Abstract
Pre-exposure prophylaxis (PrEP) is an experimental approach to HIV prevention and consists of antiretroviral drugs to be taken before potential HIV exposure in order to reduce the risk of HIV infection and continued during periods of risk. An effective PrEP could provide an additional safety net to sexually active persons at risk, when combined with other prevention strategies. Women represent nearly 60% of adults infected with HIV and PrEP can be a female-controlled prevention method for women who are unable to negotiate condom use. Two antiretroviral nucleoside analog HIV-1 reverse transcriptase inhibitor drugs are currently under trial as PrEP drugs, namely tenofovirdisoproxilfumarate (TDF) alone and TDF in combination with emricitabine (FTC), to be taken as daily single dose oral drugs. There are 11 ongoing trials of ARV-based prevention in different at risk populations across the world. The iPrex trial showed that daily use of oral TDF/FTC by MSM resulted in 44% reduction in the incidence of HIV. This led to publication of interim guidance by CDC to use of PrEP by health providers for MSM. Few other trials are Bangkok Tenofovir Study, Partners PrEP Study, FEM-PrEP study, and VOICE (MTN-003) study. Future trials are being formulated for intermittent PrEP (iPrEP) where drugs are taken before and after sex, “stand-in dose” iPrEP, vaginal or rectal PrEP, etc. There are various issues/concerns with PrEP such as ADRs and resistance to TDF/FTC, adherence to drugs, acceptability, sexual disinhibition, use of PrEP as first line of defense for HIV without other prevention strategies, and cost. The PrEP has a potential to address unmet need in public health if delivered as a part of comprehensive toolkit of prevention services, including risk-reduction, correct and consistent use of condoms, and diagnosis and treatment of sexually transmitted infections.
Keywords: HIV/AIDS, issues, pre exposure prophylaxis, trials
INTRODUCTION
There is a growing global access to antiretroviral drugs to HIV-positive patients including India. Still, approximately 7000 new infections occur daily globally; more than 50,000 new infections each week[1] and 2.7 million continue to get infected annually. Moreover, there is feminization of this HIV/AIDS pandemic with women and girls representing slightly more than half of all people living with HIV.[2] In subSaharan Africa, where the majority of new HIV infections continue to occur (1.8 million in 2009),[2] women represent nearly 60% of adults and in most cases, they acquire HIV through sexual intercourse with an infected male partner.[3] Hence, the need of the hour is to effectively prevent HIV transmission to uninfected individuals. Although behavior change programs have contributed to dramatic reductions in the number of annual infections, still at-risk population is enormous and more comprehensive strategies are needed.[4]
CONSTRAINTS IN CURRENT PREVENTION STRATEGIES
Barriers against HIV/AIDS control
Low condom acceptance in non-commercial sex
Low acceptance of circumcision (provides only partial and no protection against penile and rectal transmission, respectively)[5]
Low acceptance of testing
Low awareness of vulnerability (youth and female)
More emphasis on treatment
STRATEGIES FOR REDUCING ACQUISITION OF HIV INFECTION
Include (1) expanded HIV testing so that infected persons can be treated and their risk for transmitting infection minimized; (2) individual, small-group, and community-level behavioral interventions to reduce risk behaviors; (3) promotion of condom use; (4) detection and treatment of sexually transmitted infections; and (5) mental health and substance abuse counseling when needed.[4]
PRE-EXPOSURE PROPHYLAXIS OF HIV
Definition
PrEP is an experimental approach to HIV prevention and consists of antiretroviral drugs to be taken before potential HIV exposure in order to reduce the risk of HIV infection and continued during periods of risk. PrEP can be in the form of a pill taken by mouth or a gel applied in the vagina or rectum.[6,7]
Difference between pre-exposure prophylaxis and post-exposure prophylaxis
Post-exposure prophylaxis (PEP) is antiretroviral (ARV) drugs given to individuals within 3 days (72 hours) after possible exposure to HIV, to reduce risk of HIV infection.[1]
PrEP is the approach used in which uninfected individuals take an HIV treatment drug (ARV) in order to build a concentration of the medication in their bodies, so that if they are exposed to the virus, the medicine may reduce the chances of HIV acquisition.[1]
Scope of pre-exposure prophylaxis
An effective PrEP could provide an additional safety net to sexually active persons at risk, when combined with reduction in the number of sex partners, HIV counseling and testing, consistent and correct condom use, and other prevention strategies. And hence, it could help address the urgent need for a female-controlled prevention method for women worldwide who are unable to negotiate condom use, because of cultural and other barriers.[4]
Statistics
Mathematical modeling suggests that approximately 2.7 to 3.2 million new HIV-1 infections could be averted in southern sub-Saharan Africa over 10 years by targeting PrEP (having 90% effectiveness) to those at the highest behavioral risk and by preventing sexual disinhibition. But this benefit could be reduced to ≤50% by sexual disinhibition and by high PrEP discontinuation and lower PrEP effectiveness.[8]
Rationale of pre-exposure prophylaxis
PrEP follows the concept of providing a preventive drug before exposure to the infectious agent. Similar concept holds true for travelers being given prophylactic drugs for malaria before going to endemic countries. Theoretically, if HIV replication can be inhibited from the moment the virus enters the body, it may not be able to establish a permanent infection.[4]
DATA SUPPORTING THIS APPROACH OF PRE-EXPOSURE PROPHYLAXIS
Prevention of mother to child transmission
Single dose of nevirapine to HIV-infected women during labor and to their newborns immediately after birth reduces the risk for MTCT of HIV by about 50%.[4]
Post exposure prophylaxis
Zidovudine taken few hours after an occupational exposure e.g. needle prick and taken for 28 days decreases the chances of HIV transmission by 80%.[4]
Animal studies
Several studies on monkeys have shown that tenofovir alone or in combination with emtricitabine given before exposure to simian HIV provides significant protection to the monkeys exposed repeatedly to the virus.[4]
Animal studies where combination of tenofovir and emtricitabine was tested on humanized BLT (bone marrow liver thymic) mice, having fully developed human immune systems, showed that the drugs provided 100% protection against vaginally introduced HIV to the mice. This led to the conclusion that PrEP could be a very effective method for preventing vaginal HIV-1 transmission.[6,9]
DRUGS FOR PRE-EXPOSURE PROPHYLAXIS
Two antiretroviral nucleoside analog HIV-1 reverse transcriptase inhibitor drugs are currently under trial as PrEP drugs, namely tenofovir disoproxil fumarate (TDF) alone and TDF in combination with emricitabine (FTC).[10,11]
Mechanism of action and advantages of TDF
TDF, the orally bioavailable prodrug of tenofovir, is metabolized to a nucleotide analog HIV-1 reverse transcriptase inhibitor.[12,13]
Advantages for use as ARV and PrEP[13]
Potency against wild-type HIV and some nucleoside-resistant strains of HIV
Low potential of selecting for TDF-resistant mutants
Low likelihood of metabolic/mitochondrial toxicity
Pharmacologic profile supporting daily dosing
Dosage-1 tablet TDF [300 mg] alone or in combination with FTC [200 mg] daily with or without food[10]
ADR of TDF/FTC combination[10]
Boxed warning: Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases.
Boxed warning: Severe acute exacerbations of hepatitis B have been reported in patients who are coinfected with HBV and HIV-1. Hepatic function should thus be monitored closely.
New onset or worsening renal impairment including acute renal failure and fanconi syndrome can occur. The combination should not be used if creatinine clearance is <30 mL/min or patient is on hemodialysis. Regular serum phosphorus monitoring is also advised.
Newer drugs under trial
Two drugs, raltegravir (integrase strand transfer inhibitor) and maraviroc (CCR5 inhibitor), have been tested on humanized RAG-hu mice and results show that oral administration of either of these drugs prevents vaginal HIV-1 infection.[14]
PRE-EXPOSURE PROPHYLAXIS TRIALS
There are 11 ongoing trials of ARV-based prevention in different at risk populations across the world [Figure 1].
Figure 1.
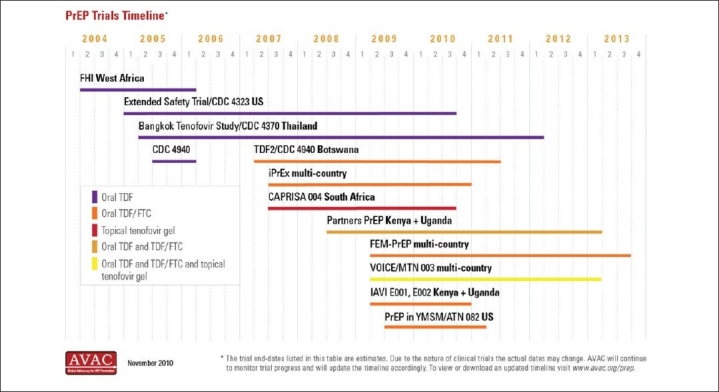
Timeline of PREP trials worldwide[15]
The iPrex trial (The pre-exposure prophylaxis initiative trial)
The iPrex study was a phase 3 study conducted in 11 sites in six countries, namely Peru, Ecuador, United States, South Africa, Brazil, and Thailand[7] from July 2007 to December 2009. A total of 2499 HIV-uninfected men including 29 male-to-female transgender adults (aged ≥18 years) who reported sex with a man and reported engaging in high-risk sexual behaviors during the preceding 6 months, and had no clinical contraindication were enrolled and were given daily oral combined formulation of 300 mg TDF and 200 mg FTC (TDF/FTC). Four weekly follow up of participants for HIV testing, risk-reduction and PrEP medication adherence counseling, dispensing of condoms was done. The subjects were followed for median 1.2 to maximum 2.8 years.[5,7,16]
Results[5,7,16]
Of these subjects, 10 were found to have been infected with HIV at enrollment, and 100 became infected during follow-up (36 in the FTC-TDF group and 64 in the placebo group), indicating a 44% reduction in the incidence of HIV.
Reduction in risk for HIV acquisition was 21% among participants with <90% adherence and 73% with ≥90% adherence, highlighting the importance of adherence to a prophylactic regimen.
Drug level testing showed a 92% reduction in risk for HIV acquisition in participants with detectable levels of TDF/FTC versus those with no drug detected.
TDF/FTC generally was well tolerated, except nausea in the first month; raised serum creatinine levels (which reversed within 4 weeks of discontinuation of drug); and unintentional weight loss.
No drug-resistant virus was found in the 100 participants infected after enrollment.
Among 10 participants who were seronegative at enrollment but later found to have been infected before enrollment, 2 cases of FTC resistance occurred; posing an important question of drug resistance in those who seroconvert.
Participants in both TDF/FTC and placebo arms reported lower total numbers of sex partners and higher percentages of partners who used condoms than reported at baseline- highlighting the importance of behavior counseling.
Interim guidance for use of pre-exposure prophylaxis
The U. S. Centers for Disease Control and Prevention (CDC) on the January 28, 2011, in Morbidity and Mortality Weekly Report published interim guidance regarding the use of tenofovir/emtricitabine for PrEP against HIV infection based on the iPrEx trial.[16–18] [Table 1].
Table 1.
CDC interim guidance for health-care providers electing to provide pre-exposure prophylaxis for the prevention of HIV infection in adult men who have sex with men (MSM) and who are at high risk for sexual acquisition of HIV[16–18]

Other current trials
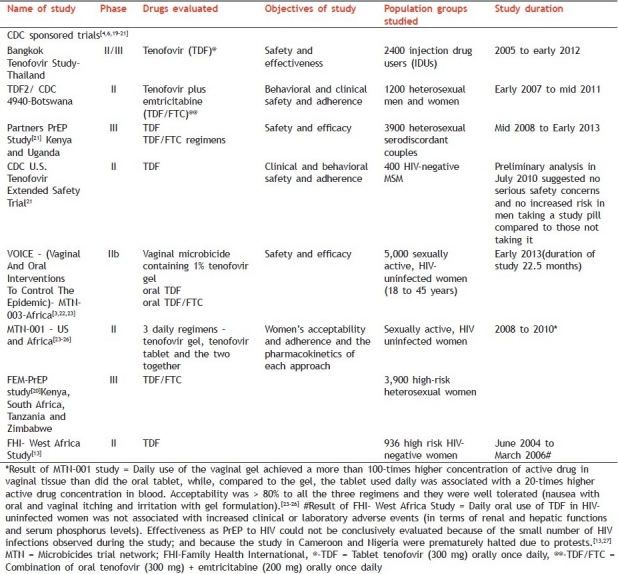
In all the trials it has been ensured that all subjects receive HIV testing, risk-reduction counseling, condoms, and management of sexually transmitted infections before starting with the oral preventive drug regimens [Table 2].
Table 2.
Current ongoing trials of pre exposure prophylaxis of HIV
Intermittent pre-exposure prophylaxis[28,29]
This study investigated the efficacy of PrEP drugs when taken before and after sex, hence named intermittent PrEP (iPrEP).
The efficacy of a two-dose oral iPrEP regimen with Truvada was evaluated in 24 male rhesus macaques (6 in each of the four groups) by exposing them with simian HIV rectally and repeating the exposure weekly for 14 weeks. The two doses were given at different time schedules, and the results are as follows [Table 3].
Table 3.

It was concluded that effective PrEP with TDF/FTC does not require daily dosing in repeat-exposure macaque model, and support the (formulation of next generation) iPrEP efficacy trials in humans.[29,30]
Future trials
Intermittent PrEP with dosing that is not exposure driven (independent of the time of exposure), known as “stand-in dose”, which is one or two doses of TDF/FTC in a week, followed by a second dose after exposure.
iPrEP trial has suggested that the modalities that are independent of the time of exposure appear to be more protective than if the doses are given right around the time of exposure.[29]
iPrEP in the macaques/ animals in vaginal transmission model to evaluate the efficacy of oral drugs in preventing vaginal transmission.[29]
Efficacy of single drug as iPrEP.[29]
Vaginal or rectal PrEP.[29]
PRE-EXPOSURE PROPHYLAXIS AND MICROBICIDES
PrEP is an oral prophylaxis for HIV. Microbicides are now referred to as “topical” PrEP.[29]
CAPRISA (Centre for the AIDS Programme of Research in South Africa) 004 study
A phase II b (proof of concept) trial in which 889 women at high risk of acquiring HIV through sexual intercourse from South Africa were enrolled and there were 39% fewer HIV infections among women who used 1% tenofovir gel within 12 h prior to sex and as soon as possible within 12 h after sex than among those who used the placebo gel.[30–32] The reduction in HIV acquisition was 54% in women with high gel adherence.[32]
ISSUES RELATED TO PRE-EXPOSURE PROPHYLAXIS
There are various issues that have cropped up with the ongoing trials for PrEP and several concerns have emerged especially with issuing of interim guidance for PrEP for MSM by CDC
The individuals becoming HIV positive later on the trials are thus providing testing for viral load, CD4 count, and HIV resistance mutations, and infected participants are followed up for an additional six months, to help guide treatment decisions and to determine whether prior exposure to tenofovir or tenofovir plus emtricitabine affects the course of disease, testing is provided[4] (CDC)
Sexual disinhibition/“risk compensation” after PrEP promoting riskier sexual behavior.[33]
What if PrEP becomes a morning after pill
ADRs of TDF/FTC because HIV prevention is not a FDA approved indication of TDF/FTC, its long-term safety in HIV-uninfected persons is not yet known.[16]
Adherence taking PrEP daily is critical, as efficacy is strongly associated with medication adherence as highlighted by iPrex study.[5,33,34]
Acceptability of a daily oral drug with various ADRs and potential for resistance.
Use of other antiretrovirals which have not proven safe for uninfected persons (e.g., more than two drugs or protease inhibitors)[16]
Use of PrEP by at risk population groups other than MSM[7,16]
Use of dosing schedules of unproven efficacy (e.g., “intermittent” dosing just before and/or after sex).[7,35]
PrEP should only be used among individuals who have been confirmed to be HIV negative. Not screening for acute infection before beginning PrEP or long intervals without retesting for HIV infection may have negative implications.[16,33]
Duration of PrEP the drugs will have to be continued till high risk sexual activity is continued.
The medication is costly, hence it is very important for the patients to understand the financial implications of starting PrEP.[7,16]
PrEP should never be seen as the first line of defense against HIV. It was only shown to be partially effective when used in combination with regular HIV testing, condoms, and other proven prevention methods. Men who have sex with men should still:
-
♦Use condoms correctly and consistently
-
♦Get tested to know their status and that of their partner(s) for certain
-
♦Get tested — and treated if needed — for other sexually transmitted infections that can facilitate HIV transmission, such as syphilis and gonorrhea
-
♦Get information and support to reduce drug use and sexual risk behavior
-
♦Reduce their number of sexual partners[33]
-
♦
PrEP must be obtained and used in close collaboration with healthcare providers to ensure regular HIV testing, risk reduction and adherence counseling, and careful safety monitoring.[33]
CONCLUSION
“Female empowerment is a distant dream. Behavioral change is not yet on the horizon. Vaccines and microbicides need more encouraging trials. Till that time, pre and post-sexual exposure prophylaxis remain important armamentaria for females.”
CDC states that “no single prevention strategy will be 100% effective against HIV transmission, abstinence and mutual monogamy with an HIV-negative partner will remain the only 100% effective ways to prevent infection.” Hence, reducing transmission will require determining how best to integrate all available prevention strategies—both biomedical and behavioral.[4,22]
PrEP has the potential to address unmet need in public health[7] and contribute to effective and safe HIV prevention, if (1) it is targeted to population groupsat high risk for HIV acquisition; (2) it is delivered as part of a comprehensive toolkit of prevention services, including risk-reduction and PrEP medication adherence counseling, correct, and consistent use of condoms, and diagnosis and treatment of sexually transmitted infections; and (3) it is accompanied by monitoring of HIV status, side effects, adherence, and risk behaviors at regular intervals.[16]
DISCLAIMER
This review article is just to introduce the readers with the concept of PrEP. Prescribing PrEP is a very sensitive issue because of obvious reasons. If at all such need arises, please refer latest guidelines available at standard sites like CDC, WHO, and UNAIDS.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Frequently Asked Questions. [Last accessed on 2011 Mar 4]. Available from: http://www.iprexnews.com/studyresults/pdfembargo/englishversion/FAQ%20complete%20Final%20PE.pdf .
- 2.UNAIDS report on the global aids epidemic 2010. [Last accessed on 2011 Mar 6]. Available from: http://www.unaids.org/globalreport/documents/20101123_GlobalReport_full_en.pdf. ISBN 978-92-9173-871-7 .
- 3.VOICE Study, a major HIV prevention trial for women, is launched in Zimbabwe. 2009. Sep 16, [Last accessed on 2011 Mar 3]. Available from: http://www.mtnstopshiv.org/node/1416 .
- 4.CDC's Clinical Studies of Pre-Exposure Prophylaxis for HIV Prevention. [Last updated2010 July 19. Last accessed on 2011 Feb 21]. Available from: http://www.cdc.gov/hiv/prep/resources/qa/index.htm .
- 5.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure Chemoprophylaxis for HIV Prevention in Men Who Have Sex with Men. N Engl J Med. 2010;363:27. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Post exposure prophylaxis and pre-exposure prophylaxis. [Last accessed on 2011 Feb 21]. Available from: http://www.avert.org/pep-prep-hiv.htm .
- 7.iPrEx: Results of a study of TRUVADA® to prevent HIV infection among MSM. [Last updated 2010 Nov30. Last accessed on 2011 Mar 2]. Available from: http://www.hvtn.org/community/iprexbackground.pdf .
- 8.Abbas UL, Anderson RM, Mellors JW. Potential Impact of Antiretroviral Chemoprophylaxis on HIV-1 Transmission in Resource-Limited Settings. PLoS ONE. 2007;2:e875. doi: 10.1371/journal.pone.0000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humanized Mouse Infected With HIV Vaginally And Rectally Allows Testing. ScienceDaily [Updated 2009 Apr 21] [Last accessed on 2011 Feb 22]. Available from: http://www.sciencedaily.com/releases/2009/04/090420103740.htm .
- 10.Food and Drug Administration. Truvada: Highlights of prescribing information (package insert) [Last accessed on 2011 Feb 26]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021752s017lbl.pdf .
- 11.Scaccabarozzi L, Milano M. Before and After: PrEP and PEP. [Last updated Summer 2007. Last accessed on 2011 Mar 6]. Available from: http://www.thebody.com/content/prev/art42460.html .
- 12.Highlights of prescribing information-Viread®(tenofovirdisoproxilfumarate) tablets. [Last updated 2010 Oct. Last accessed on 2011 Mar 4]. Available from: http://www.gilead.com/pdf/viread_pi.pdf .
- 13.Peterson L, Taylor D, Roddy R, Belai G, Phillips P, Nanda K, et al. TenofovirDisoproxilFumarate for Prevention of HIV Infection in Women: A Phase 2, Double-Blind, Randomized, Placebo-Controlled Trial. [Last accessed on 2011 Mar 4];PLoSClin Trials. 2007 2:e27. doi: 10.1371/journal.pctr.0020027. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1876601/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neff CP, Ndolo T, Tandon A, Habu Y, Akkina R. Oral Pre-Exposure Prophylaxis by Anti-RetroviralsRaltegravir and Maraviroc Protects against HIV-1 Vaginal Transmission in a Humanized Mouse Model. PLoS ONE. 2010;5:e15257. doi: 10.1371/journal.pone.0015257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.PrEP trials timeline. [Last accessed on 2011 Mar 13]. Available from: http://www.globaliprex.com/pdfs/AVAC-PrEP_timeline_graphic.November-2010.jpg .
- 16.Interim Guidance: Preexposure Prophylaxis for the Prevention of HIV Infection in Men Who Have Sex with Men. [Last accessed on 25/2/2011];MMWR Morb Mortal Wkly Rep. 2011 60:65–8. Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6003a1.htm?s_cid=mm6003a1_w . [PubMed] [Google Scholar]
- 17.CDC Interim PrEP Guidance Graphic. [Last accessed on 2011 Feb 25]. Available from: http://www.cdc.gov/nchhstp/newsroom/PrEPMSMGuidanceGraphic.html .
- 18.Pre-Exposure Prophylaxis (PrEP) [Last updated on2011 Feb 22. Last accessed on 2011 Mar 5]. Available from: http://www.cdc.gov/hiv/prep/
- 19.Ongoing Pre-Exposure Prophylaxis (PrEP) Trials. [Last updated on 2010 Nov, Last accessed on 2011 Feb 23]. Available from: http://www.avac.org/ht/a/GetDocumentAction/i/3113 .
- 20.Trial shows ARVs can prevent HIV in men who have sex with men, making VOICE Study in women more important than ever. [Last updated on 2010 Nov 23. Last accessed on 2011 Mar 3]. Available from: http://www.mtnstopshiv.org/node/2695 .
- 21.CDC FACT SHEET- CDC TRIALS: Pre-Exposure Prophylaxis for HIV Prevention. [Last updated on 2011 Feb. Last accessed on 2011 Mar 4]. Available from: http://www.cdc.gov/hiv/prep/pdf/PrEP_TrialsFactSheet.pdf .
- 22.Questions and answers. The VOICE HIV Prevention Study. [Last updated on 2009 Sep 15. Last accessed on 2011 Mar 3]. Available from: http://www.niaid.nih.gov/news/qa/pages/voiceqa.aspx .
- 23.VOICE (MTN-003) [Last accessed on 2011 Mar 3]. Available from: http://www.mtnstopshiv.org/news/studies/mtn003 .
- 24.VOICE - Vaginal And Oral Interventions To Control The Epidemic Of HIV Looks At Tablets Versus Gel. [Last updated on 2011 Mar 01. Last accessed on 2011 Mar 3]. Available from: http://www.medicalnewstoday.com/articles/217760.php .
- 25.Understanding the Results of iPrEx- A Study of Pre-exposure Prophylaxis (PrEP) [Last updated 2010 Nov23. Last accessed on 2011 Mar 3]. Available from: http://www.mtnstopshiv.org/node/2694 .
- 26.MTN-001: Adherence and Drug Absorption Study of Oral and Vaginal Gel Preparations of Tenofovir. [Last updated on 2011 Feb 28. Last accessed on 2011 Mar 13]. Available from: http://www.mtnstopshiv.org/news/studies/mtn001/backgrounder .
- 27.Singh JA, Mills EJ. The Abandoned Trials of Pre-Exposure Prophylaxis for HIV: What Went Wrong? PLoS Med. 2005;2:e234. doi: 10.1371/journal.pmed.0020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Lerma G, Cong M-E, Mitchell J, et al. Montréal, Canada: Program and abstracts of the 16th Conference on Retroviruses and Opportunistic Infections; 2009. Prevention of rectal simian HIV transmission in macaques by intermittent pre-exposure prophylaxis with oral Truvada. [Google Scholar]
- 29.Intermittent Oral PrEP May Be as Effective as Daily Oral PrEP for Prevention of Rectal HIV Transmission, Macaque Study Suggests- An Interview With Gerardo Garcia-Lerma. [Last updated on 2009 Feb 9. Last accessed on 2011 Mar 3]. Available from: http://www.thebody.com/content/treat/art51464.html .
- 30.Results of CAPRISA 004 a turning point for HIV prevention, say MTN researchers conducting VOICE. VIENNA. 2010. Jul 19, [Last accessed on 2011 Mar 13]. Available from: http://www.mtnstopshiv.org/node/2004 .
- 31.Understanding the Results of CAPRISA 004. [Last updated on 2010 July20. Last accessed on 2011 Mar 13]. Available from: http://www.mtnstopshiv.org/sites/default/files/attachments/MTN%20factsheet%20Results_CAP004July%2019_0.pdf .
- 32.Karim QA, Karim SS, Frohlich JA, et al. Effectiveness and Safety of Tenofovir Gel, an Antiretroviral Microbicide, for the Prevention of HIV Infection in Women. [Last accessed on 2011 Mar 13];Science. 2010 329:1168–74. doi: 10.1126/science.1193748. Available from: http://www.sciencemag.org/content/329/5996/1168 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.CDC FACT SHEET- Pre-exposure prophylaxis (PrEP) for HIV Prevention- Promoting Safe and Effective Use in the United States. [Last acessed on 2011 Mar5. Last updated on 2011 Feb 25]. Available from: http://www.cdc.gov/hiv/prep/pdf/PrEPfactsheet.pdf .
- 34.Interim CDC Guidance on Pre-Exposure Prophylaxis for HIV Prevention in MSM. [Last accessed on 2011 Jan 24]. Available from: http://aids-clinical-care.jwatch.org/cgi/content/full/2011/214/1 .
- 35.CDC Issues Guidance for Truvada Pre-exposure Prophylaxis. [Last accessed on 2011 Feb 25]. Available from: http://www.hivandhepatitis.com/recent/2011/0201_2011_d.html.2/1/11 .


