Abstract
Introduction:
Measurement of respiratory quotient (RQ) and resting energy expenditure (REE) has been shown to be helpful in designing nutritional regimens. There is a paucity of the literature describing the impact of a feeding regimen on the energy expenditure patterns. Therefore, we studied the effect of continuous vs. intermittent feeding regimen in head-injured patients on mechanical ventilation on RQ and REE.
Methods:
After institutional ethical approval, this randomized study was conducted in 40 adult male patients with head injury requiring controlled mode of ventilation. Patients were randomly allocated into two groups. Group C: Feeds (30 kcal/kg/day) were given for 18 h/day, with night rest for 6 h. Group I: Six bolus feeds (30 kcal/kg/day) were given three hourly for 18 h with night rest for 6 h. RQ and REE were recorded every 30 min for 24 h. Blood sugar was measured 4 hourly. Other adverse effects such as feed intolerance, aspiration were noted.
Results:
Demographic profile and SOFA score were comparable in the two groups. Base line RQ (0.8 vs. 0.86) and REE (1527 vs. 1599 kcal/day) were comparable in both the groups (P>0.05). RQ was comparable in both groups during the study period at any time of the day (P>0.05). Base line RQ was compared with all other RQ values measured every half hour and fluctuation from the base line value was insignificant in both groups (P>0.05). REE was comparable in both the groups throughout the study period (P>0.5). Adequacy of feeding as assessed by EI/MREE was 105.7% and 105.3% in group C and group I, respectively. There was no significant difference in the blood sugar levels between the two groups (P>0.05).
Conclusion:
We found from our study that RQ, REE, and blood sugar remain comparable with two regimens of enteral feeding – continuous vs. intermittent in neurosurgical patients on ventilator support in a ICU setup.
Keywords: Blood sugar, continuous enteral feeding, intermittent enteral feeding, respiratory quotient, resting energy expenditure
INTRODUCTION
Indirect calorimetry is a convenient, accessible, and highly accurate instrument for the measurement of caloric requirements. Thus, it is a valuable tool for the optimization of nutritional support in the intensive care unit (ICU).[1] Measurement of respiratory quotient (RQ) and resting energy expenditure (REE) has been shown to be helpful in designing nutritional regimens to reduce expired carbon dioxide (VCO2) in patients requiring mechanical ventilation.[2]
Malnutrition leads to impaired immunity, delayed wound healing, and decreased muscle strength, and thus, patients cope poorly with modern medical and surgical interventions leading to prolonged hospital stay.[3] Muscular weakness due to nutritional deficiency in neurosurgical patients may be errantly attributed to neurological insult. Nutritional intake affects carbon dioxide (CO2) production and in neurosurgical patients, any increased/decreased CO2 production will alter cerebral blood flow and thus affects the neurological outcome.[2–6] There is a paucity of the literature describing the impact of a feeding regimen on the energy expenditure patterns of patients.
However, we failed to find any clinical research study on the relationship among RQ, REE, and random blood sugar (RBS) in relation to the feeding regimen in head injury patients. Thus, we hypothesized that by giving continuous enteral feeding RQ, REE and blood sugar will be maintained within the physiological limit. Therefore, we studied the effect of continuous vs. intermittent feeding regimen on RQ, REE in head injury patients on mechanical ventilation.
METHODS
After obtaining Institutional Review Board approval, this randomized controlled study was conducted in the ICU of a tertiary care teaching institute. Forty adult male patients in the age group of 20-60 years with history of head injury requiring mechanical ventilatory support (controlled mode of ventilation) were included in the study. Patients were required to have functional gastrointestinal tract. Patients with signs of sepsis, fever, pneumonia, hemodynamically unstable (e.g., cardiac arrhythmia, need for vasoactive drug support), abnormal renal, hepatic, cardiac function, and receiving fraction of inspired oxygen (FiO2) of more than 0.6 were excluded from the study. The study protocol was explained and a written informed consent was obtained from next of kin for all patients.
Selected patients were randomly allocated into two groups of 20 each using computer-generated random number.
Group C: Continuous enteral feeding
Feeds were given for 18 h/day as a continuous infusion, with night rest for 6 h. Amounts of feed calculated (30 kcal/kg/day) were administered with the help of enteral feeding pump (KANGAROO™ Enteral Feeding Pump, Tyco Healthcare group LP, Manshield, MA, USA).
Group I: Intermittent enteral feeding
The calculated six bolus feeds were given three hourly for 18 h with night rest for 6 h. Amounts of feed calculated (30 kcal/kg/day) were administered using a 50 mL syringe manually.
A total caloric requirement of 30 kcal/kg body weight/day was administered with a carbohydrate-to-fat ratio of 60:40. The same formula feed with a calorie density of 1 kcal/mL was given to both the groups (Ensure, Abbott Laboratories BV, Zwolle, The Netherlands, Division of Abbott Laboratories, USA). For preparing 220 mL of feed, six level scoops (53.4 g) of feeding formula powder was gradually added and mixed in 190 mL of water in a glass which provided energy equivalent to 1 kcal/mL. A oral enteral tube of size 16G was used in all patients. Patients were kept in 30° head up position. The position of feeding tube was confirmed by aspiration of the gastric content, its type and amount, auscultation of bowel sounds and laryngoscopy if in doubt. During the study period, all ventilatory parameters were kept constant. All patients received sedation according to our institutional protocol.
Baseline parameters such as complete hemogram, liver function test, kidney function test (blood urea, serum creatinine), serum electrolyte (serum sodium, serum potassium), and sequential organ failure assessment (SOFA) scoring were recorded.[7] RQ and REE was recorded every 30 min for 24 h using the Deltatrac II metabolic monitor (Engstrom carestation, Datex-Ohmeda Inc., Madison, WI USA). Blood sugar was measured with the help of Glucometer (Ascensia ENTRUST™ Blood glucose meter/test strips, Bayer Healthcare LIC, Mishawake, IN USA)) 4 hourly for 24 h. The degree of feeding was defined as energy intake (EI) divided by measured resting energy expenditure (MREE).[8] Underfeeding was defined as a ratio lower than 90%, adequate feeding as a ratio of 90–110%, and overfeeding as a ratio higher than 110%. Underfeeding was defined as an RQ lower than 0.85 and overfeeding as an RQ higher than 1.
Signs of intolerance if present such as abdominal distention, diarrhea, absent bowel sounds were recorded. In group C, tolerance to feed was assessed every four hourly after stopping the feeding pump for half an hour and aspiration of gastric content. If aspirate volume was more than 200 mL then 100 mL aspirate was replaced, feeding was continued at same rate for next 4 h and aspirate was rechecked. If aspirate was still more than 200 mL, a clinical sign of intolerance was checked. In group I, tolerance to feed was assessed by gastric aspiration three hourly before every feed. If aspirate volume was more than 200 mL then 100 mL aspirate was replaced along with the rest of the feeding amount and aspirate was rechecked after 3 h. If again more than 200 mL or more than half the previous feed was aspirated then sign of tolerance were observed. In both the groups, if signs of intolerance were absent and volume of aspirate was more than 200 mL, then metoclopromide 0.15 mg/kg intravenous was administered twice a day. In the presence of signs of intolerance, the patient was excluded from the study and nutrition was provided as per institutional protocol. During the study period, if patients developed hemodynamic instability, accidentally extubated or needed urgent diagnostic or therapeutic procedures, they were excluded from the study.
Statistical analysis
Assuming a difference in RQ and REE of more than 20% due to different feeding regimens. i.e. continuous and intermittent enteral feeding to be of clinical significance, we required 19 patients in each group for a power of 80% and a error of 0.05. In addition, a difference in REE and EI of 250–500 kcal/day between two feeding regimens has clinical relevance. Based on this, with a power of 80% and a error of 0.05, we required 17 patients in each group. Therefore, to accommodate any dropouts of patients from study analysis, we randomized 40 patients in two groups of 20 each. Statistical evaluation was done by using SPSS version 16. The Student t-test, Paired t-test, Chi-Square test, and Mann–Whitney test was used as appropriate. The t-test was used for analyzing age, weight, height, and body surface area, RBS and RQ between the groups. The paired t-test was used for RQ and RBS to see fluctuation within the group. The Chi-Square test was used for analyzing age distribution and SOFA score. The Mann–Whitney test was used for comparison of FiO2 in two groups.
RESULTS
Seventy-two patients were recruited for the study, and 40 patients were randomly allocated to two groups because the rest of the patients did not fulfill the study criteria as described above [Consort flow chart, Figure 1]. No patient was excluded after randomization from study analysis. The demographic profile and SOFA score were comparable in the two groups (P > 0.05) [Table 1]. The mean value of fraction of inspired oxygen was comparable in the both the groups (P = 0.509).
Figure 1.
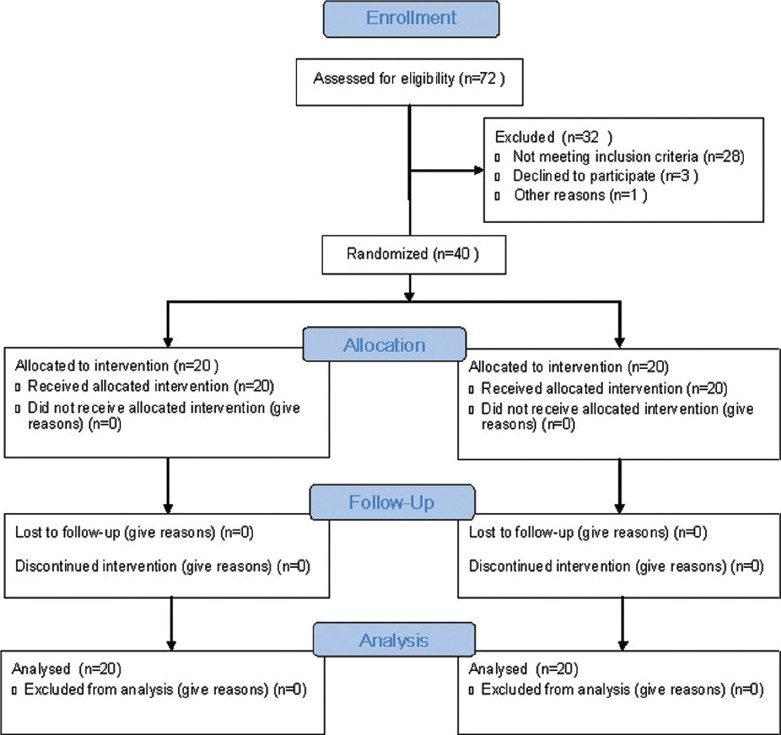
Consort flow diagram
Table 1.
Demographic profile of patients in the groups C and I
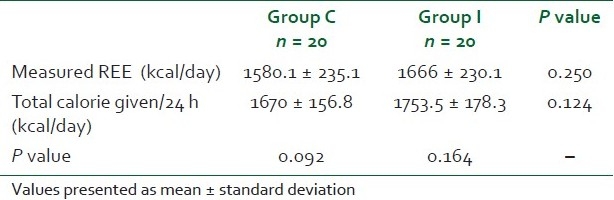
In each patient, 48 measurements, each of RQ and REE, were taken in both the groups. The base line RQ (0.8 vs. 0.86) and REE (1527 vs. 1599 kcal/day) were comparable in both the groups (P > 0.05) [Table 1]. RQ was comparable in both groups during the study period at any time of the day (P > 0.05). RQ values in both groups were within physiological limits [Figure 2]. The base line RQ was compared with all other RQ values measured every half hour, and fluctuations from the base line value were insignificant in both the groups (P > 0.05). REE was comparable in both the groups throughout the study period (P > 0.5) [Figure 3]. Measured REE was also comparable with total calorie given in both the groups [Table 2]. Adequacy of feeding as assessed by EI/MREE was 105.7% and 105.3% in groups C and I, respectively.
Figure 2.
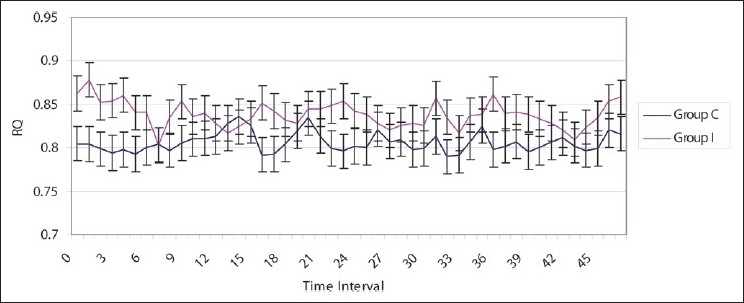
Respiratory quotient in two groups
Figure 3.
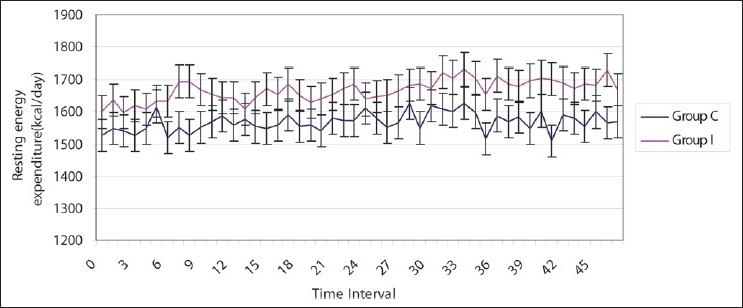
Resting energy expenditure in two groups
Table 2.
Comparison of measured resting energy expenditure and total energy intake
There was no significant difference in the blood sugar levels (measured every 4 hourly) between the two groups (P > 0.05) [Figure 4]. Blood sugar ranged from 142 to 151 mg/dL and 146 to 156 mg/dL in groups C and I, respectively. There was no incidence of hypoglycemia or hyperglycemia in either of the groups. Base line blood sugar levels was also compared with blood sugar levels at 4, 8, 12, 16, 20, 24 h, and no significant fluctuations from the base line value were observed in both the groups (P > 0.05).
Figure 4.
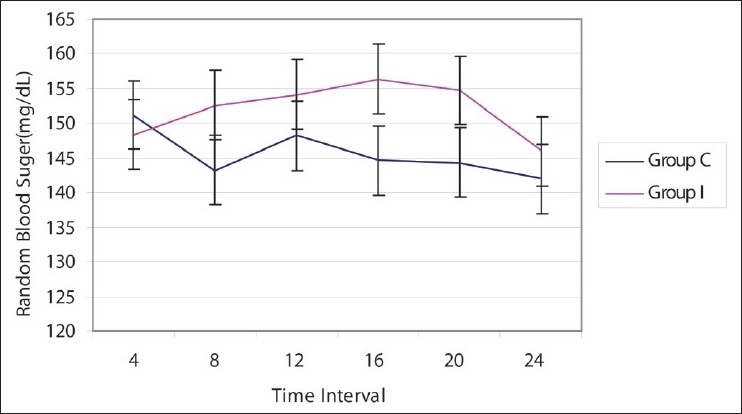
Blood sugar levels in two groups
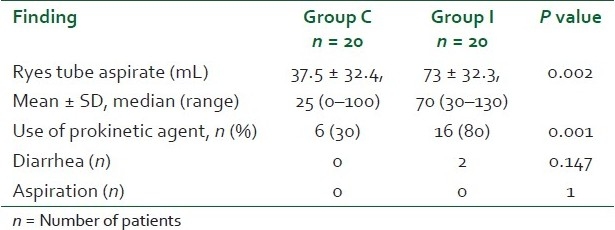
Ryles tube aspiration was performed every three hourly in both the groups as per our ICU protocol. In group C, aspirate was significantly less as compared to that of group I (P = 0.002) [Table 3]. There was significant use of metoclopramide in group I as compared to group C (P = 0.001). Two patients in group I suffered from diarrhea as compared to none in group C (P=0.147).
Table 3.
Adverse effects in the two groups
DISCUSSION
We found from our study that RQ and REE remain comparable with the two regimen of enteral feeding – continuous vs. intermittent in head injury patients on ventilator support in ICU. Irrespective of the feeding regimen, RQ was in physiological limits and in range of adequate feeding. Measured REE when compared with EI depicted adequate feeding in both types of the feeding regimen – continuous and intermittent. The blood sugar levels were also comparable in two modes of feeding regimen – continuous and intermittent.
Indirect calorimetry has been suggested for clinical use to quantitatively measure energy needs and assess nutritional status.[9] The existing REE formulae are easy to use, inexpensive, and universally available, but they have been developed based on data derived from healthy individuals.[9] Thereafter, many authors suggested modifying their equations by using correction factors to better estimate REE in different subgroups of patients. However, REE calculated from those equations varied widely from indirect calorimetrically measured REE, with estimations ranging from −38% to +43% in populations of initially ill patients at ICU admission.[10] The RQ, obtained by indirect calorimetry, is defined by the ratio of VCO2 to oxygen consumption (VO2). Underfeeding can promote the use of endogenous fat stores which decreases the RQ (RQ substrate < 0.70), whereas overfeeding, which results in lipogenesis, increases the RQ (RQ >1.0). Adequate feeding is defined as RQ ranges between 0.8 and 1.0 (11). The physiological range for the overall measured RQ exists between 0.67 and 1.3.[11] Thus, variations in the RQ in response to the feeding regimen may indicate inappropriate feeding and serve as a marker for patient intolerance.
RQ and REE are affected by various body functions and pathological states such as the presence of sepsis, abdominal, and thoracic surgery with involvement of the respiratory system.[12,13] Therefore, we excluded these patients and our study population included only head injury patients. As REE is more in males as compared to females, so we decided to select adult male patients for the study to eliminate confounding factor due to sex.[2,12,13] Moreover to avoid any other confounding variables related to body systems, we compared SOFA scoring in both the groups (P = 0.158). Inspired oxygen concentration plays an important role in evaluation of RQ and REE. It has been reported that FiO2 > 0.6 will give erroneous value of RQ and REE.[2,14,15] Therefore, we compared the FiO2 values at the time of patient recruitment for the study which was comparable in both the groups (P = 0.509). Moreover, the range of FiO2 was 0.40–0.50 in both groups C and I. Respiration of all the patients was assisted on volume-controlled mode. Ventilatory settings were not changed, and no weaning trial was performed during study period as patient-triggered ventilatory modes or weaning trial could change the energy requirement which may lead to inaccurate RQ and REE.[2,6] We decided to provide a nutritional calorie requirement of 30 kcal/kg/day as this amount of calorie is sufficient to provide the basic metabolic activity of the body.
Gravity-controlled administration of enteral nutrition has been found to have more incidence of flatulence, epigastric fullness, regurgitation, vomiting, and diarrhea as compared to pump-assisted enteral nutrition.[16] The blood glucose profile is better controlled in pump-assisted enteral nutrition.[16] Therefore, we used pump-assisted nutrition in the continuous group for enteral nutrition.
In our study baseline, RQ and REE were comparable in both the groups (P = 0.201 and 0.368, respectively). This shows that both groups were comparable with respect to energy requirement and thermogenesis. The value of RQ was within 0.8–1.0 during the study period of 24 h in both the groups. In our study, as there was no significant fluctuation of RQ from the base line in either groups (P > 0.05), we inferred that mode of nutrition did not have any impact on RQ at different time intervals. This shows that patients in both groups were adequately fed whatever was the method of enteral feeding. Therefore, it appears from our study that administering nutrition via either modes, i.e. continuous vs. intermittent (every 3 hourly) provides adequate nutrition.
Our results are in contrast to Nacht et al. who studied continuous respiratory exchange measurements on five women and five men for 1 h before and until 6 h after the administration of milkshake (53% carbohydrates, 30% lipid, and 17% protein) given either as a single bolus dose or continuously during 3 h using a nasogastric tube.[17] RQ, REE, plasma glucose, and insulin concentrations increased sooner and steeper with the meal ingested as a single dose than with continuous administration. They concluded that the mode of enteral nutrient administration influences the immediate thermogenic response as well as changes in RQ, glycemia, and insulinemia. This difference, as compared to our study, may be due to different type and composition of nutrition used. Different type of feeds may lead to different absorption rates as per its glycemic index leading to peaking of glucose levels at different times or may not show rise in glucose. Nacht et al. used the chocolate flavored milk while we used commercially available product with a different composition (formula feed). However, similar to our study, the overall nutrient balance was not affected by the mode of enteral nutrient administration.
There was no significant difference between measured REE and total calorie given during 24-h in either groups (P > 0.05). Therefore, it appears from our study that REE is suitably provided by either mode of nutrition, i.e. continuous or intermittent (three hourly) when 30 kcal/kg/day are provided. Elevated metabolic rates of 130% to more than 200% of predicted values have been well described for some disease states, including burns, sepsis, trauma, and head injury and are reflective of increased oxygen consumption associated with injury severity.[18] McClave et al. concluded from his multicentre trial that energy expenditure is difficult to predict on the basis of conventional equations and measuring a patient's energy requirement at least once by indirect calorimetry is important, because the degree of metabolism predicts how easily a patient will be underfed or overfed.[19] The amount of infused calories should be compared with caloric requirements measured by indirect calorimetry, because the accuracy or degree of underfeeding or overfeeding has an impact on ventilatory status.[19]
The blood sugar in the two groups at 4-h interval was comparable in both the groups being in physiological range, and there were no significant fluctuations. Therefore, it shows that regimen of feeding does not appear to have an effect on RBS if patients are adequately fed. In contrast to Nacht et al., there was no increased concentrations sooner and steeper in our study, most probably because of selection of different types of feed.[17] Our results are also in contrast to results of Sanz et al. who studied glucose metabolism during different feeding regimens administered either as bolus or as continuous infusion in diabetic patients.[20] They concluded that continuous feeding would be an interesting choice to improve glucose control in diabetic patients with enteral nutrition.
We observed from our study that the ryles tube aspirate was significantly more in the intermittent group as compared to the continuous group (P = 0.002) and thus requiring significant use of the prokinetic agent in the intermittent group as compared to the continuous group (P = 0.001), but no patient was excluded because of feed intolerance. This observation is comparable to earlier study by Rhoney et al. wherein they compared the effect of bolus vs. continuous gastric feeding in brain-injured patients in a retrospective cohort study.[21]
In our study, only two patients in the intermittent group and none in the continuous group had diarrhea (P = 0.147). Our results are comparable to Lee et al. who reported from their randomized controlled study that the median diarrhea scores of the continuous infusion group and the intermittent bolus group were comparable and not significant.[22] On the contrary, Jerry et al. reported statistically significant increased incidence of diarrhea in the intermittent group as compared to the continuous group of enteral feeding.[23]
In our study, no patient had any episode of pulmonary aspiration based on clinical observation. The exact incidence of aspiration and pneumonia is difficult to interpret from our study as it was a short duration study. Other authors have reported the episode of pneumonia due to aspiration in patients receiving enteral nutrition, but were comparable in patients receiving either continuous or intermittent nutrition.[24] Bonten et al. studied the influence of intermittent or continuous enteral feeding on gastric and oropharyngeal colonization and ventilator-associated pneumonia and concluded that the colonization rates of the oropharynx and trachea, the incidence of ventilator-associated pneumonia (VAP) and mortality were similar in both study groups.[25]
Our study has a few limitations. The study period was for 24h, and follow-up was not analyzed for longer duration. The indirect calorimetry was measured during the stress phase of illness, and the catabolic phase which persists for 7-10 days following the stress phase of 12-24 h was not studied. In addition, the effect of either of the feeding regimens when given for longer duration needs to be further evaluated with respect to impact on days of ventilation, ICU stay, and ease of weaning. In our study, nutritional adequacy was determined by REE only and not by anthropometric and biochemical measurements. Also, blinding was not possible in the study, but the collection of data relating to RQ and REE (primary outcome variable) was objective as obtained from the monitor and appears to be nonbiased.
CONCLUSION
Hence we conclude from our study that the two modes of nutrition – continuous feeds or intermittent feed every 3 h maintains RQ, REE, and RBS in the acceptable range in head-injured patients on ventilatory support in ICU.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.McClave SA, McClain CJ, Snider HL. Should indirect calorimetry be used as part of nutritional assessment? J Clin Gastroenterol. 2001;33:14–9. doi: 10.1097/00004836-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 2.American Association for Respiratory Care (AARC). AARC clinical practice guideline. Metabolic measurement using indirect calorimetry during mechanical ventilation. Respir Care. 1994;39:1170–5. [PubMed] [Google Scholar]
- 3.Stroud M, Duncan H, Nightingale J. Guidelines for enteral feeding in adult hospital patients. Gut. 2003;52:vii1–vii12. doi: 10.1136/gut.52.suppl_7.vii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raurich JM, Ibanez J, Marse P, Riera M, Homar X. Resting Energy Expenditure During Mechanical Ventilation and Its Relationship With the Type of Lesion. JPEN J Parenter Enteral Nutr. 2007;31:58–62. doi: 10.1177/014860710703100158. [DOI] [PubMed] [Google Scholar]
- 5.Kan MN, Chang HH, Sheu WF, Cheng CH, Lee BJ, Huang YC. Estimation of energy requirements for mechanically ventilated, critically ill patients using nutritional status. Crit Care. 2003;7:108–15. doi: 10.1186/cc2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benotti PN, Bistrian B. Metabolic and nutritional aspects of weaning from mechanical ventilation. Crit Care Med. 1989;17:181–5. doi: 10.1097/00003246-198902000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Vincent JL, De Mendonca A, Cantraine F, Moreno R, Takal J, Suter PM, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Working group om ‘sepsis-related problems’ of the european society of Intensive Care Medicine. Crit Care Med. 1998;26:1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Liusuwan MR, Palmieri TL, Greenhalgh DG. The respiratory quotient has little value in evaluating the state of feeding in burn patients. J Burn Care Res. 2008;29:655–9. doi: 10.1097/BCR.0b013e31817db9e3. [DOI] [PubMed] [Google Scholar]
- 9.Metsios GS, Stavropoulos-Kalinoglou A, Panoulas VF, Koutedakis Y, Nevill AM, Doudhlas KM, et al. New resting energy expenditure prediction equations for patients with rheumatoid arthritis. Rheumatology (Oxford) 2008;47:500–6. doi: 10.1093/rheumatology/ken022. [DOI] [PubMed] [Google Scholar]
- 10.Savard JF, Faisy C, Lerolle N, Guerot E, Diehl JL, Fagon JY. Validation of a predictive method for an accurate assessment of resting energy expenditure in medical mechanically ventilated patients. Crit Care Med. 2008;36:1175–83. doi: 10.1097/CCM.0b013e3181691502. [DOI] [PubMed] [Google Scholar]
- 11.Liusuwan MR, Palmieri TL, Greenhalgh DG. The respiratory quotient has little value in evaluating the state of fedding in burn patients. J Burn Care Res. 2008;29:655–9. doi: 10.1097/BCR.0b013e31817db9e3. [DOI] [PubMed] [Google Scholar]
- 12.McClave SA, McClain CJ, Snider HL. Should Indirect Calorimetry be used as Part of Nutritional Assessment? J Clin Gastroenterol. 2001;33:14–9. doi: 10.1097/00004836-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Faisy C, Guerot E, Diehl JL, Labrousse J, Fagon JY. Assessment of Resting Energy Expenditure in Mechanically Ventilated Patients. Am J Clin Nutr. 2003;78:241–9. doi: 10.1093/ajcn/78.2.241. [DOI] [PubMed] [Google Scholar]
- 14.Ultman JS, Bursztein S. Analysis of error in the determination of respiratory gas exchange at varying FIO2. J Appl Physiol. 1981;50:210–6. doi: 10.1152/jappl.1981.50.1.210. [DOI] [PubMed] [Google Scholar]
- 15.Browning JA, Linberg SE, Turney SZ, Chodoff P. The effects of a fluctuating FIO2 on metabolic measurements in mechanically ventilated patients. Crit Care Med. 1982;10:82–5. doi: 10.1097/00003246-198202000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Shang E, Geiger N, Sturm JW, Post S. Pump Assisted versus gravity controlled enteral nutrition in long term percutaneous endoscopic gastrostomy patients: A prospective controlled trial. JPEN J Parenter Enteral Nutr. 2003;27:216–9. doi: 10.1177/0148607103027003216. [DOI] [PubMed] [Google Scholar]
- 17.Nacht CA, Schutz Y, Vernet O, Christin L, Jequier E. Continuous versus single bolus enteral nutrition: Comparison of energy metabolism in humans. Am J Physiol. 1986;251:524–9. doi: 10.1152/ajpendo.1986.251.5.E524. [DOI] [PubMed] [Google Scholar]
- 18.Finestone HM, Greene-Finestone LS, Foley NC, Woodbury MG. Measuring longitudinally the metabolic demands of stroke patients: Resting energy expenditure is not elevated. Stroke. 2003;34:502–7. doi: 10.1161/01.str.0000053031.12332.fb. [DOI] [PubMed] [Google Scholar]
- 19.McClave SA, Lowen CC, Kleber MJ, Nicholson JF, Jimmerson SC, McConnell JW, et al. Are patients fed appropriately according to their caloric requirements? JPEN J Parenter Enteral Nutr. 1998;22:375–81. doi: 10.1177/0148607198022006375. [DOI] [PubMed] [Google Scholar]
- 20.Sanz París A, Lázaro J, Guallar A, Gracia P, Caverni A, Albero R. Continuous enteral nutrition versus single bolus: Effects on urine C peptide and nitrogen balance. Med Clin (Barc) 2005;124:613–5. doi: 10.1157/13074390. [DOI] [PubMed] [Google Scholar]
- 21.Rhoney DH, Parker D, Jr, Formea CM, Yap C, Coplin WM. Tolerability of bolus versus continuous gastric feeding in brain-injured patients. Neurol Res. 2002;24:613–20. doi: 10.1179/016164102101200456. [DOI] [PubMed] [Google Scholar]
- 22.Lee JS, Auyeung TW. A comparison of two feeding methods in the alleviation of diarrhoea in older tube-fed patients: A randomized controlled trial. Age Ageing. 2003;32:388–93. doi: 10.1093/ageing/32.4.388. [DOI] [PubMed] [Google Scholar]
- 23.Ciocon JO, Galindo-Ciocon DJ, Tiessen C, Galindo D. Continuous Compared With Intermittent Tube Feeding in the elderly. JPEN J Parenter Enteral Nutr. 1992;16:525–8. doi: 10.1177/0148607192016006525. [DOI] [PubMed] [Google Scholar]
- 24.Lee JS, Kwok T, Chui PY, Ko FW, Lo WK, Kam WC, et al. Can continuous pump feeding reduce the incidence of pneumonia in nasogastric tube-fed patients. A randomized controlled trial? Clin Nutr. 2010;29:453–8. doi: 10.1016/j.clnu.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Bonten MJ, Gaillard CA, van der Hulst R, de Leeuw PW, van der Geest S, Stobberingh EE, et al. Intermittent enteral feeding: The influence on respiratory and digestive tract colonization in mechanically ventilated intensive-care-unit patients. Am J Respir Crit Care Med. 1996;154:394–9. doi: 10.1164/ajrccm.154.2.8756812. [DOI] [PubMed] [Google Scholar]


