Abstract
Objective:
The objective was to compare the ability of norepinephrine and dopamine in reversing the hemodynamic and metabolic abnormalities of septic shock using Edwards Vigileo Monitor with Flotrac Sensor.
Design:
Prospective randomized control study.
Methods:
Fifty consecutive patients presenting with hyperdynamic septic shock who fulfilled the inclusion criteria were randomly allocated to either group I or group II. The goal of therapy was to achieve and maintain for 6 hours, all of the following - systolic blood pressure (SBP) >90 mmHg, systemic vascular resistance index (SVRI) >1800 dynes.s/cm5m2, cardiac index (CI) >4.0 lt/min/m2, index of oxygen delivery >550 ml/min/m2, index of oxygen uptake >150 ml/min/m2. The patients in group I were started on dopamine infusion at 10 μg/kg/min which was increased by 2.5 μg/kg/min, every 15 minutes till the goals were achieved. The patients in group II received norepinephrine infusion started at a dose of 0.5 μg/kg/min with a dose increment of 0.25 μg/kg/min, every 15 minutes till the goals were achieved.
Results:
Post-treatment heart rate showed an increase in the mean value in group I patients and a decrease in group II patients. The post-treatment mean SBP and SVRI in group II was significantly higher than that in group I. Patients in group I showed a significantly higher increase in post-treatment CI and index of oxygen delivery compared to patients in group II. Nineteen out of 25 patients responded to the treatment in group II while only 10 out of 25 responded in group I.
Conclusion:
Norepinephrine was more useful in reversing the hemodynamic and metabolic abnormalities of hyperdynamic septic shock compared to dopamine.
Keywords: Dopamine, hyperdynamic septic shock, norepinephrine, Vigileo monitor
INTRODUCTION
Hyperdynamic stage of septic shock is characterized by a diminished peripheral vascular tone and a rise in cardiac output (CO),[1] unless limited by hypovolemia. Agents with predominate vasoconstrictive activity play the prime role in reversing the hemodynamic and metabolic abnormalities of hyperdynamic septic shock.[2–4] Although earlier studies have shown norepinephrine to be effective,[5–7] this drug is still not widely used due to fear of excessive vasoconstriction thereby further compounding tissue hypoperfusion and leading to severe ischemia of vital organs. Dopamine though widely used,[3,4,8,9] has been wanting in restoring adequate hemodynamic conditions in many patients. Hence with a view to ascertain the efficiency and reliability of one drug over the other, the comparison of two (dopamine and norepinephrine) was taken up with predefined endpoints and continuous hemodynamic monitoring.
METHODS
This prospective randomized study was designed to compare and evaluate the ability of dopamine and norepinephrine in reversing the hemodynamic and metabolic abnormalities of septic shock. The study included patients presenting with hyperdynamic septic shock, which was defined as being hemodynamically characterized by hypotension [systolic blood pressure (SBP) <90 mmHg], systemic vasodilation and a high cardiac index (CI; >4.0 lt/min/m2) and metabolically characterized by a reduced oxygen uptake and an abnormal flow dependence of index of oxygen uptake (IVO2) on index of oxygen delivery (IDO2) even at normal or high delivery rate. Written informed consent was taken from a close relative in concordance with the guidelines laid down by institutional ethics committee. All patients were fluid resuscitated after insertion of a central venous catheter and an arterial catheter. The CO was assessed using Vigileo monitor of Edwards Life sciences and Edwards Flotrac sensor connected to arterial catheter, using the principle of arterial pressure waveform analysis. Radial artery was cannulated and it was compatible with the monitor used. Other variables displayed on monitor were arterial blood pressure (ABP), pulse rate (PR), systolic volume variation (SVV), systemic vascular resistance (SVR) and CO. However, one drawback of the monitor used is that it lacks the capability to provide data regarding pulmonary artery pressure, right-sided heart pressure, mixed venous oxygen saturation and advanced volumetrics (diastolic and systolic cardiac volumes and ejection fraction). The respiratory status was assessed and managed simultaneously with either invasive or non-invasive modes of ventilation. Variables measured and calculated at this stage included CO, CI, stroke volume and systemic vascular resistance index (SVRI). Thereafter patients were assessed as regards to inclusion criteria which was taken as SBP <90 mm of Hg, CI >4.0 lt/min/m2, SVRI <1600 dynes.s/cm5m2 and oliguria (<0.5 ml/kg/hr). Fifty consecutive patients who fulfilled the inclusion criteria were taken up for the study and then randomly allocated to either of the two groups, group I – dopamine infusion group and group II – norepinephrine infusion group.
The goal of therapy was to achieve and maintain for 6 hours, all of the following – SBP >90 mm of Hg, SVRI >1800 dynes.s/cm5m2, CI >4.0 lt/min/m2, IDO2 >550 ml/min/m2 and IVO2 >150 ml/min/m2. The patients in group I were started on dopamine infusion at 10 μg/kg/min. Thereafter, every 15 minutes the hemodynamic and metabolic parameters were compared with the preset goals of therapy. The infusion was continued at the same rate if the patient achieved and maintained the hemodynamic and metabolic goals. If, however, the patient either failed to achieve the goals or was unable to maintain them till the conclusion of the study, the infusion rate was increased by 2.5 μg/kg/min, till the goals were achieved and maintained or a maximum dose of dopamine (25μg/kg/min) was reached. Those patients who were able to achieve the goal of therapy with the dopamine infusion in the predefined range (10-25 μg/kg/min) were labelled as responders and the rest as non-responders. The patients in group II received norepinephrine infusion started at a dose of 0.5 μg/kg/min. The patients who failed to achieve or maintain the predefined goals received a dose increment of 0.25 μg/kg/min, every 15 minutes till they achieved and maintained the goals up to the end of study or a maximum infusion dose of norepinephrine (2.5 μg/kg/min) was reached. The patients who were able to achieve the goal of therapy with the norepinephrine infusion in the predefined range (0.5-2.5 μg/kg/min) were labeled as responders and the rest as non-responders.
RESULTS
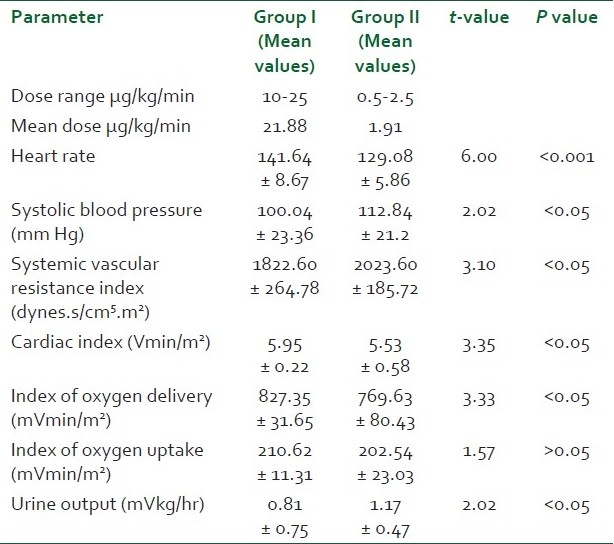
There was no significant difference between the two groups with respect to age, sex distribution, weight and Acute Physiology And Chronic Health Evaluation Score (APACHE) scores (P > 0.05) [Table 1]. Pneumonia, peritonitis and urinary tract infections were the major causes (37) of sepsis in patients. Others were pancreatitis, soft tissue infections, venous catheter-associated infections, etc. [Table 2]. The baseline hemodynamic and metabolic parameters in the two groups were recorded and compared and the difference was found to be insignificant (P>0.05) [Table 3]. Patients in group I were administered dopamine in the dose range of 10- 25 μg/kg/min. The mean dose of dopamine administered was 21.88 μg/kg/min. Post-treatment hemodynamic and metabolic parameters were recorded as values at the 6th hour in responders and the values at 25 μg/kg/min dopamine infusion in non-responders. These were then compared with their baseline values [Table 4]. Patients in group II were administered norepinephrine in the dose range of 0.5-2.5 μg/kg/min. The mean dose of norepinephrine administered was 1.91μg/kg/min. The post-treatment values were recorded and compared with their baseline values [Table 5]. Significant improvement was found in post-treatment hemodynamic and metabolic parameters in both groups.
Table 1.
Baseline characteristics
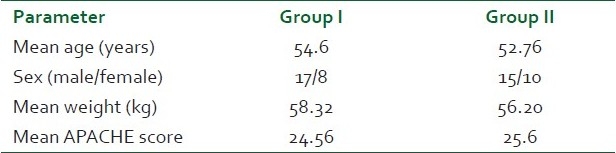
Table 2.
Etiological diagnosis
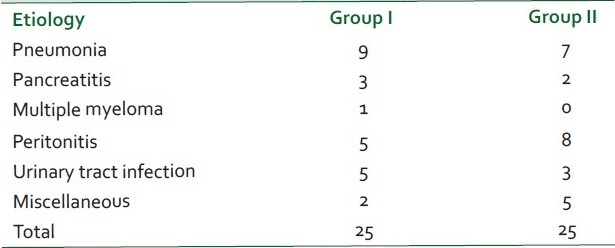
Table 3.
Comparison of baseline hemodynamic and metabolic parameters between the two groups post-patient optimization
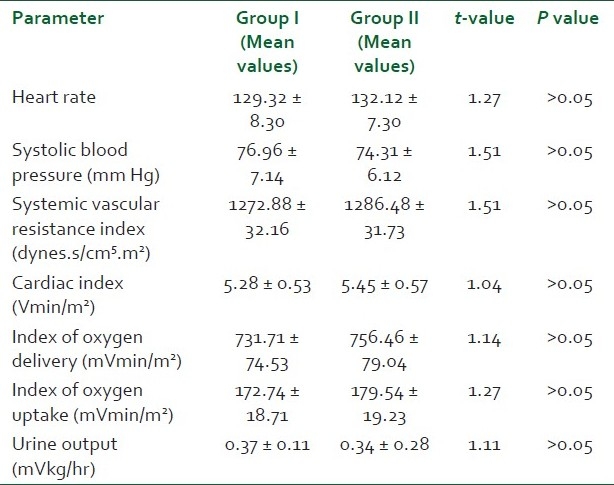
Table 4.
Comparison of post-treatment hemodynamic and metabolic parameters with the baselines in the dopamine group
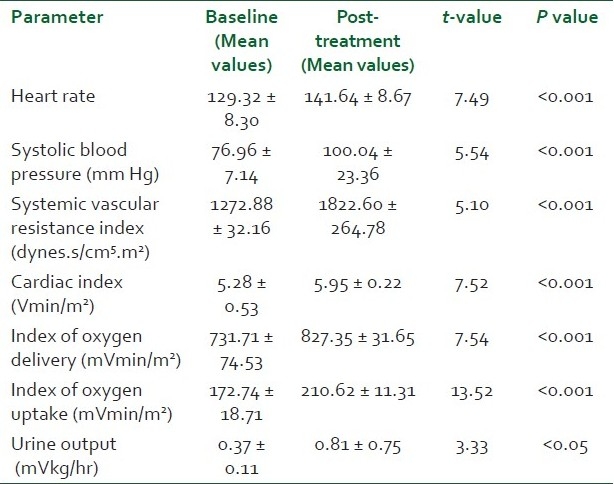
Table 5.
Comparison of post-treatment hemodynamic and metabolic parameters with the baselines in the norepinephrine group
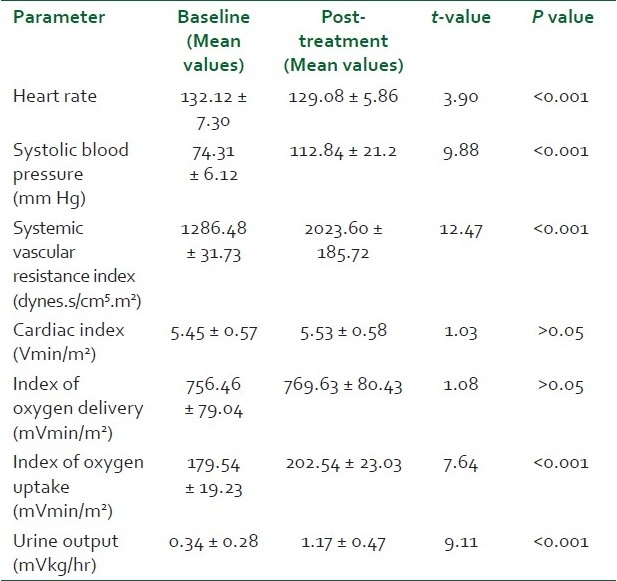
Comparison of post-treatment heart rate between the two groups showed a high statistical significance (P < 0.001) with group I patients showing an increase over their mean baseline value and group II patients a decrease from their mean baseline value [Table 6]. The post-treatment SBP in the norepinephrine group was significantly higher (P < 0.05) than that in the dopamine group. A higher mean SVRI was achieved in the norepinephrine treatment group compared to the dopamine treatment group (P < 0.05). Patients in the dopamine treatment group showed a significantly higher (P < 0.05) increase in post-treatment CI and index of oxygen delivery compared to patients in the norepinephrine treatment group. There was no statistically significant difference in the index of oxygen uptake in the two groups which increased above the baseline value in both the groups. Mean urine output increased to a much higher extent in group II patients as compared to group I patients (P < 0.05). The comparison of the responders and the non-responders of the total patients in both the groups studied showed a statistically significant response in favor of group II (P < 0.05). Nineteen out of 25 patients responded to the treatment in group II while only 10 out of 25 responded to treatment in group I, the other 15 either failed to achieve or to maintain the goals of therapy for 6 hours.
Table 6.
Comparison of post-treatment hemodynamic and metabolic parameters between group I and group II
DISCUSSION
The early state of septic shock is characterized by a decreased SVR and an increased CI together with hypotension and hypoperfusion of vital organs. One-half of non-survivors of sepsis are estimated to die of refractory hypotension.[10,11] Therefore, hemodynamic management of such patients to support blood pressure and thereby maintain perfusion to vital organs is a critical aspect of care. The hypotensive state is often not amenable to fluid resuscitation alone and requires the institution of vasoactive agents to counter the profound fall in SVR.[12] Dopamine has been widely in use and has often been advocated as the first choice vasoactive agent in septic shock. However, several studies have failed to demonstrate the restoration of adequate tissue perfusion, even with high doses of dopamine.[11] Some studies have shown norepinephrine to be more beneficial in restoring and maintaining blood pressure in patients with septic shock. However, the concern regarding excessive vasoconstriction and impairment of tissue perfusion has persisted when using norepinephrine. The study, therefore, used parameters such as delivery of oxygen and uptake of oxygen, in addition to SBP and SVRI to assess the comparative efficacy of norepinephrine and dopamine and also to allay concerns regarding their deleterious effects.[13]
Fifty consecutive patients with clinical and laboratory parameters fulfilling the diagnosis of septic shock were considered for the study and were randomized to two groups depending on whether theyp would receive dopamine (group I) or norepinephrine (group II). There was no significant difference between the two groups with respect to age, sex distribution, weight and APACHE scores (P > 0.05). Pneumonia, peritonitis and urinary tract infections were the major causes (37) of sepsis in patients. Others were pancreatitis, soft tissue infections, venous catheter-associated infections, etc. The baseline hemodynamic and metabolic parameters in the two groups were recorded and compared and the difference was found to be insignificant (P > 0.05). The post-treatment increase in heart rate in group I was attributed to the β-adrenergic properties of dopamine which predominate in patients with sepsis3 . This chronotropic effect of dopamine elevates myocardial oxygen demand.[14] Patients in group II demonstrated a favorable profile, leading to a decrease in heart rate which was attributed to increase in SVR and thereby mean arterial pressure thus leading to better organ perfusion and oxygen utilization. The post-treatment SBP in the norepinephrine group was significantly higher (P < 0.05) than that in the dopamine group, with only 10 out of 25 patients showing a sustained rise. A higher mean SVRI was achieved in the norepinephrine treatment group compared to the dopamine treatment group (P < 0.05). Fifteen out of 25 patients in group I showed a relative dopamine resistance depicted by their inability to achieve or to maintain the preset SVRI and SBP, thereby leading to continued hypoperfusion of organs. Nineteen out of 25 patients in group II were able to achieve and maintain the preset SVRI whish was attributed to the more potent vasoconstrictive action of norepinephrine as compared to dopamine. Patients in group I demonstrated a significant rise in CI which was attributed to the positive ionotropic and chronotropic effect of dopamine. However, rise in CI per se is not sufficient since studies have shown that a high CI may be present in survivors as well as non-survivors of septic shock even within a few hours of death.[15,16] Group II patients showed no significant trend in CI from baseline. This compared favorably to prior studies which have shown that a rise in blood pressure at the expense of CI leads to poor survival.[17] The mean of IDO2 increased from baseline in group I while it showed no increase in group II. This showed that in group II patients the rise in SVRI had no deleterious effect on IDO2 . The increase in IVO2 in group I was attributed to increase in IDO2 and CI rather than a fall in venous oxygen content. However, in group II patients the increase in IVO2 was not accompanied by an increase in IDO2 and CI. This may be attributed to the correction of splanchnic ischemia and can also be explained by the fact that under the influence of norepinephrine vascular reactivity is restored in sepsis and blood flow is directed towards areas of greatest oxygen demand, thereby increasing uptake and optimizing oxygen extraction. Urine output was used to demonstrate the effects of two drugs on splanchnic and renal vasculature as well as to depict normalization of hemodynamic parameters leading to adequate renal perfusion.[18] Mean urine output increased to a much higher extent in group II patients as compared to group I patients (P < 0.05). Norepinephrine by virtue of its greater effect on the efferent rather than on afferent arteriole increases the filtration fraction and helps to increase urine flow.[19] At the same time renal blood flow was maintained by normal or increased CI. Nineteen out of 25 patients responded to the treatment in group II while only 10 out of 25 responded to treatment in group I, the other fifteen either failed to achieve or to maintain the goals of therapy for 6 hours. Thus norepinephrine was more useful in reversing the hemodynamic and metabolic abnormalities of hyperdynamic septic shock compared to dopamine, at the doses tested.
CONCLUSIONS
This prospective randomized trial was undertaken to compare the ability of norepinephrine and dopamine in reversing the hemodynamic and metabolic abnormalities of hyperdynamic septic shock and it was concluded that norepinephrine was more effective and reliable than dopamine in achieving the same. Moreover, norepinephrine showed no adverse effects on peripheral blood flow or on renal blood flow, as was evidenced by normalization of urine output in patients on norepinephrine infusion.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Parrillo JE, Parker MM, Natanson C, Suffredini AF, Danner RL, Cunnion RE, et al. Septic shock in humans: Advances in understanding of pathogenesis, cardiovascular dysfunction and therapy. Ann Intern Med. 1990;113:227–42. doi: 10.7326/0003-4819-113-3-227. [DOI] [PubMed] [Google Scholar]
- 2.Müllner M, Urbanek B, Havel C, Losert H, Waechter F, Gamper G. Vasopressors for shock. Cochrane Database Syst Rev. 2004;3:CD003709. doi: 10.1002/14651858.CD003709.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Marik PE, Mohedin M. The contrasting effects of dopamine and hyperdynamic sepsis. JAMA. 1994;272:1354–7. [PubMed] [Google Scholar]
- 4.Luce JM. Pathogenesis and management of septic shock. Chest. 1987;91:883–8. doi: 10.1378/chest.91.6.883. [DOI] [PubMed] [Google Scholar]
- 5.De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, et al. Comparison of Dopamine and Norepinephrine in the Treatment of Shock. N Engl J Med. 2010;362:779–89. doi: 10.1056/NEJMoa0907118. [DOI] [PubMed] [Google Scholar]
- 6.Martin C, Papazian L, Perrin G, Saux G, Gouin F. Norepinephrine or dopamine for the treatment of hyperdynamic septic shock? Chest. 1993;103:1826–31. doi: 10.1378/chest.103.6.1826. [DOI] [PubMed] [Google Scholar]
- 7.Martin C, Viviand X, Leone M, Thirion X. Effect of norepinephrine on the outcome of septic shock. Crit Care Med. 2000;28:2758–65. doi: 10.1097/00003246-200008000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Sakr Y, Reinhart K, Vincent JL, Sprung CL, Moreno R, Ranieri VM, et al. Does dopamine administration in shock influence outcome. Results of the Sepsis Occurrence in Acutely Ill Patients (SOAP) Study? Crit Care Med. 2006;34:589–97. doi: 10.1097/01.CCM.0000201896.45809.E3. [DOI] [PubMed] [Google Scholar]
- 9.Boulain T, Runge I, Bercault N, Benzekri-Lefevre D, Wolf M, Fleury C. Dopamine therapy in septic shock: Detrimental effect on survival? J Crit Care. 2009;24:575–82. doi: 10.1016/j.jcrc.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Marik PE. Gastric intramucosal pH. A better predictor of multiorgan dysfunction syndrome and death than oxygen- derived variables in patients with sepsis. Chest. 1993;104:225–9. doi: 10.1378/chest.104.1.225. [DOI] [PubMed] [Google Scholar]
- 11.Fry DE. Multiple system organ failure. Surg Clin North Am. 1988;68:107–22. doi: 10.1016/s0039-6109(16)44435-x. [DOI] [PubMed] [Google Scholar]
- 12.Kreger BE, Craven DE, McCabe WR. Gram negative bacteremia.Reevaluation of clinical features and treatment in 612 patients. Am J Med. 1980;68:344–55. doi: 10.1016/0002-9343(80)90102-3. [DOI] [PubMed] [Google Scholar]
- 13.Varpula M, Tallgren M, Saukkonen K, Voipio-Pulkki LM, Pettilä V. Hemodynamic variables related to outcome in septic shock. Intensive Care Med. 2005;31:1066–71. doi: 10.1007/s00134-005-2688-z. [DOI] [PubMed] [Google Scholar]
- 14.Martin C, Saux C, Albanese J, Bonneru JJ, Gouin F. A new look at norepinephrine to treat hyperdynamic septic shock. Anaesthesiology. 1987;67:648. [Google Scholar]
- 15.Schreuder WO, Schneider AJ, Groenveld ABJ, Thijs LG. Effects of dopamine vs norepinephrine on hemodynamics in septic shock: Emphasis on right ventricular performance. Chest. 1989;95:1282–8. doi: 10.1378/chest.95.6.1282. [DOI] [PubMed] [Google Scholar]
- 16.Abraham E, Bland RD, Cobo JC, Shoemaker WC. Sequential cardiorespiratory patterns associated with outcome in septic shock. Chest. 1984;85:75–80. doi: 10.1378/chest.85.1.75. [DOI] [PubMed] [Google Scholar]
- 17.Groeneveld AB, Navita JJ, Thijs LG. Peripheral vascular resistance in septic shock: Its relation to outcome. Interns Care Med. 1989;14:141–7. doi: 10.1007/BF00257468. [DOI] [PubMed] [Google Scholar]
- 18.De Backer D, Creteur J, Silva E, Vincent JL. Effects of dopamine, norepinephrine, and epinephrine on the splanchnic circulation in septic shock: Which is best? Crit Care Med. 2003;31:1659–67. doi: 10.1097/01.CCM.0000063045.77339.B6. [DOI] [PubMed] [Google Scholar]
- 19.Mills LC, Moyer JH, Handley CA. Effects of various sympathomimetic drugs on renal hemodynamics in normotensive and hypotensive dogs. Am J Physiol. 1960;198:1279–83. doi: 10.1152/ajplegacy.1960.198.6.1279. [DOI] [PubMed] [Google Scholar]


