INTRODUCTION
A human papillomavirus (HPV), a member of the papillomavirus, is a double-stranded DNA virus and produces cytopathic effect in epithelium. Genital mucosal infection is persistent and multifocal and can be subclinical.
More than 30 to 40 types of HPV are typically transmitted through sexual contact and infect the anogenital region.[1] Some sexually transmitted HPV types may cause genital warts. Persistent infection with “high-risk” HPV types different from the ones that cause skin warts, may progress to precancerous lesions and invasive cancer.[2] HPV infection is a cause of nearly all cases of cervical cancer;[3] however, most infections with these types do not cause disease.
All HPV infections involve the transmission from one infected individual to another through direct skin-to skin-contact. This may occur through skin-to-skin transmission via the epidermis due to direct contact of a plantar wart virus with broken skin, sexually during intercourse, or orally during sexual activity or kissing. Symptomatic HPV infection is only the tip of the iceberg [Figure 1]. Asymptomatic shedding is much more common in women with HIV/AIDS and asymptomatic shedders carry high potential for spreading the virus. Asymptomatic HPV/DNA shedding from perianal region seems to be very common, 78.9% in AIDS patients with advanced disease stage.[4]
Figure 1.
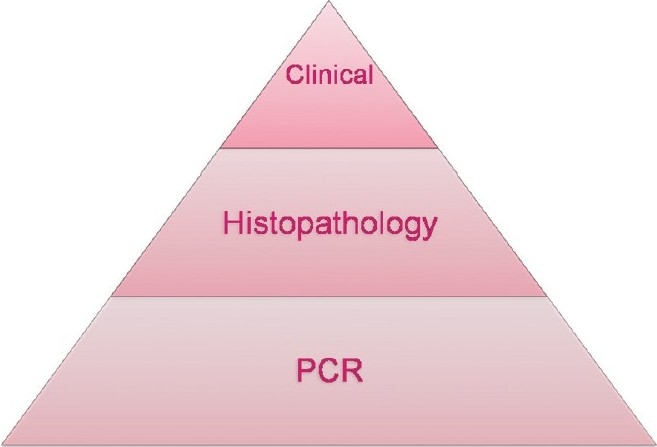
HPV- Subclinical, multifocal, and persistent
There was no consistent evidence that condom use reduces the risk of becoming HPV DNA – positive (subclinical HPV infection). On the other hand, the risk for genital warts, CIN 2-3 and invasive cervical cancer were reduced by condom use.[5]
LAB DIAGNOSIS
The traditional methods of viral diagnosis such as electron microscopy, cell culture, and certain immunological methods are not suitable for HPV detection. HPV cannot be cultured in cell cultures. The important methods to diagnose HPV infection are:
Colposcopy and acetic acid test
Biopsy
DNA test (PCR, Southern Blot Hybridization, In Situ Hybridization)
Pap smear
Colposcopy and acetic acid test
Colposcopy is a procedure performed by specially trained clinicians as an outpatient procedure using a low-powered microscope, the colposcope.[6] Colposcopy is the examination of the cervix, vagina, and in some instances the vulva after the application ofacetic acid solution; coupled with obtaining colposcopically directed biopsies of all lesions suspected of representing neoplasia.[7] Colposcopic findings are graded according to degree of acetowhite lesion, surface contour, mosaic pattern, and punctuation. Greater abnormalities of these parameters are related to severity of the lesions.
Acetic acid test
Soaking acetic acid into suspicious lesions can enhance the degree of suspicion in lesions without classic features.
The method involves applying a 3–5% acetic acid–moistened gauze pad for 5-10 minutes on suspected lesions of the penis, cervix, labia, or perianal area.
Inconspicuous, flat, genital lesions that might be difficult to assess become visible. Genital warts, dysplastic, and neoplastic tissues turn white (acetowhite).
False-positive results are common and can result from anything that causes parakeratosis (e.g., candidiasis, psoriasis, lichen planus, healing epithelium, sebaceous glands).
The acetic acid test should not be used for routine screening.
It can be used for visualizing subclinical genital HPV-associated lesions, identifying lesions for target biopsy, and for demarcating lesions during surgical therapy.[8]
The use of colposcopy screening is only to be recommended for
Immunosuppressed transplant recipients.
Human immunodeficiency virus (HIV) positive women.
Women with three consecutive inadequate samples, after three tests in a series reported as borderline nuclear change in squamous cells, and be referred for colposcopy after one test reported as borderline nuclear change in endocervical cells.
Positive cervical cytology for malignant cells or suspicious cells but clinically normal looking cervix.
Women must be referred after two tests reported as mild dyskaryosis.
Women must be referred for colposcopy after one test reported as moderate dyskaryosis or severe dyskaryosis, at least 90% of women with such test results should be seen in a colposcopy clinic within four weeks of referral. Women must be referred for colposcopy after one test reported as possible invasion or reported as glandular neoplasia, and 90% should be seen urgently within two weeks of referral
Biopsy
Colposcopy allows tissue sampling (biopsy) that is targeted to the abnormal areas. In fact, the biopsy of abnormal areas is a critical part of colposcopy because treatment will depend on how severe the abnormality is on the biopsy sample. If the biopsy results show pre-cancer (dysplasia) or cancer, then treatment is recommended. The dysplasia may be mild, moderate, or severe.
Excisional biopsy is recommended when colposcopic appearances indicate high grade abnormality, when low grade colposcopic change is associated with severe dyskaryosis or worse, or when a lesion extends into the canal.
In genital warts, the most characteristic feature is the presence of koilocytes, which are mature squamous cells with clear perinuclear zone. The nuclei of koilocytes may be enlarged and hyper chromatic, double nuclei are seen often as well.
DNA techniques
Initial methods of HPV detection used were direct probe hybridization such as dot blot and Southern blot. Besides being labor-intensive and time consuming, they had low sensitivity, required large amounts of DNA in clinical samples, and have largely been superseded by amplification technology, which has allowed detection of low-level virus copy numbers in clinical samples. The established routine method for viral detection is the hybridization of viral nucleic acids. The two main techniques are:
Hybrid capture HPV DNA Test 2 (hc2)
HC2 in conjunction with the Pap test is now approved by the FDA.[9] Since the FDA-approved Hybrid Capture 2 test can detect as little as 1 pg of HPV DNA/ml; its sensitivity and specificity are almost comparable with PCR-based detection methods. The advantages of the Hybrid Capture 2 test are the relatively simple handling and good reproducibility of results, which make this test the best standardized HPV detection method. While the exact HPV type cannot be identified, “low-risk” (6, 11, 42, 43, 44) and “high-risk” (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68) HPV genotype groups (HR HPV and LR HPV) are detected.
Polymerase chain reaction
PCR is a selective target amplification assay capable of exponential and reproducible increase in the HPV sequences present in biological specimens. The amplification process can theoretically produce one billion copies from a single double stranded DNA molecule after 30 cycles of amplification.[10]
Indications
Primary Screening in Conjunction with the Papanicolaou (Pap)
Test or as a Stand-Alone Test for women over 30 years of age
PAP smear or PAP test
It is a screening test first describe by Papanicolaou and Traut.
Apart from premalignant and malignant changes, viral infections like HPV infection and Herpes can also be detected.
Positive test requires further confirmatory tests like coloscopy, cervical biopsy, and DNA tests like PCR.
Procedure
Patient is placed in dorsal position and cervix is exposed with Cusco's speculum taking scraping from squamocolumnar junction with the help of Ayre's spatula by rotating it all around.
The scrapings are spread on glass slide fixing it with 95% ethyl alcohol and ether, or fixative spray (cytospray).
For cytological evaluation, scrapings are taken from upper lateral part of vaginal wall.
ACOG revised recommendations for Pap smears are[11]
Women from ages 21 to 30 be screened every two years instead of annually, using either the standard Pap or liquid-based cytology.
Women age 30 and older who have had three consecutive negative cervical cytology test results may be screened once every three years with either the Pap or liquid-based cytology.
Women with certain risk factors may need more frequent screening, including those who have HIV, are immunosuppressed, were exposed to diethylstilbestrol (DES) in utero, and have been treated for cervical intraepithelial neoplasia (CIN) 2, CIN 3, or cervical cancer.
HPV/HIV CO – INFECTION
Intraepithelial neoplasia (IEN) is common in HPV infected patients with HIV/AIDS. There are no clinical markers that can diagnose early IEN, so it is necessary to biopsy and genotype all HIV positive patients with condylomata accuminata before treatment.
REFERENCES
- 1.Genital HPV Infection - Fact Sheet. Centers for Disease Control and Prevention (CDC) [Last updated on 2009 Nov 24, Last accessed on 2011 Mar 15]. Available from: http://www.cdc.gov/std/HPV/STDFact-HPV.htm .
- 2.Human Papilloma Virus. [Last updated on 2011 Mar 9, Last accessed on 2011 Mar 13]. Available from: http://en.wikipedia.org/wiki/Human_papillomavirus .
- 3.Walboomers JM, Jacobs MV, Manos MM. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 4.New Colposcopy Guidelines - what are the implications for the cytology services? [Last updated on 2005, Lastaccessed on 2011 Mar 13]. Available from: http://www.curran.pwp.blueyonder.co.uk/Colpo.htm .
- 5.Sweet RL, Gibbs RS. Human papilloma virus. Infectious Diseases of the Female Genital Tract. Chapter6. [Last accessed on 2011 Mar 12]. Available from: http://books.google.co.in/books?id=wuR_ngItU5oCandpg=PA88andlpg=PA88anddq=hpv+infection+and+condomandsource=vrtandots=5p6vZOb5zOandsig=IG_TmdZYzML7KB3PY66T9jiC2VQandhl=enandei=rCd7TYyFMYXprAfGwOXCBQandsa=Xandoi=book_resultandct=resultandresnum=11andved=0CGQQ6AEwCg#v=onepageandq=hpv%20infection%20and%20condomandf=false .
- 6.Chuang TY, Brashear R. Genital Warts: Differential Diagnoses and Workup. [Last Updated on 2010 May 7, Last accessed on 2011 Mar 14]. Available from: http://emedicine.medscape.com/article/1133201-diagnosis .
- 7.NHS Cervical Screening Programme. [Last accessed on 2011 Mar 13]. Available from: http://www.cancerscreening.nhs.uk/cervical/screening.html .
- 8.Martin CK, Richardson LC, Berkman ND, Kuo TM, Yuen AN, Benard VB. Impact of the 2002 American society for Colposcopy and Cervical Pathology guidelines on cervical cancer diagnosis in a geographically diverse population of commercially insured women, 1999-2004. J Low Genit Tract Dis. 2011;15:25–32. doi: 10.1097/LGT.0b013e3181ed3c2b. [DOI] [PubMed] [Google Scholar]
- 9.Mandelblatt JS, Lawrence WF, Womack SM, Jacobson D, Yi B, Hwang YT, et al. Benefits and costs of using HPV testing to screen for cervical cancer. JAMA. 2002;287:2372–81. doi: 10.1001/jama.287.18.2372. [DOI] [PubMed] [Google Scholar]
- 10.Garland SM, Tabrizi S. Methods for HPV Detection:Polymerase Chain Reaction Assays. Monsonego J. [Last accessed on 2011 Mar 14];In emerging issue on HPV infection. Available from: http://www.eurogin.com/EmergingIssues.pdf . [Google Scholar]
- 11.ACOG Announces New Pap Smear and Cancer Screening Guidelines. [Last updated on 2009 Nov 20, Last accessed on 2011 Mar14]. Available from: http://www.acog.org/acog_districts/dist_notice.cfm?recno=13andbulletin=3161 .


