Abstract
The cytotoxic marine red algal metabolite thyrsiferol (1) was found to inhibit hypoxia-induced hypoxia-inducible factor-1 (HIF-1) activation in T47D human breast tumor cells (66% inhibition at 3 μM). Compound 1 also suppressed hypoxic induction of HIF-1 target genes (VEGF, GLUT-1) at the mRNA level, and displayed tumor cell line-selective time-dependent inhibition of cell viability/proliferation. Mechanistic studies revealed that 1 selectively suppressed mitochondrial respiration at Complex I (IC50 3 μM). Thyrsiferol represents a prototypical, structurally unique electron transport chain inhibitor. The apparent rotenone-like activity may contribute to the observed cytotoxicity of 1 and play an important role in Laurencia chemical defense.
Keywords: Thyrsiferol; Hypoxia-inducible factor-1 (HIF-1); Laurencia, Triterpene polyether; Marine natural product; Mitochondrial complex I inhibitor; NADH-ubiquinone oxidoreductase
1. Introduction
Thyrsiferol (1) was first isolated from the tropical marine red alga Laurencia thyrsifera J. Agardh (Rhodomelaceae) (Blunt et al., 1978), and was subsequently found to display potent cytotoxic activity against P-388 mouse lymphocytic leukemia and moderate activity against human solid tumor cell lines (Fernández et al., 2000). Thyrsiferol 23-acetate exhibited moderately selective serine/threonine protein phosphatase type 2A (PP2A) inhibitory activity (Matsuzawa et al., 1994; McCluskey et al., 2002). Dehydrothyrsiferol has been shown to be moderately cytotoxic to human breast tumor cell lines (Pec et al, 1999) and MDR+ (P-glycoprotein)-expressing epidermoid carcinoma KB cell lines (Pec et al., 1998). The structural uniqueness and potential anticancer activity of 1 and its analogues have inspired numerous projects aimed at their total synthesis (González and Forsyth, 1999; McDonald and Wei, 2002; Nishiguchi and Little, 2005; Nishiguchi et al., 2006, Hioki et al., 2009, etc.). However, the mechanism(s) of action for 1 to suppress tumor cells has remained unknown.
2. Results and discussion
Thyrsiferol (1) was evaluated as part of an ongoing molecular-targeted antitumor drug discovery program aimed at the discovery of natural product-derived inhibitors of hypoxiainducible factor-1 (HIF-1) (Nagle and Zhou, 2009). The transcription factor HIF-1 regulates cellular adaptation to hypoxia by activating the expression of genes that increase oxygen availability and genes that suppress oxygen consumption (Semenza 2009). Dysregulation of HIF-1 plays an important role in both the etiology and progression of cancer (Semenza 2009). Compound 1 was evaluated in a human breast tumor T47D cell-based reporter assay for HIF-1 inhibitory activity (Hodges et al., 2004). Compound 1 blocked hypoxia-induced HIF-1 activation at single-digit micromolar concentrations (Fig. 1a), but 1 was about 10-fold less potent at inhibiting chemical hypoxia (10 μM 1,10-phenanthroline)-activated HIF-1.
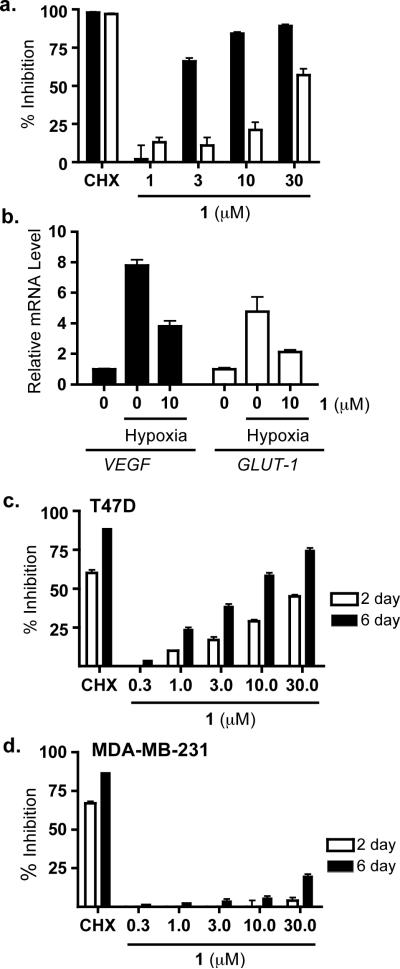
Figure 1.
Compound 1 Downregulates Hypoxia-Activated HIF-1 Signaling and Exhibits Cell Line- and Time-Dependent Inhibition of Cell Viability/Proliferation. (a) Concentration-dependent inhibition of hypoxia (1% O2, 16 h, ■) and chemical hypoxia (10 μM 1,10-phenanthroline, 16 h, □)-induced HIF-1 activation in a T47D cell-based reporter assay. Compound 1 was tested at the specified concentrations. The protein translation inhibitor cycloheximide (100 μM) was included as a positive control (CHX). Data shown are average + standard deviation from one representative experiment performed in triplicate. (b) Compound 1 inhibited hypoxic induction of HIF-1 target genes VEGF and GLUT-1 at the mRNA level. T47D cells were exposed to hypoxic conditions (1% O2, 16 h) in the presence and absence of compound 1. Cells cultured under normoxic conditions were included as an untreated normoxic control. Total RNA samples were isolated from the cells. The levels of VEGF and GLUT-1 mRNAs in each sample were quantified by real time RT-PCR, normalized to an internal control (18S rRNA), and presented as relative mRNA level of the normoxic control. (mean + SD, n = 3). (c) Compound 1 inhibited T47D cell proliferation/viability in a concentration- and time-dependent manner. T47D cells were exposed to compound 1 at the specified concentrations for 48 h (2 day, □) or 144 h (6 day, ■). Cycloheximide was tested at 100 μM as a positive control (CHX). For the 6 day exposure study, the conditioned media were replaced with fresh media that contained compounds after 3 days. Cell viability was determined using the SRB method and presented as % Inhibition of the untreated control. Data shown are averages from one representative experiment performed in triplicate and the bars represent standard deviation. (d) Compound 1 did not exert significant effects on the proliferation/viability of MDA-MB-231 cells. Experimental conditions and data presentation were the same as those described in (c).
Compound 1 was evaluated for its effects on the hypoxic induction of HIF-1 target genes VEGF (vascular endothelial growth factor) and GLUT-1 (glucose transporter-1) by real time RT-PCR. Following exposure to hypoxic conditions (1% O2, 16 h), T47D cells increased the expression of VEGF and GLUT-1 at the mRNA level and 1 suppressed the hypoxic induction of HIF-1 target genes in a concentration-dependent manner (Fig. 1b).
Most standard tumor cell cytotoxic assays are performed following a 48 h compound treatment and incubation period. However, extended exposure times (i.e., 6 d) have been reported to be required to more accurately measure the effects of mitochondrial inhibitors on cell proliferation/viability (McLaughlin, 2008). Therefore, the effects exerted by 1 on tumor cell proliferation/viability were examined in two genetically distinct breast tumor cell lines using a standard two-day incubation period and an extended six-day exposure time course (Figs. 1c and 1d). The concentration-response studies were compared to determine whether 1 required increased incubation times to exhibit antiproliferative activity. The T47D cells were significantly more sensitive to the anti-proliferative effects of 1 than the MDA-MB-231 cells were, and the extent of inhibition was exposure time-dependent.
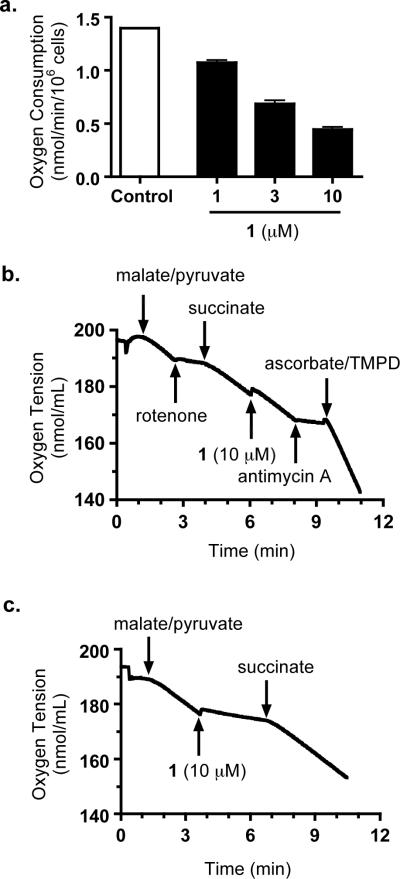
Compounds that disrupt the mitochondrial electron transport chain (ETC) have been shown to selectively suppress hypoxia-induced HIF-1 activation (Klimova and Chandel, 2008; Nagle and Zhou, 2009). To investigate if 1 inhibits mitochondrial function, a T47D cell-based respiration study was performed (Liu et al., 2009). Compound 1 suppressed oxygen consumption in a concentration-dependent manner (Fig. 2a). The effective concentrations for 1 to suppress oxygen consumption were essentially the same as those required to inhibit hypoxia-induced HIF-1 activation. In order to discern the specific site within the mitochondrial ETC that is affected by 1, further mechanistic studies were performed. These experiments were designed to investigate if 1 acts as an inhibitor of mitochondrial ETC complex I, II, III, or IV. Addition of a mixture of malate and pyruvate to digitonin-permeabilized T47D cells started respiration by initiating NADH production, thereby providing a source of electrons for NADH-ubiquinone oxidoreductase (complex I) (Fig. 2b). The electrons then pass through a series of electron carriers along the ETC to oxygen, which serves as the final electron acceptor. In general, the overall level of respiration correlates with the rate of oxygen consumption. Complex I inhibitors, such as rotenone, suppressed respiration, and thus produced a marked decrease in the oxygen consumption rate (Fig. 2b). Succinate overcame complex I inhibitor-imposed respiration blockade by providing respiratory chain complex II (succinate-ubiquinone oxidoreductase) with an alternative source of electrons. The observation that 1 (10 μM) failed to inhibit respiration in the presence of succinate suggests that 1 does not inhibit complex II, III, or IV (Fig. 2c). However, antimycin A [an inhibitor of complex III (ubiquinol-cytochrome c oxidoreductase)] suppressed respiration in the presence of succinate. A mixture of ascorbate and TMPD (N,N,N',N'-tetramethyl-p-phenylenediamine) that serves as an electron source for cytochrome c and hence for complex IV (cytochrome c oxidase) caused a resumption of respiration that was specifically blocked at complex III by antimycin. These observations indicate that 1 does not affect complexes II, III, or IV of the ETC. To test the hypothesis that 1 inhibits mitochondrial respiration by selectively targeting complex I, 1 (10 μM) was added to permeabilized T47D cells following the initiation of respiration by a mixture of malate/pyruvate. Compound 1 suppressed respiration and this inhibition was subsequently overcome by the complex II substrate succinate (Fig. 2c). These data indicate that 1 suppresses mitochondrial respiration by selectively inhibiting ETC complex I.
Figure 2.
Compound 1 Suppresses Cellular Respiration by Targeting Mitochondrial Electron Transport Chain Complex I. (a) Compound 1 inhibited cellular respiration in T47D cells in a concentration-dependent manner. The “Control” represents the rate of oxygen consumption in untreated T47D cells. Data shown are average (n = 3) + standard deviation. (b) Compound 1 inhibited mitochondrial respiration by targeting complex I. The substrates and test compound were added to digitonin-permeabilized T47D cells at the specified time point in a sequential manner. (c) Compound 1 did not affect mitochondrial ETC complex II, III, or IV.
In normal cells, most cellular oxygen is consumed to produce ATP through the process of oxidative phosphorylation. One model proposes that hypoxic conditions increase mitochondrial reactive oxygen species (ROS) and that these mitochondrial ROS provide a signal that stabilizes HIF-1α protein and activates HIF-1 (Klimova and Chandel, 2008). This study characterized the effects of thyrsiferol (1) on HIF-1 signaling pathway and established a probable mechanism of action for the bioactivities observed for 1. The finding that 1 inhibits mitochondrial function provides an explanation for the differential cytotoxicity observed between the sensitive T47D cells that rely heavily upon mitochondrial oxidative phosphorylation, and the relatively non-responsive MDA-MB-231 cells that are highly glycolytic (Figs. 1 a and 1d). Compound 1 represents the first member of a structurally novel class of mitochondrial ETC complex I inhibitors. Because of its structural uniqueness, 1 may interact with the large 45 polypeptide complex I complex at a site different from rotenone and other natural product-based complex I inhibitors (Hollerhage et al., 2009). The broader ecological importance of this metabolite appears significant, in light of our previous discovery that the related Jamaican red alga L. intricata produces the structurally unique diterpene laurenditerpenol that similarly inhibits HIF-mediated hypoxic signaling by selectively targeting mitochondrial ETC complex I (Mohammed et al., 2004, Chittiboyina et al., 2007). The ecological impact of mitchondrial ETC inhibitor production by macrophytic marine algae is potentially profound. It seems likely that the rotenone-like activity of unusual Laurencia terpenes, such as 1 and laurenditerpenol, may serve to suppress fouling and effectively deter grazing by herbivorous fish and invertebrate species.
3. Experimental section
3.1. Isolation of thyrsiferol (1)
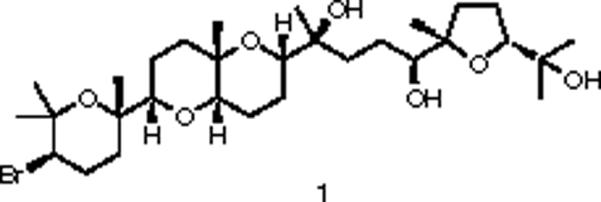
The collection of Laurencia catarinensis and the extraction procedure have been already described (Lhullier et al., 2010). The organic extract (6.9 g) was subjected to vacuum column chromatography on silica gel, using cyclohexane with increasing amounts of EtOAc, followed by EtOAc with increasing amounts of MeOH as the mobile phase, to afford seventeen fractions (A–Q). Fraction P (95% EtOAc, 35 mg) was purified by reversed-phase HPLC, using MeOH/H2O (90:10) as eluant, to afford compound 1 (17.1 mg). The structure and purity of 1 were confirmed by NMR analysis. The 1H (400 MHz) and 13C (50 MHz) NMR spectra were recorded in CDCl3 on Bruker DRX 400 and AC 200 NMR spectrometers, respectively. The spectroscopic and physical characteristics of compound 1 were in agreement with those previously reported in the literature for thyrsiferol (Manzo et al., 2007).
3.2. Cell culture and cell-based reporter and viability assays
Human breast tumor T47D (ATCC) and MDA-MB-231 (ATCC) cells were maintained in DMEM/F12 media with L-glutamine (Mediatech), supplemented with 10% (v/v) fetal bovine serum (FBS, Hyclone), 50 units/mL penicillin and 50 μg/mL streptomycin (Lonza). A previously described cell-based luciferase assay employing the pHRE3-TK-Luc reporter was performed to monitor HIF-1 activity (Hodges et al., 2004). Cell viability was determined using the sulfarhodamine B method (Liu et al., 2009). The conditioned media were replaced by fresh culture medium that contains test compound after three days for the six-day exposure study.
3.3. RNA extraction and quantitative real time RT-PCR
The plating of T47D cells, compound treatment, extraction of total RNA samples, synthesis of the first strand cDNAs, gene-specific primer sequences, quantitative real-time PCR reactions, and data analysis were previously described (Liu et al., 2009).
3.4. Mitochondria respiration assay
An Oxytherm Clarke-type electrode System (Hansatech) was used to monitor the rate of oxygen consumption in T47D cells. A non-permeabilized cell-based respiration assay was performed to determine the effects of purified compounds on cellular respiration (Liu et al., 2009). Mechanistic studies were conducted in plasma membrane permeabilized cells to manipulate the mitochondrial substrates and inhibitors. Detailed experimental procedures and reagents were the same as those already described (Liu et al., 2009).
3.5. Statistical analysis
Data were compared using one-way ANOVA followed by Bonfferoni post hoc analyses (GraphPad Prism 4). Differences were considered statistically significant when p < 0.05.
Acknowledgements
The authors thank S.L. McKnight (UT Southwestern Medical Center at Dallas) for the pHRE-TK-luc construct. This research was supported in part by NIH Grants CA98787 and NOAA/NIUST Grant NA16RU1496. The work at the University of Mississippi was conducted in a facility constructed with NIH Research Facilities Improvement Grant C06 RR-14503-01. M.F. thanks CAPES for a postdoctoral research studies scholarship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blunt JW, Hartshorn MP, McLennan TJ, Munro MHG, Robinson WT, Yorke SC. Thyrsiferol: a squalene-derived metabolite of Laurencia thyrsifera. Tetrahedron Lett. 1978;19:69–72. [Google Scholar]
- Chittiboyina AG, Gundluru MK, Carvalho P, Liu Y, Zhou Y-D, Nagle DG, Avery MA. Total synthesis and absolute configuration of laurenditerpenol: a HIF-1 activation inhibitor. J. Med. Chem. 2007;50:6299–6302. doi: 10.1021/jm7011062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández JJ, Souto ML, Norte M. Marine polyether triterpenes. Nat. Prod. Rep. 2000;17:235–246. doi: 10.1039/a909496b. [DOI] [PubMed] [Google Scholar]
- González IC, Forsyth CJ. Novel synthesis of the C1–C15 polyether domain of the thyrsiferol and venustatriol natural products. Org. Lett. 1999;1:319–322. doi: 10.1021/ol990648k. [DOI] [PubMed] [Google Scholar]
- Hioki H, Motosue M, Mizutani Y, Noda A, Shimoda T, Kubo M, Harada K, Fukuyama Y, Kodama M. Total synthesis of pseudodehydrothyrsiferol. Org. Lett. 2009;11:579–582. doi: 10.1021/ol802600n. [DOI] [PubMed] [Google Scholar]
- Hodges TW, Hossain CF, Kim Y-P, Zhou Y-D, Nagle DG. Molecular-targeted antitumor agents: the Saururus cernuus dineolignans manassantin B and 4-O-demethylmanassantin B are potent inhibitors of hypoxia-activated HIF-1. J. Nat. Prod. 2004;67:767–771. doi: 10.1021/np030514m. [DOI] [PubMed] [Google Scholar]
- Höllerhage M, Matusch A, Champy P, Lombès A, Ruberg M, Oertel WH, Höglinger GU. Natural lipophilic inhibitors of mitochondrial complex I are candidate toxins for sporadic neurodegenerative tau pathologies. Exp. Neurol. 2009;220:133–142. doi: 10.1016/j.expneurol.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Klimova T, Chandel NS. Mitochondrial complex III regulates hypoxic activation of HIF. Cell Death Differ. 2008;15:660–666. doi: 10.1038/sj.cdd.4402307. [DOI] [PubMed] [Google Scholar]
- Lhullier C, Falkenberg M, Ioannou E, Quesada A, Papazafiri P, Horta PA, Schenkel EP, Vagias C, Roussis V. Cytotoxic halogenated metabolites from the Brazilian red alga Laurencia catarinensis. J. Nat. Prod. 2010;73:27–32. doi: 10.1021/np900627r. [DOI] [PubMed] [Google Scholar]
- Liu Y, Veena CK, Morgan JB, Mohammed KA, Jekabsons MB, Nagle DG, Zhou Y-D. Methylalpinumisoflavone inhibits hypoxia-inducible factor-1 (HIF-1) activation by simultaneously targeting multiple mechanisms. J. Biol. Chem. 2009;284:5859–5868. doi: 10.1074/jbc.M806744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzo E, Gavagnin M, Bifulco G, Cimino P, Di Micco S, Ciavatta ML, Guo YW, Cimino G. Aplysiols A and B, squalene-derived polyethers from the mantle of the sea hare Aplysia dactylomela. Tetrahedron. 2007;63:9970–9978. [Google Scholar]
- McCluskey A, Sim AT, Sakoff JA. Serine-threonine protein phosphatase inhibitors: development of potential therapeutic strategies. J. Med. Chem. 2002;14:1151–1175. doi: 10.1021/jm010066k. [DOI] [PubMed] [Google Scholar]
- McDonald FE, Wei X. Concise, regioselective synthesis of the ABC tristetrahydropyran of thyrsiferol and venustatriol. Org. Lett. 2002;4:593–595. doi: 10.1021/ol0171968. [DOI] [PubMed] [Google Scholar]
- McLaughlin JL. Paw paw and cancer: annonaceous acetogenins from discovery to commercial products. J. Nat. Prod. 2008;71:1311–1321. doi: 10.1021/np800191t. [DOI] [PubMed] [Google Scholar]
- Mohammed KA, Hossain CF, Zhang L, Bruick RK, Zhou Y-D, Nagle DG. Laurenditerpenol, a new diterpene from the tropical marine alga Laurencia intricata potently inhibits HIF-1 mediated hypoxic signaling in breast tumor cells. J. Nat. Prod. 2004;67:2002–2007. doi: 10.1021/np049753f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa S, Suzuki T, Suzuki M, Matsuda A, Kawamura T, Mizuno Y, Kikuchi K. Thyrsiferyl 23-acetate is a novel specific inhibitor of protein phosphatase PP2A. FEBS Lett. 1994;356:272–274. doi: 10.1016/0014-5793(94)01281-4. [DOI] [PubMed] [Google Scholar]
- Nagle DG, Zhou Y-D. Marine Natural Products as Inhibitors of Hypoxic Signaling in Tumors. Phytochem. Rev. 2009;8:415–429. doi: 10.1007/s11101-009-9120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiguchi GA, Graham J, Bouraoui A, Jacobs RS, Little RD. 7,11-epi-thyrsiferol: completion of its synthesis, evaluation of its antimitotic properties, and the further development of an SAR model. J. Org. Chem. 2006;71:5936–5941. doi: 10.1021/jo060519z. [DOI] [PubMed] [Google Scholar]
- Nishiguchi GA, Little RD. From C-glycosides to pyranopyrans: an approach to thyrsiferol using titanium(III)-promoted redox couplings. J. Org. Chem. 2005;70:5249–5256. doi: 10.1021/jo050465d. [DOI] [PubMed] [Google Scholar]
- Pec MK, Hellan M, Moser-Their K, Fernández JJ, Souto ML, Kubista E. Inhibitory effects of a novel marine terpenoid on sensitive and multidrug resistant KB cell lines. Anticancer Res. 1998;18:3027–3032. [PubMed] [Google Scholar]
- Pec MK, Moser-Thier K, Fernández JJ, Souto ML, Kubista E. Growth inhibition by dehydrothyrsiferol - a non-Pgp modulator, derived from a marine red alga - in human breast cancer cell lines. Int. J. Oncol. 1999;14:739–743. doi: 10.3892/ijo.14.4.739. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1 inhibitors for cancer therapy: from gene expression to drug discovery. Curr. Pharm. Des. 2009;15:3839–3843. doi: 10.2174/138161209789649402. [DOI] [PubMed] [Google Scholar]