Abstract
Purpose of review
Bleeding and death from hemorrhage remain a leading cause of morbidity and mortality in the trauma population. Early resuscitation of these gravely injured patients has changed significantly over the past several years. The concept of damage control resuscitation has expanded significantly with the experience of the US military in southwest Asia. This review will focus on this resuscitation strategy of transfusing blood products (red cells, plasma, and platelets) early and often in the exsanguinating patient.
Recent findings
In trauma there are no randomized controlled trials comparing the current damage control hematology concept to more traditional resuscitation methods. But the overwhelming conclusion of the data available support the administration of a high ratio of plasma and platelets to packed red blood cells. Several large retrospective studies have shown ratios close to 1 : 1 will result in higher survival.
Summary
The current evidence supports that the acute coagulopathy of trauma is present in a high percentage of trauma patients. Patients who will require a massive transfusion will have improved outcomes the earlier that this is identified and the earlier that damage control hematology is instituted. Current evidence does not describe the best ratio but the preponderance of the data suggests it should be greater than 2 : 3 plasma-to-packed red blood cells.
Keywords: damage control resuscitation, exsanguinations, hemorrhage, massive transfusion, trauma
Introduction
Trauma is the leading cause of death in the age group of 1–44 years [1]. Hemorrhagic shock and exsanguination are responsible for a large number of these deaths, accounting for more than 80% of deaths in the operating room and nearly 50% of deaths in the first 24 h after injury [2–5]. Fortunately, less than 5% of civilian trauma patient admissions will require a massive transfusion [10 or more units of packed red blood cells (PRBCs) in the first 24 h] [6–8]. More importantly, this group of patients who require massive transfusion accounts for 75% of the blood utilization in busy urban trauma centers [9]. Several authors have demonstrated improved outcomes by using predefined ratios of blood products, early in the care of these severely injured patients [7,10••,11•,12,13•,14,15,16•]. Rapid processing and preparation of such a large amount of blood and blood products in a short period of time requires significant planning and prior coordination of personnel and dedicated resources to ensure delivery of these products in an immediate and sustained fashion (Fig. 1).
Figure 1.
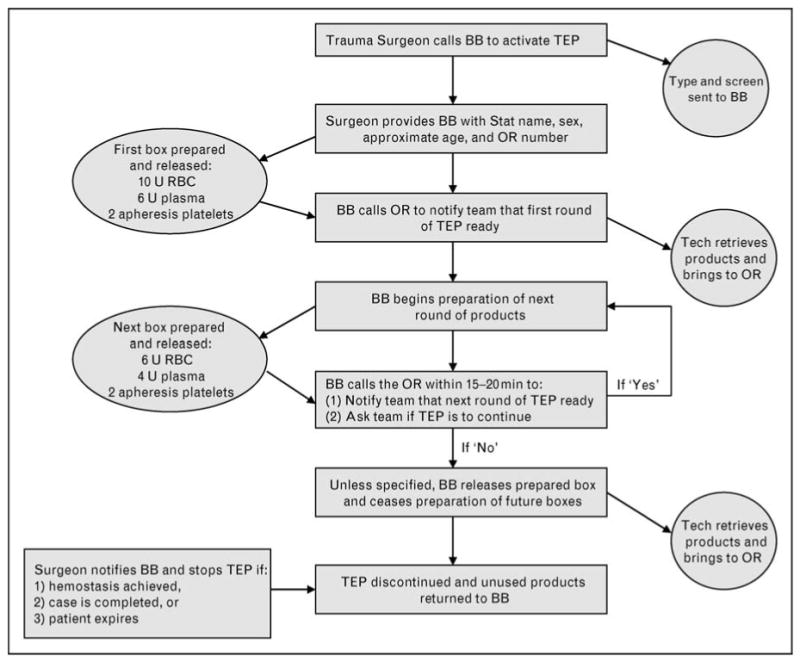
Proposed algorithm for massive transfusion protocol implementation
Acute coagulopathy of trauma shock
Over one-third of injured civilian and military patients have evidence of coagulopathy already present on admission to the trauma center [14,17–20]. Not only do available data suggest that this coagulopathy occurs very early (regardless of resuscitation), it is highly lethal [14,17–21]. In light of these findings, military and civilian researchers have increased their focus on the role of acute coagulopathy of trauma shock (ACoTS) in the early management of the exsanguinating patient. ACoTS is not a simple dilutional coagulopathy that occurs in injured patients but a complex problem with multiple factors whose mechanisms overlap with one another [22]. Whereas multiple contributing factors exist, the key initiator to the process of ACoTS is shock. This process is separate and distinct from disseminated intravascular coagulopathy with its own distinct hemostatic failure. Because of this known early coagulopathy, the current approach to managing the exsanguinating patient involves early implementation of damage control resuscitation (DCR) [6,10••]. DCR is composed of three basic components: permissive hypotension, minimizing crystalloid-based resuscitation strategies and the immediate release and administration of predefined blood products (PRBCs, plasma, and platelets) in ratios similar to that of whole blood.
Patient identification and selection for massive transfusion
Identifying patients who will benefit from DCR (only 3–5% of the injured population) is often challenging, even for experienced trauma physicians at busy level 1 trauma centers [6,7]. Whereas there are currently no uniform activation criteria for such protocols, several groups have developed scoring systems (using a variety of anatomic, physiologic, and laboratory variables) to correctly identify the patient who will likely require a massive transfusion [23–26]. Though each of these scoring systems is quite accurate, the majority of these require laboratory data and injury severity assessment. Given these limitations, Nunez and colleagues [27] developed a scoring system that relies only on data (physiology and mechanism of injury) readily available during the primary survey. The Assessment of Blood Consumption (or ABC) score correctly identifies those individuals who will or will not require massive transfusion approximately 85% of the time (Table 1). It is important to note, however, that each of these scoring systems should be used to augment, not replace, a trauma attending's clinical decision-making.
Table 1. Suggested nonweighted, nonlaboratory scoring system to predict the need for massive transfusion.
| Assessment of Blood Consumption (ABC) score |
| ED systolic blood pressure ≤90mmHg (0 = no, 1 = yes) |
| ED heart rate ≥120 b.p.m. (0 = no, 1 = yes) |
| Penetrating mechanism (0 = no, 1 =yes) |
| Positive fluid on abdominal ultrasound (0 = no, 1 =yes) |
| Score of 2 predicts 38% need for massive transfusion |
| Score of 3 predicts 45% need for massive transfusion |
| Score of 4 predicts 100% need for massive transfusion |
Products of massive transfusion
Whole blood and blood components are a precious commodity used in the resuscitation of the acutely injured patient. Clinicians need to understand the risks and benefits of the transfusion of each of these components. Allogenic blood transfusion is dependent on the millions of volunteer blood donors which accentuates the precious nature of this product.
Packed red blood cells
Component therapy has been the mainstay of transfusion therapy for the exsanguinating patient. PRBCs are the most utilized of the components in the treatment of trauma patients. Each unit has a shelf life of around 40 days. Unfortunately when given without other products it does not simulate what the trauma patient is losing. It is cold, lacks platelets, coagulation factors, and has a hematocrit of 55% [28].
Whole blood
Primarily a tool of the military, used in combat theaters with the mechanism of a walking blood bank [29]. The US military has fresh whole blood (FWB) extensively in the ongoing conflicts in southwest Asia. In a recent review of the military's experience with the transfusion of over 6000 units of FWB, Spinella [30•] was able to show a favorable risk benefit ratio in the use of FWB. FWB is warm, volume is close to 500 ml, hematocrit is 38–50%, 150–400 K platelets, 100% coagulation activity, and 1500 mg fibrinogen or exactly what the patient is losing [28,31].
Plasma
Fresh frozen plasma (FFP) has been available for transfusion since 1941 and as its name implies is kept in a frozen state at −30°C. Once the 200 ml unit of FFP is thawed in a 37°C water bath, it is available for immediate use. It is acceptable to keep this thawed plasma stored at a temperature of 4°C for up to 24 h and it will maintain its full hemostatic factor content [32–34]. After 24 h, plasma has a predictable rate of decreasing hemostatic factor content. Thawed plasma at 5 days does have adequate hemostatic activity to be used in massive transfusion protocols despite significant degradation in factor VIII activity [32,34]. Of the blood components used, plasma is severely limited in the United States; as of December 2007, 95% of the plasma issued for donation from the American Red Cross was from male donors only [35].
Liquid plasma
Another blood component option which contains essentially the same hemostatic factor content as thawed plasma is liquid plasma [34,36–38]. This form of plasma is separated from whole blood but is kept in the liquid phase as opposed to freezing [36]. It has a shelf life of almost a month and still retains enough hemostatic activity to be utilized in the face of a massive transfusion [34,38].
Platelets
Until the late 1960s platelets were not readily available for blood component therapy [39]. In most centers today the platelets are offered either as single-donor apheresis platelets or random-donor platelets which are pooled from multiple donors. Single-donor platelets are equal in volume and platelet concentration to 4–6 units of random donor platelets. Each will provide an expected rise in the platelets of about 20 000 [40]. Of the blood components platelets will have the shortest shelf life with use required within 5 days.
Adjuncts to massive transfusion
PRBCs, plasma, and platelets are the key components of a massive transfusion protocol, with some centers supplementing with cryoprecipitate replacement [8]. As well, some published protocols supplement their delivery with pharmacological adjuncts such as factor VIIa. Though arguably the most cost-effective and evidence-based adjunct, auto-transfusion/cell saver devices are considerably underutilized in the setting of massive transfusion in trauma.
Recombinant factor VIIa
Initial reports from the Israeli military on their use of recombinant factor VIIa (rfVIIa) in seven severely injured patients in 2001 were quite positive, noting cessation of diffuse bleeding and decreased blood product usage [41]. A recent review of the preclinical and clinical data available for the use of rfVIIa showed the drug to be well tolerated and possibly effective in the treatment of trauma-associated coagulopathy [42]. The US military has evaluated patients from the joint theater trauma registry and found patients who underwent massive transfusion and received rfVIIa early in their course had decreased 30-day mortality [43]. In a randomized study of exsanguinating patients, Boffard et al. [44] noted a reduction in the amount of blood transfused but did not find a mortality benefit. Similarly, Stein and colleagues [45] recently published their experience with ‘low-dose’ rfVIIa (1.2 mg) in trauma patients with evidence of coagulopathy and noted a significant reduction in prothrombin time and utilization of PRBCs and FFP.
Auto-transfusion
Two decades ago, Timberlake and McSwain [46] and Ozmen et al. [47] from Tulane showed that the use of an auto-transfusion device (such as a cell-saver) was well tolerated and effective in patients with intra-abdominal contamination and hemoperitoneum [46,47]. Smith and colleagues [48] recently noted that intraoperative blood salvage is not only well tolerated but that application of such devices is associated with a marked decrease in the use of banked blood. In a randomized controlled trial by Bowley et al. [49] there was no difference in the intraoperative blood salvage group compared to controls in regards to postoperative sepsis, survival, coagulopathy, and requirement for clotting factors. Given the proven reduction in the use of the precious commodity of banked blood, we would recommend that those centers with the capability to provide this adjunct ‘around-the-clock’, strongly consider the use of this valuable tool in the management of the exsanguinating patient.
Damage control resuscitation and the optimal ratios
An increasing number of institutions have demonstrated that a small portion of the trauma population will require a massive amount of blood products in a rapid fashion [21,28,50,51]. In light of this, it is essential that trauma centers have an established mechanism to deliver these products quickly and in the correct amounts to these critically injured patients. Several authors have shown that a trauma exsanguination protocol (TEP) can be successfully implemented and have a significant positive impact on trauma outcomes [10••,12,29,52•]. DCR is a team effort that requires teamwork, communication, and collaboration. The goal is to organize a group of individuals to think and act as a team with a common goal [53,54]. DCR evolved from the damage control surgery paradigm advocated by Stone et al. [55] and Rotondo et al. [56]. DCR involves the aggressive delivery of blood products which begins prior to any laboratory-defined anemia or coagulopathy [6,57,58]. Damage control hematology defines the process of delivering large amounts of blood products (third component of DCR) in an efficient manner in patients who have been identified as having life-threatening hemorrhage [10••,59,60].
To date, there are no prospective data informing clinicians of the optimal ratio of blood products for the massive transfusion trauma patient. Given the difficulty associated with performing a randomized controlled trial in a group of exsanguinating patients, several authors have attempted to define the optimal transfusion regimen in the absence of such a study design. Hirshberg et al. [57] created a computer-based hemodilution model to simulate the exsanguinating patient and found that current resuscitation protocols severely underestimated the need for clotting factor replacement. On the basis of their findings, the authors recommended aiming for a ratio of plasma to red blood cells (RBCs) of 2 : 3 and a ratio of platelets to RBCs of 8 : 10. Ho and colleagues [61,62] developed a mathematical model to simulate ACoTS and recommended the equivalent of whole blood be transfused. This ratio is similar to what has been proposed for DCR by the US military in the exsanguinating combat casualty [6,7,31].
An exhaustive review of the literature demonstrated no class 1 data (and little class 2 evidence) describing the ideal ratio to transfuse to the trauma patient with exsanguinating hemorrhage [7,8,11•,12,15,16•,31,51,63••,64]. On the basis of what was available, however, ratios of at least 2 : 3 for plasma : RBC and 1 : 5 for apheresis platelets : RBC seemed justifiable and were implemented [10••]. One group has found that patients receiving their massive transfusion protocol (plasma: RBC of 2 : 3 or greater and apheresis platelets : RBC of 1 : 5 or greater) have lower 30-day mortality when compared to patients receiving less than the prescribed ratios [13•]. This was similar to what the Denver group found in a retrospective review of 133 patients [65].
The clinical practice described by Beekley [31] advocates transfusing on a 1 : 1 : 1 ratio, essentially trying to recreate the transfusion of whole blood. Duchesne et al. [11•] recently evaluated their experience of patients who required a massive transfusion at their urban level 1 trauma center. The authors found that those resuscitated with plasma to RBC ratio of 1 : 1 had a distinct survival advantage over those with a ratio of 1 : 4. Holcomb et al. [63••] recently reported their findings from a multicenter, retrospective study of 466 massively transfused civilian trauma patients. The authors demonstrated that patients receiving higher ratios (>1 : 2) of plasma and platelets to RBC had decreased truncal hemorrhage and increased survival at 6 h, 24 h, and at 30 days. In an evaluation of the German Trauma Registry, Maegele and colleagues [64] evaluated outcomes in 713 critically injured patients who received a massive transfusion. They saw the greatest reduction in 24 h and 30-day mortality in the patients who achieved a high ratio of plasma to RBC. Sperry et al. [16•] recently evaluated 415 blunt trauma patients within the ‘Glue Grant’ database who received 8 units of RBC in 12 h. The authors demonstrated that in those patients who achieved a ratio of FFP : RBC greater than 1 : 1.5, a significantly lower mortality rate was observed in the first 48 h.
Outcomes of massive transfusion protocols
The TEP described by Cotton et al. [10••] has now been in place for over 3 years. After the first year, Cotton et al. published a retrospective cohort study of all TEP activations (69 patients) and compared them to a pre-TEP cohort of trauma patients who received massive transfusion (70 patients) [10••]. Given similar injuries, there was a 74% reduction in the odds of mortality in massive transfusion patients with the implementation of the TEP. This same group examined postinjury complications in the 2-year TEP group (125 patients) compared to that of the 2-year pre-TEP cohort (141 patients) [66]. Whereas there was no difference in renal failure or systemic inflammatory response syndrome, the incidence of pneumonia, pulmonary failure, open abdomens, and abdominal compartment syndrome were all lower in TEP patients. In addition, sepsis and multi-organ failure were also lower and there was a significant increase in ventilator-free days in the TEP patients. Consistent with previous findings, patients receiving the protocol had higher survival and received less blood products overall when compared to the nonprotocol cohort [66].
Other authors have also noted a significant benefit to the implementation of a massive transfusion protocol. Duchesne and colleagues [34] evaluated patients before and after establishing a massive transfusion protocol demonstrating more intraoperative blood product usage, high ratio of plasma to PRBC, less crystalloid use, and a significant reduction in mortality with patients receiving the massive transfusion protocol. Similarly, O'Keeffe et al. [52•] were able to show decrease in overall blood component usage and cost savings following massive transfusion protocol implementation. However, unlike Duchesne and Cotton they failed to demonstrate an improvement in mortality in the study group [52•].
Those injured in military conflict have also been shown to benefit from a predefined DCR protocol [7,51,67]. Fox et al. [51] looked retrospectively at two cohorts with extremity vascular injury from the Joint Trauma Theater Registry [68]. One cohort was prior to the widespread implementation of a clinical practice guideline employing DCR strategies; the other cohort was a group in which DCR was used [51]. This initial case control study showed that combat support hospitals in the combat theater were meeting the goals of DCR and furthermore showed excellent restoration of the patients' normal physiology and excellent early limb salvage [51,67].
Conclusion
Up to 5% of civilian trauma patients will require massive transfusion. This group of patients is likely to be coagulopathic on admission and require transfusion of large amounts of blood products in a relatively short period of time. Massive transfusion protocols are associated with improved survival in patients with exsanguinating hemorrhage. Much of this improvement in survival has been attributed to increased plasma and platelet to RBC ratios. However, our experience (as well as that of recent data) suggests that a well defined protocol with uniform early activation criteria, delivering of products in prespecified ratios and volumes, and a robust performance improvement/quality improvement (PI/QI) process is critical to the observed reductions in mortality. With a team effort, damage control hematology can improve patient outcomes and reduce overall blood product use.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 601–602).
- 1.Cothren CC, Moore EE, Hedegaard HB, Meng K. Epidemiology of urban trauma deaths: a comprehensive reassessment 10 years later. World J Surg. 2007;31:1507–1511. doi: 10.1007/s00268-007-9087-2. [DOI] [PubMed] [Google Scholar]
- 2.Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–193. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Acosta JA, Yang JC, Winchell RJ, et al. Lethal injuries and time to death in a level I trauma center. J Am Coll Surg. 1998;186:528–533. doi: 10.1016/s1072-7515(98)00082-9. [DOI] [PubMed] [Google Scholar]
- 4.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6 Suppl):S3–S11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 5.Demetriades D, Murray J, Charalambides K, et al. Trauma fatalities: time and location of hospital deaths. J Am Coll Surg. 2004;198:20–26. doi: 10.1016/j.jamcollsurg.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Holcomb JB, Jenkins D, Rhee P, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 7.Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 8.Malone DL, Hess JR, Fingerhut A. Massive transfusion practices around the globe and a suggestion for a common massive transfusion protocol. J Trauma. 2006;60(6 Suppl):S91–S96. doi: 10.1097/01.ta.0000199549.80731.e6. [DOI] [PubMed] [Google Scholar]
- 9.Como JJ, Dutton RP, Scalea TM, et al. Blood transfusion rates in the care of acute trauma. Transfusion. 2004;44:809–813. doi: 10.1111/j.1537-2995.2004.03409.x. [DOI] [PubMed] [Google Scholar]
- 10••.Cotton BA, Gunter OL, Isbell J, et al. Damage control hematology: the impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma. 2008;64:1177–1182. doi: 10.1097/TA.0b013e31816c5c80. discussion 1182-3. Comparison of two groups before and after implementation of a massive exsanguination protocol demonstrating improved survival and decreased blood usage. [DOI] [PubMed] [Google Scholar]
- 11•.Duchesne JC, Hunt JP, Wahl G, et al. Review of current blood transfusions strategies in a mature level I trauma center: were we wrong for the last 60 years? J Trauma. 2008;65:272–276. doi: 10.1097/TA.0b013e31817e5166. discussion 276-8. 4-year review demonstrating improved survival when high ratios of plasma to red blood cells is achieved. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez EA, Moore FA, Holcomb JB, et al. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62:112–119. doi: 10.1097/01.ta.0000250497.08101.8b. [DOI] [PubMed] [Google Scholar]
- 13•.Gunter OL, Jr, Au BK, Isbell JM, et al. Optimizing outcomes in damage control resuscitation: identifying blood product ratios associated with improved survival. J Trauma. 2008;65:527–534. doi: 10.1097/TA.0b013e3181826ddf. Demonstration of optimal ratio of plasma to red blood cells of 2 : 3. [DOI] [PubMed] [Google Scholar]
- 14.Niles SE, McLaughlin DF, Perkins JG, et al. Increased mortality associated with the early coagulopathy of trauma in combat casualties. J Trauma. 2008;64:1459–1463. doi: 10.1097/TA.0b013e318174e8bc. discussion 1463-5. [DOI] [PubMed] [Google Scholar]
- 15.Stinger HK, Spinella PC, Perkins JG, et al. The ratio of fibrinogen to red cells transfused affects survival in casualties receiving massive transfusions at an army combat support hospital. J Trauma. 2008;64(2 Suppl):S79–S85. doi: 10.1097/TA.0b013e318160a57b. discussion S85. [DOI] [PubMed] [Google Scholar]
- 16•.Sperry JL, Ochoa JB, Gunn SR, et al. An FFP:PRBC transfusion ratio >/ =1:1.5 is associated with a lower risk of mortality after massive transfusion. J Trauma. 2008;65:986–993. doi: 10.1097/TA.0b013e3181878028. Improved survival in blunt trauma patients who achieved a high ratio of plasma to red blood cells. [DOI] [PubMed] [Google Scholar]
- 17.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–1130. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 18.MacLeod JB, Lynn M, McKenney MG, et al. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55:39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 19.Maegele M, Lefering R, Yucel N, et al. Early coagulopathy in multiple injury: an analysis from the German Trauma Registry on 8724 patients. Injury. 2007;38:298–304. doi: 10.1016/j.injury.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Rugeri L, Levrat A, David JS, et al. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. J Thromb Haemost. 2007;5:289–295. doi: 10.1111/j.1538-7836.2007.02319.x. [DOI] [PubMed] [Google Scholar]
- 21.Tieu BH, Holcomb JB, Schreiber MA. Coagulopathy: its pathophysiology and treatment in the injured patient. World J Surg. 2007;31:1055–1064. doi: 10.1007/s00268-006-0653-9. [DOI] [PubMed] [Google Scholar]
- 22.Hess JR, Brohi K, Dutton RP, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. 2008;65:748–754. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 23.McLaughlin DF, Niles SE, Salinas J, et al. A predictive model for massive transfusion in combat casualty patients. J Trauma. 2008;64(2 Suppl):S57–S63. doi: 10.1097/TA.0b013e318160a566. discussion S63. [DOI] [PubMed] [Google Scholar]
- 24.Schreiber MA, Perkins J, Kiraly L, et al. Early predictors of massive transfusion in combat casualties. J Am Coll Surg. 2007;205:541–545. doi: 10.1016/j.jamcollsurg.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Yucel N, Lefering R, Maegele M, et al. Trauma Associated Severe Hemorrhage (TASH)-Score: probability of mass transfusion as surrogate for life threatening hemorrhage after multiple trauma. J Trauma. 2006;60:1228–1236. doi: 10.1097/01.ta.0000220386.84012.bf. discussion 1236-7. [DOI] [PubMed] [Google Scholar]
- 26.Kuhne CA, Zetti RP, Fischbacher M, et al. Emergency Transfusion Score (ETS): a useful instrument for prediction of blood transfusion requirement in severely injured patients. World J Surg. 2008;32:1183–1188. doi: 10.1007/s00268-007-9425-4. [DOI] [PubMed] [Google Scholar]
- 27.Nunez TC, Voskresensky IV, Dossett LA, et al. Early prediction of massive transfusion in trauma: simple as ABC (assessment of blood consumption)? J Trauma. 2009;66:346–352. doi: 10.1097/TA.0b013e3181961c35. [DOI] [PubMed] [Google Scholar]
- 28.Armand R, Hess JR. Treating coagulopathy in trauma patients. Transfus Med Rev. 2003;17:223–231. doi: 10.1016/s0887-7963(03)00022-1. [DOI] [PubMed] [Google Scholar]
- 29.Repine TB, Perkins JG, Kauvar DS, Blackbourne L. The use of fresh whole blood in massive transfusion. J Trauma. 2006;60(6 Suppl):S59–S69. doi: 10.1097/01.ta.0000219013.64168.b2. [DOI] [PubMed] [Google Scholar]
- 30•.Spinella PC. Warm fresh whole blood transfusion for severe hemorrhage: U.S. military and potential civilian applications. Crit Care Med. 2008;36(7 Suppl):S340–S345. doi: 10.1097/CCM.0b013e31817e2ef9. Retrospective review of fresh whole blood transfusion in current conflicts demonstrating safety of its use and potential benefits compared to component therapy. [DOI] [PubMed] [Google Scholar]
- 31.Beekley AC. Damage control resuscitation: a sensible approach to the exsanguinating surgical patient. Crit Care Med. 2008;36:S267–S274. doi: 10.1097/CCM.0b013e31817da7dc. [DOI] [PubMed] [Google Scholar]
- 32.O'Shaughnessy DF, Atterbury C, Bolton Maggs P, et al. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol. 2004;126:11–28. doi: 10.1111/j.1365-2141.2004.04972.x. [DOI] [PubMed] [Google Scholar]
- 33.Stainsby D, MacLennan S, Hamilton PJ. Guidelines on the management of massive blood loss. Br J Haematol. 2006;135:634–641. doi: 10.1111/j.1365-2141.2006.06355.x. [DOI] [PubMed] [Google Scholar]
- 34.Duchesne JC, Barbeau JM, Islam TM, et al. eastern association for the surgery of trauma. Lake Buena Vista, Florida: 2009. Damage control resuscitation: making sense out of non-sense. [Google Scholar]
- 35.Eder AF, Benjamin RJ. TRALI risk reduction: donor and component management strategies. J Clin Apher. 2009;24:122–129. doi: 10.1002/jca.20198. [DOI] [PubMed] [Google Scholar]
- 36.Valeri CR. Blood components in the treatment of acute blood loss: use of freeze-preserved red cells, platelets, and plasma proteins. Anesth Analg. 1975;54:1–14. doi: 10.1213/00000539-197501000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Nilsson L, Hedner U, Nilsson IM, Robertson B. Shelf-life of bank blood and stored plasma with special reference to coagulation factors. Transfusion. 1983;23:377–381. doi: 10.1046/j.1537-2995.1983.23584018713.x. [DOI] [PubMed] [Google Scholar]
- 38.Duchesne JC, Holcomb JB. Damage control resuscitation: addressing trauma-induced coagulopathy. Br J Hosp Med (Lond) 2009;70:22–25. doi: 10.12968/hmed.2009.70.1.37690. [DOI] [PubMed] [Google Scholar]
- 39.Aster RH, Jandl JH. Platelet sequestration in man. I Methods. J Clin Invest. 1964;43:843–855. doi: 10.1172/JCI104970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bishop JF, McGrath K, Wolf MM, et al. Clinical factors influencing the efficacy of pooled platelet transfusions. Blood. 1988;71:383–387. [PubMed] [Google Scholar]
- 41.Martinowitz U, Kenet G, Segal E, et al. Recombinant activated factor VII for adjunctive hemorrhage control in trauma. J Trauma. 2001;51:431–438. doi: 10.1097/00005373-200109000-00002. discussion 438-9. [DOI] [PubMed] [Google Scholar]
- 42.Holcomb JB. Use of recombinant activated factor VII to treat the acquired coagulopathy of trauma. J Trauma. 2005;58:1298–1303. doi: 10.1097/01.ta.0000169871.29748.95. [DOI] [PubMed] [Google Scholar]
- 43.Spinella PC, Perkins JG, McLaughlin DF, et al. The effect of recombinant activated factor VII on mortality in combat-related casualties with severe trauma and massive transfusion. J Trauma. 2008;64:286–293. doi: 10.1097/TA.0b013e318162759f. discussion 293-4. [DOI] [PubMed] [Google Scholar]
- 44.Boffard KD, Riou B, Warren B, et al. Recombinant factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: two parallel randomized, placebo-controlled, double-blind clinical trials. J Trauma. 2005;59:8–15. doi: 10.1097/01.ta.0000171453.37949.b7. discussion 15-8. [DOI] [PubMed] [Google Scholar]
- 45.Stein DM, Dutton RP, Hess JR, Scalea TM. Low-dose recombinant factor VIIa for trauma patients with coagulopathy. Injury. 2008;39:1054–1061. doi: 10.1016/j.injury.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 46.Timberlake GA, McSwain NE., Jr Autotransfusion of blood contaminated by enteric contents: a potentially life-saving measure in the massively hemorrhaging trauma patient? J Trauma. 1988;28:855–857. doi: 10.1097/00005373-198806000-00026. [DOI] [PubMed] [Google Scholar]
- 47.Ozmen V, McSwain NE, Jr, Nichols RL, et al. Autotransfusion of potentially culture-positive blood (CPB) in abdominal trauma: preliminary data from a prospective study. J Trauma. 1992;32:36–39. doi: 10.1097/00005373-199201000-00008. [DOI] [PubMed] [Google Scholar]
- 48.Smith LA, Barker DE, Burns RP. Autotransfusion utilization in abdominal trauma. Am Surg. 1997;63:47–49. [PubMed] [Google Scholar]
- 49.Bowley DM, Barker P, Boffard KD. Intraoperative blood salvage in penetrating abdominal trauma: a randomised, controlled trial. World J Surg. 2006;30:1074–1080. doi: 10.1007/s00268-005-0466-2. [DOI] [PubMed] [Google Scholar]
- 50.Hess JR, Zimrin AB. Massive blood transfusion for trauma. Curr Opin Hematol. 2005;12:488–492. doi: 10.1097/01.moh.0000177828.85904.70. [DOI] [PubMed] [Google Scholar]
- 51.Fox CJ, Gillespie DL, Cox ED, et al. The effectiveness of a damage control resuscitation strategy for vascular injury in a combat support hospital: results of a case control study. J Trauma. 2008;64(2 Suppl):S99–S106. doi: 10.1097/TA.0b013e3181608c4a. discussion S106-7. [DOI] [PubMed] [Google Scholar]
- 52•.O'Keeffe T, Refaai M, Tchorz K, et al. A massive transfusion protocol to decrease blood component use and costs. Arch Surg. 2008;143:686–690. doi: 10.1001/archsurg.143.7.686. discussion 690-1. Demonstrated decrease blood usage after implementation of a massive transfusion protocol without improved survival. [DOI] [PubMed] [Google Scholar]
- 53.McConaughey E. Crew resource management in healthcare: the evolution of teamwork training and MedTeams. J Perinat Neonatal Nurs. 2008;22:96–104. doi: 10.1097/01.JPN.0000319095.59673.6c. [DOI] [PubMed] [Google Scholar]
- 54.Helmreich RL, Merritt AC, Wilhelm JA. The evolution of Crew Resource Management training in commercial aviation. Int J Aviat Psychol. 1999;9:19–32. doi: 10.1207/s15327108ijap0901_2. [DOI] [PubMed] [Google Scholar]
- 55.Stone HH, Strom PR, Mullins RJ. Management of the major coagulopathy with onset during laparotomy. Ann Surg. 1983;197:532–535. doi: 10.1097/00000658-198305000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rotondo MF, Schwab CW, McGonigal MD, et al. ‘Damage control’: an approach for improved survival in exsanguinating penetrating abdominal injury. J Trauma. 1993;35:375–382. discussion 382-3. [PubMed] [Google Scholar]
- 57.Hirshberg A, Dugas M, Banez EI, et al. Minimizing dilutional coagulopathy in exsanguinating hemorrhage: a computer simulation. J Trauma. 2003;54:454–463. doi: 10.1097/01.TA.0000053245.08642.1F. [DOI] [PubMed] [Google Scholar]
- 58.Lucas CE, Ledgerwood AM, Saxe JM, et al. Plasma supplementation is beneficial for coagulation during severe hemorrhagic shock. Am J Surg. 1996;171:399–404. doi: 10.1016/S0002-9610(97)89618-3. [DOI] [PubMed] [Google Scholar]
- 59.Bormanis J. Development of a massive transfusion protocol. Transfus Apher Sci. 2008;38:57–63. doi: 10.1016/j.transci.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 60.Stainsby D, MacLennan S, Hamilton PJ. Management of massive blood loss: a template guideline. Br J Anaesth. 2000;85:487–491. doi: 10.1093/bja/85.3.487. [DOI] [PubMed] [Google Scholar]
- 61.Ho AM, Dion PW, Cheng CA, et al. A mathematical model for fresh frozen plasma transfusion strategies during major trauma resuscitation with ongoing hemorrhage. Can J Surg. 2005;48:470–478. [PMC free article] [PubMed] [Google Scholar]
- 62.Ho AM, Karmakar MK, Dion PW. Are we giving enough coagulation factors during major trauma resuscitation? Am J Surg. 2005;190:479–484. doi: 10.1016/j.amjsurg.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 63••.Holcomb JB, Wade CE, Michalek JE, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–458. doi: 10.1097/SLA.0b013e318185a9ad. Restrospective review of 16 civilian level I trauma centers demonstrating improved survival when high ratio of plasma to red blood cells is achieved. [DOI] [PubMed] [Google Scholar]
- 64.Maegele M, Lefering R, Paffrath T, et al. Red-blood-cell to plasma ratios transfused during massive transfusion are associated with mortality in severe multiple injury: a retrospective analysis from the Trauma Registry of the Deutsche Gesellschaft fur Unfallchirurgie. Vox Sang. 2008;95:112–119. doi: 10.1111/j.1423-0410.2008.01074.x. [DOI] [PubMed] [Google Scholar]
- 65.Kashuk JL, Moore EE, Johnson JL, et al. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008;65:261–270. doi: 10.1097/TA.0b013e31817de3e1. discussion 270-1. [DOI] [PubMed] [Google Scholar]
- 66.Cotton BA, Au BK, Nunez TC, et al. Predefined massive transfusion protocols are associated with a reduction in organ failure and postinjury complications. J Trauma. 2009;66:41–48. doi: 10.1097/TA.0b013e31819313bb. discussion 48-9. [DOI] [PubMed] [Google Scholar]
- 67.Fox CJ, Gillespie DL, Cox ED, et al. Damage control resuscitation for vascular surgery in a combat support hospital. J Trauma. 2008;65:1–9. doi: 10.1097/TA.0b013e318176c533. [DOI] [PubMed] [Google Scholar]
- 68.Eastridge BJ, Jenkins D, Flaherty S, et al. Trauma system development in a theater of war: experiences from Operation Iraqi Freedom and Operation Enduring Freedom. J Trauma. 2006;61:1366–1372. doi: 10.1097/01.ta.0000245894.78941.90. discussion 1372-3. [DOI] [PubMed] [Google Scholar]


