Abstract
Gastric submucosal tumors (SMTs) are a rather frequent finding, occurring in about 0.36% of routine upper GI-endoscopies. EUS has emerged as a reliable investigative procedure for evaluation of these lesions. Diagnostic Endoscopic ultrasonography (EUS) has the ability to differentiate intramural tumors from extraluminal compressions and can also show the layer of origin of gastric SMTs. Tumors can be further characterized by their layer of origin, echo pattern and margin. EUS-risk criteria of their malignant potential are presented, although the emergence of EUS-guided fne needle aspiration (EUS-FNA) has opened new indications for transmural tissue diagnosis and expanded the possibilities of EUS in SMTs of the stomach. Tissue diagnosis should address whether the SMT is a Gastrointestinal stromal tumour (GIST) or another tumor type and evaluate the malignant potential of a given GIST. However, there seems to be a lack of data on the optimal strategy in SMTs suspected to be GISTs with a negative EUS-FNA tissue diagnosis. The current management strategies, as well as open questions regarding their treatment are also presented.
Keywords: Endoscopic ultrasound, Gastric submucosal tumors, EUS-guided fne needle aspiration, Gastrointestinal stromal tumours
INTRODUCTION
Endoscopic ultrasonography (EUS) became a part of clinical practice at the beginning of the eighties and has become an excellent tool for the imaging of the gastrointestinal wall and its surrounding structures. Various studies have highlighted the value of EUS, especially in the diagnosis and staging of gastric diseases. Development of EUS-guided fine needle aspiration (EUS-FNA) in the early nineties broadened the applicability of this method by allowing tissue sampling of lesions within or accessible from the gastrointestinal tract and established EUS as an important tool in the management of patients with gastrointestinal diseases, including those of the stomach.
In this review, we evaluate the role of EUS in the diagnosis and management of gastric submucosal lesions.
PERFORMING EUS IN THE STOMACH: EXAMINATION TECHNIQUE
The variety of echoendoscopes and probes used for endosonography precludes a detailed analysis of instrument types and specifications currently in use. Aspects of EUS-instrumentarium have been recently reviewed[1]. In principal, EUS-imaging is currently performed with radial (360°) or linear echoendoscopes. In their latest version, these scopes are video-endoscopes coupled to electronic ultrasound processors for generation of electronic EUS-images, and are endowed with special features including Doppler, contrast, and harmonic imaging. Standard EUS usually utilizes high ultrasound frequencies, varying between 5 and 20 MHz (with 7.5 MHz being the most commonly used frequency). They produce a high-resolution image in the near field with limited penetration depth, which ranges from 1-2 to 5-6 cm, depending of the ultrasound frequency used. Gastric EUS is performed with the patient in the left lateral position, usually under conscious sedation (mostly with benzodiazepines), sometimes in conjunction with a central analgesic and, more recently, with propofol. The technique is associated with very low complication rates[2].
The transducer in most radial echoendoscopes generates radial images of 360°, which are oriented perpendicular to the shaft axis of the instrument, while linear echoendoscopes produce images directed parallel to the shaft axis of the endoscope, thus allowing for an effective and safe performance of EUS-guided fine-needle aspiration puncture (FNA) when needed. Review of the literature suggests similar performance for both types of endoscopes. However, the authors’ personal experience is that this mainly applies to pancreatobiliary imaging, whereas complete gastric and perigastric scanning appears to be more difficult with linear instruments. Here, radial imaging offers a better overview of the gastrointestinal wall and paramural structures[3-5].
Acoustic coupling of the ultrasonic transducer to the gastrointestinal wall requires application of fluid as interface between the transducer and the wall. This can be achieved by either a water-filled balloon around the instrument tip or by filling of luminal organs with fluid. When performing EUS in the stomach the following scanning principles should be adhered to, in order to avoid artifacts and misinterpretation: (1) Scanning of target lesions should be perpendicular, as oblique scanning may lead to broadening and blurring of normal and pathological structures (and give rise to erroneous diagnoses or overstaging); (2) An adequate focal distance (0.5-1.0 cm, depending on the ultrasonic frequencies) should be kept; and (3) Use of higher frequencies may be help in better visualization of structures and lesions close to the EUS transducer.
The proper technique for gastric EUS-scanning generally includes conventional upper endoscopy initially, to determine the morphology and possibly identify the lesions. This is followed by the echoendoscope, which is positioned at an identified lesion and moved slightly and slowly backward and forward, with fine movements of the instrument tip. Such a technique will help depict the full extent of the lesion and its relation to neighboring organs and structures. Gastric EUS also permits evaluation of the wall-layers of the stomach, analyses of mucosal or submucosal lesions and imaging of perigastric structures. The water-filling method is the most frequently used technique to evaluate the gastric wall. The stomach is initially collapsed by aspiration, followed by introduction of 200-400 mL water into the lumen up to the fundus. The examination is done from the antrum, while the instrument is slowly withdrawn and all parts of the gastric circumference are visualized as far as possible with perpendicular scanning. However, there are challenging aspects in EUS of the stomach, especially in the prepyloric region and the gastric angle where maintaining the water level and the probe scanning perpendicular to the wall can sometimes be hard to achieve. In these cases rotating the patient may help to keep the water level constant, whereas pushing the scope in, pulling it out and then rotating it may help to achieve a perpendicular position[6]. The alternative balloon-inflation method is usually used for rapid screening of submucosal lesions and perigastric structures. The gastrointestinal wall normally consists of 5 distinct layers (Figure 1). The two inner layers (echo-rich and echo-poor) represent the interface/superficial mucosa and deep mucosa/muscularis mucosa. The third, echo-rich, layer corresponds to the submucosa, the fourth (echo-poor) to the muscularis propria, and the fifth (echo-rich) to the serosa, which is usually not easily distinguishable from the surrounding echo-rich tissue. Surrounding organs, vessels, and other structures are important for orientation and for other diagnostic purposes (e.g. tumor infiltration depth). These consist of various organs including the pancreatic body and tail, parts of the liver (especially the left lobe) and parts of the left kidney and spleen, as well as vessels such as the aorta, the vena cava (proximal stomach), the celiac trunk and the splenic and left renal veins. In everyday practice, the water-filling and the balloon-inflation methods can be combined for better imaging. There are no established values for the thickness of normal gastrointestinal wall, but a figure of 2-4 mm is usually considered to be the normal range, as well as a 1:1:1 relation between the mucosa, submucosa, and muscularis propria[6,7].
Figure 1.
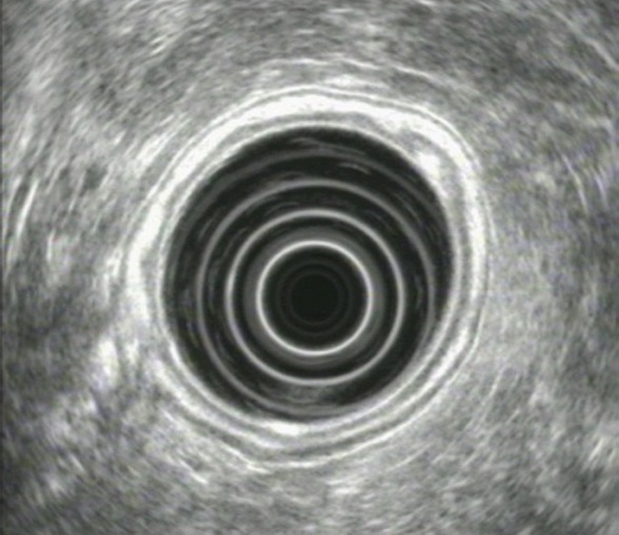
Gastric endoscopic ultrasonography. Note the 5 distinct layers that comprise the gastric wall.
SUBMUCOSAL LESIONS
Introduction
The term submucosal lesion (SML) or submucosal tumor (SMT) includes a wide spectrum of non-neoplastic and neoplastic conditions (benign and malignant) and is used to define an intramural growth underneath the mucosa, the exact nature of which cannot be definitely determined either by standard luminal endoscopy or by barium contrast radiography[8]. Despite the fact that the incidence of SMTs in the whole GI-tract is unknown and precise epidemiologic data are scarce, it seems that the stomach is the organ most frequently affected and it has been reported that gastric SMTs occur with an incidence of about 0.36% of routine upper GI-endoscopies (i.e. roughly one in every 300 endoscopies)[9-11]. Such findings are usually asymptomatic and incidental during various diagnostic procedures requiring endoscopy. However, when a physician encounters such a lesion, decisions of high clinical significance have to be made concerning the lesion’s nature (e.g. compression on the gastric wall from the outside versus a tumor deep in the wall, under the overlying normal mucosa and if so, a benign or a malignant tumor requiring treatment). The physicians armamentarium for successfully answering these questions and adequately treating the affected patients includes transabdominal ultrasonography, CT and MRI scans, as well as diagnostic EUS carried out when indicated by EUS-guided fine-needle aspiration (EUS-FNA), (leading to treatment options such as close follow-up, endoscopic resection or surgical removal)[12].
SMLs in EUS: EUS, EUS-FNA and other diagnostic modalities?
The diagnostic ability of EUS to clearly demonstrate the gastric wall and its layers makes it a great tool for the clinician to make the differential diagnosis of “real” SMTs (i.e. intramural tumors) from extraluminal compressions caused by either normal or pathological structures. Moreover, it can also show the layer of origin of gastric SMTs and can therefore assist in their exact characterization[11]. If EUS shows that a suspected submucosal bulge is an impression caused by a normal organ (e.g., the spleen or gallbladder), further diagnostic steps are not necessary. If the lesion is intramural, the differential diagnosis includes SMTs, cysts and vessels. Cysts usually present as anechoic, round or ovular lesions which arise from the 3rd gastric wall layer and vessels (most importantly varices) present as tubular or serpiginous anechoic formations, also usually arising from the 3rd layer, that produce a “positive” signal at electronic (Doppler-endowed) EUS. Additional information regarding the nature of tumors can be extracted from their layer of origin, echo pattern, and margin. The most frequent myogenic tumors (leiomyomas) are characteristically located in the second or forth echo-poor layer; they have an echo-poor pattern, and are more or less homogeneous and more or less well demarcated. Other lesions (granular-cell tumors, aberrant pancreas, fibroma, lipoma) have different echo patterns and usually originate from the third, echo-rich layer (submucosa), though sometimes from other layers as well (see the relevant paragraphs that follow)[7,12,13].
The identification of large SMTs can also be achieved by other imaging modalities such as barium studies, CT, MRI and even careful transabdominal ultrasound scanning with gastric water filling, the later being dependent on the experience of the examiner. Although there is a theoretical advantage of CT and MRI over EUS in staging, therapeutic planning and follow-up, i.e. the possibility to depict the full extension of a large SMT[14], nevertheless both of these methods are unable to determine the organ of origin of an SMT when dealing with significantly exophytic tumors, and have limited contribution in SMT classification in more than 50% of cases (especially gastrointestinal stromal-cell tumors). Furthermore, they cannot differentiate between malignant and benign lesions (unless in cases of obvious locally advanced or metastatic disease)[8,14]. Therefore, EUS is commonly agreed to be the best imaging modality for diagnosing and differentiating between SMLs in the GI-tract and has been shown to be consistently superior to other imaging tests[8,11,13,15]. Histopathological diagnosis cannot be made by (diagnostic) EUS alone, nor can benign lesions definitely be differentiated from malignant ones. Nevertheless, certain risk criteria have been established on EUS (size > 3 cm, inhomogeneous echo pattern, irregular margins, presence of lymph nodes) that may suggest malignancy; the most reliable of these probably being size[16]. If CT or MRI are to pose a threat to the leading role of EUS in diagnostics of SMTs, this will be with the help of new scanners which combine CT (or possibly MRI in the future) with positron emission tomography (PET), thus uniting functional and morphologic imaging. The latter depicts metabolic changes in tissue and has shown favorable results not only in the early evaluation of response of gastrointestinal stromal cell tumors (GIST) to treatment with imatinib, but seems also to be promising in the diagnosis, staging and assessment of disease recurrence in these cases[8]. Another advantage of EUS is that it can easily be combined with conventional endoscopy and EUS-guided fine-needle aspiration and biopsy (EUS-FNA). The advent of EUS-FNA, some 15 years ago, led to limited use of EUS as a mere imaging test, with the combination opening new possibilities for transmural tissue diagnosis and expanding the indications of EUS in pathologies of various organs, including SMTs of the stomach. Lately the characterization of GISTs with their inherent malignant potential has triggered a renewed interest in differential diagnosis of gastric SMTs. In this case, a final diagnosis using EUS-FNA with adequate tissue sampling and histological (aided by immunohistochemical) studies, is an attractive possibility. Tissue diagnosis of SMTs should address two questions: a) GIST versus another histology and b) malignant potential of a given GIST. The efficacy of EUS-FNA to accurately diagnose SMTs had some initial encouraging reports[17], only to be followed by the doubts of others. The tissue sampled from lesions at EUS-FNA was initially examined cytologically, but it has been recently shown that acquiring a core specimen for histological assessment is possible, even with a small number of needle passes[18]. When cytological examination is the aim, the presence of a cytopathologist during EUS-FNA, in order to obtain an adequate sample, has been strongly recommended especially in reports from the U.S.. This is virtually impossible in Europe, due to cost and personnel issues but the problem has been overcome by increasing the number of needle passes through the lesion in question; however, there is still lack of firm data supporting this option. Furthermore, there are different options in processing the cytological samples, including smears and cell-blocks. It is logical and desirable to have close contact with the cytopathologist and discuss the EUS-FNA procedure, in order to optimize the process of EUS-FNA tissue sampling by avoiding possible mistakes or weaknesses in the technique that are apparent to the cytopathologist but not to the clinician. For example, mitotic counts and immunohistochemistry cannot be performed on smears; thus requiring cell blocks from the cytological sample[8]. Although the diagnosis of an SMT was initially made by using cytological analysis exclusively, histological tissue analysis seems to be preferable[19], e.g. when wishing to differentiate between a benign and a malignant SMT of the smooth muscles. Histology offers the possibility of immunohistochemistry and mitotic counts (necessary for differentiation of GISTs from other SMTs and for the assessment of their malignant potential) and some distinct advantages over cytology, such as standardization of tissue acquisition (defined number of biopsies, formalin fixation), analysis (later assessment, no on-site analysis, decreasing the number of diagnoses such as “indeterminate or suspicious”, second opinion established) and availability of expertise. A number of studies have reported on the tissue acquisition yield and the accuracy rates of EUS-FNA. Results vary between 50% and 93%[12,17,19-21] and seem to be influenced by various factors including the lesion’s size (diagnostic rate for GISTs < 2cm, 2-4cm and 4cm or more were 71%, 86% and 100%, respectively)[19], cytological versus histological assessment[12] and needle size. Recently, newer advanced types of needle aiming at larger specimens or offering other advantages have become available. The Trucut needle, previously shown to offer a limited benefit was tested and compared with conventional 22 gauge (G) needles in a small series (only 10 cases) with SMLs. Although the Trucut needle (19 G size) was inferior in terms of final diagnostic yield (70% vs 90%), determination of the marker c-kit to diagnose GISTs was possible in all 6 cases in whom in was indicated[22]. A larger prospective, uncontrolled study using the Trucut needle, involving 49 consecutive patients with hypoechoic gastric SMTs also showed a moderate diagnostic yield for the needle (tumor tissue adequate for diagnosis obtained in 63% of patients; 95% CI 49%-75%), whereas the samples were too small to reliably determine the mitotic index[23]. However, another study on SMTs, presented in abstract form, used a 19 G prototype needle with a mean number of 4 passes and reached a tissue yield of 74%, and this only included repeated procedures in 2 cases[24]. It seems that a obtaining a definite tissue diagnosis in SMTs can be rather difficult. For example, although differentiation between a myogenic tumor and a lipoma or a fibroma can be made even by EUS-FNA cytology alone, this is complicated, as a large tissue sample is needed and differentiation can even be difficult on frozen sections during surgery, especially when dealing with myogenic tumors. One should also have in mind possible complications such as bleeding and sepsis. Doppler-EUS examination performed prior to EUS-FNA may prevent rupture of a possible varice and antibiotic prophylaxis should be considered[8,23]. Despite the fact that the aforementioned results with EUS-FNA are at best moderate, one must keep in mind that another non-surgical alternative, namely forceps biopsy during standard endoscopy, faired significantly worse in trials than EUS-FNA (in one study 35% was submucosal representation achieved, in spite of the endoscopist’s efforts to obtain submucosal tissue)[9].
To summarize, EUS (with or without EUS-FNA) remains the gold standard of non surgical diagnosis and classification of SMTs and allows decision-making regarding therapy and management of patient’s with SMTs. There seems to be a lack of evidence regarding the optimal strategy in SMTs suspected to be GISTs with a negative EUS-FNA tissue diagnosis, what the optimal decision should be (i.e. EUS-FNA versus surgery) in cases of large SMTs, and also the role (and the intervals) of follow-up in cases with a small/intermediate suspicion of malignancy or an equivocal histology. These issues simply stress the need for prospective, randomized trials (possibly multi-center, in order to recruit greater numbers of patients), which will answer these and similar questions.
Appearance of various SMLs in EUS
For EUS-imaging of all SMLs there should be an initial endoscopic localization of the lesion followed by focus on the transition zone of the normal gastric wall and the SML. Here, it is easier to precisely locate the wall layer of origin. This should be followed by careful inspection and determination of the size and shape of the lesion, the regularity of its borders, its echogenic characteristsics, presence of vessels (facilitated by the Doppler-imaging possibilities of modern electronic echoendoscopes). Finally, the perigastric area should be searched for signs of infiltration of adjacent organs, metastatic disease and especially lymph nodes.
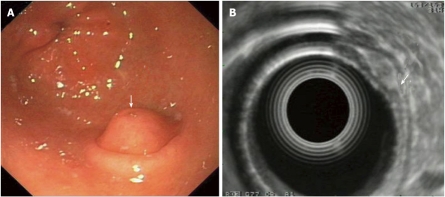
GISTs in EUS: The origin of GISTs is thought to be from multipotential mesenchymal stem cells. Therefore, myogenic and neurogenic features may be present in these tumors, which are the commonest mesenchymal tumors in the GI-tract. 65% of GISTs occur in the stomach and at upper GI-endoscopy appear as submucosal, intramural, or sometimes serosal nodules covered by an intact normal mucosa, but may also present as umbilicated lesions with a central ulceration (Figure 2A). At EUS, they are characteristically located in the fourth echo-poor layer (which corresponds to the muscularis propria) or (less often) to the second echo-poor layer (muscularis mucosae). They appear with an echo-poor pattern, and are more or less homogeneous and more or less well demarcated (Figure 2B). Signs of suspected malignancy include a large size (e.g. > 4 cm, although this cutoff is rather arbitrary), irregular borders, lobulations, anechoic spaces or echogenic foci. The malignancy potential of a given GIST increases in parallel with the presence of these imaging criteria. However, as previously pointed-out, these features are only suggestive of malignant potential and only tissue diagnosis with immunohistochemistry (most GISTs are c-kit, - CD 117 and CD34 positive) and mitotic counts are diagnostic, a fact that highlights the importance of EUS-FNA in the diagnosis of these tumors (Figures 2C and 2D). About 10-30% of GISTs have a malignant behavior. However, it should be stressed that according to the current suggested terminology for GISTs, the diagnoses “benign” or “malignant” should be avoided, due to the inherent malignant potential of all GISTs and that definitions including “low”, “intermediate” or “high” risk are preferred instead[7,8,12,20]. As previously mentioned, there is lack of evidence on treatment algorithms, when encountering possible gastric GISTs at endoscopy. Options could include surgical resection, EUS-FNA and close surveillance with repeat EUS-examinations. It seems that the first of these should be followed in cases of large GISTs or cases where EUS features change at follow-up, with appearance of necrosis, change of echogenicity, or increase in size. EUS-FNA (and decisions on further management according to the results of histology or cytology) is usually advocated in cases of intermediate GIST size without changes at surveillance-EUS.
Figure 2.
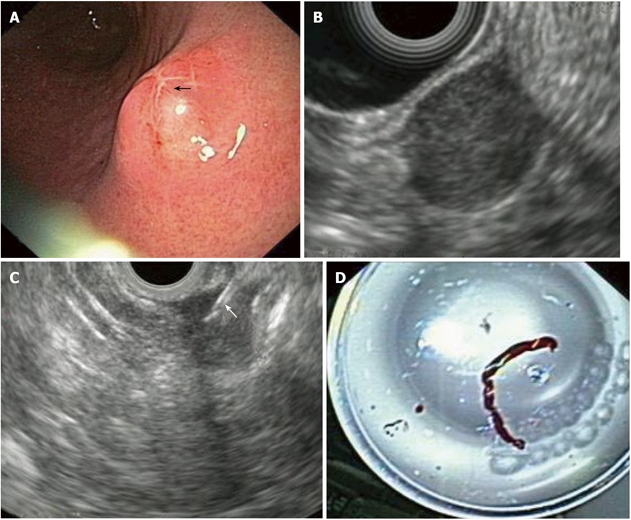
Gastric gastrointestinal stromal cell tumors: Endoscopic aspects, endoscopic ultrasonography-imaging and tissue sampling. A: Endoscopic image of the lesion; note that the lesion is covered by a normal mucosa with a central umbilication (black arrow); B: EUS imaging of the lesion, which is located in the 4th echo-poor layer (muscularis propria); C: EUS-FNA of the lesion; note the presence of the needle (white arrow); D: Histological specimen of the EUS-FNA. EUS:Endoscopic ultrasonography; EUS-FNA: EUS-guided fne needle aspiration.
Pancreatic rests (aberrant pancreas) in EUS: Pancreatic rests or aberrant pancreas (or ectopic, or heterotopic pancreas) are foci of ectopic pancreatic tissue i.e. pancreatic tissue in other locations, lacking anatomic or vascular connection with the normal pancreas. In a surgical series they were found in about 0.25% of explorative laparotomies[24]. They can be encountered throughout the GI-tract, with the stomach being the most usual site where they are diagnosed and are usually asymptomatic, but may also manifest with symptoms including (acute or chronic) pancreatitis, bleeding ulceration or obstruction. Rarely, pancreatic rests may even mimic a malignant GIST, although EUS can usually differentiate these lesions[24]. Endoscopically, they usually present as sessile SMLs, possibly with a duct opening on their surface (Figure 3A), from which fluid may exit on pressure[8]. Aberrant pancreas normally originates in the third layer (submucosa), but may sometimes originate from other layers (i.e. second or forth wall layer) and is usually hypoechoic or of mixed echogenicity, including anechoic structures that correspond to ductal formations (Figure 3B). Because of their endosonographic appearance they may cause difficulties in differential diagnosis from carcinoid tumors, which have similar endosonographic characteristics[7,8,12,24,25].
Figure 3.
Pancreatic rest of the stomach: Endoscopic and endoscopic ultrasonography -imaging. A: Endoscopic image of a pancreatic rest. Note the duct opening on the surface of lesion is covered by a normal mucosa with a central umbilication (arrow); B: EUS imaging of the lesion which originates from the 3rd layer, i.e. the submucosa (arrow); note the lesion’s mixed echogenicity. EUS: Endoscopic ultrasonography.
Lipomas in EUS: Lipomas are benign tumors which can appear throughout the GI-tract, their most common location being the colon. Endoscopically, they usually present as solitary, yellow-colored, well-circumscribed, smooth SMLs, with a very slow (if any) rate of growth when repeat endoscopies are performed. These lesions are characteristically soft when pressure is exercised on them. In EUS, a lipoma usually presents as a hyperechoic homogenous mass, which originates from the third wall layer (submucosa)[7,8,12].
Neuroendocrine tumors: carcinoids in EUS: Carcinoid tumors are rare, slow-growing neuroendocrine tumors arising from the enterochromaffin cells disseminated throughout the bronchopulmonary system and the GI-tract, which however carry malignant potential. They are the most common type of neuroendocrine tumor located in the stomach. Gastric carcinoids are usually asymptomatic and may be incidentally discovered at GI-endoscopy. Their size is a good predictor of their risk for malignancy (with carcinoids smaller than the cutoff size of 2cm rarely being malignant). Endoscopically, carcinoids usually have the appearance of small polyps and present either as solitary lesions or in clusters. Endosonographically, gastric carcinoids are homogeneous, well demarcated, mildly hypoechoic SMLs, that originate from the first, second and/or third layer[12,13,26,27].
Granular cell tumors in EUS: Granular cell tumors are SMTs that are believed to be of neural origin (immunohistochemichal studies indicate that they originate from Schwann cells). They are rarely encountered in the GI-tract (about 8% of all granular cell tumors), whereas approximately 30% of all GI-tract granular cell tumors are located in the middle to distal esophagus. Localization in the stomach is very rare. Gastric granular cell tumors can be solitary or, more frequently, are associated with another GI-localization. In endoscopy they appear as small yellowish nodules (< 4 cm and -in about 95% of cases- < 2 cm). Granular cell tumors are usually benign in behavior, although some malignant cases (a single gastric case) have also been reported. Endosonographically they present as a hypoechoic, heterogeneous well-demarcated mass with smooth borders, arising from the second or third wall layer[8,12,13,28].
Schwannomas in EUS: Schwannomas are well-demarcated, benign nerve sheath tumors usually of the soft tissue, rarely encountered in the GI-tract, where they are often discovered incidentally as small polypoid intraluminal lesions covered by intact normal mucosa. GI-tract Schwannomas, though rare, are mostly encountered in the stomach (0.2% of all gastric tumors). The tumors are generally asymptomatic or manifest with non-specific symptoms including abdominal discomfort or as a palpable epigastric mass when exophytic growth has occurred. Bleeding may occasionally occur, in the case of deep ulceration. In standard endoscopy, gastric schwannomas may present as round or oval (multinodular) SMLs. As they usually and principally involve the submucosa and muscularis propria, endosonographically they appear as homogenous, hypoechoic, small SMLs with distinct borders, arising from the third and/or forth gastric wall layer[8,29].
Cysts in EUS: Cysts in the GI-tract are usually the result of a resolved inflammatory process, or derive from embryological development, including foregut and duplication cysts. Cystic SMLs in the GI-tract may appear as simple cysts, multicystic or solid cystic lesions. Foregut cysts are usually located in the mediastinum and categorized as bronchogenic or neurenteric, according to their embryogenic origin; EUS and EUS-FNA play a pivotal role in their diagnosis. On the other hand, gastroenteric duplication cysts arise from abnormal development of the part of the dorsal foregut that becomes the GI-tract[12,13,30].
Localization of cysts in the stomach is rare and they may be either asymptomatic, or present (especially when dealing with children) with obstructive symptoms, pain, or bleeding. In standard endoscopy, cysts appear as compressible nodule structures, which protrude (to a greater or smaller extent) into the lumen of the GI-tract. Endosonograhically, they present as well-demarcated, round or oval anechoic lesions, located in the third gastric wall layer. The wall of inflammatory cysts is a single hyperechoic layer.
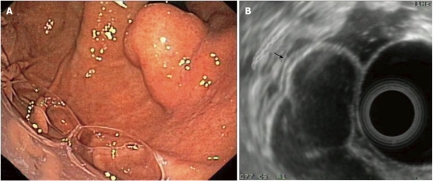
Duplication cysts are rare congenital abnormalities. They can occur anywhere throughout the GI-tract, with gastric duplication cysts being the most uncommon, representing only 2%-8% of all duplication cysts located in the GI-tract[31]. Characteristically, in EUS the walls of a duplication cyst may appear as a 3- or 5-layer structure due to the presence of a submucosa and a muscularis layer (Figure 4). Diagnosis of duplication cysts in adulthood is uncommon and is usually an incidental finding in clinical settings. They are usually benign, although rare cases of malignant transformation have also been described[12,13,30,31].
Figure 4.
Duplication cyst of the stomach. A: Endoscopic image of the lesion (retrograde view); B: EUS imaging of the same lesion; note the lesion’s 3-layer structure which originates from the 3rd layer, i.e. the submucosa (arrow). EUS: Endoscopic ultrasonography.
Gastric varices in EUS: EUS in combination with the color Doppler technique is a noninvasive method which allows us not only to definitely differentiate gastric varices from thickened gastric folds or SMLs in the stomach, but also to study the progression of hemodynamic changes in the portal venous system of affected patients and also to objectively assess the effect of pharmacological agents (or other therapies, e.g. TIPPS) on portal hypertension. EUS has also found a role in the treatment and follow up of esophageal and gastric varices[12,32]. Gastric varices usually present at the fundus or the body of the stomach as serpiginous or oval structures covered by normal mucosa, that retreat when pressed by a biopsy forceps. Tissue sampling is risky when gastric varises are suspected and therefore the diagnosis is made by means of EUS. Endosonographically, they appear as round, oval, tortuous or tubular anechoic structures within the third gastric wall layer (i.e. the submucosa). A positive signal in Doppler examination is diagnostic[32,33] (Figure 5). A thickening of the gastric wall layers, as well as the presence of gastric (or paragastric) collateral varises may also be seen[12,32,34]. The latter (together with their esophageal counterparts) may correlate with the risk of variceal bleeding[34].
Figure 5.
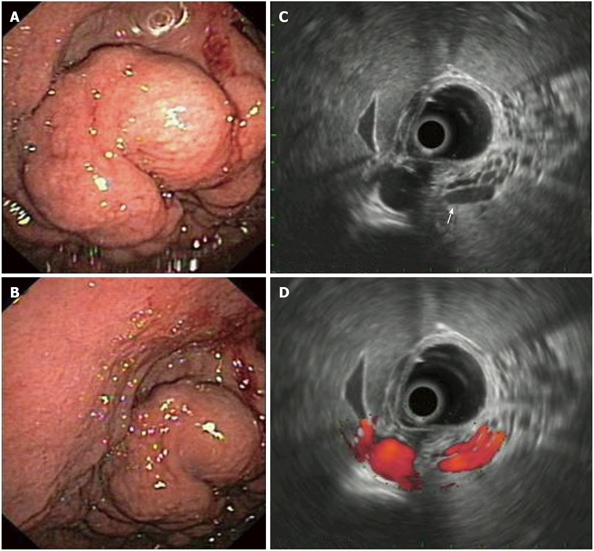
Gastric varices in endoscopy and endoscopic ultrasonography. A, B: Endoscopic image of gastric varices presenting as thick serpiginous structures, covered by normal mucosa; C: EUS imaging of the varices; note their tortuous, anechoic structure which originates from the 3rd layer, i.e. the submucosa (arrow); D: EUS Doppler imaging of the same varices; the positive signal denotes their vascular origin. EUS: Endoscopic ultrasonography.
Miscellaneous SMLs in EUS: (1) Gastric leiomyomas: Leiomyomas are the most common SMTs of mesenchymal origin in the esophagus, but are very rare in the stomach. Contrary to “real” GISTs, leiomyomas are almost invariably benign and therefore differential diagnosis between these two conditions is vital to therapeutic decisions. As previously mentioned, differential diagnosis of GISTs from leiomyomas is not always easy, even with help from EUS-FNA. Studies have attempted to differentiate leiomyomas from “true” (bearing a malignant potential) GISTs based on their EUS features. Leiomyomas appear endosonographically as small (< 5 cm) homogenous, hypoechoic SMTs, with smooth/distinct borders, originating from the forth or second wall layer[8]. Signs like inhomogenicity, hyperechogenic spots, a marginal halo and higher echogenicity compared to the surrounding muscle layer might appear more frequently in GISTs than in leiomyomas[35], but differentiation based merely on imaging is risky and therefore should be done only in specific conditions and with the informed of the patient. For larger lesions, surgical resection seems to be the best alternative; (2) Extrinsic compressions: Compressions on the gastric wall from organs neighboring the stomach may occasionally present as SMLs and sometimes can cause diagnostic problems. The spleen, the left hepatic lobe or even the gallbladder can produce impressions on the gastric fundus and upper body or antrum, which may appear as SMLs in standard endoscopy; in these cases, EUS has been shown to be a valuable diagnostic tool[12,13,15]. Furthermore, pathological structures, (including pancreatic pseudocysts and tumors or enlarged lymph nodes), structures of cardiovascular origin (e.g. the left atrium, or aneurysms of the aorta or the splenic artery) may also compress (or, in the case of malignant entities, infiltrate) the gastric wall. Therefore, EUS-based differential diagnosis should be performed carefully, possibly combining “usual” ultrasound frequencies of 7.5 MHz (which allow a “deeper” view and can better assess the correlation of the gastric wall with source of the impression) with higher frequencies, such as 12 MHz (for a more detailed “scanning” of the interface/gastric serosal wall and extrinsic compression) and Doppler scanning (which will present a positive signal in case of vascular lesions). These measures can help in ruling-out an infiltration of the gastric wall. Care should be taken to look for possible pathological lymph nodes[12,13]; (3) Submucosal metastases: Carcinomas or lymphomas may, although rarely, metastasize to the GI-tract (including the stomach) and appear as submucosal masses[10,12]. Their endosonographic appearance generally is that of hypoechoic, heterogeneous masses, which may originate from any (or all) of the wall layers[12,13]; and (4) Fundic gland polyps: Finally, fundic gland polyps are usually recognized by their macroscopic appearance in endoscopy. However, in doubtful cases, they can be easily removed with a biopsy forceps and be sent for histology. EUS is rarely necessary and may be difficult to perform in these cases, as optimal acoustic coupling of the ultrasonic transducer to the lesions is extremely difficult to achieve, due to the small size of the latter. However, if EUS is performed, the lesions are usually observed as hyperechoic structures originating (and remaining) in the first layer [12,36].
Footnotes
Peer reviewer: Majid Abdulrahman Almadi, MD, FRCPC, Department of Gastroenterology Division, McGill University Health Center, McGill University, Royal Victoria Hospital, 687 Pine Ave West, Montreal H3A 1A1, Canada
S- Editor Zhang HN L- Editor Hughes D E- Editor Zhang L
References
- 1.Röesch T. Endoscopic ultrasonography: equipment and technique. Gastrointest Endosc Clin N Am. 2005;15:13–31, vii. doi: 10.1016/j.giec.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Papanikolaou IS, Fockens P, Hawes R, Rösch T. Update on endoscopic ultrasound: how much for imaging, needling, or therapy? Scand J Gastroenterol. 2008;43:1416–1424. doi: 10.1080/00365520701737252. [DOI] [PubMed] [Google Scholar]
- 3.Anderson MA, Scheiman JM. Initial experience with an electronic radial array echoendoscope: randomized comparison with a mechanical sector scanning echoendoscope in humans. Gastrointest Endosc. 2002;56:573–577. doi: 10.1067/mge.2002.127761. [DOI] [PubMed] [Google Scholar]
- 4.Noh KW, Woodward TA, Raimondo M, Savoy AD, Pungpapong S, Hardee JD, Wallace MB. Changing trends in endosonography: linear imaging and tissue are increasingly the issue. Dig Dis Sci. 2007;52:1014–1018. doi: 10.1007/s10620-006-9491-8. [DOI] [PubMed] [Google Scholar]
- 5.Rösch T. The radial echoendoscope: here to stay or gone tomorrow? Gastrointest Endosc. 2009;69:S159–S162. doi: 10.1016/j.gie.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 6.Sabbagh LC. The gut: esophagus, stomach, and rectum. Gastrointest Endosc. 2009;69:S90–S92. doi: 10.1016/j.gie.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Rösch T, Classen T. Gastroenterologic Endosonography. New York: Thieme Stuttgart; 1992. [Google Scholar]
- 8.Ponsaing LG, Kiss K, Loft A, Jensen LI, Hansen MB. Diagnostic procedures for submucosal tumors in the gastrointestinal tract. World J Gastroenterol. 2007;13:3301–3310. doi: 10.3748/wjg.v13.i24.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc. 1991;5:20–23. doi: 10.1007/BF00591381. [DOI] [PubMed] [Google Scholar]
- 10.Polkowski M. Endoscopic ultrasound and endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy. 2005;37:635–645. doi: 10.1055/s-2005-861422. [DOI] [PubMed] [Google Scholar]
- 11.Rösch T, Lorenz R, Dancygier H, von Wickert A, Classen M. Endosonographic diagnosis of submucosal upper gastrointestinal tract tumors. Scand J Gastroenterol. 1992;27:1–8. doi: 10.3109/00365529209011157. [DOI] [PubMed] [Google Scholar]
- 12.Kim EY (A) Submucosal lesions. In: Hawes RH, Fockens P. Endosonography. Thieme Stuttgart; 2006. pp. 99–110. [Google Scholar]
- 13.Willmen HR, Kogel H. [Diagnosis and therapy of sigmavolvulus in the adult] Zentralbl Chir. 1975;100:1198–1199. [PubMed] [Google Scholar]
- 14.Lau S, Tam KF, Kam CK, Lui CY, Siu CW, Lam HS, Mak KL. Imaging of gastrointestinal stromal tumour (GIST) Clin Radiol. 2004;59:487–498. doi: 10.1016/j.crad.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Rösch T, Kapfer B, Will U, Baronius W, Strobel M, Lorenz R, Ulm K. Accuracy of endoscopic ultrasonography in upper gastrointestinal submucosal lesions: a prospective multicenter study. Scand J Gastroenterol. 2002;37:856–862. [PubMed] [Google Scholar]
- 16.Caletti G, Odegaard S, Rösch T, Sivak MV, Tio TL, Yasuda K. Endoscopic ultrasonography (EUS): a summary of the conclusions of the Working Party for the Tenth World Congress of Gastroenterology Los Angeles, California October, 1994. The Working Group on Endoscopic Ultrasonography. Am J Gastroenterol. 1994;89:S138–S143. [PubMed] [Google Scholar]
- 17.Matsui M, Goto H, Niwa Y, Arisawa T, Hirooka Y, Hayakawa T. Preliminary results of fine needle aspiration biopsy histology in upper gastrointestinal submucosal tumors. Endoscopy. 1998;30:750–755. doi: 10.1055/s-2007-1001416. [DOI] [PubMed] [Google Scholar]
- 18.Akahoshi K, Sumida Y, Matsui N, Oya M, Akinaga R, Kubokawa M, Motomura Y, Honda K, Watanabe M, Nagaie T. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007;13:2077–2082. doi: 10.3748/wjg.v13.i14.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087–1095. doi: 10.1016/s0016-5085(97)70164-1. [DOI] [PubMed] [Google Scholar]
- 20.Wiech T, Walch A, Werner M. Histopathological classification of nonneoplastic and neoplastic gastrointestinal submucosal lesions. Endoscopy. 2005;37:630–634. doi: 10.1055/s-2005-870127. [DOI] [PubMed] [Google Scholar]
- 21.Ginès A, Fernández-Esparrach G, Pellisé M, Argüello L, Solé M, Colomo L. L, Sendino O, Moura L, Gimeno A, Mata A, Llach J, Bordas J. M.Comparison of EUS-Guided Trucut with EUS-Guided Fine-Needle Aspiration in Subepithelial Tumors: Preliminary Results of a Prospective Study. Endoscopy. 2006;39:FR43. [Google Scholar]
- 22.Polkowski M, Gerke W, Jarosz D, Nasierowska-Guttmejer A, Rutkowski P, Nowecki ZI, Ruka W, Regula J, Butruk E. Diagnostic yield and safety of endoscopic ultrasound-guided trucut [corrected] biopsy in patients with gastric submucosal tumors: a prospective study. Endoscopy. 2009;41:329–334. doi: 10.1055/s-0029-1214447. [DOI] [PubMed] [Google Scholar]
- 23.Polkowski M, Gerke W, Nasierowska-Guttmejer A, Nowecki Z, Rutkowski P, RukaW , ButrukE EUS-guided trucut biopsy of hypoechoic intramural tumors of the stomach: a single-center experience in 17 cases. Endoscopy. 2006;39:FR32. [Google Scholar]
- 24.Tanaka K, Tsunoda T, Eto T, Yamada M, Tajima Y, Shimogama H, Yamaguchi T, Matsuo S, Izawa K. Diagnosis and management of heterotopic pancreas. Int Surg. 1993;78:32–35. [PubMed] [Google Scholar]
- 25.Akaraviputh T, Manuyakorn A, Lohsiriwat V. Diagnosis by endoscopic ultrasound of a large aberrant pancreas mimicking malignant gastrointestinal stromal tumor of the stomach. Endoscopy. 2009;41 Suppl 2:E63–E64. doi: 10.1055/s-0028-1119457. [DOI] [PubMed] [Google Scholar]
- 26.Pinchot SN, Holen K, Sippel RS, Chen H. Carcinoid tumors. Oncologist. 2008;13:1255–1269. doi: 10.1634/theoncologist.2008-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burkitt MD, Pritchard DM. Review article: Pathogenesis and management of gastric carcinoid tumours. Aliment Pharmacol Ther. 2006;24:1305–1320. doi: 10.1111/j.1365-2036.2006.03130.x. [DOI] [PubMed] [Google Scholar]
- 28.Patti R, Almasio PL, Di Vita G. Granular cell tumor of stomach: a case report and review of literature. World J Gastroenterol. 2006;12:3442–3445. doi: 10.3748/wjg.v12.i21.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon HY, Kim CB, Lee YH, Kim HG. Gastric schwannoma. Yonsei Med J. 2008;49:1052–1054. doi: 10.3349/ymj.2008.49.6.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hizawa K, Matsumoto T, Kouzuki T, Suekane H, Esaki M, Fujishima M. Cystic submucosal tumors in the gastrointestinal tract: endosonographic findings and endoscopic removal. Endoscopy. 2000;32:712–714. doi: 10.1055/s-2000-9025. [DOI] [PubMed] [Google Scholar]
- 31.Seijo Ríos S, Lariño Noia J, Abdulkader Nallib I, Lozano León A, Vieites Pérez-Quintela B, Iglesias García J, Domínguez Muñoz JE. [Adult gastric duplication cyst: diagnosis by endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA)] Rev Esp Enferm Dig. 2008;100:586–590. doi: 10.4321/s1130-01082008000900011. [DOI] [PubMed] [Google Scholar]
- 32.El-Saadany M, Jalil S, Irisawa A, Shibukawa G, Ohira H, Bhutani MS. EUS for portal hypertension: a comprehensive and critical appraisal of clinical and experimental indications. Endoscopy. 2008;40:690–696. doi: 10.1055/s-2008-1077400. [DOI] [PubMed] [Google Scholar]
- 33.Wong RC, Farooq FT, Chak A. Endoscopic Doppler US probe for the diagnosis of gastric varices (with videos) Gastrointest Endosc. 2007;65:491–496. doi: 10.1016/j.gie.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 34.Faigel DO, Rosen HR, Sasaki A, Flora K, Benner K. EUS in cirrhotic patients with and without prior variceal hemorrhage in comparison with noncirrhotic control subjects. Gastrointest Endosc. 2000;52:455–462. doi: 10.1067/mge.2000.107297. [DOI] [PubMed] [Google Scholar]
- 35.Kim GH, Park do Y, Kim S, Kim DH, Kim DH, Choi CW, Heo J, Song GA. Is it possible to differentiate gastric GISTs from gastric leiomyomas by EUS? World J Gastroenterol. 2009;15:3376–3381. doi: 10.3748/wjg.15.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giovannini M, Bernardini D, Moutardier V, Monges G, Houvenaeghel G, Seitz JF, Derlpero JR. Endoscopic mucosal resection (EMR): results and prognostic factors in 21 patients. Endoscopy. 1999;31:698–701. doi: 10.1055/s-1999-79. [DOI] [PubMed] [Google Scholar]