarkers, including high sensitivity C-reactive protein (hsCRP), tumor necrosis factor-α (TNFα), and monocyte chemoattractant protein 1 (MCP-1). TNFα and MCP-1 were measured on a Luminex system using kits from Millipore. High sensitivity C-reactive protein was measured by automated immunoassay as assessed on a Siemens 2000 instrument. In addition, the serum lipid profile was measured (triglycerides, total cholesterol, LDL-cholesterol, and HDL-cholesterol). Triglycerides and total cholesterol were measured by using a Beckman Coulter DXC600 and HDL-cholesterol was measured by using a Trinity DXC600. LDL-cholesterol was based on a calculation [cholesterol − (1/5 triglycerides) – HDL].
Food records and questionnaires.
At the screening visits, a registered dietitian instructed participants to record a detailed 3-d food record (i.e., 2 weekdays and 1 weekend day). Participants were asked to provide labels and/or recipes for accuracy of the food records. The dietitian reviewed the food records for accuracy and completeness. Based on their eating patterns and usual intake, participants were counseled by the dietitian on ways to remove ∼2000 kJ/d (500 kcal/d) from their daily intake to compensate for the energy consumed in the blueberry and placebo smoothies. Food records were also administered at the midpoint and end of the study. The food records were analyzed using the Pennington Biomedical Research Center’s Food Diary Program (Pennington Biomedical Research Foundation). Participants were asked to maintain their current body weight and physical activity or they would be eliminated from the study. The participants’ body weights were measured weekly to monitor weight maintenance. A change of ≥1 kg of body weight was addressed by the dietitian and proper counseling was provided. They also reported adverse events and changes in medication during the study.
The smoothie rating and fruit/wine questionnaires were also used in the study. Before starting the study, participants were given the opportunity to taste the smoothie for acceptability. The fruit/wine questionnaire was administered at each visit as a reminder to abstain from berries, grapes, juices that contained berries and grapes, and wine throughout the study. The rationale for these questionnaires was to eliminate consumption of anthocyanin-containing foods and drinks.
Statistical analysis.
All analyses were performed using SAS version 9.2. Repeated-measures ANOVA with week as the repeated factor was used to compare the blueberry with placebo groups. Differences between the blueberry and placebo baseline characteristics were analyzed by a 2-sample t test (continuous data) and within groups analyzed by a paired t-test. Categorical data were summarized as counts and analyzed by chi-square tests. Nutritional value of food intake was analyzed by mixed-model ANOVA. P ≤ 0.05 indicated a significant difference between the groups. Data were expressed as means ± SEM.
Results
At baseline, the groups did not differ in age, body composition, lipid profile, blood pressure, and inflammatory biomarkers (Table 1).
TABLE 1.
Anthropometrics and serum biochemistry of obese, insulin-resistant participants before (pre) and after (post) the blueberry and placebo treatments1
| Blueberry |
Placebo |
|||
| Variables | Pre | Post | Pre | Post |
| Race (African American/Caucasian), n/n | 8/7 | — | 8/9 | — |
| Gender (male/female), n/n | 2/13 | — | 3/14 | — |
| Age, y | 54 ± 3 | — | 49 ± 3 | — |
| Body weight, kg | 98.7 ± 3.1 | 99.1 ± 3.1 | 102.9 ± 3.4 | 103.4 ± 3.5 |
| BMI, kg/m2 | 36.8 ± 0.9 | 37.0 ± 0.9 | 38.0 ± 0.9 | 38.2 ± 1.0 |
| Body fat, % | 40.9 ± 1.3 | 40.9 ± 1.3 | 42.5 ± 1.4 | 42.8 ± 1.4 |
| Fat mass, kg | 40.8 ± 2.0 | 40.8 ± 2.0 | 44.2 ± 2.3 | 44.7 ± 2.3 |
| Lean mass, kg | 58.7 ± 2.1 | 58.7 ± 2.1 | 59.2 ± 2.0 | 59.4 ± 2.1 |
| Systolic blood pressure, mm Hg | 116.9 ± 3.2 | 115.2 ± 3.2 | 122.6 ± 3.7 | 118.5 ± 3.2 |
| Diastolic blood pressure, mm Hg | 73.5 ± 2.3 | 73.2 ± 1.9 | 75.7 ± 1.9 | 76.6 ± 2.1 |
| Serum biochemistry2 | ||||
| Glucose, mmol/L | 5.7 ± 0.1 | 5.7 ± 0.1 | 5.9 ± 0.1 | 5.9 ± 0.1 |
| Insulin, pmol/L | 132 ± 15 | 140 ± 17 | 142 ± 15 | 148 ± 16 |
| Triglycerides, mmol/L | 1.53 ± 0.18 | 1.66 ± 0.17 | 1.44 ± 0.21 | 1.67 ± 0.26 |
| Cholesterol, mmol/L | 5.34 ± 0.21 | 4.76 ± 0.24 | 5.18 ± 0.19 | 4.65 ± 0.18 |
| LDL cholesterol, mmol/L | 3.28 ± 0.21 | 2.88 ± 0.19 | 3.22 ± 0.18 | 2.84 ± 0.17 |
| HDL cholesterol, mmol/L | 1.35 ± 0.08 | 1.12 ± 0.06 | 1.30 ± 0.07 | 1.05 ± 0.06 |
| C-reactive protein, mg/L | 5.3 ± 1.3 | 6.9 ± 1.8 | 6.9 ± 1.1 | 8.5 ± 1.9 |
| TNFα, ng/L | 7.4 ± 1.5 | 6.2 ± 1.0 | 11.5 ± 4.3 | 6.5 ± 0.5 |
| MCP-1, ng/L | 358 ± 37 | 377 ± 44 | 401 ± 58 | 396 ± 38 |
Values are means ± SEM, = 15 (blueberry) or 17 (placebo) except TNFα, where n = 11 or 13, respectively.
Blood was drawn from participants after a 10-h fast.
Energy intake, body composition, and metabolic variables.
Throughout the study, the groups did not differ in energy and macronutrient (protein, carbohydrate, and fat) consumption (data not shown) or in body weight or adiposity (Table 1). In addition, the inflammatory biomarkers, lipid profile, and blood pressure did not differ between the study groups from the beginning to the end of the study (Table 1). None of these variables changed within each group during the treatment period (Table 1).
Insulin sensitivity.
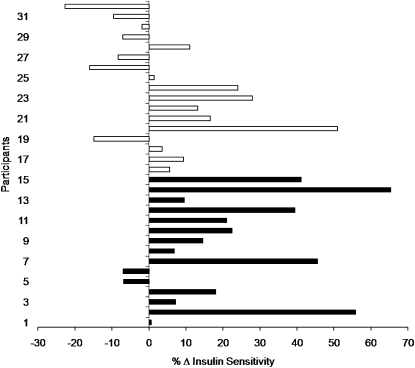
When evaluating the percent change of insulin sensitivity, 67% of the participants (10 of 15) randomized to the blueberry group had at least a 10% or greater favorable change in insulin sensitivity, whereas only 41% of the placebo participants (7 of 17) demonstrated this change (Fig. 1). The mean change in insulin sensitivity was improved significantly more in the blueberry group compared to the placebo group (Fig. 2). Also, the percent change in insulin sensitivity was greater in the blueberry group (22.2 ± 5.8%) than in the placebo group (4.9 ± 4.5%) (P = 0.02).
FIGURE 1.
Percent change in insulin sensitivity in individual obese, insulin-resistant men and women who consumed the blueberry (black bars) or placebo (white bars) smoothies for 6 wk. % Δ = [(postintervention − preintervention)/preintervention] × 100. Values are means ± SEM, n = 15 (blueberry) or 17 (placebo).
FIGURE 2.
Mean change in insulin sensitivity in the obese, insulin-resistant men and women who consumed either the blueberry or placebo smoothies for 6 wk. Δ = postintervention − preintervention. Values are means ± SEM, n = 15 (blueberry) or 17 (placebo).
Discussion
To our knowledge, this is the first reported human study that evaluated the effect of daily dietary supplementation with bioactives in blueberries on whole-body insulin sensitivity in obese, nondiabetic, and insulin-resistant men and women. The uniqueness of this study relates to the design, which was randomized, double blinded, and placebo controlled. By design, the blueberry and placebo smoothies were identical in physical appearance and macronutrient content with the exception of adding the blueberry bioactives to the blueberry smoothie. Another strength of the study was the use of the most precise metabolic technique for assessing whole-body insulin sensitivity, i.e., hyperinsulinemic-euglycemic clamps. The major finding was that daily consumption of whole blueberry bioactives for 6 wk improved insulin sensitivity in a population at high risk for type 2 diabetes compared with ad libitum dietary intake alone.
Consumption of smoothies (in the case of this study, bioactives in blueberries) may be a more attractive and convenient dietary approach for those adults who do not consume the recommended daily amounts of fruits and vegetables. In the current study, we made sure that the energy in the smoothies did not contribute to any body weight gain. Specifically, our study dietitian worked with the participants during the weekly visits to eliminate 2000 kJ/d (1000 kJ/smoothie) from their diets to compensate for the energy provided by the smoothies. As such, the participants were able to maintain a constant body weight throughout the study. The observation that insulin sensitivity increased without a change in body weight suggests that the blueberry bioactives had a direct effect on increasing whole-body insulin action.
The current study evaluated the synergistic effect of all the bioactive compounds in blueberries. Limited data exist on using whole blueberries as the intervention. In a previous preclinical study, DeFuria et al. (3) used a comparable dose of an identical freeze-dried whole blueberry powder and observed similar health effects to the current clinical trial. The study showed that mice who consumed a high-fat diet with blueberries for 8 wk had a lower plasma glucose AUC during a 90-min intraperitoneal insulin tolerance test compared with the mice fed the high-fat diet alone. Plasma insulin concentrations were unchanged. These results suggest that blueberries improved the high-fat diet–induced hyperglycemia. However, Prior et al. (11) found that freeze-dried whole blueberry powder did not affect the plasma glucose AUC during a 120-min intraperitoneal glucose tolerance test in high-fat diet–induced obese mice. Perhaps the null finding was due to the type of freeze-dried blueberry powder used in the experiment, which was different from the current and previous (3) studies or the specific technique used could have potentially lacked the precision to adequately assess carbohydrate metabolism.
It is well established that any change in adiposity can greatly alter whole-body insulin sensitivity (12). In the current study, body weight was kept constant throughout the study, so that it would not be a confounding factor that contributed to the improved insulin sensitivity. Furthermore, participants were instructed not to alter their physical activity during the study. Even after controlling for certain variables, as expected for human studies, there was variability in insulin sensitivity values for both treatment groups. However, compared with the placebo group overall, insulin sensitivity improved significantly more in the blueberry group without any changes in body weight, adiposity, or energy intake. Also, no changes in body composition were observed in diet-induced obese mice fed whole blueberries (3). Another study (11) found the opposite in that whole blueberry supplementation increased body weight and adiposity in mice that were fed a high-fat diet with added blueberries compared with mice fed only a high-fat diet. The increase in the body weight and adiposity of the mice throughout the study could have potentially affected the outcome of unobserved improvements in glucose tolerance with whole blueberry supplementation, as discussed previously.
Emerging data have clearly linked inflammation to adiposity with significant reports on the mechanisms by which inflammation at a whole-body level attenuates insulin action (13). Specifically, DeFuria et al. (3) found that supplementing obese mice with blueberries reduced the gene expression for inflammatory biomarkers TNFα and interleukin-10. Unfortunately, significant changes were not observed in all the measured inflammatory biomarkers (MCP-1, interleukin-6, and inducible nitric oxide synthase). In the current study, consumption of the daily dose of bioactives in blueberries did not alter the participants’ inflammatory biomarker profile, which consisted of hsCRP, TNFα, and MCP-1. The previous study (3) and current study cannot be compared because of the different research species and evaluations of inflammatory biomarkers [gene expression (3) vs. serum (current study)].
Given the enhanced insulin sensitivity in the group randomized to the blueberry bioactives, a determination of insulin-dependent or -independent signaling pathways in muscle would provide a cellular basis contributing to the understanding of the clinical effect. However, muscle biopsies were not obtained in the current study and cellular mechanisms were not evaluated. Some may view this as a study limitation, but we did evaluate whole-body insulin sensitivity, which is a critical step before evaluating cellular mechanisms. Furthermore, an in vitro study showed (4) that 21-h incubation of the blueberry extract in muscle cells enhanced glucose uptake only in the presence of insulin. Another study (5) found that 6-h treatment of fermented blueberry juice with and without insulin increased glucose uptake into the muscle and adipocyte cells. However, the nonfermented blueberry juice had no effect on glucose uptake. The fermented blueberry juice also increased the phosphorylation/activation of proteins in the insulin-independent pathway (i.e., AMP-activated protein kinase) and did not phosphorylate/activate proteins in the insulin-dependent pathway (i.e., AKT and ERK1/2). These results suggest that the addition of fermented blueberry bioactives increased glucose uptake into the cells in an insulin-independent mechanism. More cellular mechanistic studies are warranted to elucidate the specific cellular pathway involved in the improvement of insulin sensitivity that was observed when blueberries were consumed in our study.
In conclusion, our double-blinded and placebo-controlled study showed that daily dietary supplementation of bioactives in freeze-dried whole blueberry powder improved insulin sensitivity over 6 wk in obese, nondiabetic, and insulin-resistant participants. The bioactives in blueberries enhanced insulin sensitivity independent of any changes in inflammatory biomarkers or adiposity. This study is not conclusive, but it strongly suggests a need to further explore the cellular mechanism for the effect. In addition, our study suggests the need for studies of longer duration that will evaluate blueberries and their potential role in improving insulin sensitivity in an insulin-resistant human population.
Supplementary Material
Acknowledgments
A.J.S. designed research, conducted research, collected and analyzed the data, and wrote the manuscript; C.M.C. and K.C.C. designed dietary research, conducted dietary research, and collected and analyzed dietary data; W.D.J. performed statistical analysis; and W.T.C. was the principal investigator who designed research and had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported in part by the NIH training grant T32 AT004094 (supporting A.J.S.), by the United States Highbush Blueberry Council, and P50AT002776-01 from the National Center for Complementary and Alternative Medicine and the Office of Dietary Supplements (W.T.C.), which funds the Botanical Research Center of Pennington Biomedical Research Center and The Biotech Center of Rutgers University. This project used facilities that are supported in part by Centers of Biomedical Research Excellence (NIH P20-RR021945) and Clinical Nutrition Research Unit (NIH 1P30-DK072476) center grants from the NIH.
This trial was registered at clinicaltrials.gov as NCT01005420.
Supplemental Figure 1 and Table 1 are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: AUC, area under the curve; hsCRP, high sensitivity C-reactive protein; MCP-1, monocyte chemoattractant protein 1; TNFα, tumor necrosis factor-α.
Literature Cited
- 1.Bagchi D, Sen CK, Bagchi M, Atalay M. Anti-angiogenic, antioxidant, and anti-carcinogenic properties of a novel anthocyanin-rich berry extract formula. Biochemistry (Mosc). 2004;69:75–80, 1 p preceding 75 [DOI] [PubMed] [Google Scholar]
- 2.Papandreou MA, Dimakopoulou A, Linardaki ZI, Cordopatis P, Klimis-Zacas D, Margarity M, Lamari FN. Effect of a polyphenol-rich wild blueberry extract on cognitive performance of mice, brain antioxidant markers and acetylcholinesterase activity. Behav Brain Res. 2009;198:352–8 [DOI] [PubMed] [Google Scholar]
- 3.DeFuria J, Bennett G, Strissel KJ, Perfield JW II, Milbury PE, Greenberg AS, Obin MS. Dietary blueberry attenuates whole-body insulin resistance in high fat-fed mice by reducing adipocyte death and its inflammatory sequelae. J Nutr. 2009;139:1510–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martineau LC, Couture A, Spoor D, Benhaddou-Andaloussi A, Harris C, Meddah B, Leduc C, Burt A, Vuong T, et al. Anti-diabetic properties of the Canadian lowbush blueberry Vaccinium angustifolium Ait. Phytomedicine. 2006;13:612–23 [DOI] [PubMed] [Google Scholar]
- 5.Vuong T, Martineau LC, Ramassamy C, Matar C, Haddad PS. Fermented Canadian lowbush blueberry juice stimulates glucose uptake and AMP-activated protein kinase in insulin-sensitive cultured muscle cells and adipocytes. Can J Physiol Pharmacol. 2007;85:956–65 [DOI] [PubMed] [Google Scholar]
- 6.Youdim KA, Shukitt-Hale B, MacKinnon S, Kalt W, Joseph JA. Polyphenolics enhance red blood cell resistance to oxidative stress: in vitro and in vivo. Biochim Biophys Acta. 2000;1523:117–22 [DOI] [PubMed] [Google Scholar]
- 7.Hosseinian FS, Beta T. Saskatoon and wild blueberries have higher anthocyanin contents than other Manitoba berries. J Agric Food Chem. 2007;55:10832–8 [DOI] [PubMed] [Google Scholar]
- 8.Faria A, Oliveira J, Neves P, Gameiro P, Santos-Buelga C, de Freitas V, Mateus N. Antioxidant properties of prepared blueberry (Vaccinium myrtillus) extracts. J Agric Food Chem. 2005;53:6896–902 [DOI] [PubMed] [Google Scholar]
- 9.Blanck HM, Gillespie C, Kimmons JE, Seymour JD, Serdula MK. Trends in fruit and vegetable consumption among U.S. men and women, 1994–2005. Prev Chronic Dis. 2008;5:A35. [PMC free article] [PubMed] [Google Scholar]
- 10.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–23 [DOI] [PubMed] [Google Scholar]
- 11.Prior RL, Wu X, Gu L, Hager TJ, Hager A, Howard LR. Whole berries versus berry anthocyanins: interactions with dietary fat levels in the C57BL/6J mouse model of obesity. J Agric Food Chem. 2008;56:647–53 [DOI] [PubMed] [Google Scholar]
- 12.Reaven GM. Insulin resistance: the link between obesity and cardiovascular disease. Endocrinol Metab Clin North Am. 2008;37:581–601 [DOI] [PubMed] [Google Scholar]
- 13.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.